Abstract
The mammalian genome is replete with various classes of non-coding (nc) RNA genes. Many of them actively transcribe, and their relevance to CNS diseases is just beginning to be understood. CNS is one of the organs in the body that shows very high ncRNAs activity. Recent studies demonstrated that cerebral ischemia rapidly changes the expression profiles of different classes of ncRNAs: including microRNA, long noncoding RNA and piwi-interacting RNA. Several studies further showed that post-ischemic neuronal death and/or plasticity/regeneration can be altered by modulating specific microRNAs. These studies are of interest for therapeutic development as they may contribute to identifying new ncRNA targets that can be modulated to prevent secondary brain damage after stroke.
Keywords: Cerebral ischemia, microRNA, protein-coding RNA, Pathophysiology, Molecular mechanisms, Transcription, Translation
1. Introduction
Non-coding RNAs (ncRNAs) are functional RNAs which will not translate to form proteins like mRNAs (Berezikov, 2011). In mammals, >98% of the transcriptional output is comprised of various classes of ncRNAs that range from 17 to >9,000 nucleotides in length (Ketting, 2011; Wright and Bruford, 2011) (Table 1). While transfer RNAs and ribosomal RNAs that play significant roles in protein translation and ribosomal integrity are well-known, many other classes of ncRNAs with distinct regulatory functions are transcribed from the intergenic as well as intragenic regions of the genome (Guil and Esteller, 2012; Ishizu et al., 2012; Lee, 2012). For several decades, ncRNAs have been considered transcriptional non-sense or genomic dark matter. However, recent studies indicate significant functions for many classes of ncRNAs, particularly in controlling transcription and translation to maintain normal cellular physiology (Ambros, 2004; Dharap et al., 2009; Fire et al., 1998; Place et al., 2008). Several studies also suggest that altered expression and function of ncRNAs modulate the pathophysiology of CNS disorders (Dharap et al., 2009; Dharap et al., 2011, 2012; Harries, 2012; Krichevsky et al., 2003; Lee, 2012; Qureshi and Mehler, 2012; Salta and De Strooper, 2012; Saugstad, 2010; Schonrock and Gotz, 2012). These studies have provided impetus for further evaluating ncRNAs in detail, to identify them as biomarkers of stroke risk as well as mediators of post-stroke pathologic changes.
Table 1.
Various classes of ncRNAs
| Size (bp) | Current number* |
Putative functions | |
|---|---|---|---|
| Small ncRNAs | |||
| tiRNA | 17–18 | >5,000 | Transcriptional initiation |
| miRNA | 18–25 | 2042 | Translational repression; Transcriptional activation |
| siRNA | 19–25 | >20,000 | mRNA degradation |
| tasiRNA | 20–22 | unknown | Gene silencing in plants |
| tel-sRNA | 23–28 | unknown | Epigenetic regulation of telomerase |
| rasiRNA | 24–29 | >1,000 | Transposon silencing |
| piRNA | 26–31 | >60,000 | Transposon silencing |
| CRISPR | 24–48 | Unknown | Prokaryotic immune control |
| crasiRNA | 34–42 | Unknown | Heterochromatin recruitment |
| Medium-size ncRNAs | |||
| TSS-aRNA | 20–90 | >10,000 | Transcriptional regulation |
| PASR | 22–200 | >10,000 | Transcriptional regulation |
| snoRNA | 60–300 | >300 | Maturation of other ncRNAs |
| scaRNA | 83–330 | >26 | Guiding spliceosomal RNAs |
| Long ncRNAs | |||
| lncRNA | >200 | >10,000 | Transcriptional regulation |
| T-UCR | 200–779 | 481 | Antisense inhibition of mRNAs and ncRNAs |
| CUT | 200–800 | >900 | Chromatin regulation |
| SUT | 200–800 | >800 | Transposon silencing |
| TERRA | 100–9,000 | unknown | Regulation of telomere length |
| PROMPT | >200 | unknown | Promoter control |
Current number in humans is given as of today. New ncRNAs of all classes are still being discovered. tiRNA, transcription initiation RNA; miRNA, microRNA; siRNA, short interfering RNA; tasiRNA, trans-acting siRNA; tel-sRNA, telomere small RNA; rasiRNA, repeat-associated siRNA; piRNA, piwi-interacting RNA; CRISPR, clustered regularly interspaced short palindromic repeats; crasiRNA, centromere repeat associated short interfering RNA; TSS-aRNA, transcription start site associated RNA; PASR, promoter associated small RNA; snoRNA, small nucleolar RNA; scaRNA, small Cajal body-specific RNA; lncRNA, long noncoding RNA; T-UCR, transcribed ultraconserved region; CUT, cryptic unstable transcript; SUT, stable unannotated transcript; TERRA, telomere-associated ncRNA; PROMPT, promoter associated pervasive transcript.
While the list of various classes of ncRNAs given in Table 1 is exhaustive, as of today only the expression profiles of microRNAs (miRNAs), piwi-interacting RNAs (piRNAs) and long noncoding RNAs (lncRNAs) are known to be altered after stroke (Dharap et al., 2009; Dharap et al., 2011, 2012; Dharap and Vemuganti, 2010). The goal of this review is to discuss the studies that show the functional significance of ncRNAs and in particularly miRNAs in secondary brain damage after acute insults to CNS.
2. Stroke alters cerebral protein-coding gene expression
Following stroke, the secondary brain damage progresses rapidly during the first 3 days and then at a much slower pace up to 2 weeks. The core of the ischemic insult undergoes irreversible damage very quickly. Whereas, the area surrounding the core known as penumbra can be protected with therapy (Olson, 1985). The extent of the core and penumbra depends on several factors including the severity and duration of the ischemic insult. In most cases, the volume of the core is smaller than penumbra to start with, but as the time progress, the infarct grows by encroaching penumbra. The secondary neuronal death is a major cause of infarct growth which leads to the long-term neurological dysfunction after stroke. Many pathological mechanisms including excitotoxicity, ionic imbalance leading to edema, inflammation, oxidative stress, endoplasmic reticulum stress and transcriptional failure act synergistically to mediate the neuronal death in the post-ischemic brain (Dirnagl et al., 1999).
Many studies documented that strokes extensively alter the mRNA expression profiles in both the blood and brain of humans, as well as in experimental animals. These studies showed that following a stroke, the major classes of transcripts altered are those related to inflammation, immune response, apoptotic, autophagic and necrotic cell death, ionic balance, oxidative metabolism, transcriptional control, neurotransmitter function, immediate early genes and protein chaperones (Carmichael, 2003; Eltzschig and Eckle, 2011; Giffard and Yenari, 2004; Read et al., 2001; Sharp et al., 2011b; Weinstein et al., 2004; Yi et al., 2007). All these pathways are thought to participate either in secondary neuronal death or plasticity/reorganization in the post-ischemic brain (Kapadia et al., 2008; King et al., 2010; Lipton, 1999; Onteniente et al., 2003; Sharp et al., 2011a; Vemuganti and Dempsey, 2005; Yi et al., 2007).
One highly important observation for genomic studies has been that the expression of many transcription factors that promote either neuronal death or neuroprotection change rapidly after stroke and post-ischemic outcome can be altered by modulating these transcription factors (Bernaudin et al., 2002; Collino et al., 2006; Iadecola et al., 1999; Kapadia et al., 2006; Satriotomo et al., 2006; Shih et al., 2005; Song et al., 2011; Tanaka et al., 2000a; Tanaka et al., 2000b; Tureyen et al., 2008; Tureyen et al., 2007; Venna et al., 2012).
3. Stroke influences non-coding RNA profiles
The mRNAs and proteins altered after a stroke have been used as major targets for stroke therapeutic development in the past few decades. However, recent studies on ncRNAs have changed our perspective of stroke pathophysiology. Because different classes of ncRNAs operate above the level of mRNA transcription and protein translation, they need to be studied in depth to understand stroke pathology, and to design new stroke therapies. Our recent studies showed that focal ischemia significantly alters the expression profiles of miRNAs, piRNAs and lncRNAs in rodent brain (Dharap et al., 2009; Dharap et al., 2011, 2012; Dharap and Vemuganti, 2010).
3.1. Stroke-induced changes in microRNAs
The miRNAs are the most studied of all ncRNAs. Functionally, miRNAs bind to consensus 8-bp seed sequences in the 3’UTRs of mRNAs to prevent their translation (Jackson and Standart, 2007; Lewis et al., 2005; Lewis et al., 2003; Pillai et al., 2007). The miRNA seed sequences have also been observed in the promoters of many protein-coding genes, and their binding has been shown to induce the expression of those genes (Dharap et al., 2009; Orom et al., 2008; Place et al., 2008; Vasudevan et al., 2007). The sequences of miRNAs are conserved among various species, indicating their evolutionary importance. Furthermore, the miRNA function is highly redundant, as the 3’UTRs of most mRNAs contain binding sites for multiple miRNAs, and a specific miRNA binding site can be found in the 3’UTRs of several mRNAs. Hence, miRNAs influence both transcription and translation.
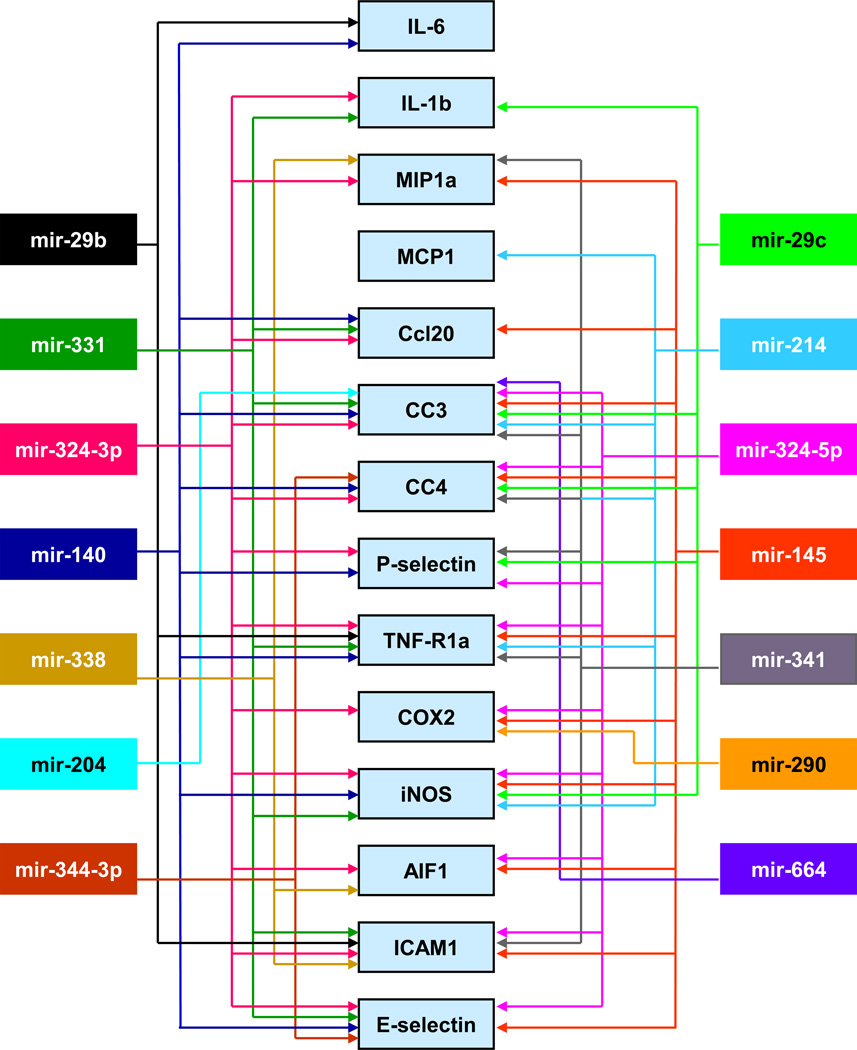
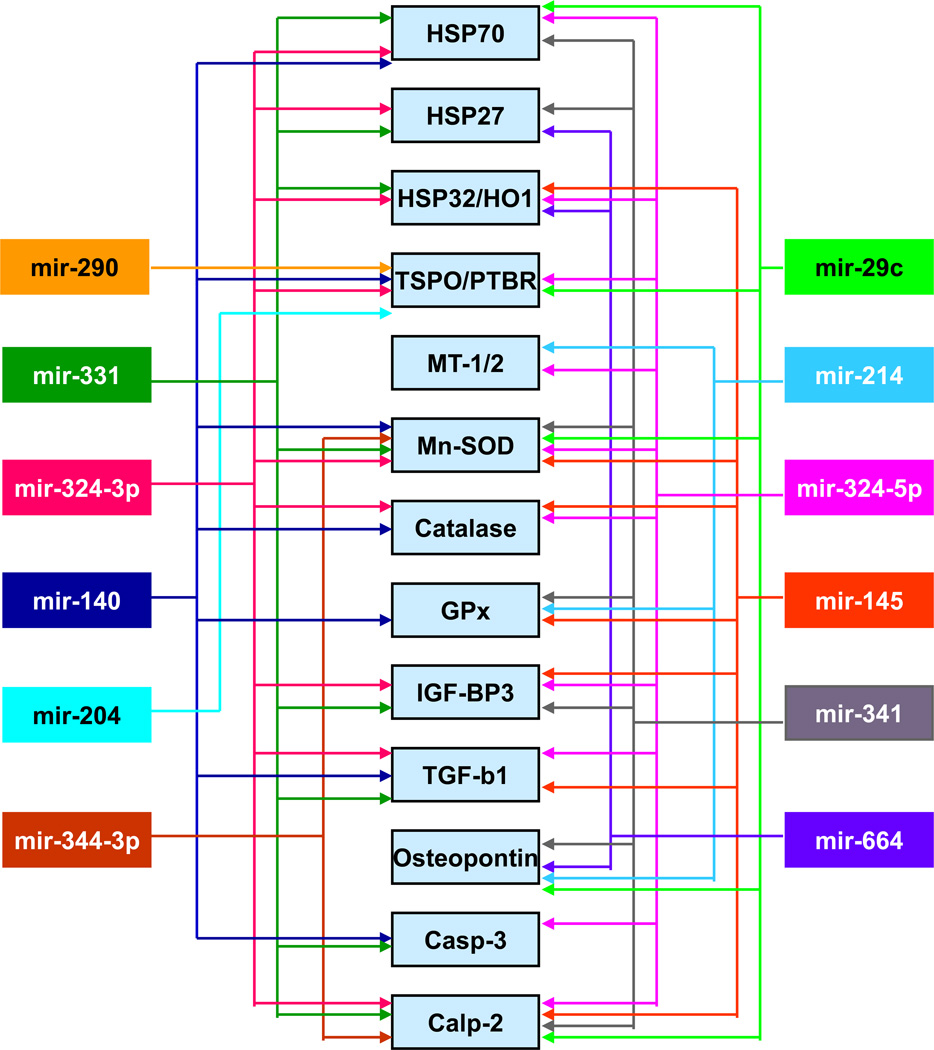
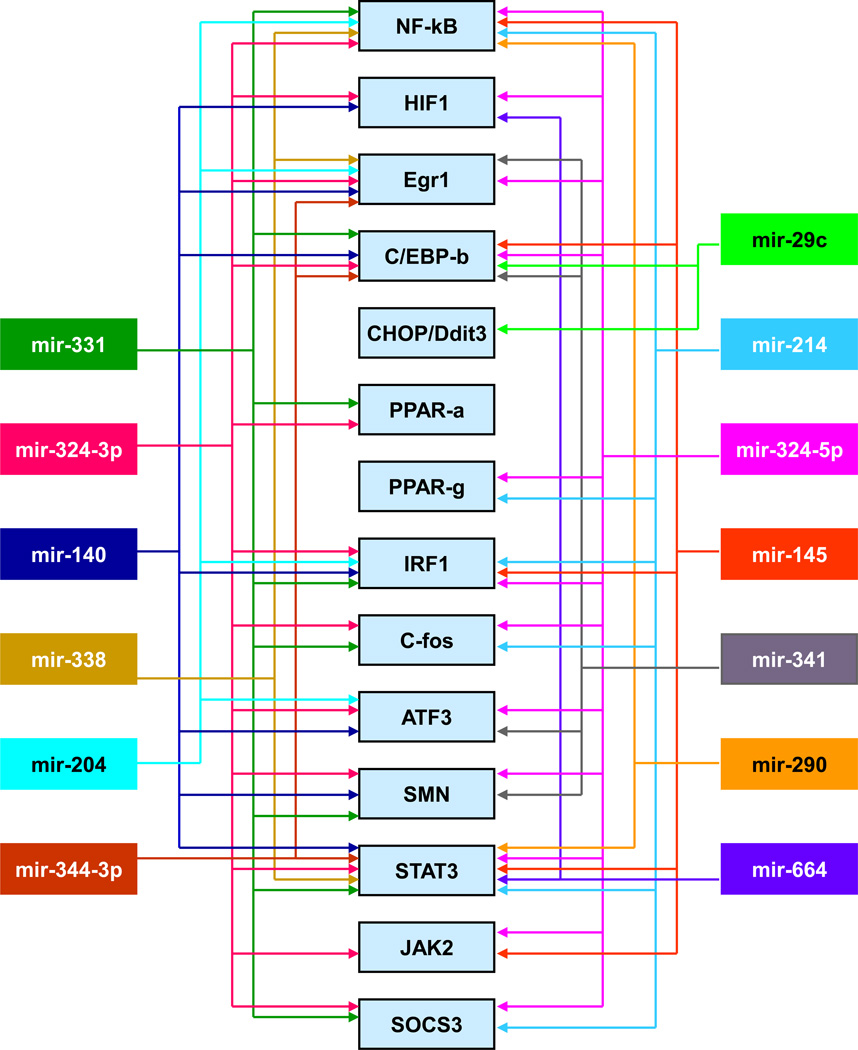
Many labs have showed that cerebral ischemia alters miRNA profiles in the blood and brain of rodents and humans (Dharap et al., 2009; Jeyaseelan et al., 2008; Liu et al., 2010a; Tan et al., 2009). In particular, microarray studies from our laboratory showed that cerebral miRNAome responds rapidly to focal ischemia (Dharap et al., 2009). In the ischemic cortex, 11 miRNAs altered as early as 3h of reperfusion, and many of them showed long-term expression changes of up to 3 days of reperfusion (Dharap et al., 2009). Interestingly, the number of miRNAs that altered increased progressively and 46 miRNAs showed altered expression by 3 days of reperfusion. Because miRNAs does not code for any proteins, their actions will be mediated by the mRNAs they target. Using bioinformatics, we analyzed the targets common to miRNAs altered after focal ischemia and interestingly, many of the mRNAs targeted by these miRNAs are also known to be altered after stroke (Vemuganti et al., 2002). Furthermore, many of those fit into pathophysiologic mechanisms that modulate either secondary brain damage or neuroprotection after ischemia. For example, many cytokines, chemokines, adhesion molecules and complement components that modulate inflammation were observed to be targeted by multiple miRNAs altered after stroke (Fig. 1). Furthermore, several heat shock proteins (HSPs), growth factors and anti-oxidant enzymes that minimize the secondary neuronal death are also targeted by the set of stroke-sensitive miRNAs (Fig. 2). Stroke is a known modulator of transcription factors that either positively or negatively impact the post-ischemic outcome. We observed that the 3’UTRs of several transcription factor coding mRNAs including HIF-1, NF-kB, C/EBPβ, PPARγ, Egr1, IRF1 and STAT3 have binding sites for stroke-responsive miRNAs (Fig. 3). The miRNAs that target transcription factors have wide-ranging functional implications in post-stroke outcome as transcription factors can alter the expression of hundreds of down-stream target genes.
Fig. 1. Bioinformatics correlation of the miRNAs and the inflammatory mRNAs altered after focal ischemia.
Stroke-responsive miRNAs can modulate mRNAs that mediate the cerebral pro-inflammatory response including cytokines, chemokines, cell adhesion molecules and free radical generating enzymes. This figure appeared previously as supplemental information in Dharap et al. (2009).
Fig. 2. Bioinformatics correlation of the miRNAs and the neuroprotective mRNAs altered after focal ischemia.
Many neuroprotective and neurorestorative mRNAs are also the predicted targets of the stroke-responsive miRNAs. These include protein chaperones, antioxidant enzymes and growth factors. This figure appeared previously as supplemental information in Dharap et al. (2009).
Fig. 3. Bioinformatics correlation of the miRNAs and the transcription factor mRNAs altered after focal ischemia.
Transcription factor mRNAs are a major group of predicted targets of the miRNAs altered in the post-ischemic brain. Some of them are known promoters of inflammation and neuronal death, while some are upstream to neuroprotective pathways. This figure appeared previously as supplemental information in Dharap et al. (2009).
Other groups have also identified extensive changes in miRNA expression profiles in the post-ischemic brain.Jeyaseelan et al. (2008) reported extensive changes in the miRNA expression in rat cerebral cortex after focal ischemia and the targets of the stroke-responsive miRNAs include those that mediate excitotoxicity, oxidative stress, inflammation and apoptosis. This study further showed that blood miRNA profiles also change following focal ischemia (Jeyaseelan et al., 2008). Another study showed that permanent focal ischemia as well as intracerebral hemorrhage alters the miRNA expression profiles in rodent brains (Liu et al., 2010a). Using pathways analysis, they showed that the down-stream targets of the ischemia-sensitive miRNAs are associated with biological functions: including cell cycle regulation, cellular growth and proliferation, posttranslational modification, cardiovascular function and cell death.
3.2. Effect of intracerebral hemorrhage (ICH) on microRNAs
Although ICH accounts only for 10% to 15% of all strokes, the associated mortality rate is very high (~50% within the first 30 days) (Gonzales, 2013). Recent studies showed that in humans, the blood miRNA profiles change significantly following ICH (Guo et al., 2013; Liu et al., 2010a; Zheng et al., 2012). Liu et al. (2010) showed that in the blood of rats subjected to ICH, 21 miRNAs were upregulated and 20 miRNAs were down-regulated compared to sham control (Liu et al., 2010a). Zheng et al. (2012) analyzed the miRNA profiles in the plasma of 32 ICH patients and showed that 30 miRNAs are differentially expressed between the 14 patients with hematoma enlargement and 18 patients without hematoma enlargement (Zheng et al., 2012). Interestingly, a recent study showed significant differences between the male and female patients; while 70 miRNAs were altered in males, only 42 were altered in females following ICH compared to the respective healthy controls (Guo et al., 2013). This study also observed that 13 out of the 30 miRNAs upregulated in ICH were unique and not changed in ischemic stroke patients. All these studies indicate that specific miRNAs in blood can be used as biomarkers for identifying the ICH among stroke patients as well as can distinguish those with hematoma progression from those without among the ICH patients.Liu et al. (2010) showed that miRNA profiles also alter in the brain tissue after ICH in rats and interestingly 3 miRNAs miR-298, miR-155 and miR-362-3p were altered in both blood and brain of these animals (Liu et al., 2010a). Further studies are needed to evaluate the functional significance of miRNAs in ICH-mediated brain damage.
3.3 Stroke-induced changes in piRNAs and lncRNAs
The piRNAs are one of the most expressed classes of the ncRNAs in eukaryotes. They target and silence RNAs formed by different classes of transposons to maintain genetic equilibrium (Cordaux and Batzer, 2009; Gogvadze and Buzdin, 2009; Halic and Moazed, 2009; O'Donnell and Boeke, 2007). A recent study from our lab showed that stroke rapidly alters the cerebral piRNA profiles in rodents (Dharap et al., 2011). We observed that by 1 day of reperfusion, expression levels of 105 piRNAs were altered in the cerebral cortex of rats subjected to transient focal ischemia. Interestingly, 25 of those showed >5 fold change. With bioinformatics, we identified that the stroke-sensitive piRNAs target retrotransposons and hence might play an important role in preventing mutations after a stroke. Our studies also showed that a set of transcription factors redundantly target the piRNA promoters and those might be responsible for the piRNA changes observed after a stroke. At this point, the functional significance of the piRNAs in post-stroke pathophysiology needs to be experimentally validated. Those studies will pave ways to design new therapies to prevent post-stroke brain damage.
Although many lncRNAs are known to be transcribed from the mammalian genome, their functional significance in normal physiology and in disease pathologies is poorly understood. Unlike other ncRNAs, no common function as a group is identified so far for lncRNAs. However, lncRNA homeostasis is essential for normal brain function as many of them regulate transcription by acting as scaffolds for the chromatin modifying proteins (Gupta et al., 2010; Hung et al., 2011; Pasmant et al., 2007; Tsai et al., 2010; Yu et al., 2008). A recent microarray profiling study from our lab showed that stroke in rodents significantly influences cerebral lncRNAome (Dharap et al., 2012). Bioinformatics showed that many of these stroke-responsive lncRNAs have >90% sequence homology with the exons of protein-coding genes, but none of the lncRNAs translate into protein products due to the absence of open-reading frames (Dharap et al., 2012). These lncRNAs might act as mimics to control the function of the homologous mRNAs.
4. Functional implications of microRNAs in post-ischemic brain
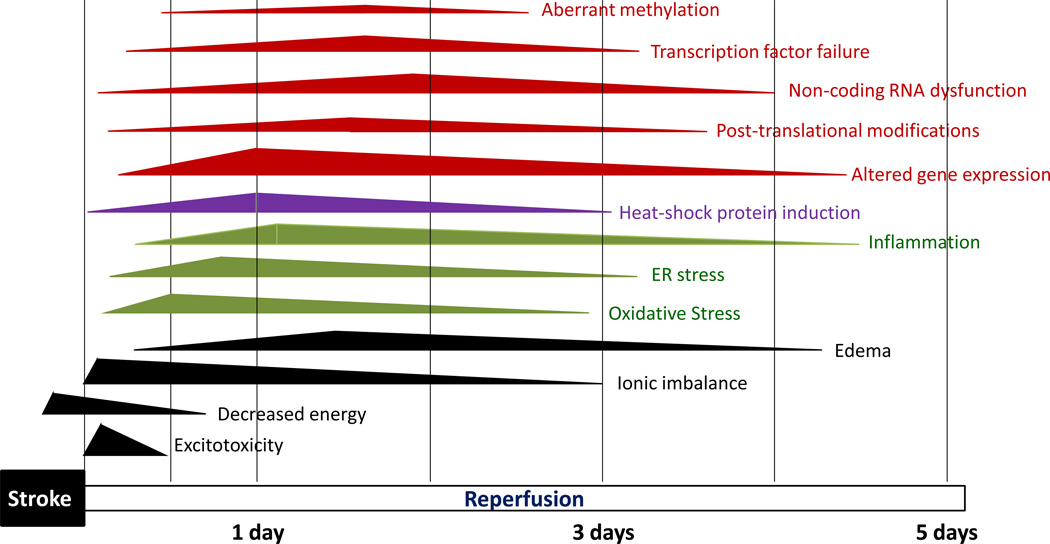
While several studies showed that stroke significantly alters the expression profiles of various classes of ncRNAs, the functional significance of these changes has been evaluated only in a limited manner. The miRNAs are the only class of ncRNAs that have been analyzed for their role in post-ischemic pathophysiology. Secondary neuronal death after stroke is known to be synergistically mediated by several mechanisms including excitotoxicity, energy failure, ionic imbalance leading to edema, oxidative stress, endoplasmic reticulum (ER) stress, inflammation, transcription factor failure and aberrant methylation (Fig. 4). As discussed above, miRNA dysfunction might be a contributor to most of these post-stroke pathologic changes.
Fig. 4.
Many pathophysiologic mechanisms synergistically contribute to neuronal death and neurologic dysfunction after stroke. Excitotoxicity, energy failure and ionic imbalance start within minutes after stroke and continue for days. Immediately after the insult, increased glutamate release combined with a failure of the glutamate transporters lead to elevated glutamate levels in the synaptic cleft, contributing to excitotoxic neuronal death. The energy failure leads to ionic imbalance that induces water to rush in leading to edema. Oxidative stress, ER stress and inflammation that start within hours after stroke are major events that lead to neuronal death if not controlled. A massive induction of HSPs in the post-ischemic brain might be an endogenous effort of self-protection. In addition, transcriptional and translational failure that encompasses altered expression of transcription factors, epigenetic changes like altered promoter methylation, post-translational modifications and altered ncRNA function also play a role in post-stroke pathophysiology.
Our lab observed that miR-145 was upregulated in a rapid and sustained manner in rat brain after focal ischemia, and miR-145 knockdown increased its target antioxidant enzyme superoxide dismutase-2 protein levels in neurons leading to decreased infarct size (Dharap et al., 2009). The miRNAs were also shown to modulate the inflammatory responses in the post-ischemic brain. Our studies showed that miRNAs altered in the ischemic brain target a set of pro-inflammatory molecules including cytokines (IL-6 and IL-1β), chemokines (MIP1α and MCP-1), complement components (CC3 and CC4), adhesion molecules (ICAM-1, P-selectin and E-selectin) and enzymes like COX2 and iNOS that form free radicals (Dharap et al., 2009) (Fig. 1). The miR-181c and miR-21 were shown to minimize the oxygen-glucose deprivation induced neuronal apoptosis by suppressing the pro-inflammatory response mediated by TNF-α and FasL in activated microglia (Zhang et al., 2012b, c). The miR-146a known to be upregulated after ischemia was thought to potentiate the Toll-like receptor signaling, leading to neuroprotection after stroke (Zhang et al., 2012a).
Several miRNAs have been shown to target the pro-apoptotic pathways leading to neuroprotection after stroke. Neurons in the ischemic penumbra are known to survive after stroke, and these cells showed increased miR-21 levels for days after ischemia (Buller et al., 2010). Furthermore, in one study, when cortical neuronal cultures were transfected with miR-21, they showed reduced expression of the pro-apoptotic FAS ligand and curtailed cell death when subjected to oxygen-glucose deprivation (Buller et al., 2010). A recent study showed that miR-15b induction suppresses the down-stream anti-apoptotic Bcl-2, and knockdown of miR-15b decreases post-ischemic neuronal death (Shi et al., 2013). The miR-15a also targets Bcl-2 mRNA. Interestingly, miR-15a expression was shown to be under the control of the transcription factor PPAR-δ and when PPAR-δ was overexpressed, post-ischemic miR-15a levels decreased leading to increased Bcl-2 protein levels and neuroprotection (Yin et al., 2010a). Another study demonstrated that the miR-497 known to be induced after ischemia also targets Bcl-2 mRNA and post-ischemic knockdown of miR-497 led to increased Bcl-2 protein levels in neurons with decreased infarction (Yin et al., 2010b).
Glutamate excitotoxicity is a known promoter of ischemic neuronal death. The miR-223 has been shown to protect hippocampal neurons after global cerebral ischemia by targeting down-stream glutamate receptors GluR2 and NR2B, thus reducing calcium influx (Harraz et al., 2012). Cerebral edema is dependent on the water movement across cell membranes controlled by a family of aquaporin (AQP) proteins and AQP-4 knockout mice showed reduced cerebral edema after focal ischemia (Manley et al., 2000). The 3’-UTR of AQP-4 mRNA contains a binding site for miR-320a, and treatment with anti-miR-320a decreases aquaporin-4 protein levels and curtailed edema and infarction when rodents were subjected to focal ischemia (Sepramaniam et al., 2010).
Cerebral ischemia is known to induce HSPs, which act as protein chaperones to minimize neuronal damage (Barreto et al., 2012; Stetler et al., 2009; Zhan et al., 2010). The miR-181 induced in the ischemic core targets the HSP70 family members, particularly GRP78/BIP which is essential for protein folding in ER (Ouyang et al., 2012). Conversely, miR-18, when down-regulated in the ischemic penumbra, contributes to neuronal survival. Furthermore, in one study, miR-181a inhibition was observed to be neuroprotective after focal ischemia (Ouyang et al., 2012).
During the chronic phase after cerebral ischemia, angiogenesis and neurogenesis increases, which might be an endogenous effort to promote plasticity (Ergul et al., 2012; Wiltrout et al., 2007). The miR-210 induced after cerebral ischemia contributes to post-ischemic angiogenesis by modulating the Notch signaling pathway (Lou et al., 2012). The miR-17/92 cluster was shown to mediate the postischemic increased proliferation and survival of the neural progenitor cells in the subventricular zone of the lateral ventricles (Bellenchi et al., 2013; Liu et al., 2013; Liu et al., 2009b). In one study, down-regulation of miR-124a in the progenitor cells of SVZ was shown to lead to depression of Jagged-1 (JAG1), which is a Notch ligand that participates in the post-ischemic neurogenesis (Liu et al., 2011). Furthermore, these authors also showed that transfection of neural progenitors with miR-124a decreases JAG1, leading to inactivation of Notch signaling (Liu et al., 2011). A combination therapy with VELCADE and tissue-plasminogen activator was shown to induce neuroprotection by targeting miR-146a and TLR signaling (Zhang et al., 2012a).
The extent of ischemic brain damage is dependent on sex and age (Lewis et al., 2012; Liu and McCullough, 2012; Manwani and McCullough, 2012). It is well-known that the secondary brain damage after stroke is less in young female rodents than in young males (Nordell et al., 2003; Wilson, 2013). The miR-23a has been shown to play a role in this dimorphism as it targets the X-linked inhibitor of apoptosis (XIAP) which is a major caspase inhibitor (Siegel et al., 2011). Interestingly, following stroke, miR-23a levels are very different in the brains of males and females; XIAP mRNA decreases in female brains after stroke, and the inhibition of miR-23a increases XIAP, leading to neuroprotection (Siegel et al., 2011). Furthermore, middle-aged female rats are known to develop larger infarcts than younger female rats. Knockdown of the miRNA Let-7f which targets IGF-1 pathway has been shown to promote significant neuroprotection in middle-aged female rats following focal ischemia (Selvamani et al., 2012).
5. microRNAs as biomarkers of ischemia
Identifying patients who have had silent strokes as well as classifying different subtypes of strokes are challenges for proper treatment options. RNAs are known to be released into blood, and their profiles change in brain disorders (Scholer et al., 2010; Sharp et al., 2011b). Many studies showed that mRNA profiles in human blood can serve as a sensitive index to identify various stroke subtypes and disease progression (Jickling et al., 2013; Jickling et al., 2012a; Jickling et al., 2011; Jickling et al., 2010; Jickling et al., 2012b; Liu et al., 2010a; Stamova et al., 2010; Xu et al., 2010).
As miRNAs released into the blood are stable for days, blood miRNA profiles are emerging as good biomarkers for myocardial infarction as well as stroke (Adachi et al., 2010; Wang et al., 2010; Weber et al., 2010a). Many miRNAs known to participate in stroke-related pathologies including endothelial dysfunction, angiogenesis and erythropoiesis have been shown to be altered in the blood of young and older stroke patients compared to age-appropriate healthy controls (Gan et al., 2012; Tan et al., 2009; Zeng et al., 2011). The blood miRNA profiles have also been shown to be altered in rodents following focal ischemia and cerebral hemorrhage (Dharap et al., 2009; Jeyaseelan et al., 2008; Liu et al., 2010a; Weng et al., 2011).
6. microRNAs and ischemic tolerance
While a stroke of sufficient duration kills neurons, a brief ischemic episode preconditions the brain and promotes ischemic tolerance (Dhodda et al., 2004; Pignataro et al., 2008). This phenomenon of ischemic preconditioning (PC) is known to be associated with increased protein synthesis and altered expression of many protein-coding genes (Barone et al., 1998; Meller and Simon, 2013; Stenzel-Poore et al., 2003). Several chemicals like 3-nitropropionic acid (3-NP), sevoflurane and isoflurane, as well as exogenous stimuli like brief hypoxia, enriched environment, hyperbaric oxygen therapy and remote limb PC have also been shown to induce the cerebral ischemic tolerance (Della-Morte et al., 2012; Galle and Jones, 2012; Nunes et al., 2013; Yan et al., 2013).
Cerebral ischemic tolerance has been shown to change the expression of many miRNAs in rodent brain (Dharap and Vemuganti, 2010; Lee et al., 2010; Liu et al., 2012a; Lusardi et al., 2010). Following a brief PC insult, cerebral ischemic tolerance is known to develop quickly, within 1 to 3 days and expression of several miRNAs alters during this critical period, starting as early as 6h after PC (Dharap and Vemuganti, 2010). The down-stream targets of the PC-responsive miRNAs are known to be critical for the acquisition of ischemic tolerance, including the members of TGF-β signaling, mTOR signaling, MAP kinase signaling, ubiquitin-proteasomal system, JAK-STAT signaling and Notch signaling (Dharap and Vemuganti, 2010). The protein levels of methyl CpG binding protein 2 (MeCP2) which is a global transcriptional activator/repressor (Chahrour et al., 2008; Nan et al., 1998) increases during the development of ischemic tolerance and interestingly this effect seems to be mediated by the down-regulation of many miRNAs that target the 3’-UTR of MeCP2 mRNA (Lusardi et al., 2010). Several miRNAs including miR-615-3p are involved in hypoxia-induced ischemic PC by targeting the protein kinase C family members (Liu et al., 2012b). Cerebral ischemic tolerance induced by 3-NP treatment was shown to be mediated by the down-regulation of miR-199a leading to depression of its target sirt1 protein which is a known neuroprotectant (Xu et al., 2012b). In another study, sevoflurane preconditioning was shown to induce miR-15b, which in turn suppresses the translation of its target Bcl-2 mRNA leading to reduced apoptosis and neuroprotection (Shi et al., 2013). Increased protein conjugation to ubiquitin-like modifiers (ULMs) mediates neuroprotection during torpor in rodents, which was shown to be associated with decreased levels of cerebral miR-200 and miR-182 family members that target various ULM proteins (Lee et al., 2012). Inhibition of miR-200 family and/or miR-182 family increased protein conjugation to ULMs and made SH-SY5Y cells tolerant to oxygen-glucose deprivation-induced cell death (Lee et al., 2012).
7. Non-coding RNAs as future stroke therapeutic targets
The therapeutic applicability of ncRNAs is not yet fully understood. Currently no drugs that modulate ncRNAs are in clinical use. However, all the above recent studies show their promise in controlling various pathological features that promote post-stroke neuronal death and/or neurological dysfunction. Many reagents that either increase (premiRs, miRNA mimics and viral vectors that encode miRNAs) or decrease (antagomiRs and miRNA sponges) the levels of miRNAs are currently being tested in animals for various pathologies (Bhalala et al., 2012; Dharap et al., 2009; Krutzfeldt et al., 2005; Pandi et al., 2013; Yin et al., 2010b). To use them in a clinical setting, it is essential to develop the vehicles/transporting agents to efficiently transfer the miRs to the brain from systemic circulation. Modifications such as polyamination and phosphorothioation, and using fectamines, nanoparticles and microvesicles are few current strategies for efficient transfer and prevention of degradation of miRNAs (Chen et al., 2010b; Elmen et al., 2008a; Noguchi et al., 2012; Rahbek et al., 2010). Furthermore, it is also essential to study the toxicity and long-term effects of miRNA therapeutics before they can be used as drugs.
Some recent studies tested miRNAs for their therapeutic efficacy to prevent tumor growth. Efficient silencing of miR-122 in the liver was achieved by the administration of cholesterol-conjugated antagomiR-122, which decreased the hepatic tumor growth within days (Krutzfeldt et al., 2005). Systemic administration of antagomiR-122 modified with the locked nucleic acid chemistry was also shown to silence the miR-122 in non-human primates and this compound entered Phase II clinical trials (Elmen et al., 2008b; Haussecker and Kay, 2010). Treatment with 2′-O-methyl modified antagomiR-21 was shown to reduce breast cancer growth (Si et al., 2007). A miRNA sponge was used to silence the OncomiR-17-92 cluster which is implicated in the growth of various types of tumors (Ebert et al., 2007). These studies might take the center stage in the next few years of ncRNA research. However, extensive toxicity testing is needed to use the ncRNA reagents in humans to understand their non-specific actions.
8. Carotid atherosclerosis alters mRNA and non-coding RNA expression profiles
Although carotid atherosclerotic (CA) plaque rupture is a major cause of stroke in humans, the mechanisms responsible for this are not completely understood. Our lab analyzed the gene expression profiles of CA plaques from symptomatic and asymptomatic patients to understand the mechanisms of plaque stability and embolization. We chose the patients with clinically identifiable symptoms in contrast to those with no symptoms based on the asymptomatic carotid stenosis study (ACAS) (Baker et al., 2000). We observed that the expression levels of 236 of the 44,860 mRNAs analyzed were higher in the symptomatic patients compared to the asymptomatic patients and 90% of those transcripts belong to the functional classes that promote plaque growth including signal transduction, ionic homeostasis, nucleotide and protein metabolism, organogenesis, cell growth, cell maintenance and cell adhesion (Dempsey et al., 2010; Vemuganti and Dempsey, 2005, 2006). We also observed that symptomatic plaques show significantly higher enrichment of many mRNAs that are related to angiogenesis indicating an active capillary formation leading to the development of stroke symptoms (Tureyen et al., 2006).
Several miRNAs have also been shown to be associated with CA plaque maturation and rupture. In macrophages, miR-155 silences Bcl-6 and hence miR-155 induction was shown to promote atherosclerosis (Nazari-Jahantigh et al., 2012). The miR-145 is known to be expressed at high levels in the vascular smooth muscle cells (SMC) and SMC-targeted miR-145 overexpression has been shown to reduce plaque size as well as necrotic core area indicating that miR-145 is a potential therapeutic target to limit CA plaque rupture, and thus stroke (Santovito et al., 2013). It has also been shown that deficiency of hematopoietic miR-155 leads to increased inflammatory monocytes, and thus enhancing CA plaque development concurrently decreasing plaque stability (Donners et al., 2012). Many monocyte-specific miRNAs like miR-99b, miR-152 and miR-422a were shown to be expressed in plaques indicating that these miRNAs can be therapeutic targets to prevent monocyte recruitment to plaques (Bidzhekov et al., 2012). A recent expression-profiling study (Raitoharju et al., 2011) showed increased expression of several miRNAs in the CA plaque samples in comparison to non-atherosclerotic arterial samples indicating the possibility of these miRNAs playing a role in plaque growth and/or rupture. Furthermore, 5 miRNAs (miR-100, miR-127, miR-145, miR-133a and miR-133b) were shown to be expressed at significantly different levels between symptomatic and asymptomatic plaques, which indicate their prognosis to identify plaque instability (Cipollone et al., 2011). The miR-33 which is located within the SREBF2 gene, suppresses expression of the cholesterol transporter ABC transporter A1 leading to lowered HDL levels and treatment with anti-miR-33 has been shown to regress atherosclerosis in mice deficient in LDL receptors (Rayner et al., 2011).
Few polymorphisms associated with miRNA function have been shown to promote stroke in humans. An “A” to “T” single nucleotide polymorphism known as rs2507800 in the miR-211 binding site in the 3’-UTR of the angiopoietin-1 (Angpt1) mRNA was shown to increase Angpt1 protein levels that indicate a decreased stroke risk (Chen et al., 2010a; Jeansson et al., 2011). Down-regulation of aortic miR-155 was shown to correlate with the development of hypertension in rats (Xu et al., 2008). In humans, a polymorphism known as A1166C was shown to modify the miR-155 binding site in the 3’-UTR of the angiotensin II type 1 receptor (AT1R) mRNA, leading to increased AT1R protein levels in homozygous patients (Ceolotto et al., 2011; Martin et al., 2007). The A1166C polymorphism is known to be associated with hypertension, which promotes stroke (de Oliveira-Sales et al., 2010; Mettimano et al., 2002). Other studied indicate that miR-146aG allele and miR-146aG/-149T/-196a2C/-499G allele combinations are associated with ischemic stroke pathogenesis in humans (Rah et al., 2013).
The miR-210 inactivation has been shown to prevent angiogenesis, which is a proponent of plaque rupture (Fasanaro et al., 2008; Fasanaro et al., 2009). In addition to hypertension, type-2 diabetes is also a stroke risk factor in humans. Down-regulation of miR-126 in endothelial cells increases the stroke susceptibility in diabetics by de-repressing its target vascular cell adhesion molecule-1 that facilitates the macrophage adhesion to endothelium, which is a prognostic factor in CA plaque rupture (Harris et al., 2008; Zampetaki et al., 2010). The miR-21 is also a neuroprotective miRNA induced by PC-mediated ischemic tolerance (Dharap and Vemuganti, 2010) and miR-21 knockdown exacerbates and overexpression decreases neuronal death after stroke (Buller et al., 2010). miR-21 is known to be induced in CA plaques leading to modulation of its target Bcl-2 that changes the vascular smooth muscle cell survival and plaque formation (Raitoharju et al., 2011). Paradoxically, other studies indicate that CA plaque rupture is increased by miR-21 indicating that it might be a pro-stroke miRNA under certain conditions (Weber et al., 2010b).
9. microRNAs alter after traumatic injury to CNS
Other acute insults to CNS including traumatic brain injury (TBI) and spinal cord injury (SCI) also share many common features with post-stroke pathophysiology. Some studies showed that these conditions are also associated with altered profiles and functionality of miRNAs.
9.1. TBI and microRNA
In rodents, traumatic injuries to the cerebral cortex is known to induce cell death in both the cortex and the hippocampus, followed by altered cognitive and motor functions (Bales et al., 2009; Blennow et al., 2012; Xiong et al., 2013; Yi et al., 2008). Following controlled cortical impact (CCI)-induced TBI in adult rats, many miRNAs were altered in the hippocampus in the first day after the injury and mRNAs that code proteins involved in signal transduction, transcriptional regulation, and cell proliferation/differentiation, the processes important for post-TBI pathophysiology are the targets of the TBI-responsive miRNAs (Redell et al., 2009).Hu et al. (2012) showed that 2 different sets of miRNAs were altered in the rodent hippocampus at 1 to 7 days after CCI injury. At the earlier time points, miRNAs that modulate mRNAs involved in apoptosis, inflammation and transcriptional failure were altered, while at the later time points, miRNAs that regulate intracellular trafficking, cytoskeleton and cell adhesion to allow cellular remodeling and synaptogenesis were altered (Hu et al., 2012). Another showed that fluid-percussion (FP) injury to brain also alters the miRNA profiles in the cerebral cortex of rats between 6h to 3 days (Lei et al., 2009).
Hypothermia is known to minimize the secondary neuronal death as well as prolong the therapeutic window after TBI (Dietrich and Bramlett, 2010). Hypothermia after FP injury in rats was shown to significantly modulate the post-TBI miRNA profiles indicating their possible role in hypothermia-mediated neuroprotection (Truettner et al., 2011). A recent study showed that hypothermia inhibits the proliferation of endogenous neural progenitors in the hippocampus, probably by negating the post-TBI miR-34a down-regulation and induction of its target Notch signaling pathway (Wang et al., 2012). Exposure of adult rats to blast-induced TBI altered the serum levels of let-7i, indicating it as a sensitive biomarker of brain injury (Balakathiresan et al., 2012). A further study showed that miRNA levels also alter in the blood following TBI in humans indicating their potential to act as biomarkers for brain injury (Redell et al., 2010).
9.2. SCI and microRNA
Following SCI in adult rats, expression levels of many miRNAs that control inflammation, apoptosis and oxidative stress were altered in the first 7 days (Liu et al., 2009a; Yunta et al., 2012). Strickland (2011) found that miR-124, miR-129 and miR-1 were down-regulated with concomitant induction of their target SNORD2, which is a translation-initiation factor following SCI in adult rats. They further showed that miR-21 was significantly induced in the contused spinal cord indicating an adaptive anti-apoptotic response (Strickland et al., 2011). SCI in adult mice was shown to increase miR-223 and decrease miR-124a levels and these changes were thought to promote inflammation and cell death (Nakanishi et al., 2010). Using in situ hybridization, this group further showed that miR-223 was expressed in the neutrophils that extravasated the spinal cord parenchyma after an injury (Izumi et al., 2011).
The miRNAs also seem to participate in the plasticity and regeneration after SCI. The pattern of miRNA changes coincide with the appearance of SOX2, nestin, and REST protein expression, suggesting that some of the SCI-responsive miRNAs may reflect the emergence of stem cell niches (Strickland et al., 2011). To understand the role of miRNAs in activity-dependent plasticity, miRNA profiles have been analyzed in adult rats subjected to cycling exercise after SCI (Liu et al., 2010b). While SCI induced Let-7a and miR-16 expression, exercise increased miR-21 and decreased miR-15b. These miRNA changes further correlated with the expression of their target genes that control apoptosis, suggesting that the benefits of post-SCI exercise might be mediated in part by miRNAs that modulate apoptosis (Liu et al., 2010b). Exercise after SCI was also shown to modulate the miRNAs that control PTEN and mTOR signaling, indicating an increased regenerative potential of the neurons (Liu et al., 2012c). In another study, when zebrafish spinal cord was transected, the regenerating neurons showed increased expression of miR-133b and its antisense knockdown mitigated axonal regeneration and locomotor recovery (Yu et al., 2011). This study also showed that miR-133b targets RhoA which is an inhibitor of axonal growth and thus miR-133b induction might be a useful adaptation after SCI. In adult mice, SCI was shown to induce miR-486 which represses its target mRNA NeuroD6 leading to oxidative stress and poor outcome, and knocking down miR-486 was shown to promote significant post-SCI motor function recovery (Jee et al., 2012a). The miR-20a was another miRNA induced after SCI that blocks the translation of neurogenin 1 (Ngn1) and as Ngn1 participates in plasticity and regeneration, knocking-down miR-20a led to motor neuron survival and neurogenesis followed by decreased functional deficit after SCI (Jee et al., 2012b). A recent study showed that neural stem cells transfected with miR-124 differentiate more efficiently into adult neurons leading to decreased secondary cavitation and increased motor function in rats subjected to SCI (Xu et al., 2012a). The miR-21 is a prototypic miRNA that is consistently reported to be induced after an acute injury to CNS including ischemia, TBI and SCI and the astrocytes adjacent to the post-SCI lesion expressed high levels of miR-21 (Bhalala et al., 2012). The hypertrophic response to SCI was observed to be attenuated in the astrocytic miR-21 overexpressing transgenic mice while augumented by the expression of miR-21 sponge indicating this miRNA as a potential future therapeutic target to improve outcome after an injury (Bhalala et al., 2012).
10. Conclusions
Various classes of ncRNAs control the transcription and translation to maintain normal cellular homeostasis in mammals. Acute injuries to CNS including stroke, TBI and SCI significantly alter ncRNA profiles. Many studies showed that miRNAs altered after CNS injury modulate processes that promote neuronal death including inflammation, apoptosis and oxidative stress as well as processes that promote plasticity and regeneration. Furthermore, miRNAs can act as sensitive biomarkers of secondary brain damage.
Highlights.
Several classes of non-coding RNAs are actively transcribed in mammals.
Non-coding RNAs are considered as master controllers of transcription and translation.
The role of non-coding RNAs in post-stroke brain pathology by controlling multiple pathophysiologic mechanisms including inflammation and oxidative stress is discussed.
Particular focus of this review is the studies on microRNA, a class of non-coding RNA.
The review discussed the use of microRNAs in blood as biomarkers of stroke.
The review discussed the translational potential and therapeutic implications of microRNAs to protect brain after stroke.
Acknowledgments
Supported by NIH grants NS079585 and NS074444.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest: No duality of interest to declare.
REFERENCES
- Adachi T, Nakanishi M, Otsuka Y, Nishimura K, Hirokawa G, Goto Y, Nonogi H, Iwai N. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clinical chemistry. 2010;56:1183–1185. doi: 10.1373/clinchem.2010.144121. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Baker WH, Howard VJ, Howard G, Toole JF. Effect of contralateral occlusion on long-term efficacy of endarterectomy in the asymptomatic carotid atherosclerosis study (ACAS). ACAS Investigators. Stroke. 2000;31:2330–2334. doi: 10.1161/01.str.31.10.2330. [DOI] [PubMed] [Google Scholar]
- Balakathiresan N, Bhomia M, Chandran R, Chavko M, McCarron RM, Maheshwari RK. MicroRNA let-7i is a promising serum biomarker for blast-induced traumatic brain injury. Journal of neurotrauma. 2012;29:1379–1387. doi: 10.1089/neu.2011.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales JW, Wagner AK, Kline AE, Dixon CE. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neuroscience and biobehavioral reviews. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–1950. doi: 10.1161/01.str.29.9.1937. discussion 1950-1931. [DOI] [PubMed] [Google Scholar]
- Barreto GE, White RE, Xu L, Palm CJ, Giffard RG. Effects of heat shock protein 72 (Hsp72) on evolution of astrocyte activation following stroke in the mouse. Experimental neurology. 2012;238:284–296. doi: 10.1016/j.expneurol.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellenchi GC, Volpicelli F, Piscopo V, Perrone-Capano C, di Porzio U. Adult neural stem cells: an endogenous tool to repair brain injury? Journal of neurochemistry. 2013;124:159–167. doi: 10.1111/jnc.12084. [DOI] [PubMed] [Google Scholar]
- Berezikov E. Evolution of microRNA diversity and regulation in animals. Nature reviews. Genetics. 2011;12:846–860. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Tang Y, Reilly M, Petit E, Sharp FR. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J Biol Chem. 2002;277:39728–39738. doi: 10.1074/jbc.M204619200. [DOI] [PubMed] [Google Scholar]
- Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA. microRNA-21 regulates astrocytic response following spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17935–17947. doi: 10.1523/JNEUROSCI.3860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidzhekov K, Gan L, Denecke B, Rostalsky A, Hristov M, Koeppel TA, Zernecke A, Weber C. microRNA expression signatures and parallels between monocyte subsets and atherosclerotic plaque in humans. Thrombosis and haemostasis. 2012;107:619–625. doi: 10.1160/TH11-09-0607. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Buller B, Liu X, Wang X, Zhang RL, Zhang L, Hozeska-Solgot A, Chopp M, Zhang ZG. MicroRNA-21 protects neurons from ischemic death. The FEBS journal. 2010;277:4299–4307. doi: 10.1111/j.1742-4658.2010.07818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Gene expression changes after focal stroke, traumatic brain and spinal cord injuries. Current opinion in neurology. 2003;16:699–704. doi: 10.1097/01.wco.0000102621.38669.77. [DOI] [PubMed] [Google Scholar]
- Ceolotto G, Papparella I, Bortoluzzi A, Strapazzon G, Ragazzo F, Bratti P, Fabricio AS, Squarcina E, Gion M, Palatini P, Semplicini A. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. American journal of hypertension. 2011;24:241–246. doi: 10.1038/ajh.2010.211. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yang T, Yu H, Sun K, Shi Y, Song W, Bai Y, Wang X, Lou K, Song Y, Zhang Y, Hui R. A functional variant in the 3'-UTR of angiopoietin-1 might reduce stroke risk by interfering with the binding efficiency of microRNA 211. Human molecular genetics. 2010a;19:2524–2533. doi: 10.1093/hmg/ddq131. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Molecular therapy : the journal of the American Society of Gene Therapy. 2010b;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollone F, Felicioni L, Sarzani R, Ucchino S, Spigonardo F, Mandolini C, Malatesta S, Bucci M, Mammarella C, Santovito D, de Lutiis F, Marchetti A, Mezzetti A, Buttitta F. A unique microRNA signature associated with plaque instability in humans. Stroke; a journal of cerebral circulation. 2011;42:2556–2563. doi: 10.1161/STROKEAHA.110.597575. [DOI] [PubMed] [Google Scholar]
- Collino M, Aragno M, Mastrocola R, Benetti E, Gallicchio M, Dianzani C, Danni O, Thiemermann C, Fantozzi R. Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free radical biology & medicine. 2006;41:579–589. doi: 10.1016/j.freeradbiomed.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Cordaux R, Batzer MA. The impact of retrotransposons on human genome evolution. Nature reviews. Genetics. 2009;10:691–703. doi: 10.1038/nrg2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT, Campos RR. Upregulation of AT1R and iNOS in the rostral ventrolateral medulla (RVLM) is essential for the sympathetic hyperactivity and hypertension in the 2K-1C Wistar rat model. American journal of hypertension. 2010;23:708–715. doi: 10.1038/ajh.2010.64. [DOI] [PubMed] [Google Scholar]
- Della-Morte D, Guadagni F, Palmirotta R, Ferroni P, Testa G, Cacciatore F, Abete P, Rengo F, Perez-Pinzon MA, Sacco RL, Rundek T. Genetics and genomics of ischemic tolerance: focus on cardiac and cerebral ischemic preconditioning. Pharmacogenomics. 2012;13:1741–1757. doi: 10.2217/pgs.12.157. [DOI] [PubMed] [Google Scholar]
- Dempsey RJ, Vemuganti R, Varghese T, Hermann BP. A review of carotid atherosclerosis and vascular cognitive decline: a new understanding of the keys to symptomology. Neurosurgery. 2010;67:484–493. doi: 10.1227/01.NEU.0000371730.11404.36. discussion 493-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:675–687. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Nakka VP, Vemuganti R. Altered expression of PIWI RNA in the rat brain after transient focal ischemia. Stroke; a journal of cerebral circulation. 2011;42:1105–1109. doi: 10.1161/STROKEAHA.110.598391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Nakka VP, Vemuganti R. Effect of Focal Ischemia on Long Noncoding RNAs. Stroke; a journal of cerebral circulation. 2012;43:2800–2802. doi: 10.1161/STROKEAHA.112.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Vemuganti R. Ischemic pre-conditioning alters cerebral microRNAs that are upstream to neuroprotective signaling pathways. Journal of neurochemistry. 2010;113:1685–1691. doi: 10.1111/j.1471-4159.2010.06735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. Journal of neurochemistry. 2004;89:73–89. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends in neurosciences. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Donners MM, Wolfs IM, Stoger LJ, van der Vorst EP, Pottgens CC, Heymans S, Schroen B, Gijbels MJ, de Winther MP. Hematopoietic miR155 deficiency enhances atherosclerosis and decreases plaque stability in hyperlipidemic mice. PLoS One. 2012;7:e35877. doi: 10.1371/journal.pone.0035877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nature methods. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjarn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008a;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- Elmen J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjarn M, Hansen JB, Hansen HF, Straarup EM, McCullagh K, Kearney P, Kauppinen S. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic acids research. 2008b;36:1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nature medicine. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke; a journal of cerebral circulation. 2012;43:2270–2274. doi: 10.1161/STROKEAHA.111.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. The Journal of biological chemistry. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanaro P, Greco S, Lorenzi M, Pescatori M, Brioschi M, Kulshreshtha R, Banfi C, Stubbs A, Calin GA, Ivan M, Capogrossi MC, Martelli F. An integrated approach for experimental target identification of hypoxia-induced miR-210. The Journal of biological chemistry. 2009;284:35134–35143. doi: 10.1074/jbc.M109.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Galle AA, Jones NM. The neuroprotective actions of hypoxic preconditioning and postconditioning in a neonatal rat model of hypoxic-ischemic brain injury. Brain research. 2012 doi: 10.1016/j.brainres.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Gan CS, Wang CW, Tan KS. Circulatory microRNA-145 expression is increased in cerebral ischemia. Genetics and molecular research : GMR. 2012;11:147–152. doi: 10.4238/2012.January.27.1. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. Journal of neurosurgical anesthesiology. 2004;16:53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- Gogvadze E, Buzdin A. Retroelements and their impact on genome evolution and functioning. Cell Mol Life Sci. 2009;66:3727–3742. doi: 10.1007/s00018-009-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales NR. Ongoing clinical trials in intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2013;44:S70–S73. doi: 10.1161/STROKEAHA.111.000563. [DOI] [PubMed] [Google Scholar]
- Guil S, Esteller M. Cis-acting noncoding RNAs: friends and foes. Nature structural & molecular biology. 2012;19:1068–1075. doi: 10.1038/nsmb.2428. [DOI] [PubMed] [Google Scholar]
- Guo D, Liu JN, Wang WH, Hao F, Sun XD, Wu X, Bu PL, Zhang Y, Liu Y, Liu FQ, Zhang QY, Jiang F. Alteration in Abundance and Compartmentalization of Inflammation-Related miRNAs in Plasma After Intracerebral Hemorrhage. Stroke. 2013;44:1739–1742. doi: 10.1161/STROKEAHA.111.000835. [DOI] [PubMed] [Google Scholar]
- Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halic M, Moazed D. Transposon silencing by piRNAs. Cell. 2009;138:1058–1060. doi: 10.1016/j.cell.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraz MM, Eacker SM, Wang X, Dawson TM, Dawson VL. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18962–18967. doi: 10.1073/pnas.1121288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries LW. Long non-coding RNAs and human disease. Biochemical Society transactions. 2012;40:902–906. doi: 10.1042/BST20120020. [DOI] [PubMed] [Google Scholar]
- Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussecker D, Kay MA. miR-122 continues to blaze the trail for microRNA therapeutics. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:240–242. doi: 10.1038/mt.2009.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Yu D, Almeida-Suhett C, Tu K, Marini AM, Eiden L, Braga MF, Zhu J, Li Z. Expression of miRNAs and their cooperative regulation of the pathophysiology in traumatic brain injury. PLoS One. 2012;7:e39357. doi: 10.1371/journal.pone.0039357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P, Kong B, Langerod A, Borresen-Dale AL, Kim SK, van de Vijver M, Sukumar S, Whitfield ML, Kellis M, Xiong Y, Wong DJ, Chang HY. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nature genetics. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Salkowski CA, Zhang F, Aber T, Nagayama M, Vogel SN, Ross ME. The transcription factor interferon regulatory factor 1 is expressed after cerebral ischemia and contributes to ischemic brain injury. J Exp Med. 1999;189:719–727. doi: 10.1084/jem.189.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizu H, Siomi H, Siomi MC. Biology of PIWI-interacting RNAs: new insights into biogenesis and function inside and outside of germlines. Genes & development. 2012;26:2361–2373. doi: 10.1101/gad.203786.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi B, Nakasa T, Tanaka N, Nakanishi K, Kamei N, Yamamoto R, Nakamae T, Ohta R, Fujioka Y, Yamasaki K, Ochi M. MicroRNA-223 expression in neutrophils in the early phase of secondary damage after spinal cord injury. Neuroscience letters. 2011;492:114–118. doi: 10.1016/j.neulet.2011.01.068. [DOI] [PubMed] [Google Scholar]
- Jackson RJ, Standart N. How do microRNAs regulate gene expression? Science's STKE : signal transduction knowledge environment 2007. 2007:re1. doi: 10.1126/stke.3672007re1. [DOI] [PubMed] [Google Scholar]
- Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. The Journal of clinical investigation. 2011;121:2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee MK, Jung JS, Choi JI, Jang JA, Kang KS, Im YB, Kang SK. MicroRNA 486 is a potentially novel target for the treatment of spinal cord injury. Brain : a journal of neurology. 2012a;135:1237–1252. doi: 10.1093/brain/aws047. [DOI] [PubMed] [Google Scholar]
- Jee MK, Jung JS, Im YB, Jung SJ, Kang SK. Silencing of miR20a is crucial for Ngn1-mediated neuroprotection in injured spinal cord. Human gene therapy. 2012b;23:508–520. doi: 10.1089/hum.2011.121. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- Jickling GC, Ander BP, Stamova B, Zhan X, Liu D, Bsc LR, Verro P, Khoury J, Jauch EC, Pancioli A, Broderick JP, Sharp FR. RNA in blood is altered prior to hemorrhagic transformation in ischemic stroke. Annals of neurology. 2013 doi: 10.1002/ana.23883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickling GC, Stamova B, Ander BP, Zhan X, Liu D, Sison SM, Verro P, Sharp FR. Prediction of cardioembolic, arterial, and lacunar causes of cryptogenic stroke by gene expression and infarct location. Stroke; a journal of cerebral circulation. 2012a;43:2036–2041. doi: 10.1161/STROKEAHA.111.648725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickling GC, Stamova B, Ander BP, Zhan X, Tian Y, Liu D, Xu H, Johnston SC, Verro P, Sharp FR. Profiles of lacunar and nonlacunar stroke. Annals of neurology. 2011;70:477–485. doi: 10.1002/ana.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickling GC, Xu H, Stamova B, Ander BP, Zhan X, Tian Y, Liu D, Turner RJ, Mesias M, Verro P, Khoury J, Jauch EC, Pancioli A, Broderick JP, Sharp FR. Signatures of cardioembolic and large-vessel ischemic stroke. Annals of neurology. 2010;68:681–692. doi: 10.1002/ana.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickling GC, Zhan X, Stamova B, Ander BP, Tian Y, Liu D, Sison SM, Verro P, Johnston SC, Sharp FR. Ischemic transient neurological events identified by immune response to cerebral ischemia. Stroke; a journal of cerebral circulation. 2012b;43:1006–1012. doi: 10.1161/STROKEAHA.111.638577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia R, Tureyen K, Bowen KK, Kalluri H, Johnson PF, Vemuganti R. Decreased brain damage and curtailed inflammation in transcription factor CCAAT/enhancer binding protein beta knockout mice following transient focal cerebral ischemia. Journal of neurochemistry. 2006;98:1718–1731. doi: 10.1111/j.1471-4159.2006.04056.x. [DOI] [PubMed] [Google Scholar]
- Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Frontiers in bioscience : a journal and virtual library. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketting RF. microRNA Biogenesis and Function : An overview. Advances in experimental medicine and biology. 2011;700:1–14. doi: 10.1007/978-1-4419-7823-3_1. [DOI] [PubMed] [Google Scholar]
- King MD, Laird MD, Ramesh SS, Youssef P, Shakir B, Vender JR, Alleyne CH, Dhandapani KM. Elucidating novel mechanisms of brain injury following subarachnoid hemorrhage: an emerging role for neuroproteomics. Neurosurgical focus. 2010;28:E10. doi: 10.3171/2009.10.FOCUS09223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 2003;9:1274–1281. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338:1435–1439. doi: 10.1126/science.1231776. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK, Kim M, Lee SK, Roh JK. MicroRNAs induced during ischemic preconditioning. Stroke; a journal of cerebral circulation. 2010;41:1646–1651. doi: 10.1161/STROKEAHA.110.579649. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Johnson KR, Hallenbeck JM. Global protein conjugation by ubiquitin-like-modifiers during ischemic stress is regulated by microRNAs and confers robust tolerance to ischemia. PLoS One. 2012;7:e47787. doi: 10.1371/journal.pone.0047787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei P, Li Y, Chen X, Yang S, Zhang J. Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain research. 2009;1284:191–201. doi: 10.1016/j.brainres.2009.05.074. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Lewis DK, Thomas KT, Selvamani A, Sohrabji F. Age-related severity of focal ischemia in female rats is associated with impaired astrocyte function. Neurobiology of aging. 2012;33:1123, e1121-1116. doi: 10.1016/j.neurobiolaging.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiological reviews. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu C, Peng Z, Zhang N, Yu L, Han S, Li D, Li J. Identification of differentially expressed microRNAs and their PKC-isoform specific gene network prediction during hypoxic preconditioning and focal cerebral ischemia of mice. Journal of neurochemistry. 2012a;120:830–841. doi: 10.1111/j.1471-4159.2011.07624.x. [DOI] [PubMed] [Google Scholar]
- Liu CL, Li X, Hu GL, Li RJ, He YY, Zhong W, Li S, He KL, Wang LL. Salubrinal protects against tunicamycin and hypoxia induced cardiomyocyte apoptosis via the PERK-eIF2alpha signaling pathway. Journal of geriatric cardiology : JGC. 2012b;9:258–268. doi: 10.3724/SP.J.1263.2012.02292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010a;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, McCullough LD. Interactions between age, sex, and hormones in experimental ischemic stroke. Neurochemistry international. 2012;61:1255–1265. doi: 10.1016/j.neuint.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Detloff MR, Miller KN, Santi L, Houle JD. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Experimental neurology. 2012c;233:447–456. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Keeler BE, Zhukareva V, Houle JD. Cycling exercise affects the expression of apoptosis-associated microRNAs after spinal cord injury in rats. Experimental neurology. 2010b;226:200–206. doi: 10.1016/j.expneurol.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Experimental neurology. 2009a;219:424–429. doi: 10.1016/j.expneurol.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Wang XL, Zhang L, Hozeska-Solgot A, Tang T, Kassis H, Zhang RL, Chen C, Xu J, Zhang ZG. MicroRNA-17/92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. The Journal of biological chemistry. 2013 doi: 10.1074/jbc.M112.449025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, Zhang ZG. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One. 2011;6:e23461. doi: 10.1371/journal.pone.0023461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YP, Lang BT, Baskaya MK, Dempsey RJ, Vemuganti R. The potential of neural stem cells to repair stroke-induced brain damage. Acta neuropathologica. 2009b;117:469–480. doi: 10.1007/s00401-009-0516-1. [DOI] [PubMed] [Google Scholar]
- Lou YL, Guo F, Liu F, Gao FL, Zhang PQ, Niu X, Guo SC, Yin JH, Wang Y, Deng ZF. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Molecular and cellular biochemistry. 2012;370:45–51. doi: 10.1007/s11010-012-1396-6. [DOI] [PubMed] [Google Scholar]
- Lusardi TA, Farr CD, Faulkner CL, Pignataro G, Yang T, Lan J, Simon RP, Saugstad JA. Ischemic preconditioning regulates expression of microRNAs and a predicted target, MeCP2, in mouse cortex. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:744–756. doi: 10.1038/jcbfm.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nature medicine. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Manwani B, McCullough LD. Estrogen in ischaemic stroke: the debate continues. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2012;19:1276–1277. doi: 10.1111/j.1468-1331.2012.03746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MM, Buckenberger JA, Jiang J, Malana GE, Nuovo GJ, Chotani M, Feldman DS, Schmittgen TD, Elton TS. The human angiotensin II type 1 receptor +1166 A/C polymorphism attenuates microrna-155 binding. The Journal of biological chemistry. 2007;282:24262–24269. doi: 10.1074/jbc.M701050200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Meller R, Simon RP. Tolerance to Ischemia - an increasingly complex biology. Translational stroke research. 2013;4:40–50. doi: 10.1007/s12975-012-0246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettimano M, Romano-Spica V, Ianni A, Specchia M, Migneco A, Savi L. AGT and AT1R gene polymorphism in hypertensive heart disease. International journal of clinical practice. 2002;56:574–577. [PubMed] [Google Scholar]
- Nakanishi K, Nakasa T, Tanaka N, Ishikawa M, Yamada K, Yamasaki K, Kamei N, Izumi B, Adachi N, Miyaki S, Asahara H, Ochi M. Responses of microRNAs 124a and 223 following spinal cord injury in mice. Spinal cord. 2010;48:192–196. doi: 10.1038/sc.2009.89. [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, Weber C, Schober A. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. The Journal of clinical investigation. 2012;122:4190–4202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S, Mori T, Hoshino Y, Yamada N, Nakagawa T, Sasaki N, Akao Y, Maruo K. Comparative study of anti-oncogenic microRNA-145 in canine and human malignant melanoma. The Journal of veterinary medical science / the Japanese Society of Veterinary Science. 2012;74:1–8. doi: 10.1292/jvms.11-0264. [DOI] [PubMed] [Google Scholar]
- Nordell VL, Scarborough MM, Buchanan AK, Sohrabji F. Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiology of aging. 2003;24:733–743. doi: 10.1016/s0197-4580(02)00193-8. [DOI] [PubMed] [Google Scholar]
- Nunes RR, Duval Neto GF, Garcia de Alencar JC, Franco SB, de Andrade NQ, Holanda Dumaresq DM, Cavalcante SL. Anesthetics, cerebral protection and preconditioning. Revista brasileira de anestesiologia. 2013;63:119–138. [Google Scholar]
- O'Donnell KA, Boeke JD. Mighty Piwis defend the germline against genome intruders. Cell. 2007;129:37–44. doi: 10.1016/j.cell.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson ST. Heparin and ionic strength-dependent conversion of antithrombin III from an inhibitor to a substrate of alpha-thrombin. The Journal of biological chemistry. 1985;260:10153–10160. [PubMed] [Google Scholar]
- Onteniente B, Rasika S, Benchoua A, Guegan C. Molecular pathways in cerebral ischemia: cues to novel therapeutic strategies. Molecular neurobiology. 2003;27:33–72. doi: 10.1385/MN:27:1:33. [DOI] [PubMed] [Google Scholar]
- Orom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5'UTR of ribosomal protein mRNAs and enhances their translation. Molecular cell. 2008;30:460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX, White RE, Sun X, Giffard RG. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiology of disease. 2012;45:555–563. doi: 10.1016/j.nbd.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi G, Nakka VP, Dharap A, Roopra A, Vemuganti R. MicroRNA miR-29c Down-Regulation Leading to De-Repression of Its Target DNA Methyltransferase 3a Promotes Ischemic Brain Damage. PLoS One. 2013;8:e58039. doi: 10.1371/journal.pone.0058039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasmant E, Laurendeau I, Heron D, Vidaud M, Vidaud D, Bieche I. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer research. 2007;67:3963–3969. doi: 10.1158/0008-5472.CAN-06-2004. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, Gala R, Simon RP. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:232–241. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends in cell biology. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1608–1613. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nature reviews. Neuroscience. 2012;13:528–541. doi: 10.1038/nrn3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rah H, Jeon YJ, Shim SH, Cha SH, Choi DH, Kwon H, Kim JH, Shin JE, Kim NK. Association of miR-146aC>G, miR-196a2T>C, and miR-499A>G polymorphisms with risk of premature ovarian failure in Korean women. Reproductive sciences. 2013;20:60–68. doi: 10.1177/1933719112450341. [DOI] [PubMed] [Google Scholar]
- Rahbek UL, Nielsen AF, Dong M, You Y, Chauchereau A, Oupicky D, Besenbacher F, Kjems J, Howard KA. Bioresponsive hyperbranched polymers for siRNA and miRNA delivery. Journal of drug targeting. 2010;18:812–820. doi: 10.3109/1061186X.2010.527982. [DOI] [PubMed] [Google Scholar]
- Raitoharju E, Lyytikainen LP, Levula M, Oksala N, Mennander A, Tarkka M, Klopp N, Illig T, Kahonen M, Karhunen PJ, Laaksonen R, Lehtimaki T. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219:211–217. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. The Journal of clinical investigation. 2011;121:2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read SJ, Parsons AA, Harrison DC, Philpott K, Kabnick K, S OB, Clark S, Brawner M, Bates S, Gloger I, Legos JJ, Barone FC. Stroke genomics: approaches to identify, validate, and understand ischemic stroke gene expression. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21:755–778. doi: 10.1097/00004647-200107000-00001. [DOI] [PubMed] [Google Scholar]
- Redell JB, Liu Y, Dash PK. Traumatic brain injury alters expression of hippocampal microRNAs: potential regulators of multiple pathophysiological processes. Journal of neuroscience research. 2009;87:1435–1448. doi: 10.1002/jnr.21945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redell JB, Moore AN, Ward NH, 3rd, Hergenroeder GW, Dash PK. Human traumatic brain injury alters plasma microRNA levels. Journal of neurotrauma. 2010;27:2147–2156. doi: 10.1089/neu.2010.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salta E, De Strooper B. Non-coding RNAs with essential roles in neurodegenerative disorders. Lancet neurology. 2012;11:189–200. doi: 10.1016/S1474-4422(11)70286-1. [DOI] [PubMed] [Google Scholar]
- Santovito D, Mandolini C, Marcantonio P, De Nardis V, Bucci M, Paganelli C, Magnacca F, Ucchino S, Mastroiacovo D, Desideri G, Mezzetti A, Cipollone F. Overexpression of 37 microRNA-145 in atherosclerotic plaques from hypertensive patients. Expert opinion on therapeutic targets. 2013;17:217–223. doi: 10.1517/14728222.2013.745512. [DOI] [PubMed] [Google Scholar]
- Satriotomo I, Bowen KK, Vemuganti R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J Neurochem. 2006;98:1353–1368. doi: 10.1111/j.1471-4159.2006.04051.x. [DOI] [PubMed] [Google Scholar]
- Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1564–1576. doi: 10.1038/jcbfm.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholer N, Langer C, Dohner H, Buske C, Kuchenbauer F. Serum microRNAs as a novel class of biomarkers: a comprehensive review of the literature. Experimental hematology. 2010;38:1126–1130. doi: 10.1016/j.exphem.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Schonrock N, Gotz J. Decoding the non-coding RNAs in Alzheimer's disease. Cellular and molecular life sciences : CMLS. 2012;69:3543–3559. doi: 10.1007/s00018-012-1125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sathyan P, Miranda RC, Sohrabji F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PLoS One. 2012;7:e32662. doi: 10.1371/journal.pone.0032662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepramaniam S, Armugam A, Lim KY, Karolina DS, Swaminathan P, Tan JR, Jeyaseelan K. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. The Journal of biological chemistry. 2010;285:29223–29230. doi: 10.1074/jbc.M110.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Jickling GC, Stamova B, Tian Y, Zhan X, Ander BP, Cox C, Kuczynski B, Liu D. RNA expression profiles from blood for the diagnosis of stroke and its causes. Journal of child neurology. 2011a;26:1131–1136. doi: 10.1177/0883073811408093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Jickling GC, Stamova B, Tian Y, Zhan X, Liu D, Kuczynski B, Cox CD, Ander BP. Molecular markers and mechanisms of stroke: RNA studies of blood in animals and humans. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011b;31:1513–1531. doi: 10.1038/jcbfm.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Sun BL, Zhang J, Lu S, Zhang P, Wang H, Yu Q, Stetler RA, Vosler PS, Chen J, Gao Y. miR-15b Suppression of Bcl-2 Contributes to Cerebral Ischemic Injury and is Reversed by Sevoflurane Preconditioning. CNS & neurological disorders drug targets. 2013;12:381–391. doi: 10.2174/1871527311312030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- Siegel C, Li J, Liu F, Benashski SE, McCullough LD. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11662–11667. doi: 10.1073/pnas.1102635108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DY, Oh KM, Yu HN, Park CR, Woo RS, Jung SS, Baik TK. Role of activating transcription factor 3 in ischemic penumbra region following transient middle cerebral artery occlusion and reperfusion injury. Neuroscience research. 2011;70:428–434. doi: 10.1016/j.neures.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Stamova B, Xu H, Jickling G, Bushnell C, Tian Y, Ander BP, Zhan X, Liu D, Turner R, Adamczyk P, Khoury JC, Pancioli A, Jauch E, Broderick JP, Sharp FR. Gene expression profiling of blood for the prediction of ischemic stroke. Stroke; a journal of cerebral circulation. 2010;41:2171–2177. doi: 10.1161/STROKEAHA.110.588335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Stetler RA, Gao Y, Signore AP, Cao G, Chen J. HSP27: mechanisms of cellular protection against neuronal injury. Current molecular medicine. 2009;9:863–872. doi: 10.2174/156652409789105561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland ER, Hook MA, Balaraman S, Huie JR, Grau JW, Miranda RC. MicroRNA dysregulation following spinal cord contusion: implications for neural plasticity and repair. Neuroscience. 2011;186:146–160. doi: 10.1016/j.neuroscience.2011.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Expression profile of MicroRNAs in young stroke patients. PLoS One. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Ito D, Suzuki S, Dembo T, Kosakai A, Fukuuchi Y. Activated phosphorylation of cyclic AMP response element binding protein is associated with preservation of striatal neurons after focal cerebral ischemia in the rat. Neuroscience. 2000a;100:345–354. doi: 10.1016/s0306-4522(00)00289-x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Nagata E, Ito D, Suzuki S, Dembo T, Kosakai A, Fukuuchi Y. Persistent CREB phosphorylation with protection of hippocampal CA1 pyramidal neurons following temporary occlusion of the middle cerebral artery in the rat. Experimental neurology. 2000b;161:462–471. doi: 10.1006/exnr.1999.7313. [DOI] [PubMed] [Google Scholar]
- Truettner JS, Alonso OF, Bramlett HM, Dietrich WD. Therapeutic hypothermia alters microRNA responses to traumatic brain injury in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:1897–1907. doi: 10.1038/jcbfm.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tureyen K, Brooks N, Bowen K, Svaren J, Vemuganti R. Transcription factor early growth response-1 induction mediates inflammatory gene expression and brain damage following transient focal ischemia. Journal of neurochemistry. 2008;105:1313–1324. doi: 10.1111/j.1471-4159.2008.05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Vemuganti R, Salamat MS, Dempsey RJ. Increased angiogenesis and angiogenic gene expression in carotid artery plaques from symptomatic stroke patients. Neurosurgery. 2006;58:971–977. doi: 10.1227/01.NEU.0000210246.61817.FE. discussion 971-977. [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Vemuganti R, Bowen K, Dhodda VK, Song G, Franklin JL, Gavva NR, Dempsey RJ. Gene expression analysis of spontaneously hypertensive rat cerebral cortex following transient focal cerebral ischemia. J Neurochem. 2002;83:1072–1086. doi: 10.1046/j.1471-4159.2002.01208.x. [DOI] [PubMed] [Google Scholar]
- Vemuganti R, Dempsey RJ. Carotid atherosclerotic plaques from symptomatic stroke patients share the molecular fingerprints to develop in a neoplastic fashion: a microarray analysis study. Neuroscience. 2005;131:359–374. doi: 10.1016/j.neuroscience.2004.08.058. [DOI] [PubMed] [Google Scholar]
- Vemuganti R, Dempsey RJ. Increased expression of genes that control ionic homeostasis, second messenger signaling and metabolism in the carotid plaques from patients with symptomatic stroke. Journal of neurochemistry. 2006;97(Suppl 1):92–96. doi: 10.1111/j.1471-4159.2005.03516.x. [DOI] [PubMed] [Google Scholar]
- Venna VR, Weston G, Benashski SE, Tarabishy S, Liu F, Li J, Conti LH, McCullough LD. NF-kappaB contributes to the detrimental effects of social isolation after experimental stroke. Acta neuropathologica. 2012;124:425–438. doi: 10.1007/s00401-012-0990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. European heart journal. 2010;31:659–666. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo F, Pan C, Lou Y, Zhang P, Guo S, Yin J, Deng Z. Effects of low temperatures on proliferation-related signaling pathways in the hippocampus after traumatic brain injury. Experimental biology and medicine. 2012;237:1424–1432. doi: 10.1258/ebm.2012.012123. [DOI] [PubMed] [Google Scholar]
- Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clinical chemistry. 2010a;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochemical and biophysical research communications. 2010b;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein PR, Hong S, Sharp FR. Molecular identification of the ischemic penumbra. Stroke; a journal of cerebral circulation. 2004;35:2666–2670. doi: 10.1161/01.STR.0000144052.10644.ed. [DOI] [PubMed] [Google Scholar]
- Weng H, Shen C, Hirokawa G, Ji X, Takahashi R, Shimada K, Kishimoto C, Iwai N. Plasma miR-124 as a biomarker for cerebral infarction. Biomedical research. 2011;32:135–141. doi: 10.2220/biomedres.32.135. [DOI] [PubMed] [Google Scholar]
- Wilson ME. Stroke: understanding the differences between males and females. Pflugers Archiv : European journal of physiology. 2013 doi: 10.1007/s00424-013-1260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltrout C, Lang B, Yan Y, Dempsey RJ, Vemuganti R. Repairing brain after stroke: a review on post-ischemic neurogenesis. Neurochemistry international. 2007;50:1028–1041. doi: 10.1016/j.neuint.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Wright MW, Bruford EA. Naming 'junk': human non-protein coding RNA (ncRNA) gene nomenclature. Human genomics. 2011;5:90–98. doi: 10.1186/1479-7364-5-2-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nature reviews. Neuroscience. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CC, Han WQ, Xiao B, Li NN, Zhu DL, Gao PJ. [Differential expression of microRNAs in the aorta of spontaneously hypertensive rats] Sheng li xue bao : [Acta physiologica Sinica] 2008;60:553–560. [PubMed] [Google Scholar]
- Xu H, Stamova B, Jickling G, Tian Y, Zhan X, Ander BP, Liu D, Turner R, Rosand J, Goldstein LB, Furie KL, Verro P, Johnston SC, Sharp FR, Decarli CS. Distinctive RNA expression profiles in blood associated with white matter hyperintensities in brain. Stroke; a journal of cerebral circulation. 2010;41:2744–2749. doi: 10.1161/STROKEAHA.110.591875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Wang X, Li P, Qin K, Jiang X. miR-124 regulates neural stem cells in the treatment of spinal cord injury. Neuroscience letters. 2012a;529:12–17. doi: 10.1016/j.neulet.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Xu WH, Yao XY, Yu HJ, Huang JW, Cui LY. Downregulation of miR-199a may play a role in 3-nitropropionic acid induced ischemic tolerance in rat brain. Brain research. 2012b;1429:116–123. doi: 10.1016/j.brainres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Yan W, Fang Z, Yang Q, Dong H, Lu Y, Lei C, Xiong L. SirT1 mediates hyperbaric oxygen preconditioning-induced ischemic tolerance in rat brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:396–406. doi: 10.1038/jcbfm.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain research. 2008;1244:164–172. doi: 10.1016/j.brainres.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Park SW, Kapadia R, Vemuganti R. Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem Int. 2007;50:1014–1027. doi: 10.1016/j.neuint.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, Jiang X, Wang Y, Chen YE. Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci. 2010a;30:6398–6408. doi: 10.1523/JNEUROSCI.0780-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010b;38:17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Gius D, Onyango P, Muldoon-Jacobs K, Karp J, Feinberg AP, Cui H. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature. 2008;451:202–206. doi: 10.1038/nature06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YM, Gibbs KM, Davila J, Campbell N, Sung S, Todorova TI, Otsuka S, Sabaawy HE, Hart RP, Schachner M. MicroRNA miR-133b is essential for functional recovery after spinal cord injury in adult zebrafish. The European journal of neuroscience. 2011;33:1587–1597. doi: 10.1111/j.1460-9568.2011.07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunta M, Nieto-Diaz M, Esteban FJ, Caballero-Lopez M, Navarro-Ruiz R, Reigada D, Pita-Thomas DW, del Aguila A, Munoz-Galdeano T, Maza RM. MicroRNA dysregulation in the spinal cord following traumatic injury. PLoS One. 2012;7:e34534. doi: 10.1371/journal.pone.0034534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circulation research. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- Zeng L, Liu J, Wang Y, Wang L, Weng S, Tang Y, Zheng C, Cheng Q, Chen S, Yang GY. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Frontiers in bioscience. 2011;3:1265–1272. doi: 10.2741/e330. [DOI] [PubMed] [Google Scholar]
- Zhan X, Ander BP, Liao IH, Hansen JE, Kim C, Clements D, Weisbart RH, Nishimura RN, Sharp FR. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke; a journal of cerebral circulation. 2010;41:538–543. doi: 10.1161/STROKEAHA.109.572537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chopp M, Liu X, Teng H, Tang T, Kassis H, Zhang ZG. Combination therapy with VELCADE and tissue plasminogen activator is neuroprotective in aged rats after stroke and targets microRNA-146a and the toll-like receptor signaling pathway. Arteriosclerosis, thrombosis, and vascular biology. 2012a;32:1856–1864. doi: 10.1161/ATVBAHA.112.252619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dong LY, Li YJ, Hong Z, Wei WS. The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor. Journal of neuroinflammation. 2012b;9:211. doi: 10.1186/1742-2094-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dong LY, Li YJ, Hong Z, Wei WS. miR-21 represses FasL in microglia and protects against microglia-mediated neuronal cell death following hypoxia/ischemia. Glia. 2012c;60:1888–1895. doi: 10.1002/glia.22404. [DOI] [PubMed] [Google Scholar]
- Zheng HW, Wang YL, Lin JX, Li N, Zhao XQ, Liu GF, Liu LP, Jiao Y, Gu WK, Wang DZ, Wang YJ. Circulating MicroRNAs as Potential Risk Biomarkers for Hematoma Enlargement after Intracerebral Hemorrhage. Cns Neuroscience & Therapeutics. 2012;18:1003–1011. doi: 10.1111/cns.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]