Abstract
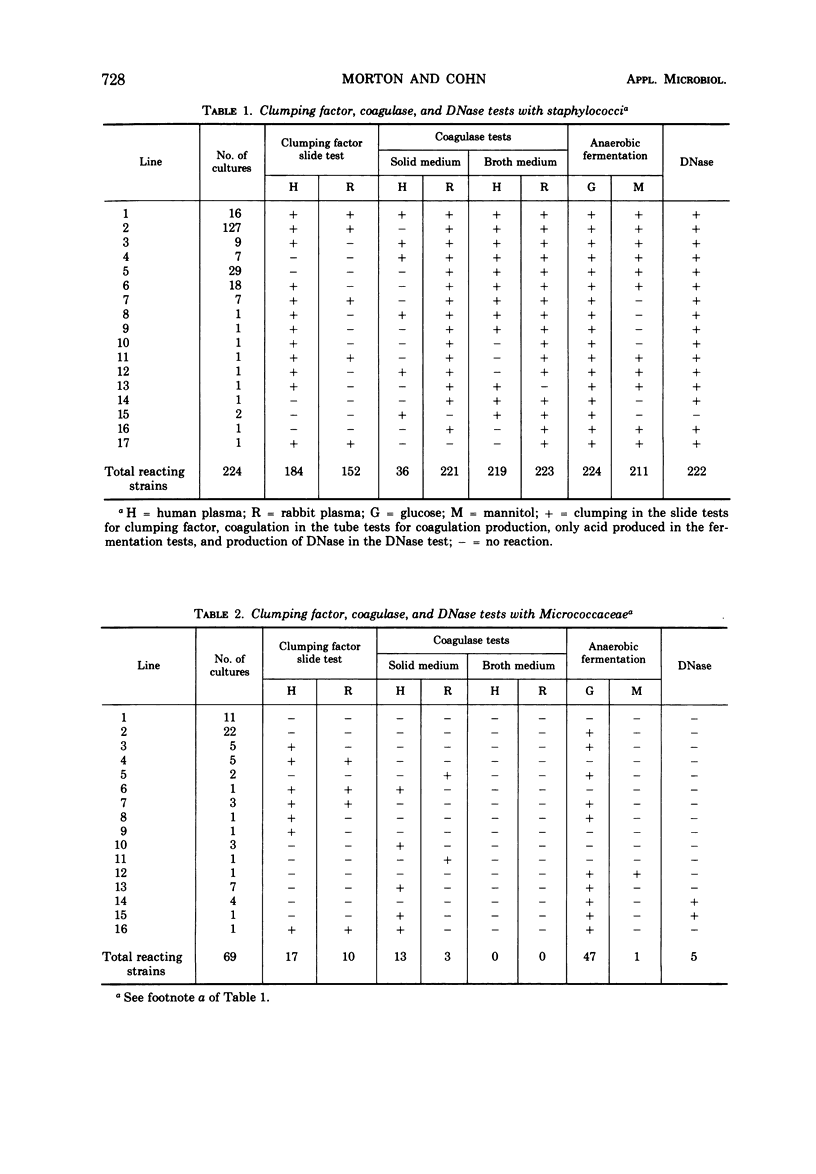
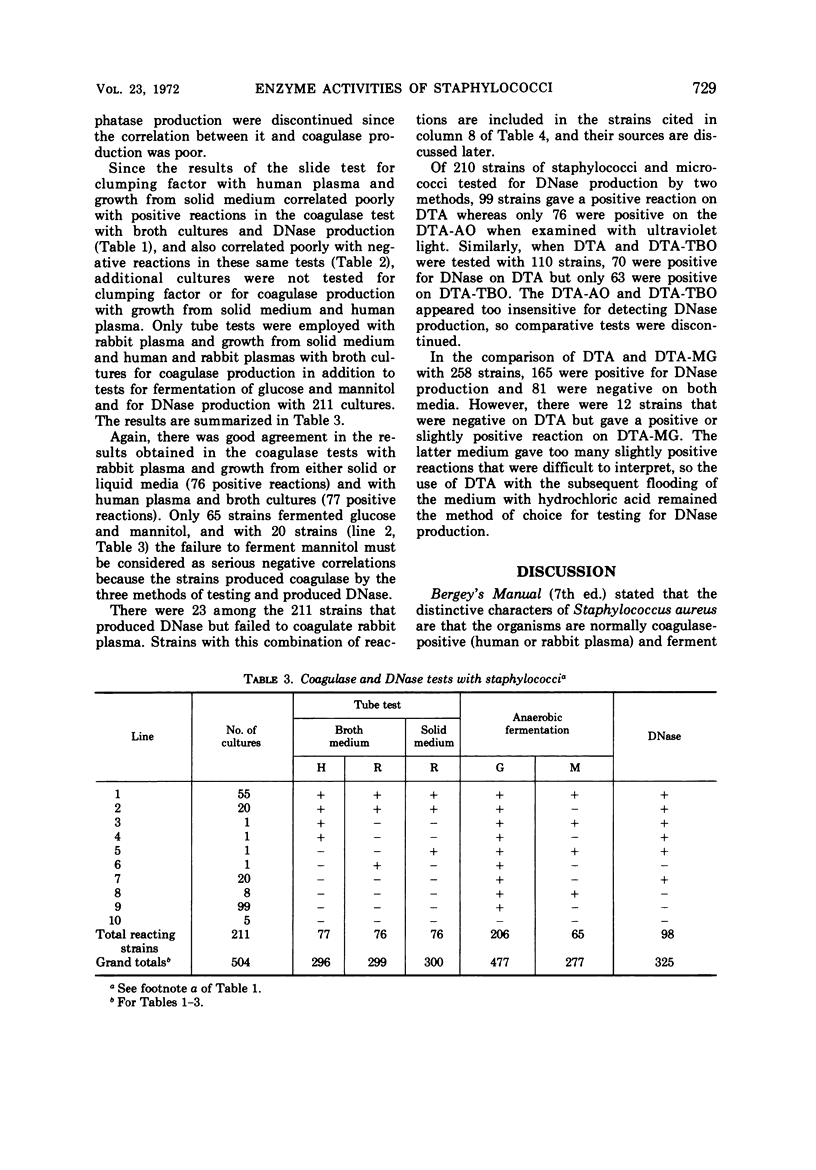
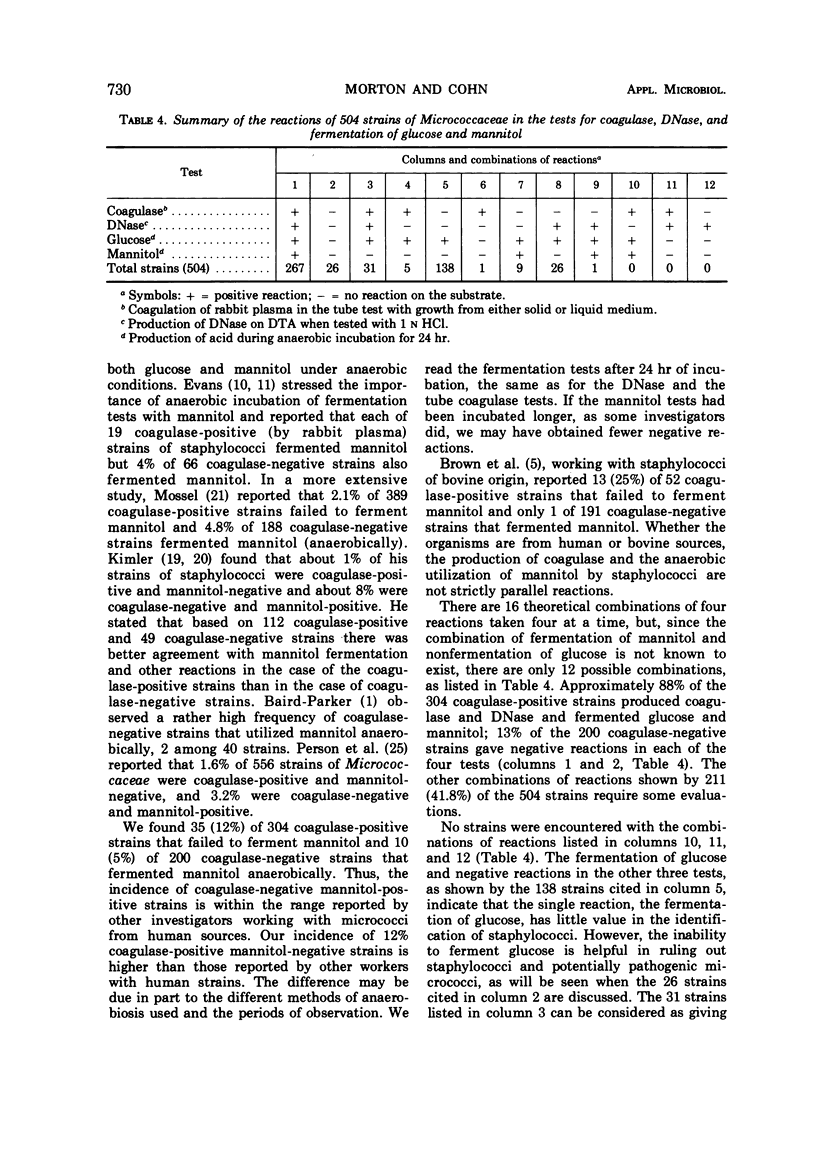
A total of 504 clinical isolates of the family Micrococcaceae were tested for coagulase, deoxyribonuclease, clumping factor, and phosphatase to determine whether there is a correlation between the results of these tests and the pathogenicity of staphylococci. In the tests for coagulase production, it was found that either human or rabbit plasma could be used with broth cultures, whereas rabbit but not human plasma was satisfactory when microorganisms removed from solid culture medium were used. Deoxyribonuclease production correlated better than the fermentation of mannitol with coagulase production. The use of methyl green, Toluidine Blue O, or acridine orange offered no advantage over the use of HCl for detecting the production of deoxyribonuclease. Neither the presence of clumping factor nor the production of phosphatase correlated well with coagulase production. Strains of staphylococci that did not produce coagulase and deoxyribonuclease were isolated as frequently as, and from a greater variety of clinical sources than, strains which produced these substances. It is concluded that the production of coagulase and deoxyribonuclease are properties of staphylococci which are not necessarily indicative of potential pathogenicity of the organisms for man.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAIRD-PARKER A. C. A classification of micrococci and staphylococci based on physiological and biochemical tests. J Gen Microbiol. 1963 Mar;30:409–427. doi: 10.1099/00221287-30-3-409. [DOI] [PubMed] [Google Scholar]
- BARBER M., KUPER S. W. A. Identification of Staphylococcus pyogenes by the phosphatase reaction. J Pathol Bacteriol. 1951 Jan;63(1):65–68. doi: 10.1002/path.1700630108. [DOI] [PubMed] [Google Scholar]
- Breckinridge J. C., Bergdoll M. S. Outbreak of food-borne gastroenteritis due to a coagulase-negative enterotoxin-producing staphylococcus. N Engl J Med. 1971 Mar 11;284(10):541–543. doi: 10.1056/NEJM197103112841010. [DOI] [PubMed] [Google Scholar]
- Brown R. W., Sandvik O., Scherer R. K., Rose D. L. Differentiation of strains of Staphylococcus epidermidis isolated from bovine udders. J Gen Microbiol. 1967 May;47(2):273–287. doi: 10.1099/00221287-47-2-273. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz N. J., Elliott D. F., Zarco R. Serum Bacteriostasis of Staphylococcus aureus. Infect Immun. 1971 May;3(5):664–670. doi: 10.1128/iai.3.5.664-670.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. B. Anaerobic Fermentation of Mannitol by Staphylococci. J Bacteriol. 1947 Aug;54(2):266–266. doi: 10.1128/jb.54.2.266-266.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. B. Studies of Staphylococci with Special Reference to the Coagulase-positive Types. J Bacteriol. 1948 Jun;55(6):793–800. doi: 10.1128/jb.55.6.793-800.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER S. The antistaphylococcal activity of human sera in vitro and its relationship to passive protective potency. Aust J Exp Biol Med Sci. 1960 Aug;38:339–346. doi: 10.1038/icb.1960.36. [DOI] [PubMed] [Google Scholar]
- JEFFRIES C. D., HOLTMAN D. F., GUSE D. G. Rapid method for determining the activity of microorganisms on nucleic acids. J Bacteriol. 1957 Apr;73(4):590–591. doi: 10.1128/jb.73.4.590-591.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINS C. J., Jr, METZGER W. I. Evalution of different substrates for the staphylococcal coagulase test and a comparison of the tube and the slide techniques. J Lab Clin Med. 1959 Jul;54(1):141–144. [PubMed] [Google Scholar]
- Jarvis J. D., Wynne C. D. A short survey of the reliability of deoxyribonuclease as an adjunct in the determination of staphylococcal pathogenicity. J Med Lab Technol. 1969 Apr;26(2):131–133. [PubMed] [Google Scholar]
- KIMLER A. Evaluation of mediums for identification of Staphylococcus aureus. Am J Clin Pathol. 1962 Jun;37:593–596. doi: 10.1093/ajcp/37.6.593. [DOI] [PubMed] [Google Scholar]
- KIMLER A. Some clinical laboratory briefs on staphylococci. J Bacteriol. 1962 Jan;83:207–208. doi: 10.1128/jb.83.1.207-208.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSSEL D. A. Attempt in classification of catalase-positive staphylococci and micrococci. J Bacteriol. 1962 Dec;84:1140–1147. doi: 10.1128/jb.84.6.1140-1147.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenn G. S., Friedman J. Isolation of mutants of Staphylococcus aureus lacking extracellular nuclease activity. J Bacteriol. 1970 Mar;101(3):921–924. doi: 10.1128/jb.101.3.921-924.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person D. A., Yu P. K., Washington J. A., 2nd Characterization of Micrococcaceae isolated from clinical sources. Appl Microbiol. 1969 Jul;18(1):95–97. doi: 10.1128/am.18.1.95-97.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn E. L., Cox F., Fisher M. The problem of associating coagulase-negative staphylococci with disease. Ann N Y Acad Sci. 1965 Jul 23;128(1):428–442. doi: 10.1111/j.1749-6632.1965.tb11652.x. [DOI] [PubMed] [Google Scholar]
- Rames L., Wise B., Goodman J. R., Piel C. F. Renal disease with Staphylococcus albus bacteremia. A complication in ventriculoatrial shunts. JAMA. 1970 Jun 8;212(10):1671–1677. [PubMed] [Google Scholar]
- SCHIMKE R. T., BLACK P. H., MARK V. H., SWARTZ M. N. Indolent Staphylococcus albus or aureus bacteremia after ventriculoatriostomy. Role of foreign body in its initiation and perpetuation. N Engl J Med. 1961 Feb 9;264:264–270. doi: 10.1056/NEJM196102092640602. [DOI] [PubMed] [Google Scholar]
- STEWARD E. E., KELLY F. C. Variation of bound coagulase of Staphylococcus aureus. J Bacteriol. 1959 Jan;77(1):101–103. doi: 10.1128/jb.77.1.101-103.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREITFELD M. M., SALLMAN B., SHOELSON S. M. Staphylocoagulase inhibition by pooled human gamma-globulin. Nature. 1959 Nov 21;184(Suppl 21):1665–1666. doi: 10.1038/1841665a0. [DOI] [PubMed] [Google Scholar]
- Schreier J. B. Modification of deoxyribonuclease test medium for rapid identification of Serratia marcescens. Am J Clin Pathol. 1969 Jun;51(6):711–716. doi: 10.1093/ajcp/51.6.711. [DOI] [PubMed] [Google Scholar]
- Smith H. B., Farkas-Himsley H. The relationship of pathogenic coagulase-negative staphylococci to Staphylococcus aureus. Can J Microbiol. 1969 Aug;15(8):879–890. doi: 10.1139/m69-157. [DOI] [PubMed] [Google Scholar]
- Smith P. B., Hancock G. A., Rhoden D. L. Improved medium for detecting deoxyribonuclease-producing bacteria. Appl Microbiol. 1969 Dec;18(6):991–993. doi: 10.1128/am.18.6.991-993.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor R., Lachica F., Weiss K. F., Deibel R. H. Relationships among coagulase, enterotoxin, and heat-stable deoxyribonuclease production by Staphylococcus aureus. Appl Microbiol. 1969 Jul;18(1):126–127. doi: 10.1128/am.18.1.126-127.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierdt C. H., Golde D. W. Deoxyribonuclease-positive Staphylococcus epidermidis strains. Appl Microbiol. 1970 Jul;20(1):54–57. doi: 10.1128/am.20.1.54-57.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


