Abstract
The AP2/ERF proteins are plant-specific transcription factors involved in multiple regulatory pathways, from plant organ development to response to various environmental stresses. One of the mechanisms that regulates the AP2-like genes involves the microRNA miR172, which controls their activity at the post-transcriptional level. Extensive studies on AP2-like genes are available in many different species; however, in orchids, one of the largest plant families, studies are restricted to a few species, all belonging to the Epidendroideae subfamily. In the present study, we report the isolation of an AP2-like gene in the Mediterranean orchid Orchis italica (Orchidoideae). The OitaAP2 locus includes 10 exons and 9 introns, and its transcript is alternatively spliced, resulting in the long OitaAP2 and the short OitaAP2_ISO isoforms, with the latter skipping exon 9. Both isoforms contain the conserved target site for miR172, whose action is demonstrated by the presence of cleaved OitaAP2 mRNA. The OitaAP2 and OitaAP2_ISO mRNAs are present in the tepals and lip before and after anthesis at different expression levels. In addition, the OitaAP2_ISO isoform is expressed in the ovary before pollination and in the root and stem. The isoform-specific expression pattern suggests a functional differentiation of the OitaAP2 alternatively spliced transcripts. The expression profile of miR172 is complementary to that of the OitaAP2 isoforms in inflorescence tissues before anthesis, whereas after anthesis and in ovary tissue before and after pollination, this relationship disappears, suggesting the existence of OitaAP2 inhibitory mechanisms in these tissues that differ from that involving miR172.
Introduction
The large plant-specific superfamily APETALA2/Ethylene-Responsive element-binding Factor (AP2/ERF) includes transcription factors involved in various regulatory pathways. The highly conserved DNA-binding domain AP2 is shared by all members of the superfamily [1], [2]. Based on the number of AP2 domains and the presence of other functional domains, the superfamily is divided into three families: AP2-like/AINTEGUMENTA (ANT), ERF-like and RAV. The AP2-like/ANT family, in which two AP2 domains are present [3], includes proteins that act in multiple stages and tissues during plant development [4], [5], [6], [7]. Sequence comparison revealed that the AP2-like group can be further divided into AP2 and the RELATED TO AP2 (RAP2) groups [2]. The ERF-like family and the RAV family are both characterized by a single AP2 domain, and their members are involved in the response to various environmental stresses [8], [9], [10], [11]. In addition to the single AP2 domain, the RAV family contains the DNA-binding domain B3 [12].
The AP2-like proteins (both AP2 and RAP2) are encoded by mRNAs containing a conserved target site for the microRNA miR172 that regulates their activity at the post-transcriptional level, predominantly through mRNA cleavage and translational inhibition [13], [14], [15]. To date, a number of studies have analyzed the AP2-like genes; the majority of studies have focused on dicots and occasionally monocots (mainly Poaceae) [16], [17], [18], gymnosperms [19], [20], [21] and ferns [3].
The AP2-like genes are involved in reproductive phase transition and flower development. Within the ABCDE model of flower development [22], [23], [24], [25], the AP2-like genes belong to the A-class and, alone or together with the B-class genes, are mainly involved in the specification of the identity of outer floral whorls (sepals and petals) [4], [22], [26], [27], [28], in ovule, seed and post-embryonic development [4], [29], [30], [31], [32]. The A- and C-class functions are mutually exclusive, even though recent studies conducted in Arabidopsis demonstrated that the balance in expression of these two gene classes, moreso than the presence/absence of their gene products, regulates the correct formation of outer and inner floral whorls [27]. AP2-like proteins interact with their specific binding sites located within intron 2 of the C-class AG gene, repressing its expression [33]. In addition to their role in flower development, some AP2-like genes are also expressed in vegetative tissues [2], [34], [35].
The analysis of genes involved in the flower development pathway in model species is of great relevance (e.g., Arabidopsis and Oryza) to the understanding of the general mechanisms that govern the formation of the floral organs. However, as revealed by the extension of the study of the B- and C-class genes to non-model species (such as orchids), gene functions often do not overlap, completely or in part, between these species and model species [36], [37], [38], [39].
The family Orchidaceae is one of the largest among the flowering plants and is characterized by highly diversified flowers and reproductive strategies. The orchid flower is zygomorphic, with three outer tepals, two inner lateral tepals and an inner median tepal called labellum or lip. The male and female reproductive tissues are fused into a single structure known as column, at the base of which the ovary is located. The pollen grains (pollinia) are located at its top.
To date, studies of the AP2-like genes in orchids are restricted to species belonging to the subfamily Epidendroideae. The DcruAP2 gene of Dendrobium crumenatum is expressed in all floral organs [40], and the twelve EpAP2-like genes of Erycina pusilla are expressed at different levels in flower and vegetative tissues [41]. The function of the microRNA mir172 in the cleavage of AP2-like mRNA was also demonstrated in E. pusilla and Phalaenopsis aphrodite (Epidendroideae) [41], [42].
In this study we report the isolation, genomic characterization and expression analysis of the AP2-like gene OitaAP2 in the orchid Orchis italica (Orchidoideae) and demonstrate that it is subjected to alternative splicing. In addition, we verified the presence of cleaved products generated by the interaction of miR172 with its specific target site within the OitaAP2 mRNA, analyzed the expression pattern of miR172 within the floral tissues of O. italica and compared it to that of the OitaAP2 gene.
Materials and Methods
Isolation of the OitaAP2 cDNA
Total RNA was extracted from floral buds of O. italica using the TRIzol Reagent (Ambion). After DNase treatment (Ambion), RNA was quantified using a Nanodrop 2000c spectrophotometer (ThermoScientific) and reverse-transcribed (1 µg) using the Advantage RT-PCR kit (Clontech) and oligo dT primer. The degenerate forward primer AP2F2 (Table 1), which matches a region of the nucleotide sequence encoding the conserved AP2 domain, and the oligo dT primer were used to amplify 1 µl of cDNA using the LongAmp Taq PCR Kit (New England Biolabs), following the manufacturer instructions. The amplification products were cloned into the pGEM-T Easy vector (Promega), and several clones were sequenced using the plasmid primers T7 and SP6 and an ABI 310 Automated Sequencer (Applied Biosystems). Based on the obtained nucleotide sequences, two specific reverse primers (AP2R1 and AP2R2, Table 1) that anneal downstream of the region encoding the AP2 domains were designed to amplify the 5′-coding and UTR terminus of the putative AP2-like cDNA using the FirstChoice RLM-RACE Kit (Ambion). The amplification product was cloned and sequenced as described above. The nucleotide sequences of the 5′- and 3′- regions of the putative AP2-like gene were overlapped using the software BIOEDIT [43], and the resulting full length sequence (called OitaAP2) was used to perform BLAST analysis.
Table 1. Nucleotide sequences of the primers used to amplify the cDNA and the genomic DNA of the OitaAP2 locus of Orchis italica.
| Name | Direction | Sequence (5′-3′) | Position |
| AP2F8 | F | ATGGTGCTAGATCTCAACGTGTCAT | 1–25 |
| AP2F4new | F | CATCAAACTCCTCTGTTCTCAATG | 89–112 |
| AP2F1 | F | GATGGGARTCKCAYATYTGGGA | 509–530 |
| AP2F6 | F | GGAAGAATTTGTGCATATTCTTCGG | 690–714 |
| AP2F5 | F | CTTCACAAATGTGGGCGGTG | 766–785 |
| AP2R5 | R | CACCGCCCACATTTGTGAAG | 785–766 |
| AP2F2 | F | TGGGARGCTMGNATGGGNCARTT | 780–806 |
| AP2RealF_iso | F | CAAGAAATTGAAGGAAAGGGCCATGG | 1089–1101; 1207–1219 |
| AP2F4old | F | GAGCATCCTCATGTTTGGGGCAG | 1153–1175 |
| AP2R4old | R | CTGCCCCAAACATGAGGATGCTC | 1175–1153 |
| AP2RealF | F | TGTGTACCCCGGATTATTTCCT | 1176–1197 |
| AP2R2 | R | AGGAAATAATCCGGGGTACACA | 1197–1176 |
| AP2R1 | R | TTTCTGGGGCCAAGTGGTCATGGT | 1260–1237 |
| APF3 | F | TGCAGCATCATCAGGATTC | 1296–1314 |
| APR3 | R | GAATCCTGATGATGCTGCA | 1314–1296 |
| AP2R9 | R | TCAGCTCTGAAAGAAGTGATGACG | 1431–1407 |
| AP2R4 | R | CCTCTGGCTTCATTTGATATTGAG | 1558–1535 (5′-UTR) |
The primer positions are numbered starting from the first base of the ATG start codon (position 1) of the OitaAP2 cDNA.
Based on the BLASTX results, the amino acid sequences of AP2-like proteins were downloaded and aligned to the virtual translation of the OitaAP2 cDNA using the CLUSTAL OMEGA online tool. The resulting alignment was manually adjusted and used to construct the Maximum Likelihood tree using the software MEGA5 [44].
Several primers pairs were designed and used in PCR amplifications of the OitaAP2 cDNA to verify the existence of alternative splicing events (Table 1). All the obtained amplification fragments were cloned and sequenced as described above. The alternatively spliced form of the OitaAP2 gene was named OitaAP2_ISO.
Amplification of genomic DNA
Genomic DNA was extracted from O. italica leaf tissue [45] and used as a template in amplification reactions using several primer pairs (Table 1). PCR amplifications were performed using the LongAmp Taq PCR Kit (New England Biolabs) following the manufacturer instructions. The genomic region that includes intron 9 was particularly difficult to amplify. Different approaches were used, producing partially successful results that allowed us to map the position of intron 9. In brief, starting from 200 ng of genomic DNA of O. italica, a single strand elongation reaction was conducted using the AP2R4 primer (Table 1, Figure 1b). A poly-C tail was added to the 3′-OH end of the obtained single stranded DNA using Terminal deoxynucleotidyl transferase (Invitrogen). The reaction product was PCR-amplified using the forward primer 5′-GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG-3′ and the nested OitaAP2-specific reverse primer AP2R9 (Table 1). All amplification products were cloned and sequenced as described above. The obtained nucleotide sequences were aligned and compared to the sequence of the OitaAP2 and OitaAP2_ISO cDNAs.
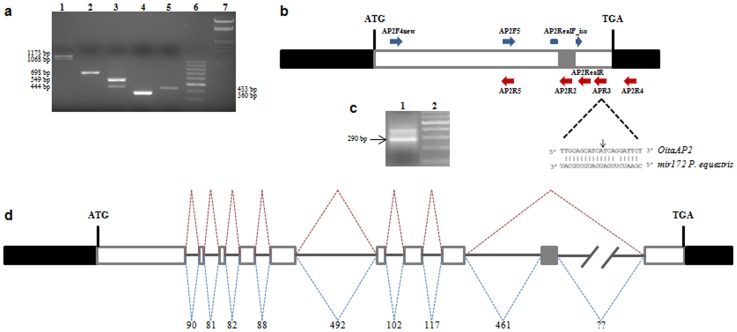
Figure 1. Alternative splicing, mir172 target site and genomic organization of the OitaAP2 gene of O. italica.
(a) Agarose gel electrophoresis of the PCR amplification products of the OitaAP2 cDNA with different primer pairs: lane 1, AP2F4new/AP2RealR; lane 2, AP2F4new/AP2R5; lane 3, AP2F5/APR3; lane 4, AP2RealF_iso/AP2R4; lane 5, AP2F5/AP2R2; lane 6, 100 bp DNA ladder (Thermo Scientific); lane 7, DNA Marker III (Thermo Scientific). (b) Schematic representation of the OitaAP2 cDNA with the relative positions of the primers used in the PCR amplifications. Black boxes represent the 5′- and 3′-UTRs; white boxes represent the exons; gray box represents the alternatively spliced region; dotted lines highlight the region corresponding to the miR172 target site with the black arrow indicating the cleavage point. (c) Agarose gel electrophoresis of the PCR amplification product of the OitaAP2 cDNA fragment cleaved by miR172 (lane 1); lane 2, 100 bp DNA ladder (Thermo Scientific). (d) Schematic representation of the genomic organization of the OitaAP2 gene. Black boxes represent the 5′- and 3′-UTRs; white boxes represent the exons; gray box represents the alternatively spliced exon; continuous lines represent introns; dotted lines indicate the two alternative splicing events; numbers indicate the length of introns (bp); question marks indicate the unknown size of intron 9.
Identification of the miR172-cleaved OitaAP2 mRNA
The 5′-end of the OitaAP2 mRNA cleavage product produced by miR172 was determined by a modified 5′-RACE [46] using the RLM-RACE GeneRace kit (Invitrogen). In brief, starting from 500 ng of total RNA extracted from early column tissue, the 5′ adaptor was ligated to the 5′-terminus of the RNA without any enzymatic treatment to remove the 5′ cap. After reverse transcription, cDNA was amplified with the GeneRace 5′ Primer and the OitaAP2 gene-specific reverse primer AP2R4 (Table 1) that anneals within the 3′-UTR, downstream of the miR172 cleavage site. The amplification product was cloned and sequenced as described above.
Expression analysis of OitaAP2 and miR172
Total RNA was extracted as described above from outer and inner tepal, lip and column dissected from inflorescence of O. italica at two different stages: before anthesis (early, ∼9 mm diameter size) and after anthesis (late). In the early stage (the bud stage), cell division and flower organ formation are already completed; however, cell elongation is still occurring. Total RNA was extracted from unpollinated ovary tissue and, after manual fertilization, from ovary tissue collected 3, 7 and 10 days after pollination (dap). Before pollination, the ovules of O. italica are immature, and the megaspore mother cell is undergoing the first meiotic division. At 3 days after pollination, the female gametophyte is completely developed; at 7 days after pollination, fertilization has occurred and the seeds are in early developmental stages; at 10 days after pollination, the seeds are almost mature with seed coats completely developed (Barone Lumaga, personal communication). In addition, total RNA was extracted from root, stem and leaf tissue. After DNase treatment, RNA was quantified as described above.
For expression analysis of the OitaAP2 gene, 350 ng of total RNA from each tissue was reverse transcribed as described above. The isoform-specific primer pairs AP2RealF/APR3 and AP2RealF_iso/APR3 (Table 1) that selectively amplify a fragment of the two alternatively spliced OitaAP2 mRNAs were used in the Real Time RT-PCR experiments.
For expression analysis of the microRNA miR172, the Poly(T) Adaptor RT-PCR method was used [47]. Starting from 350 ng of total RNA from each tissue, a poly-T adaptor was ligated to the 3′-terminus of the miRNAs and subsequently, reverse transcription was performed. The forward primer specific for miR172 was designed based on the nucleotide sequence of miR172 in P. aphrodite [42] and was used in combination with the poly-T adaptor reverse primer during the Real Time PCR experiments [47]. The Poly(T) Adaptor RT-PCR product of several samples was cloned and sequenced, confirming the specificity of the amplification reaction.
Real time PCR experiments were performed on 30 ng of first strand cDNA from each tissue using the actin OitaAct as an endogenous control gene (GenBank accession number AB630020) using the conditions previously described [38]. Reactions were run in technical and biological triplicates. The Real Time PCR Miner online tool [48] was used to calculate PCR efficiency and optimal threshold cycle (CT) for each well. The mean relative expression ratio (rER) and standard deviation of the OitaAP2 gene and of the miR172 microRNA in the different tissues were calculated within each technical triplicate using OitaAct as the endogenous control gene and leaf cDNA as the reference sample [49]. Subsequently, the mean rER relative to each tissue of each biological replicate was averaged and standard deviation was calculated. Differences in the relative expression levels of the OitaAP2 isoforms and mir172 between and/or among the different tissues were assessed by the two-tailed t test and ANOVA followed by the Tukey HSD post-hoc test, respectively.
In situ hybridization
Inflorescences before anthesis were fixed in 4% (v/v) paraformaldehyde, 0.5% (v/v) glutaraldehyde, 0.1% Triton X-100 and 4% dimethylsulfoxide in phosphate-saline buffer 1X for 16 h at 4°C [50] and then dehydrated through an ethanol series, paraffin embedded and sectioned at 7 µm.
Two different digoxigenin-labeled sense and antisense RNA probes, one corresponding to exon 9 and specific for the OitaAP2 isoform and the other including the 3′-end of exon 8 followed by the 5′-end of exon 10 and specific for the OitaAP2_ISO isoform, were synthesized using the T7 and SP6 RNA polymerases and the DIG RNA Labeling kit (Roche). Hybridization and immunological detection of the signals with alkaline phosphatase was performed using the DIG Nucleic Acid Detection kit (Roche) following the manufacturer's instructions.
Results and Discussion
The OitaAP2 cDNA: alternative splicing
The isolation of OitaAP2 cDNA was performed using first strand cDNA from the inflorescence of O. italica as a template in PCR reactions conducted in the presence of a degenerate forward primer that anneals within the region encoding the AP2 domain 1 and the poly-T reverse primer. This reaction produced two different amplification fragments of ∼1200 and 1100 bp that were cloned and sequenced. Alignment of the two sequenced fragments revealed their full nucleotide identity, excluding a 105 bp region that was absent in the shorter fragment. BLAST analysis showed that both fragments encode a partial AP2-like protein. Based on these preliminary results, specific reverse primers were designed to obtain the 5′-terminus of these cDNAs, and, subsequently, a number of forward and reverse specific primers were designed (Table 1) to verify whether the observed fragments could be the product of an alternative splicing event. PCR amplifications of the cDNA from O. italica inflorescence were conducted with different primer pairs. When the forward primer AP2F4new (which anneals 88 bp downstream of the ATG start codon) was used in combination with the AP2RealR primer (which anneals 191 bp upstream of the TGA stop codon), two amplification fragments (1173 and 1068 bp) were produced (Figure 1a, lane 1), whereas a single fragment (698 bp) was obtained when the AP2F4new primer was used in combination with the AP2R5 reverse primer (which anneals within the region encoding the AP2 domain 2) (Figure 1a, lane 2). Two amplification fragments (549 and 444 bp) were produced when the forward primer AP2F5 (reverse and complement of AP2R5) was used in combination with the APR3 reverse primer (which anneals ∼60 bp downstream of AP2RealR) (Figure 1a, lane 3). A single amplification fragment (360 bp) was obtained when the primer AP2RealF_iso, which anneals 13 bp upstream and 13 bp downstream of the region missing in the shortest fragment, was used in combination with AP2R4 (which anneals within the 3′-UTR) (Figure 1a, lane 4). A single amplification fragment (433 bp) was obtained when the forward primer AP2F5 was used in combination with the AP2R2 primer (that anneals within the region absent in the shortest fragment) (Figure 1a, lane 5). The relative primer positions are shown in Figure 1b.
These results might be explained by a number of different phenomena (e.g. gene duplication, transposition events, etc). However, all the evidences strongly suggest the existence of an alternative splicing event resulting in two isoforms of different sizes named OitaAP2 and OitaAP2_ISO. The deletion of 105 bp, which differentiates the two isoforms, is in frame with the main ORF. The entire cDNA sequence of the OitaAP2 cDNA of O. italica is 2264 bp (GenBank accession number KF152921), whereas the size of the OitaAP2_ISO cDNA is 2159 bp (accession number KF152922). Both OitaAP2 and OitaAP2_ISO include identical 5′- and 3′-UTRs of 560 and 273 bp, respectively.
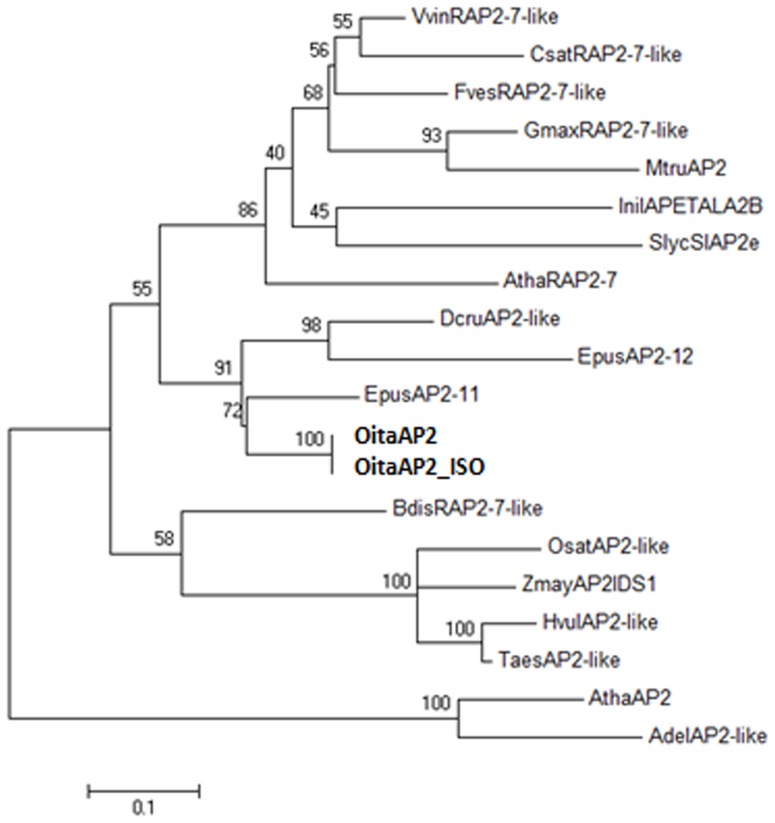
BLAST analysis revealed the highest similarity with the AP2-like loci EpAP2-11 of the orchid Erycina pusilla (74% nucleotide and 68% amino acid identity) and DcruAP2 of Dendrobium crumenatum (72% nucleotide and 60% amino acid identity), followed by the RAP2-7-like genes of Glycine max, Vitis vinifera and other species. The amino acid alignment of the virtually translated sequence of the OitaAP2 (476 residues) and OitaAP2_ISO (441 residues) cDNAs with the AP2-like sequences of different species retrieved from GenBank shows the presence of the two conserved AP2 domains (AP2R1 and AP2R2). In addition, upstream of the AP2 domains are the canonical Motifs 1 and 2 and a nuclear localization signal. A short conserved amino acid stretch located at the C-terminus corresponds to the target site of the microRNA miR172 on the AP2 mRNA (data not shown). The maximum likelihood (ML) tree shows that the OitaAP2 and OitaAP2_ISO sequences are positioned within the RAP2 group of the AP2-like transcription factors, strictly related to the sequences of the other orchid species (Figure 2). All these features indicate that the OitaAP2 locus of O. italica is member of the AP2-like gene subfamily, where it seems to be orthologous to RAP2-7.
Figure 2. Maximum likelihood tree constructed on the alignment of the AP2-like amino acid sequences belonging to both AP2 and RAP2 groups.
Numbers indicate the bootstrap percentages (on 1000 replicates). The abbreviations used (those obtained in the present study are in bold) are in agreement with the GenBank definitions. Actinidia deliciosa AdelAP2-like (AER60526); Arabidopsis thaliana AthaRAP2-7 and AthaAP2 (NP_001189625 and NP_195410, respectively); Brachypodium distachyon BdisRAP2-7-like (XP_003569031); Cucumis sativus CsatRAP2-7-like (XP_004148250); Dendrobium crumenatum DcruAP2-like (AAZ95247); Erycina pusilla EpAP2-11 and EpAP2-12 (AGI62047 and AGI62048, respectively); Fragaria vesca FvesRAP2-7-like (XP_004295997); Glycine max GmaxRAP2-7-like (XP_003542008); Hordeum vulgare HvulAP2-like (AAL50205); Ipomea nil InilAPETALA2B (BAD36744); Medicago truncatula MtruAP2 (XP_003606515); Orchis italica OitaAP2 and OitaAP2_ISO (KF152921 and KF152922, respectively); Oryza sativa OsatAP2-like (AAO65862); Solanum lycopersicum SlycSlAP2e (NP_001233891); Triticum aestivum TaesAP2-like (AAU88192); Vitis vinifera VvinRAP2-7-like (XP_002284749); Zea mays ZmayAP2IDS1 (NP_001104904).
Both the OitaAP2 and OitaAP2_ISO cDNAs contain the conserved 21 bp sequence that represents the target site of miR172 on the mRNA. In Arabidopsis, miR172 represents a negative post-transcriptional regulator of AP2; it cleaves the AP2 mRNA and acts predominantly by translational inhibition [14], [15], [51]. This regulatory mechanism seems to be conserved among plant species [52], [53], [54]. Using a modified 5′RACE reaction [46], the miR172 5′-end cleavage product of the OitaAP2/OitaAP2_ISO mRNA was successfully amplified (Figure 1c). Cloning and sequencing of this fragment (∼290 bp) revealed the position of the cleavage site within the miR172 target site (Figure 1b). The nucleotide sequence of the other fragment (∼390 bp) detectable in Figure 1c showed it is a PCR artifact. According to the results obtained in P. aphrodite and E. pusilla [41], [42], this finding demonstrates that in orchids, the regulatory mechanism that determines the translational repression of OitaAP2 through miR172 is conserved.
Genomic structure of the OitaAP2 locus
Based on the differential splicing observed for the OitaAP2 mRNA, it was necessary to characterize the genomic organization of the OitaAP2 locus of O. italica. To evaluate and compare the structure of the OitaAP2 gene with that of known AP2-like genes, the OitaAP2 locus was amplified from genomic DNA using multiple primer pairs. Sequence comparison of the OitaAP2 and OitaAP2_ISO cDNAs with the genomic sequence of the OitaAP2 locus (accession number KF152923) revealed the presence of 10 exons and 9 introns (Figure 1d).
This gene structure appears conserved for the AP2 genes of Arabidopsis, grapevine [55] and apple [56], all of which constitute 10 exons and 9 introns. The twelve AP2-like genes of the orchid E. pusilla show an intron number ranging from 7 to 11; however, the specific structure of the EpAP2-11 gene, the putative ortholog of OitaAP2, is not reported [41]. The size of the exons and the position of the introns are quite conserved among the AP2-like genes. All the introns of the OitaAP2 gene have a relatively small size (ranging from 81 to 492 bp), with the only exception possibly represented by the intron 9. Numerous attempts to amplify this intron were unsuccessful; however, the only approach that resulted in a positive result allowed us to map the position and obtain a partial sequence of intron 9 (∼340 bp). Two hypotheses could explain the difficulties in PCR amplification of this genomic region: the great size of intron 9 and/or its base composition, which might inhibit the polymerase activity. As the amplification condition tested allowed us to amplify large introns (up to 16,000 bp) in other orchid genes (e.g., OitaAG and OitaSTK) [39] and the single strand DNA extension was interrupted a few hundred nucleotides upstream of the 3′-end of intron 9, the hypothesis of a complex base composition seems more likely than that of an intron size greater than 16,000 bp.
All the introns identified contain the canonical 5′GT and 3′AT, with the only exception being intron 4, which presents a non-canonical 5′GC donor splicing site. CENSOR analysis of the intron sequences showed traces of mobile elements belonging to class I and II transposable elements within the longest intron 5 (Chapaev-16_HM), intron 8 (Copia-10_TC-I) and part of the sequence of intron 9 (CR1-23_CQ) of OitaAP2. This result further confirms the abundance of mobile elements within orchid genomes, as detected for the OitaAG and OitaSTK genes [39].
Nucleotide comparison of the genomic sequence of the OitaAP2 locus with the OitaAP2 and OitaAP2_ISO cDNA reveals that the isoform OitaAP2_ISO is produced through differential splicing of the region spanning intron 8 to intron 9, with skipping of exon 9 (105 bp) that preserves the correct reading frame (Figure 3). Alternative splicing of the AP2-like genes was reported in Arabidopsis within the ANT group [41] and more recently in kiwifruit within the AP2 group [54]. To date, alternative splicing of the RAP2-like genes has not been described. Similarly to O. italica, the region involved in the alternative splicing of the AdAP2 locus of kiwifruit spans from intron 8 to 9 and includes an unusually large intron. However, the alternative kiwifruit isoform AdAP2Δ, whose functional significance is unknown, retains an additional fragment (named exon 9a) that alters the main reading frame and introduces a stop codon and a putative polyadenylation site, excluding the miR172 target site from the transcript.
Figure 3. Region of the OitaAP2 locus of O. italica involved in the alternative splicing.
Nucleotide alignment spans from exon 8 to exon 10 and includes intron sequences. Boxes indicate exons; Ns indicate unknown nucleotides of intron 9.
Expression pattern of the OitaAP2 isoforms
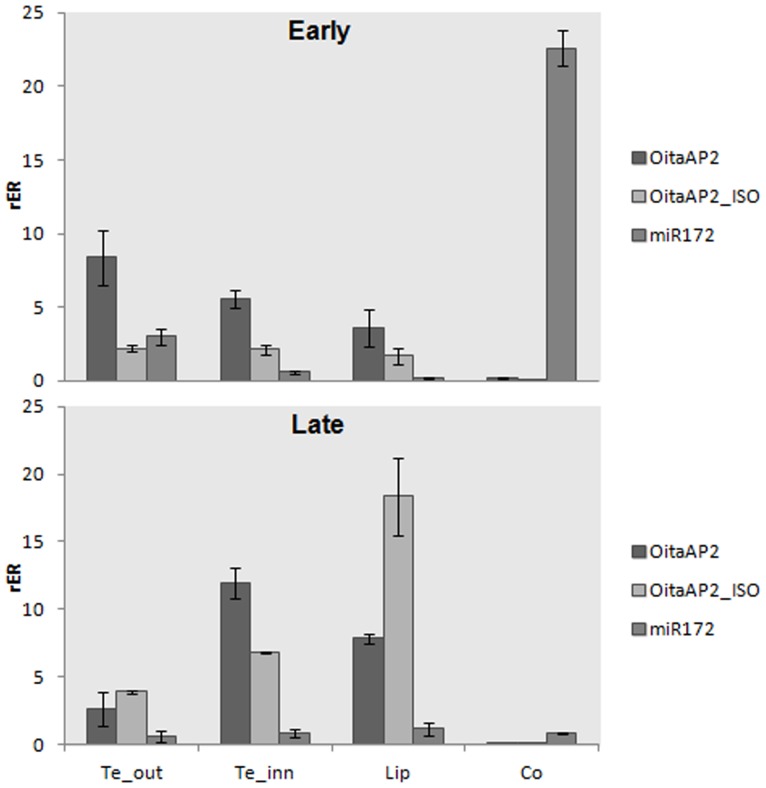
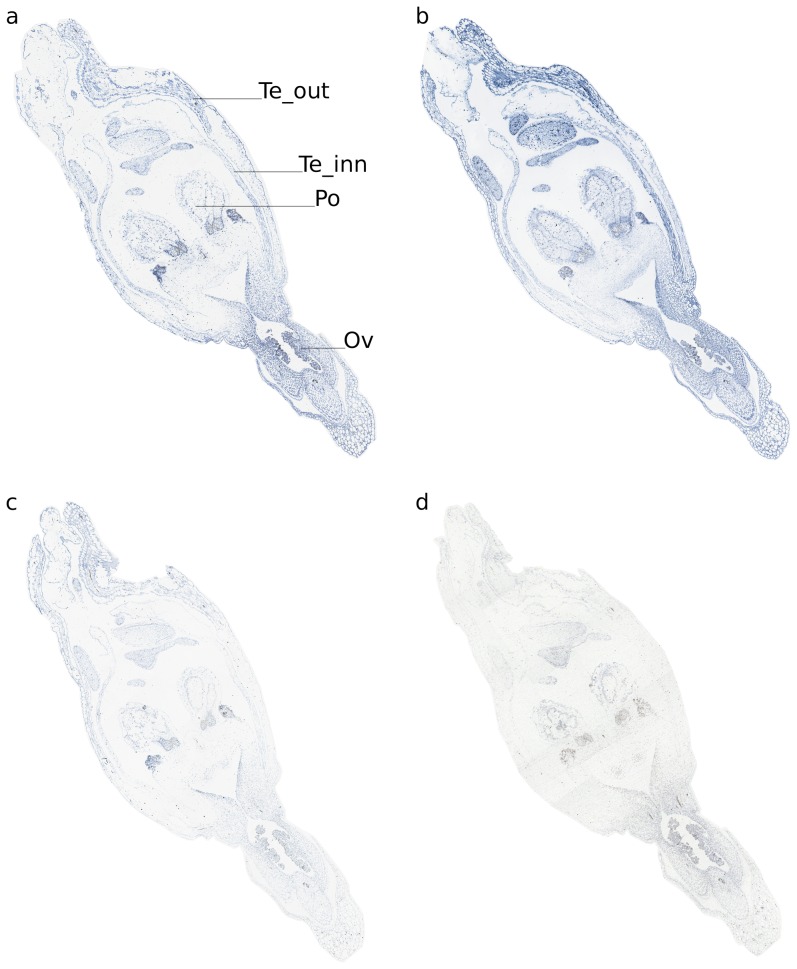
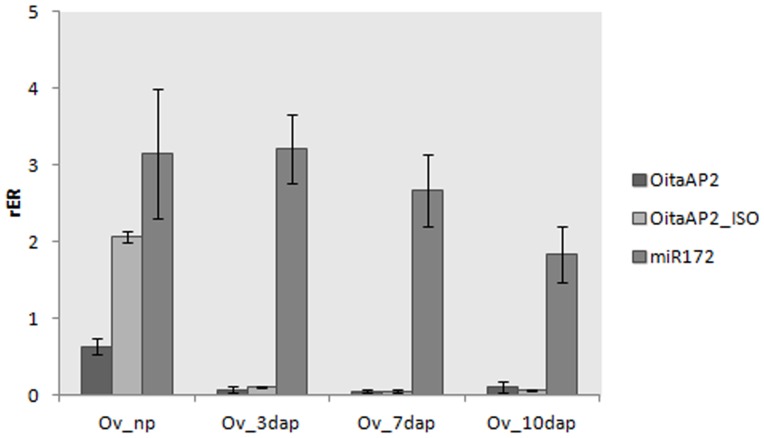
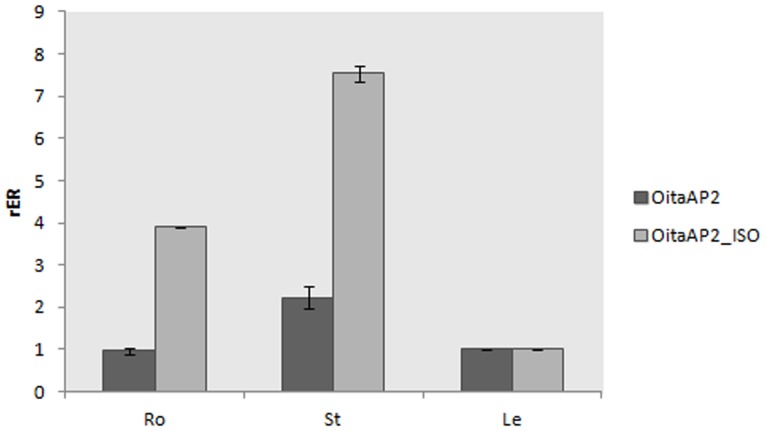
Real-time RT-PCR experiments were performed to reveal the expression pattern of the OitaAP2 gene in O. italica. Isoform-specific reverse primers were used to distinguish between the expression profile of OitaAP2 and OitaAP2_ISO in floral tissues from early and late inflorescence. The melting curve plots demonstrated the effectiveness of the primers used. The mean PCR efficiency for each gene (the target isoforms OitaAP2 and OitaAP2_ISO and the endogenous control OitaAct) showed comparable values (data not shown). Both isoforms are ubiquitously expressed in the perianth in early and late inflorescence and are absent in the column (Figure 4). Specifically, in the early inflorescence OitaAP2 is more highly expressed in the outer and inner tepals than in the lip, whereas in the same tissues OitaAP2_ISO is expressed at levels always significantly lower than those detected for OitaAP2. In late inflorescence, the expression level of OitaAP2 decreases in the outer tepals and increases in the inner tepals and lip, whereas the amount of OitaAP2_ISO mRNA increases in outer and inner tepals and, more strongly, in the lip (Figure 4). RNA in situ hybridization confirms the expression of the OitaAP2 and presumably of the OitaAP2_ISO isoform in all the organs of the perianth (Figure 5). The relative expression ratio of OitaAP2 and OitaAP2_ISO was evaluated also in ovary tissue before and after pollination (at 3, 7 and 10 dap) (Figure 6). Both isoforms are expressed only in ovary before pollination, where the OitaAP2_ISO is significantly more abundant than OitaAP2. RNA in situ hybridization confirms the expression of the OitaAP2 and presumably of the OitaAP2_ISO isoform in the ovary tissue before pollination (Figure 5). Relative expression analysis of OitaAP2 and OitaAP2_ISO in vegetative tissues revealed very low amounts of OitaAP2 mRNA, with a slight increase in stem tissue. In contrast, the expression of the OitaAP2_ISO isoform is significantly higher in root and stem tissue (Figure 7).
Figure 4. Relative expression ratio (rER) of OitaAP2, OitaAP2_ISO and mir172 in different tissues of O. italica at early and late stages.
Te_out, outer tepal; Te_inn, inner tepal; Co, column. Bars represent standard deviation of the biological replicates.
Figure 5. RNA in situ hybridization of OitaAP2 and OitaAP2_ISO in inflorescence tissue of O. italica.
Sections were hybridized with OitaAP2 and OitaAP2_ISO antisense (a, b) and sense (c, d) probes. Te_out, outer tepal; Te_inn, inner tepal; Co, column; Ov, ovary; Po, pollinia.
Figure 6. Relative expression ratio (rER) of OitaAP2, OitaAP2_ISO and mir172 in ovary tissue of O. italica before and after pollination.
Ov_np, unpollinated ovary; Ov_3dap, Ov_7dap, and Ov_10dap, ovary 3, 7 and 10 days after pollination, respectively. Bars represent standard deviation of the biological replicates.
Figure 7. Relative expression ratio (rER) of OitaAP2 and OitaAP2_ISO in vegetative tissues of O. italica.
Ro, root; St, stem; Le, leaf. Bars represent standard deviation of the biological replicates.
In orchids, the DcruAP2 gene of D. crumenatum is expressed in all floral organs and leaves without differential splicing [40], and ML phylogenetic analysis revealed that this gene is orthologous to the EpAP2-12 gene of E. pusilla, whereas the OitaAP2 gene of O. italica is orthologous to the EpAP2-11 gene (Figure 2), homologous to the RAP2.7 gene of Arabidopsis and expressed both in floral and vegetative tissues [41]. Studies conducted in Arabidopsis, rice and grapevine confirmed the expression of the RAP2-7-like genes in floral and vegetative tissues [2], [34], [35]. However, differential splicing has never been described for these genes. In O. italica, the expression pattern of both isoforms, OitaAP2 and OitaAP2_ISO, is restricted to the outer and inner tepals and lip and is absent in the fused reproductive organs (column). The different expression level of the two isoforms, in particular in the lip from the late inflorescence, where the highest expression of the OitaAP2_ISO isoform is detected, might reflect a possible functional partition of the two isoforms in the development and maintenance of the perianth organs. The expression profile detected in the ovary before pollination and in vegetative tissues seems to confirm non-redundant functions of OitaAP2 and OitaAP2_ISO, with the latter specifically involved in ovary formation (but not in post-pollination processes) and in root- and stem-specific functions. However, further studies are needed to confirm this hypothesis and to verify the presence of two AP2 isoforms in other orchid species.
Comparative expression analysis of the OitaAP2 isoforms and miR172
AP2-like genes are negatively regulated by the microRNA miR172. To evaluate the relationship existing between OitaAP2 and mir172 in O. italica, their relative expression patterns in different floral tissues was compared (Figure 4).
In the tissues from early inflorescence, the expression profile of miR172 was clearly complementary to that observed for the OitaAP2 isoforms. In particular, miR172 appears to be expressed mainly in the column from early inflorescence and is almost absent in the other tissues. This pattern fully agrees with the repressive action of mir172 on the OitaAP2 isoforms. Surprisingly, in the floral tissues from late inflorescence, miR172 is expressed at very low levels in all tissues, including the column. This behavior suggests that in O. italica the inhibitory role of miR172 on the OitaAP2 transcripts is exerted until the stages preceding the anthesis, whereas the absence of OitaAP2 mRNA in the column after anthesis might be related to different regulatory mechanisms.
In the ovary tissue, miR172 is expressed from pre-pollinated ovary to 10 days after pollination, as observed in Arabidopsis [27], showing its possible involvement in ovary development. However, in the ovary tissue of O. italica, the miR172 expression pattern is not complementary to that observed for the OitaAP2 isoforms (Figure 6). These results also suggest that during ovary maturation the inhibition of the OitaAP2 isoforms might not be directly realized through miR172-mediated cleavage.
Acknowledgments
The authors are grateful to Mrs. Rosaria Terracciano and Mr. Enzo Iacueo for their technical support. The authors wish to thank Prof. Salvatore Cozzolino for supplying the plant material and performing the manual pollination of ovaries in O. italica and Dr. Maria Rosaria Barone Lumaga for the description of the ovary development stages.
Funding Statement
This work was supported by the 2009 Regione Campania Grant L.R. N5/2002. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Weigel D (1995) The APETALA2 domain is related to a novel type of DNA binding domain. Plant Cell 7: 388–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Okamuro JK, Caster B, Villarroel R, Van Montagu M, Jofuku KD (1997) The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc Natl Acad Sci U S A 94: 7076–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim S, Soltis PS, Wall K, Soltis DE (2006) Phylogeny and domain evolution in the APETALA2-like gene family. Mol Biol Evol 23: 107–120. [DOI] [PubMed] [Google Scholar]
- 4. Jofuku KD, den Boer BG, Van Montagu M, Okamuro JK (1994) Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6: 1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klucher KM, Chow H, Reiser L, Fischer RL (1996) The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. Plant Cell 8: 137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riechmann JL, Meyerowitz EM (1998) The AP2/EREBP family of plant transcription factors. Biol Chem 379: 633–646. [DOI] [PubMed] [Google Scholar]
- 7. Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, et al. (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14: 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, et al. (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10: 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haake V, Cook D, Riechmann JL, Pineda O, Thomashow MF, et al. (2002) Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol 130: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, et al. (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J 33: 751–763. [DOI] [PubMed] [Google Scholar]
- 11. Gutterson N, Reuber TL (2004) Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr Opin Plant Biol 7: 465–471. [DOI] [PubMed] [Google Scholar]
- 12. Kagaya Y, Ohmiya K, Hattori T (1999) RAV1, a novel DNA-binding protein, binds to bipartite recognition sequence through two distinct DNA-binding domains uniquely found in higher plants. Nucleic Acids Res 27: 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, et al. (2002) Prediction of plant microRNA targets. Cell 110: 513–520. [DOI] [PubMed] [Google Scholar]
- 14. Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell 15: 2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen X (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303: 2022–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chuck G, Meeley R, Hake S (2008) Floral meristem initiation and meristem cell fate are regulated by the maize AP2 genes ids1 and sid1. Development 135: 3013–3019. [DOI] [PubMed] [Google Scholar]
- 17. Gil-Humanes J, Piston F, Martin A, Barro F (2009) Comparative genomic analysis and expression of the APETALA2-like genes from barley, wheat, and barley-wheat amphiploids. BMC Plant Biol 9: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sharoni AM, Nuruzzaman M, Satoh K, Shimizu T, Kondoh H, et al. (2011) Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol 52: 344–360. [DOI] [PubMed] [Google Scholar]
- 19. Vahala T, Oxelman B, von Arnold S (2001) Two APETALA2-like genes of Picea abies are differentially expressed during development. J Exp Bot 52: 1111–1115. [DOI] [PubMed] [Google Scholar]
- 20. Nilsson L, Carlsbecker A, Sundas-Larsson A, Vahala T (2007) APETALA2 like genes from Picea abies show functional similarities to their Arabidopsis homologues. Planta 225: 589–602. [DOI] [PubMed] [Google Scholar]
- 21. Shigyo M, Ito M (2004) Analysis of gymnosperm two-AP2-domain-containing genes. Dev Genes Evol 214: 105–114. [DOI] [PubMed] [Google Scholar]
- 22. Bowman JL, Smyth DR, Meyerowitz EM (1991) Genetic interactions among floral homeotic genes of Arabidopsis. Development 112: 1–20. [DOI] [PubMed] [Google Scholar]
- 23. Coen ES, Meyerowitz EM (1991) The War of the Whorls - Genetic Interactions Controlling Flower Development. Nature 353: 31–37. [DOI] [PubMed] [Google Scholar]
- 24. Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405: 200–203. [DOI] [PubMed] [Google Scholar]
- 25. Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, et al. (2003) Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424: 85–88. [DOI] [PubMed] [Google Scholar]
- 26. Kunst L, Klenz JE, Martinez-Zapater J, Haughn GW (1989) AP2 Gene Determines the Identity of Perianth Organs in Flowers of Arabidopsis thaliana. Plant Cell 1: 1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wollmann H, Mica E, Todesco M, Long JA, Weigel D (2010) On reconciling the interactions between APETALA2, miR172 and AGAMOUS with the ABC model of flower development. Development 137: 3633–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yant L, Mathieu J, Dinh TT, Ott F, Lanz C, et al. (2010) Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22: 2156–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jofuku KD, Omidyar PK, Gee Z, Okamuro JK (2005) Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc Natl Acad Sci U S A 102: 3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohto MA, Fischer RL, Goldberg RB, Nakamura K, Harada JJ (2005) Control of seed mass by APETALA2. Proc Natl Acad Sci U S A 102: 3123–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wurschum T, Gross-Hardt R, Laux T (2006) APETALA2 regulates the stem cell niche in the Arabidopsis shoot meristem. Plant Cell 18: 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guillaumot D, Lelu-Walter MA, Germot A, Meytraud F, Gastinel L, et al. (2008) Expression patterns of LmAP2L1 and LmAP2L2 encoding two-APETALA2 domain proteins during somatic embryogenesis and germination of hybrid larch (Larix x marschlinsii). J Plant Physiol 165: 1003–1010. [DOI] [PubMed] [Google Scholar]
- 33. Dinh TT, Girke T, Liu X, Yant L, Schmid M, et al. (2012) The floral homeotic protein APETALA2 recognizes and acts through an AT-rich sequence element. Development 139: 1978–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Licausi F, Giorgi FM, Zenoni S, Osti F, Pezzotti M, et al. (2010) Genomic and transcriptomic analysis of the AP2/ERF superfamily in Vitis vinifera. BMC Genomics 11: 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rashid M, Guangyuan H, Guangxiao Y, Hussain J, Xu Y (2012) AP2/ERF Transcription Factor in Rice: Genome-Wide Canvas and Syntenic Relationships between Monocots and Eudicots. Evol Bioinform Online 8: 321–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zahn LM, Leebens-Mack J, DePamphilis CW, Ma H, Theissen G (2005) To B or Not to B a flower: the role of DEFICIENS and GLOBOSA orthologs in the evolution of the angiosperms. J Hered 96: 225–240. [DOI] [PubMed] [Google Scholar]
- 37. Mondragon-Palomino M, Theissen G (2011) Conserved differential expression of paralogous DEFICIENS- and GLOBOSA-like MADS-box genes in the flowers of Orchidaceae: refining the ‘orchid code’. Plant J 66: 1008–1019. [DOI] [PubMed] [Google Scholar]
- 38. Salemme M, Sica M, Gaudio L, Aceto S (2011) Expression pattern of two paralogs of the PI/GLO-like locus during Orchis italica (Orchidaceae, Orchidinae) flower development. Dev Genes Evol 221: 241–246. [DOI] [PubMed] [Google Scholar]
- 39. Salemme M, Sica M, Gaudio L, Aceto S (2013) The OitaAG and OitaSTK genes of the orchid Orchis italica: a comparative analysis with other C- and D-class MADS-box genes. Mol Biol Rep 40: 3523–3535. [DOI] [PubMed] [Google Scholar]
- 40. Xu Y, Teo LL, Zhou J, Kumar PP, Yu H (2006) Floral organ identity genes in the orchid Dendrobium crumenatum. Plant J 46: 54–68. [DOI] [PubMed] [Google Scholar]
- 41. Lin CS, Chen JJ, Huang YT, Hsu CT, Lu HC, et al. (2013) Catalog of Erycina pusilla miRNA and categorization of reproductive phase-related miRNAs and their target gene families. Plant Mol Biol 82: 193–204. [DOI] [PubMed] [Google Scholar]
- 42. An FM, Hsiao SR, Chan MT (2011) Sequencing-based approaches reveal low ambient temperature-responsive and tissue-specific microRNAs in phalaenopsis orchid. PLoS One 6: e18937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41: 95–98. [Google Scholar]
- 44. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small amounts of leaf tissue. Phytochem Bull 19: 11–15. [Google Scholar]
- 46. Llave C, Xie Z, Kasschau KD, Carrington JC (2002) Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297: 2053–2056. [DOI] [PubMed] [Google Scholar]
- 47. Shi R, Sun YH, Zhang XH, Chiang VL (2012) Poly(T) adaptor RT-PCR. Methods Mol Biol 822: 53–66. [DOI] [PubMed] [Google Scholar]
- 48. Zhao S, Fernald RD (2005) Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol 12: 1047–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H (2006) Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression's CT difference” formula. J Mol Med 84: 901–910. [DOI] [PubMed] [Google Scholar]
- 50.Javelle M, Marco CF, Timmermans M (2011) In situ hybridization for the precise localization of transcripts in plants. J Vis Exp: e3328. [DOI] [PMC free article] [PubMed]
- 51. Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, et al. (2005) Specific effects of microRNAs on the plant transcriptome. Dev Cell 8: 517–527. [DOI] [PubMed] [Google Scholar]
- 52. Lauter N, Kampani A, Carlson S, Goebel M, Moose SP (2005) microRNA172 down-regulates glossy15 to promote vegetative phase change in maize. Proc Natl Acad Sci U S A 102: 9412–9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chuck G, Meeley R, Irish E, Sakai H, Hake S (2007) The maize tasselseed4 microRNA controls sex determination and meristem cell fate by targeting Tasselseed6/indeterminate spikelet1. Nat Genet 39: 1517–1521. [DOI] [PubMed] [Google Scholar]
- 54. Varkonyi-Gasic E, Lough RH, Moss SM, Wu R, Hellens RP (2012) Kiwifruit floral gene APETALA2 is alternatively spliced and accumulates in aberrant indeterminate flowers in the absence of miR172. Plant Mol Biol 78: 417–429. [DOI] [PubMed] [Google Scholar]
- 55. Velasco R, Zharkikh A, Troggio M, Cartwright DA, Cestaro A, et al. (2007) A high quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS One 2: e1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, et al. (2010) The genome of the domesticated apple (Malus x domestica Borkh.). Nat Genet 42: 833–839. [DOI] [PubMed] [Google Scholar]