Abstract
Purpose
The aim of this study was to assess clinical correlations with postoperative alteration of p16 DNA methylation, and to clarify whether postoperative changes in the serum DNA methylation status of p16 could be used as a reliable prognostic factor for gastric cancer.
Materials and Methods
Fifty-three consecutive gastric adenocarcinoma patients who underwent gastric resection (Chung-Ang University Hospital, Seoul, Korea) were included. DNA methylation of p16 was evaluated by methylation-specific polymerase chain reaction using serum DNA preoperatively and at the 10th postoperative day. The correlation between changes in methylation status and patients' prognosis was analyzed.
Results
p16 was methylated in 79.2% of preoperative serum DNA and in 54.7% of postoperative serum DNA, respectively. Methylation in p16 disappeared more frequently in patients who underwent standard D2 lymphadenectomy compared to those who underwent modified D1+ lymphadenectomy (P=0.016). Whereas methylation of preoperative serum DNA was not correlated with survival, patients with postoperative disappearance of p16 methylation showed longer survival than those without postoperative disappearance of p16 methylation in the patients who had gastric cancer with lymph node metastasis (P=0.042).
Conclusions
Postoperative disappearance of p16 methylation could be an available prognostic factor for node-positive gastric cancer.
Keywords: Stomach neoplasms; Methylation; Genes, p16
Introduction
The most effective method for treatment of gastric cancer is surgical resection including radical lymph node dissection and combined resection of the invaded organ. Improved surgical management has played an important role in treatment outcomes.1 Many studies have indicated that surgical curability is one of the most valuable prognostic factors together with the pathologic stage.2,3 However, patients may experience tumor recurrence after curative resection, and the recurrence pattern and survival outcomes differ among individual patients even at the same tumor stage. Thus, metastasis may have already occurred in many gastric cancer patients at the time of surgery, presenting a limitation to assessment of the prognosis with surgical curability and pathologic stage. Strategic management of adjuvant treatment is important for patients who have the potential for cancer recurrence after curative surgery. A variety of parameters have been studied to select the appropriate candidate for adjuvant treatment and to detect cancer recurrence earlier. DNA methylation is one such proposed prognostic factor for gastric cancer.4,5
Epigenetic silencing of tumor-related genes by promoter hypermethylation is increasingly recognized as having an instrumental role in the development of cancers.4 Gastric cancer is one of the tumors with a high frequency of aberrant methylation.6,7 DNA methylation of tumor-related genes occurs in the early stages of disease6,8,9 and increases in parallel with the multistep pathway of gastric carcinogenesis.10 DNA methylation has also been reported to be associaed with the tumor stage in gastric cancer.11 However, DNA of cancer tissue from surgical or endoscopic biopsy can be obtained only before surgery or at the time of surgery. Thus, DNA analysis from tissue biopsy is not applicable to a continuing treatment strategy, but serum DNA methylation of tumor-related genes could be used as a method for cancer screening and surveillance for cancer recurrence. However, to date, there have been no reports of postoperative changes in methylation in gastric cancer patients.
Here we used methylation-specific polymerase chain reaction (PCR) (MSP) to evaluate p16 methylation, one of cancer-associated genes of gastric cancer,12,13 in preoperative and postoperative serum from gastric cancer patients. Furthermore, we sought to determine the clinical correlations of the change of serum p16 methylation with the survival and prognosis of patients with gastric adenocarcinoma.
Materials and Methods
1. Patient population
Fifty-three consecutive patients with histologically confirmed gastric adenocarcinoma were examined. All patients underwent gastric resection primarily at Chung-Ang University Hospital (Seoul, Korea) from October 2007 to December 2010. No patient had received preoperative chemotherapy with neoadjuvant or palliative intent. Patients who underwent explorative laparotomy or palliative bypass surgery and one patient who had no residual cancer in the final pathologic specimen were excluded. Patients who had other malignant disease except gastric cancer were also excluded. The follow-up intervals during the study period ranged from 1 to 48 months (median, 29). All patients provided informed consent according to institutional guidelines, and the study was approved by the Institutional Review Board at Chung-Ang University Hospital (IRB No: 09-015-04-09-G4).
Tumors were staged according to the 7th edition of Union for International Cancer Control (UICC) TNM classification.14 Surgical curability was stratified by residual tumor classification according to the American Joint Committee on Cancer (AJCC) and UICC staging system14: R0 for no residual tumor; R1 for microscopic residual tumor; and R2 macroscopic residual tumor.
2. DNA extraction from serum sample
Blood samples were obtained at the time of diagnosis before surgery and at the 10th postoperative day to avoid the effect of adjuvant chemotherapy. Serum was isolated by centrifugation and stored at -80℃ until further processing.
3. Bisulfite modification and methylation-specific polymerase chain reaction
Aberrant DNA methylation in the CpG islands of the genes was determined by chemical modification of genomic DNA with sodium bisulfite followed by MSP. The bisulfite modification procedure was carried out with the EZ DNA Methylation-Gold Kit™ (Zymo Research, Irvine, CA, USA). A total of 20 µl of genomic DNA was denatured at 98℃ for 10 minutes and then chemically modified with 130 µl of computed tomography conversion reagent at 64℃ for 2.5 hours. The unmethylated cytosine was converted to thymine, whereas methylated cytosine remains unchanged.
The primer sequence of p16 was based on the previous report and are listed in Table 1.15 Bisulfite-modified DNA was denatured in a total volume of 25 µl containing 1 µl of each primer, 2 µl of deoxynucleotide triphosphate, 2.5 µl of 10× PCR buffer, and 0.4 µl of hot-taq polymerase at 95℃ for 10 minutes. This was followed by 40 cycles of 95℃ for 30 seconds, incubation at the primerspecific annealing temperature for 45 seconds, and 72℃ for 45 seconds. Samples were finally incubated at 72℃ for 10 minutes. Annealing temperature was 63.3℃. In vitro methylated DNA from the H460 cell line and known methylated DNA from the H1299 cell line were used as positive controls for methylation, and DNA from H460 and NHBE cells was used as a control for unmethylated DNA. Distilled water was used as a negative control.
Table 1.
Primer sequences used in methylation-specific PCR
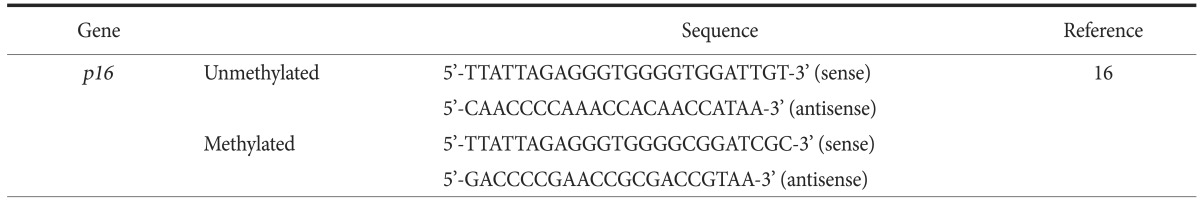
PCR = polymerase chain reaction.
PCR products were electrophoresed on 2% agarose gels and visualized by ethidium bromide staining. Samples were scored as methylated when there was a clearly visible band on the gel with the methylation-specific primers. The sizes of PCR product in this study were 150 bp for methylation and 151 bp for unmethylation respectively. All samples were examined by one experimenter who was unaware of the patient's clinical features.
4. Statistical analysis
Statistical analyses were performed with SPSS statistical software, version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Categorical data were analyzed by chi-square or Fisher's exact tests, and numerical data were analyzed by the Student's t test. Correlations between the methylation status were analyzed by the Spearman Correlation Coefficient. Survival curves were calculated by the Kaplan-Meier method. Differences in the survival curves were measured using log-rank tests. Statistical significance was defined as P<0.05.
Results
1. Patients and tumor characteristics
Fifty-three consecutive patients with gastric adenocarcinoma were examined. Their ages ranged from 30 to 85 years (median, 64 years). Patients included 38 men (72.0%) and 15 women (28.0%). There were 28 (52.8%) distal, 18 (34.0%) middle, and 7 (13.2%) proximal cancers. Subtotal gastrectomy was performed in 37 patients (69.8%) and total gastrectomy in 16 patients (30.2%). In 37 patients (70.0%), D2 lymph node dissection was completed and the median number of retrieved lymph nodes was 36.5 (range: 8 to 58). There were 21 early (T1) gastric cancers (EGC) and 32 advanced gastric cancers (AGC).
According to the residual tumor classification, R0 (no residual tumor) resection was performed in 50 patients (94.3%) and 3 patients (5.7%) had macroscopic or microscopic residual tumor (R1, R2).
2. Methylation of p16 in the serum DNA of gastric cancer patients
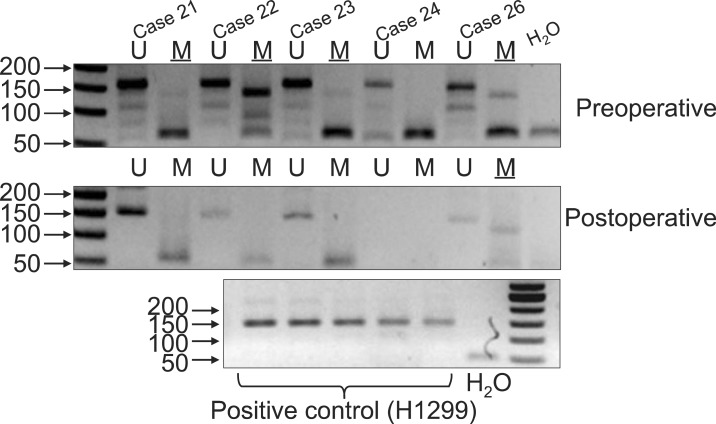
Representative examples of the MSP products analyzed by electrophoresis on an agarose gel are shown in Fig. 1.
Fig. 1.
Representative gel electrophoresis pictures demonstrating aberrant methylation in p16 for preoperative and postoperative serum DNA. M and U, the methylated and unmethylated primers, respectively. DNA from the H1299 cell line was used as positive controls for methylation. Water was used as negative control for each amplification.
Methylation in p16 was detected in 79.2% of preoperative serum DNA, and in 54.7% of postoperative serum DNA. Methylation of p16 was detected postoperatively in 13.2% of the patients with no preoperative methylation, whereas methylation had disappeared postoperatively in 37.7% of the patients (Table 2).
Table 2.
The frequency of methylation and postoperative change of methylaiton status of p16 in serum DNA of gastric cancer patients (n=53)

Values are presented as number (%).
The relationship between clinicopathologic parameters and p16 methylation in preoperative serum DNA was evaluated. Methylation of p16 was more frequently detected in the patients with nodenegative gastric cancer than in node-positive patients (P=0.010). Methylation in p16 disappeared more frequently in the patients who underwent standard D2 lymphadenectomy than in those who underwent modified D1+ lymphadenectomy (P=0.016) (Table 3).
Table 3.
Relationship between clinicopathologic parameters and preoperative status and postoperative disappearance of serum p16 methylation
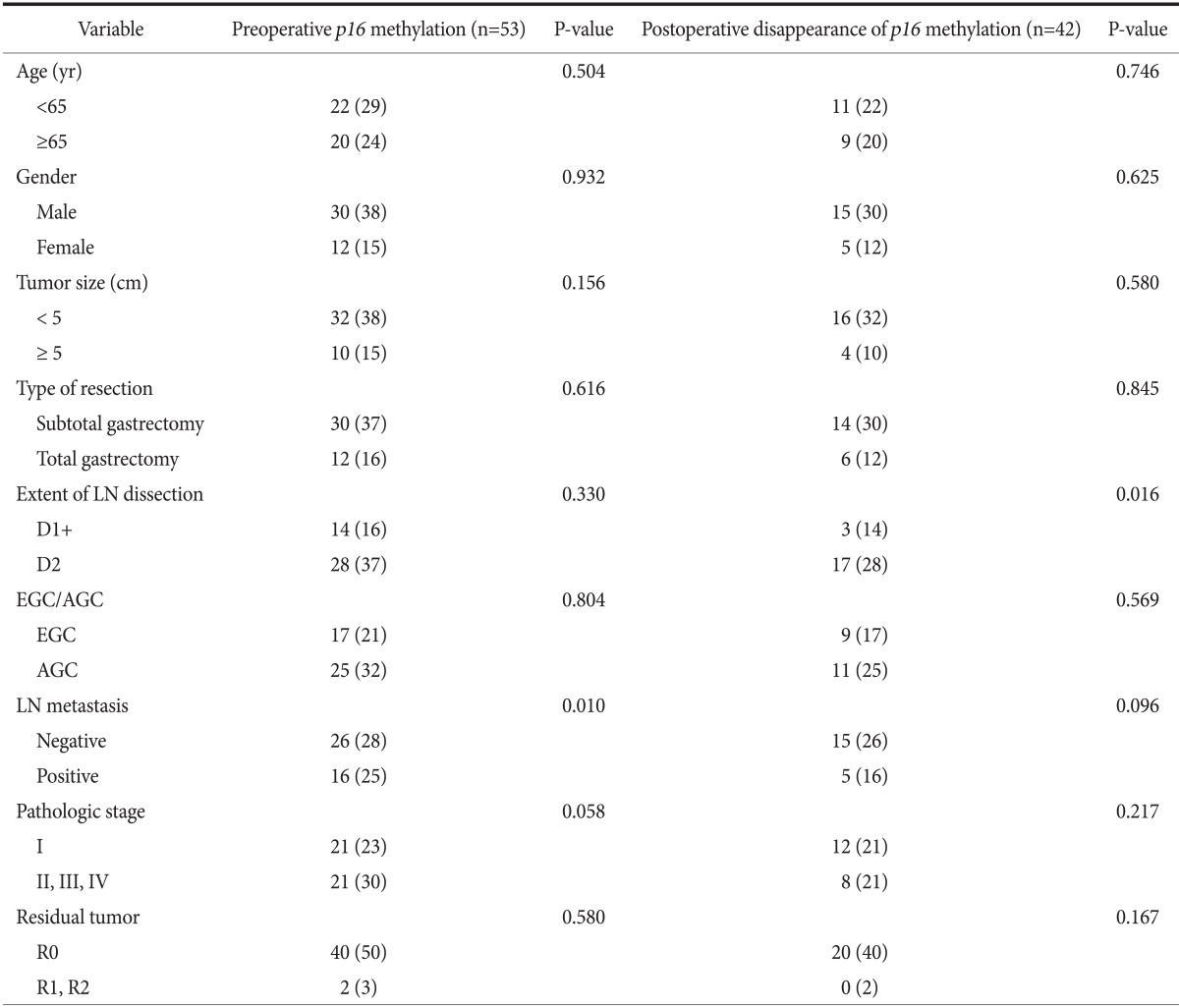
Values are presented as number (total number). LN = lymph node; EGC = early gastric cancer; AGC = advanced gastric cancer.
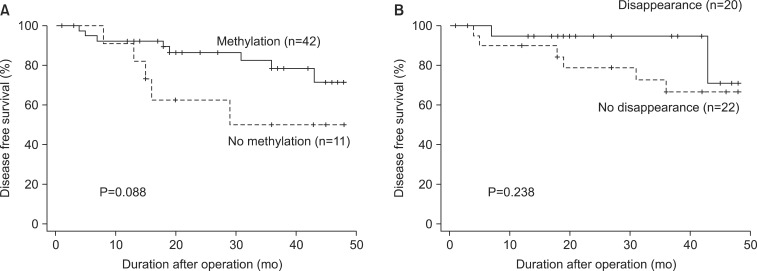
In the survival analysis for 53 patients, the disease free survival (DFS) of patients with preoperative p16 methylation was not different from that of patients without such methylation (Fig. 2A; P=0.088).
Fig. 2.
Kaplan-Meier survival curves of 53 gastric cancer patients according to the preoperative hypermethylation of p16 gene (A) and Kaplan-Meier survival curves of 42 gastric cancer patients who had preoperative p16 methylation according to the postoperative disappearance of p16 hypermethylation (B).
The 3-year survival rate of patients with postoperative disappearance of p16 methylation was 94.7%, whereas the 3-year survival rate of patients without postoperative disappearance of p16 methylation was 66.9%. However, this difference was not statistically significant (Fig. 2B, P=0.238).
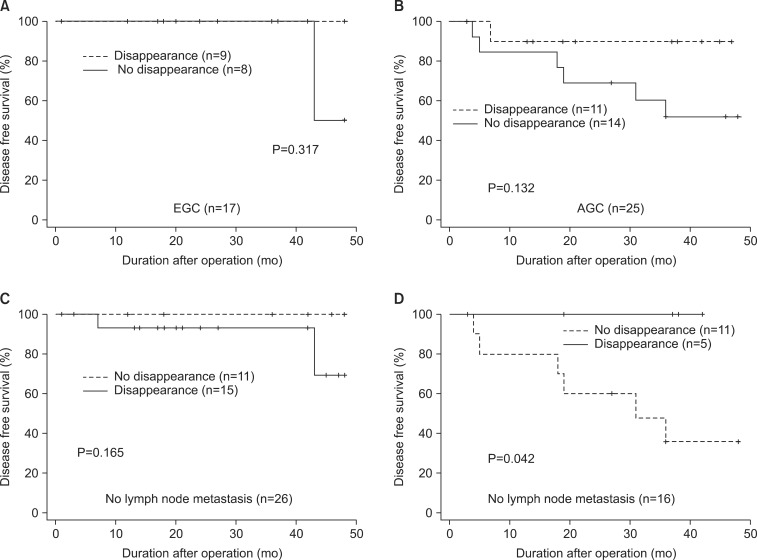
We further stratified the patients according to the depth of tumor (EGC or AGC) or lymph node metastasis, and compared DFS rates of patients with or without postoperative disappearance of p16 methylation (Fig. 3). Our analysis showed that postoperative disappearance of p16 methylation was associated with a better survival rate of patients in the AGC group. However, this difference of survival rate was not statistically significant (P=0.132, Fig. 3B). On the other hand, in the node-positive group, patients with postoperative disappearance of p16 methylation had a significantly longer survival rate than those without it (P=0.042, Fig. 3D). All 5 patients in the node-positive group have survived without tumor recurrence when p16 methylation disappeared after surgery.
Fig. 3.
Kaplan-Meier survival curves of gastric cancer patients who had preoperative p16 methylation according to the postoperative disappearance of p16 hypermethylation. (A) Seventeen early gastric cancer (EGC) patients. (B) Twenty-five advanced gastric cancer (AGC) patients. (C) Twentysix gastric cancer patients without lymph node metastasis. (D) Sixteen gastric cancer patients with lymph node metastasis.
Discussion
Epigenetic silencing of tumor-related genes due to CpG island hypermethylation has emerged as one of the most important epigenetic factors in cancer development. Promoter hypermethylation usually results in transcriptional silencing of genes. If tumor suppressor genes are involved, hypermethylation may result in their inactivation, thus conferring a growth advantage to the cells and favoring cancer development. Here we analyzed the relationship between the postoperative change in serum p16 methylation and clinicopathologic factors presenting as the surgical curability and patient survival to clarify the usefulness of serum p16 methylation as a prognostic factor after gastric cancer surgery.
The DNA methylation analysis in this study was conducted not from the cancer tissue but from patient's serum. Unlike tumor tissue samples, serum is usually more accessible for sampling at any time after surgery. Although many studies have suggested that tumor tissue DNA methylation has prognostic value,11,12,16-18 studies using serum DNA have failed to show the prognostic value of methylation in colon cancer, lung cancer, and liver cell cancer.19-22 DNA methylation usually has a lower detection rate in serum as compared with tumor tissue.22 Lee et al.22 investigated hypermethylation in 53 gastric cancer patients. They found hypermethylation rates of DAP-kinase, E-cadherin, GSTP1, p15, and p16 cancer-related genes of 70.3%, 75.9%, 18.5%, 68.5%, and 66.7% in tumor tissue and 48.1%, 57.4%, 14.8%, 55.6%, and 51.9% in serum, whereas no hypermethylated DNA was detected in serum of normal control subjects. Despite these data, there was no correlation between serum methylation and clinical staging. On the other hand, Leung et al.23 reported serum DNA methylation of 60 gastric cancer patients and found patients with hypermethylation had a worse prognosis, suggesting that serum DNA methylation can be correlated with tumor DNA methylation and may be a potential prognostic factor for predicting tumor stage and cancer recurrence.
The convenience, non-invasiveness, and simplicity of detecting aberrantly methylated DNA in serum are major advantages of this test. The focus of detection of gastric cancer by DNA methylation has moved from tissue DNA to serum DNA. Here we extended the detection of serum DNA methylation from preoperative to postoperative samples, and investigated postoperative methylation changes. To our knowledge, this is the first report in which patients' survival has been analyzed in terms of the postoperative change in DNA methylation.
We found that the DNA methylation frequency for p16 was high in preoperative serum and had a tendency towards decreased frequency postoperatively. Thus, disappearance of methylation of p16 may be correlated with surgical curability and patient survival, making it a candidate prognostic factor. Previous studies of tumor tissue DNA indicated that methylation of p16 is a potential biomarker for early diagnosis and prognostic evaluation in gastric cancer.17,18 Methylation of p16 gene is frequently detected in lymphatic invasive gastric cancer and correlated with chemosensitivity.12,13 In this study, we found that p16 was useful gene for evaluation of serum DNA methylation of gastric cancer patients.
The disappearance of cancer-related DNA methylation after surgical resection of gastric cancer may indicate the curative removal of cancer tissue with no residual cancer tissue in the blood stream. It can be the reason that explains why the methylation disappeared more frequently in the patients who underwent standard D2 lymphadenectomy than in those who underwent modified D1+ lymphadenectomy in this study. Thus, postoperative disappearance of DNA methylation could be an available prognostic factor to supplement TNM cancer staging to decide which patients should be candidates for special postoperative adjuvant treatment. Our data showed that postoperative alteration of p16 methylation had prognostic significance, particularly in node-positive gastric cancer patients. That means the prediction of outcome by postoperative detection of DNA methylation of p16 could be valuable especially for node-positive gastric cancer patients, not for node-negative gastric cancer patients.
Nevertheless, the multivariate analysis in this study was not suitable to confirm the disappearance of p16 methylation as the independent prognostic factor for patients with node-positive gastric cancer because patient number and follow up length in this study were not enough to be evaluated by multivariate analysis model. That is the limitation of this study. However, disappearance of p16 methylation was uniquely correlated with DFS of node-positive gastric cancer patients who had preoperative hypermethylation. Although the large scaled and long-term follow up study is needed to conclude our result, the survival difference according to the disappearance of p16 methylation for node-positive gastric cancer could be meaningful in itself.
In East Asia, where the incidence of gastric cancer is high, the need for a more reliable noninvasive screening test is obvious. Serum DNA methylation has been suggested as a new screening test by several reports.22,23 However, in Korea and Japan, gastroscopy is a well established cancer screening test and gastroscopic biopsy provides high accuracy. Therefore, serum DNA methylation would be useful for postoperative surveillance and as a prognostic indicator of recurrence, rather than a screening test. Our results suggest methylation of p16 as a reliable biomarker for predicting prognosis by postoperative assessment.
In conclusion, we found that postoperative disappearance of methylation in the p16 gene in serum DNA was related to patients' survival after gastric cancer surgery. These findings suggest that the postoperative disappearance of methylation in p16 could be used as prognostic factor for node-positive gastric cancer. Additional studies are necessary to clarify the prognostic value of DNA methylation for gastric cancer patients in terms of long-term survival.
Acknowledgments
This study was supported by a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean Government (MEST) (2010-0005333) and Chung-Ang University Research Institute for Biomedical and Pharmaceutical Sciences.
References
- 1.Desai AM, Pareek M, Nightingale PG, Fielding JW. Improving outcomes in gastric cancer over 20 years. Gastric Cancer. 2004;7:196–201. doi: 10.1007/s10120-004-0289-0. [DOI] [PubMed] [Google Scholar]
- 2.Park JM, Ryu WS, Kim JH, Park SS, Kim SJ, Kim CS, et al. Prognostic factors for advanced gastric cancer: stage-stratified analysis of patients who underwent curative resection. Cancer Res Treat. 2006;38:13–18. doi: 10.4143/crt.2006.38.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–461. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 5.Ikoma H, Ichikawa D, Daito I, Nobuyuki T, Koike H, Okamoto K, et al. Clinical application of methylation specific-polymerase chain reaction in serum of patients with gastric cancer. Hepatogastroenterology. 2007;54:946–950. [PubMed] [Google Scholar]
- 6.Kang GH, Shim YH, Jung HY, Kim WH, Ro JY, Rhyu MG. CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res. 2001;61:2847–2851. [PubMed] [Google Scholar]
- 7.Toyota M, Ahuja N, Suzuki H, Itoh F, Ohe-Toyota M, Imai K, et al. Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res. 1999;59:5438–5442. [PubMed] [Google Scholar]
- 8.Oue N, Sentani K, Yokozaki H, Kitadai Y, Ito R, Yasui W. Promoter methylation status of the DNA repair genes hMLH1 and MGMT in gastric carcinoma and metaplastic mucosa. Pathobiology. 2001;69:143–149. doi: 10.1159/000048769. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Park SJ, Abraham SC, Seo JS, Nam JH, Choi C, et al. Frequent CpG island methylation in precursor lesions and early gastric adenocarcinomas. Oncogene. 2004;23:4646–4654. doi: 10.1038/sj.onc.1207588. [DOI] [PubMed] [Google Scholar]
- 10.Kang GH, Lee S, Kim JS, Jung HY. Profile of aberrant CpG island methylation along the multistep pathway of gastric carcinogenesis. Lab Invest. 2003;83:635–641. doi: 10.1097/01.lab.0000067481.08984.3f. [DOI] [PubMed] [Google Scholar]
- 11.Oue N, Mitani Y, Motoshita J, Matsumura S, Yoshida K, Kuniyasu H, et al. Accumulation of DNA methylation is associated with tumor stage in gastric cancer. Cancer. 2006;106:1250–1259. doi: 10.1002/cncr.21754. [DOI] [PubMed] [Google Scholar]
- 12.Goto T, Mizukami H, Shirahata A, Yokomizo K, Kitamura YH, Sakuraba K, et al. Methylation of the p16 gene is frequently detected in lymphatic-invasive gastric cancer. Anticancer Res. 2010;30:2701–2703. [PubMed] [Google Scholar]
- 13.Mitsuno M, Kitajima Y, Ide T, Ohtaka K, Tanaka M, Satoh S, et al. Aberrant methylation of p16 predicts candidates for 5-fluorouracil-based adjuvant therapy in gastric cancer patients. J Gastroenterol. 2007;42:866–873. doi: 10.1007/s00535-007-2113-1. [DOI] [PubMed] [Google Scholar]
- 14.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th ed. West Sussex, Hoboken: Blackwell Publisging Ltd.; 2010. International Union against Cancer. [Google Scholar]
- 15.Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An C, Choi IS, Yao JC, Worah S, Xie K, Mansfield PF, et al. Prognostic significance of CpG island methylator phenotype and microsatellite instability in gastric carcinoma. Clin Cancer Res. 2005;11:656–663. [PubMed] [Google Scholar]
- 17.Shi J, Zhang G, Yao D, Liu W, Wang N, Ji M, et al. Prognostic significance of aberrant gene methylation in gastric cancer. Am J Cancer Res. 2012;2:116–129. [PMC free article] [PubMed] [Google Scholar]
- 18.Tsujie M, Yamamoto H, Tomita N, Sugita Y, Ohue M, Sakita I, et al. Expression of tumor suppressor gene p16(INK4) products in primary gastric cancer. Oncology. 2000;58:126–136. doi: 10.1159/000012089. [DOI] [PubMed] [Google Scholar]
- 19.Hibi K, Robinson CR, Booker S, Wu L, Hamilton SR, Sidransky D, et al. Molecular detection of genetic alterations in the serum of colorectal cancer patients. Cancer Res. 1998;58:1405–1407. [PubMed] [Google Scholar]
- 20.Esteller M, Sanchez-Cespedes M, Rosell R, Sidransky D, Baylin SB, Herman JG. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from nonsmall cell lung cancer patients. Cancer Res. 1999;59:67–70. [PubMed] [Google Scholar]
- 21.Wong IH, Lo YM, Zhang J, Liew CT, Ng MH, Wong N, et al. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 1999;59:71–73. [PubMed] [Google Scholar]
- 22.Lee TL, Leung WK, Chan MW, Ng EK, Tong JH, Lo KW, et al. Detection of gene promoter hypermethylation in the tumor and serum of patients with gastric carcinoma. Clin Cancer Res. 2002;8:1761–1766. [PubMed] [Google Scholar]
- 23.Leung WK, To KF, Chu ES, Chan MW, Bai AH, Ng EK, et al. Potential diagnostic and prognostic values of detecting promoter hypermethylation in the serum of patients with gastric cancer. Br J Cancer. 2005;92:2190–2194. doi: 10.1038/sj.bjc.6602636. [DOI] [PMC free article] [PubMed] [Google Scholar]