Abstract
Objectives
To evaluate bone invasion, survival, and expression of BMP-6 in oral cavity cancer in the context of known biomarkers indicative of poor prognosis.
Study Design
Molecular expression study combined with retrospective chart review of corresponding patients at a tertiary care center.
Methods
Between 2000 and 2009, 197 patients underwent resection for oral cavity squamous cell carcinoma. Of these, 30 pathologic specimens were chosen for further molecular analysis. These 30 patients were separated into three groups (n=10 per group) based on American Joint Committee on Cancer staging and staging based on size alone (TAJCC/SIZE). The first group consisted of tumors staged as T2/2 based on size less than 4 centimeters and that had no evidence of bone invasion. The T2/4 group consisted of tumors that were upstaged from T2 based on bone invasion. The T4/4 group consisted of tumors that were large with and without bone invasion. The expression of extracellular matrix metalloproteinase inducer (EMMPRIN), bone morphogenetic protein-6 (BMP-6), and epidermal growth factor receptor (EGFR) was examined using immunohistochemistry techniques. Patient demographics, tumor characteristics, survival, and recurrence were compared.
Results
Average follow-up was 21 months. Expression of BMP-6 was significantly higher in the T2/4 cohort (tumor less than 4 centimeters with bony invasion) than the larger tumors without bone invasion (T4/4 cohort, p=0.05). Additionally, increased BMP-6 expression correlated with aggressive behavior in the smaller tumors. Furthermore, increased EGFR expression positively correlated with increased levels of BMP-6.
Conclusions
Increased expression of BMP-6 in oral cavity cancer may affect bone invasion.
Keywords: Head and neck, BMP-6, immunohistochemistry, tumor biomarkers, oral cavity cancer, squamous cell carcinoma
Introduction
Oral cavity squamous cell carcinoma (OCSCC) represents a significant therapeutic challenge because of its aggressive local and regional behavior. Surgical resection is often the mainstay of therapy, but advanced stage disease often requires adjuvant medical therapy, compounding the deficits associated with surgery. This can result in poor functional outcomes for this patient population, including xerostomia, mucositis, osteoradionecrosis, ulceration, dysphagia, aspiration, and need for other surgery1. Predictors for requiring adjuvant therapy are critical to ensure the appropriate treatment plan and duration. OCSCC with bone invasion often portends a poor prognosis as well as confers a need for additional therapy. Interestingly, patients with similar T classification based on size can have substantially different prognosis based on the presence of bone invasion1-4. Although this has been well recognized to carry a poor prognosis in the literature, the underlying biology of this relationship remains largely unknown. It is thought that this may be related to surgical margins, implications of the size of tumor, and access to bone marrow. Ebrahimi et al showed that patients with medullary invasion of the mandible did not suffer from a locoregional recurrence but rather from distant metastases (which is often predictive of worsened survival). Access to the cancellous bone of the mandible may grant these tumors a mode of hematogenous spread or alternatively could signify that these tumors are more aggressive in general2.
Investigations into biomarkers predictive of bone invasion have included circulating C-reactive protein, VEGF, EGFR, and EMMPRIN.5-9 Despite its known relationship to bone remodeling and its expression in cancer, the role of bone morphogenetic protein 6 (BMP-6) in OCSCC has not been evaluated in oral cavity cancer. It has, however, been demonstrated to be upregulated in esophageal cancer.10 Furthermore, expression of several members of the BMP family has been shown to induce ectopic bone formation in vivo in breast and prostate cancers. In particular, bone invasion in the presence of increased BMP-6 expression has been extensively documented11,12,13,14. In order to investigate the potential role of BMP-6 in oral cavity tumors, we evaluated thirty patients with tumors of comparable size, and compared molecular expression of BMP-6 in those tumors with and without mandibular invasion. To provide a comparison of well known biomarkers of aggressive behavior in OCSCC, BMP-6 was evaluated in conjunction with EMMPRIN and EGFR.
METHODS
Patient Population
Tumor specimens were collected from 197 patients with oral cavity squamous cell carcinoma between 2000 and 2009. At random, thirty patient specimens were chosen from this group based on their American Joint Committee on Cancer staging (AJCC) and a classification criteria based on size alone (TAJCC/SIZE). Given the large span of time from which these patients were pulled, the AJCC classifications are congruent with the most recent edition at the time of diagnosis. The T-classification criteria based on size was defined by lesions length: T2 represents small tumors, less than 4 cm that did not have bone invasion, regardless of bone invasion, and T4 consisted tumors larger than or equal to 4 cm. Demographics were compared across the groups including age, gender, and race. Tumor and disease characteristics were also evaluated including bone invasion, perineural invasion, presence of metastatic disease, margin status after definitive resection, and recurrence. Immunohistochemistry was performed to assess presence and upregulation of biomarkers including BMP-6, EGF-R, and EMMPRIN as well as to assess whether there were any relationships between these biomarkers as well.
Immunohistochemistry
Embedded paraffin slides containing patient tumor tissue were obtained from the UAB Department of Surgical Pathology. Slides were incubated at 60°C for 1 hour. Slides were then immersed in EZ-DEWAX solution (Biogenex cat #H) for 5 minutes with occasional agitation, then transferred to a fresh container of EZ-DEWAX for an additional 5 minutes. Antigen retrieval was performed using a 1:100 solution of citrate buffer (Fisher Scientific TA-050-CBX) and incubated at 90°C for 10 minutes. Slides were rinsed with deionized distilled water for 5 minutes, then blocked with a 5% (w/v) solution of bovine serum albumin in TBST buffer for 5 minutes at room temperature. Excess blocking solution was removed from the slides, and samples were then incubated with primary antibody in a humidified chamber at room temperature for 1 hour. Following hybridization, the slides were rinsed once with TBST then placed in a bath of TBST 2 times for five minutes each. Excess buffer was removed and samples were then incubated with Alexa Fluor 488 secondary antibody (Invitrogen A11029, A11008) in a humidified chamber for 40 minutes. Following incubation, the slides were again rinsed twice in TBST bath for 5 minutes each. Excess buffer was removed, and coverslips were mounted using Dapi-Fluoromount-G (Southern Biotech 0100-20) and slides were allowed to dry overnight. Samples were visualized using an Olympus IX70 fluorescence microscope.
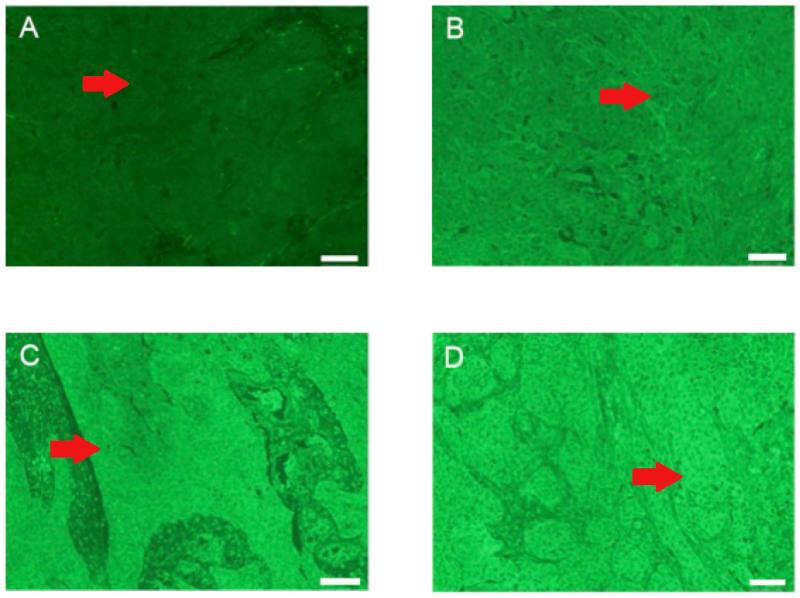
Expression of BMP-6, EMMPRIN, and EGFR was assayed by scoring fluorescence intensity and distribution throughout the tissue samples. Fluorescence was scored independently and blindly by two investigators. Scores were assigned descriptively using an Allred scoring method which was then modified by assigning a relative expression of 0 (Low), 1 (Mid-Low), 2 (High-Mid), or 3 (High) based on the distribution of scores among the biomarker in question15 (Figure 1, Supplementary Figure 1, Supplementary Figure 2). Aggregated scores were then used for statistical analysis.
Figure 1. Relative Expression of BMP-6 in Oral Cavity SCC: Benchmarks for Score Assignment.
IHC of BMP-6 in patient tumor samples. Relative expression was assigned a score of 0 (Low), 1 (Mid-Low), 2 (High-Mid), or 3 (High). Samples were scored blindly by two investigators. The red arrow indicates a representative nest of tumor cells.
Statistical Analyses
Descriptive variables were summarized by mean (± SD) for continuous variables and n (%) for categorical variables. A one-way ANOVA was used to analyze relationships between categorical factors and continuous responses. A student’s t-test was used to compare differences in means between groups. A contingency analysis was used to analyze relationships between categorical factors and responses. A multivariate analysis was performed to analyze the relationship between multiple variables. A p-value of < 0.05 was considered statistically significant. Statistical analysis was performed using Jmp 10 software (SAS, Cary, NC).
RESULTS
Patient Outcomes
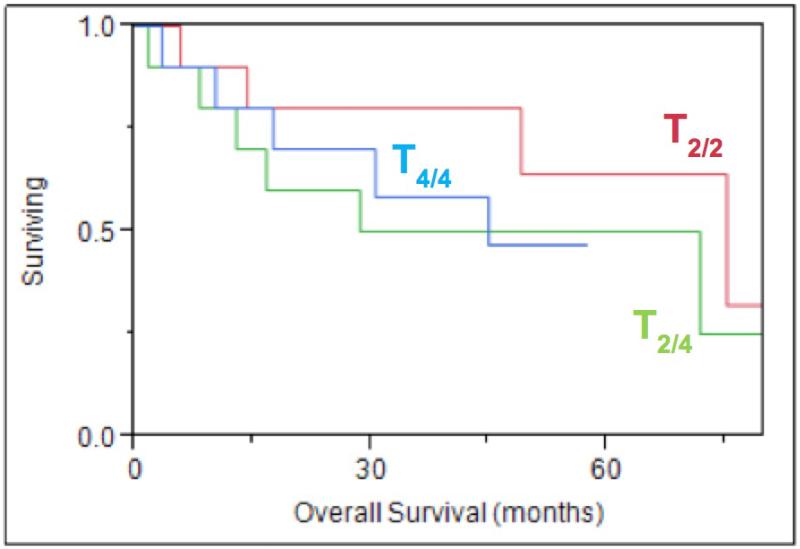
Thirty patients were included in this study and were separated into three cohorts based on their TAJCC/SIZE category. The first group represents small tumors, less than 4 cm that did not have bone invasion (T2/2), the second group represented small tumors which were upstaged based on bone invasion (T2/4), and the third group consisted of large tumors both with and without bone invasion (T4/4). The average follow-up time was 21 months (range 9 days - 71 months). Patient demographics were not significantly different between any of the groups (Table 1). At the conclusion of the study period, 16 patients were deceased with 8 dying from their disease. Survival did not differ significantly between the three cohorts (Figure 2). Age, race, and gender were not independent predictors of decreased survival. Patients with bone invasion and smaller tumors were more likely to undergo adjuvant radiation therapy (p=0.03) than those with small tumors alone.
Table 1. Patient Demographics and Tumor Characteristics.
| T2/2 % (n=10) | T2/4 % (n=10) | T4/4 % (n=10) | p-value | |
|---|---|---|---|---|
| Gender | 0.11 | |||
| Male | 50 | 80 | 90 | |
| Female | 50 | 20 | 10 | |
| Ethnicity | 0.40 | |||
| White | 90 | 90 | 70 | |
| Black | 10 | 10 | 30 | |
| Bone Invasion | 0.0001 | |||
| Yes | 0 | 100 | 40 | |
| No | 100 | 0 | 60 | |
| Metastatic Disease Y/N | 1.00 | |||
| Yes | 20 | 20 | 20 | |
| No | 80 | 80 | 80 | |
|
Perineural Invasion
Y/N |
0.38 | |||
| Yes | 10 | 10 | 30 | |
| No | 90 | 90 | 70 | |
| Recurrence Y/N | 0.86 | |||
| Yes | 30 | 30 | 40 | |
| No | 70 | 70 | 60 | |
| Margins Positive Y/N | 0.24 | |||
| Yes | 10 | 40 | 40 | |
| No | 90 | 60 | 60 |
Figure 2. Overall Survival, TAJCC/SIZE.
Kaplan-Meier overall survival curve based on staging by AJCC and by size alone, p=0.50
Tumor Characteristics
Tumor characteristics associated with a poorer prognosis and decreased survivals have been well studied and include: perineural invasion, lymphovascular invasion, margin status, invasion of surrounding structures, degree of squamous differentiation, locoregional and distant metastasis16. Interestingly, in this small cohort, metastatic disease, differentiation of the tumor, and upregulation of specific biomarkers were not associated with any decrease in survival. Furthermore, there was no significant impact on survival when looking at bone invasion between the three cohorts, although there was a trend towards decreased survival for the T2/4 cohort. Evidence of recurrent disease and perineural invasion were the only two tumor characteristics associated with significant decrease in survival (p=0.019 and 0.007).
Biomarkers
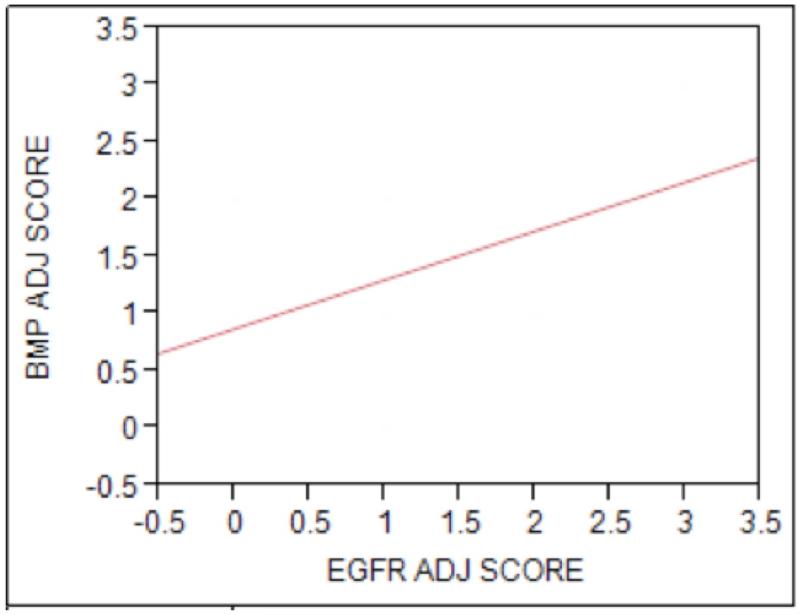
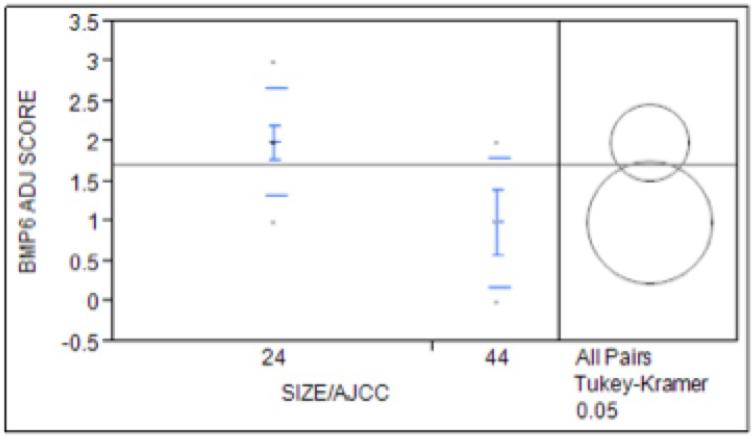
Expression of BMP-6 was significantly higher in the smaller tumors with bone invasion (T2/4 cohort) compared to the larger tumors with bone invasion (T4/4 cohort, p=0.05, Figure 3). There was a non-significant increase in bony invasion in patients with increased expression of BMP-6 between the T2/2 and the T2/4 cohort, although it did trend towards significance. There was no increase in the incidence of perineural invasion, metastatic disease, or recurrence with increasing BMP-6 expression. When examining the relationship of BMP-6 to EGFR expression, we found an increased level of EGFR expression correlated with increased levels of BMP-6 (p=0.019, Figure 4).
Figure 3. Relationship Between Cancer Staging and BMP-6 Expression.
Expression of BMP-6 in patients with confirmed bony invasion showed significantly increased expression in upstaged tumors (T2/T4) than in the larger tumor cohort (T4/4) (p=0.05)
Figure 4. BMP-6 and EGFR Expression are Positively Correlated in Oral Cavity SCC.
Comparative analysis of aggregated scores of all three patient cohorts revealed a positive correlation between BMP-6 and EGFR expression (p=0.019)
Expression of EMMPRIN was not significantly different between the three cohorts. EMMPRIN expression had no correlation with perineural invasion or overall survival; although, there was a trend towards decreased survival with increased EMMPRIN expression. Higher expression levels of EMMPRIN were, however, associated with decreased incidence of metastatic disease (p=0.04) as well as a trend towards decreased incidence of recurrence (p=0.06). An increase in EMMPRIN expression trended toward a correlation with increased BMP-6 expression (p=0.20).
Expression of EGFR was not significantly different between the three cohorts. There was no significant association between EGFR expression level and overall survival. However, increased EGFR expression did correlate with medullary invasion of bone versus cortical invasion. Additionally, increased expression of EGFR significantly correlated with perineural invasion (p=0.01) (Supplementary Figure 3).
In patients who had bony invasion, higher EGFR scores were correlated with medullary invasion (p=0.03). This was not seen with any of the other markers. When comparing the relationship between cortical and medullary bone invasion, there was no difference in overall survival.
DISCUSSION
This is the first paper that examines the role of BMP-6 in the pathogenesis of bone invasion in oral cavity cancers. Using thirty tumor specimens of oral cavity squamous cell carcinoma we examined the relationship between BMP-6 expression and bone invasion. This comparison was achieved by dividing tumors based on their American Joint Committee on Cancer staging as well as a classification based on size alone (TAJCC/SIZE). Several known biomarkers associated with poor prognosis of oral cavity squamous cell carcinoma were assessed in this investigation. Additionally, standard predictors of poor prognosis were also examined in the context of biomarker expression. It was hypothesized that these standard predictors would correlate with the size-based and AJCC-based classification.1, 2. Additionally, expression of BMP-6 has previously been proven to correlate with bone invasion and found to be upregulated in squamous cell carcinoma of the esophagus. Given these relationships, we hypothesized it would also play a role in the invasive nature of OSCC and subsequently be a prognostic indicator. In this study, increased expression of BMP-6 was found to be associated with elevated EGFR expression levels, a known marker of poor prognosis.
In addition, in this study, EGFR expression was found to be associated with perineural invasion, a known predictor of significantly decreased overall survival. The relationship between perineural invasion and EGFR expression is still in its nascence in the otolaryngology literature although this relationship has been shown in colorectal cancer17. Given these relationships, it might be hypothesized that BMP-6 may be related to perineural invasion, although this was not captured in this small subset of patients.
Among the three groups, (T2/2, T2/4, T4/4), there was no statistically significant difference in overall survival. Known predictors of poor prognosis (recurrence, perineural invasion, poor differentiation) showed a significant impact on overall survival, although metastatic disease and bone invasion did not. Our studies indicate that tumors that were small in size, but also had aggressive phenotypes including bone invasion, had increased expression of BMP-6 and had a prognosis similar to those patients with larger tumors which confirms previous studies2.
Given the well-studied relationship between bone invasion and prognosis, tumors with bone invasion in the oral cavity are automatically upstaged, regardless of size, in the current AJCC staging guideline. Our analysis found those patients with small tumors and positive bone invasion exhibit a slightly poorer overall survival, although this was not found to be statistically significant. This finding is in support of the current AJCC staging criteria. Our data further suggests that the expression of BMP-6 may promote bone invasion.
With regard to survival, patients whose tumors demonstrated bone invasion on final pathologic specimen were more likely to undergo adjuvant therapy (70% versus 20%, p=0.03); this too, is consistent with current guidelines.1 However, our data suggest that patients with small tumors and increased BMP-6 expression may benefit from more aggressive therapy, as they may be more predisposed towards invasive phenotypes. One of the three patients in the T2/2 cohort who also had high BMP-6 expression, but no bone invasion, developed metastatic disease as well as recurrence.
BMP-6 has been shown to not only be a marker for bony invasion, but also can be used as a possible prognostic indicator. In esophageal SCC, Raida et al showed that BMP-6 protein content is correlated with the grade of tumor-cell differentiation and may add to indications of poor prognosis. Molecular data has shown that BMP-6, when found in high levels and in conjunction with noggin and Sost in squamous cell carcinoma, can predict cancer progression. Importantly, this relationship was confirmed in prostate, bladder, and colorectal cancers18.
CONCLUSION
Despite a limited sample size, this study suggest that there exists a potential correlation between BMP-6 and aggressive tumor phenotypes. Our data supports the need for further investigation into the biological role of BMP-6 in bone invasion and metastasis in oral cavity squamous cell carcinoma. Predicting a tumor’s propensity for bone invasion is a useful tool for the head and neck surgeon as well as for the medical oncologist; expression levels of biomarkers may provide predictors towards more aggressive phenotypes requiring a more intensive treatment plan as well as be a potential therapeutic target. While only three biomarkers were investigated in this study, several interesting conclusions were found. These included the correlation between BMP-6 expression levels and more aggressive behaviors of the smaller lesions as well as increased EGFR expression. This study presents several interesting theories and highlights the need for additional research into the mechanisms associated with OCSCC bony invasion as well as the clinical correlation between these markers and clinical outcomes.
Supplementary Material
Supplemental Figure 1: Relative Expression of EGFR in Oral Cavity SCC: Benchmarks for Score Assignment
IHC of EGFR in patient tumor samples. Relative expression was assigned a score of 0 (Low), 1 (Mid-Low), 2 (High-Mid), or 3 (High). Samples were scored blindly by two investigators. The red arrow indicates a representative nest of tumor cells.
Supplemental Figure 2: Relative Expression of EMMPRIN in Oral Cavity SCC: Benchmarks for Score Assignment
IHC of EMMPRIN in patient tumor samples. Relative expression was assigned a score of 0 (Low), 1 (Mid-Low), 2 (High-Mid), or 3 (High). Samples were scored blindly by two investigators. The red arrow indicates a representative nest of tumor cells.
Supplemental Figure 3: Expression of Biomarkers within Area of Perineural Invasion
Expression of BMP-6, EGFR, and EMMPRIN are compared within the same are of perineural invasion via IHC.
ACKNOWLEDGMENTS
Yolanda Hartman, BS
Taylor M Deal, MD
Financial Disclosures:
National Institutes of Health R01CA142637-0A1
National Cancer Institute 5R25CA76023
Abbreviations
- BMP-6
Bone morphogenetic protein 6
- SCC
squamous cell carcinoma
- AJCC
American Joint Committee on Cancer
- EGFR
Endothelial growth factor receptor
- EMMPRIN
Extracellular matrix metalloproteinase inducer (CD-147)
- IHC
Immunohistochemistry
Footnotes
PRESENTED AT THE COMBINED SECTIONS MEETING, TRIOLOGICAL SOCIETY 2013 ON JANUARY 24TH, 2013 SCOTTSDALE, ARIZONA, UNITED STATES OF AMERICA
No Conflicts of Interest
REFERENCES
- 1.Wang CC, Cheng MH, Hao SP, Wu CC, Huang SS. Osteoradionecrosis with combined mandibulotomy and marginal mandibulectomy. Laryngoscope. 2005;115:1963–1967. doi: 10.1097/01.mlg.0000178374.29219.5e. [DOI] [PubMed] [Google Scholar]
- 2.Ebrahimi A, Murali R, Gao K, Elliott MS, Clark JR. The prognostic and staging implications of bone invasion in oral squamous cell carcinoma. Cancer. 2011;117:4460–4467. doi: 10.1002/cncr.26032. [DOI] [PubMed] [Google Scholar]
- 3.Bilodeau EA, Chiosea S. Oral squamous cell carcinoma with mandibular bone invasion: intraoperative evaluation of bone margins by routine frozen section. Head Neck Pathol. 2011;5:216–220. doi: 10.1007/s12105-011-0264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abd El-Hafez YG, Chen CC, Ng SH, et al. Comparison of PET/CT and MRI for the detection of bone marrow invasion in patients with squamous cell carcinoma of the oral cavity. Oral Oncol. 2011;47:288–295. doi: 10.1016/j.oraloncology.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Chen HH, Chen IH, Liao CT, Wei FC, Lee LY, Huang SF. Preoperative circulating C-reactive protein levels predict pathological aggressiveness in oral squamous cell carcinoma: a retrospective clinical study. Clin Otolaryngol. 2011;36:147–153. doi: 10.1111/j.1749-4486.2011.02274.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang SF, Cheng SD, Chien HT, et al. Relationship between epidermal growth factor receptor gene copy number and protein expression in oral cavity squamous cell carcinoma. Oral Oncol. 2012;48:67–72. doi: 10.1016/j.oraloncology.2011.06.511. [DOI] [PubMed] [Google Scholar]
- 7.Seki S, Fujiwara M, Matsuura M, et al. Prediction of outcome of patients with oral squamous cell carcinoma using vascular invasion and the strongly positive expression of vascular endothelial growth factors. Oral Oncol. 2011;47:588–593. doi: 10.1016/j.oraloncology.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Erdem NF, Carlson ER, Gerard DA, Ichiki AT. Characterization of 3 oral squamous cell carcinoma cell lines with different invasion and/or metastatic potentials. J Oral Maxillofac Surg. 2007;65:1725–1733. doi: 10.1016/j.joms.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Chen YJ, Jin YT, Shieh DB, Tsai ST, Wu LW. Molecular characterization of angiogenic properties of human oral squamous cell carcinoma cells. Oral Oncol. 2002;38:699–705. doi: 10.1016/s1368-8375(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 10.Raida M, Sarbia M, Clement JH, Adam S, Gabbert HE, Hoffken K. Expression, regulation and clinical significance of bone morphogenetic protein 6 in esophageal squamous-cell carcinoma. Int J Cancer. 1999;83:38–44. doi: 10.1002/(sici)1097-0215(19990924)83:1<38::aid-ijc8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Autzen P, Robson CN, Bjartell A, et al. Bone morphogenetic protein 6 in skeletal metastases from prostate cancer and other common human malignancies. Br J Cancer. 1998;78:1219–1223. doi: 10.1038/bjc.1998.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darby S, Cross SS, Brown NJ, Hamdy FC, Robson CN. BMP-6 over-expression in prostate cancer is associated with increased Id-1 protein and a more invasive phenotype. J Pathol. 2008;214:394–404. doi: 10.1002/path.2292. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Yan JD, Zhang L, et al. Activation of bone morphogenetic protein-6 gene transcription in MCF-7 cells by estrogen. Chin Med J (Engl) 2005;118:1629–1636. [PubMed] [Google Scholar]
- 14.Dai J, Keller J, Zhang J, Lu Y, Yao Z, Keller ET. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res. 2005;65:8274–8285. doi: 10.1158/0008-5472.CAN-05-1891. [DOI] [PubMed] [Google Scholar]
- 15.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 16.Jerjes W, Upile T, Petrie A, Riskall A, Hamdoon Z, Vourvachis M, Karavidas K, et al. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1-T2 oral squamous cell carcinoma patients. Head &Neck Oncology. 2010;2(1):9–30. doi: 10.1186/1758-3284-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viana L, Affonso R, Silva S, Denadai M, Matos D, Salinas de Souza C, Waisberg J. Relationship between the expression of the extracellular matrix genes SPARC, SPP1, FN1, ITGA5 and ITGAV and clinicopathological parameters of tumor progression and colorectal cancer dissemination. Oncology. 2013;84(2):81–91. doi: 10.1159/000343436. [DOI] [PubMed] [Google Scholar]
- 18.Yuen H, McCrudden C, Grills C, Zhang S, Huang Y, Chan K, Chan Y, et al. Combinatorial use of bone morphogenetic protein 6, noggin, and Sost significantly predicts cancer progression. Cancer Sci. 2012;103(6):1145–1154. doi: 10.1111/j.1349-7006.2012.02252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Relative Expression of EGFR in Oral Cavity SCC: Benchmarks for Score Assignment
IHC of EGFR in patient tumor samples. Relative expression was assigned a score of 0 (Low), 1 (Mid-Low), 2 (High-Mid), or 3 (High). Samples were scored blindly by two investigators. The red arrow indicates a representative nest of tumor cells.
Supplemental Figure 2: Relative Expression of EMMPRIN in Oral Cavity SCC: Benchmarks for Score Assignment
IHC of EMMPRIN in patient tumor samples. Relative expression was assigned a score of 0 (Low), 1 (Mid-Low), 2 (High-Mid), or 3 (High). Samples were scored blindly by two investigators. The red arrow indicates a representative nest of tumor cells.
Supplemental Figure 3: Expression of Biomarkers within Area of Perineural Invasion
Expression of BMP-6, EGFR, and EMMPRIN are compared within the same are of perineural invasion via IHC.