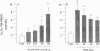Abstract
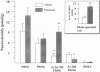
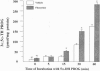
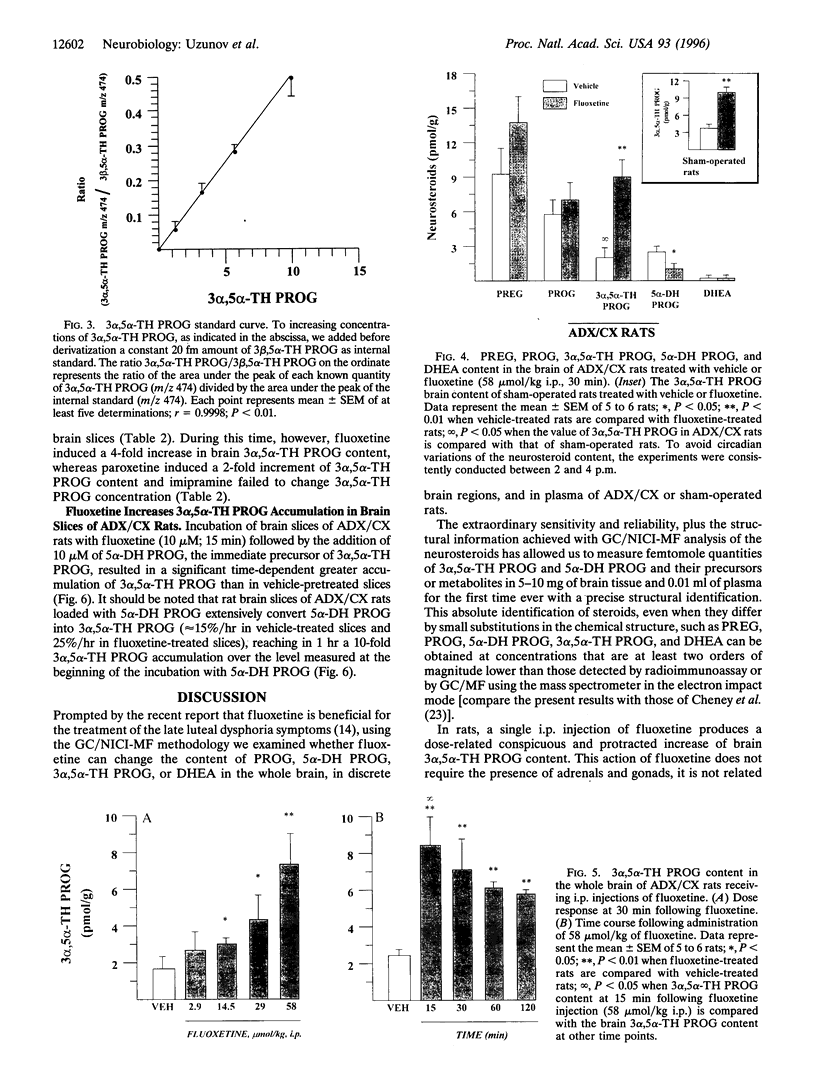
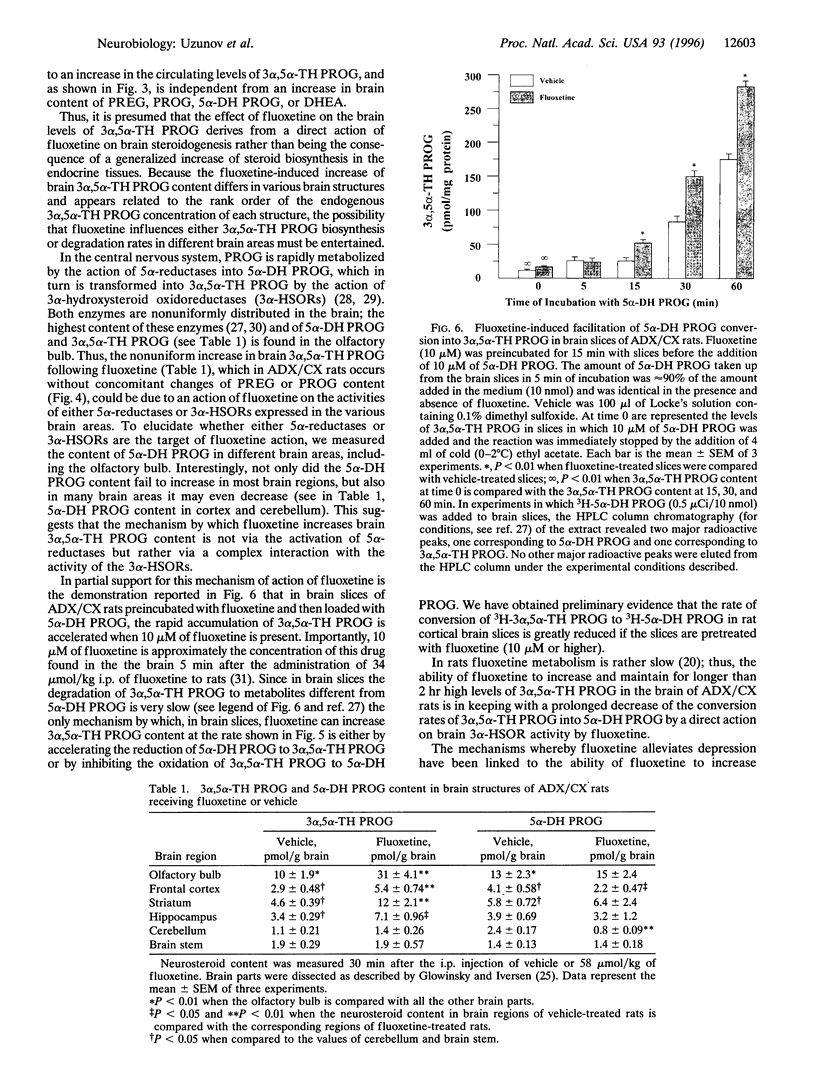
Fluoxetine administered intraperitoneally to sham-operated or adrenalectomized/castrated (ADX/CX) male rats dose-dependently (2.9-58 mumol/kg i.p.) increased the brain content of the neurosteroid 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone, 3 alpha, 5 alpha-TH PROG). The increase of brain 3 alpha, 5 alpha-TH PROG content elicited by 58 mumol/kg fluoxetine lasted more than 2 hr and the range of its extent was comparable in sham-operated (approximately 3-10 pmol/g) and ADX/CX rats (2-9 pmol/g) and was associated with a decrease (from 2.8 to 1.1 pmol/g) in the 5 alpha-pregnan-3,20-dione (5 alpha-dihydroprogesterone, 5 alpha-DH PROG) content. The pregnenolone, progesterone, and dehydroepiandrosterone content failed to change in rats receiving fluoxetine. The extent of 3 alpha, 5 alpha-TH PROG accumulation elicited by fluoxetine treatment differed in various brain regions, with the highest increase occurring in the olfactory bulb. Importantly, fluoxetine failed to change the 3 alpha, 5 alpha-TH PROG levels in plasma, which in ADX/CX rats were at least two orders of magnitude lower than in the brain. Two other serotonin re-uptake inhibitors, paroxetine and imipramine, in doses equipotent to those of fluoxetine in inhibiting brain serotonin uptake, were either significantly less potent than fluoxetine (paroxetine) or failed to increase (imipramine) 3 alpha, 5 alpha-TH PROG brain content. The addition of 10 microM of 5 alpha-DH PROG to brain slices of ADX/CX rats preincubated with fluoxetine (10 microM, 15 min) elicited an accumulation of 3 alpha, 5 alpha-TH PROG greater than in slices preincubated with vehicle. A fluoxetine stimulation of brain 3 alpha, 5 alpha-TH PROG biosynthesis might be operative in the anxiolytic and antidysphoric actions of this drug.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulieu E. E., Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990 Nov 20;37(3):395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- Caccia S., Bizzi A., Coltro G., Fracasso C., Frittoli E., Mennini T., Garattini S. Anorectic activity of fluoxetine and norfluoxetine in rats: relationship between brain concentrations and in-vitro potencies on monoaminergic mechanisms. J Pharm Pharmacol. 1992 Mar;44(3):250–254. doi: 10.1111/j.2042-7158.1992.tb03592.x. [DOI] [PubMed] [Google Scholar]
- Cheney D. L., Uzunov D., Costa E., Guidotti A. Gas chromatographic-mass fragmentographic quantitation of 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone) and its precursors in blood and brain of adrenalectomized and castrated rats. J Neurosci. 1995 Jun;15(6):4641–4650. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpéchot C., Young J., Calvel M., Wehrey C., Veltz J. N., Touyer G., Mouren M., Prasad V. V., Banner C., Sjövall J. Neurosteroids: 3 alpha-hydroxy-5 alpha-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993 Sep;133(3):1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Costa E., Cheney D. L., Grayson D. R., Korneyev A., Longone P., Pani L., Romeo E., Zivkovich E., Guidotti A. Pharmacology of neurosteroid biosynthesis. Role of the mitochondrial DBI receptor (MDR) complex. Ann N Y Acad Sci. 1994 Nov 30;746:223–242. doi: 10.1111/j.1749-6632.1994.tb39240.x. [DOI] [PubMed] [Google Scholar]
- Friedman L., Gibbs T. T., Farb D. H. Gamma-aminobutyric acidA receptor regulation: chronic treatment with pregnanolone uncouples allosteric interactions between steroid and benzodiazepine recognition sites. Mol Pharmacol. 1993 Jul;44(1):191–197. [PubMed] [Google Scholar]
- Fuller R. W., Wong D. T., Robertson D. W. Fluoxetine, a selective inhibitor of serotonin uptake. Med Res Rev. 1991 Jan;11(1):17–34. doi: 10.1002/med.2610110103. [DOI] [PubMed] [Google Scholar]
- Gee K. W., McCauley L. D., Lan N. C. A putative receptor for neurosteroids on the GABAA receptor complex: the pharmacological properties and therapeutic potential of epalons. Crit Rev Neurobiol. 1995;9(2-3):207–227. [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Khanna M., Qin K. N., Cheng K. C. Distribution of 3 alpha-hydroxysteroid dehydrogenase in rat brain and molecular cloning of multiple cDNAs encoding structurally related proteins in humans. J Steroid Biochem Mol Biol. 1995 Jun;53(1-6):41–46. doi: 10.1016/0960-0760(95)00019-v. [DOI] [PubMed] [Google Scholar]
- Khanna M., Qin K. N., Wang R. W., Cheng K. C. Substrate specificity, gene structure, and tissue-specific distribution of multiple human 3 alpha-hydroxysteroid dehydrogenases. J Biol Chem. 1995 Aug 25;270(34):20162–20168. doi: 10.1074/jbc.270.34.20162. [DOI] [PubMed] [Google Scholar]
- Koenig H. L., Schumacher M., Ferzaz B., Thi A. N., Ressouches A., Guennoun R., Jung-Testas I., Robel P., Akwa Y., Baulieu E. E. Progesterone synthesis and myelin formation by Schwann cells. Science. 1995 Jun 9;268(5216):1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- Korneyev A., Guidotti A., Costa E. Regional and interspecies differences in brain progesterone metabolism. J Neurochem. 1993 Dec;61(6):2041–2047. doi: 10.1111/j.1471-4159.1993.tb07440.x. [DOI] [PubMed] [Google Scholar]
- Korneyev A., Pan B. S., Polo A., Romeo E., Guidotti A., Costa E. Stimulation of brain pregnenolone synthesis by mitochondrial diazepam binding inhibitor receptor ligands in vivo. J Neurochem. 1993 Oct;61(4):1515–1524. doi: 10.1111/j.1471-4159.1993.tb13647.x. [DOI] [PubMed] [Google Scholar]
- Lambert J. J., Belelli D., Hill-Venning C., Peters J. A. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995 Sep;16(9):295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Demirgören S., Spivak C. E., London E. D. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990 Aug 27;526(1):143–146. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Harrison N. L., Schwartz R. D., Barker J. L., Paul S. M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986 May 23;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mienville J. M., Vicini S. Pregnenolone sulfate antagonizes GABAA receptor-mediated currents via a reduction of channel opening frequency. Brain Res. 1989 Jun 5;489(1):190–194. doi: 10.1016/0006-8993(89)90024-3. [DOI] [PubMed] [Google Scholar]
- Paul S. M., Purdy R. H. Neuroactive steroids. FASEB J. 1992 Mar;6(6):2311–2322. [PubMed] [Google Scholar]
- Puia G., Santi M. R., Vicini S., Pritchett D. B., Purdy R. H., Paul S. M., Seeburg P. H., Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990 May;4(5):759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Rupprecht R., Reul J. M., Trapp T., van Steensel B., Wetzel C., Damm K., Zieglgänsberger W., Holsboer F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993 Sep;11(3):523–530. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- Shaskan E. G., Snyder S. H. Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther. 1970 Nov;175(2):404–418. [PubMed] [Google Scholar]
- Steiner M., Steinberg S., Stewart D., Carter D., Berger C., Reid R., Grover D., Streiner D. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med. 1995 Jun 8;332(23):1529–1534. doi: 10.1056/NEJM199506083322301. [DOI] [PubMed] [Google Scholar]
- Wang M., Seippel L., Purdy R. H., Bãckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J Clin Endocrinol Metab. 1996 Mar;81(3):1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- Yu R., Ticku M. K. Chronic neurosteroid treatment produces functional heterologous uncoupling at the gamma-aminobutyric acid type A/benzodiazepine receptor complex in mammalian cortical neurons. Mol Pharmacol. 1995 Mar;47(3):603–610. [PubMed] [Google Scholar]