Abstract
Purpose
Surgical resection of the primary tumour in patients with advanced colorectal cancer (crc) remains controversial. This review compares survival in patients with advanced crc who underwent surgical resection of the primary tumour with that in patients not undergoing resection, and determines rates of post-operative mortality and nonfatal complications, the primary tumour complication rate, the non-resection surgical procedures rate, and quality of life (qol).
Methods
Reports in the central, medline, and embase databases were searched for relevant studies, which were selected using pre-specified eligibility criteria. The search was also restricted to publication dates from 1980 onward, the English language, and studies involving human subjects. Screening, evaluation of relevant articles, and data abstraction were performed in duplicate, and agreement between the abstractors was assessed. Articles that met the inclusion criteria were assessed for quality using the Newcastle–Ottawa Scale. Data were collected and synthesized per protocol.
Results
From among the 3379 reports located, fifteen retrospective observational studies were selected. Of the 12,416 patients in the selected studies, 8620 (69%) underwent surgery. Median survival was 15.2 months (range: 10–30.7 months) in the resection group and 11.4 months (range: 3–22 months) in the non-resection group. Hazard ratio for survival was 0.69 [95% confidence interval (ci): 0.61 to 0.79] favouring surgical resection. Mean rates of postoperative mortality and nonfatal complications were 4.9% (95% ci: 0% to 9.7%) and 25.9% (95%ci: 20.1% to 31.6%) respectively. The mean primary tumour complication rate was 29.7% (95% ci: 18.5% to 41.0%), and the non-resection surgical procedures rate in the non-resection group was 27.6% (95 ci: 15.4% to 39.9%). No study provided qol data.
Conclusions
Although this review supports primary tumour resection in advanced crc, the results have significant biases. Randomized trials are warranted to confirm the findings.
Keywords: Primary tumour resection, stage iv colorectal cancer, palliative surgery, survival
1. INTRODUCTION
Colorectal cancer (crc) is one of the leading causes of cancer death in North America1. The median overall survival of patients with stage iv crc managed with best supportive care alone is about 5–6 months2. Systemic therapy provides meaningful improvements in median survival and progression-free survival. Overall, with the judicious use of novel cytotoxic and biologic agents, the median overall survival of patients with stage iv crc has been extended to approximately 2 years3–5.
The optimal surgical management of stage iv crc that is not amenable to curative resection is unknown. Although administration of systemic therapy in patients with stage iv crc may convert unresectable into resectable disease, the principal goal of treatment in most patients is to prolong survival, and only about 10%–15% patients are alive at 5 years. Consequently, in patients with stage iv crc, the potential morbidity of treatment and the treatment’s impact on quality of life (qol) for the patient must be considered.
Resection of the primary tumour in patients with stage iv cancer is often performed to deal with presenting primary tumour symptoms and to prevent future primary tumour complications. Potential advantages of resection of the primary tumour are prevention of obstruction and major bleeding, better pain control, and a potential reduction in serious adverse effects—such as bleeding and perforation—related to novel targeted therapy. Resection may facilitate treatment tolerance (with better response) and potentially improve survival. Conversely, newer-generation chemotherapy in combination with targeted therapy has been associated with response rates of 40%–60%3–5. Systemic therapy not only reduces the size of metastatic lesions, but also shrinks the primary tumour, thereby potentially reducing local complications, such as bowel obstruction, related to primary tumours. Complications after resection of a primary tumour in patients with advanced crc can delay or prevent initiation of systemic therapy and thereby preclude the associated benefit. Whether resection of the primary tumour improves disease control by reducing tumour bulk remains unknown.
The available data about the potential benefit of resection of the primary tumour—and otherwise unresectable metastatic lesions—in patients with stage iv crc are limited. Some authors have advocated for surgery6–8, but others have failed to demonstrate a survival benefit for resection9–12. Whether a similar benefit can be achieved in the era of second- and third-generation anticancer agents, which are associated with higher response rates and better overall survival in patients with stage iv crc, is not known. In spite of uncertain survival benefit, a high rate of surgical resection of the primary tumour has been reported in patients with unresectable metastatic disease13,14.
We undertook the present comprehensive and critical analysis of the available literature to assess if surgical resection of the primary tumour in patients with advanced crc improves outcomes.
2. OBJECTIVES AND OUTCOMES OF INTEREST
2.1. Primary Objective
The primary objective was to compare survival in patients with stage iv crc who did and did not undergo surgical resection of the primary tumour.
2.2. Secondary Objectives
Secondary objectives included determining
the rates of 30-day postoperative mortality and nonfatal complications in the intervention group.
the rate of primary tumour complications in the control group.
the rate of non-resection procedures and the qol in both groups.
the survival benefit of surgical intervention in the subgroup of patients treated with second- and third-generation anticancer therapy.
the survival benefit of surgical intervention in the subgroup of minimally symptomatic patients.
3. DEFINITIONS
All outcomes of interest were pre-specified and defined. “Primary tumour complications” was defined as development of bleeding, obstruction, or perforation during the study period. “Fatal primary tumour complications” was defined as death within 30 days of bleeding, obstruction, or perforation secondary to an intact primary tumour. “Surgical mortality” was defined death within 30 days of surgery, and “nonfatal surgical complications” was defined as a postoperative infection, anastomotic leak, or any other complication recorded 30 days after resection of primary tumour. “Non-resection procedures” included bypass surgery with colostomy formation, endoscopic laser therapy, or placement of endoluminal stents. “Modern chemotherapy” was defined as use of third-generation agents (bevacizumab or the anti-epidermal growth factor receptor monoclonal antibodies cetuximab or panitumumab) alone or in combination with second-generation agents (irinotecan or oxaliplatin, or both). Second-generation chemotherapy became available for clinical use in most centres in the early 2000s. Individual patient data were not available, and so for the purposes of this analysis, all studies that specified the use of second- and third-generation therapy or those that were conducted in whole or in part (≥50%) after the year 1999 were considered studies using second- and third-generation anticancer therapy.
4. METHODS
Our methods conformed to the prisma statement guidelines15. Two investigators (SA, RKS) independently evaluated the abstracts, selected relevant articles matching the selection criteria, and independently extracted the data. The Cohen kappa coefficient was used to assess agreement between the two investigators16. Disagreements were resolved by discussion.
4.1. Inclusion and Exclusion Criteria
Studies involving patients with histologically documented adenocarcinoma of colon and rectum and evidence of metastases were included. Only studies with comparative data on the survival of patients with advanced crc with and without resection of the primary tumour were included. Studies that included data from patients who underwent upfront metastasectomy or from a comparison group of patients with nonsurgical procedures or curative resection were excluded.
4.2. Information Sources, Search Strategies, and Selection of Studies
An extensive search of reports in the medline (1946 to February 2012), embase (1947 to February 2012), and central (Cochrane Central Register of Controlled Trials, The Cochrane Library, Issue 3, 2012) databases was conducted. Studies were selected using the pre-specified criteria, with restriction to publication dates from 1980 onward, the English language, and studies involving human subjects.
The keywords, synonyms, and controlled vocabulary (mesh, emtree) used for the literature search are described in Appendix a. The computerized literature search was augmented by a manual review of citations from relevant studies to identify additional articles for assessment. The reference lists of all retrieved articles and relevant reviews and clinical practice guidelines were retrieved for identification of additional studies. In cases of duplicate publications, the most recent or most complete study was included. A standardized form was used during full-text screening to assess eligibility of studies for inclusion in the present review.
4.3. Data Collection
The data extracted from the included studies were these: study eligibility, design and characteristics, baseline patient characteristics (age, sex, comorbid illnesses, Eastern Cooperative Oncology Group performance status, etc.), primary tumour location, disease burden (extent of liver involvement, extra-hepatic disease, etc.), co-interventions (radiation therapy, chemotherapy, second- and third-generation chemotherapy, metastasectomy rate, etc.), and primary and secondary outcomes (median overall survival, 2-year survival, 30-day postoperative mortality, primary tumour complications, nonsurgical procedures, and qol). Attempts were made to contact the corresponding authors of all eligible studies for relevant missing information.
4.4. Validity Assessment
Study designs were evaluated according to whether they were retrospective or prospective, and randomized or observational. Two authors independently evaluated all the included studies using a list of selected quality items assessing components of validity and bias. Disagreements were resolved by discussion. Risk of bias in the eligible studies was assessed by each reviewer using the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions17. For observational studies, the Newcastle–Ottawa Quality Assessment Scale was applied18. The Newcastle–Ottawa Scale consists of 9 items grouped into 3 sections that are relevant to the quality of an observational study (Appendix b). For each outcome of interest, validity scores were evaluated as follows: ≤5, low quality; 6–7, medium quality; 8–9, high quality. The Cohen kappa coefficient was used to assess agreement between the two investigators with respect to the outcomes of interest16.
4.5. Analysis and Synthesis of Result
Results of the included studies for the primary outcome were combined in a formal meta-analysis to produce an overall analysis of surgical intervention. For quantitative pooling, the DerSimonian and Laird random-effects model was used, and all calculations were performed using the Review Manager analysis software (RevMan, version 5.1.2: The Cochrane Collaboration, http://ims.cochrane.org/revman). Treatment effects are expressed as hazard ratios (hrs) with corresponding 95% confidence intervals (cis). For studies that did not provide numeric information about time to events, the hr and variance were estimated using Kaplan–Meier survival curves19. A p value of 0.05 was used as the cut-off value for statistical significance. Funnel plots were constructed to evaluate potential publication bias. Heterogeneity across studies was assessed using a statistical test, with the proportion of variation being expressed as I2. All other outcomes are presented descriptively, and results are presented as the mean or median of variables in the analyzed studies. Single-group analyses were done for surgical mortality and complication rates (intervention group) and for the primary tumour complication rate (control group), because those outcomes were not applicable to both groups. A sensitivity analysis was performed if appropriate.
Pre-specified subgroup analyses were performed to assess the survival of patients with minimally symptomatic primary tumours and of patients involved in trials that offered treatment with second- and third-generation anticancer therapy. Risk of bias for all outcomes was assessed across the analyzed studies in duplicate by the two reviewers and reported using the grade scale20.
5. RESULTS
5.1. Study Selection
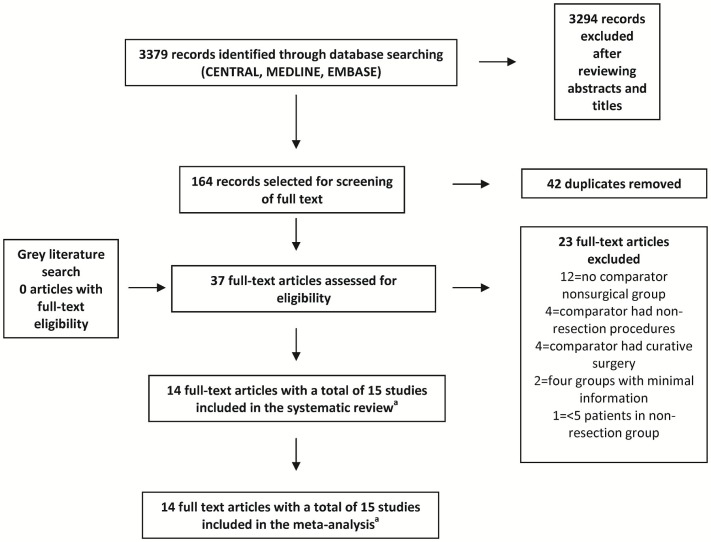
Figure 1 shows a flow chart of the search procedure, which identified 3379 citations. Publications not meeting the inclusion criteria and duplicate publications were excluded after a review of titles and abstracts. Thirty-seven potentially eligible articles underwent full-text assessment to determine their eligibility for inclusion in the final analysis. Fifteen studies (reported in fourteen articles) were identified as meeting the eligibility criteria6–12,21–26. Kappa agreement scores between the two abstractors with respect to “screening for the citations” and “full-text screening” were 0.68 and 0.86 respectively, suggesting substantial-to-excellent agreement.
FIGURE 1.
Flow of information through the phases of the literature search.a One article reported a retrospective analysis of two large randomized controlled trials (see text).
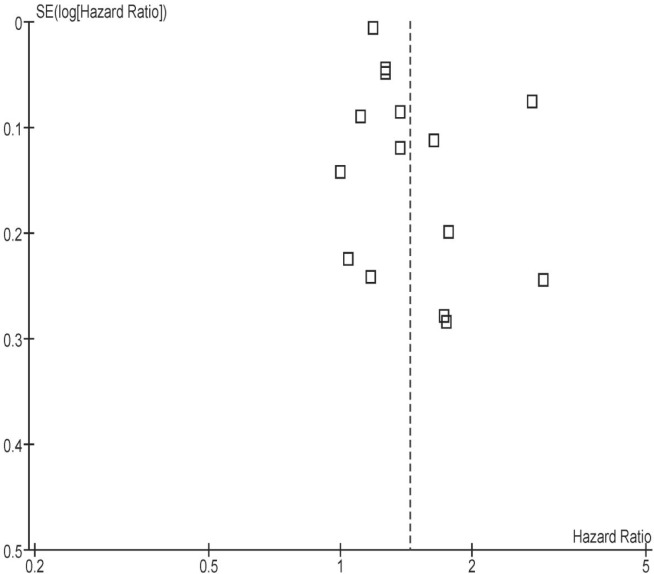
Of twenty-three full-text articles that were excluded, twelve had no comparator nonsurgical group27–38; four used a non-resection group (that is, ostomy procedures) as comparators39–42; and another four used patients who underwent curative surgery as the comparator group43–46. Two studies, each with four comparator groups, provided minimal information about those groups, and one had a patient population that overlapped with the population of another study included in the present review14,47. One study whose non-resection group contained fewer than 5 patients was excluded after discussion between the reviewers48. The asymmetry of the funnel plot around the point estimate suggests an element of publication bias (Appendix c, Figure c.1)
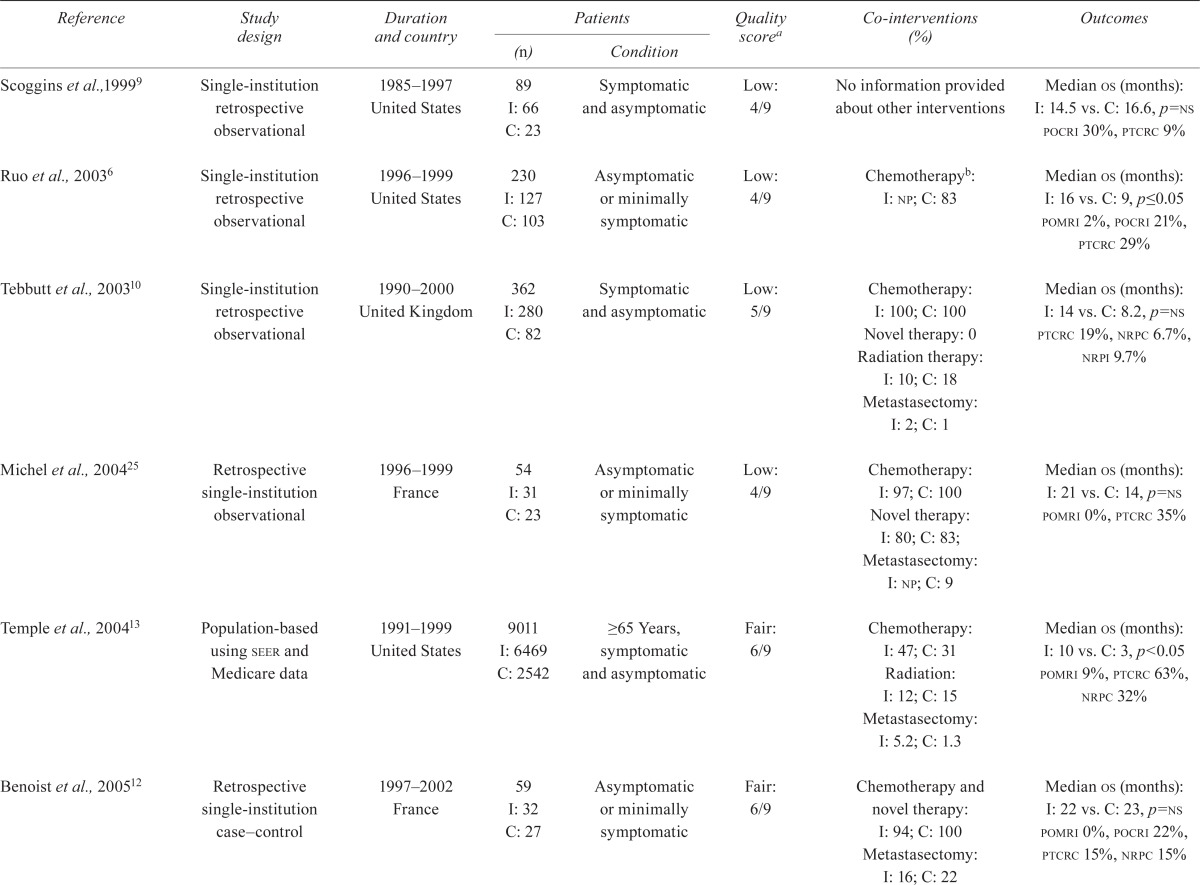
5.2. Study Characteristics and Risk of Bias
Table i describes the characteristics and risks of bias of the included studies. As anticipated, no prospective trial describing randomization between surgical and nonsurgical treatment was found. The study by Venderbosch et al.7 was a retrospective analysis of two randomized studies reported by Koopman et al.49 and Tol et al.50 (cairo and cairo ii).
TABLE I.
Study characteristics: design, quality, population, interventions, and outcomes of interest
| Reference | Study design | Duration and country |
Patients
|
Quality scorea | Co-interventions (%) | Outcomes | |
|---|---|---|---|---|---|---|---|
| (n) | Condition | ||||||
| Scoggins et al.,19999 | Single-institution retrospective observational | 1985–1997 United States |
89 I: 66 C: 23 |
Symptomatic and asymptomatic | Low: 4/9 | No information provided about other interventions | Median os (months): I: 14.5 vs. C: 16.6, p=ns pocri 30%, ptcrc 9% |
| Ruo et al., 20036 | Single-institution retrospective observational | 1996–1999 United States |
230 I: 127 C: 103 |
Asymptomatic or minimally symptomatic | Low: 4/9 | Chemotherapyb: I: np; C: 83 | Median os (months): I: 16 vs. C: 9, p≤0.05 pomri 2%, pocri 21%, ptcrc 29% |
| Tebbutt et al., 200310 | Single-institution retrospective observational | 1990–2000 United Kingdom |
362 I: 280 C: 82 |
Symptomatic and asymptomatic | Low: 5/9 | Chemotherapy: I: 100; C: 100 Novel therapy: 0 Radiation therapy: I: 10; C: 18 Metastasectomy: I: 2; C: 1 |
Median os (months): I: 14 vs. C: 8.2, p=ns ptcrc 19%, nrpc 6.7%, nrpi 9.7% |
| Michel et al., 200425 | Retrospective single-institution observational | 1996–1999 France |
54 I: 31 C: 23 |
Asymptomatic or minimally symptomatic | Low: 4/9 | Chemotherapy: I: 97; C: 100 Novel therapy: I: 80; C: 83; Metastasectomy: I: np; C: 9 |
Median os (months): I: 21 vs. C: 14, p=ns pomri 0%, ptcrc 35% |
| Temple et al., 200413 | Population-based using seer and Medicare data | 1991–1999 United States |
9011 I: 6469 C: 2542 |
≥65 Years, symptomatic and asymptomatic | Fair: 6/9 | Chemotherapy: I: 47; C: 31 Radiation: I: 12; C: 15 Metastasectomy: I: 5.2; C: 1.3 |
Median os (months): I: 10 vs. C: 3, p<0.05 pomri 9%, ptcrc 63%, nrpc 32% |
| Benoist et al., 200512 | Retrospective single-institution case–control | 1997–2002 France |
59 I: 32 C: 27 |
Asymptomatic or minimally symptomatic | Fair: 6/9 | Chemotherapy and novel therapy: I: 94; C: 100 Metastasectomy: I: 16; C: 22 |
Median os (months): I: 22 vs. C: 23, p=ns pomri 0%, pocri 22%, ptcrc 15%, nrpc 15% |
| Konyalian et al., 200724 | Retrospective single-institution cohort | 1991–2002 United States |
109 I: 62 C: 47 |
Symptomatic and asymptomatic | Low: 5/9 | Chemotherapyb: I: 71; C: 60 Radiation therapy: I: 27; C: 34 |
Median os (months): I: 12.5 vs. C: 4.6, p<0.05 pomri 5%, pocri 20%, ptcrc 57% |
| Galizia et al., 200811 | Retrospective single-institution observational | 1995–2005 Italy |
65 I: 42 C: 23 |
Asymptomatic or minimally symptomatic | Fair: 6/9 | Chemotherapy: I: 100; C: 100b
Metastasectomy: I: 12; C: 4 |
Median os (months): I: 15.2 vs. C: 12.3, p=0.003 pomri 0%, pocri 21%, ptcrc 31%, nrpc 22% |
| Evans et al., 200923 | Retrospective single-institution observational | 1999–2006 United Kingdom |
97 I: 45 C: 52 |
Symptomatic and asymptomatic | Low: 4/9 | Chemotherapy: I: np; C: 42b Radiation therapy: I: np; C: 8 |
Median os (months): I: 11 vs. C: 7, p=ns pomri 16%, ptcrc 23%, nrpc 50% |
| Aslam et al., 201021 | Retrospective multicentre observational | 1998–2007 United Kingdom |
647 I: 366 C: 281 |
Minimally symptomatic | Low: 5/9 |
Chemotherapy: I: 63; C: 36b | Median os (months): I: 14.5 vs. C: 5.8, p<0.05 pomri 7%, pocri 32%, nrpc 46% |
| Chan et al., 201022 | Retrospective population-based | 2000–2002 Canada |
411 I: 286 C: 125 |
Symptomatic and asymptomatic | Low: 5/9 | Chemotherapy: I: 61; C: 58 Novel therapy: I: 57; C: 36 Metastasectomy: I: 10; C: 0 |
Median os (months): I: 14 vs. C: 6, p<0.05 nrpc 22%, nrpi 4% |
| Seo et al., 201026 | Single-institution retrospective observational | 2001–2008 South Korea |
227 I: 144 C: 83 |
Asymptomatic or minimally symptomatic | Fair: 6/9 | Chemotherapy: I: 100; C: 100 Novel therapy: I: 85; C: 82 Radiation therapy: I: 10; C: 12 |
Median os (months): I: 22 vs. C: 14, p=ns pomri 0%, pocri 35%, ptcrc 19%, nrpc 7%, nrpi 2% |
| Karoui et al., 20118 | Retrospective multicentre observational | 1998–2007 France |
208 I: 123 C: 85 |
Symptomatic and asymptomatic | Low: 5/9 | Chemotherapy: I: 100; C: 99 Novel therapy: I: 89; C: 93 Metastasectomy I: 23; C: 29 |
Median os (months): I: 30.7 vs. C: 21.9, p=0.004 ptcrc 27%, nrpc 27% |
| Venderbosch et al., 20117 and Koopman et al., 200749 (cairo) | Retrospective multicentre cohort of a rcta | 2003–2004 (recruitment period) Netherlands |
399 I: 258 C: 141 |
Symptomatic and asymptomatic | Fair: 6/9 | Novel therapy: 100 in both groups | Median os (months): I: 16.7 vs. C: 11.4 |
| Venderbosch et al., 20117 and Tol et al., 200950 (cairo 2) | Retrospective multicentre cohort of a rcta | 2005–2006 (recruitment period) Netherlands |
448 I: 289 C: 159 |
Symptomatic and asymptomatic | Fair: 6/9 | Novel therapy: 100 in both groups | Median os (months): I: 20.7 vs. C: 13.4 |
Assessed using the Ottawa–Newcastle scale for nonrandomized studies (see Appendix b for score details).
Information about novel therapy (second- and third-generation anticancer therapy) was not provided. Six studies did not report postoperative mortality; eight studies did not provide postoperative morbidity; four studies did not provide information on primary tumour complication rates; six studies did not provide information on non-resection procedures in the control group, and twelve studies did not provide information on the intervention group; no study provided information about quality of life.
I = intervention group; C = control group; os = overall survival; ns = nonsignificant; pocri = postoperative nonfatal complication rate, intervention group; ptcrc = primary tumour complication rate, control group; np = not provided; pomri = postoperative mortality rate, intervention group; nrpc = non-resection procedures, control group; nrpi = non-resection procedures, intervention group; seer = Surveillance, Epidemiology and End Results; rct = randomized controlled trial.
Eight studies originated in Europe; five, in North America; and one, in Asia. Six studies exclusively involved minimally symptomatic patients, and ten studies met the pre-specified criteria for use of modern anticancer therapy. All but one study13 imposed no age restrictions.
All included publications reported retrospective observational studies. Using validity scoring for observational studies, no study met the criteria for good quality for any outcome of interest. For the primary outcome of overall survival, nine of fifteen studies were of low quality, and the remaining six were of fair quality (Appendix b).
With respect to secondary outcomes, the quality of evidence was lower overall than it had been for the primary outcome. Reporting bias was noted for all the secondary outcomes: six studies did not report the postoperative mortality rate7–10,22; eight did not provide data for postoperative complications or morbidity7,8,10,13,22,23,25; four lacked information about the rate of primary tumour complications7,21,22; six provided no information on non-resection procedures6,7,9,24,25; and no study provided information about qol.
5.3. Patient Characteristics
The included studies involved a total of 12,416 patients, among whom 8620 (69%) underwent surgery as initial treatment, and 3796 (31%) initially received systemic therapy. Several studies did not provide information about the baseline characteristics of the patients. All except one study9 provided information about systemic therapy; eight studies provided information about performance status7,8,10,11,22,25,26; and only four studies provided information about comorbid illnesses6,8,13,25.
Table ii describes the baseline characteristics of the patients in the analyzed studies. Median age was 61 years (range: 19–96 years), and 41% (95% ci: 36.8% to 45.1%) were women. Of the patients overall, 16.6% (95% ci: 7.9% to 25.2%) had an Eastern Cooperative Oncology Group performance status greater than 1, and 54% (95% ci: 40% to 69%) had more than 25% liver involvement. The tumour was located in the rectum in 26% of the patients (95% ci: 19.7% to 32.2%): 21.9% (95% ci: 14.3% to 29.5%) in the resection group, and 31% (95% ci: 22.8% in 39.2%) in the control group. With respect to systemic therapy, 82% of the patients (95% ci: 73% to 92%) received chemotherapy, and 64% (95%ci: 35% to 84%) received second- or third-generation therapy. Only in the cairo and cairo ii trials did patients in both groups uniformly receive second-generation (cairo) and third-generation therapy (cairo ii)49,50. The mean rate of metastasectomy was 11.4% (95% ci: 3.5% to 19.3%) in the intervention group and 9.3% (95% ci: 0% to 18.2%) in the control group.
TABLE II.
Baseline characteristics of the patients in the control and intervention groups from fifteen studies
| Characteristic |
Patient group
|
|||||
|---|---|---|---|---|---|---|
|
Overall
|
Control
|
Intervention
|
||||
| Mean | 95% ci | Mean | 95% ci | Mean | 95% ci | |
| Patients [n (%)] | 12,416 | 3796 (30.6) | 8620 (69.4) | |||
| Age (years) | ||||||
| Median | 61 | 63 | 60 | |||
| Range | 19–96 | 19–96 | 22–92 | |||
| Sex (% women)a | 41 | 36.8 to 45.1 | 38.7 | 31.3 to 46.2 | 43.5 | 38.5 to 49.0 |
| Rectal tumour (%)b | 26 | 19.7 to 32.2 | 31 | 22.8 to 39.2 | 21.9 | 14.3 to 29.5 |
| ecog performance status > 1 (%)c | 16.6 | 7.9 to 25.2 | 18.1 | 2.5 to 33.7 | 15 | 3.0 to 27 |
| Liver involvement > 25% (%)d | 54 | 40 to 69 | 54.7 | 25.6 to 83.7 | 54.2 | 34.4 to 73.9 |
| Peritoneal disease (%)e | 21.6 | 13.3 to 30 | 22 | 2.3 to 41.7 | 21.4 | 6.5 to 36.3 |
| Received radiation (%)f | 16.2 | 9.5 to 23.0 | 17.4 | 5.0 to 29.8 | 14.8 | 1.7 to 27.8 |
| Received chemotherapy (%)g | 82 | 73 to 92 | 79.2 | 63.3 to 95.1 | 86.1 | 73.6 to 98.6 |
| Single-agent fluoropyrimidineh | 88.6 | 80 to 97.1 | 88.1 | 73.6 to 100 | 89.0 | 76.2 to 100 |
| Second- or third-generation therapyh | 64 | 35 to 84 | 66 | 33.4 to 98.6 | 67.2 | 36.3 to 98.2 |
| Underwent metastasectomy (%)i | 10.3 | 4.6 to 16.1 | 9.3 | 0 to 18.2 | 11.4 | 3.5 to 19.3 |
One study did not provide information about chemotherapy9.
ci = confidence interval; ecog = Eastern Cooperative Oncology Group.
5.4. Overall Survival
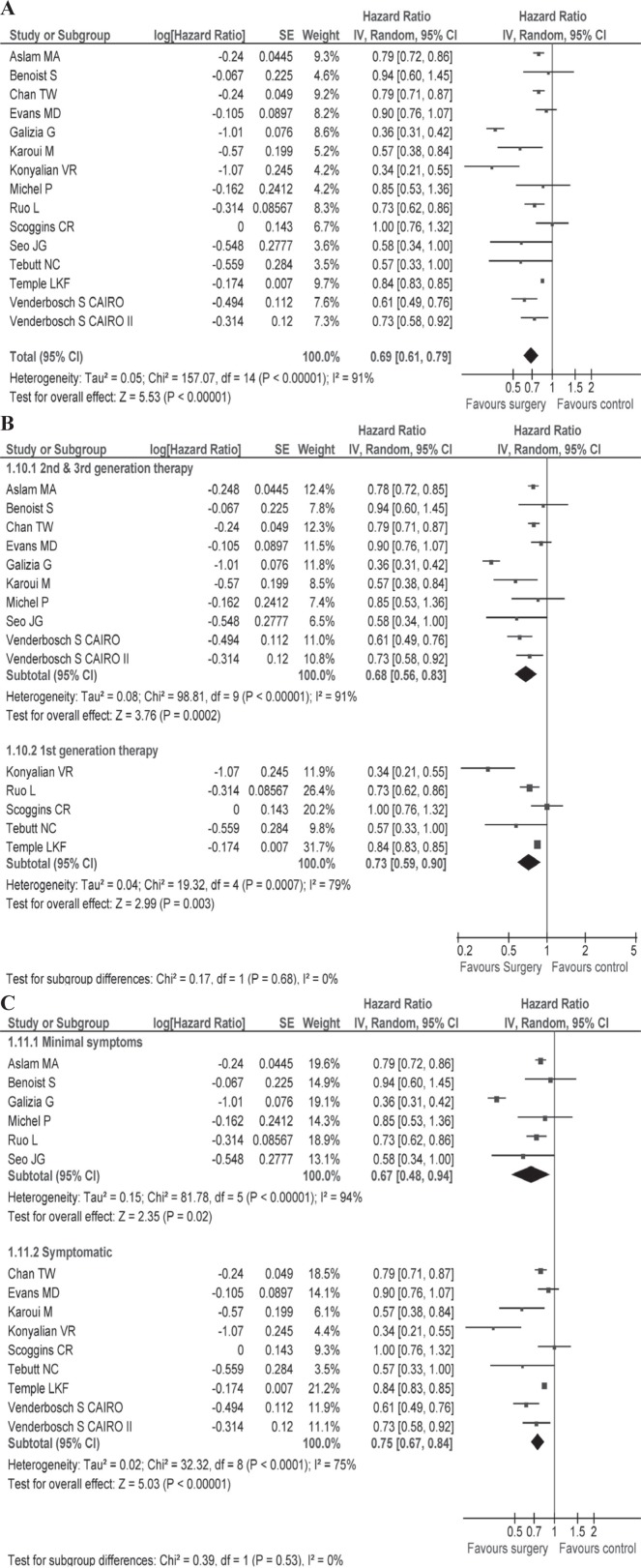
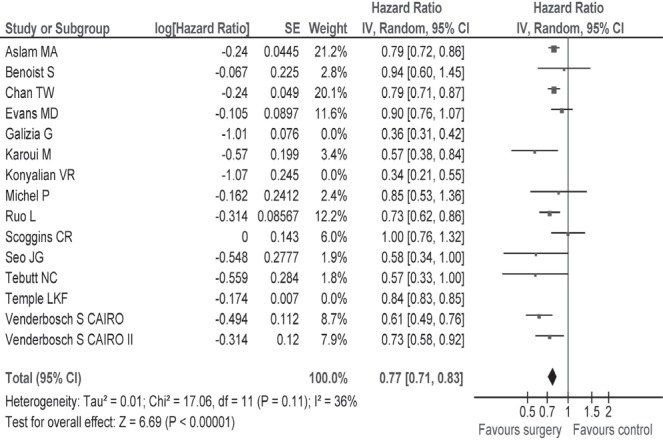
Table i describes survival and secondary outcomes for the individual studies, and Table iii describes summary findings and risk of bias for the studies overall. Median survival was 15.2 months (range: 10–30.7 months) in the resection group and 11.4 months (range: 3–22 months) in the non-resection group. A quantitative meta-analysis using the data from all fifteen studies revealed that, compared with no surgery, resection of the primary tumour was associated with a significant improvement in survival (hr: 0.69; 95% ci: 0.61 to 0.79; p < 0.00001; Figure 2). Subgroup analyses were performed for more homogenous patient populations with respect to symptoms and type of systemic therapy (see the Subgroup Analyses subsection).
TABLE III.
Evidence profile and summary of primary and secondary outcomes
| Outcome | Patients (n) | Studies (n) |
Patient groups
|
Quality of evidence20 | Comments | |
|---|---|---|---|---|---|---|
| Control | Intervention | |||||
| Overall survival | 12,416 | 15 | Very low | Hazard ratio for survival: 0.69 [95% confidence interval (ci): 0.61 to 0.79] favouring the intervention group. Significant imbalance in patients characteristics. Most studies did not provide information on performance status and comorbid illnesses. | ||
| Median | 11.4 | 15.2 | ||||
| Range | 3–22 | 10–30.7 | ||||
| Quality of life (qol) | 0 | (See comments) | (See comments) | na | All studies were retrospective and qol was not measured in any study. | |
| Surgical mortality rate (%) | 10,499 | 9 | Low | Four studies reported 0 postoperative mortality; a mortality rate of >5% was noted in the older studies. | ||
| Mean | na | 4.9 | ||||
| 95% ci | 0 to 9.7 | |||||
| Surgical morbidity rate | 1,426 | 7 | Very low | Most studies did not distinguish between major and minor postoperative complications. | ||
| Mean | na | 25.9 | ||||
| 95% ci | 20.1 to 31.6 | |||||
| Primary tumour complications rate (%) | 10,511 | 11 | Very low | Of twelve studies, six had both symptomatic and asymptomatic patients. | ||
| Mean | 29.7 | na | ||||
| 95% ci | 18.5 to 41.0 | |||||
| Mean nonsurgical procedure rate (%)a | 10,725 | 9 | Very low | Only three studies (n=862) provided a non-resection procedure rate in the intervention group. | ||
| Mean | 27.6 | 4.2 | ||||
| 95% ci | 15.4 to 39.9 | 0 to 10.1 | ||||
| (See comments) | ||||||
Nonsurgical procedures include bypass surgery and placement of stents.
na = not applicable.
FIGURE 2.
(A) Hazard ratio with 95% confidence interval (ci) for overall survival, all reviewed studies, favours the intervention group6–13,21–26,49. (B) Hazard ratio with 95% ci for overall survival, subgroups based on type of chemotherapy. (C) Hazard ratio with 95% ci for overall survival, subgroups based on extent of symptoms. se = standard error; iv = intervention.
5.5. Sensitivity Analyses
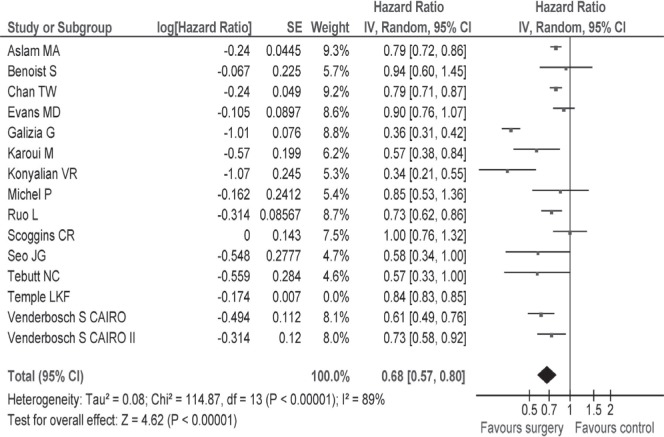
Only seven studies reported hrs and 95% cis; for the remaining 8 studies, we used the methods suggested by Tierney et al.19 to estimate hrs and variances. In a sensitivity analysis pooling the data of seven studies7,8,10,11,24,26, the hr for survival was 0.52 (95% ci: 0.40 to 0.68) favouring the resection group (Appendix c, Figure c.2). Among fifteen studies reviewed, the study by Temple et al. was conducted in patients more than 65 years of age. A sensitivity analysis that excluded the Temple et al. study revealed a hr for survival of 0.68 (95% ci: 0.57 to 0.80; Appendix c, Figure c.3).
5.6. Secondary Endpoints
The surgical mortality rate was reported in nine studies. The mean 30-day postoperative mortality rate was 4.9% (95% ci: 0% to 9.7%) in the intervention group. Only seven studies reported nonfatal surgical complications, including anastomotic leaks, wound infection, and other complications. The mean surgical morbidity rate was 25.9% (95% ci: 20.1% to 31.6%). Most studies did not separate major and minor complications. The mean rate of anastomotic leak, a serious postoperative complication, was 3.2% (95% ci: 0% to 8.3%)
The mean rates of primary tumour complications and intestinal obstruction secondary to the primary tumour were 29.7% (95%ci: 18.5% to 41.0%) and 23.4% (95% ci: 14.1% to 32.7%) respectively. Most studies failed to specify major and minor bleeding. No study specifically reported the rate of fatal primary tumour complications. The non-resection surgical procedures rate in the control group was 27.6% (95% ci: 15.4% to 39.9%). Only three studies reported rates of non-resection surgical procedures in the intervention group, for whom the rate was 4.2% (95% ci: 0% to 10.1%). Because all studies were retrospective, none assessed qol.
5.7. Subgroup Analyses
Table d.i (Appendix d) presents information about various outcomes in the patient subgroups of interest.
5.7.1. Studies Using Second- and Third-Generation Anticancer Therapy
In the subgroup of patients receiving modern chemotherapy, median overall survival in the group undergoing surgery was 18.7 months (range: 11–30.7 months); it was 12.85 months (range: 5.8–22 months) in the control group. The hr for survival in this subgroup was 0.68 (95% ci: 0.56 to 0.83) compared with a hr of 0.73 (95% ci: 0.59 to 0.90) in patients treated with an older regimen, which favours surgical intervention [Figure 2(B)]. A test for interaction between the groups was nonsignificant. The mean 30-day postoperative mortality rate in the group treated with modern chemotherapy was 3.9% (95% ci: 0% to 11%). The mean rates of primary tumour complications and non-resection procedures in the control group were 27.4% (95% ci: 16.4% to 38.5%) and 27% (95% ci: 12.5% to 41.6%).
5.7.2. Studies with Minimally Symptomatic Patients
The median overall survival in the group receiving surgery was 18.5 months (range: 14.5–23 months); it was 13.2 months (range: 5.8–22 months) in the control group. The hr for survival in minimally symptomatic patients was 0.67 [95% ci: 0.48 to 0.94; Figure 2(C)] compared with a hr of 0.75 (95% ci: 0.67 to 0.84) in symptomatic patients (test for subgroup interaction: p = 0.53), which favours the intervention group.
In minimally symptomatic patients, the mean 30-day postoperative mortality rate was 1.6% (95% ci: 0% to 74.8%), with four of six studies reporting 0% surgical mortality. The mean rates of primary tumour complications and non-resection procedures in the control group were 25.6% (95% ci: 5.9% to 45.2%) and 22.2% (95% ci: 0% to 49.1%).
6. DISCUSSION
Our review demonstrates a consistent trend favouring noncurative surgical management of primary tumours in patients with stage iv crc. Overall, the group treated with surgery experienced a 31% relative improvement in survival, with an absolute survival difference of approximately 4 months. A survival benefit of similar magnitude was demonstrated in the other reviews51,52; however, a recent review did not support the surgical intervention53.
We found comparable survival benefits in studies using newer-generation chemotherapies and in minimally symptomatic patients. Notably, the pooled estimate for survival revealed considerable heterogeneity across studies. Conceivably, those studies involved clinically heterogeneous groups with respect to patient population (age, performance status, comorbid illnesses, disease burden, primary tumour–related symptoms, for example) and co-interventions (type of systemic therapy, differing rate of metastasectomy). Likewise, considerable variability was noted across the different study designs, and the risk of bias was suggestive of methodologic diversity. Despite those limitations, we opted to report the pool result, because the direction of the effect was consistent across the studies and subgroups—albeit of varying magnitude.
Of special interest, a quantitative analysis excluding low-quality studies revealed a hr for survival of 0.64 (95% ci: 0.45 to 0.92) favouring surgical intervention (Appendix c, Figure c.4). Because of selective reporting and a lack of explicit information in some studies, examination of heterogeneity with respect to important clinical variables (with the exception of underlying symptoms and type of treatment) was not feasible. Notably, the test for heterogeneity was no longer significant after exclusion from the pool of three studies that had either larger effect sizes or a very narrow confidence interval, suggesting statistical heterogeneity (Appendix c, Figure c.5)11,13,24. Likewise, in the subgroup analysis, the test for heterogeneity was nonsignificant after exclusion of the study by Galizia et al.11. Because of the concern of publication bias, overestimation of the intervention effect relative to the true outcome is quite plausible.
A high rate of postoperative complications can offset the survival benefit associated with surgery. Our review was limited by selective reporting of surgical mortality and morbidity across the included studies. Compared with patients having localized disease, those with advanced crc tended to experience increased mortality after resection of the primary tumour. Although four of nine studies reported no postoperative mortality, the rate in some studies was not trivial, reaching up to 16%. As anticipated, a higher mortality rate has been associated with emergency surgery21,23. Fewer than half the included studies reported nonfatal operative complications, and many failed to distinguish between major and minor complications, limiting the clinical relevance of the information.
The mean rate of primary tumour complications was 27%, but reached as high as 63%. Complication rates of more than 50% were noted mostly in older studies. Realistically, there is no evidence to suggest that response rates for the primary tumour are inferior to those for metastases. Three retrospective studies specifically investigated the risk of primary tumour complications in patients with non-resection management and reported complication rates between 3% and 17%54–56.
When anti–vascular endothelial growth factor therapy is combined with cytotoxic agents in patients with an intact primary tumour, a concern about perforation risk arises57. Two recent uncontrolled prospective studies did not support prophylactic resection of the primary tumour in minimally symptomatic patients treated with targeted therapy58,59. In one cohort of 233 patients with intact primary tumours, only 7% of patients required emergency palliative surgery58. Use of bevacizumab, primary tumour location, and metastatic disease burden were not associated with an increased intervention rate. The other phase ii trial, which used an oxaliplatin and bevacizumab combination regimen, reported a 14% major complication rate related to the intact primary tumour59. Median overall survival of the treated cohort was 19.9 months. The authors concluded that survival is not compromised by leaving the primary colon tumour intact. The mean non-resection procedure rate in our review was 28% in patients with an intact primary tumour, which accorded with the primary tumour complication rate of 30% reported by McCahill et al.59. Only three studies reported non-resection procedures in the intervention group, and as expected, the numbers were much lower than those in the control group.
Quality of life is an important outcome that helps patients and their physicians choose appropriate treatment. No study in our review reported qol. Because major intestinal complications such as obstruction, perforation, and hemorrhage related to the primary tumour and postoperative complications are likely to be associated with a significant adverse effect on qol, qol can be indirectly assessed by reviewing the rates of surgical and primary tumour complications. A surgical intervention with a low complication rate could potentially result in a favorable qol as a result of fewer non-resection interventions, lack of primary tumour complications, and better tolerance for systemic therapy.
Potential limitations of the present review are the substantial number of low-quality studies, publication bias, and selective reporting. Importantly, all outcomes in the review were evaluated retrospectively, and patients were not randomized to surgery or non-surgical management. Several studies did not provide baseline prognostic characteristics for their groups, and others showed significant imbalances in baseline characteristics. Furthermore, few studies provided detailed information about the use and type of systemic therapy in each group, making it difficult to assess the relative contribution of resection to outcomes. Those concerns affect the validity of the survival benefit observed in our review, which might simply reflect the selection of younger and healthier patients with good performance status and low disease burden for surgery.
7. CONCLUSIONS
The retrospective data favour resection of the primary tumour in patients with advanced crc. However, the very low quality of the current evidence requires that good-quality cohort studies and adequately powered, well-designed randomized trials be conducted to assess all the important outcomes in this patient population. We have begun a large population-based cohort study in the province of Saskatchewan, and European investigators are currently working on several randomized trials, including cairo 4, to resolve this matter.
8. ACKNOWLEDGMENTS
We thank Drs. Galizia, Gill, and Koopman for correspondence; Me-Linh and Kaitryn Campbell for assistance in developing the search strategy; and Edel Murphy and Reitzer Rodseth for helpful comments. A special thanks goes to Dr. Jan Brozek at McMaster University, Ontario, for valuable feedback and comments and to Drs. Fields, Janzen, and Muhajarine for their supervision.
Portions of these data were presented in September 2012 at the European Society of Medical Oncology Annual Meeting in Vienna, Austria, and in June 2013 at the American Society of Clinical Oncology Annual Meeting in Chicago, Illinois, U.S.A.
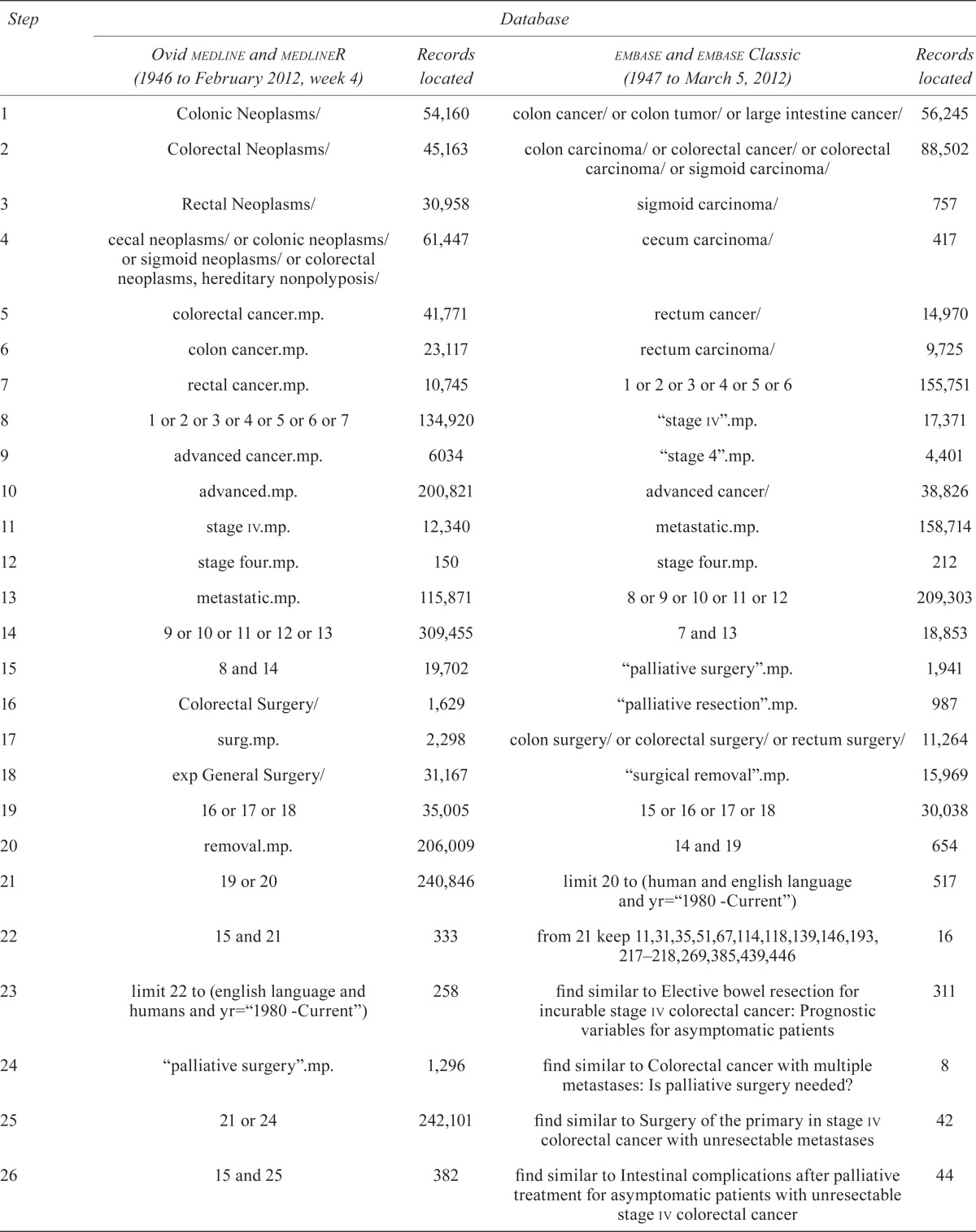
APPENDIX A. SEARCH STRATEGY
| Step |
Database
|
|||
|---|---|---|---|---|
| Ovid medline and medlineR (1946 to February 2012, week 4) | Records located | embase and embase Classic (1947 to March 5, 2012) | Records located | |
| 1 | Colonic Neoplasms/ | 54,160 | colon cancer/ or colon tumor/ or large intestine cancer/ | 56,245 |
| 2 | Colorectal Neoplasms/ | 45,163 | colon carcinoma/ or colorectal cancer/ or colorectal carcinoma/ or sigmoid carcinoma/ | 88,502 |
| 3 | Rectal Neoplasms/ | 30,958 | sigmoid carcinoma/ | 757 |
| 4 | cecal neoplasms/ or colonic neoplasms/ or sigmoid neoplasms/ or colorectal neoplasms, hereditary nonpolyposis/ | 61,447 | cecum carcinoma/ | 417 |
| 5 | colorectal cancer.mp. | 41,771 | rectum cancer/ | 14,970 |
| 6 | colon cancer.mp. | 23,117 | rectum carcinoma/ | 9,725 |
| 7 | rectal cancer.mp. | 10,745 | 1 or 2 or 3 or 4 or 5 or 6 | 155,751 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 134,920 | “stage iv”.mp. | 17,371 |
| 9 | advanced cancer.mp. | 6034 | “stage 4”.mp. | 4,401 |
| 10 | advanced.mp. | 200,821 | advanced cancer/ | 38,826 |
| 11 | stage iv.mp. | 12,340 | metastatic.mp. | 158,714 |
| 12 | stage four.mp. | 150 | stage four.mp. | 212 |
| 13 | metastatic.mp. | 115,871 | 8 or 9 or 10 or 11 or 12 | 209,303 |
| 14 | 9 or 10 or 11 or 12 or 13 | 309,455 | 7 and 13 | 18,853 |
| 15 | 8 and 14 | 19,702 | “palliative surgery”.mp. | 1,941 |
| 16 | Colorectal Surgery/ | 1,629 | “palliative resection”.mp. | 987 |
| 17 | surg.mp. | 2,298 | colon surgery/ or colorectal surgery/ or rectum surgery/ | 11,264 |
| 18 | exp General Surgery/ | 31,167 | “surgical removal”.mp. | 15,969 |
| 19 | 16 or 17 or 18 | 35,005 | 15 or 16 or 17 or 18 | 30,038 |
| 20 | removal.mp. | 206,009 | 14 and 19 | 654 |
| 21 | 19 or 20 | 240,846 | limit 20 to (human and english language and yr=“1980 -Current”) | 517 |
| 22 | 15 and 21 | 333 | from 21 keep 11,31,35,51,67,114,118,139,146,193, 217–218,269,385,439,446 | 16 |
| 23 | limit 22 to (english language and humans and yr=“1980 -Current”) | 258 | find similar to Elective bowel resection for incurable stage iv colorectal cancer: Prognostic variables for asymptomatic patients | 311 |
| 24 | “palliative surgery”.mp. | 1,296 | find similar to Colorectal cancer with multiple metastases: Is palliative surgery needed? | 8 |
| 25 | 21 or 24 | 242,101 | find similar to Surgery of the primary in stage iv colorectal cancer with unresectable metastases | 42 |
| 26 | 15 and 25 | 382 | find similar to Intestinal complications after palliative treatment for asymptomatic patients with unresectable stage iv colorectal cancer | 44 |
| 27 | “palliative resection”.mp. | 658 | find similar to How Aggressive Should We Be in Patients with Stage iv Colorectal Cancer? | 9 |
| 28 | 25 or 27 | 242,706 | find similar to Is there a survival advantage for elective primary tumor resection in asymptomatic patients with incurable stage iv colorectal cancer? | 1,344 |
| 29 | 15 and 28 | 433 | ||
| 30 | limit 29 to (english language and humans and yr=“1980 -Current”) | 328 | ||
| 31 | find similar to Elective palliative resection of incurable stage iv colorectal cancer: who really benefits from it?. | 364 | ||
| 32 | find similar to The role of primary tumour resection in patients with stage iv colorectal cancer. | 68 | ||
| 33 | find similar to Surgery of the primary in stage iv colorectal cancer with unresectable metastases. | 27 | ||
| 34 | from 33 keep 9–10 | 2 | ||
| 35 | find similar to Elective bowel resection for incurable stage iv colorectal cancer: prognostic variables for asymptomatic patients. | 209 | ||
APPENDIX B. NEWCASTLE–OTTAWA QUALITY ASSESSMENT SCALE AND STUDY SCORES
B.1. Cohort Studies—Primary Outcome: Mortality
For a cohort study, these items are assessed:
- Selection
- ○ True representativeness of the exposed cohort in the community
- ○ Non-exposed cohort drawn from the same community
- ○ Ascertainment of exposure
- ○ Demonstration that outcome of interest was not present at start of study.
- Comparability of cohorts
- ○ Control for symptoms
- ○ Control for systemic therapy
- Outcome
- ○ Assessment of outcome
- ○ Follow –up was long enough for outcomes to occur
- ○ Adequacy of follow-up of cohorts
A study can be awarded a maximum of 1 star for each item within the Selection and Outcome categories. A maximum of 2 stars can be given for Comparability:
Selection (0–4)
Comparability (0–2)
Outcome (0–3)
TOTAL: 0–9
TABLE B.I.
Scoring form for cohort studies

|
Selection | ||||
|
Comparability
| ||||
Outcome
| ||||
|
| ||||
| _____ /4 | +_____/2 | +_____/3 | =_____/9 | TOTAL SCORE |
1 point.
B.2. Case–Control Studies
For a case–control study, these items are assessed:
- Selection
- ○ Case definition
- ○ Representativeness of the cases
- ○ Selection of controls
- ○ Definition of controls
- Comparability of cohorts
- ○ Control for symptoms
- ○ Control for systemic therapy
- Exposure
- ○ Assessment of exposure
- ○ Same method of ascertainment for cases and controls
- ○ Nonresponse rate
A study can be awarded a maximum of 1 star for each item within the Selection and Outcome categories. A maximum of 2 stars can be given for Comparability:
Selection (0–4)
Comparability (0–2)
Outcome (0–3)
TOTAL: 0–9
TABLE B.II.
Scoring form for case–control studies

Selection
| ||||
|
Comparability
| ||||
| Exposure | ||||
|
| ||||
| _____/4 | +_____/2 | +_____/3 | =_____/9 | TOTAL SCORE |
1 point.
B.3. Newcastle–Ottawa Scores for the Studies Included in the Analysis
TABLE B.III.
Scores for observational studies
| Reference |
Score
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | TOTAL | |||||||
|
| ||||||||||
| 1 Representativeness of the exposed cohort | 2 Selection of the non-exposed cohort | 3 Ascertainment of exposure | 4 Demonstration that outcome of interest was not present at start of (yes=1, no=0) | 5 Study controls for symptoms (yes=1, no=0) | 6 Study controls for systemic therapy (yes=1, no=0) | 7 Assessment of outcome | 8 Was follow-up long enough for outcomes to occur (yes=1, no=0) | 9 Adequacy of follow up of cohorts | ||
| Aslam et al., 201021 | 0a | 0b | 1c | 1 | 1 | 0 | 1d | 1e | 0f | 5 |
| Chan et al., 201022 | 1g | 1h | 1c | 1 | 0 | 0 | 1d | 0i | 0f | 5 |
| Evans et al., 200923 | 0a | 0b | 1c | 1 | 0 | 0 | 1d | 1e | 0f | 4 |
| Galizia et al., 200811 | 0a | 0j | 1c | 1 | 1 | 1 | 1d | 1e | 0f | 6 |
| Karoui et al., 20118 | 0k | 0j | 1c | 1 | 0 | 1 | 1d | 1e | 0f | 5 |
| Konyalian et al., 200724 | 0a | 0j | 1c | 1 | 0 | 1 | 1d | 1e | 0f | 5 |
| Michel et al., 200425 | 0a | 0j | 1c | 1 | 1 | 0 | 1d | 0i | 0f | 4 |
| Ruo et al., 20036 | 0a | 0b | 1c | 1 | 1 | 0 | 1d | 0i | 0f | 4 |
| Scoggins et al.,19999 | 0a | 0b | 1c | 1 | 0 | 0 | 1d | 1e | 0f | 4 |
| Seo et al., 201026 | 0a | 0j | 1c | 1 | 1 | 1 | 1d | 1e | 0f | 6 |
| Tebutt et al., 200310 | 0a | 0b | 1c | 1 | 1 | 0 | 1d | 1e | 0f | 5 |
| Temple et al., 200413 | 1g | 1h | 1c | 1 | 0 | 0 | 1d | 1e | 0f | 6 |
| Venderbosch et al., 20117,49 | 1g,l | 1h,l | 1c | 1 | 0 | 1 | 1d | 1e | 0f | 6 |
| Venderbosch et al., 20117,50 | 1g,l | 1h,l | 1c | 1 | 0 | 1 | 1d | 1e | 0f | 6 |
Selected group (single institution).
Source not known (non-community).
Secure record.
Record linkage.
>5 Years’ duration.
No statement.
Somewhat representative of the patients with advanced colorectal cancer in the community.
Drawn from the same community as the exposed cohort.
<5 Years’ duration.
Source not known; could be from a different source (non-community).
Selected group.
Subgroup of randomized patients across the Netherlands.
TABLE B.IV.
Score for case–control study
| Reference |
Score
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Exposure | TOTAL | |||||||
|
| ||||||||||
| 1 Is the case definition adequate? | 2 Representativeness of the cases | 3 Selection of controls | 4 Definition of controls | 5 Study controls for symptoms (yes=1, no=0) | 6 Study controls for systemic therapy (yes=1, no=0) | 7 Ascertainment of exposure | 8 Same method of ascertainment for cases and controls (yes=1, no=0) | 9 Nonresponse rate | ||
| Benoist et al., 200512 | 0a | 1b | 0c | 1d | 1 | 1 | 1e | 1 | 0f | 6 |
Record linkage or based on self-report.
Consecutive or obviously representative series of cases.
Hospital controls.
No history of disease (endpoint).
Secure record.
Rate different and no designation.
APPENDIX C. ADDITIONAL FIGURES
FIGURE C.1.
Funnel plot shows asymmetry of studies around the point estimate, suggestive of publication bias.
FIGURE C.2.
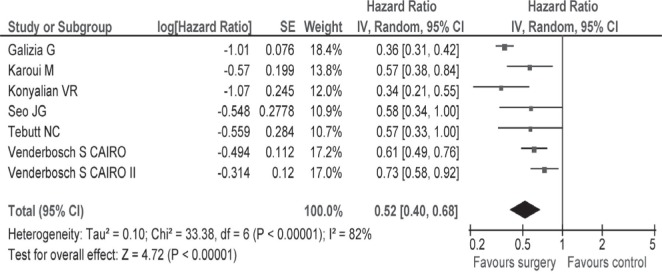
Sensitivity analysis of overall survival for seven studies reported a hazard ratio favouring the intervention group.
FIGURE C.3.
Sensitivity analysis of overall survival, excluding the study by Temple et al.13, favours the intervention group.
FIGURE C.4.
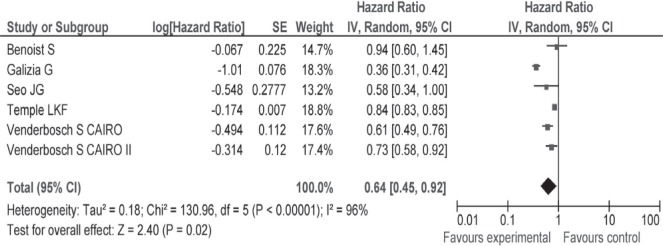
Hazard ratios with 95% confidence intervals for overall survival in subgroups, based on a score of “ fair” on the Ottawa–Newcastle Scale.
FIGURE C.5.
Hazard ratios with 95% confidence intervals for overall survival, twelve studies (excluding three with a large effect size or narrow confidence interval), favour the intervention group with low heterogeneity.
APPENDIX D. ADDITIONAL TABLE
TABLE D.I.
Primary and secondary outcomes in subgroups of patients, by population and intervention
| Subgroup | Survival | Postoperative, intervention group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Mean | 95% ci | hr | 95% ci |
Mortality
|
Nonfatal complications
|
Primary tumour complications, control group
|
Nonsurgical procedure, control group
|
|||||
| (months) | (%) | (95% ci) | (%) | (95% ci) | (%) | (95% ci) | (%) | (95% ci) | ||||
| Asymptomatic or minimally symptomatic patients | ||||||||||||
| Intervention | 18.6 | 14.6 to 22.6 | 0.67 | 0.48 to 0.94a | 1.6 | 0 to 4.8 | 25.6 | 5.9 to 45.2 | na | 2b | ||
| Control | 12.8 | 7.1 to 18.6 | 0.78 | 0.72 to 0.84c | na | na | 25 | 15.3 to 36.1 | 22.2 | 0 to 49.1 | ||
| Symptomatic and asymptomatic patients | ||||||||||||
| Intervention | 16.0 | 11.1 to 20.9 | 0.75 | 0.67 to 0.84d | 10 | 0 to 23.8 | 25 | 12.6 to 37.4 | na | 5.3 | 2 to 8.7 | |
| Control | 10.0 | 5.1 to 14.9 | 0.79 | 0.70 to 0.90e | na | na | 27 | 7.5 to 46.5 | 32.8 | 13.3 to 52.2 | ||
| Studies using 2nd- and 3rd-generation therapy | ||||||||||||
| Intervention | 18.9 | 14.8 to 23.0 | 0.68 | 0.56 to 0.83f | 3.9 | 0 to 11 | 27.4 | 16.4 to 38.5 | na | 3 | 0 to 5.5 | |
| Control | 12.8 | 8.6 to 17.0 | 0.75 | 0.67 to 84g | na | na | 25 | 17 to 33 | 27 | 12.5 to 41.6 | ||
| Studies using 1st-generation therapy | ||||||||||||
| Intervention | 13.4 | 10.6 to 16.2 | 0.73 | 0.59 to 0.90h | 5.3 | 0 to 14.1 | 23.7 | 10 to 37.4 | 28.2 | 2.5 to 53.9 | 6.7i | |
| Control | 8.1 | 1.7 to 14.8 | 0.81 | 0.72 to 0.92j | na | na | na | 32i | ||||
Test for heterogeneity was significant (data from six studies).
Only 1 of 6 studies reported on non-resection surgical procedures in the intervention group.
Test for heterogeneity was nonsignificant after data from the study by Galizia et al., 200811 were excluded.
Test for heterogeneity was significant (data from nine studies).
Test for heterogeneity was nonsignificant after data from the studies by Konyalian et al., 200724, Temple et al., 200413, and Venderbosch et al., 20117 were excluded.
Test for heterogeneity was significant (data from ten studies).
Test for heterogeneity was nonsignificant after data from the studies by Aslam et al., 201021, and Galizia et al., 200811 were excluded.
Test for heterogeneity was significant (data from five studies).
Only one of five studies reported non-resection surgical procedures in the intervention and control groups.
Test for heterogeneity was nonsignificant after data from the study by Konyalian et al., 200724 were excluded.
ci = confidence interval; hr = hazard ratio; na = not applicable.
9. CONFLICT OF INTEREST DISCLOSURES
This review was part of a project funded by the Saskatchewan Cancer Agency. The authors have no financial conflicts of interest to declare.
10. REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306:752–5. doi: 10.1136/bmj.306.6880.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tournigand C, André T, Achille E, et al. folfiri followed by folfox6 or the reverse sequence in advanced colorectal cancer: a randomized gercor study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 6.Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage iv colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg. 2003;196:722–8. doi: 10.1016/S1072-7515(03)00136-4. [DOI] [PubMed] [Google Scholar]
- 7.Venderbosch S, Wilt JH, Teerenstra S, et al. Prognostic value of resection of primary tumor in patients with stage iv colorectal cancer: retrospective analysis of two randomized studies and a review of the literature. Ann Surg Oncol. 2011;18:3252–60. doi: 10.1245/s10434-011-1951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karoui M, Roudot–Thoraval F, Mesli F, et al. Primary colectomy in patients with stage iv colon cancer and unresectable distant metastases improves overall survival: results of a multicentric study. Dis Colon Rectum. 2011;54:930–8. doi: 10.1097/DCR.0b013e31821cced0. [Erratum in: Dis Colon Rectum 2011;54:1338] [DOI] [PubMed] [Google Scholar]
- 9.Scoggins CR, Meszoely IM, Blanke CD, Beauchamp RD, Leach SD. Nonoperative management of primary colorectal cancer in patients with stage iv disease. Ann Surg Oncol. 1999;6:651–7. doi: 10.1007/s10434-999-0651-x. [DOI] [PubMed] [Google Scholar]
- 10.Tebbutt NC, Norman AR, Cunningham D, et al. Intestinal complications after chemotherapy for patients with unresected primary colorectal cancer and synchronous metastases. Gut. 2003;52:568–73. doi: 10.1136/gut.52.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galizia G, Lieto E, Orditura M, et al. First-line chemotherapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch Surg. 2008;143:352–8. doi: 10.1001/archsurg.143.4.352. [DOI] [PubMed] [Google Scholar]
- 12.Benoist S, Pautrat K, Mitry E, Rougier P, Penna C, Nordlinger B. Treatment strategy for patients with colorectal cancer and synchronous irresectable liver metastases. Br J Surg. 2005;92:1155–60. doi: 10.1002/bjs.5060. [DOI] [PubMed] [Google Scholar]
- 13.Temple LK, Hsieh L, Wong WD, Saltz L, Schrag D. Use of surgery among elderly patients with stage iv colorectal cancer. J Clin Oncol. 2004;22:3475–84. doi: 10.1200/JCO.2004.10.218. [DOI] [PubMed] [Google Scholar]
- 14.Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage iv colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol. 2005;12:637–45. doi: 10.1245/ASO.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A, Altman DG, Tetzlaff J, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 16.Fleiss JL, Cohen J. The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educ Psychol Meas. 1973;33:613–19. doi: 10.1177/001316447303300309. [DOI] [Google Scholar]
- 17.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, U.K.: The Cochrane Collaboration; 2011. Ver. 5.1.0. [Available online at http://www.cochrane.org/training/cochrane-handbook; cited February 16, 2012] [Google Scholar]
- 18.Wells GA, Shea B, O’Connell D, et al. Ottawa, ON: Ottawa Hospital Research Institute; Home > Our Research > Research Programs > Clinical Epidemiology > The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses [Web page] n.d. [Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp; February 10, 2012] [Google Scholar]
- 19.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Vist GE, et al. grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aslam MI, Kelkar A, Sharpe D, Jameson JS. Ten years experience of managing the primary tumours in patients with stage iv colorectal cancers. Int J Surg. 2010;8:305–13. doi: 10.1016/j.ijsu.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Chan TW, Brown C, Ho CC, Gill S. Primary tumor resection in patients presenting with metastatic colorectal cancer: analysis of a provincial population-based cohort. Am J Clin Oncol. 2010;33:52–5. doi: 10.1097/COC.0b013e31819e902d. [DOI] [PubMed] [Google Scholar]
- 23.Evans MD, Escofet X, Karandikar SS, Stamatakis JD. Outcomes of resection and non-resection strategies in management of patients with advanced colorectal cancer. World J Surg Oncol. 2009;7:28. doi: 10.1186/1477-7819-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konyalian VR, Rosing DK, Haukoos JS, et al. The role of primary tumour resection in patients with stage iv colorectal cancer. Colorectal Dis. 2007;9:430–7. doi: 10.1111/j.1463-1318.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- 25.Michel P, Roque I, Di Fiore F, et al. Colorectal cancer with non-resectable synchronous metastases: should the primary tumor be resected? Gastroenterol Clin Biol. 2004;28:434–7. doi: 10.1016/S0399-8320(04)94952-4. [DOI] [PubMed] [Google Scholar]
- 26.Seo GJ, Park JW, Yoo SB, et al. Intestinal complications after palliative treatment for asymptomatic patients with unresectable stage iv colorectal cancer. J Surg Oncol. 2010;102:94–9. doi: 10.1002/jso.21577. [DOI] [PubMed] [Google Scholar]
- 27.Yamamura T, Tsukikawa S, Akaishi O, et al. Multivariate analysis of the prognostic factors of patients with unresectable synchronous liver metastases from colorectal cancer. Dis Colon Rectum. 1997;40:1425–9. doi: 10.1007/BF02070706. [DOI] [PubMed] [Google Scholar]
- 28.Stelzner S, Hellmich G, Koch R, Ludwig K. Factors predicting survival in stage iv colorectal carcinoma patients after palliative treatment: a multivariate analysis. J Surg Oncol. 2005;89:211–17. doi: 10.1002/jso.20196. [DOI] [PubMed] [Google Scholar]
- 29.Rosen SA, Buell JF, Yoshida A, et al. Initial presentation with stage iv colorectal cancer: how aggressive should we be? Arch Surg. 2000;135:530–4. doi: 10.1001/archsurg.135.5.530. [DOI] [PubMed] [Google Scholar]
- 30.Nash GM, Saltz LB, Kemeny NE, et al. Radical resection of rectal cancer primary tumor provides effective local therapy in patients with stage iv disease. Ann Surg Oncol. 2000;9:954–60. doi: 10.1007/BF02574512. [DOI] [PubMed] [Google Scholar]
- 31.Mäkelä J, Haukipuro K, Laitinen S, Kairaluoma MI. Palliative operations for colorectal cancer. Dis Colon Rectum. 1990;33:846–50. doi: 10.1007/BF02051920. [DOI] [PubMed] [Google Scholar]
- 32.Law WL, Chan WF, Lee YM, Chu KW. Non-curative surgery for colorectal cancer: critical appraisal of outcomes. Int J Colorectal Dis. 2004;19:197–202. doi: 10.1007/s00384-003-0551-7. [DOI] [PubMed] [Google Scholar]
- 33.Kleespies A, Füessl KE, Seeliger H, et al. Determinants of morbidity and survival after elective non-curative resection of stage iv colon and rectal cancer. Int J Colorectal Dis. 2009;24:1097–109. doi: 10.1007/s00384-009-0734-y. [DOI] [PubMed] [Google Scholar]
- 34.Harris GJ, Senagore AJ, Lavery IC, Church JM, Fazio VW. Factors affecting survival after palliative resection of colorectal carcinoma. Colorectal Dis. 2002;4:31–5. doi: 10.1046/j.1463-1318.2002.00304.x. [DOI] [PubMed] [Google Scholar]
- 35.Cummins ER, Vick KD, Poole GV. Incurable colorectal carcinoma: the role of surgical palliation. Am Surg. 2004;70:433–7. [PubMed] [Google Scholar]
- 36.Costi R, Mauro DD, Veronesi L, et al. Elective palliative resection of incurable stage iv colorectal cancer: who really benefits from it? Surg Today. 2011;41:222–9. doi: 10.1007/s00595-009-4253-9. [DOI] [PubMed] [Google Scholar]
- 37.Bajwa A, Blunt N, Vyas S, et al. Primary tumour resection and survival in the palliative management of metastatic colorectal cancer. Eur J Surg Oncol. 2009;35:164–7. doi: 10.1016/j.ejso.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Frago R, Kreisler E, Biondo S, Salazar R, Dominguez J, Escalante E. Outcomes in the management of obstructive unresectable stage iv colorectal cancer. Eur J Surg Oncol. 2010;36:1187–94. doi: 10.1016/j.ejso.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Al-Sanea N, Isbister WH. Is palliative resection of the primary tumor in the presence of advanced rectal cancer, a safe and useful technique for symptom control? ANZ J Surg. 2004;74:229–32. doi: 10.1111/j.1445-2197.2004.02946.x. [DOI] [PubMed] [Google Scholar]
- 40.Costi R, Mazzeo A, Di Mauro D, et al. Palliative resection of colorectal cancer: does it prolong survival? Ann Surg Oncol. 2007;14:2567–76. doi: 10.1245/s10434-007-9444-2. [DOI] [PubMed] [Google Scholar]
- 41.Mik M, Dziki L, Galbfach P, Trzcinski R, Sygut A, Dziki A. Resection of the primary tumour or other palliative procedures in incurable stage iv colorectal cancer patients? Colorectal Dis. 2010;12:e61–7. doi: 10.1111/j.1463-1318.2009.01825.x. [DOI] [PubMed] [Google Scholar]
- 42.Tanoue Y, Tanaka N, Nomura Y. Primary site resection is superior for incurable metastatic colorectal cancer. World J Gastroenterol. 2010;16:3561–6. doi: 10.3748/wjg.v16.i28.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cellini C, Hunt SR, Fleshman JW, Birnbaum EH, Bierhals AJ, Mutch MG. Stage iv rectal cancer with liver metastases: is there a benefit to resection of the primary tumor? World J Surg. 2010;34:1102–8. doi: 10.1007/s00268-010-0483-7. [DOI] [PubMed] [Google Scholar]
- 44.Kuo LJ, Leu SY, Liu MC, et al. How aggressive should we be in patients with stage iv colorectal cancer? Dis Colon Rectum. 2003;46:1646–52. doi: 10.1007/BF02660770. [DOI] [PubMed] [Google Scholar]
- 45.Yun HR, Lee WY, Lee WS, Cho YB, Yun SH, Chun HK. The prognostic factors of stage iv colorectal cancer and assessment of proper treatment according to the patient’s status. Int J Colorectal Dis. 2007;22:1301–10. doi: 10.1007/s00384-007-0315-x. [DOI] [PubMed] [Google Scholar]
- 46.Verheijen PM, Stevenson AR, Lumley JW, Clark AJ, Stitz RW, Clark DA. Laparoscopic resection of advanced colorectal cancer. Br J Surg. 2011;98:427–30. doi: 10.1002/bjs.7329. [DOI] [PubMed] [Google Scholar]
- 47.Kauffman MS, Radharkrishnan N, Roy R, et al. Influence of palliative surgical resection on overall survival in patients with advanced colorectal cancer: a retrospective single institutional study. Colorectal Dis. 2008;10:498–502. doi: 10.1111/j.1463-1318.2007.01384.x. [DOI] [PubMed] [Google Scholar]
- 48.Liu SK, Church JM, Lavery IC, Fazio VW. Operation in patients with incurable colon cancer—is it worthwhile? Dis Colon Rectum. 1997;40:11–14. doi: 10.1007/BF02055675. [DOI] [PubMed] [Google Scholar]
- 49.Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (cairo): a phase iii randomised controlled trial. Lancet. 2007;370:135–42. doi: 10.1016/S0140-6736(07)61086-1. [DOI] [PubMed] [Google Scholar]
- 50.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–72. doi: 10.1056/NEJMoa0808268. [Erratum in: N Engl J Med 2010;363:2573] [DOI] [PubMed] [Google Scholar]
- 51.Faron M, Bourredjem A, Pignon JP, et al. Impact on survival of primary tumor resection in patients with colorectal cancer and unresectable metastasis: pooled analysis of individual patients’ data from four randomized trials [abstract 3507] J Clin Oncol. 2012;30 doi: 10.1016/j.ejca.2014.10.023. [Available online at: http://meetinglibrary.asco.org/content/98587-114; cited September 15, 2013] [DOI] [PubMed] [Google Scholar]
- 52.Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage iv colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg. 2010;34:797–807. doi: 10.1007/s00268-009-0366-y. [DOI] [PubMed] [Google Scholar]
- 53.Cirocchi R, Trastulli S, Abraha I, et al. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage iv colorectal cancer. Cochrane Database Syst Rev. 2012;8:CD008997. doi: 10.1002/14651858.CD008997.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muratore A, Zorzi D, Bouzari H, et al. Asymptomatic colorectal cancer with unresectable iver metastases: immediate colorectal resection or up-front systemic chemotherapy? Ann Surg Oncol. 2007;14:766–70. doi: 10.1245/s10434-006-9146-1. [DOI] [PubMed] [Google Scholar]
- 55.Sarela AI, Guthrie JA, Seymour MT, Ride E, Guillou PJ, O’Riordain DS. Non-operative management of the primary tumour in patients with incurable stage iv colorectal cancer. Br J Surg. 2001;88:1352–6. doi: 10.1046/j.0007-1323.2001.01915.x. [DOI] [PubMed] [Google Scholar]
- 56.Clements D, Dhruva Rao P, Ramanathan D, Adams R, Maughan TS, Davies MM. Management of the asymptomatic primary in the palliative treatment of metastatic colorectal cancer. Colorectal Dis. 2009;11:845–8. doi: 10.1111/j.1463-1318.2008.01695.x. [DOI] [PubMed] [Google Scholar]
- 57.Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol. 2007;14:1860–9. doi: 10.1245/s10434-006-9337-9. [DOI] [PubMed] [Google Scholar]
- 58.Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumor in patients with synchronous stage iv colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379–84. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCahill LE, Yothers G, Sharif S, et al. Primary mfolfox6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of nsabp trial C-10. J Clin Oncol. 2012;30:3223–8. doi: 10.1200/JCO.2012.42.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]