Abstract
Synthesis of the gonadotropin β-subunits is tightly controlled by a complex network of hormonal signaling pathways that may be modulated by metabolic cues. Recently, we reported that insulin regulates FOXO1 phosphorylation and cellular localization in pituitary gonadotropes and that FOXO1 overexpression inhibits Lhb transcription. In the current study, we investigated whether FOXO1 modulates Fshb synthesis. Here, we demonstrate that FOXO1 represses basal and GnRH-induced Fshb transcription in LβT2 cells. In addition, we show that PI3K inhibition, which increases FOXO1 nuclear localization, results in decreased Fshb mRNA levels in murine primary pituitary cells. FOXO1 also decreases transcription from the human FSHB promoter, suggesting that FOXO1 regulation of FSHB transcription may be conserved between rodents and humans. Although the FOXO1 DNA-binding domain is necessary for suppression of Fshb, we do not observe direct binding of FOXO1 to the Fshb promoter, suggesting that FOXO1 exerts its effect through protein-protein interactions with transcription factors required for Fshb synthesis. FOXO1 suppression of basal Fshb transcription may involve PITX1 because PITX1 interacts with FOXO1, FOXO1 repression maps to the proximal Fshb promoter containing a PITX1-binding site, PITX1 induction of Fshb or a PITX1 binding element in CV-1 cells is decreased by FOXO1, and FOXO1 suppresses Pitx1 mRNA and protein levels. GnRH induction of an Fshb promoter containing a deletion at −50/−41 or −30/−21 is not repressed by FOXO1, suggesting that these two regions may be involved in FOXO1 suppression of GnRH-induced Fshb synthesis. In summary, our data demonstrate that FOXO1 can negatively regulate Fshb transcription and suggest that FOXO1 may relay metabolic hormonal signals to modulate gonadotropin production.
Produced by gonadotrope cells in the anterior pituitary, FSH plays a key role in mammalian fertility. In females, it is required for ovarian folliculogenesis, while in males it promotes spermatogenesis in conjunction with testosterone (1, 2). Female Fshb knockout mice exhibit an arrest in ovarian folliculogenesis prior to the antral stage, whereas males are fertile but have impaired reproductive function (3). In contrast, the human FSHB gene appears to be critical for reproductive function in both genders. Nonsynonymous mutations result in absent or incomplete pubertal development and infertility in women, as well as azoospermia and infertility in men (4).
FSH is a heterodimeric glycoprotein composed of a common α-subunit, shared with LH and TSH, as well as a unique β-subunit that confers biological specificity. Transcription of the Fshb gene is dynamically regulated during the estrous cycle. Alterations in Fshb mRNA levels are observed prior to changes in serum FSH levels, indicating that transcription of Fshb is a rate-limiting step for production of the mature hormone (5, 6). Proper regulation of FSH levels is important for fertility. Low FSH levels are associated with defective follicular growth, whereas high levels are associated with premature ovarian failure (7).
Basal Fshb transcription involves multiple transcription factors, including LIM homeobox gene (LHX), nuclear transcription factor Y (NFY), and paired-like homeodomain transcription factor (PITX) (8). LHX3 was reported to bind the porcine Fshb promoter at several binding sites, including one element that is highly conserved among mammals (9). In addition, NFY was shown to bind to a site at −76/−72 in the murine Fshb promoter and mutation of this site decreased basal Fshb synthesis by 30% (10). Furthermore, Lamba et al showed that PITX1/2 binds to a conserved element at −54/−49 of the murine Fshb promoter and that mutation of this binding element reduced basal Fshb gene expression (11).
Hormonal regulation of Fshb gene expression has been shown to occur via multiple signaling pathways (12). GnRH increases Fshb transcription via protein kinase C and MAPK signaling pathways through the induction of the activator protein 1 (AP1) transcription factors, c-FOS and JUNB, which bind and induce the Fshb promoter, along with c-JUN and FOSB (13–18). AP1 also interacts with factors involved in basal expression of Fshb, such as NFY, in the mouse and USF1, in the rat to integrate GnRH responsiveness (16, 19). Activin is also an inducer of the Fshb gene (20). Activin signaling through SMAD and FOXL2 proteins also appears to be critical for transcriptional regulation of the Fshb gene (8, 21).
In addition to reproductive hormones, metabolic hormones may regulate FSH production at the level of the gonadotrope. Although insulin, insulin-like growth factor 1, and leptin treatment of rat primary pituitary cells have been reported to increase FSH, as well as LH levels (22–25), it is not clear whether this is due to an effect of these hormones on Fshb transcription vs production and/or secretion of the mature hormone. One possibility is that metabolic hormones regulate Fshb transcription in gonadotropes through the activity of downstream effectors such as the FOXO subfamily of forkhead transcription factors. This family is composed of FOXO1, 3, 4 and 6. The transcriptional activity of FOXOs is controlled by posttranslational modifications in multiple cell and tissue types (26–28). FOXO phosphorylation has been shown to be regulated by insulin, insulin-like growth factor 1, and leptin (29–31). Insulin stimulation induces PI3K/AKT activation, triggering AKT phosphorylation of FOXO1 on residues Thr24, Ser256, and Ser319 (32). This results in FOXO1 export from the nucleus to the cytoplasm, where it is sequestered by 14-3-3 proteins, preventing FOXO1 transcriptional activity. Although considerable progress has been made in understanding the role of FOXOs in reproductive tissues such as the ovary and uterus (33, 34), FOXO regulation of gonadotropin hormone production in pituitary gonadotrope cells remains largely unexplored.
Recently, we reported that insulin signaling regulated FOXO1 phosphorylation in a PI3K-dependent manner in immortalized gonadotropes, similarly to what has been observed in other cells (26, 35, 36). Because we also discovered that overexpression of FOXO1 suppressed basal and GnRH-induced Lhb transcription, the purpose of this study was to determine whether FOXO1 can regulate Fshb synthesis. We show that overexpression of FOXO1 in LβT2 cells results in decreased basal and GnRH-induced transcription from murine and human FSHB-luc reporters as well as endogenous Fshb mRNA. In addition, we show that inhibition of PI3K, which increases FOXO1 nuclear localization (35), results in decreased basal and GnRH-induced Fshb mRNA in murine primary pituitary cells. We also demonstrate that the FOXO1 suppression maps to the proximal Fshb promoter. Moreover, our data suggest that the FOXO1 suppression occurs through protein-protein interactions with factors necessary for Fshb transcription such as PITX1, because the FOXO1 DNA binding domain (DBD) is required for the suppression but FOXO1 does not appear to bind directly to the proximal Fshb promoter. This idea is further supported by the fact that PITX1 induction of Fshb or a PITX1-binding element in CV-1 cells is suppressed by FOXO1, that PITX1 interacts with FOXO1, and that two regions of the proximal Fshb promoter (−50/−41 and −30/−21) appear to be necessary for FOXO1 suppression of GnRH-induced Fshb transcription.
Materials and Methods
Murine primary pituitary cell culture
Seven-week-old, male C57 Black 6 mice (Harlan Laboratories) were housed in the University of California, San Diego (UCSD) vivarium for 1 week under standard conditions. All animal procedures were conducted in accordance with the UCSD Institutional Animal Care and Use Committee requirements. Eight mice were killed and their pituitaries were collected in ice-cold Dulbecco's A PBS. After a PBS rinse, the pituitaries were minced on ice with fine scissors and then placed in dissociation media containing phosphate buffered 0.25% trypsin-EDTA (Gibco/Life Technologies) and 0.25% collagenase (Life Technologies). The pituitaries were shaken for 30 minutes at 37°C in a water bath; then an equal volume of DMEM (Mediatech Inc) containing 10% fetal bovine serum (FBS) (Omega Scientific, Inc) was added along with Deoxyribonuclease I (Worthington Biochemical) at a final concentration of 25 μg/mL and incubated for another 15 minutes at 37°C. After removal of tissue debris, the cells were pelleted by centrifugation and plated at a density of 6 × 105/2 cm2 well in Primaria plates (BD Biosciences). After 24 hours in 10% FBS DMEM, the cells were changed to serum-free media 18 hours before treatment. Cells were pretreated with dimethylsulfoxide (DMSO) or 50 μM LY294002 (EMD Biosciences) for 1 hour, then treated with 0.1% BSA vehicle or 30 nM GnRH (Sigma-Aldrich Co) along with DMSO or LY294002 for an additional 5 hours, after which the cells were lysed to obtain total RNA.
Immortalized cell culture
Cell culture was performed using the LβT2 cell line, which has many characteristics of a mature, differentiated gonadotrope (37, 38). CV-1 cells lacking PITX1 were also used (39, 40). The cells were maintained in 10-cm plates in DMEM with 10% FBS and penicillin/streptomycin antibiotics (Gibco/Invitrogen) at 37°C and 5% CO2.
Adenoviral infection
Adenoviral vectors containing cDNA of green fluorescent protein (Ad-GFP) and constitutively active FOXO1 (T24A/S256A/S319A) (Ad-FOXO1-CA) were generously provided by Dr. Domenico Accili. LβT2 cells were seeded at 2 × 106 cells per well on six-well plates. The next morning, cells were transduced with a multiplicity of infection of 200 of Ad-GFP or Ad-FOXO1-CA for 6 hours and then switched to serum-free DMEM. Twenty-four hours after adenoviral infection, cells were treated with vehicle (0.1% BSA) or 10 nM GnRH for 1 or 6 hours as noted.
Quantitative RT-PCR
Total RNA was extracted from LβT2 cells with TRIzol Reagent (Life Technologies) following the manufacturer's protocol. Contaminating DNA was removed with DNA-free reagent (Life Technologies). Two micrograms of RNA was reverse-transcribed using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc) according to the manufacturer's protocol. Quantitative real-time PCR was performed in an iQ5 iCycler using iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc) and the following primers: Fshb forward, GCCGTTTCTGCATAAGC; Fshb reverse, CAATCTTACGGTCTCGTATACC; Pitx1 forward, GTCCGACGCTGATCTGCCA; Pitx1 reverse, TTGCCGCCGCTGTTTCTTCTT; c-Fos forward, GGCAAAGTAGAGCAGCTATCTCCT; c-Fos reverse GGCAAAGTAGAGCAGCTATCTCCT; c-Jun forward, GTCCCCTATCGACATGGAGTCT; c-Jun reverse, GAGTTTTGCGCTTTCAAGGTTT; Gapdh forward, TGCACCACCAACTGCTTAG; Gapdh reverse, GGATGCAGGGATGATGTTC; under the following conditions: 95°C for 5 minutes, followed by 40 cycles at 95°C for 45 seconds, 56°C for 45 seconds, and 72°C for 45 seconds except for an annealing temperature of 61°C for Pitx1. Each sample was assayed in triplicate and the experiment was conducted at least three times. Standard curves with dilutions of a plasmid containing Fshb, Pitx1, c-Fos, c-Jun, or Gapdh cDNA were generated with the samples in each run. In each experiment, the amount of Fshb, Pitx1, c-Fos, or c-Jun was calculated by comparing the threshold cycle obtained for each sample with the standard curve generated in the same run. Replicates were averaged and divided by the mean value of Gapdh in the same sample. After each run, a melting curve analysis was performed to confirm that a single amplicon was generated for each primer pair.
Plasmid constructs
The pcDNA3 Flag human FOXO1 and pcDNA3 Flag FOXO1-CA expression vectors were previously described (32). We obtained the pcDNA3 FOXO1-ΔDBD (Δ208–220) from Dr. William Sellers (Addgene plasmid 10694). The pcDNA3 murine PITX1 expression vector was described previously (41). The construction of the −1000 murine Fshb luciferase (luc) reporter plasmid and 5′ truncations were described previously (16, 42). The 10-bp deletions of the −398 Fshb promoter from −70/−11 were generously provided by Dr. Djurdjica Coss. The −1028/+7 human FSHB-luc reporter plasmid was provided by Dr. Daniel Bernard. The 4xFBE-luc was constructed by inserting four repeats of a consensus forkhead binding element (FBE) (CCGTAAACAACT) upstream of a minimal −81 Herpes simplex virus thymidine kinase promoter in pGL3 using KpnI and NheI restriction enzyme sites, whereas the 4xHDBE-luc contains four repeats of a consensus bicoid-related homeodomain binding element (HDBE) (ACTAATCCCT) (39). The elements are underlined. Sequences were confirmed by dideoxyribonucleotide sequencing.
Mutagenesis
The QuikChange Site-Directed Mutagenesis Kit (Stratagene) was used to generate mutations in a plasmid containing the murine Fshb promoter. Mutagenesis was performed with the following mutations in the −54/−49 PITX1 and −72/−69 AP1 binding elements, respectively: 5′-GTGGATCGAC-3′ and 5′-ATTGGTCCCG-3′ (mutated bases are indicated in bold). The sequences of the promoters were confirmed by dideoxyribonucleotide sequencing.
Transient transfection
LβT2 cells were seeded at 4.5 × 105 cells per well on 12-well plates and transfected 18 hours later, using Polyjet (SignaGen Laboratories) following the manufacturer's instructions. CV-1 cells were seeded at 1.5 × 105 cells per well. For all experiments, the cells were transfected with 400 ng of the indicated luc reporter plasmid and 200 ng of a β-galactosidase (β-gal) reporter plasmid driven by the Herpes Virus thymidine kinase promoter to control for transfection efficiency. The cells were switched to serum-free DMEM containing 0.1% BSA, 5 mg/L transferrin, and 50 mM sodium selenite 6 hours after transfection. After overnight incubation in serum-free media, the cells were treated with vehicle (0.1% BSA) or 10 nM GnRH for 6 hours, as indicated.
Luciferase and β-gal assays
To harvest the cells, they were washed with 1× PBS and lysed with 0.1 M K-phosphate buffer pH 7.8 containing 0.2% Triton X-100. Lysed cells were assayed for luc activity using a buffer containing 100 mM Tris-HCl pH 7.8, 15 mM MgSO4, 10 mM ATP, and 65 μM luciferin. β-Gal activity was assayed using the Tropix Galacto-light assay (Applied Biosystems), according to the manufacturer's protocol. Both assays were measured using a Veritas Microplate Luminometer (Promega).
Statistical analyses
Quantitative RT-PCR and transient transfection experiments were performed in triplicate and each experiment was repeated at least three times. For the transient transfections, the data were normalized for transfection efficiency by expressing luc activity relative to β-gal and relative to the empty reporter plasmid to control for hormone effects on the vector DNA. The data were analyzed by Student's t test for independent samples or one-way ANOVA followed by post-hoc comparisons with the Tukey-Kramer honestly significant difference (HSD) test using the statistical package JMP 10.0 (SAS). Significant differences were designated as P < .05.
Immunofluorescence
LβT2 cells were seeded onto poly l-lysine coverslips (BD Biosciences) at 4.5 × 105 cells per well in 12-well plates and then transfected 18 hours later with pcDNA3 Flag human FOXO1 or pcDNA3 Flag FOXO1-CA expression vectors. Six hours after transection, the cells were switched to serum-free DMEM containing 0.1% BSA, 5 mg/L transferrin, and 50 mM sodium selenite for overnight incubation. The next day, the cells were washed twice with PBS and fixed with 4% paraformaldehyde for 10 minutes. Cells were then washed in PBS twice and permeabilized with Nonidet P-40 solution (PBS containing 0.2% Nonidet P-40, 20% goat serum, 1% BSA) for 1 hour at room temperature. Cells were washed twice in PBS and then incubated with mouse anti-Flag (Sigma, 1:100, F3165) primary antibody in blocking buffer (PBS containing 20% goat serum, 1% BSA) for 24 hours at 4°C. Cells were washed three times with PBS for 5 minutes and incubated with Alexa488-conjugated goat antimouse secondary antibody (Invitrogen; 1:300, A11001) in blocking buffer for 1 hour at room temperature. Cells were washed three times with PBS for 5 minutes and incubated with 300 nM 4′,6-diamidino-2-phenylindole (Invitrogen) for 4 minutes, then washed three times with PBS for 5 minutes each. Coverslips were mounted using Prolong Gold (Invitrogen) and cells were viewed using a Nikon Eclipse TE 2000-U inverted fluorescence microscope. Digital images were collected using a CoolSNAP EZ cooled CCD camera (Roper Scientific) and analyzed with the Version 2.3 NIS-elements image analysis system.
EMSA
Flag-FOXO1-CA was transcribed and translated using a TnT Coupled Reticulolysate System (Promega Corp). The oligonucleotides were end-labeled with T4 polynucleotide kinase and [γ-32P] ATP. Four microliters of TnT lysate was incubated with 1 fmol of 32P-labeled oligo at 4°C for 30 minutes in a DNA-binding buffer (10 mM HEPES pH 7.8, 50 mM KCl, 5 mM MgCl2, 0.1% Nonidet P-40, 1 mM dithiothreitol, 2 μg poly[dI-dC], and 10% glycerol). After 30 minutes, the DNA binding reactions were run on a 5% polyacrylamide gel (30:1 acrylamide: bisacrylamide) containing 2.5% glycerol in a 0.25× Tris-borate, EDTA buffer. Murine anti-Flag M2 antibody was used to supershift Flag-FOXO1-CA; murine IgG was used as a control for nonspecific binding; 100× cold oligo was used as a competitor. The following oligonucleotides were used for EMSA: −95/−61 5′-CAGCAGGCTTTATGTTGGTATTGGTCATGTTAACA-3′, −65/−31 5′-TAACACCCAGTAAATCCACAGGGTTTTAAGTTTGT-3′, −35/−1 5′-TTTGTATAAAAGATGAGGTGTAACTTGACTCAGTG-3′, the consensus FBE (43) 5′-CTAGATGGTAAACAACTGTGACTAGTAGAACACGG-3′ and the mutated consensus FBE 5′-CTAGATGGTGGGCAACTGTGACTAGTAGAACACGG-3′ with the mutations underlined.
Glutathione S-transferase (GST) interaction assay
GST-PITX1 was provided by Dr Jacques Drouin and the GFP expression vector by Dr Douglass Forbes. 35S-labeled proteins were produced using the TnT Coupled Reticulolysate System. Bacteria transformed with GST plasmids were grown to OD of 0.6 and induced with isopropyl β-D-1-thiogalactopyranoside overnight at 30°C (44). Bacterial pellets were sonicated in 0.1% Triton X-100, 5 mM EDTA in 1× PBS and centrifuged, and the supernatant was bound to glutathione sepharose 4B resin (Amersham Pharmacia Biotech). Beads were washed 4× in PBS and in HND buffer (10 mg/mL BSA, 20 mM HEPES pH 7.8, 50 mM NaCl, 5 mM DTT, and 0.1% NP-40). For the interaction assay, 20 μL of 35S-labeled in vitro transcribed and translated FOXO1, FOXO1-ΔDBD, or GFP was added to the beads with 400 μL of HND buffer. Beads were incubated overnight at 4°C, washed twice with HND buffer and twice with 0.1% NP-40 in PBS. Thirty microliters of twice Laemmli load buffer was added; the samples were boiled and electrophoresed on a 10% SDS-polyacrylamide gel. One-fourth of the 35S-labeled in vitro transcribed-translated product was loaded onto the gel as input.
Western blot analysis
Whole cell extracts from adenoviral-infected LβT2 cells were analyzed by Western for PITX1, c-JUN, c-FOS, and FOXO1. Cells were washed twice with ice-cold PBS and then harvested by incubating in lysis buffer (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, complete protease inhibitor mixture pellet [Roche Molecular Biochemical] and phosphatase inhibitor mixture pellet [Roche]) for 10 minutes at 4°C. Lysates were centrifuged at 20 000g for 30 minutes. The protein concentration was determined by Bradford assay. An equal amount of protein per sample was loaded on a 10% SDS-PAGE gel. Proteins were resolved by electrophoresis and transferred onto a polyvinylidene difluoride membrane (Millipore). Membranes were blocked overnight in 5% nonfat milk and then incubated overnight at 4°C with goat antihuman PITX1 (1:500, sc-18922), rabbit antihuman c-JUN (1:1000; sc-1694), rabbit antihuman c-FOS (1:1000; sc-52), rabbit antihuman FOXO1 (1:1000; sc-11350), or rabbit antihuman β-Tubulin (1:3000, sc-9104) primary antibodies (Santa Cruz Biotechnology). Blots were incubated with an antirabbit or antigoat horseradish peroxidase-linked secondary antibody (1:10 000; Santa Cruz Biotechnology) and bands were visualized using the SuperSignal West Dura Substrate (Thermo Scientific). Densitometric analysis of band intensity was performed with ImageJ software (National Institutes of Health).
Results
Overexpression of FOXO1-CA and PI3K inhibition decreases basal and GnRH-induced Fshb mRNA levels
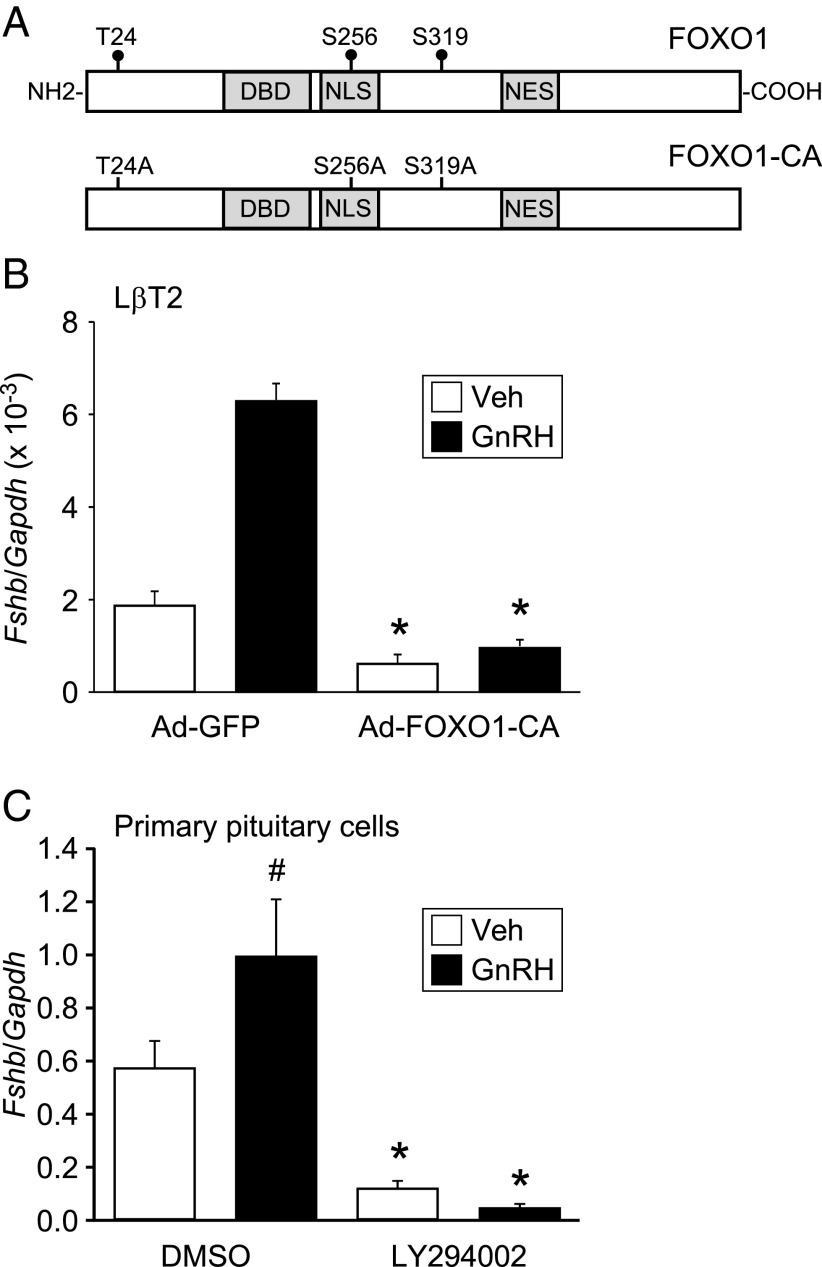
To ascertain whether the FOXO1 transcription factor can suppress Fshb gene expression, we transduced LβT2 cells with Ad expressing GFP or FOXO1-CA and measured Fshb mRNA levels using quantitative RT-PCR. FOXO1-CA is localized in the nucleus due to the inability of insulin/growth factor signaling to phosphorylate the mutated residues (Figure 1A). As observed previously (45), GnRH induced Fshb transcription by three-fold (Figure 1B). We also demonstrated that Fshb mRNA synthesis was significantly decreased in cells infected with Ad-FOXO1-CA vs Ad-GFP (Figure 1B). Basal Fshb mRNA levels were reduced by 67%, whereas GnRH-induced Fshb transcription was decreased by 84%. These results indicate that FOXO1 can suppress endogenous Fshb synthesis in the context of the native chromatin.
Figure 1.
Constitutively active FOXO1 and inhibition of PI3K reduces basal transcription and GnRH induction of Fshb mRNA in gonadotropes. (A) Diagram illustrating wild-type FOXO1 and FOXO1-CA (T24A/S256A/S319A). NES, nuclear export signal; NLS, nuclear localization signal. (B) LβT2 cells were transduced with Ad-GFP or Ad-FOXO1-CA for 6 hours, then switched to serum-free media. Twenty-four hours after adenoviral infection, cells were treated with 0.1% BSA vehicle (Veh) or 10 nM GnRH for 6 hours, as indicated. The results represent the mean ± SEM of three experiments performed in triplicate and are presented as amount of Fshb mRNA relative to Gapdh. *, Fshb transcription is significantly repressed by FOXO1-CA compared to GFP using Student's t test. (C) After overnight incubation in serum-free media, dispersed murine primary pituitary cells were pretreated with DMSO or 50 μM LY294002 for 1 hour, then treated with Veh or 30 nM GnRH along with DMSO or LY294002 for an additional 5 hours. The results represent the mean ± SEM of four experiments performed in triplicate and are presented as amount of Fshb mRNA relative to Gapdh. #, GnRH significantly increased Fshb mRNA levels compared to Veh using Student's t test; *, Fshb transcription is significantly repressed by LY294002 compared to DMSO using Student's t test.
We then determined whether repression of Fshb transcription could be observed in murine primary pituitary cells. Because there is no pharmacological activator of FOXO1, we used a PI3K inhibitor to mimic FOXO1-CA because inhibition of PI3K results in FOXO1 nuclear localization in gonadotropes (35). As shown in Figure 1C, treatment of dispersed pituitary cells with 30 nM GnRH resulted in a significant 1.7-fold induction of Fshb. Inhibition of PI3K with 50 μM LY294002 resulted in a substantial decrease in basal and GnRH-induced Fshb mRNA levels (79% and 95%, respectively). Although pharmacological inhibition of PI3K may affect multiple PI3K/AKT targets (46), these results demonstrate, for the first time, that inhibition of PI3K results in decreased basal and GnRH-induced Fshb transcription in murine primary pituitary cells, potentially through increased nuclear localization of FOXO1.
FOXO1 suppresses basal transcription and GnRH induction of murine Fshb-luc
Since FOXO1-CA suppressed endogenous Fshb mRNA levels, FOXO1 or FOXO1-CA was overexpressed in LβT2 cells to determine whether FOXO1 regulates the Fshb promoter. Initially, we determined the cellular localization of pcDNA3 Flag human FOXO1 and pcDNA3 Flag human FOXO1-CA transiently transfected in LβT2 cells and incubated in serum-free media. As shown in Figure 2A, FOXO1 is predominantly localized in the cytoplasm with some nuclear localization, whereas FOXO1-CA is localized in the nucleus. We then tested whether FOXO1 transfected into LβT2 cells could induce transcription from a minimal promoter containing a characterized FBE. FOXO1 increased transcription of four copies of a consensus FBE linked to a luc reporter (4xFBE-luc) (Figure 2B). In contrast, when 200 ng FOXO1 or FOXO1-CA was transiently transfected with the −1000 murine Fshb promoter linked to a luc reporter (mFshb-luc), we observed repression of Fshb synthesis (Figure 2C). More specifically, basal Fshb transcription was decreased by 50% and 99%, respectively (Figure 2D), whereas GnRH-induced Fshb was reduced by 63% and 29%, respectively (Figure 2E).
Figure 2.
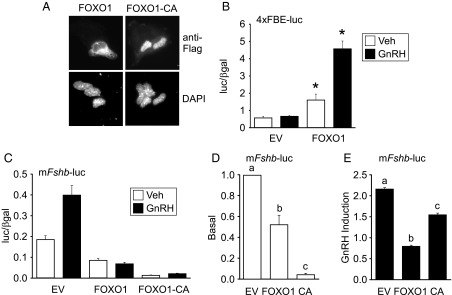
FOXO1 suppresses basal transcription and GnRH induction of the murine Fshb-luc in LβT2 cells. (A) The pcDNA3 Flag human FOXO1 and the pcDNA3 Flag human FOXO1-CA expression vectors were transfected into LβT2 cells and then incubated overnight in serum-free media. Immunofluorescence was performed with a mouse anti-Flag primary antibody and an Alexa488-conjugated goat antimouse secondary antibody. 4′,6-diamidino-2-phenylindole (DAPI) was used as a nuclear marker. Representative images were obtained using a Nikon Eclipse TE2000-U inverted fluorescence microscope at 60× magnification. (B) The 4xFBE-luc reporter was transiently transfected into LβT2 cells along 200 ng of pcDNA3 empty vector (EV) or FOXO1 expression vectors, as indicated. After overnight incubation in serum-free media, cells were treated for 6 hours with 0.1% BSA vehicle (Veh) or 10 nM GnRH. The results represent the mean ± SEM of three experiments performed in triplicate and are presented as luc/β-gal. *, induction by FOXO1 is significantly different from the empty vector using Student's t test. (C–E) The −1000 murine Fshb-luc reporter was transfected into LβT2 cells along with EV, FOXO1, or FOXO1-CA (CA), as indicated. After overnight incubation in serum-free media, cells were treated for 6 hours with Veh or 10 nM GnRH. The results represent the mean ± SEM of three experiments performed in triplicate and are presented as luc/β-gal (C), basal transcription relative to EV (D), or fold GnRH induction relative to the vehicle control (E). The different lowercase letters indicate that Fshb-luc transcription is significantly repressed by FOXO1 or FOXO1-CA compared to EV using one-way ANOVA followed by Tukey's HSD post-hoc test.
FOXO1 suppresses transcription of human FSHB-luc
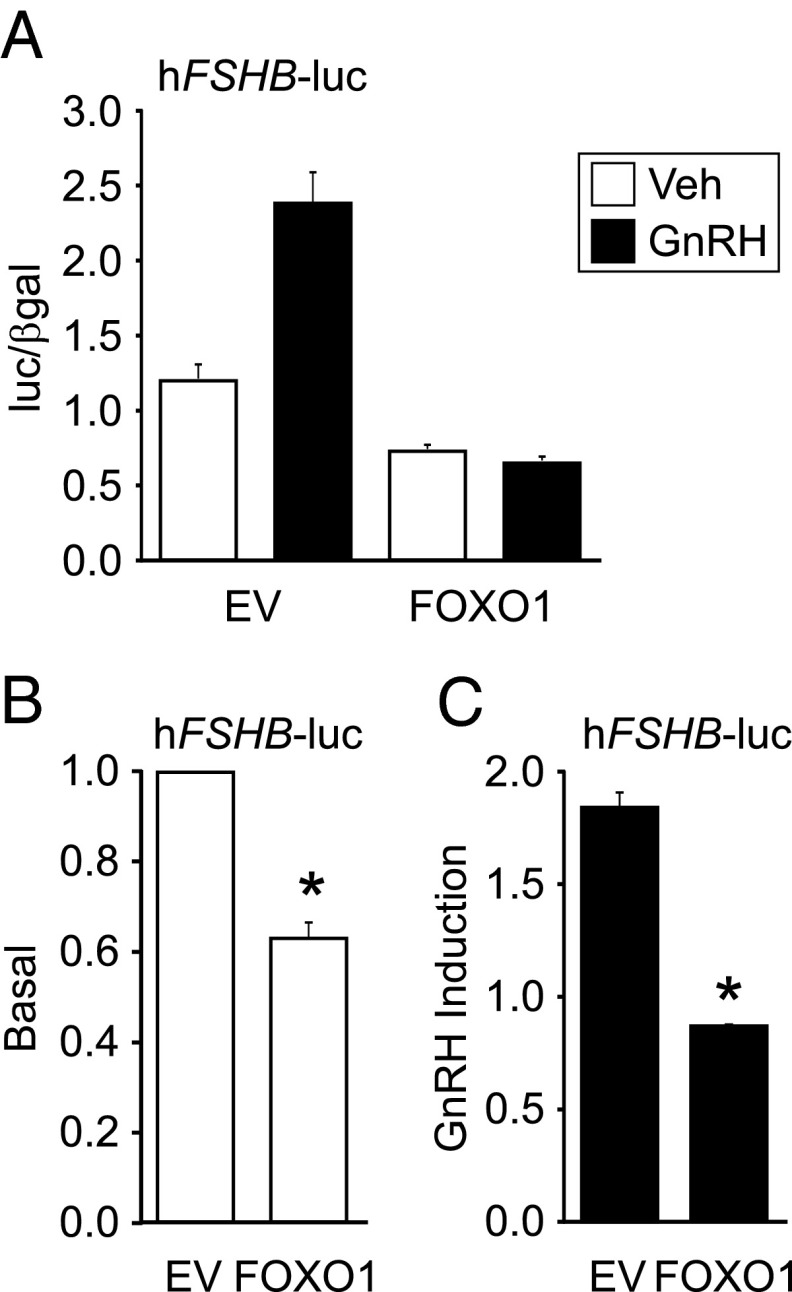
Given that the proximal murine and human FSHB promoters are highly conserved (8), we hypothesized that FOXO1 would have a similar repressive effect on human FSHB gene expression as on murine Fshb. When 200 ng of FOXO1 was transiently transfected with the −1028/+7 human FSHB promoter linked to a luciferase reporter (hFSHB-luc), we observed repression of basal and GnRH-induced FSHB synthesis (Figure 3, A–C). Basal FSHB transcription was reduced 38% by FOXO1, whereas GnRH-induced FSHB gene expression was reduced by 52%.
Figure 3.
FOXO1 suppresses basal transcription and GnRH induction of human FSHB-luc. (A–C) The −1028/+7 human FSHB-luc reporter was transfected into LβT2 cells along with pcDNA3 empty vector (EV) or FOXO1, as indicated. After overnight incubation in serum-free media, cells were treated for 6 hours with 0.1% BSA vehicle (Veh) or 10 nM GnRH. The results represent the mean ± SEM of three experiments performed in triplicate and are presented as luc/β-gal (A), basal transcription relative to empty vector (B), or fold GnRH induction relative to the vehicle control (C). *, FSHB-luc transcription is significantly repressed by FOXO1 compared to EV using Student's t test.
FOXO1 suppression maps to the proximal Fshb promoter
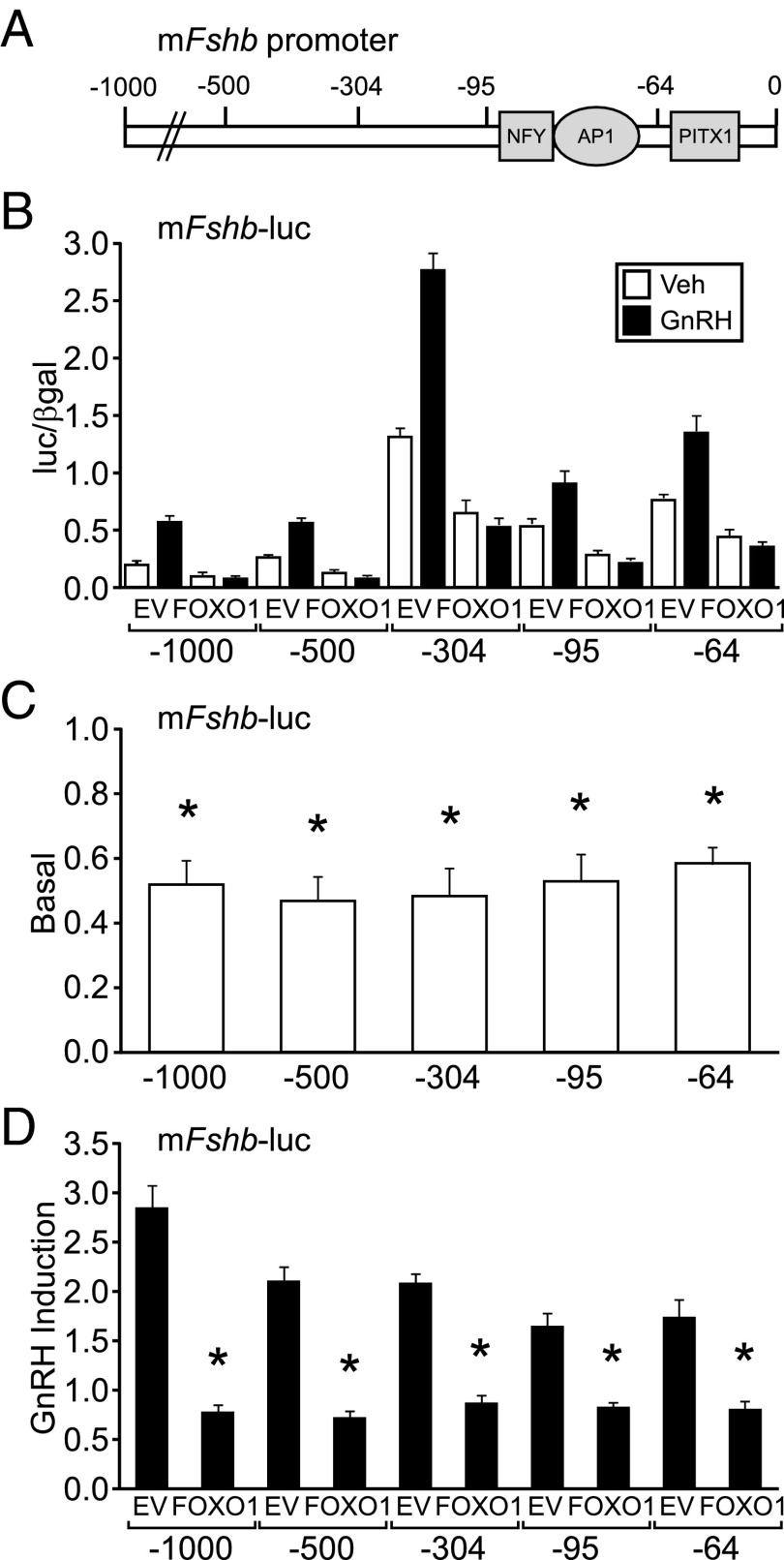
Since the proximal Fshb promoter contains PITX1, NFY, and AP-1 binding elements necessary for basal and GnRH induction of Fshb (Figure 4A), we determined which regions of the Fshb promoter were required for FOXO1 suppression using 5′ truncation analysis of the promoter. Basal transcription and GnRH induction were measured using −1000, −500, −304, −95, and −64 Fshb-luc reporter plasmids (Figure 4B). FOXO1 suppression of basal Fshb gene expression and GnRH induction still occurred with the −64 Fshb-luc (Figure 4, C and D), suggesting that elements present in the 64 bp upstream of the transcription start site are sufficient for FOXO1 repression of basal and GnRH-induced Fshb transcription.
Figure 4.
FOXO1 suppression maps to the proximal Fshb promoter. (A) Diagram illustrating the location of binding elements for NFY, AP1, and PITX1 transcription factors on the murine Fshb Promoter. (B–D) The −1000, −500, −304, −95, and −64 murine Fshb-luc reporters were transiently transfected into LβT2 cells along with pcDNA3 empty vector (EV) or FOXO1, as indicated. After overnight incubation in serum-free media, cells were treated for 6 hours with 0.1% BSA vehicle (Veh) or 10 nM GnRH. The results represent the mean ± SEM of three experiments performed in triplicate and are presented as luc/β-gal (B), basal transcription relative to empty vector (C), or fold GnRH induction relative to the vehicle control (D). *, Fshb-luc transcription is significantly repressed by FOXO1 compared to EV using one-way ANOVA followed by Tukey's HSD post-hoc test.
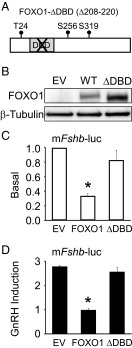
The DNA-binding domain of FOXO1 is required for suppression of Fshb synthesis
To determine whether FOXO1 repression requires the FOXO1 DBD, we tested whether a DNA-binding deficient FOXO1, termed FOXO1-ΔDBD (Δ208–220) (Figure 5A), could elicit a repressive response. As a control for the level of protein expression, we showed that FOXO1 and FOXO1-ΔDBD are stably expressed when transfected into LβT2 cells (Figure 5B). We then demonstrated that while overexpression of FOXO1 reduced Fshb gene expression, the FOXO1-ΔDBD did not alter basal Fshb synthesis (Figure 5C) or suppress GnRH-induced Fshb gene expression (Figure 5D). These results indicate that the DBD of FOXO1 is necessary for FOXO1 repression of Fshb basal transcription and GnRH induction on the Fshb promoter and suggest that FOXO1 exerts an effect by either binding to the Fshb promoter or indirectly through FOXO1 DBD interaction with other factors.
Figure 5.
DNA binding domain of FOXO1 is required to suppress basal and GnRH-induced Fshb gene expression. (A) Diagram illustrating FOXO1-ΔDBD (Δ208–220). (B) LβT2 cells were transfected with pcDNA3 empty vector (EV), pcDNA3-FOXO1 (WT), or pcDNA3-FOXO1-ΔDBD for 6 hours, then switched to serum-free media. Twenty-four hours after transfection, the cells were harvested. Western blot analysis was performed on whole cell extracts using FOXO1 and β-Tubulin primary antibodies and a horseradish peroxidase–linked secondary antibody. A representative image is shown. (C–D) The −1000 murine Fshb-luc reporter was transfected into LβT2 cells along with EV, FOXO1, or FOXO1-ΔDBD, as indicated. After overnight incubation in serum-free media, cells were treated for 6 hours with 0.1% BSA or 10 nM GnRH. The results represent the mean ± SEM of three experiments performed in triplicate and are presented as basal transcription relative to empty vector (C) or fold GnRH induction relative to the vehicle control (D). *, Fshb-luc transcription is significantly repressed by FOXO1 compared to EV or FOXO1-ΔDBD using one-way ANOVA followed by Tukey's HSD post-hoc test.
FOXO1 does not bind to the proximal Fshb promoter via a high-affinity binding site
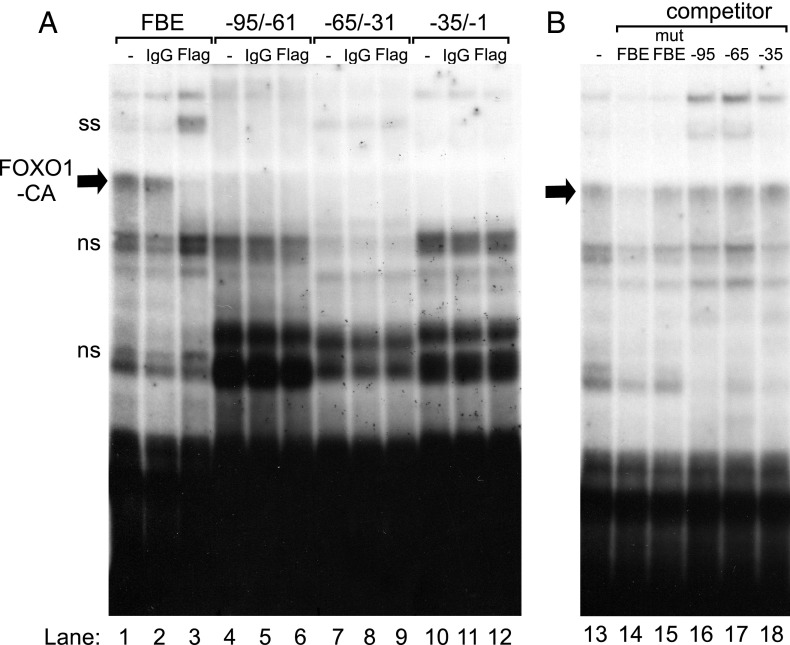
Because the FOXO1 repression mapped to the proximal Fshb promoter and required the FOXO1 DBD, we performed EMSA to determine whether FOXO1 could bind to the proximal promoter in vitro. Three 35-mer oligonucleotide probes were designed to span the −95/−1 region relative to the transcription start site. Flag-FOXO1-CA, synthesized with TnT rabbit reticulocyte lysate, bound to an oligonucleotide probe containing a consensus FBE (Figure 6A, lane 1) but not to probes encompassing the −95/−1 region of the Fshb promoter (Figure 6A, lanes 4, 7, and 10). TnT Flag-FOXO1 also bound to a consensus FBE (data not shown). To identify which complex contained the Flag-FOXO1-CA bound to the FBE, we supershifted the complex with an anti-Flag antibody (Figure 6A, lane 3) but not with control IgG (Figure 6A, lane 2). This complex was not present when rabbit reticulocyte lysate containing the pcDNA3 empty vector was used (data not shown). This complex also showed evidence of self-competition (Figure 6B, lane 14) but did not compete with a mutated consensus FBE (Figure 6B, lane 15). Incubation with oligos encompassing the −95/−1 region of the Fshb promoter did not result in competition (Figure 6B, lanes 16–18). These results suggest that, in contrast to the consensus FBE, FOXO1 does not bind to the proximal Fshb promoter via a high affinity binding site.
Figure 6.
FOXO1 does not bind directly to the proximal Fshb promoter. TnT Flag-FOXO1-CA was incubated with a consensus FBE, −95/−61, −65/31, or −35/−1 Fshb probes and tested for complex formation in EMSA. (A) FOXO1-CA-DNA complex on the FBE is shown in lane 1, while anti-Flag supershift is shown in lane 3 and IgG control in lane 2. The FOXO1-CA-DNA complex (arrow), antibody supershift (ss), and nonspecific binding of proteins (ns) are indicated on the left of the gel. (B) Self-competition with excess cold FBE is shown in lane 14, lack of competition with mutant FBE, −95/−61, −65/31, or −35/−1 Fshb oligos in lanes 15–18.
FOXO1 suppression of basal Fshb transcription may involve PITX1
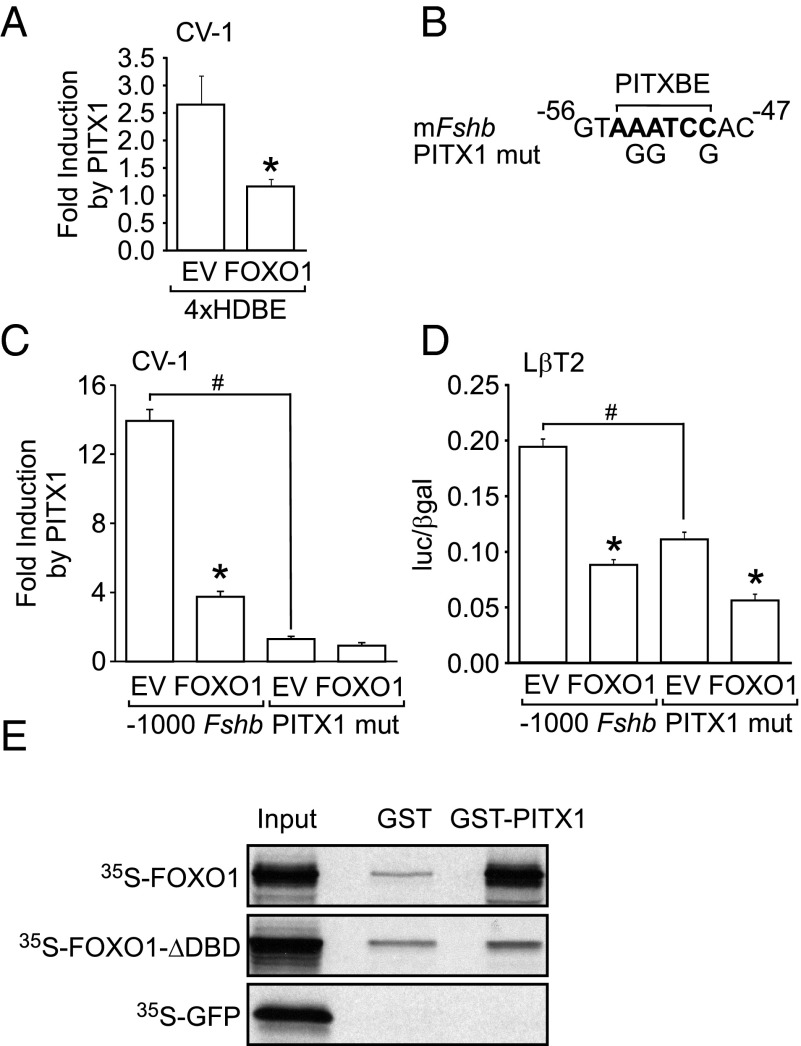
Since the −64 Fshb-luc containing the PITX1 binding site was sufficient to elicit FOXO1 suppression, we tested whether Fshb transcription due to PITX1 overexpression was reduced by FOXO1. We performed these experiments in CV-1 cells lacking PITX1 because LβT2 cells contain PITX1 (39, 40). We first tested whether a multimer of a bicoid-related HDBE was sufficient to elicit the FOXO1 suppressive effect. As shown in Figure 7A, PITX1 induction of the 4xHDBE was significantly decreased by FOXO1. We then showed that overexpression of PITX1 resulted in a 14-fold induction of Fshb gene expression, which was reduced 73% by FOXO1 (Figure 7C). In addition, we tested whether the PITX1 binding element in the proximal Fshb promoter shown in Figure 7B was necessary for the PITX1 induction. Our results demonstrated that the PITX1 binding element was absolutely required for PITX1 induction of Fshb gene expression in CV-1 cells (Figure 7C). We also tested whether the PITX1 binding element was required in LβT2 cells. As previously reported (11), mutation of the PITX1 binding element reduced basal Fshb transcription (Figure 7D) but had no effect on GnRH induction (data not shown). Interestingly, overexpression of FOXO1 reduced transcription of Fshb from the mutated promoter by 49% compared to 64% for the wild-type promoter (Figure 7D), suggesting that the PITX1 binding element is not necessary for basal FOXO1 suppression of Fshb. In addition, mutation of the PITX1 binding element did not prevent FOXO1 suppression of GnRH-induced Fshb transcription (data not shown).
Figure 7.
FOXO1 suppression of basal Fshb transcription may involve PITX1. (A) The 4xHDBE-luc reporter was transiently transfected into CV-1 cells along with pcDNA3 empty vector (EV), FOXO1, and PITX1, as indicated. The results represent the mean ± SEM of three experiments performed in triplicate and are presented as fold PITX1 induction relative to pcDNA3 empty vector. *, transcription is significantly repressed by FOXO1 compared to EV using Student's t test. (B) Diagram illustrating the mutations in the PITX1 binding element (PITXBE) in the murine Fshb promoter. (C–D) The −1000 Fshb-luc reporter and the Fshb PITX1 mutant (mut) were transiently transfected into CV-1 (C) or LβT2 (D) cells along with EV, FOXO1, and PITX1, as indicated. The results represent the mean ± SEM of three experiments performed in triplicate and are presented as fold PITX1 induction relative to pcDNA empty vector (C) or luc/β-gal (D). *, Fshb-luc transcription is significantly repressed by FOXO1 compared to EV using Student's t test; #, PITX1 mut was significantly repressed compared to the wild-type Fshb promoter. (E) GST interaction assays were performed using bacterially expressed GST-fusion proteins (indicated above each lane) and 35S-labeled in vitro transcribed and translated FOXO1, FOXO1-ΔDBD, and GFP (indicated on the left of the panels). GFP was used as a negative control. The GST-fusion proteins included GST alone and GST-PITX1. One-quarter of the protein used in the interaction assay was loaded in the lane marked input. The experiment was repeated several times with the same results and a representative experiment is shown.
FOXO1 interacts with PITX1
Because FOXO1 can suppress PITX1 induction of Fshb and the 4xHDBE in CV-1 cells, we investigated whether FOXO1 can physically interact with PITX1. We tested whether FOXO1 or DNA-binding deficient FOXO1 interacts with PITX1 by incubating a GST-PITX1 fusion protein with in vitro transcribed and translated 35S-labeled FOXO1 or FOXO1-ΔDBD in pull-down experiments. As shown in Figure 7E, there was minimal interaction between the GST-PITX1 fusion protein and the negative control (35S-GFP) or with GST alone incubated with FOXO1 or FOXO1-ΔDBD. In addition, FOXO1 bound to PITX1 while there was no detectable interaction between FOXO1-ΔDBD and PITX1, indicating that the interaction between FOXO1 and PITX1 requires the FOXO1 DBD.
FOXO1 reduces Pitx1 mRNA and protein levels
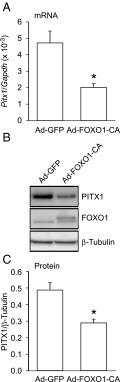
Given that the FOXO1 DBD is required for suppression of Fshb but FOXO1 does not appear to bind to the proximal Fshb promoter, we hypothesized that FOXO1 may modulate Fshb transcription indirectly by regulating the amount of PITX1 in the cells. In these experiments, LβT2 cells were transduced with Ad-GFP or Ad-FOXO1-CA and Pitx1 mRNA levels were measured using quantitative RT-PCR (Figure 8A). FOXO1-CA significantly decreased Pitx1 mRNA levels by 57%. We also tested whether the decrease in Pitx1 mRNA levels translated into lower PITX1 protein levels. As shown in Figure 8, B and C, FOXO1-CA transduction of LβT2 cells resulted in a 40% reduction in PITX1 protein levels. These results suggest that FOXO1 may suppress Fshb transcription indirectly through regulation of Pitx1 mRNA and protein levels.
Figure 8.
FOXO1 suppression of basal Fshb transcription may involve FOXO1 regulation of Pitx1 mRNA and protein levels. LβT2 cells were transduced with Ad-GFP or Ad-FOXO1-CA for 6 hours, then switched to serum-free media. Twenty-four hours after adenoviral infection, cells were harvested for total mRNA (A) or protein (B–C). (A) The results represent the mean ± SEM of seven experiments performed in triplicate and are presented as amount of Pitx1 mRNA relative to Gapdh. *, Pitx1 transcription is significantly repressed by FOXO1-CA compared to GFP using Student's t test. (B–C) Western blot analysis was performed on whole cell extracts using PITX1, FOXO1, and β-Tubulin primary antibodies and a horseradish peroxidase–linked secondary antibody. A representative image is shown (B) or graph representing the mean ± SEM of four experiments presented as the amount of PITX1 protein relative to β-Tubulin (C). *, PITX1 protein levels are significantly decreased by FOXO1-CA compared to GFP using Student's t test.
FOXO1 does not regulate c-Fos or c-Jun mRNA and protein levels
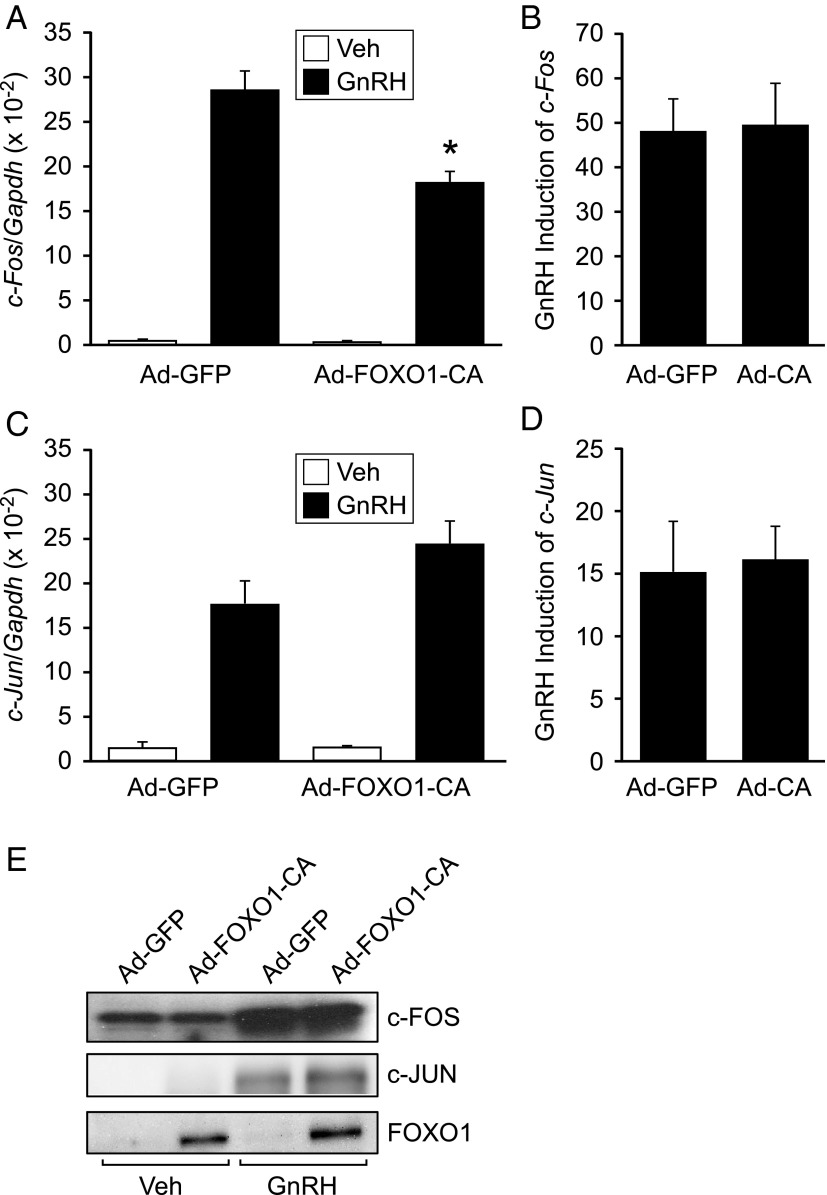
Since GnRH induction of the murine Fshb promoter involves the intermediate early genes, c-FOS and c-JUN, that comprise the transcriptional AP1 complex, we determined whether FOXO1-CA overexpression alters production of these two genes. LβT2 cells were transduced with Ad-GFP or Ad-FOXO1-CA and c-fos and c-jun mRNA levels were measured using quantitative RT-PCR (Figure 9, A and C). Similar to previous reports (47, 48), 1 hour of GnRH treatment substantially increased c-fos and c-jun mRNA levels (Figure 9, B and D). However, FOXO1-CA overexpression had little or no effect on c-fos and c-jun mRNA. We also demonstrated that there was no effect of FOXO1-CA overexpression on c-FOS and c-JUN protein levels (Figure 9E).
Figure 9.
FOXO1 suppression of GnRH-induced Fshb transcription does not involve regulation of c-Fos or c-Jun mRNA or protein levels. LβT2 cells were transduced with Ad-GFP or Ad-FOXO1-CA for 6 hours, then switched to serum-free media. Twenty-four hours after adenoviral infection, cells were treated with 0.1% BSA vehicle (Veh) or 10 nM GnRH for 1 hour, as indicated. Cells were harvested for total mRNA (A–D) or protein (E). (A–D) The results represent the mean ± SEM of three experiments performed in triplicate and are presented as amount of c-Fos mRNA relative to Gapdh (A), fold GnRH induction of c-Fos relative to Veh (B), c-Jun mRNA relative to Gapdh (C), fold GnRH induction of c-Jun relative to Veh (D). *, transcription is significantly repressed by FOXO1-CA compared to GFP using Student's t test. (E) Western blot analysis was performed on whole cell extracts using c-FOS, c-JUN, and FOXO1 primary antibodies and a horseradish peroxidase–linked secondary antibody. A representative image is shown.
FOXO1 Suppression of GnRH-induced Fshb transcription involves two regions of the proximal Fshb promoter at −50/−41 and −30/−21
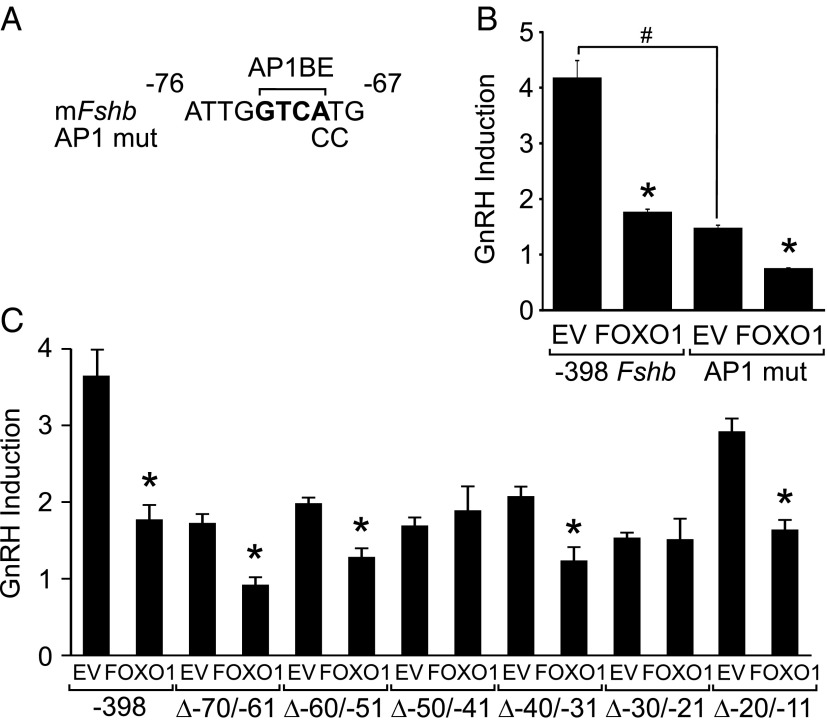
Because GnRH induction involves an AP1 binding element in the proximal Fshb promoter, we tested whether this site was necessary for FOXO1 suppression of Fshb transcription. Mutation of the AP1 binding element shown in Figure 10A reduced GnRH-induced Fshb transcription (Figure 10B), as previously reported (16). However, overexpression of FOXO1 reduced transcription of Fshb from the mutated promoter by 50% compared to 59% for the wild-type promoter (Figure 10B), indicating that the AP1 binding element is not required for FOXO1 suppression. Because FOXO1 suppression of GnRH-induced Fshb transcription occurred within the −64 Fshb promoter lacking the AP1 binding element, we sought to map which regions of the proximal promoter were required for the suppression. Ten base-pair deletions of the Fshb promoter ranging from −70/−61 to −20/−11 within the −398 Fshb-luc were compared to wild-type −398 Fshb-luc. Basal transcription of Fshb from either wild-type or mutated promoters was suppressed by FOXO1 (data not shown), suggesting that none of the 10-bp regions individually were required for FOXO1 suppression of basal Fshb synthesis. Interestingly, GnRH induction of Fshb was reduced with the mutated promoters compared to the wild-type promoter (Figure 10C), indicating that GnRH induction involves other regions of the Fshb promoter in addition to the AP1 binding element. Moreover, FOXO1 was unable to repress GnRH induction of Fshb from promoters containing deletions at −50/−41 or −30/−21 (Figure 10C), indicating that these regions may play a role in FOXO1 suppression of Fshb gene expression induced by GnRH.
Figure 10.
FOXO1 suppression of GnRH-induced Fshb transcription involves two elements in the proximal Fshb promoter. (A) Diagram illustrating the mutations in the AP1 binding element (AP1BE) in the murine Fshb promoter. (B) The −398 Fshb-luc reporter and the Fshb AP1 mutation (mut) were transiently transfected into LβT2 cells along with pcDNA3 empty vector (EV) or FOXO1, as indicated. After overnight incubation in serum-free media, cells were treated for 6 hours with 0.1% BSA vehicle or 10 nM GnRH. The results represent the mean ± SEM of three experiments performed in triplicate and are presented as fold GnRH induction relative to the vehicle control. *, Fshb-luc transcription is significantly repressed by FOXO1 compared to EV using Student's t test; #, the AP1 mut was significantly repressed compared to the wild-type Fshb promoter. (C) The −398 Fshb-luc reporter and 10-bp deletions ranging from −70/−61 to −20/−11 were transiently transfected into LβT2 cells along with EV or FOXO1, as indicated. After overnight incubation in serum-free media, cells were treated for 6 hours with 0.1% BSA or 10 nM GnRH. The results represent the mean ± SEM of three experiments performed in triplicate and are presented as fold GnRH induction relative to the vehicle control. *, Fshb-luc transcription is significantly repressed by FOXO1 compared to EV using one-way ANOVA followed by Tukey's HSD post-hoc test.
Discussion
Our studies suggest that the FOXO1 transcription factor may regulate fertility through modulation of gonadotropin β-subunit gene expression in pituitary gonadotrope cells. Overexpression of wild-type or FOXO1-CA greatly diminished basal and GnRH-induced Fshb mRNA levels and Fshb-luc expression in LβT2 cells (Figures 1 and 2), similarly to the repressive effect of FOXO1 on Lhb gene expression (35). Although wild-type FOXO1 is predominantly localized in the cytoplasm under serum-free media conditions, the level of nuclear FOXO1 appears to be sufficient to suppress Fshb transcription (Figure 2) (35). Our results demonstrating that inhibition of PI3K results in increased nuclear localization of FOXO1 (35) and decreased basal and GnRH induction of Fshb mRNA in primary pituitary cells (Figure 1) provides support for the idea that FOXO1 is a repressor of Fshb transcription in a more physiological context. The suppression of Fshb and Lhb transcription by FOXO1 is not due to a global repressive mechanism because reporter plasmids containing the insulin response element from the IGFBP1 gene (35) or a consensus FBE (Figure 2) are induced by FOXO1 in LβT2 cells while endogenous c-fos and c-jun mRNA levels are not altered by adenoviral transduction of LβT2 cells with FOXO1-CA (Figure 9). In addition, FOXO1 repression of transcription is not an artifact of the reporter plasmids because substantial repression of endogenous Fshb and Lhb mRNA was observed in LβT2 cells transduced with FOXO1-CA (Figure 1) (35).
At this time, it remains to be determined whether FOXO1 regulates transcription of the gonadotropin β- subunits in human gonadotropes. There is one report that FOXO1 colocalized with Lhb-staining gonadotropes in a human male pituitary section (49). Our results also suggest that FOXO1 regulation of gonadotropin levels may occur in humans because FOXO1 suppressed transcription of a reporter plasmid containing either the human FSHB or the LHB promoter in LβT2 cells (Figure 3) (35).
Nevertheless, how does FOXO1 regulate gonadotropin gene expression via transcriptional repression? Although there are several similarities between FOXO1 repressive effects on the Fshb and Lhb promoters, there are promoter-specific mechanisms that are also required for FOXO1 suppression of the individual promoters. First, the DBD of FOXO1 is required for the repression of both genes (Figure 5) (35), although the specific residues involved have not yet been mapped and it is unknown whether residues required for the repression differ from those necessary for binding to DNA. Second, there is no evidence that FOXO1 binds directly to either the Fshb or Lhb proximal promoters (Figure 6) (35). It is possible that FOXO1 binds to these promoters with such low affinity that it cannot be detected in a gel-shift assay, although FOXO1 binding to a consensus FBE is not altered by 100-fold excess of cold oligonucleotides from the proximal Fshb or Lhb promoters. Thus, our data support the idea that FOXO1 elicits a repressive effect on the gonadotropin β-subunit promoters through indirect mechanisms involving the FOXO1 DBD. This idea is not altogether unexpected because FOXO1 has been shown to interact with other proteins such as SMADs through its DBD (50).
Given that FOXO1 has been reported to interact physically with other proteins to regulate transcription of specific target genes (51), it is plausible that FOXO1 represses basal transcription and GnRH induction of the Fshb and Lhb promoters through specific protein-protein interactions with transcription factor(s) or cofactors necessary for gonadotropin synthesis. Although basal transcription of Fshb involves several factors, including LHX3 and NFY, FOXO1 suppression of basal Fshb gene expression mapped to the −64/−1 region of the Fshb promoter (Figure 4), suggesting that FOXO1 interaction with factors binding to this region (eg, PITX1) may play a role in the repression of transcription. In comparison, FOXO1 suppression of basal Lhb transcription mapped to the −150/−87 region of the Lhb promoter, which contains PITX1 and steroidogenic factor 1 (SF1) binding sites (35). Because the PITX1 binding site in the proximal FSHB promoter is conserved among mammals and mutation of this site in the murine and human promoters reduced FSHB transcription (11), it is interesting to speculate that FOXO1 suppression of basal FSHB transcription via PITX1 may also be conserved.
The hypothesis that PITX1 plays a role in FOXO1 suppression of the Fshb promoter is supported by our results demonstrating that PITX1 induction of the Fshb promoter in CV-1 cells is repressed by FOXO1, that PITX1 induction of a consensus HDBE is also decreased by FOXO1, and that PITX1 physically interacts with FOXO1 (Figure 7). The fact that the FOXO1 DBD is required for the interaction between FOXO1 and PITX1 is also in agreement with our data showing that the FOXO1 DBD is necessary for FOXO1 repression of Fshb. Although this is the first report of a physical interaction between PITX1 and FOXO1, PITX2 interacts with another member of the forkhead transcription factor family, FOXC1, via the C-terminal activation domain of FOXC1 and the homeodomain of PITX2 (52). Interestingly, the PITX1 binding element in the proximal Fshb promoter was not required for FOXO1 suppression in LβT2 cells, suggesting that the repression can occur without PITX1 binding to DNA. It is noteworthy that PITX1 was reported to activate the rat or human Fshb promoter even when the PITX1 binding element was mutated, suggesting that PITX1 activation can occur through protein-protein interactions with factors such as SF1, in addition to direct DNA binding (11, 53–55). Altogether, our studies imply that FOXO1 interaction with PITX1 may be important for suppression of basal Fshb and possibly Lhb transcription.
In addition to the protein-protein interaction between FOXO1 and PITX1, which may regulate Fshb transcription, it is possible that FOXO1 modulates Fshb mRNA levels through regulation of Pitx1 mRNA and protein levels. Both PITX1/2 are expressed in Rathke's pouch at embryonic day 10.5 and in the developing anterior lobe at e11.5 (56, 57). PITX1/2 are also highly expressed in adult gonadotrope cells and have been reported to regulate transcription of the α-subunit, Fshb, Lhb, and the GnRH receptor (39, 57). Although knockout of PITX1 had little effect on pituitary gland development, lack of PITX1/2 resulted in a more severe defect in pituitary growth and differentiation than the PITX2 knockout alone (58). These studies indicate that PITX1/2 may have distinct and overlapping functions in pituitary development and gonadotropin hormone production in the adult. Interestingly, little is known about the factors that regulate PITX1/2 expression during pituitary development and in the adult (59). Because overexpression of FOXO1 in LβT2 cells resulted in a significant reduction of Pitx1 mRNA and PITX1 protein levels (Figure 8), it is possible that FOXO1 may repress Pitx1 synthesis through regulation of the Pitx1 promoter.
GnRH responsiveness of the Fshb and Lhb promoters occurs due to the induction and binding of gene-specific transcription factors such as AP1 to the Fshb promoter and early growth response protein 1 (EGR1) to the Lhb promoter. Our results suggest that FOXO1 suppression of GnRH-induced Fshb gene expression does not occur through regulation of AP1. Transduction of LβT2 cells with FOXO1-CA did not alter c-Fos and c-Jun mRNA or protein levels (Figure 9). In addition, FOXO1 suppression of the GnRH induction of Fshb still occurred on the −64 Fshb promoter lacking the characterized AP1 binding element (Figure 4). We also demonstrated that the AP1 binding element was not necessary for FOXO1 to elicit a repressive effect on Fshb transcription (Figure 10). These results are not that surprising, given that GnRH responsiveness on the murine Fshb promoter appears to involve additional regions outside the AP1 binding element (16). This is also illustrated by the fact that the −64 Fshb promoter was still responsive to GnRH, that mutation of the AP1 element reduced, but did not abolish, the GnRH induction, and that 10-bp deletions of the proximal Fshb promoter from −70/−61 to −30/−21 all resulted in reduction of GnRH responsiveness. Although other factors involved in the GnRH induction of murine Fshb have not yet been identified, our studies highlight two regions between −50/−41 and −30/−21 that are required for FOXO1 suppression (Figure 10) and suggest that factors other than AP1 are targeted by FOXO1. It should be noted that the −50/−41 region partially overlaps the PITX1 binding element, whereas the −30/−21 region overlaps the TATA box.
In contrast to the Fshb promoter, FOXO1 suppression of GnRH responsiveness on the Lhb promoter mapped to the −87/−1 region, which contains an EGR1 binding element (35). Our previous studies also demonstrated that FOXO1 suppressed Lhb transcription induced by EGR1 overexpression in LβT2 cells. Overexpression of FOXO1 with PITX1 or SF1 also suppressed EGR1 induction of Lhb in CV-1 cells. These results provide support for the idea that an interaction between FOXO1 and EGR1 may be responsible for FOXO1 suppression of GnRH-induced Lhb transcription, whereas FOXO1 interactions with as yet unknown factors are responsible for FOXO1 suppression of GnRH-induced Fshb gene expression.
In summary, we demonstrate that FOXO1 suppresses basal transcription and GnRH induction of the Fshb gene, potentially through protein-protein interactions between FOXO1 and transcription factors recruited to the proximal Fshb promoter such as PITX1 as well as FOXO1 regulation of Pitx1 mRNA and protein levels. If FOXO1 is regulated by metabolic hormone signaling in gonadotropes and represses Fshb and Lhb transcription, as our studies suggest, then it may prove to be an important factor in the regulation of gonadotropin production in situations of caloric insufficiency or excess. Further studies are necessary to comprehend how interactions among FOXO1, PITX1, EGR1, and other factors contribute to the regulation of Fshb and Lhb transcription in pituitary gonadotropes. Investigation of the regulation of PITX1 synthesis may also provide insight into the mechanisms of FOXO1 repression. Additional studies will also help elucidate how FOXO1 integrates input from multiple hormonal signaling pathways to regulate reproduction under favorable or adverse environmental conditions.
Acknowledgments
The authors thank Djurdjica Coss, Pamela Mellon, Kellie Breen, and Scott Kelley for helpful discussions and critical reading of the manuscript.
This work was supported by National Institutes of Health Grants K01 DK080467 and R01 HD067448 to V.G.T. as well as T32 HD007203 and F32 HD074414 to D.V.S. This work was also supported by a pilot and feasibility grant from the UCSD/University of California, Los Angeles Diabetes Research Center (P30 DK063491), by National Institute of Child Health and Human Development /National Institutes of Health through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, and by a UCSD Academic Senate Health Sciences Research Grant.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ad
- adenoviral
- AP1
- activator protein 1
- β-gal
- β-galactosidase
- CA
- constitutively active
- DBD
- DNA-binding domain
- DMSO
- dimethylsulfoxide
- EGR1
- early growth response protein 1
- FBE
- forkhead binding element
- FBS
- fetal bovine serum
- GFP
- green fluorescent protein
- GST
- Glutathione S-transferase
- HDBE
- homeodomain binding element
- HSD
- honestly significant difference
- LHX
- LIM homeobox gene
- luc
- luciferase
- NFY
- nuclear transcription factor Y
- PITX
- paired-like homeodomain transcription factor
- SF1
- steroidogenic factor 1
- UCSD
- University of California, San Diego.
References
- 1. Apter D. Development of the hypothalamic-pituitary-ovarian axis. Ann N Y Acad Sci. 1997;816:9–21 [DOI] [PubMed] [Google Scholar]
- 2. Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22(6):764–786 [DOI] [PubMed] [Google Scholar]
- 3. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201–204 [DOI] [PubMed] [Google Scholar]
- 4. Lamminen T, Jokinen P, Jiang M, Pakarinen P, Simonsen H, Huhtaniemi I. Human FSHβ subunit gene is highly conserved. Mol Hum Reprod. 2005;11(8):601–605 [DOI] [PubMed] [Google Scholar]
- 5. Ortolano GA, Haisenleder DJ, Dalkin AC, et al. Follicle-stimulating hormone β subunit messenger ribonucleic acid concentrations during the rat estrous cycle. Endocrinology. 1988;123(6):2946–2948 [DOI] [PubMed] [Google Scholar]
- 6. Halvorson LM, Weiss J, Bauer-Dantoin AC, Jameson JL. Dynamic regulation of pituitary follistatin messenger ribonucleic acids during the rat estrous cycle. Endocrinology. 1994;134(3):1247–1253 [DOI] [PubMed] [Google Scholar]
- 7. Chand AL, Harrison CA, Shelling AN. Inhibin and premature ovarian failure. Hum Reprod Update. 2010;16(1):39–50 [DOI] [PubMed] [Google Scholar]
- 8. Bernard DJ, Fortin J, Wang Y, Lamba P. Mechanisms of FSH synthesis: what we know, what we don't, and why you should care. Fertil Steril. 2010;93(8):2465–2485 [DOI] [PubMed] [Google Scholar]
- 9. West BE, Parker GE, Savage JJ, et al. Regulation of the follicle-stimulating hormone β gene by the LHX3 LIM-homeodomain transcription factor. Endocrinology. 2004;145(11):4866–4879 [DOI] [PubMed] [Google Scholar]
- 10. Jacobs SB, Coss D, McGillivray SM, Mellon PL. Nuclear factor Y and steroidogenic factor 1 physically and functionally interact to contribute to cell-specific expression of the mouse Follicle-stimulating hormone-β gene. Mol Endocrinol. 2003;17(8):1470–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamba P, Khivansara V, D'Alessio AC, Santos MM, Bernard DJ. Paired-like homeodomain transcription factors 1 and 2 regulate follicle-stimulating hormone β-subunit transcription through a conserved cis-element. Endocrinology. 2008;149(6):3095–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thackray VG, Mellon PL, Coss D. Hormones in synergy: regulation of the pituitary gonadotropin genes. Mol Cell Endocrinol. 2010;314(2):192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dalkin AC, Burger LL, Aylor KW, et al. Regulation of gonadotropin subunit gene transcription by gonadotropin- releasing hormone: measurement of primary transcript ribonucleic acids by quantitative reverse transcription-polymerase chain reaction assays. Endocrinology. 2001;142(1):139–146 [DOI] [PubMed] [Google Scholar]
- 14. Vasilyev VV, Pernasetti F, Rosenberg SB, et al. Transcriptional activation of the ovine follicle-stimulating hormone-β gene by gonadotropin-releasing hormone involves multiple signal transduction pathways. Endocrinology. 2002;143(5):1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. GnRH activates ERK1/2 leading to the induction of c-fos and LHβ protein expression in LβT2 cells. Mol Endocrinol. 2002;16(3):419–434 [DOI] [PubMed] [Google Scholar]
- 16. Coss D, Jacobs SB, Bender CE, Mellon PL. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem. 2004;279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu F, Ruiz MS, Austin DA, Webster NJ. Constitutively active Gq impairs gonadotropin-releasing hormone-induced intracellular signaling and luteinizing hormone secretion in LβT2 cells. Mol Endocrinol. 2005;19(8):2074–2085 [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Fortin J, Lamba P, et al. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone β-promoter activity. Endocrinology. 2008;149(11):5577–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ciccone NA, Lacza CT, Hou MY, et al. A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone β gene. Mol Endocrinol. 2008;22(8):1908–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weiss J, Guendner MJ, Halvorson LM, Jameson JL. Transcriptional activation of the follicle-stimulating hormone β-subunit gene by activin. Endocrinology. 1995;136(5):1885–1891 [DOI] [PubMed] [Google Scholar]
- 21. Coss D, Mellon PL, Thackray VG. A FoxL in the Smad house: activin regulation of FSH. Trends Endocrinol Metab. 2010;21:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adashi EY, Hsueh AJ, Yen SS. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology. 1981;108(4):1441–1449 [DOI] [PubMed] [Google Scholar]
- 23. Kanematsu T, Irahara M, Miyake T, Shitsukawa K, Aono T. Effect of insulin-like growth factor I on gonadotropin release from the hypothalamus-pituitary axis in vitro. Acta Endocrinol. 1991;125(2):227–233 [DOI] [PubMed] [Google Scholar]
- 24. Ogura K, Irahara M, Kiyokawa M, et al. Effects of leptin on secretion of LH and FSH from primary cultured female rat pituitary cells. Eur J Endocrinol. 2001;144(6):653–658 [DOI] [PubMed] [Google Scholar]
- 25. Tezuka M, Irahara M, Ogura K, et al. Effects of leptin on gonadotropin secretion in juvenile female rat pituitary cells. Eur J Endocrinol. 2002;146(2):261–266 [DOI] [PubMed] [Google Scholar]
- 26. Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380(Pt 2):297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem. 2003;278(38):35959–35967 [DOI] [PubMed] [Google Scholar]
- 28. Anderson MJ, Viars CS, Czekay S, Cavenee WK, Arden KC. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics. 1998;47(2):187–199 [DOI] [PubMed] [Google Scholar]
- 29. Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512 [DOI] [PubMed] [Google Scholar]
- 30. Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20(2):126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13(12):1079–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274(24):16741–16746 [DOI] [PubMed] [Google Scholar]
- 33. Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120(4):963–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brosens JJ, Wilson MS, Lam EW. FOXO transcription factors: from cell fate decisions to regulation of human female reproduction. Adv Exp Med Biol. 2009;665:227–241 [DOI] [PubMed] [Google Scholar]
- 35. Arriola DJ, Mayo SL, Skarra DV, Benson CA, Thackray VG. FOXO1 transcription factor inhibits luteinizing hormone β gene expression in pituitary gonadotrope cells. J Biol Chem. 2012;287(40):33424–33435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27(16):2276–2288 [DOI] [PubMed] [Google Scholar]
- 37. Graham KE, Nusser KD, Low MJ. LβT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to activin A. J Endocrinol. 1999;162:R1–R5 [DOI] [PubMed] [Google Scholar]
- 38. Pernasetti F, Vasilyev VV, Rosenberg SB, et al. Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology. 2001;142(6):2284–2295 [DOI] [PubMed] [Google Scholar]
- 39. Tremblay JJ, Lanctôt C, Drouin J. The pan-pituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol Endocrinol. 1998;12(3):428–441 [DOI] [PubMed] [Google Scholar]
- 40. Jiang Q, Jeong KH, Horton CD, Halvorson LM. Pituitary homeobox 1 (Pitx1) stimulates rat LHβ gene expression via two functional DNA-regulatory regions. J Mol Endocrinol. 2005;35(1):145–158 [DOI] [PubMed] [Google Scholar]
- 41. Rosenberg SB, Mellon PL. An Otx-related homeodomain protein binds an LHβ promoter element important for activation during gonadotrope maturation. Mol Endocrinol. 2002;16:1280–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thackray VG, McGillivray SM, Mellon PL. Androgens, progestins and glucocorticoids induce follicle-stimulating hormone β-subunit gene expression at the level of the gonadotrope. Mol Endocrinol. 2006;20(9):2062–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang X, Gan L, Pan H, et al. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem. 2002;277(47):45276–45284 [DOI] [PubMed] [Google Scholar]
- 44. Zappavigna V, Sartori D, Mavilio F. Specificity of HOX protein function depends on DNA-protein and protein-protein interactions, both mediated by the homeo domain. Genes Dev. 1994;8(6):732–744 [DOI] [PubMed] [Google Scholar]
- 45. Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL. p38 mitogen-activated protein kinase is critical for synergistic induction of the FSHβ gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol Endocrinol. 2007;21(12):3071–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4(9):a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ely HA, Mellon PL, Coss D. GnRH Induces the c-Fos gene via phosphorylation of SRF by the calcium/calmodulin kinase II pathway. Mol Endocrinol. 2011;25(4):669–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lindaman LL, Yeh DM, Xie C, Breen KM, Coss D. Phosphorylation of ATF2 and interaction with NFY induces c-Jun in the gonadotrope. Mol Cell Endocrinol. 2013;365(2):316–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stavrou E. Regulation of FOXO transcription factors by gonadotropin-releasing hormone. Doctoral Thesis, University of Edinburgh, Edinburgh, Scotland: 2011 [Google Scholar]
- 50. Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117(2):211–223 [DOI] [PubMed] [Google Scholar]
- 51. Monsalve M, Olmos Y. The complex biology of FOXO. Curr Drug Targets. 2011;12(9):1322–1350 [DOI] [PubMed] [Google Scholar]
- 52. Berry FB, Lines MA, Oas JM, et al. Functional interactions between FOXC1 and PITX2 underlie the sensitivity to FOXC1 gene dose in Axenfeld-Rieger syndrome and anterior segment dysgenesis. Hum Mol Genet. 2006;15(6):905–919 [DOI] [PubMed] [Google Scholar]
- 53. Suszko MI, Antenos M, Balkin DM, Woodruff TK. Smad3 and Pitx2 cooperate in stimulation of FSHβ gene transcription. Mol Cell Endocrinol. 2008;281(1–2):27–36 [DOI] [PubMed] [Google Scholar]
- 54. Tremblay JJ, Marcil A, Gauthier Y, Drouin J. Ptx1 regulates SF-1 activity by an interaction that mimics the role of the ligand-binding domain. EMBO J. 1999;18(12):3431–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zakaria MM, Jeong KH, Lacza C, Kaiser UB. Pituitary homeobox 1 activates the rat FSHβ (rFSHβ) gene through both direct and indirect interactions with the rFSHβ gene promoter. Mol Endocrinol. 2002;16(8):1840–1852 [DOI] [PubMed] [Google Scholar]
- 56. Lanctôt C, Gauthier Y, Drouin J. Pituitary homeobox 1 (Ptx1) is differentially expressed during pituitary development. Endocrinology. 1999;140(3):1416–1422 [DOI] [PubMed] [Google Scholar]
- 57. Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol. 2005;19(7):1893–1903 [DOI] [PubMed] [Google Scholar]
- 58. Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development. 2002;129(2):329–337 [DOI] [PubMed] [Google Scholar]
- 59. Goodyer CG, Tremblay JJ, Paradis FW, et al. Pitx1 in vivo promoter activity and mechanisms of positive autoregulation. Neuroendocrinology. 2003;78(3):129–137 [DOI] [PubMed] [Google Scholar]