Abstract
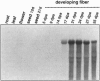
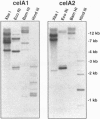
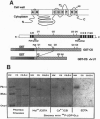
In spite of much effort, no one has succeeded in isolating and characterizing the enzyme(s) responsible for synthesis of cellulose, the major cell wall polymer of plants. We have characterized two cotton (Gossypium hirsutum) cDNA clones and identified one rice (Oryza sativa) cDNA that are homologs of the bacterial celA genes that encode the catalytic subunit of cellulose synthase. Three regions in the deduced amino acid sequences of the plant celA gene products are conserved with respect to the proteins encoded by bacterial celA genes. Within these conserved regions, there are four highly conserved subdomains previously suggested to be critical for catalysis and/or binding of the substrate UDP-glucose (UDP-Glc). An overexpressed DNA segment of the cotton celA1 gene encodes a polypeptide fragment that spans these domains and binds UDP-Glc, while a similar fragment having one of these domains deleted does not. The plant celA genes show little homology at the N- and C-terminal regions and also contain two internal insertions of sequence, one conserved and one hypervariable, that are not found in the bacterial gene sequences. Cotton celA1 and celA2 genes are expressed at high levels during active secondary wall cellulose synthesis in developing cotton fibers. Genomic Southern blot analyses in cotton demonstrate that celA forms a small gene family.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amor Y., Haigler C. H., Johnson S., Wainscott M., Delmer D. P. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9353–9357. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor Y., Mayer R., Benziman M., Delmer D. Evidence for a cyclic diguanylic acid-dependent cellulose synthase in plants. Plant Cell. 1991 Sep;3(9):989–995. doi: 10.1105/tpc.3.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Amor Y. Cellulose biosynthesis. Plant Cell. 1995 Jul;7(7):987–1000. doi: 10.1105/tpc.7.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Pear J. R., Andrawis A., Stalker D. M. Genes encoding small GTP-binding proteins analogous to mammalian rac are preferentially expressed in developing cotton fibers. Mol Gen Genet. 1995 Jul 22;248(1):43–51. doi: 10.1007/BF02456612. [DOI] [PubMed] [Google Scholar]
- Delmer D. P., Solomon M., Read S. M. Direct Photolabeling with [P]UDP-Glucose for Identification of a Subunit of Cotton Fiber Callose Synthase. Plant Physiol. 1991 Feb;95(2):556–563. doi: 10.1104/pp.95.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut D. M., Carpita N. C. Biosynthesis of plant cell wall polysaccharides. FASEB J. 1994 Sep;8(12):904–915. doi: 10.1096/fasebj.8.12.8088456. [DOI] [PubMed] [Google Scholar]
- Inoue S. B., Takewaki N., Takasuka T., Mio T., Adachi M., Fujii Y., Miyamoto C., Arisawa M., Furuichi Y., Watanabe T. Characterization and gene cloning of 1,3-beta-D-glucan synthase from Saccharomyces cerevisiae. Eur J Biochem. 1995 Aug 1;231(3):845–854. doi: 10.1111/j.1432-1033.1995.tb20770.x. [DOI] [PubMed] [Google Scholar]
- Jacob S. R., Northcote D. H. In vitro glucan synthesis by membranes of celery petioles: the role of the membrane in determining the type of linkage formed. J Cell Sci Suppl. 1985;2:1–11. doi: 10.1242/jcs.1985.supplement_2.1. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin F. C., Brown R. M., Jr, Drake R. R., Jr, Haley B. E. Identification of the uridine 5'-diphosphoglucose (UDP-Glc) binding subunit of cellulose synthase in Acetobacter xylinum using the photoaffinity probe 5-azido-UDP-Glc. J Biol Chem. 1990 Mar 25;265(9):4782–4784. [PubMed] [Google Scholar]
- Maltby D., Carpita N. C., Montezinos D., Kulow C., Delmer D. P. beta-1,3-Glucan in Developing Cotton Fibers: Structure, Localization, and Relationship of Synthesis to That of Secondary Wall Cellulose. Plant Physiol. 1979 Jun;63(6):1158–1164. doi: 10.1104/pp.63.6.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G., Thomas D. L., White A. R. Mechanism of cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol. 1995 Feb;177(4):1076–1081. doi: 10.1128/jb.177.4.1076-1081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse A. G., White S., Lightfoot R. Genes required for cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol. 1995 Feb;177(4):1069–1075. doi: 10.1128/jb.177.4.1069-1075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R., Ross P., Weinhouse H., Amikam D., Volman G., Ohana P., Calhoon R. D., Wong H. C., Emerick A. W., Benziman M. Polypeptide composition of bacterial cyclic diguanylic acid-dependent cellulose synthase and the occurrence of immunologically crossreacting proteins in higher plants. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5472–5476. doi: 10.1073/pnas.88.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert M. C., Delmer D. P. Changes in biochemical composition of the cell wall of the cotton fiber during development. Plant Physiol. 1977 Jun;59(6):1088–1097. doi: 10.1104/pp.59.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 1987;153:461–481. doi: 10.1016/0076-6879(87)53072-5. [DOI] [PubMed] [Google Scholar]
- Oikonomakos N. G., Acharya K. R., Stuart D. I., Melpidou A. E., McLaughlin P. J., Johnson L. N. Uridine(5')diphospho(1)-alpha-D-glucose. A binding study to glycogen phosphorylase b in the crystal. Eur J Biochem. 1988 May 2;173(3):569–578. doi: 10.1111/j.1432-1033.1988.tb14037.x. [DOI] [PubMed] [Google Scholar]
- Ross P., Mayer R., Benziman M. Cellulose biosynthesis and function in bacteria. Microbiol Rev. 1991 Mar;55(1):35–58. doi: 10.1128/mr.55.1.35-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena I. M., Brown R. M., Jr, Fevre M., Geremia R. A., Henrissat B. Multidomain architecture of beta-glycosyl transferases: implications for mechanism of action. J Bacteriol. 1995 Mar;177(6):1419–1424. doi: 10.1128/jb.177.6.1419-1424.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena I. M., Kudlicka K., Okuda K., Brown R. M., Jr Characterization of genes in the cellulose-synthesizing operon (acs operon) of Acetobacter xylinum: implications for cellulose crystallization. J Bacteriol. 1994 Sep;176(18):5735–5752. doi: 10.1128/jb.176.18.5735-5752.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena I. M., Lin F. C., Brown R. M., Jr Cloning and sequencing of the cellulose synthase catalytic subunit gene of Acetobacter xylinum. Plant Mol Biol. 1990 Nov;15(5):673–683. doi: 10.1007/BF00016118. [DOI] [PubMed] [Google Scholar]
- Saxena I. M., Lin F. C., Brown R. M., Jr Identification of a new gene in an operon for cellulose biosynthesis in Acetobacter xylinum. Plant Mol Biol. 1991 Jun;16(6):947–954. doi: 10.1007/BF00016067. [DOI] [PubMed] [Google Scholar]
- Wong H. C., Fear A. L., Calhoon R. D., Eichinger G. H., Mayer R., Amikam D., Benziman M., Gelfand D. H., Meade J. H., Emerick A. W. Genetic organization of the cellulose synthase operon in Acetobacter xylinum. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8130–8134. doi: 10.1073/pnas.87.20.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]