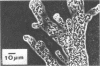
Abstract
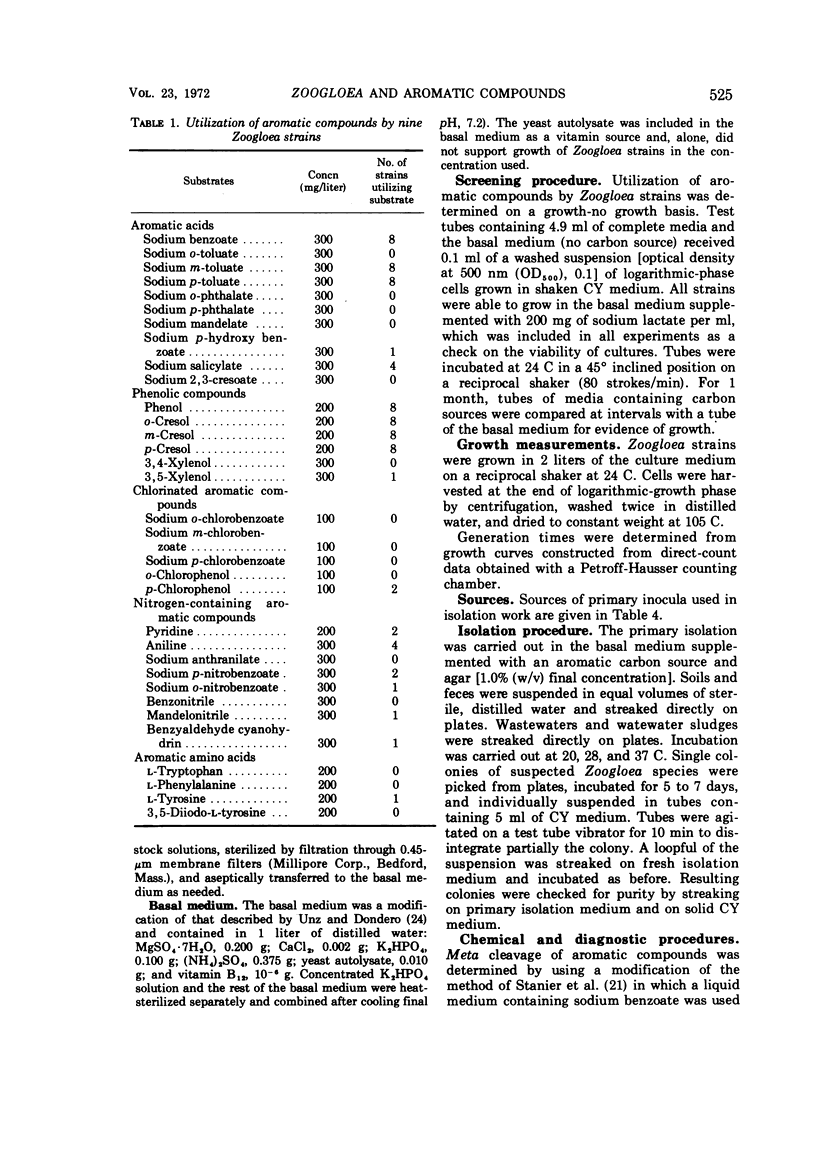
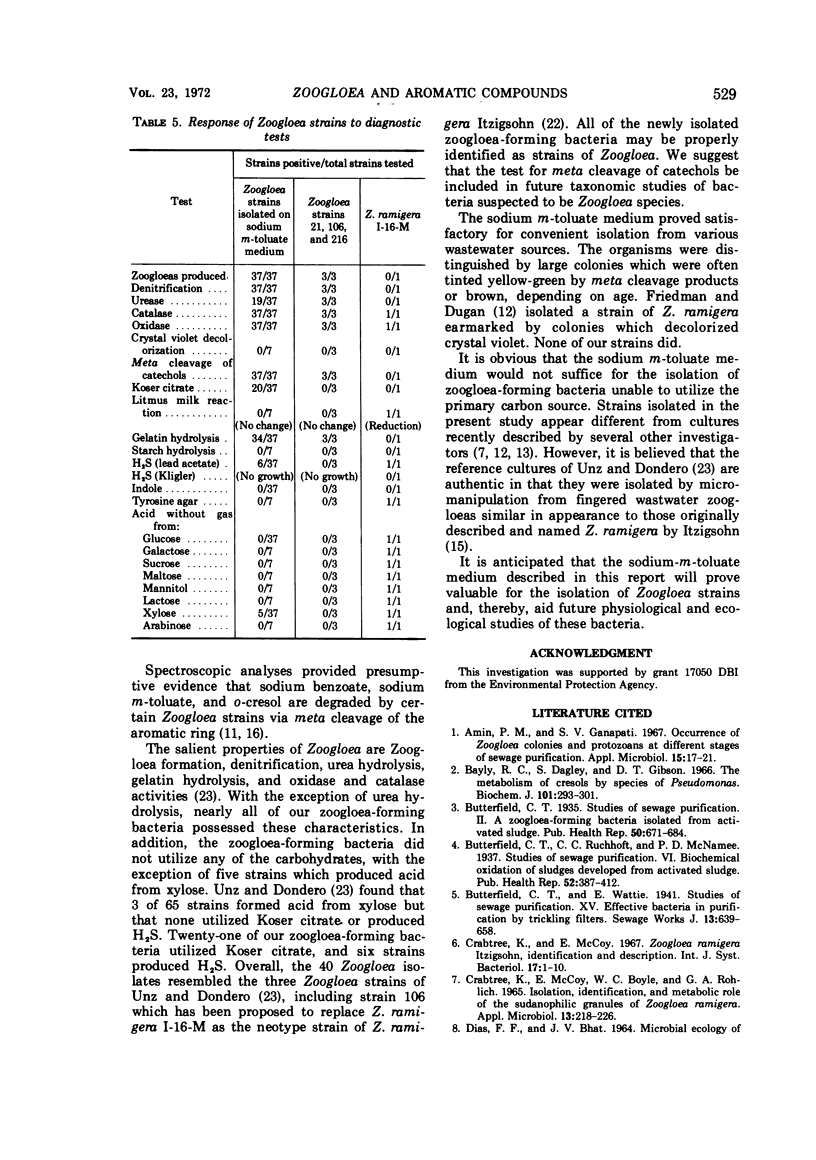
Nine Zoogloea strains, were examined for their ability to utilize 35 aromatic compounds. Benzoate, m-toluate, and p-toluate, as well as phenol, o-cresol, m-cresol, and p-cresol, were utilized by eight strains. These strains exhibited meta cleavage of catechol and of methyl-substituted catechols. With the exception of L-tyrosine, none of the aromatic compounds tested supported growth of Z. ramigera ATCC 19623. A medium containing sodium m-toluate was used to isolate 37 zoogloea-forming bacteria from various polluted environments. The isolates were identified as strains of Zoogloea.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin P. M., Ganapati S. V. Occurrence of zoogloea colonies and protozoans at different stages of sewage purification. Appl Microbiol. 1967 Jan;15(1):17–21. doi: 10.1128/am.15.1.17-21.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABTREE K., MCCOY E., BOYLE W. C., ROHLICH G. A. ISOLATION, IDENTIFICATION, AND METABOLIC ROLE OF THE SUDANOPHILIC GRANULES OF ZOOGLOEA RAMIGERA. Appl Microbiol. 1965 Mar;13:218–226. doi: 10.1128/am.13.2.218-226.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONDERO N. C. SIMPLE AND RAPID METHOD FOR DEMONSTRATING MICROBIAL CAPSULES BY PHASE-CONTRAST MICROSCOPY. J Bacteriol. 1963 May;85:1171–1173. doi: 10.1128/jb.85.5.1171-1173.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGAN P. R., LUNDGREN D. G. Isolation of the floc-forming organism Zoogloea ramigera and its culture in complex and synthetic media. Appl Microbiol. 1960 Nov;8:357–361. doi: 10.1128/am.8.6.357-361.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr D. R., Cain R. B. Catechol oxygenase induction in Pseudomonas aeruginosa. Biochem J. 1968 Feb;106(4):879–885. doi: 10.1042/bj1060879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B. A., Dugan P. R. Identification of Zoogloea species and the relationship to zoogloeal matrix and floc formation. J Bacteriol. 1968 May;95(5):1903–1909. doi: 10.1128/jb.95.5.1903-1909.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOJIMA Y., ITADA N., HAYAISHI O. Metapyrocatachase: a new catechol-cleaving enzyme. J Biol Chem. 1961 Aug;236:2223–2228. [PubMed] [Google Scholar]
- Public Health Weekly Reports for APRIL 2, 1937. Public Health Rep. 1937 Apr 2;52(14):387–426. [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Unz R. F., Dondero N. C. The predominant bacteria in natural zoogloeal colonies. I. Isolation and identification. Can J Microbiol. 1967 Dec;13(12):1671–1682. doi: 10.1139/m67-217. [DOI] [PubMed] [Google Scholar]
- Unz R. F., Dondero N. C. The predominant bacteria in natural zoogloeal colonies. II. Physiology and nutrition. Can J Microbiol. 1967 Dec;13(12):1683–1694. doi: 10.1139/m67-218. [DOI] [PubMed] [Google Scholar]