This investigation of the association between body mass index at the time of multiple myeloma diagnosis and overall survival in a cohort of patients within the Veterans Health Administration system showed that disease-related weight loss may be an important and heretofore unknown indicator of poor prognosis in multiple myeloma. Weight loss ≥10% of baseline in the year before diagnosis was associated with significantly increased mortality.
Keywords: Multiple myeloma, Survival, Body mass index, Obesity, Overweight, Mortality
Learning Objectives
Describe the association between baseline BMI, weight loss, and survival in MM.
Explain the importance of BMI and baseline weight loss as part of the standard history obtained in patients with MM.
Abstract
Purpose.
We investigated the association between body mass index (BMI) at the time of multiple myeloma (MM) diagnosis and overall survival in a cohort of patients within the Veterans Health Administration system. We also evaluated the association between weight loss in the year prior to diagnosis and survival.
Patients and Methods.
Prospective analysis was performed on a retrospectively assembled cohort of 2,968 U.S. veterans diagnosed and treated for MM between September 1, 1999, and September 30, 2009, with follow-up information through October 22, 2011. Cox modeling controlling for patient- and disease-related prognostic variables was used to analyze the data.
Results.
Underweight patients (BMI <18.5 kg/m2) had increased mortality, whereas patients who were overweight (BMI 25–29.9 kg/m2) and obese (BMI ≥30 kg/m2) had lower mortality compared with healthy-weight patients (BMI 18.5–24.9 kg/m2). Weight loss ≥10% of baseline in the year before diagnosis was also associated with increased mortality and made the association between increased BMI and survival nonsignificant.
Conclusion.
Disease-related weight loss may be an important and heretofore unknown indicator of poor prognosis in MM. Assessment of weight loss prior to MM diagnosis should become a standard component of the clinical history in patients with newly diagnosed MM. Further research may identify relationships between disease-related weight loss and currently used prognostic factors in MM, further defining the role of this clinical factor in prognostic stratification.
Implications for Practice:
Weight loss of 10% or more in the year leading up to multiple myeloma diagnosis was seen in nearly one out of four United States veterans with newly diagnosed multiple myeloma. Patients who lost weight had increased mortality compared to those who did not lose weight. This is similar to what is seen in other malignancies, such as non-Hodgkin's lymphoma, where weight loss before diagnosis is routinely determined. Assessment of weight loss in the year leading up to a diagnosis of multiple myeloma should become part of the standard medical history in patients with multiple myeloma. Further research will identify if weight loss is associated with known prognostic factors in multiple myeloma, such as disease cytogenetics and severity.
Introduction
In 2010, it was estimated that more than two-thirds of the adult population of the U.S. was either overweight or obese based on body mass index (BMI) [1]. Although opinions differ on whether the population prevalence of obesity is stable or increasing [1, 2], for the foreseeable future, most adult cancer patients will be overweight or obese. Elevated BMI has been associated previously with an increased risk of death, with many of the excess deaths attributable to cancer [3, 4].
Along with an increased risk of solid tumor malignancies, elevated BMI increases the risk of death from many hematologic malignancies, including the common leukemias, non-Hodgkin lymphoma (NHL), and multiple myeloma (MM) [5]. In general, elevated BMI can influence disease-specific survival by modifying disease incidence, survival after diagnosis, or both [4]. Consequently, the influence of BMI on mortality in the hematologic malignancies may vary at different time points in the disease process. In acute promyelocytic leukemia, for example, elevated BMI before diagnosis is associated with increased disease incidence, and elevated BMI is associated with an increased risk of relapse after treatment [6–8]. Elevated BMI is also associated with an increased incidence of diffuse large B-cell lymphoma [9], but patients who are overweight or obese at the time of diagnosis demonstrate improved survival compared with those with a healthy-weight BMI [10].
Substantial evidence demonstrates an association between elevated BMI before diagnosis and increased MM incidence and death, but the impact of BMI at the time of diagnosis has not been clearly defined [4, 11–15]. Because MM is generally considered to be an incurable malignancy, it is conceivable that increased incidence alone drives the increased risk of death observed in cohort studies. Given the high population prevalence of obesity, a clearer understanding of how BMI influences survival after MM diagnosis would provide insight into optimizing care of MM patients. We investigated the association between BMI at the time of MM diagnosis and overall survival (OS) in a cohort of U.S. veterans diagnosed and treated within the Veterans Health Administration (VHA) system.
Materials and Methods
Study Population and Design
We obtained data on patients with newly diagnosed MM between September 1, 1999, and September 30, 2009, from the VHA central cancer registry. Institutional review boards at both the St. Louis VHA Medical Center and Washington University School of Medicine approved the study prior to identification of the study cohort. A total of 5,013 patients with a diagnosis of MM were identified based on the International Classification of Diseases for Oncology third revision code 9732/3. Patients with extramedullary or solitary plasmacytoma were not included. Data were obtained from all 21 regional VHA districts throughout the U.S.
Patient records were linked to administrative data on height, weight, International Classification of Diseases ninth revision (ICD-9) codes for comorbid conditions, vital status, and pharmaceutical utilization. The Romano adaptation of the Charlson comorbidity index was calculated using ICD-9 codes based on comorbid conditions present at the time of diagnosis [16]. Patients without date-of-death information were assumed to be alive at the time of the last death recorded within the cohort, October 22, 2011. This assumption is supported by previous work demonstrating that more than 97% of death events are captured in VHA vital status files [17, 18].
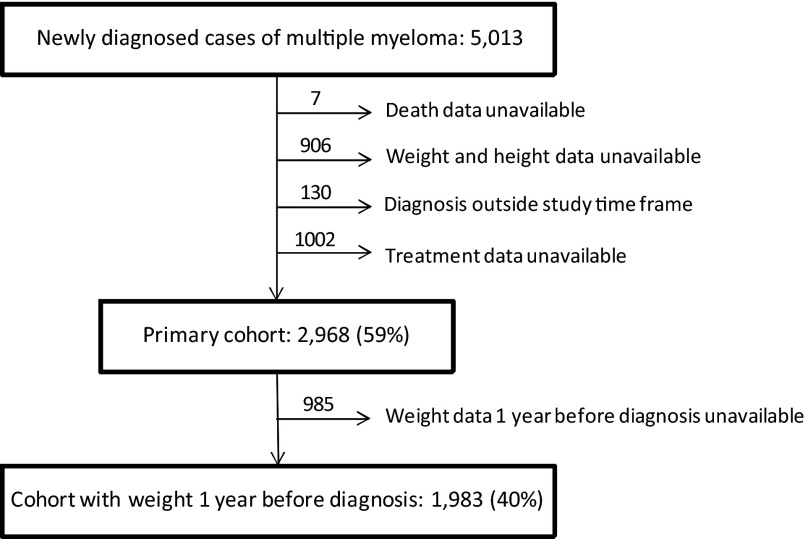
Because of concerns that some patients with a diagnosis of MM may have been misclassified in the setting of only monoclonal gammopathy of undetermined significance or smoldering myeloma, those patients who did not receive treatment at a VHA facility within 6 months of diagnosis were excluded. Patients without weight measurement within 1 month of diagnosis or laboratory and/or biopsy confirmation of MM were also excluded, resulting in an analytic cohort of 2,968 patients (Fig. 1).
Figure 1.
Consort diagram for multiple myeloma cases diagnosed in U.S. veterans between 1998 and 2009.
Measurements
Height was determined based on the most frequently recorded height. Weight at diagnosis was defined as the value measured closest to but within 1 month of MM diagnosis. Outlier weights of <45 kg or >180 kg were either verified or corrected based on review of patient records using the U.S. Department of Veterans Affairs Compensation and Pension Records Interchange software system. BMI was calculated as weight in kilograms divided by height in meters squared and categorized as defined by the World Health Organization as “underweight” (BMI <18.5 kg/m2), “normal weight” (BMI 18.5 to 24.9 kg/m2), “overweight” (BMI 25.0 to 29.9 kg/m2), and “obese” (BMI ≥30.0 kg/m2) [19]. The normal-weight BMI category was considered the referent group in all analyses. Age at diagnosis was used as a continuous variable in all analyses. OS was defined as the time between the date of MM diagnosis and the date of death or the end of the study follow-up period.
Statistical Analyses
Univariate analyses were performed using chi-square tests for categorical variables and analysis of variance tests for continuous variables. OS was estimated using Kaplan-Meier methods, and comparisons were made using the log-rank statistic. Cox proportional hazards modeling was used to estimate the association between BMI at diagnosis and OS while controlling for potential confounding variables. The proportional hazards assumption was tested using Schoenfeld residuals. Violations of the proportional hazards assumption were addressed with the addition of a time-dependent covariate. An α significance level of <0.05 was considered statistically significant. To address potential variable interaction effects, interaction terms were inserted into each model and tested for significance. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC, http://www.sas.com).
Results
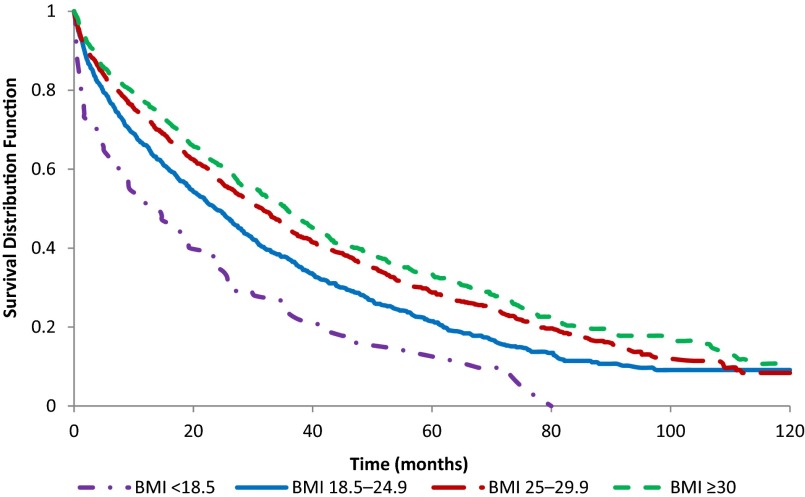
Baseline demographic and clinical characteristics of the analytic cohort are presented in Table 1. Kaplan-Meier analysis demonstrated increased mortality in the underweight category and decreased mortality in the overweight and obese categories, compared with the normal-weight BMI group (Fig. 2). Median survival time for the entire cohort was 28.6 months. When considered by BMI category, median survival was 14.3 months for the underweight group, 23.7 months for the normal-weight group, 31.7 months for the overweight group, and 35.7 months for the obese group. Obese patients were significantly younger than those in the other BMI groups, and there were more black patients in the underweight and normal-weight BMI groups compared with the overweight and obese groups. Charlson comorbidity score demonstrated a trend toward a higher mean score in the obese group (p = .057).
Table 1.
Demographic and clinical characteristics stratified by body mass index among U.S. veterans with multiple myeloma diagnosed from 1998 to 2009
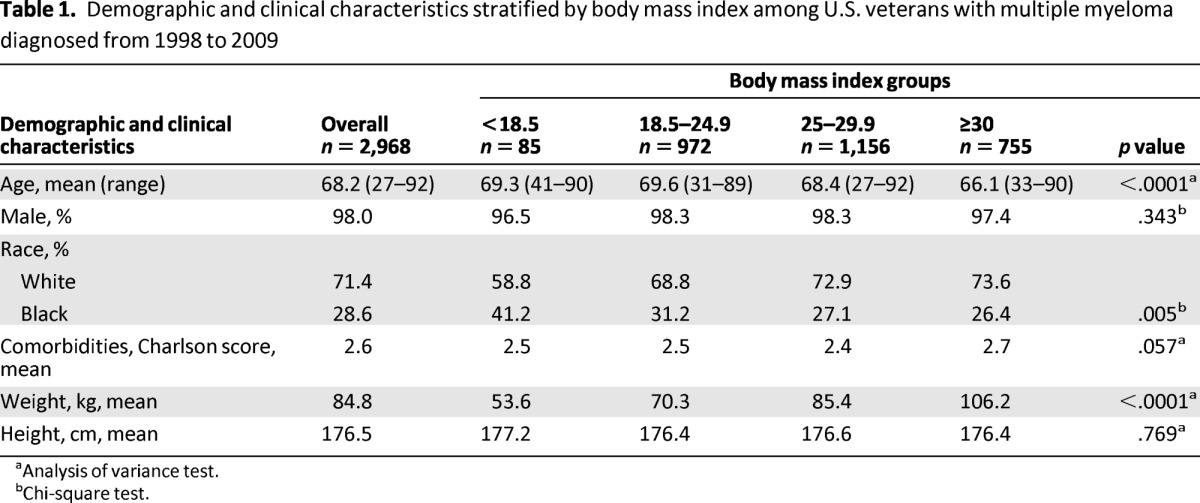
aAnalysis of variance test.
bChi-square test.
Figure 2.
Kaplan-Meier curves of U.S. veterans diagnosed with multiple myeloma from 1998 to 2009, body mass index at diagnosis and overall survival (n = 2,968). Log-rank p < .001.
Abbreviation: BMI, body mass index.
Cox modeling was then performed to evaluate the influence of BMI on mortality while controlling for age, race, and Charlson comorbidity score. Comorbidity score violated the proportional hazards assumption and was considered as a time-dependent variable stratified at 12 months from diagnosis date. The proportional hazards assumption was tested for all other variables, and no others were in violation. Hazard ratios (HRs) and 95% confidence intervals (CIs) from the Cox analysis are presented in Table 2. After controlling for other variables, the underweight group had higher mortality (HR: 1.64; 95% CI: 1.30–2.08), whereas the overweight group (HR: 0.82; 95% CI: 0.75–0.91) and the obese group (HR: 0.75; 95% CI: 0.67–0.84) had lower mortality, compared with the normal-weight BMI group.
Table 2.
Hazard ratios for mortality among multiple myeloma cases diagnosed among U.S. veterans from 1998 to 2009 with body mass index at diagnosis (n = 2,968)
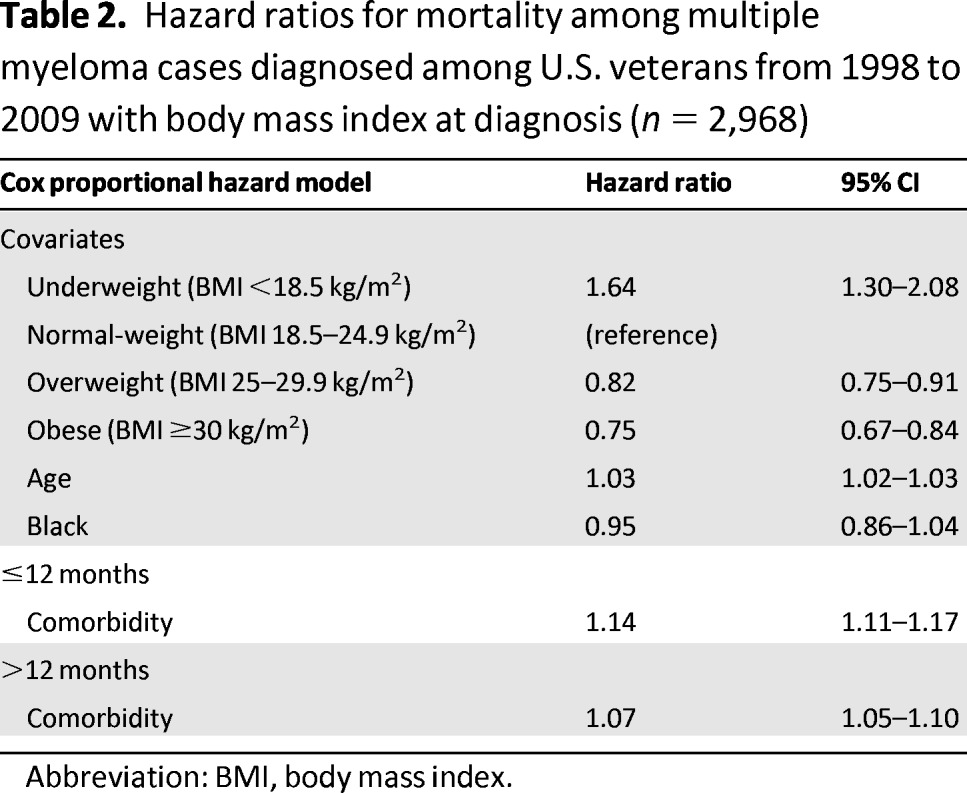
Abbreviation: BMI, body mass index.
To examine whether the inverse association between BMI and OS was constant during the time leading up to diagnosis, we identified patients with weight information available 1 year before diagnosis ±3 months (n = 1,983) and performed a Cox analysis using BMI measured 1 year before diagnosis. These patients had a higher median age of 71 years (range: 27–92 years) and had a higher mean comorbidity score (3.0) compared with the overall cohort. This finding was not unexpected because older patients with more comorbidities visit the physician more often. Results of the Cox analysis are presented in Table 3. Patients who were underweight 1 year before MM diagnosis had a nonsignificant increase in mortality compared with those with a normal-weight BMI 1 year before diagnosis (HR: 1.27; 95% CI: 0.71–2.27), and those who were overweight 1 year before diagnosis had decreased mortality of borderline significance (HR: 0.87; 95% CI: 0.76–0.99). Survival among those who were obese 1 year before diagnosis was not significantly different from those in the normal-weight group (HR: 0.93; 95% CI: 0.81–1.07). To further understand why obese BMI 1 year before diagnosis was not significantly associated with improved survival, we performed a second Cox analysis on the same subgroup (n = 1,983) using BMI at diagnosis. The underweight group had increased mortality (HR: 1.71; 95% CI: 1.23–2.40), the overweight group showed a nonsignificant trend toward decreased mortality (HR: 0.90; 95% CI: 0.79–1.01), and the obese group had significantly decreased mortality compared with the normal-weight group (HR: 0.80; 95% CI: 0.70–0.92). Taken together, these findings suggest that the association between BMI and survival is not constant during the time leading up to MM diagnosis. A potential explanation is that disease-related weight loss may cause migration of poorer prognosis patients into lower BMI groups by the time of diagnosis.
Table 3.
Hazard ratios for mortality among multiple myeloma cases diagnosed among US veterans from 1998 to 2009 with BMI 1 year before diagnosis (n = 1,983).

Abbreviations: BMI, body mass index; CI, confidence interval.
An additional analysis was performed to evaluate the influence of clinically significant weight loss (≥10% of baseline weight in the year leading up to diagnosis) on OS. Of the 1,983 patients with weight information 1 year before diagnosis, 451 (23%) lost ≥10% of their baseline weight in the year leading up to diagnosis. Those who demonstrated this degree of disease-related weight loss had significantly higher mortality (HR: 1.52; 95% CI: 1.34–1.72) compared with those who did not. When stratified by BMI at diagnosis, 57% of the underweight group, 36% of the normal-weight group, 18% of the overweight group, and 12% of the obese group lost ≥10% of baseline weight in the year prior to diagnosis. After controlling for disease-related weight loss, age, race, and comorbidities in a Cox analysis, being overweight or obese at the time of diagnosis was no longer significantly associated with decreased mortality (overweight: HR: 0.98; 95% CI: 0.86–1.11; obese: HR: 0.89; 95% CI: 0.77–1.02).
In recent years, survival for patients with newly diagnosed MM has improved with the addition of novel agents such as immunomodulating drugs (thalidomide and lenalidomide) and the proteasome inhibitor bortezomib to treatment paradigms. Consequently, we performed sensitivity analyses to examine how treatment affected our observations through insertion of treatment variables into the multivariate Cox model while controlling for age, race, and Charlson comorbidity score. Treatments were considered in the following categories: dexamethasone monotherapy; low-dose melphalan; high-dose melphalan with autologous transplantation; bortezomib; thalidomide; lenalidomide; and a composite variable of doxorubicin, etoposide, or vincristine. The associations between BMI and mortality in the primary cohort (n = 2,968) changed trivially because underweight patients continued to have increased mortality (HR: 1.58; 95% CI: 1.25–2), whereas overweight patients (HR: 0.84; 95% CI: 0.76–0.92) and obese patients (HR: 0.76; 95% CI: 0.68–0.85) had reduced mortality. Among the 1,983 patients with weight information from 1 year before diagnosis, after controlling for treatment, disease-related weight loss continued to be associated with increased mortality (HR: 1.5; 95% CI: 1.32–1.69). This suggests that disease-related weight loss remains a significant predictor of mortality regardless of treatment assignment.
Discussion
This study is the first to analyze the association between BMI at the time of diagnosis and OS in MM patients. Disease-related weight loss may be relatively common in patients with newly diagnosed MM [20]. We observed that MM patients who are overweight or obese at the time of diagnosis had better OS compared with those who have a normal-weight BMI. After we controlled for disease-related weight loss through insertion of a weight loss variable into the multivariate Cox model, the primary predictor of outcome was disease-related weight loss. This suggests that disease-related weight loss is a major driver of the poorer survival seen in patients with underweight or normal-weight BMI. Similar to patients with lymphoma or lung cancer [21–23], patients with newly diagnosed MM and significant disease-related weight loss have a poorer prognosis.
From a clinical perspective, weight is often first assessed at the time of MM diagnosis, and it is clear that elevated BMI at the time of MM diagnosis is associated with a better overall prognosis. This observation is true after controlling for potential confounding by age, race, and comorbidity score but only if BMI is considered at that single point in time. When BMI at diagnosis is considered along with weight loss in the year leading up to diagnosis, disease-related weight loss dominates the association and BMI loses significance. This suggests that disease-related weight loss may be an important prognostic variable in patients with MM. Moreover, significant changes in BMI were observed in 23% of patients, suggesting that disease-related weight loss is a common manifestation of poor-prognosis disease in veterans with MM.
The advantage of the clinical use of weight loss as a prognostic variable is the ease with which it can be assessed when obtaining a clinical history, similar to the routine assessment of weight loss in patients with lymphoma. In contrast, some of the prognostic variables in the Durie-Salmon staging system and the International Staging System (ISS) are associated with considerable cost or delay related to their assessment [24, 25]. We do not know whether the association between disease-related weight loss and survival would remain significant after controlling for disease stage using one of these staging systems. Answering that question will require further study. Retrospective assessment of ISS in a large, multiyear observational cohort such as this one is not possible because of the reliance on laboratory testing performed at diagnosis.
The mechanism by which disease-related weight loss drives a poorer prognosis cannot be determined from a population-based study; however, because MM is a malignancy of terminally differentiated B-cells, it is logical to consider whether the mechanisms are similar to those seen in the B-cell lymphomas. In both Hodgkin lymphoma and NHL, significant weight loss in the months leading up to diagnosis is known as a “B-symptom” and is associated with poorer prognosis [21, 22]. B-symptoms in NHL are associated with elevated serum levels of inflammatory cytokines, such as tumor necrosis factor and interleukin-6 (IL-6) [26–28]. In turn, both tumor necrosis factor and IL-6 have been independently associated with poorer progression-free survival and OS in NHL. The association between IL-6 levels and poorer survival in MM is well established [29]. It seems logical that patients with disease-related weight loss in MM may have more aggressive disease or higher disease burden associated with higher levels of inflammatory cytokines, which are also associated with cancer cachexia [30].
Our study has several strengths. First, the VHA database allows determination of objective measurements of weight 1 year before diagnosis; these data are not available in most cohorts constructed at the time of MM diagnosis. Second, the large number of patients treated at multiple facilities represents a diversity of practice patterns, enhancing the generalizability of results. Finally, the VHA maintains detailed and accurate records on patient vital status, even when care is provided outside the VHA system, ensuring adequate ascertainment of death events.
This study also has several limitations. First, our results may not be generalizable to women because a considerable majority of MM patients treated in the VHA system (and included in this study) are men. Second, we were unable to measure ISS for the patients who met study inclusion criteria because many did not have information on the baseline β2 microglobulin level. Third, although we attempted to reduce the inclusion of patients who would now be diagnosed with smoldering myeloma or monoclonal gammopathy of undetermined significance by including only those who received treatment for MM, it is possible that patients with these disorders were treated based on older disease definitions, particularly those diagnosed before 2003 [31]. We also did not have information on cytogenetics, an important prognostic factor in MM [32]. There are no studies looking at how BMI and/or disease-related weight loss may be related to differences in the cytogenetic abnormalities associated with survival in MM, and the relationship between weight loss and disease cytogenetics could be explored further. Third, the VHA provides care to veterans regardless of ability to pay, eliminating the differences in insurance status that are common outside the VHA system. Finally, we did not have data on other possible confounders that may influence survival such as education, marital status, employment status, current occupation, and income.
An incidental finding in this study was the time-variable nature of the association between comorbidity and mortality. The stronger association between comorbidities and death seen in the first year after diagnosis suggests interaction with treatment. Further study is warranted to determine whether specific comorbidities are responsible for the higher mortality in the first year after diagnosis, particularly if that higher mortality is seen in conjunction with specific treatments. If such an interaction is noted, it could guide treatment choice in patients with significant comorbid conditions at the time of diagnosis.
Conclusion
BMI at diagnosis is a significant predictor of OS in patients with newly diagnosed MM, although this association is largely the result of migration of higher risk patients into lower BMI groups because of disease-related weight loss in the months prior to diagnosis. Assessment of weight loss prior to diagnosis should be considered a standard part of the clinical history taken for patients with MM. Further study of the association between disease-related weight loss and existing prognostic variables is warranted. As has been suggested for other malignancies, combining clinical, laboratory, and biologic data may improve risk stratification for patients with MM [33].
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This study was funded by the Barnes-Jewish Hospital Foundation, the American Cancer Society Grant IRG 58–010-52, and the National Cancer Institute at the National Institutes of Health, Grants U54CA155496 and KM1CA15608. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of National Institutes of Health.
Author Contributions
Conception/Design: Tracey S. Beason, Kristen M. Sanfilippo, Graham A. Colditz, Kenneth R. Carson
Provision of study material or patients: Tracey S. Beason
Collection and/or assembly of data: Tracey S. Beason, Su-Hsin Chang, Kristen M. Sanfilippo, Suhong Luo, Arun Ganti, Kenneth R. Carson
Data analysis and interpretation: Tracey S. Beason, Su-Hsin Chang, Kristen M. Sanfilippo, Suhong Luo, Ravi Vij, Michael H. Tomasson, John F. Dipersio, Keith Stockerl-Goldstein, Arun Ganti, Tanya Wildes, Kenneth R. Carson
Manuscript writing: Tracey S. Beason, Kristen M. Sanfilippo, Ravi Vij, Michael H. Tomasson, John F. Dipersio, Keith Stockerl-Goldstein, Tanya Wildes, Kenneth R. Carson
Final approval of manuscript: Tracey S. Beason, Su-Hsin Chang, Kristen M. Sanfilippo, Suhong Luo, Graham A. Colditz, Ravi Vij, Michael H. Tomasson, John F. Dipersio, Keith Stockerl-Goldstein, Arun Ganti, Tanya Wildes, Kenneth R. Carson
Disclosures
Ravi Vij: Onyx, Celgene (C/A); Onyx, Celgene, Millennium (H); Onyx, Celgene (RF); Keith E. Stockerl-Goldstein: Celgene, Onyx, Millennium (H); Kenneth Carson: Celgene (C/A). The other authors indicated no financial relationships.
Section editor: Powel Brown: None
Reviewer “A”: None
Reviewer “B”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Hill AL, Rand DG, Nowak MA, et al. Infectious disease modeling of social contagion in networks. PLoS Comput Biol. 2010;6:e1000968. doi: 10.1371/journal.pcbi.1000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Lichtman MA. Obesity and the risk for a hematological malignancy: Leukemia, lymphoma, or myeloma. The Oncologist. 2010;15:1083–1101. doi: 10.1634/theoncologist.2010-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estey E, Thall P, Kantarjian H, et al. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia. 1997;11:1661–1664. doi: 10.1038/sj.leu.2400783. [DOI] [PubMed] [Google Scholar]
- 7.Wong O, Harris F, Wang Y, et al. A hospital-based case-control study of non-Hodgkin lymphoid neoplasms in Shanghai: Analysis of personal characteristics, lifestyle, and environmental risk factors by subtypes of the WHO classification. J Occup Environ Med. 2010;52:39–53. doi: 10.1097/JOM.0b013e3181c5c399. [DOI] [PubMed] [Google Scholar]
- 8.Breccia M, Mazzarella L, Bagnardi V, et al. Increased BMI correlates with higher risk of disease relapse and differentiation syndrome in patients with acute promyelocytic leukemia treated with the AIDA protocols. Blood. 2012;119:49–54. doi: 10.1182/blood-2011-07-369595. [DOI] [PubMed] [Google Scholar]
- 9.Larsson SC, Wolk A, Larsson SC, et al. Obesity and risk of non-Hodgkin's lymphoma: A meta-analysis. Int J Cancer. 2007;121:1564–1570. doi: 10.1002/ijc.22762. [DOI] [PubMed] [Google Scholar]
- 10.Carson KR, Barlett NL, McDonald JR, et al. Increased body mass index is associated with improved survival in United States veterans with diffuse large B-cell lymphoma. J Clin Oncol. 2012;30:3217–3222. doi: 10.1200/JCO.2011.39.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallin A, Larsson S. Body mass index and the risk of multiple myeloma: A meta-analysis of prospective studies. Eur J Cancer. 2011;47:1606–1615. doi: 10.1016/j.ejca.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Reeves GK, Pirie K, Beral V, et al. Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu BC, Gapstur SM, Greenland P, et al. Body mass index, abnormal glucose metabolism, and mortality from hematopoietic cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2348–2354. doi: 10.1158/1055-9965.EPI-06-0007. [DOI] [PubMed] [Google Scholar]
- 14.Khan MM, Mori M, Sakauchi F, et al. Risk factors for myeloma: Evidence from the Japan Collaborative Cohort (JACC) study. Asian Pac J Cancer Prev. 2006;7:575–581. [PubMed] [Google Scholar]
- 15.Parr C, Batty GD, Lam TH, et al. Body-mass index and cancer mortality in the Asian-Pacific Cohort Studies Collaboration: Pooled analyses of 424,519 participants. Lancet Oncol. 2010;11:741–752. doi: 10.1016/S1470-2045(10)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 17.Savas LS, del Junco DJ, Bastian LA, et al. Mortality ascertainment of women veterans: A comparison of sources of vital status information, 1979–2002. Med Care. 2009;47:125–128. doi: 10.1097/MLR.0b013e3181809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Global database on body mass index. [Accessed April 27, 2012]. Avalable at http://apps.who.int/bmi/index.jsp?introPage=intro_3.htm.
- 20.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 21.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 22.Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol. 2002;20:2101–2108. doi: 10.1200/JCO.2002.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65:25–32. [PubMed] [Google Scholar]
- 24.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 26.Salles G, Bienvenu J, Bastion Y, et al. Elevated circulating levels of TNFalpha and its p55 soluble receptor are associated with an adverse prognosis in lymphoma patients. Br J Haematol. 1996;93:352–359. doi: 10.1046/j.1365-2141.1996.5181059.x. [DOI] [PubMed] [Google Scholar]
- 27.Rathore VB, Advani SH, Nadkarni JJ. Altered release of tumor necrosis factor and its soluble receptor in non-Hodgkin's lymphoma patients. Cancer Detect Prev. 1999;23:226–231. doi: 10.1046/j.1525-1500.1999.99024.x. [DOI] [PubMed] [Google Scholar]
- 28.Seymour JF, Talpaz M, Cabanillas F, et al. Serum interleukin-6 levels correlate with prognosis in diffuse large-cell lymphoma. J Clin Oncol. 1995;13:575–582. doi: 10.1200/JCO.1995.13.3.575. [DOI] [PubMed] [Google Scholar]
- 29.Bataille R, Boccadoro M, Klein B, et al. C-reactive protein and beta-2 microglobulin produce a simple and powerful myeloma staging system. Blood. 1992;80:733–737. [PubMed] [Google Scholar]
- 30.Cahlin C, Korner A, Axelsson H, et al. Experimental cancer cachexia: The role of host-derived cytokines interleukin (IL)-6, IL-12, interferon-gamma, and tumor necrosis factor alpha evaluated in gene knockout, tumor-bearing mice on C57 Bl background and eicosanoid-dependent cachexia. Cancer Res. 2000;60:5488–5493. [PubMed] [Google Scholar]
- 31.Durie BG, Kyle RA, Belch A, et al. Myeloma management guidelines: A consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol J. 2003;4:379–398. [PubMed] [Google Scholar]
- 32.Fonseca R, Blood E, Rue M, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003;101:4569–4575. doi: 10.1182/blood-2002-10-3017. [DOI] [PubMed] [Google Scholar]
- 33.Perry AM, Cardesa-Salzmann TM, Meyer PN, et al. A new biologic prognostic model based on immunohistochemistry predicts survival in patients with diffuse large B-cell lymphoma. Blood. 120:2290–2296. doi: 10.1182/blood-2012-05-430389. [DOI] [PMC free article] [PubMed] [Google Scholar]