Abstract
In many organisms, the methylation of cytosine in DNA has a key role in silencing ‘parasitic’ DNA elements, regulating transcription and establishing cellular identity. The recent discovery that ten-eleven translocation (TET) proteins are 5-methylcytosine oxidases has provided several chemically plausible pathways for the reversal of DNA methylation, thus triggering a paradigm shift in our understanding of how changes in DNA methylation are coupled to cell differentiation, embryonic development and cancer.
Since the initial description of their enzymatic activity in 2009 (REFS 1,2), proteins of the TET family have become a focus of substantial interest. TET proteins are named after the ten-eleven translocation (t(10;11)(q22;q23)) that is found in rare cases of acute myeloid and lymphocytic leukaemia. This translocation fuses the mixed-lineage leukaemia 1 (MLL1) gene located on human chromosome 10 with the TET1 gene on human chromosome 11 (REFS 3,4). The three mammalian TET proteins, namely TET1, TET2 and TET3, are Fe2+- and 2-oxoglutarate-dependent dioxygenases that successively oxidize 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) in DNA1,2,5,6 (FIG. 1a). All three forms of oxidized methylcytosine are now known to be present in numerous mammalian tissues1,5,7–9. TET proteins have roles in diverse biological processes, including epigenetic regulation of gene transcription, embryonic development, stem cell function and cancer, but the mechanisms underlying these roles are still poorly defined.
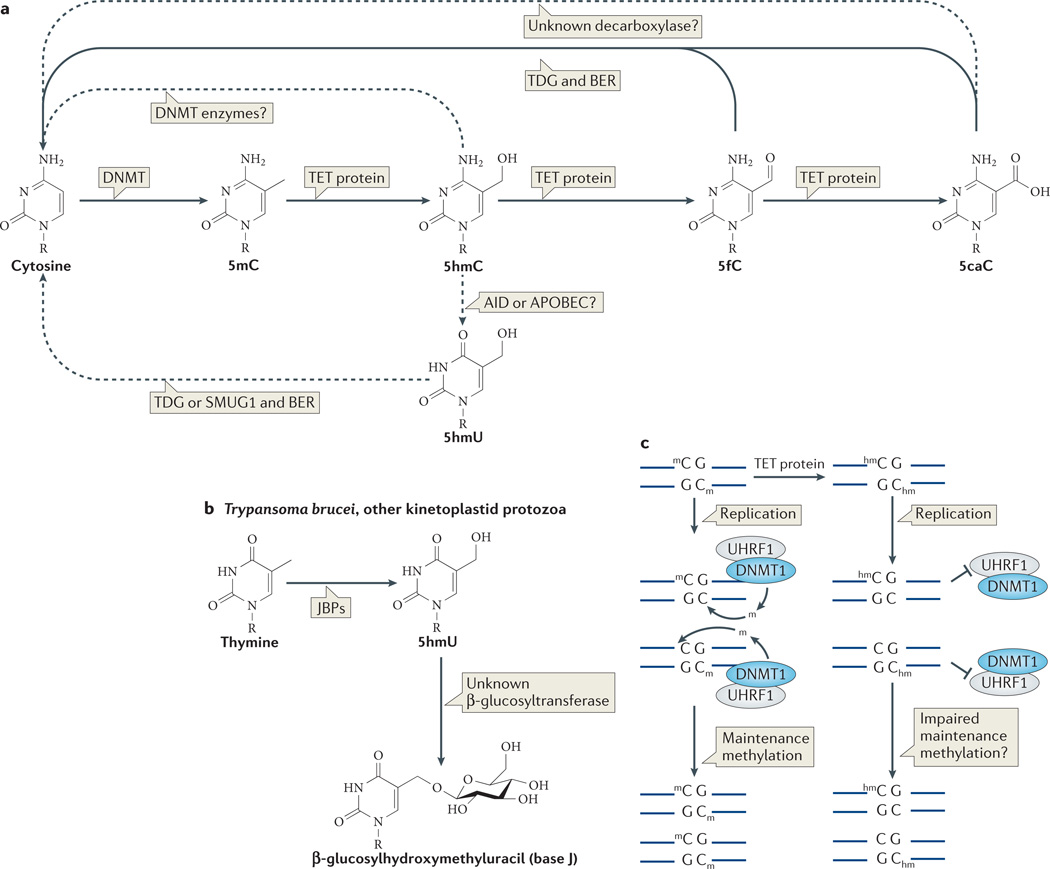
Figure 1. Mechanisms of TET-mediated demethylation.
a | Known and putative pathways of DNA demethylation that involve oxidized methylcytosine intermediates. Ten-eleven translocation (TET) proteins sequentially oxidize 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC). 5fC and 5caC can be removed by thymine DNA glycosylase (TDG) and replaced by cytosine via base excision repair (BER), although the extent to which this mechanism operates in specific cell types during development is unknown. Other proposed mechanisms of demethylation are less well established, including decarboxylation of 5caC, DNA methyltransferase (DNMT)-mediated removal of the hydroxymethyl group of 5hmC and deamination of 5hmC (and 5mC) (see main text) by the cytidine deaminases AID (activation-induced cytidine deaminase) and APOBEC (apolipoprotein B mRNA editing enzyme, catalytic polypeptide). AID enzymes deaminate cytosine bases in DNA to yield uracil. AID and the larger family of APOBEC enzymes have been proposed to effect DNA demethylation by deaminating 5mC and 5hmC in DNA to yield thymine and 5hmU, respectively. As these are present in mismatched T:G and 5hmU:G basepairs, they have been proposed to be excised by SMUG1 (single-strand-selective monofunctional uracil DNA glycosylase) or TDG. This mechanism is controversial, however (see main text). b | The mechanism of base J (β-d-glucosyl-hydroxymethyluracil) biosynthesis. The thymidine oxidation step mediated by J-binding protein 1 (JBP1) or JBP2, to produce 5-hydroxyuracil (5hmU), is analogous to the 5mC oxidation mediated by TET proteins. JBPs are the founding members of the TET–JBP superfamily: the predicted oxygenase domains of JBP1 and JBP2 were used as the starting point for the sequence profile searches that recovered the homologous domains of the three mammalian TET proteins. c | Mechanism by which 5hmC could facilitate replication-dependent DNA demethylation. A symmetrically-methylate d CpG sequence is converted during DNA replication into two asymmetrically methylated DNA strands (left panel). Hemimethylated CpG sites are recognized by UHRFI, the obligate partner of the maintenance DNA methyltransferase DNMT1, which restores symmetrical methylation. TET proteins act at methylated CpG sites to generate symmetrically hydroxymethylated CpG sequences. 5hmC and other oxizided methylcytosines may impair maintenance methylation by inhibiting UHRF1 binding, DNMT1 activity, or both (right panel). As a result, the CpG sequence progressively loses DNA methylation through successive DNA replication cycles.
Here, we review our current understanding of TET enzymes and their biological functions, focusing on recent studies not covered in previous reviews10–14. We discuss the established and controversial roles of TET proteins and methylcytosine oxidation products in DNA demethylation, gene regulation and embryonic development. The role of TET proteins in haematopoietic differentiation and oncogenesis has been reviewed elsewhere10,15–17; their role in neurons is only briefly discussed here owing to space limitations.
TET proteins are 5mC oxidases
The prediction that TET proteins might have DNA-modifying activity was based on the analysis of J-binding protein 1 (JBP1) and JBP2 from Trypanosoma brucei, the causative parasite of African sleeping sickness in humans18. Leishmania species, trypanosomes such as T.brucei and other kinetoplastids contain a modified thymine known as ‘base J’ (β-d-glucosyl-hydroxymethyluracil)19. JBP1 and JBP2 generate base J by oxidizing the methyl group of thymine to yield 5-hydroxymethyluracil, and the resulting hydroxyl group is then glucosylated by an unknown glucosyltransferase20 (FIG. 1b). JBP enzymes are members of a large family of Fe2+- and 2-oxoglutarate-dependent dioxygenases21,22; AlkB enzymes, which remove aberrant methylation from damaged DNA bases by an oxidative mechanism, are distantly related members of the same superfamily21–23. In T.brucei , base J is present at subtelomeric repeats, at inactive copies of the variant surface glycoprotein that is used by the parasite to evade host immune defence and at other silenced regions of the genome24. In Leishmania species, base J was recently shown to restrain elongation of the unique polycistronic transcripts of kineto plastids beyond transcription stop sites25.
Computational screens to identify additional homologues of JBP enzymes revealed a large family of predicted nucleic acid-modifying dioxygenases from diverse eukaryotes and bacteriophages, which included the metazoan TET enzymes2,18. A gene encoding an enzyme of the TET–JBP family entered the common ancestor of the metazoan lineage and fused with a second gene containing a CXXC domain (described below), forming the TET subfamily2. TET enzymes are present in all metazoans that have retained cytosine methylation but are absent in organisms such as Caenorhabditis elegans in which methylation has been unambiguously lost2. The coexistence of TET proteins with DNA methylation, their association with CXXC domains which frequently bind unmethylated CpG sequences (see below) and the chemical similarity of thymine and 5mC oxidation all led to the proposal that TET proteins might function as 5mC oxidases and potentially as DNA demethylases2,18.
Indeed, ectopic expression of TET proteins in cell lines reduces 5mC levels and causes the appearance of 5hmC, and this activity is abrogated by mutation of the signature His-Xaa-Asp motif (where Xaa represents any amino acid) of these dioxygenases1,26,27 (see below). Recombinant TET catalytic domains and full-length TET proteins efficiently convert 5mC to 5hmC in vitro in the presence of the essential cofactors 2-oxoglutarate and Fe2+ (REFS 1,27). TET proteins also produce the further oxidation products 5fC and 5caC5,6,28 (FIG. 1a). Thus, the successive actions of DNA methyltransferases (DNMTs) and TET proteins produce four distinct cytosine modifications, bringing the total number of cytosine species to five (FIG. 1a).
5hmC is found at different levels in mammalian cells: it is present at 1% of the total level of 5mC in some immune cell populations26, ~5–10% of the level of 5mC in embryonic stem (ES) cells1 and as high as 40% of 5mC in Purkinje neurons9. Consistently, the highest reported levels of 5hmC are in the brain5,7,8. An early report of 5hmC in mammalian DNA29 is questionable, as in this study 5mC was not detected in mouse brain and liver DNA, whereas the level of 5hmC was unrealistically high (15–17% of all cytosines), suggesting massive oxidation of 5mC during the unconventional DNA extraction procedure devised by this group29. 5fC and 5caC are present in mammalian cells at much lower levels than 5hmC (0.03% and 0.01%, respectively, of the level of 5mC in mouse ES cells)5,6,28, at least partly because there are enzymatic mechanisms for their removal. These include base excision by thymine DNA glycosylase (TDG)6,30 and possibly decarboxylation of 5caC by unknown enzymes present in ES cell lysates31 (FIG. 1a).
Domain structure of metazoan TETs
Most metazoan TET proteins contain an amino-terminal CXXC domain (~60 amino acids) and a carboxy-termina l catalytic domain (FIG. 2a), the core of which adopts a double-stranded β-helix (DSBH) fold1,2. Metazoan TET proteins can be distinguished from all other members of the TET–JBP family by a Cys-rich domain in the N-terminal region of the DSBH domain. In jawed vertebrates, the TET gene underwent triplication, giving rise to TET1, TET2 and TET3. TET2 then underwent a chromosomal inversion event in which the exon containing the CXXC domain was detached and became a separate gene that encodes IDAX (inhibition of the Dvl and axin complex; also known as CXXC4)2,32 (FIG. 2a, b).
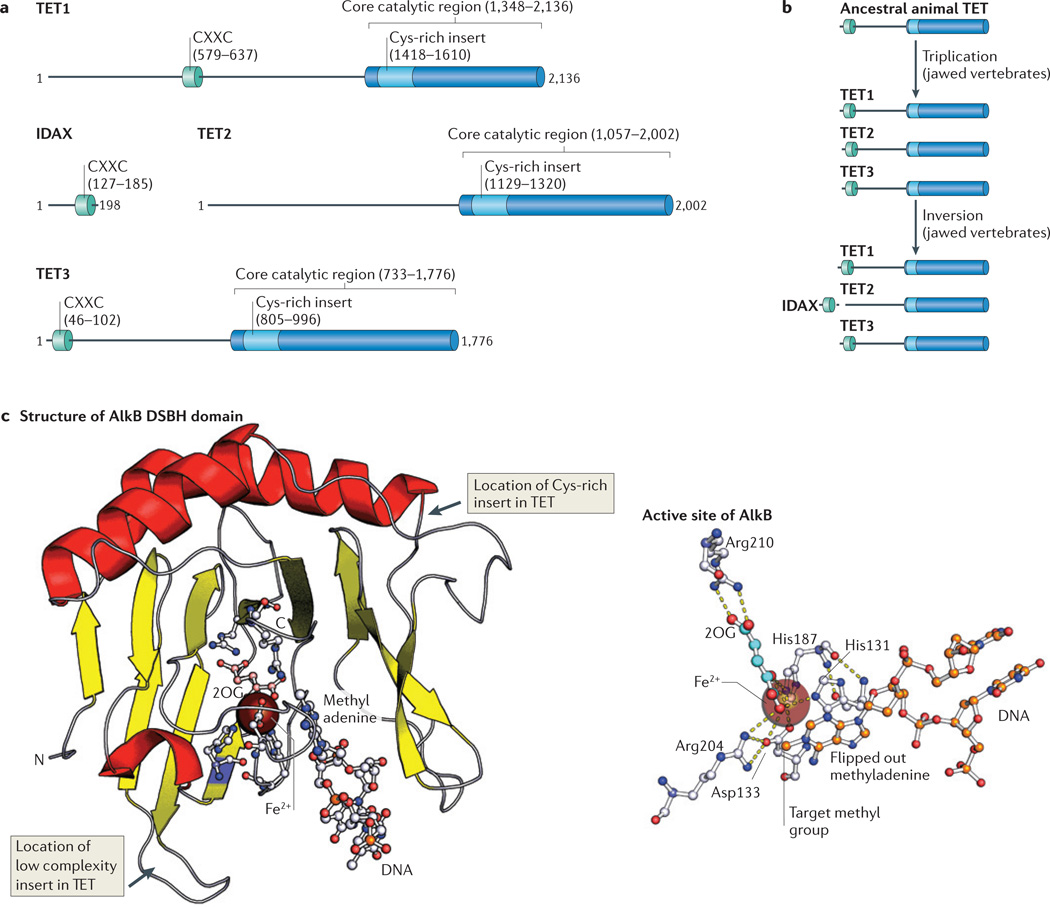
Figure 2. Known protein domains of TET family members.
a | Ten-eleven translocation (TET) proteins contain a DNA-binding CXXC domain towards the ami no terminus and a carboxy-terminal catalytic core region that includes a Cys-rich insert and a larger double-stranded β-helix (DSBH) domain. The number of amino acids is indicated, and the numbering corresponds to the human proteins. b | Evolutionary changes in the domain structure of TET proteins. A gene triplication event that occurred in jawed vertebrates resulted in the generation of three TET family members. A chromosomal inversion then detached the catalytic domain of TET2 from its CXXC domain, which became a separate gene (which encodes IDAX (inhibition of the Dvl and axin complex)). c | A cartoon representation of AlkB (Protein Databank (PDB) identifier: 2FD8), a protein that belongs to the same superfamily as the TET proteins and shares a common fold with them. AlkB is shown as a complex with its substrate methyladenine and its cofactor 2-oxoglutarate (2OG). In the 2OG structure, carbon atoms are shown pink, in the rest of the structure carbons are shown in grey. Nitrogens are shown in blue, oxygens in red and phosphates in orange (left panel). A stripped-down view of the active site of AlkB in complex with its substrate methlyadenine. Note the series of interactions, including pi–pi stacking interactions, between the His residues and the target base, and cation–pi interaction with the active site metal. Such interactions are likely to be retained in the TET– J-binding protein (JBP) family. In the 2OG structure, carbon atoms are shown in cyan and oxygens in red. In the protein structure, carbons are shown in grey, nitrogens in blue and oxygens in red. In methyladenine, carbons are orange and nitrogens are blue. Dashed yellow lines represent hydrogen bonds (right panel).
CXXC domains occur in many chromatin-associated proteins. Three distinct subfamilies of CXXC domains can be identified by their sequence33 (Supplementary information S1 (figure)). Subfamily 1 includes the CXXC domains of CXXC1 (also known as CFP1), DNMT1, MLL and Lys-specific demethylase (KDMA) family proteins, as well as the third CXXC domain of methyl CpG-binding protein 1 (MBD1); these CXXC domains specifically recognize unmodified CG sequences and target subfamily 1 proteins to CpG-rich sequences in DNA34–38. Subfamily 2 consists of the first two CXXC domains of MBD1; these CXXC domains have not yet been demonstrated to bind DNA and have no documented function36. Subfamily 3 includes the CXXC domains of TET1, TET3, IDAX and RINF (retinoid-inducible nuclear factor; also known as CXXC5)2,39. The IDAX CXXC domain preferentially binds unmethylated CpG sequences in vitro, and is found at CpG islands and CpG-rich promoters (which are predominantly unmethylated) in cells (Ref. 41). Curiously, however, the TET1 CXXC domain is reported not to bind DNA33 or alternatively to bind CpG sequences regardless of whether the cytosine is modified39,40, and the TET3 CXXC domain is reported to bind unmodified cytosine irrespective of whether it is followed by a guanine, a finding supported by affinity measurements and X-ray crystallography42. As subfamily 3 CXXC domains have a strong positive charge that increases their tendency to bind nonspecifically to DNA, an analysis of binding sites in vivo is needed to reconcile these in vitro findings.
Surprisingly, IDAX (a reported inhibitor of WNT signalling43) targets TET2, the protein from which it was separated during evolution, for destruction via a caspase-dependent mechanism41. Depletion of IDAX from ES cells prevented the downregulation of TET2 that normally occurs upon differentiation, whereas its depletion from a human myeloid cell line increased TET2 and 5hmC levels. IDAX cannot negatively regulate TET2 if key DNA-binding residues in the IDAX CXXC domain are mutated, suggesting that IDAX recruits TET2 to DNA before the caspase-dependent degradation mechanism is activated. The implication is that TET2, which like other TET proteins is likely to also bind DNA through the cysteine-rich region of its catalytic domain (see below), binds different sets of genomic regions depending on whether IDAX is present. The CXXC domain of TET3 negatively regulates TET3 catalytic activity perhaps through an autoinhibitory mechanism that involves a physical interaction between the CXXC and catalytic domains or, alternatively, by tethering TET3 to particular DNA elements and thus limiting its genome-wide activity.
The catalytic domains of TET proteins are characteristic of Fe2+- and 2-oxoglutarate-dependent dioxygenases (reviewed in REF. 22). The DSBH fold, which comprises the catalytic domain of all these dioxygenases, contains an His-Xaa-Asp/Glu signature motif, a C-terminal conserved His residue that is involved in coordinating Fe2+ and a conserved Arg residue that binds 2-oxoglutarate via a salt bridge21,22. Because a crystal structure of the TET catalytic domain is not yet available, the structure of the AlkB DSBH domain and a detailed view of the active site44 are shown in FIG. 2c. As is the case for AlkB, the substrate 5mC is likely to be flipped out of the DNA double helix into the catalytic cavity of the TET protein, where it is brought in close proximity to the catalytic Fe2+ ion (FIG. 2c).
The inserted Cys-rich domain in the catalytic region of TET proteins is likely to chelate two or more Zn2+ ions via nine conserved Cys residues and one His residue, and has been postulated to be part of a DNA-binding surface that might help in target recognition2. Indeed, the same region contains comparable insertions in the distantly related Jumonji-like family45 (for example, the AT-rich interaction domain (ARID; also known as BRIGHT) in Jumonji and ARID domain-containing 1 (JARID1) and the histone-binding PHD finger in SMCX (also known as KDM5C or JARID1C)). Metazoan TET proteins also contain a large low-complexity insert predicted to be on the exterior surface of the DSBH fold2,32 (FIG. 2c). The sequence and size of this unstructured insert varies greatly between TET family members, but its continued presence indicates a function, perhaps as a site of protein–protein interaction2,32.
TET-mediated DNA demethylation: mechanisms
Interest in TET proteins has primarily centred around the possibility that oxidized methylcytosines could serve as intermediates in one or more ‘DNA demethylation’ pathways. In such a pathway, TET would oxidize 5mC to 5hmC, 5fC or 5caC, which would then be replaced with cytosine, the net result thus being ‘demethylation’. There are at least four mechanisms by which TET proteins could mediate DNA demethylation (FIG. 1a).
Facilitation of passive DNA demethylation
Because CG sites are palindromes that in general are symmetrically methylated46, DNA replication yields two strands with hemimethylated CG sites. Maintenance of DNA methylation patterns requires the DNA methyltransferase DNMT1 and its obligate partner UHRF1 (REF. 47) (FIG. 1c). UHRF1 binds to hemimethylated CpG sites via its SAD/ SRA (SET associated Deinococcus domain (SAD)/SET and RING associated (SRA) domain) domain and recruits DNMT1. DNMT1 then methylates the CG sites on the nascent DNA strand, thus maintaining methylation patterns through cell division47,48. In vitro, UHRF1–hemi-5hmC binding is tenfold less efficient than UHRF1–hemi-5mC binding49. In addition, the activity of recombinant DNMT1 is reduced 12-fold50 or 50-fold49 at sites of hemi-5hmC in vitro. Together, these results imply that the TET-mediated hydroxymethylation of a methylated CG site in vivo can block maintenance methylation during cell division and eliminate 5mC in a ‘passive’, replication-dependent manner. However, hydroxymethylated plasmids stably transfected into transformed human cells retain maintenance methylation through cycles of plasmid division as efficiently as a methylated plasmid51, suggesting that 5hmC does not completely block maintenance methylation in cells.
Active DNA demethylation through DNA repair
Two replication-independent (‘active’) demethylation mechanisms have been reported to couple the methylcytosine oxidase activity of TET proteins with base excision repair (BER). The first, which involves 5fC, 5caC and TDG, has been confirmed by multiple laboratories6,30,53. The second mechanism, which involves AID (activation-induced cytidine deaminase) and APOBEC (apolipo-protein B mRNA editing enzyme, catalytic polypeptide), is still controversial52,54. In the first mechanism, TET proteins further oxidize 5hmC to generate 5fC and 5caC5,6,28. 5fC and 5caC can be excised by TDG6,30; their replacement with cytosine results in demethylation (FIG. 1a). Electrophoretic mobility shift assays (EMSAs) and the structure of TDG in complex with 5caC53 indicate that TDG binds 5caC:G mismatches with higher affinity than T:G mismatches (its conventional substrate). Depletion of TDG causes a 2–10-fold increase in the levels of 5fC and 5caC in ES cells6,55,56, consistent with a model in which these bases are short-lived demethylation intermediates that are removed by TDG. However, even in a TDG-deficient cell, 5fC is rare compared with 5mC (0.2–0.3% of the total methylcytosines)5,55,56, and 5caC is even less abundant6,56, indicating that this demethylation pathway has limited throughput in ES cells.
The second DNA repair-based mechanism has been reported by one laboratory in transfected human cells and in mouse brain52. They proposed that 5hmC is deaminated to 5-hydroxyuracil (5hmU) by AID and APOBEC family enzymes, removed by SMUG1 (single-strand-selective monofunctional uracil DNA glyco-sylase 1) or TDG glycosylases and ultimately replaced by cytosine (FIG. 1a). This sequential deamination and removal of 5hmC is similar to the deamination of 5mC and the removal of the resulting T:G mismatches by TDG, a mechanism previously proposed to occur in zebrafish57,58, human cancers58, primordial germ cells59 and fused cells undergoing reprogramming60. However, there is considerable controversy about the significance of deamination-based demethylation mechanisms in general. In support of such mechanisms, TDG can excise T:G mismatches and 5hmU:G mismatches in vitro54,61, and both PGCs derived from Aid-deficient mice and mouse embryonic fibroblasts (MEFs) derived from Tdg -deficient mice have modestly increased levels of methylation at some CpG island promoters61,62. Arguing against a deamination mechanism, the AID enzyme primarily acts on single-stranded DNA63, and recombinant AID and APOBEC enzymes have their strongest activity against unmodified cytosine with reduced activity against 5mC and no detectable activity against 5hmC54,64. It therefore seems unlikely that AID and APOBEC enzymes have a role in 5hmC-dependent demethylation pathways, although their involvement in specific situations cannot be ruled out.
Enzymatic decarboxylation of 5caC
There is some evidence that 5caC may be decarboxylated by protein(s) present in ES cell lysates. An oligonucleotide containing 5caC that was labelled with 15N at both positions of the pyrimidine ring was synthesized and incubated with ES cell lysate. Following the recovery and digestion of the oligonucleotide, a small but detectable quantity of [15N2]-deoxycytosine was detected, indicating direct conversion of 5caC to cytosine without BER31 (FIG. 1a). The factors that catalyse this decarboxylation reaction remain to be identified.
Dehydroxymethylation by DNMT enzymes
Remarkably, DNMT enzymes can remove the hydroxymethyl group of 5hmC in vitro, directly converting 5hmC to cytosine65,66 (FIG. 1a). Reducing conditions favour the methyl-transferase activity of DNMT3A, whereas oxidizing conditions favour dehydroxymethylation66. Whether this reaction occurs in cells is still unknown. Two papers reported cyclical changes in the DNA methylation status of several oestrogen-responsive genes upon the addition of oestrogen67,68, and one of these also showed cyclical recruitment of all three DNMTs67. It would be interesting to revisit this system to determine whether reciprocal actions of TET proteins and DNMTs were involved.
When a biological role is observed for a TET protein, there has been a strong tendency in the literature to invoke DNA demethylation as a major mechanism. However, an unequivocal demonstration of demethylation can be difficult, especially as the changes observed in most studies have been subtle. At a given locus, TET may simply be converting 5mC to 5hmC, or it may be converting 5mC to cytosine via an oxidized 5mC intermediate by one of the pathways described in FIG. 1a. Actual DNA demethylation only occurs when 5mC is converted to cytosine via an oxidized 5mC intermediate. TET depletion will necessarily result in decreased 5hmC, 5fC and 5caC, and a corresponding increase in 5mC from which these oxidized methylcytosines are derived. Thus, a simple increase in 5mC upon TET depletion is not enough to prove that a TET protein is causing demethylation. Rather, the increase in 5mC has to be greater than the attendant decrease in 5hmC, 5fC and 5caC. If TET is demethylating a locus, TET depletion will result in a decrease in unmodified cytosine as judged by bisulphite sequencing. Even here, caveats apply: 5fC and 5caC will appear as unmodified cytosine. Another point to bear in mind is that binding of transcription factors to a TET-regulated locus may antagonize DNA methylation (REFS 69–72), thus if TET depletion results in altered transcription factor binding, the resulting changes in cytosine species could lead to incorrect interpretations of whether TET is causing demethylation at that locus or not. Methylated DNA immunoprecipitation (MeDIP) and other methods of measuring 5mC abundance are less useful than bisulphite sequencing, as simple conversion of 5mC to 5hmC will produce a drop in measured methylation even if TET does not mediate demethylation at the locus.
Genomic distribution of oxidized 5mC
Large-scale mapping of 5hmC, and more recently 5fC and 5caC, has been performed for mammalian genomes. The available methods for mapping these modified bases are outlined in BOX 1 and BOX 2. Commercial kits are available in many cases.
Box 1|Detecting and mapping modified cytosines by DNA precipitation.
5hmC immunoprecipitation
This is the most straightforward method for detecting 5-hydroxymethylcytosine (5hmC) and has been used by many laboratories39,84,93,126,141. However, the efficiency of precipitation strongly depends on the density of 5hmC in the samples26,76; there are notable variations in the results obtained in different laboratories39,84,93,126, and there is evidence that the 5hmC-specific antibody precipitates poly-CA repeats, resulting in spurious peaks immediately adjacent to such repeats142.
CMS immunoprecipitation
When 5hmC is treated with sodium bisulphite, it is converted into a compound called cytosine methylene sulphonate (CMS)143. CMS is highly immunogenic, and CMS immuno precipitation recovers 5hmC-containing DNA with high specificity and low background76,144.
GLIB (glucosylation, periodate oxidation, biotinylation)
Glucose is attached to the hydroxyl group of 5hmC using the enzyme β-glucosyl transferase (BGT) from phage T4. Glucose is then oxidized with sodium periodate and treated with aldehyde reactive probe (ARP) to generate an adduct containing two biotins at the site of every 5hmC76,145. The hydroxymethylated DNA is then precipitated with streptavidin beads.
JBP1
5hmC is glucosylated by BGT The glucosylated base is bound by J-binding protein 1 (JBP1), which is conjugated to a bead, allowing for selective precipitation of hydroxymethylated DNA146. The precipitation of 5hmC is highly specific, but not very efficient. This method has not yet been used for the genome-wide mapping of 5hmC.
5-hmC selective chemical labelling (hMe-Seal)
BGT adds a glucose-azide conjugate to 5hmC, which can then be biotinylated under gentle conditions using click chemistry73,147.
5fC-DNA pulldown
An ARP molecule biotinylates the formyl group of 5fC83. This method was used to generate the first published genome-wide map of 5fC83, but it also biotinylates abasic sites to some extent55.
5fC-Seal
BGT is used to protect 5hmC by linking it to an unmodified glucose. 5fC is then reduced to 5hmC using sodium borohydride (NaBH4), and the product then reacts with gluc ose-azide and is biotinylated as in hMe-Seal. Thus, 5fC is selectively biotinylated55.
5fC and 5caC immunoprecipitation
Antibodies specific for 5fC and 5caC have been used to precipitate these modifications for sequencing, although the efficiency of precipitation is fairly low56.
Box 2|Mapping 5hmC at base pair resolution.
The simplest and most widespread method for distinguishing cytosine from 5-methylcytosine (5mC) — treatment with sodium bisulphite followed by PCR amplification and bisulphite sequencing (BS-seq)148–150 — conflates the five cytosine species into two groups. 5mC and 5-hydroxymethylcytosine (5hmC) are resistant to deamination by sodium bisulphite and thus are read as cytosine, whereas cytosine, 5-carboxylcytosine (5caC) and 5-formylcytosine (5fC) are deaminated and so are read as thymine6,89,151 (see the table). Methods have been developed that distinguish 5hmC or 5fC from each other and from other cytosine species at base resolution, although there is no method yet that allows all five bases to be distinguished simultaneously.
Single molecule real time (SMRT) sequencing
Using chemistry similar to 5hmC selective chemical labelling (hMe-Seal) (BOX 1), a bulky conjugate is added to sites of 5hmC. The conjugate stalls a specially designed polymerase during DNA replication, and this stalling is monitored in real time to identify 5hmC152. This method, as well as the oxidative bisulphite sequencing (Ox-BS-seq) and TET-assisted bisulphite sequencing (TAB-seq) methods (see below), provide single-base resolution mapping of 5hmC. 5fC and 5caC produce substantial delays in replication even without the addition of a conjugate, although it is hard to distinguish them from each other153. SMRT sequencing can handle long DNA fragments (6–10 kb) but does not yet exhibit the throughput required for genome-wide mapping in mammalian cells.
OxBS-seq
5hmC is oxidized to 5fC using potassium perruthenate151. Bisulphite sequencing is performed on the same sample both before and after oxidation. As 5hmC is unaffected by sodium bisulphite treatment and 5fC is deaminated, the extent of 5hmC at a site can be approximated by subtracting the frequency of deamination after perruthenate oxidation from the frequency of deamination before oxidation. 5hmC is a rare base, so very high sequencing coverage is required to determine the extent of hydroxymethylation.
TAB-seq
5hmC is protected from oxidation by β-glucosyl transferase (3BGT)-mediated glucosylation, and then all 5mC bases are oxidized to 5caC using a recombinant ten-eleven translocation (TET) enzyme89. After bisulphite treatment, all cytosine species are therefore deaminated, except those that were omC, so these can be read specifically. TAB-seq has revealed that 5hmC is often asymmetric with cytosine or 5mC at CpG sites. Like Ox-BS, this method requires very high sequencing coverage.
5fC chemically assisted bisulphite sequencing (fCAB-seq)
5fC is normally deaminated by sodium bisulphite treatment, but when it reacts with O-ethylhydroxylamine, deamination is prevented. The extent of 5fC at a site can be approximated by subtracting the frequency of deamination after the bisulphite treatment of unreacted samples from the frequency of deamination after the bisulphite treatment of O-ethylhydroxylamine-treated sample. Although the technique is selective, 5fC is very rare, so extremely high sequencing coverage (>1000 fold) must be used to accurately map the base.
| Base | Read by BS-seq |
Read by oxBS-seq |
Read by TAB-seq |
Read by fCAB-seq |
| Cytosine | T | T | T | T |
| 5mC | C | C | T | C |
| 5hmC | C | T | C | C |
| 5fC | T | T | T | C |
| 5caC | T | T | T | T |
Oxidized methylcytosine enrichment at promoters
There is contention as to whether 5hmC is enriched at promoters, owing largely to how ‘promoter’ and ‘enrichment’ are defined. First, the definition of promoter varies between laboratories. Many promoters that are CpG-rich contain peaks of hydroxymethylation 500–2000 bases before and after the transcription start site (TSS) but are depleted for 5hmC at the TSS itself, where there is typically almost no modified cytosine73,74. In this Review, we classify these promoters as 5hmC-enriched. Second, the definition of enrichment varies depending on whether precipitation-based or single-base resolution methods are used. Precipitation-based methodologies compare how many times a given region of DNA is sequenced in a 5hmC pulldown compared with a negative control sample. As a result, regions that contain a higher than average number of 5hmC residues in a span of several hundred bases will be classified as enriched. In contrast, single-base resolution studies typically measure the fraction of total reads in which an individual CpG site is hydroxymethylated. Thus, promoters with a high CpG density, but in which each CpG site has a relatively low frequency of 5hmC, may be classified as 5hmC-enriched by a p recipitation-based methodology but as 5hmC-depleted by a single-base methodology. We will predominantly discuss results from precipitation-based studies, although it is unclear which definition of enrichment is more biologically relevant. Furthermore, any given cytosine modification is either present or absent at any given CpG site at the level of a single allele, but can seem infrequent when a large, potentially hetero-genous population of cells is examined.
In human73,75 and mouse11,74,76 ES cells, mouse neural progenitor cells77, mouse neurons77 and the cerebellum of 7-day-old mice78 (which contains a significant population of neural progenitor cells), 5hmC is enriched at promoters in absolute terms and especially when compared with 5mC, which is generally depleted at promoters79. In these cell types, genes with hydroxymethylated promoters are expressed at lower levels than other genes, although this does not prove a silencing role for 5hmC; 5hmC can only be formed at promoters that have some underlying level of methylation, and promoter methyl-ation correlates with silencing. In other cell types, including PGCs80, adult nervous tissue78, liver cells81 and benign nevi82, 5hmC is depleted from TSSs. The discrepancies in 5hmC distribution among cell types could be caused by differences in the underlying distribution of 5mC and TET proteins.
In ES cells, 5fC is found at largely the same set of promoters as 5hmC83, and levels of 5fC55 and 5caC56 increase at these promoters upon TDG depletion, indicating that at least some TET-mediated demethylation occurs at these loci.
Oxidized methylcytosine enrichment at gene bodies
In virtually all mammalian cell types studied, including mouse and human ES cells, mouse liver and brain and human melanomas, 5hmC is enriched at gene bodies11,73,77,78,81,82. In mouse brain, liver and ES cells (but not in human ES cells), gene expression positively correlates with gene body hydroxymethylation78,84,85, although the reason for this correlation is unclear. While promoter methylation strongly correlates with gene silencing, how gene body methylation correlates with gene expression varies with cell type and whether the methylated cyto-sine exists in a CpG or non-CpG context86–88, making it unclear how 5mC oxidation and/or DNA demethylation by TET proteins in gene bodies affects gene expression.
Oxidized methylcytosine enrichment at enhancers
In all mammalian cells in which both 5hmC and enhancers have been mapped, 5hmC is strongly enriched at enhanc-ers72,73,75,81,89, which are often regions of low CpG density with reduced levels of DNA methylation compared with neighbouring regions72. Single-base resolution mapping using TET-assisted bisulphite sequencing (TAB-seq) (BOX 2) indicates that 5hmC is strongly enriched immediately adjacent to transcription factor-binding sites in enhancers, but is depleted at the precise site of binding89. Due to the absence of data on the binding of transcription factors to enhancers in TET-deficient cells, it is difficult to know whether methylcytosine oxidation ‘opens’ chromatin structure, allowing transcription factors to bind, whether transcription factors recruit TET proteins or whether both mechanisms operate and reinforce one another. Moreover, the reduction in 5mC and 5hmC at transcription factor-binding sites may reflect demethyl-ation mediated by TET proteins, but could also be because transcription factors physically block access of DNMT69,90 and TET proteins. Probably, both mechanisms are at work.
Notably, in differentiating ES cells, 5hmC levels increase sharply at activated enhancers91. 5hmC levels rise almost immediately with the onset of differentiation and either precede or accompany acetylation of Lys27 of histone H3 (H3K27), which is a mark of active enhancers. Furthermore, 5fC and 5caC are enriched at enhancers in TDG-depleted ES cells, consistent with TET-mediated demethylation of enhancers55,56. However, the correlation between the 5fC levels at enhancers and hypomethylation is weak55. Further studies will be necessary to determine the extent to which TET proteins contribute to enhancer hypomethylation and function.
Mapping TETs and TET-interacting proteins
The distributions of TET proteins and oxidized 5mC species do not overlap neatly. All three TET proteins and IDAX are strongly enriched at promoters, especially promoters that are CpG-rich39,41,92–95. In the case of TET1 (REF. 39) and TET3 (REF. 42), this preference is partially determined by the presence of CXXC domains, which most likely bind CpG sequences, as discussed above.
The data support a repressive role for TET1 in ES cells. In three studies in mouse ES cells, there is a strong statistical correlation between the physical presence of TET1 at a promoter and increased gene expression from this promoter upon TET1 knockdown39,92,93. The correlation between the presence of TET1 at a promoter and decreased expression from this promoter upon TET1 knockdown is much weaker. TET2 shows precisely the opposite trend: there is a correlation between the physical presence of TET2 at a promoter and decreased expression from this promoter upon TET2 depletion, which indicates that TET2 is generally a positive regulator of gene expression95. The likely explanation for the different activities of TET1 and TET2 is that, in ES cells, TET1 primarily modulates transcription by recruiting the repressive histone deacetylase-containing MBD3–NURD (nucleosome remodelling and deacetylase)96 and SIN3A (switch-independent 3A) complexes13,94,97 to promoters. TET2 shows no association with these complexes in ES cells93 and, when exogenously expressed in 293T cells, TET1 and TET3 associate with SIN3A, whereas TET2 does not94. Furthermore, in contrast to somatic cells in which methyl-ation has an impact on the expression of thousands of genes98, methylation only regulates a handful of genes in ES cells and is dispensable for ES cell growth and survival99. Hence, the demethylating properties ascribed to TET proteins may be of limited importance in regulating transcription in ES cells.
Recently, three groups have reported a physical association between TET proteins and the enzyme OGT (O-linked β-d-N-acetylglucosamine (O-GlcNAc) transferase)94,95,97. Despite some contradictions in these studies, it is probable that all three TET proteins associate with OGT100. OGT adds a GlcNAc sugar to Ser and Thr residues of numerous proteins, including histone H2B and other chromatin modifiers. The impact of this sugar modification on the target protein is highly contextual. For instance, O-GlcNAcylation can serve to antagonize phosphorylation, direct proteolysis or allow protein– protein interaction101. OGT, like TET proteins, is strongly enriched at promoters. In ES cells, TET proteins recruit OGT to promoters, but not vice versa. Depletion of TET proteins diminishes the amount of OGT at promoters, but depletion of OGT does not influence the association of TET1 or TET2 with chromatin, although it does alter the distribution of 5hmC at certain loci95, 97. There are various mechanisms by which OGT could influence transcription. For example, levels of O-GlcNAcylation at Ser112 of H2B, a mark associated with transcriptional activation102, drop sharply upon TET2 depletion providing a mechanism whereby TET2 increases transcription through OGT. Likewise, OGT glycosylates HCF1 (host cell factor 1), a component of the H3K4 methyltransferase SET1 complex (also known as COMPASS) associated with active RNA polymerase II (Pol II), providing a link between H3K4 trimethylation and TET proteins94. Given the many protein targets of OGT, it is likely that there will be additional consequences of TET-mediated OGT recruitment.
TET proteins in embryonic development
TET proteins are implicated in several stages of mouse development, particularly those in which mass demethyl-ation and de novo methylation take place, namely the zygote, the inner cell mass of the blastula and PGCs (FIG. 3). The phenotypes of TET-deficient mice are described in BOX 3.
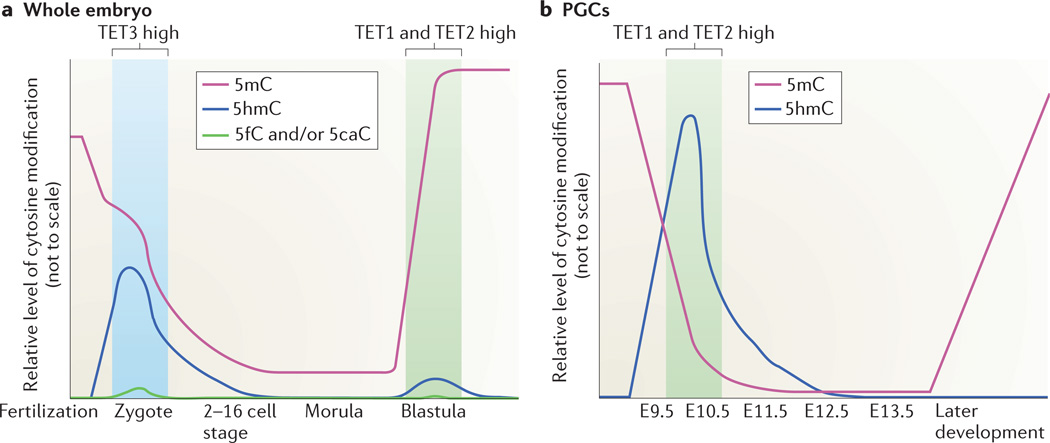
Figure 3. Methylation dynamics in mammalian development.
a | Immediately after fertilization, the male pronucleus undergoes mass cytosine oxidation111–113, mediated by ten-eleventranslocation 3 (TET3). B-methylcytosine (5mC) and oxidized cytosines are then lost from the early embryo in a ‘passive’ or replication-dependent manner, resulting in the loss of nearly all modified cytosines by the 16-cell stage103,105. Imprinted loci retain methylation183 and some repetitive element classes184 retain partial methylation. Approximately when the blastula implants into the uterus, the inner cell mass, which gives rise to the embryo, undergoes mass de novo DNA methylation105,183.TET1 and TET2 are highly expressed at this stage, potentially fine-tuning methylation patterns, b | Demethylat ion also occurs in primordial germ cells (PGCs) between embryonic days E9.B and E13.B of embryonic development80,120,128. This event also entails both mass BmC oxidation by TETl and TET2 and loss of modified cytosine by passive demethylation, resulting in the loss of imprints. A similar process of 5mC oxidation and demethylation occurs more slowly in human germ cells185. This demethylat ion of imprints is critical because whereas somatic cells of an organism contain male and female imprints, the germ cells of an organism contain the imprints that correspond exclusively to the gender of the organism. Germ cells are then gradually re-methylated and imprints placed, starting at E1B in males and after birth in females186.
Box 3|Phenotypes of TET-deficient mice.
Ten-eleven translocation (Tet1) −/− mice on a mixed C57BL/6 × 129 inbred mouse strain background are born at Mendelian ratio and appear healthy despite a low birth weight123. Mouse Tet1 gene-trap mutant animals show embryonic lethality on a 129P2/OlaHsd mouse strain background, with noticeable abnormalities by embryonic day E8.5, but they are viable and fertile on a C57BL/6 background129. Female Tet1 gene-trap mutants have smaller litters, similarly to Tet1−/− mice. In female Tet1 gene-trap mutants, which have smaller ovaries, about half of the developing female gametes display a defect in meiotic synapsis and suffer a developmental arrest between E16.5 and E18.5 (REF. 129). Whether Tet1−/− mice have a similar meiotic defect is unknown.
Tet2−/− mice on C57BL/6 or mixed backgrounds are born at Mendelian ratios and are fertile, but display clear haematological abnormalities. They have more haematopoietic stem cells (HSCs) than normal mice, and their HSCs have enhanced self-renewal and proliferative potential in culture and in experiments in which progenitor cells are serially transferred in mice154–157. At 4 months old, one strain of Tet2−/− mice has been reported to develop a condition similar to human chronic myelomonocytic leukaemia (CMML), a malignancy typified by a gross abundance of monocytes155, 157. A Tet2 gene-trap strain shows perinatal lethality158, but because this phenotype is not observed in several conventional Tet2 gene-disrupted strains, it is most likely an artefact.
Tet1−/− Tet2−/− mice on a mixed C57BL/6 × 129 background are born at near Mendelian ratios and have fully developed organs, but roughly half of these animals die perinatally, often with visible defects in head development124. Surviving mice have a smaller birth and adult weight, and females have reduced ovary size and fertility, producing litters of about one fourth the normal litter size. Tet1−/− Tet2−/− male mice crossed with wild-type females produced healthy progeny, but more than half of the progeny of Tet1−/− Tet2−/− females crossed with wild-type males died perinatally, probably due to an imprinting defect in the Tet1− Tet2− oocytes (see main text).
Roughly half of the embryos that arise from Tet3− oocytes, regardless of the sperm genotype, arrest around E11.5 and do not survive. The Tet3−/− mice that survive embryonic development die perinatally for unknown reasons113.
TET3 in the zygote
Immediately after fertilization, before the genetic material from the sperm and egg have fused to form one nucleus, the male pronucleus loses almost all methylation as assessed by staining with antibodies specific for 5mC103–105 (FIG. 3). This process occurs before replication and is not inhibited by DNA polymerase inhibitors. In contrast, bisulphite sequencing, which does not distinguish between 5mC and 5hmC106–108 (BOX 2), indicates that this loss of 5mC is much slower and less complete than the abrupt loss measured by antibody staining104,109–111. These results are reconciled by the observation that the zygotic demethylation event is in fact a mass 5mC oxidation event; the apparent loss of 5mC stems from the fact that 5mC-specific antibodies do not recognize 5hmC or other oxidized methylcytosines. Instead, the male pronucleus shows a selective increase in 5hmC111–113 and other oxidized methylcytosines114, as observed by staining with appropriate antibodies. The increase in 5hmC is abrogated by siRNA-mediated knockdown of Tet3 (REF. 112 ), and is not observed in fertilizations that involve TET3-deficient oocytes113. This indicates that TET3 oxidizes 5mC in the male pronucleus.
The protein DPPA3 (development pluripotency-associated 3; also known as Stella or PGC7) protects maternal DNA and a few imprinted loci in the paternal genome from TET3-mediated oxidation115. DPPA3 is reported to be recruited to the protected regions by dimethylation of H3 at Lys9 (H3K9me2), a mark that is found selectively at these sites115. In the absence of DPPA3, both paternal and maternal genomes become hydroxymethylated112,115 and most show abnormalities by the 2-cell or 4-cell stage116–118. In contrast, Tet 3 -deficient oocytes fertilized with wild-type sperm have reduced expression of OCT4 during the morula stage but form blastocysts normally. Moreover, about half of these animals show serious abnormalities by embryonic day E11.5 and fail to develop113. Thus, methylcytosine oxidation in the male pronucleus enhances, but is not required for, survival of the embryo. It is not clear why some zygotes survive, whereas others do not, and why it takes 11 days for the failure of zygotic oxidation to result in lethality.
The bulk of the evidence suggests that cytosine modification is lost through a replication-dependent process that may be enhanced by, but is not completely dependent on, TET enzymes or BER. In fact, maintenance methyl-ation is very inefficient in the early embryo because of the cytoplasmic localization of DNM1O, which is a special splice variant of DNMT1 present in the early mouse embryo80,103,105. As a result, 5mC on the maternal DNA and 5hmC on the paternal DNA are diluted out through a passive replication-dependent process (FIG. 3). Substantial levels of 5hmC are retained at least through the 8-cell stage111,119, arguing against the possibility that it is excised or converted directly to cytosine. However, some loss of 5mC is observed by bisulphite sequencing before any cell replication event113. A likely explanation for this is that TET3 is oxidizing 5mC to 5fC and 5caC114, creating the appearance of demethylation by bisulphite sequencing (BOX 2). It is also plausible that some proportion of 5fC and 5caC is removed by TDG and replaced by cytosine30. Evidence showing BER in the male pronucleus supports this possibility110,120. Arguing against direct removal of 5fC and 5caC is evidence demonstrating that the modified bases remain in the embryo at least until the 4-cell stage114 and the fact that large-scale BER would be expected to cause increased rates of mutations in the zygote, which would be deleterious to the organism and its progeny.
As methylation is lost passively anyway, why are methylcytosines oxidized in the mammalian zygote? The most likely possibility is that methylcytosine oxidation in the male pronucleus accelerates global DNA demethylation, either through 5fC and 5caC formation and the subsequent removal of these modified bases or by ensuring that maintenance methylation is especially inefficient in the earliest cell divisions. No proof of either mechanism has been demonstrated. It is also possible that cytosine oxidation promotes broad transcriptional activation previously observed in the male pronucleus121. However, depletion of TET3 in the early zygote is reported to have no effect on global transcriptional levels122. In short, it is clear that TET3 oxidizes the male pronucleus and that this oxidation event enhances survival of the early embryo, but why this oxidation occurs remains unknown.
TET1 and TET2 in ES cells
When pluripotent ES cells are injected into immunocompromised mice, they form tumours called teratomas that contain tissues from all three germ layers (ectoderm, mesoderm and endoderm). Tet1−/− ES cells123, Tet1−/− Tet2r−/− ES cells124 and ES cells stably expressing Tet1 short hairpin RNA (shRNA)125 form large haemorrhagic teratomas that are enriched for trophoblast cells, suggesting that TET1 regulates the first lineage commitment step in the embryo by suppressing differentiation towards the extra-embryonic lineage. Notably, however, Tet1−/− Tet2−/− ES cells contribute efficiently to chimaeras when injected into blastocysts124, indicating that the skewing towards trophoblast differentiation observed in Tet1−/− and Tet1−/− Tet2−/− teratomas can be overcome by other regulatory influences during embryonic development. TET1 depletion influences gene expression and differentiation in mouse ES cells by negatively regulating the expression of key trophectoderm regulators, such as caudal-type homeo box 2 (CDX2)27,125,126, eomesodermin (EOMES)93,123,125 and ELF5 (REF. 125), and positively regulating expression of the neuroectoderm factors paired box 6 (PAX6)39,93,123,125,126 and neurogenic differentiation factor 2 (NeuroD2)39,93,123. However, neural progenitor cells can be derived from Tet1−/− embryoid bodies123, and Tet1−/− and Tet1−/− Tet2−/− mice develop all three germ layers and survive, suggesting that the role of TET1 and TET2 in differentiation is minor. Overall, the data indicate that, despite the abundance of 5hmC in ES cells, TET1 and TET2 have modulatory but not essential roles in mouse development, ES cell survival or pluripotency. Whether TET3 can compensate for the loss of TET1 and TET2 has not yet been established.
Nevertheless, the catalytic activity of TET proteins may be important in ES cells and the inner cell mass from which they are derived, because of the underlying biology of these cells. ES cells approximately recapitulate the stage of development in which large-scale de novo methylation of the genome occurs (FIG. 3). Because transcriptional initiation antagonizes methylation71,87, genes with lower levels of transcription are presumably more susceptible to aberrant increases in DNA methylation. As methylation is generally maintained through cell divisions, aberrant methylation may be inconsequential at early embryonic stages but deleterious during later development. Thus, the function of TET proteins in the inner cell mass may be to repress lineage-specific genes, while simultaneously antagonizing methylation to permit activation of these genes later in development. Accordingly, in ES cells, both TET1 and 5hmC are enriched at promoters that contain dual H3K4me3 and H3K27me3 marks, which are so-called ‘poised’ promoters that can be activated later in development74,76. Loci with high levels of 5fC in ES cells show increased cyto-sine methylation in TDG-deficient MEFs83, even though TET1 and TET2 are almost absent from MEFs. Thus, a demethylation defect in early development seems to result in excess methylation at a later stage.
Generally, the data support a real but limited role for TET1 and TET2 in demethylation in ES cells. Bisulphite sequencing of selected loci in TET1-deficient ES cells does indicate a very modest increase in methylation levels39. Mass spectrometry indicates that cells deficient in both TET1 and TET2 show an increase in 5mC levels (from 5.3% to 5.8% of all cytosines) and a smaller absolute drop in 5hmC levels (from 0.13% of all cytosine to undetectable)124. Thus, there is a ~1.05-fold increase in the total levels of cytosine modification (5.43% to 5.8%). If the increase in cytosine modification is concentrated at promoters at which CpG levels are high and TET proteins are most active, this could have a strong impact on transcription and development. However, caution must be used in extrapolating changes in methylation observed in ES cells, which undergo many generations of division in culture, to the inner cell mass that differentiate s quickly.
Most tissues in Tet1−/− Tet2−/− mice show modest increases in total cytosine modification levels as measured by mass spectrometry, although it is unclear whether this is due to inadequate demethylation in the blastula, during later embryonic development or in adult tissue. The incompletely penetrant lethal phenotype of Tet−/−Tet2−/− mice (BOX 3) could reflect a model in which, by stochastic variation, some mice acquire excess methylation at key promoters in early development, leading to subsequent lethality, whereas other mice do not. However, the perinatal lethality of Tet1−/−Tet2−/− mice cannot be assumed to be caused by alterations in DNA methylation. Furthermore, virtually all of the Tet1−/−Tet2−/− mice develop to birth with fully developed organs, so any dysregulation of methylation is necessarily fairly mild.
TET proteins in PGCs
PGCs undergo a rapid drop in 5mC levels between E9.5 and E10.5 (FIG. 3). As in zygotes, this apparent demethylation event corresponds to mass conversion of 5mC to 5hmC, in this case mediated by TET1 and TET2 (REFS 80,127). There is no detectable formation of 5fC or 5caC as measured by immunocytochemistry80. Measurement of individual loci during germ cell development indicates that 5mC is replaced by 5hmC between E9.5 and E11.5, and that both marks are subsequently lost at a rate consistent with dilution by replication, as opposed to active removal80. Thus, if TET proteins are mediating demethylation in PGCs, it is by facilitated passive demethylation. However, even this mechanism may not be critical, as UHRF1 and DNMT3B levels are quite low in PGCs128 and thus passive demethylation could occur without TET activity.
Germ cells derived from mouse Tet1 gene-trap mutants (BOX 3) have almost normal methylation levels129. At E13.5, when demethylation is complete, an increase in methylation corresponding to less than 1% of the total cytosines at CpG sites is observed in TET1-deficient cells129. A much greater effect is seen at the level of gene expression: loss of TET1 causes dys regulation of ~1000 genes, 90% of which are positively regulated by TET1, indicating that TET1 primarily functions to activate transcription in PGCs. A number of critical meiosis-related genes are targets of TET1 activation, including malate dehydrogenase 1 (Mae1), synaptonemal complex protein 1 (Sycp1) and Sycp3. Half of the developing TET1-deficient female gametes subsequently display a defect in meiotic synapsis and suffer a developmental arrest between E16.5 and E18.5 (REF. 129), and accordingly Tet1 gene-trap and Tet1−/− mice have reduced litter size. Although it is possible that TET1 facilitates critical demethylation events at key genes, it seems unlikely that such a modest change in methylation levels has such a drastic effect on gene expression. It is more likely that TET1 modulates gene expression by recruiting other proteins or that oxidized cytosines are influencing transcription in a way that does not involve their removal.
The simultaneous shRNA-mediated depletion of TET1 and TET2 (REF. 80), or the depletion of TET1 from Tet2−/− cells127, seems to partly antagonize demethyl-ation at a few loci in PGC-like cells derived in vitro from precursors. Furthermore, there is evidence that oocytes deficient in TET1 and TET2 may not completely erase all parental imprints during PGC demethyl-ation. Although Tet1+/−Tet2+/− mice had no intrinsic survival defect, half of the progeny of Tet1−/−Tet2−/− females crossed with wild-type males display perinatal lethality124. This indicates that some fraction of the T e t 1− Te t2− oocytes completed meiosis successfully but were nevertheless defective, as judged by the perinatal lethal phenotype observed in mice developing from the Tet1−Tet2− oocytes. Accordingly, some imprinted loci in the progeny of Tet1−/− Tet2−/− mice show aberrant methylation. The occurrence and degree of increased methylation varies across loci and between individual mice. This is consistent with mass genomic demethyl-ation proceeding normally in TET-deficient animals, but the subsequent erasure of imprints being variably impaired to cause fatal defects in some, but not all, progeny.
Thus, in the two instances of mass demethylation in mammals, essentially the same pattern occurs. 5mC is oxidized enmasse by TET3 in zygotes and TET1 and TET2 in PGCs, predominantly producing 5hmC, and there is little evidence that the oxidized methylcytosines are excised. Rather, all modified cytosines are diluted out during subsequent cell divisions, due to the expression of DNMT1O in zygotes and the downregulation of UHRF1 in PGCs. Insofar as oxidized methylcytosines are promoting demethylation in these systems, they probably achieve this by antagonizing maintenance methylation under conditions in which this process is already in efficient. Potentially, the oxidized methylcytosines have important functions that are not related to their removal as, for example, epigenetic marks.
TET proteins in reprogramming
The transfection of developmentally-committed cells with certain combinations of transcription factors can reprogramme them, with low efficiency, into induced pluripotent stem (iPS) cells that are phenotypically similar to ES cells130. The classic combination of transcription factors used in these experiments is OCT4 (also known as POU5F1), SOX2, Krüppel-like factor 4 (KLF4) and MYC (collectively referred to as OSKM), although other combinations have also been used successfully131. For efficient reprogramming, endogenous pluripotency factors must be activated and their promoters and enhancers demethylated132. Although TET proteins are apparently dispensable for pluripotency in ES cells and in vivo, several recent publications indicate roles for TET1 and/or TET2 in the generation of iPS cells, although they do not report a common mechanism. Specifically, one study showed that the depletion of TET2 by shRNA completely ablated reprogramming of fibroblasts by OSKM, and that the Nanog promoter was hydroxymethylated and demethylated during the first 4 days after reprogramming133. These findings, together with the fact that TET1 and TET3 expression was not observed during this time frame, suggest that TET2 has a role in generating iPS cells. In another study, TET1 and TET2 were shown to physically interact with Nanog in ES cells, and co-transfection of Nanog with either TET1 or TET2 greatly enhanced OCT4, KLF4 and MYC-mediated reprogramming of neural stem cells134. The authors proposed that Nanog and TET1 or TET2 could co-activate the endogenous Oct4 and Esrrb (oestrogen-related receptor beta) genes via a mechanism that involves at least some demethylation. A third study showed that co-transfecting TET1 with OSKM factors greatly enhances the reprogramming of fibroblasts135. These data suggest that TET1 mediates the oxidation and demethylation of the Oct4 promoter and proximal enhancer, and that TET1 can substitute for OCT4 in OSKM-dependent reprogramming transfection. In all of the above cases, demethylation of the target loci is slow enough to be caused by a passive mechanism. The requirement for TET proteins in reprogramming clearly varies depending on the precise conditions and status of the starting cell lines. However, the ability of TET protein s to generate oxidized methylcytosines and antagonize methylation of select loci may help to establis h the pluripotent state.
TET proteins in the nervous system
The brain consistently has the highest levels of 5hmC of any mammalian tissue. This may in part reflect the abundance of long-lived post-mitotic cells in the nervou s system, such that 5hmC can accumulate in neurons without being depleted through replication. It is still largely unknown how TET deficiency influences mouse brain development. Increased 5hmC and decreased DNA methylation clearly correlate with higher gene expression levels and a more open chromatin state in neural cells85 but causality has not been established. Depletion of TET2 and TET3 in the developing mouse cortex, by in utero electroporation of shRNA, causes a block in the differentiation of neural progenitor cells into neurons77.
Xenopus laevis embryos injected with morpholinos targeting tet3 develop a gross defect in early development, that features loss of expression of critical eye and neural markers and results in small heads and no eyes42. The phenotype was partly rescued by injection of mRNA coding for a catalytically inactive TET3, whereas injection with a TET3 CXXC domain mutant provided no rescue at all. Thus, TET3 has a role in normal head development in X. laevis. It is unknown whether the perinatal lethality observed in Tet3−/− mice113 is linked to a neurological development defect.
Interpreters of 5mC oxidation
As the extent to which 5mC oxidation products are demethylation intermediates is unclear, other mechanisms by which they could influence transcription should be considered.
Some transcriptional regulators and chromatin-associate d proteins specifically recognize 5hmC or other oxidized methylcytosines. Mass spectrometry has been used to identify proteins bound selectively to 5hmC-containing DNA oligonucleotides in ES cells, neural progenitor cells and mouse adult brain136. Proteins that were found to bind to 5hmC included the neural progenitor cell-specific protein UHRF2, which presumably recognizes 5hmC-containing DNA via its SAD/SRA domain, transcription factors such as zinc-fingers and homeoboxes protein 1 (ZHX1), ZHX2 and THAP domain-containing protein 11 (THAP11), as well as several uncharacterized proteins. An important limitation of this study is that it does not distinguish between proteins that bind directly and indirectly to 5hmC. Despite the inability of glycosylases to cleave 5hmC in vitro52, several glycosylases and DNA repair proteins were found to associate with 5hmC in this study, suggesting that 5hmC may be a target of DNA repair136. In addition, two methyl-binding proteins, methyl CpG binding-protein 2 (MeCP2) and methyl-CpG binding domain protein 4 (MBD4), were shown in this and other studies to bind to 5hmC85,136,137. However, an initial finding that MBD3 ;REF 96) preferentially binds to 5hmC over 5mC has not been reproducible49,136.
TDG specifically binds 5fC and 5caC in vitro53 and might therefore be a reader of 5fC and 5caC. Indeed, 5fC and 5caC were found to bind TDG in mouse ES cell, as well as a substantial population of DNA repair proteins, several transcription factors and a number of miscellaneous proteins, some of which may simply bind formyl or carboxyl groups nonspecifically136. A suggestion that TDG recruits the histone acetyltransferase p300 ;REF 138) is countered by the finding that the number of p300-binding sites in the genome actually increases in TDG-deficient ES cells55. However, TDG could nevertheless be a transcriptional regulator, as it has been reported to interact with multiple DNA-binding transcription factors139.
Oxidized methylcytosines can affect Pol II processivity directly. 5mC and 5hmC have a relatively small effect on polymerase processivity in vitro, but a single 5fC or 5caC can drastically stall the progression of Pol II on a recombinant substrate140. This phenomenon is functionally and perhaps also mechanistically analogous to that observed with base J, which has a crucial role in transcription termination in kinetoplastids25.
Finally, methylcytosine oxidation could antagonize 5mC-mediated silencing. Six different mechanisms for methylation-based silencing have been demonstrated; BOX 4). The relative importance of each mechanism is unclear, but their relevance clearly varies across cell types and loci. The impact of methylcytosine oxidation on these silencing mechanisms is also likely to be variable. Thus, in order to understand how 5hmC influences transcription at a locus, it may first be necessary to determine how methylation mediates silencing at that locus.
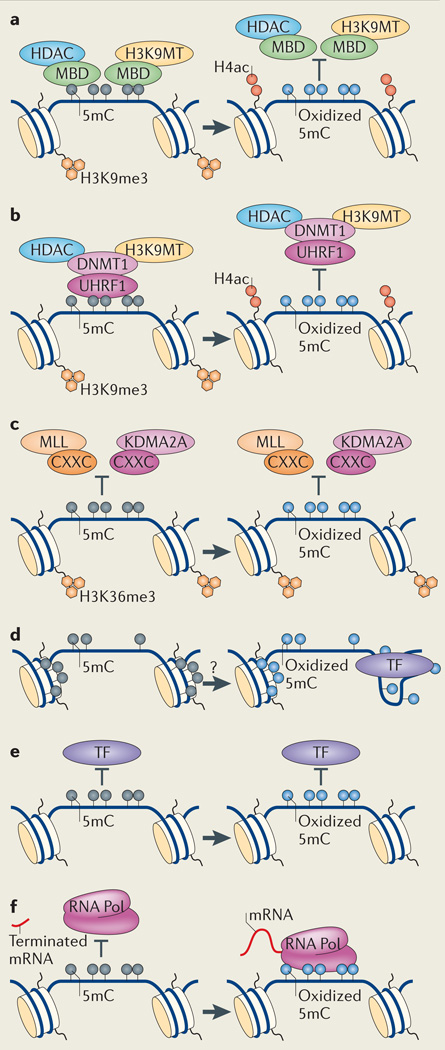
Box 4|Effects of hydroxymethylation on methylation-based silencing.
Methyl CpG-binding protein 1 (MBD1), MBD2 and Kaiso specifically recognize methylated DNA and recruit histone deacetylases or repressive histone H3 Lys9 methyltransferases (H3K9MTs) to 5mC159–164. 5-hydroxy methylcytosine (5hmC) strongly inhibits the binding of these MBD proteins to DNA49,108,165 and therefore is thought to induce transcriptional activation (see the figure, part a). However the role of MBD proteins in regulating gene expression is relatively subtle. Mice deficient in Kaiso166, MBD1 (REF. 167) and MBD2 (REF 168) develop and grow normally, although MBD1- and MBD2-deficient mice have mild neurological impairments. Methyl CpG binding-protein 2 (MeCP2), an MBD protein with a more complex regulatory role169–171, may bind 5hmC in addition to 5-methylcytosine (5mC)49,85. Various other transcriptional modulators bind 5mC selectively, and whether and how 5mC oxidation perturbs their activity is unclear136.
DNA methyltransferases (DNMTs), including DNMT1, recruit histone deacetylases (HDACs) and H3K9 methyltransferases to DNA. At promoters, this perpetuates a repressive chromatin state through cell division172 (see the figure, part b). As 5hmC potentially prevents DNMT1 recruitment, hydroxymethylation could block this mechanism of methylation-mediated silencing in dividing cells (FIG. 1c).
5mC can prevent chromatin remodellers that possess CXXC domains from binding to DNA (see the figure, part c)34. These include the H3K4 methyltransferase mixed-lineage leukaemia (MLL)38, CXXC1, a component of the SET1 H3K4 methyltransferase complex173, and Lys-specific demethylase 2A (KDM2A), which removes H3K36 methylation marks37. It is unlikely that these proteins could bind oxidized 5mCs.
Methylation increases nucleosome compaction (see the figure, part d), and this is likely to reduce the accessibility of adjacent DNA to transcription factors in vitro174. How hydroxymethylation affects nucleosome compaction is unknown, although 5hmC-rich Purkinje neurons have markedly decondensed nuclei.
In some instances, cytosine methylation physically blocks the binding of a transcription factor (TF) to its target sequence175, 176 (see the figure, part e). Alternatively, by blocking binding of repressors or insulators, methylation can actually increase the expression of a locus177–180. If the binding of a transcription factor is inhibited by methylation, it is unlikely that methylcytosine oxidation would restore binding. Furthermore, as 5hmC is bulkier than 5mC, it may block the binding of proteins that tolerate 5mC.
Finally, quantum mechanical calculations indicate an inherent role for 5mC in stabilizing DNA duplexes by promoting base stacking interactions181. Recent experiments confirm that methylated DNA has a higher melting temperature than unmodified DNA, suggesting that methylation may directly inhibit transcriptional initiation or elongation by RNA polymerase (RNA Pol) by preventing necessary melting of the template (see the figure, part f)182. By contrast, 5hmC reduces the melting temperature of DNA duplexes to promote transcriptional elongation.
Conclusions and future directions
The discovery of TET proteins has provided a new perspective on how DNA modification influences gene expression. There are now several potential mechanisms by which TET proteins and oxidized methylcytosines might mediate DNA demethylation, but whether and how these mechanisms contribute to gene regulation in different cell types remains to be elucidated. It will be important to determine how proteins that bind oxidized methylcytosines contribute to gene regulation and how the enzymatic activities and biological roles of TET proteins are influenced by interactions with other protein complexes. The ongoing development of improved methods for mapping oxidized methylcytosines promises to advance the field substantially, especially if it can be applied at single-base resolution to small numbers of differentiating cells. A careful analysis of changes in the distribution of oxidized methylcytosines, transcription levels, transcription factor binding and chromatin modifications in cells from animals lacking one or multiple TET proteins will also be essential to further our understanding of how TET proteins modulate gene expression, cell differentiation and function.
Supplementary Material
Acknowledgements
The authors thank S. Evans and A. Clark for comments on the manuscript. This work was supported by US National Institutes of health (NIH) R01 grants AI44432, HD065812 and CA151535, grant RM-01729 from the California Institute of Regenerative Medicine, and Translational Research grant TRP 6187–12 from the Leukemia and Lymphoma Society (to A.R). Work in the laboratory of L.A. is supported by intramural funds of the National Library of Medicine, NIH. W.A.P. was supported by a predoctoral graduate research fellowship from the National Science Foundation and is currently supported by a postdoctoral fellow ship from the Jane Coffin Childs Memorial Fund for Medical Research.
Glossary
- Dioxygenases
Enzymes that catalyse the addition of both oxygen atoms from molecular oxygen to one or two organic substrates
- DNA demethylation
Here, defined as replacement of 5-methylcytosine, the major methylated base in mammalian DNA, with unmodified cytosine, either directly or through intermediates
- CXXC domain
A Zn2+-chelating domain typified by the signature amino acid sequence CGXCXXC(X)NC, in which X represents any amino acid. CXXC domains in metazoans always contain two such sequences
- CpG sequences
Any instance of a cytosine followed immediately by a guanine on the same strand of DNA. Most 3DNA methylation in mammals occurs at CpG sites
- Base excision repair
(BER). A DNA repair pathway in which a DNA base is removed by a glycosylase enzyme and ultimately replaced by a new basel
- Primordial germ cells
(PGCs). Precursors of mature germ cells (egg in female and sperm in male)
- Click chemistry
Chemistry involving high-yield, highly specific reactions that are compatible with physiological conditions and maintain the integrity of biological molecules
- Sequencing coverage
Average number of times that a genome or a DNA region is sequenced using a next-generation sequencing instrument
- Zygote
Cell formed by fertilization of the oocyte (egg) with a sperm cell
- Imprinted locus
In epigenetics this describes a genomic region with a methylation mark that is present only on the maternally or paternally derived copy of an allele
- Trophectoderm
Cells that give rise to the placenta and other extra-embryonic tissue
- Embryoid bodies
Aggregates of cells formed by allowing embryonic stem cells to differentiate without contact with a solid surface
- Inbred mouse strain
Experiments are typically conducted using inbred mouse strains, in which all mice are genetically extremely similar. C57BL/6 is one of the most frequently used strains. 129P2/OlaHsd is another inbred mouse strain. The same mutation can have different effects in different backgrounds
- Gene-trap
A mutant in which a gene is disrupted by the random insertion of transgenic DNA that contains a splice acceptor site followed by stop codons
- Meiotic synapsis
The event in meiosis prophase I in which homologous chromosomes align to allow recombination of genetic material (known as ‘crossing over’)
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Anjana Rao’s homepage: http://www.liai.org/pages/faculty-rao
Protein Data Bank: http://www.rcsb.org/pdb/home/home.do.2FD8
SUPPLEMENTARY INFORMATION
See online article: S1 (figure)
References
- 1. Tahiliani M, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET 1. Science. 2009;324:930–935. doi: 10.1126/science.1170116.Discovery that TET proteins oxidize 5mC to 5hmC
- 2. Iyer LM, Tahiliani M, Rao A, Aravind L. Prediction of novel families of enzymes involved in oxidative and other complex modifications of bases in nucleic acids. Cell Cycle. 2009;8:1698–1710. doi: 10.4161/cc.8.11.8580.Describes the evolution of TET proteins and the presence of TET homologues in non-metazoan species
- 3.Ono R, et al. LCX, leukemia-associated protein with a CXXC domain, is fused to MLL in acute myeloid leukemia with trilineage dysplasia having t( 10; 11] (q22;q23) Cancer Res. 2002;62:4075–4080. [PubMed] [Google Scholar]
- 4.Lorsbach RB, et al. TET1, pa member of a novel protein family, is fused to MLL in acute myeloid leukemia containing the t(10; 11)(q22;q23) Leukemia. 2003;17:637–641. doi: 10.1038/sj.leu.2402834. [DOI] [PubMed] [Google Scholar]
- 5. Ito S, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597.Shows that TET proteins can produce 5fC and 5caC and quantifies the level of modified cytosines in a range of cell types
- 6. He YF, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Sdence. 2011;333:1303–1307. doi: 10.1126/science.1210944.Together with references 30 and 53, shows that TDG excises 5fC and 5caC. Demonstrates the presence of 5caC in mammalian DNA and shows that 5caC levels increase upon TDG depletion
- 7.Szwagierczak A, Bultmann S, Schmidt CS, Spada F, Leonhardt H. Sensitive enzymatic quantification of 5-hydroxymethylcytosine in genomic DNA. Nucleic Adas Res. 2010;38:el81. doi: 10.1093/nar/gkq684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Globisch D, et al. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PloS one. 2010;5:e15367. doi: 10.1371/journal.pone.0015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786.Together with reference 1, convincingly demonstrates the presence of 5hmC in mammals
- 10.Cimmino L, Abdel-Wahab O, Levine RL, Aifantis I. TET family proteins and their role in stem cell differentiation and transformation. Cell Stem Cell. 2011;9:193–204. doi: 10.1016/j.stem.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011;25:2436–2452. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nature Rev. Genet. 2012;13:7–13. doi: 10.1038/nrg3080. [DOI] [PubMed] [Google Scholar]
- 13.Williams K, Christensen J, Helin K. DNA methylation: TET proteins-guardians of CpG islands? EMBO Rep. 2012;13:28–35. doi: 10.1038/embor.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan L, Shi YG. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development. 2012;139:1895–1902. doi: 10.1242/dev.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bejar R, Levine R, Ebert BL. Unraveling the molecular pathophysiology of myelodysplastic syndromes. J. Clin. Oncol. 2011;29:504–515. doi: 10.1200/JCO.2010.31.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pronier E, Delhommeau F. Role of TET2 mutations in myeloproliferative neoplasms. Curr. Hematol. Malignancy Rep. 2012;7:57–64. doi: 10.1007/s11899-011-0108-8. [DOI] [PubMed] [Google Scholar]
- 17.Mercher T, et al. TET2, a tumor suppressor in hematological disorders. Biochim. Biophys. Acta. 2012;1825:173–177. doi: 10.1016/j.bbcan.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 18. Iyer LM, Anantharaman V, Wolf MY, Aravind L. Comparative genomics of transcription factors and chromatin proteins in parasitic protists and other eukaryotes. Int. J. Parasitol. 2008;38:1–31. doi: 10.1016/j.ijpara.2007.07.018.First paper to predict a 5mC oxidase and demethylase activity for TET1 (which was then termed CXXC6)
- 19.Gommers-Ampt JH, et al. β-D-glucosyl-hydroxymethyluracil: a novel modified base present in the DNA of the parasitic protozoan. T. brucei. Cell. 1993;75:1129–1136. doi: 10.1016/0092-8674(93)90322-h. [DOI] [PubMed] [Google Scholar]
- 20.Yu Z, et al. The protein that binds to DNA base J in trypanosomatids has features of a thymidine hydroxylase. Nucleic Acids Res. 2007;35:2107–2115. doi: 10.1093/nar/gkm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loenarz C, Schofield CJ. Expanding chemical biology of 2-oxoglutarate oxygenases. Nature Chern Biol. 2008;4:152–156. doi: 10.1038/nchembio0308-152. [DOI] [PubMed] [Google Scholar]
- 22.Loenarz C, Schofield CJ. Physiological and biochemical aspects of hydroxylations and demethylations catalyzed by human 2-oxoglutarate oxygenases. Trends Biochem Sci. 2011;36:7–18. doi: 10.1016/j.tibs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Aravind L, Koonin EV. The DNA-repair protein AlkB, EGL-9, and leprecan define new families of 2-oxoglutarate- and iron-dependent dioxygenases. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-research0007. RESEARCH0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borst P, Sabatini R. Base J: discovery, biosynthesis, and possible functions. Annu. Rev. Microbiol. 2008;62:235–251. doi: 10.1146/annurev.micro.62.081307.162750. [DOI] [PubMed] [Google Scholar]
- 25.van Luenen HG, et al. Glucosylated hydroxymethyluracil, DNA base j, prevents transcriptional readthrough in leishmania. Cell. 2012;150:909–921. doi: 10.1016/j.cell.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko M, et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–843. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito S, et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaffeneder T, et al. The discovery of 5-formylcytosine in embryonic stem cell DNA. Angewandte Chemie. 2011;50:7008–7012. doi: 10.1002/anie.201103899. [DOI] [PubMed] [Google Scholar]
- 29.Penn NW, Suwalski R, O’Riley C, Bojanowski K, Yura R. The presence of 5-hydroxymethylcytosine in animal deoxyribonucleic aciddeoxyribonucleic acid. Biochem. J. 1972;126:781–790. doi: 10.1042/bj1260781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiti A, Drohat AC.Thymine DNAglycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: potential implications for active demethylation of CpG sites J. Biol. Chern 201128635334–35338.Together with references 6 and 53, shows that TDG excises 5fC and 5caC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiesser S, et al. Mechanism and stem-cell activity of 5-carboxycytosine decarboxylation determined by isotope tracing. Angewandte Chemie. 2012;51:6516–6520. doi: 10.1002/anie.201202583. [DOI] [PubMed] [Google Scholar]
- 32.Iyer LM, Abhiman S, Aravind L. Natural history of eukaryotic DNA methylation systems. Prog. Mol. Biol. Trans!. Sci. 2011;101:25–104. doi: 10.1016/B978-0-12-387685-0.00002-0. [DOI] [PubMed] [Google Scholar]
- 33.Frauer C, et al. Different binding properties and function of CXXC zinc finger domains in Dnmtl and Tet 1. PloS one. 2011;6:e16627. doi: 10.1371/journal.pone.0016627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JH, Voo KS, Skalnik DG. Identification and characterization of the DNA binding domain of CpG-binding protein. J. Biol. Chem. 2001;276:44669–44676. doi: 10.1074/jbc.M107179200. [DOI] [PubMed] [Google Scholar]
- 35.Birke M, et al. The MT domain of the proto-oncoprotein MLL binds to CpG-containing DNA and discriminates against methylation. Nucleic Acids Res. 2002;30:958–965. doi: 10.1093/nar/30.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jorgensen HF, Ben-Porath I, Bird AP. Mbdl is recruited to both methylated and nonmethylated CpGs via distinct DNA binding domains. Mol. Cell. Biol. 2004;24:3387–3395. doi: 10.1128/MCB.24.8.3387-3395.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blackledge NP, et al. CpG islands recruit a histone H3 lysine 36 demethylase. Mol. Cell. 2010;38:179–190. doi: 10.1016/j.molcel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen MD, et al. Solution structure of the nonmethyl-CpG-binding CXXC domain of the leukaemia-associated MLL histone methyl transferase. EMBO J. 2006;25:4503–4512. doi: 10.1038/sj.emboj.7601340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, et al. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tetl hydroxylase in mouse embryonic stem cells. Mol. Cell. 2011;42:451–464. doi: 10.1016/j.molcel.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, et al. TET 1 is a DNA-binding protein that modulates DNA methylation and gene transcription via hydroxylation of 5-methylcytosine. Cell Res. 2010;20:1390–1393. doi: 10.1038/cr.2010.156. [DOI] [PubMed] [Google Scholar]
- 41. Ko M, et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature. 2013;497:122–126. doi: 10.1038/nature12052.Describes the role of IDAX in regulating TET2
- 42.Xu Y, et al. Tet3 CXXC domain and dioxygenase activity cooperatively regulate key genes for Xenopus eye and neural development. Cell. 2012;151:1200–1213. doi: 10.1016/j.cell.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hino S, et al. Inhibition of the Wnt signaling pathway by Idax, a novel Dvl-binding protein. Mol. Ceil. Biol. 2001;21:330–342. doi: 10.1128/MCB.21.1.330-342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu B, et al. Crystal structures of catalytic complexes of the oxidative DNA/RNA repair enzyme AlkB. Nature. 2006;439:879–884. doi: 10.1038/nature04561. [DOI] [PubMed] [Google Scholar]
- 45.Iyer LM, Abhiman S, de Souza RF, Aravind L. Origin and evolution of peptide-modifying dioxygenases and identification of the wybutosine hydroxylase/hydroperoxidase. Nucleic Acids Res. 2010;38:5261–5279. doi: 10.1093/nar/gkq265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bird A. The dinucleotide CG as a genomic signalling module. J. Mol. Biol. 2011;409:47–53. doi: 10.1016/j.jmb.2011.01.056. [DOI] [PubMed] [Google Scholar]
- 47.Bostick M, et al. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317:1760–1764. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- 48.Sharif J, et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmtl to methylated DNA. Nature. 2007;450:908–912. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto H, et al. Recognition and potential mechanisms for replication and erasure of cytosine hydroxymethylation. Nucleic Acids Res. 2012;40:4841–4849. doi: 10.1093/nar/gks155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valinluck V, Sowers LC. Endogenous cytosine damage products alter the site selectivity of human DNA maintenance methyltransferase DNMT1. Cancer Res. 2007;67:946–950. doi: 10.1158/0008-5472.CAN-06-3123. [DOI] [PubMed] [Google Scholar]
- 51.Kubosaki A, et al. CpG site-specific alteration of hydroxymethylcytosine to methylcytosine beyond DNA replication. Biochem. Biophys. Res. Commn. 2012;426:141–147. doi: 10.1016/j.bbrc.2012.08.053. [DOI] [PubMed] [Google Scholar]
- 52.Guo JU, Su Y, Zhong C, Ming GL, Song H. Hydroxylation of 5-methylcytosine by TET 1 promotes active DNA demethylation in the adult brain. Cell. 2011;145:423–434. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang L, et al. Thymine DNA glycosylase specifically recognizes 5-carboxylcytosine-modified DNA. Nature Chem. Biol. 2012;8:328–330. doi: 10.1038/nchembio.914.Together with references 6 and 30, shows that TDG excises 5fC and 5caC. This paper also shows a crystal structure of TDG with 5caC
- 54.Nabel CS, et al. AID/APOBEC deaminases disfavor modified cytosines implicated in DNA demethylation. Nature Chem. Biol. 2012;8:751–758. doi: 10.1038/nchembio.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song CX, et al. Genome-wide profiling of 5-formylcytosine reveals its roles in epigenetic priming. Cell. 2013;153:678–691. doi: 10.1016/j.cell.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen L, et al. Genome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics. Cell. 2013;153:692–706. doi: 10.1016/j.cell.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rai K, et al. DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell. 2008;135:1201–1212. doi: 10.1016/j.cell.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rai K, et al. DNA demethylase activity maintains intestinal cells in an undifferentiated state following loss of APC. Cell. 2010;142:930–942. doi: 10.1016/j.cell.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Popp C, et al. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463:1101–1105. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhutani N, et al. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature. 2010;463:1042–1047. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cortellino S, et al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell. 2011;146:67–79. doi: 10.1016/j.cell.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cortazar D, et al. Embryonic lethal phenotype reveals a function of TDG in maintaining epigenetic stability. Nature. 2011;470:419–423. doi: 10.1038/nature09672. [DOI] [PubMed] [Google Scholar]
- 63.Bransteitter R, Pham P, Scharff MD, Goodman MF. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc. Natl Acad Sci. USA. 2003;100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rangam G, Schmitz KM, Cobb AJ, Petersen-Mahrt SK. AID enzymatic activity is inversely proportional to the size of cytosine C5 orbital cloud. PloS one. 2012;7:e43279. doi: 10.1371/journal.pone.0043279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liutkeviciute Z, Lukinavicius G, Masevicius V, Daujotyte D, Klimasauskas S. Cytosine-5-methyltransferases add aldehydes to DNA. Nature Chem. Biol. 2009;5:400–402. doi: 10.1038/nchembio.172. [DOI] [PubMed] [Google Scholar]
- 66.Chen CC, Wang KY, Shen CK. The mammalian de novo DNA. methyltransferases, DNMT3A and DNMT3B are also DNA 5-hydroxymethylcytosine dehydroxy me myiases. J. Biol. Chem. 2012;287:33116–33121. doi: 10.1074/jbc.C112.406975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Metivier R, et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- 68.Kangaspeska S, et al. Transient cyclical methylation of promoter DNA. Nature. 2008;452:112–115. doi: 10.1038/nature06640. [DOI] [PubMed] [Google Scholar]
- 69.Hsieh CL. Evidence that protein binding specifies sites of DNA demethylation. Mol. Cell. Biol. 1999;19:46–56. doi: 10.1128/mcb.19.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin IG, Tomzynski TJ, Ou Q, Hsieh CL. Modulation of DNA binding protein affinity directly affects target site demethylation. Mol. Cell. Biol. 2000;20:2343–2349. doi: 10.1128/mcb.20.7.2343-2349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brandeis M, et al. Sp 1 elements protect a CpG island from de novo methylation. Nature. 1994;371:435–438. doi: 10.1038/371435a0. [DOI] [PubMed] [Google Scholar]
- 72.Stadler MB, et al. DNA-binding factors shape the mouse methylome at distal regulatory regions. Nature. 2011;480:490–495. doi: 10.1038/nature10716. [DOI] [PubMed] [Google Scholar]
- 73.Szulwach KE, et al. Integrating 5-Hydroxymethylcytosine into the epigenomic landscape of human embryonic stem cells. PLoS Genet. 2011;7:el002154. doi: 10.1371/journal.pgen.1002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu H, Zhang Y. Tetl and 5-hydroxymethylation: a genome-wide view in mouse embryonic stem cells. Cell Cycle. 2011;10:2428–2436. doi: 10.4161/cc.10.15.16930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stroud H, Feng S, Morey Kinney S, Pradhan S, Jacobsen SE. 5-hydroxymethylcytosine is associated with enhancers and gene bodies in human embryonic stem cells. Genome Biol. 2011;12:R54. doi: 10.1186/gb-2011-12-6-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pastor WA, et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Mature. 2011;473:394–397. doi: 10.1038/nature10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hahn MA, et al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in mammalian neurogenesis. Cell Rep. 2013;3:291–300. doi: 10.1016/j.celrep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szulwach KE, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nature Neurosci. 2011;14:1607–1616. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weber M, et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nature Genet. 2007;39:457–466. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- 80.Hackett JA, et al. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine Science 2013339448–452.Demonstrates that TET1 and TET2 oxidize 5mC en masse in PGCs [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomson JP, et al. Non-genotoxic carcinogen exposure induces defined changes in the 5-hydroxymethylome. Genome Biol. 2012;13:R93. doi: 10.1186/gb-2012-13-10-r93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lian CG, et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150:1135–1146. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raiber EA, et al. Genome-wide distribution of 5-formylcytosine in ES cells is associated with transcription and depends on thymine DNA glycosylase. Genome Biol. 2012;13:R69. doi: 10.1186/gb-2012-13-8-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu H, et al. Genome-wide analysis of 5-hydroxymethylcytosine distribution reveals its dual function in transcriptional regulation in mouse embryonic stem cells. Genes Dev. 2011;25:679–684. doi: 10.1101/gad.2036011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mellen M, Ayata P, Dewell S, Kriaucionis S, Heintz N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151:1417–1430. doi: 10.1016/j.cell.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lister R, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Straussman Rv, et al. Developmental programming of CpG island methylation profiles in the human genome. Nature Struct. Mol. Biol. 2009;16:564–571. doi: 10.1038/nsmb.1594. [DOI] [PubMed] [Google Scholar]
- 88.Weber M, et al. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nature Genet. 2005;37:853–862. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- 89. Yu M, et al. Base-re solution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell. 2012;149:1368–1380. doi: 10.1016/j.cell.2012.04.027.First genome-wide map of 5hmC at single-molecule resolution
- 90.Hsieh CL. Dependence of transcriptional repression on CpG methylation density. Mol. Cell. Biol. 1994;14:5487–5494. doi: 10.1128/mcb.14.8.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Serandour AA, et al. Dynamic hydroxymethylation of deoxyribonucleic acid marks differentiation-associated enhancers. Nucleic Acids Res. 2012;40:8255–8265. doi: 10.1093/nar/gks595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu H, et al. Dual functions of Tet 1 in transcriptional regulation in mouse embryonic stem cells. Nature. 2011;473:389–393. doi: 10.1038/nature09934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Williams K, et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473:343–348. doi: 10.1038/nature10066.Describes the distribution of TET1 and 5hmC in ES cells and demonstrates, using several methods, that TET1 recruits SIN3A to target genes
- 94.Deplus R, et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET 1 /COMPASS. EMBO J. 2013;32:645–655. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Q, Chen Y, Bian C, Fujiki R, Yu X. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature. 2013;493:561–564. doi: 10.1038/nature11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yildirim O, et al. Mbd3/NURD complex regulates expression of 5-hydroxymethylcytosine marked genes in embryonic stem cells. Cell. 2011;147:1498–1510. doi: 10.1016/j.cell.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vella P, et al. Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Mol. Cell. 2013;49:645–656. doi: 10.1016/j.molcel.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 98.Jackson-Grusby L, et al. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nature Genet. 2001;27:31–39. doi: 10.1038/83730. [DOI] [PubMed] [Google Scholar]
- 99.Fouse SD, et al. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex, and histone H3 K4/K27 trimethylation. Cell Stem Cell. 2008;2:160–169. doi: 10.1016/j.stem.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Balasubramani A, Rao A. O-GlcNAcylation and 5-methylcytosine oxidation: an unexpected association between OGT and TETs. Mol. Cell. 2013;49:618–619. doi: 10.1016/j.molcel.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling transcription, and chronic disease. Annu. Rev. Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fujiki R, et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480:557–560. doi: 10.1038/nature10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403:501–502. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]
- 104.Oswald J, et al. Active demethylation of the paternal genome in the mouse zygote. Curr. Biol. 2000;10:475–478. doi: 10.1016/s0960-9822(00)00448-6. [DOI] [PubMed] [Google Scholar]
- 105.Santos F, Hendrich B, Reik W, Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev. Biol. 2002;241:172–182. doi: 10.1006/dbio.2001.0501. [DOI] [PubMed] [Google Scholar]
- 106.Huang Y, et al. The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PLoS ONES. 2010:e8888. doi: 10.1371/journal.pone.0008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nestor C, Ruzov A, Meehan R, Dunican D. Enzymatic approaches and bisulfite sequencing cannot distinguish between 5-methylcytosine and 5-hydroxymethylcytosine in DNA. Biotechniques. 2010;48:317–319. doi: 10.2144/000113403. [DOI] [PubMed] [Google Scholar]
- 108.Jin SG, Kadam S, Pfeifer GP. Examination of the specificity of DNA methylation profiling techniques towards 5-methylcytosine and 5-hydroxymethylcytosine. Nucleic Acids Res. 2010;38:el25. doi: 10.1093/nar/gkq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Okada Y, Yamagata K, Hong K, Wakayama T, Zhang Y. A role for the elongator complex in zygotic paternal genome demethylation. Nature. 2010;463:554–558. doi: 10.1038/nature08732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wossidlo M, et al. Dynamic link of DNA demethylation, DNA strand breaks and repair in mouse zygotes. EMBO J. 2010;29:1877–1888. doi: 10.1038/emboj.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iqbal K, Jin SG, Pfeifer GP, Szabo PE. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc. Natl Acad. Sci. USA. 2011;108:3642–3647. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wossidlo M, et al. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nature Commun. 2011;2:241. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- 113. Gu TP, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477:606–610. doi: 10.1038/nature10443.Together with reference 112, proves that TET3 is responsible for mass methylcytosine oxidation in the male pronucleus. Reference 113 also establishes the phenotype of TET3-deficient oocytes
- 114.Inoue A, Shen L, Dai Q, He C, Zhang Y. Generation and replication-dependent dilution of 5fC and 5caC during mouse preimplantation development. Cell Res. 2011;21:1670–1676. doi: 10.1038/cr.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nakamura T, et al. PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature. 2012;486:415–419. doi: 10.1038/nature11093.Shows that the maternal pronucleus of the zygote and select loci on the paternal pronucleus are protected from TET3-mediated oxidation by DPPA3
- 116.Nakamura T, et al. PGC7/Stella protects against DNA demethylation in early embryogenesis. Nature Cell Biol. 2007;9:64–71. doi: 10.1038/ncb1519. [DOI] [PubMed] [Google Scholar]
- 117.Bortvin A, Goodheart M, Liao M, Page DC. Dppa3/ Pgc7 / stella is a maternal factor and is not required for germ cell specification in mice. BMC Dev. Biol. 2004;4:2. doi: 10.1186/1471-213X-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Payer B, et al. Stella is a maternal effect gene required for normal early development in mice. Curr. Biol. 2003;13:2110–2117. doi: 10.1016/j.cub.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 119.Inoue A, Zhang Y. Replication-dependent loss of 5-hydoxymethylcytosine in mouse preimplantation embryos. Science. 2011;334:194. doi: 10.1126/science.1212483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hajkova P, et al. Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway. Science. 2010;329:78–82. doi: 10.1126/science.1187945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aoki F, Worrad DM, Schultz RM. Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Dev. Biol. 1997;181:296–307. doi: 10.1006/dbio.1996.8466. [DOI] [PubMed] [Google Scholar]
- 122.Inoue A, Matoba S, Zhang Y. Transcriptional activation of transposable elements in mouse zygotes is independent of Tet3-mediated 5-methylcytosine oxidation. Cell Res. 2012;22:1640–1649. doi: 10.1038/cr.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dawlaty MM, et al. Tet 1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell. 2011;9:166–175. doi: 10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dawlaty MM, et al. Combined deficiency of Tet 1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev. Cell. 2013;24:310–323. doi: 10.1016/j.devcel.2012.12.015.Reports the phenotype of Tet1−/− Tet2−/− mice and cells and demonstrates defective imprinting in these mice
- 125.Koh KP, et al. Tetl and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Ceil Stem Cell. 2011;8:200–213. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ficz G, et al. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011;473:398–402. doi: 10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- 127.Vincent JJ, et al. Stage-specific roles for Tetl and Tet2 in DNA demethylation in primordial germ cells. Cell stem cell. 2013;12:470–478. doi: 10.1016/j.stem.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hackett JA, Zylicz JJ, Surani MA. Parallel mechanisms of epigenetic reprogramming in the germline. Trends Genet. 2012;28:164–174. doi: 10.1016/j.tig.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 129. Yamaguchi S, et al. Tetl controlsmeiosis by regulating meiotic gene expression. Nature. 2012;492:443–447. doi: 10.1038/nature11709.Demonstrates that TET1 positively regulates gene expression in PGCs and that TET 1 deficiency impairs meiosis
- 130.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 131.Cox JL, Rizzino A. Induced pluripotent stem cells: what lies beyond the paradigm shift. Exp. Biol. Med. 2010;235:148–158. doi: 10.1258/ebm.2009.009267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mikkelsen TS, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Doege CA, et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp 1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Costa Y, et al. NANOG-dependent function of TET 1 and TET2 in establishment of pluripotency. Nature. 2013;495:370–374. doi: 10.1038/nature11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gao Y, et al. Replacement of Oct4 by Tetl during iPSC induction reveals an important role of DNA methylation and hydroxymethylation in reprogramming. Cell Stem Cell. 2013;12:453–469. doi: 10.1016/j.stem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 136.Spruijt CG, et al. Dynamic readers for 5-(hydroxy) methylcytosine and its oxidized derivatives. Cell. 2013;152:1146–1159. doi: 10.1016/j.cell.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 137.Otani J, et al. Structural basis of the versatile DNA recognition ability of the methyl-CpG binding domain of methyl-CpG binding domain protein 4. J. Biol. Chem. 2013;288:6351–6362. doi: 10.1074/jbc.M112.431098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tini M, et al. Association of CBP/p300 acetylase and thymine DNA glycosylase links DNA repair and transcription. Mol. Cell. 2002;9:265–277. doi: 10.1016/s1097-2765(02)00453-7. [DOI] [PubMed] [Google Scholar]
- 139.Cortazar D, Kunz C, Saito Y, Steinacher R, Schar P. The enigmatic thymine DNA glycosylase. DNA Repair. 2007;6:489–504. doi: 10.1016/j.dnarep.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 140.Kellinger MW, et al. 5-formylcytosine 5-carboxylcytosine reduce the rate and substrate specificity of RNA polymerase II transcription. Nature Struct. Mol. Biol. 2012;19:831–833. doi: 10.1038/nsmb.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jin SG, Wu X, Li AX, Pfeifer GP. Genomic mapping of 5-hydroxymethylcytosine in the human brain. Nucleic Acids Res. 2011;39:5015–5024. doi: 10.1093/nar/gkr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Matarese F, Carrillo-de Santa Pau E, Stunnenberg HG. 5-Hydroxymethylcytosine: a new kid on the epigenetic block? Mol. Systems Biol. 2011;7:562. doi: 10.1038/msb.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hayatsu H, Shiragami M. Reaction of bisulfite with the 5-hydroxymethyl group in pyrimidines and in phage DNAs. Biochemistry. 1979;18:632–637. doi: 10.1021/bi00571a013. [DOI] [PubMed] [Google Scholar]
- 144.Huang Y, Pastor WA, Zepeda-Martinez JA, Rao A. The anti-CMS technique for genome-wide mapping of 5-hydroxymethylcytosine. Nature Protoc. 2012;7:1897–1908. doi: 10.1038/nprot.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pastor WA, Huang Y, Henderson HR, Agarwal S, Rao A. The GLIB technique for genome-wide mapping of 5-hydroxymethylcytosine. Nature Protoc. 2012;7:1909–1917. doi: 10.1038/nprot.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Robertson AB, et al. A novel method for the efficient and selective identification of 5-hydroxymethylcytosine in genomic DNA. Nucleic Acids Res. 2011;39:e55. doi: 10.1093/nar/gkr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Song CX, et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nature Biotechnol. 2011;29:68–72. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zilberman D, Henikoff S. Genome-wide analysis of DNA methylation patterns. Development. 2007;134:3959–3965. doi: 10.1242/dev.001131. [DOI] [PubMed] [Google Scholar]
- 149.Krueger F, Kreck B, Franke A, Andrews SR. DNA methylome analysis using short bisulfite sequencing date . Nature Methods. 2012;9:145–151. doi: 10.1038/nmeth.1828. [DOI] [PubMed] [Google Scholar]
- 150.Rein I, DePamphilis ML, Zorbas H. Identifying 5-methylcytosine and related modifications in DNA genomes. Nucleic Acids Res. 1998;26:2255–2264. doi: 10.1093/nar/26.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Booth MJ, et al. Quantitative sequencing of 5-methylcytosine and 5-hydroxymethylcytosine at single-base resolution. Science. 2012;336:934–937. doi: 10.1126/science.1220671. [DOI] [PubMed] [Google Scholar]
- 152.Song CX, et al. Sensitive and specific single-molecule sequencing of 5-hydroxymethylcytosine. Nature Methods. 2012;9:75–77. doi: 10.1038/nmeth.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Clark TA, et al. Enhanced 5-methylcytosine detection in single-molecule, real-time sequencing via Tetl oxidation. BMC Biol. 2013;11:4. doi: 10.1186/1741-7007-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Quivoron C, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20:25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 155.Moran-Crusio K, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ko M, et al. Ten-eleven-translocation 2 (TET2] negatively regulates homeostasis differentiation of hematopoietic stem cells in mice. Proc. Natl Acad. Sci. USA. 2011;108:14566–14571. doi: 10.1073/pnas.1112317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li Z, et al. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011;118:4509–4518. doi: 10.1182/blood-2010-12-325241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shide K, et al. TET2 is essential for survival and hematopoietic stem cell homeostasis. Leukemia. 2012;26:2216–2223. doi: 10.1038/leu.2012.94. [DOI] [PubMed] [Google Scholar]
- 159.Ng HH, et al. MBD2 is a transcriptional repressor belonging to the MeCP 1 histone deacetylase complex. Nature Genet. 1999;23:58–61. doi: 10.1038/12659. [DOI] [PubMed] [Google Scholar]
- 160.Kass SU, Landsberger N, Wolffe AP. DNA methylation directs a time-dependent repression of transcription initiation. Curr. Biol. 1997;7:157–165. doi: 10.1016/s0960-9822(97)70086-1. [DOI] [PubMed] [Google Scholar]
- 161.Ng HH, Jeppesen P, Bird A. Active repression of methylated genes by the chromosomal protein MBD1. Mol. Cell. Biol. 2000;20:1394–1406. doi: 10.1128/mcb.20.4.1394-1406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fujita N, et al. MCAF mediates MBD1-dependent transcriptional repression. Mol. Cell. Biol. 2003;23:2834–2843. doi: 10.1128/MCB.23.8.2834-2843.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sarraf SA, Stancheva I. Methyl-CpG binding protein MBD 1 couples histone H3 methylation at lysine 9 by SETDB1 to DNA replication and chromatin assembly. Mol. Cell. 2004;15:595–605. doi: 10.1016/j.molcel.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 164.Prokhortchouk A, et al. The p120 catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor. Genes Dev. 2001;15:1613–1618. doi: 10.1101/gad.198501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Valinluck V, et al. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2) Nucleic Acids Res. 2004;32:4100–4108. doi: 10.1093/nar/gkh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Prokhortchouk A, et al. Kaiso-deficient mice show resistance to intestinal cancer. Mol. Cell. Biol. 2006;26:199–208. doi: 10.1128/MCB.26.1.199-208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhao X, et al. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc. Natl Acad. Sci USA. 2003;100:6777–6782. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hendrich B, Guy J, Ramsahoye B, Wilson VA, Bird A. Closely related proteins MBD2 and MBD3 play distinctive but interacting roles in mouse development. Genes Dev. 2001;15:710–723. doi: 10.1101/gad.194101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jones PL, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 170.Ben-Shachar S, Chahrour M, Thaller C, Shaw CA, Zoghbi HY. Mouse models of MeCP2 disorders share gene expression changes in the cerebellum and hypothalamus. Hum. Mol. Genet. 2009;18:2431–2442. doi: 10.1093/hmg/ddp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Skene PJ, et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol. Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 173.Lee JH, Skalnik DG. CpG-binding protein (CXXC finger protein 1) is a component of the mammalian Set1 histone H3-Lys4 methyltransferase complex, the analogue of the yeast Set1/COMPASS complex. J. Biol. Chem. 2005;280:41725–41731. doi: 10.1074/jbc.M508312200. [DOI] [PubMed] [Google Scholar]
- 174.Choy JS, et al. DNA methylation increases nucleosome compaction and rigidity. J. Am Chem. Soc. 2010;132:1782–1783. doi: 10.1021/ja910264z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Watt F, Molloy PL. Cytosine methylation prevents binding to DNA of a HeLa cell transcription factor required for optimal expression of the adenovirus major late promoter. Genes Dev. 1988;2:1136–1143. doi: 10.1101/gad.2.9.1136. [DOI] [PubMed] [Google Scholar]
- 176.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bell AC, Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature. 2000;405:482–485. doi: 10.1038/35013100. [DOI] [PubMed] [Google Scholar]
- 178.Miranda TB, Jones PA. DNA methylation: the nuts bolts of repression. J. Cell. Physiol. 2007;213:384–390. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- 179.Wu H, et al. Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science. 2010;329:444–448. doi: 10.1126/science.1190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brinkman AB, et al. Sequential ChIP–bisulfite sequencing enables direct genome-scale investigation of chromatin and DNA methylation cross-talk. Genome Res. 2012;22:1128–1138. doi: 10.1101/gr.133728.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Acosta-Silva C, Branchadell V, Bertran J, Oliva A. Mutual relationship between stacking and hydrogen bonding in DNA. Theoretical study of guanine-cytosine, guanine-5-methylcytosine, and their dimers. J. Phys. Chem. B. 2010;114:10217–10227. doi: 10.1021/jp103850h. [DOI] [PubMed] [Google Scholar]
- 182.Thalhammer A, Hansen AS, El-Sagheer AH, Brown T, Schofield CJ. Hydroxylation of methylated CpG dinucleotides reverses stabilisation of DNA duplexes by cytosine 5-methylation. Chem. Commun. (Camb.) 2011;47:5325–5327. doi: 10.1039/c0cc05671e. [DOI] [PubMed] [Google Scholar]
- 183.Borgel J, et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nature Genet. 2010;42:1093–1100. doi: 10.1038/ng.708. [DOI] [PubMed] [Google Scholar]
- 184.Lane N, et al. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35:88–93. doi: 10.1002/gene.10168. [DOI] [PubMed] [Google Scholar]
- 185.Gkountela S, et al. The ontogeny of cKIT+ human primordial germ cells proves to be a resource for human germ line reprogramming, imprint erasure and in vitro differentiation. Nature Cell Biol. 2013;15:113–122. doi: 10.1038/ncb2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.