Abstract
GH is a potent anabolic and metabolic factor that binds its cell surface receptor (GHR), activating the GHR-associated tyrosine kinase, Janus kinase 2, which phosphorylates and activates the latent transcription factor, signal transducer and activator of transcription 5 (STAT5). Some GH actions are mediated by the elaboration of IGF-1, which exerts effects by binding and activating the heterotetrameric tyrosine kinase growth factor receptor, IGF-1R. In addition to this GH-GHR-IGF-1-IGF-1R scheme, we have demonstrated in primary osteoblasts and in islet β-cells that then deletion or silencing of IGF-1R results in diminished GH-induced STAT5 phosphorylation, suggesting that the presence of IGF-1R may facilitate GH signaling. In this study, we explore potential roles for protein tyrosine phosphatase activity in modulating GH-induced signaling, comparing conditions in which IGF-1R is present or diminished. We confirm that in mouse primary osteoblasts harboring loxP sites flanking the IGF-1R gene, infection with an adenovirus that expresses the Cre recombinase results in IGF-1R deletion and diminished acute GH-induced STAT5 phosphorylation. Furthermore, we present a new model of IGF-1R silencing, in which expression of short hairpin RNA directed at IGF-1R greatly reduces IGF-1R abundance in LNCaP human prostate cancer cells. In both models, treatment with a chemical inhibitor of protein tyrosine phosphatase-1B (PTP-1B), but not one of src homology region 2 domain-containing phosphotase-1 (SHP-1) and SHP-2, reverses the loss of GH-induced STAT5 phosphorylation in cells lacking IGF-1R but has no effect in cells with intact IGF-1R. Furthermore, expression of either a dominant-negative PTP-1B or the PTP-1B-interacting inhibitory protein, constitutive photomorphogenesis 1, also rescues acute GH-induced STAT5 signaling in IGF-1R-deficient cells but has no effect in IGF-1R replete cells. By expressing a substrate-trapping mutant PTP-1B, we demonstrate that tyrosine phosphorylated Janus kinase-2 is a PTP-1B substrate only in cells lacking IGF-1R. Collectively, our data suggest that IGF-1R positively regulates acute GH signaling by preventing access of PTP-1B activity to Janus kinase 2 and thereby preventing PTP-1B-mediated suppression of GH-induced STAT5 activation.
GH, produced and secreted largely by the anterior pituitary, promotes growth and exerts multiple important metabolic effects (1, 2). GH signals at the cellular level by interaction with the cell surface GH receptor (GHR), a single membrane-spanning protein that binds GH in the extracellular domain, causing activation of the intracellular GHR-associated tyrosine kinase, Janus kinase (JAK)-2, and phosphorylation of the latent cytoplasmic transcription factor, signal transducer and activator of transcription (STAT)-5 as well as other proteins (3, 4). Among the important genes regulated by GH is that encoding IGF-1, which is produced in and secreted from GH target tissues in part in response to GH-induced STAT5 activation (5, 6). IGF-1, in turn, can cause anabolic effects by interacting with its signaling receptor (the type I IGF-1R), an intrinsic tyrosine kinase growth factor receptor composed of two α- and two β-chains in a disulfide-linked assemblage that binds IGF-1 in its extracellular domain and signals via the intracellular portion of its β-chain (7–9).
The degree to which GH action is mediated by IGF-1 is imperfectly understood. The original somatomedin hypothesis of GH action was articulated more than half a century ago and suggested that GH triggers the hepatic production of IGF-1 (somatomedin-C), which then exerts endocrine growth-promoting actions at target tissues (10, 11). Aspects of this hypothesis have stood the test of time, but it is increasingly understood that IGF-1 emanating from sources other than liver can importantly regulate growth and that GH may also act directly in an IGF-1-independent fashion to exert some anabolic and metabolic effects (12–15). Indeed, some studies have suggested that GH and IGF-1 may act collaboratively at the level of cellular signaling, perhaps by virtue of the formation of a GH-induced complex that includes GHR, JAK2, and IGF-1R (16–18). Our recent studies in primary mouse osteoblasts indicate that the deletion of IGF-1R renders cells less sensitive to GH in terms of acute STAT5 activation and subsequent IGF-1 gene expression (19). Because GH does not promote IGF-1R activation or phosphorylation in the osteoblast system, these observations suggest that the presence of IGF-1R positively influences GH's ability to activate JAK2 and/or negatively regulates the activity of a protein tyrosine phosphatase(s) (PTP) that suppresses GH-induced JAK2-mediated STAT5 activation.
The involvement of PTPs in GH signaling has been explored in previous work. Several PTPs have been suggested as regulators of GH-induced STAT5 activity (20–29). In most instances, PTP activity is believed to negatively regulate GH action by tyrosine dephosphorylation of a proximal element(s) of the GHR-mediated GH signaling cascade. In the current study, we explore the impact of PTP activity in conferring the desensitization to GH that arises with deficiency of IGF-1R. We use two separate systems to approach these issues: 1) our mouse osteoblast system in which Cre-mediated excision of the loxP-flanked IGF-1R gene renders the cells IGF-1R deficient; and 2) a new complementary system in which human LNCaP prostate cancer cells are depleted of IGF-1R by RNA interference methods. In both systems, our data suggest that the reduction of IGF-1R abundance allows PTP-1B to exert negative effects on GH-induced STAT5 phosphorylation and thereby desensitize cells to GH. Our data suggest novel mechanisms whereby the level of IGF-1R may modulate acute GH signaling.
Materials and Methods
Materials
Recombinant human GH was kindly provided by Eli Lilly & Co. The PTP-1B inhibitor, 3-(3,5-dibromo-4-hydroxy-benzoyl)-2-ethyl-benzofuran-6-sulfonicacid-(4-(thiazol-2-ylsulfamyl)-phenyl)-amide, and the src homology region 2 domain-containing phosphatace (SHP)-1/2 inhibitor (NSC-87877) were from Calbiochem. Other routine reagents were from Sigma-Aldrich Co, unless otherwise noted. Cell culture media, α-MEM, and RPMI 1640, were obtained from Cellgro-Mediatech, and fetal bovine serum was from Atlanta Biologicals.
Antibodies
Polyclonal anti-STAT5 and anti-IGF-Rα antibodies were purchased from Santa Cruz Biotechnology, Inc. Polyclonal antiphospho-STAT5 was purchased from Cell Signaling Technology. Monoclonal antiphosphotyrosine antibody 4G10 was obtained from Upstate Biotechnology. Monoclonal anti-PTP-1B antibody was obtained from Biovision. Polyclonal anti-GHR (anti-GHRcyt-AL47) against the intracellular domain of GH receptor (30) and anti-JAK2 (anti-JAK2AL33) (31) were previously described.
Cells and cell culture
Osteoblasts were isolated from calvaria of newborn Igf1rflox/flox mice as described previously (19, 32). In general, a single newborn mouse calvaria preparation produced a yield of primary osteoblasts sufficient for approximately 10 samples of 1 × 106 cells each in the experiments outlined below. To achieve a reduction of IGF-1R in LNCaP cells, we prepared pRNAU6.1/Neo-shIGF-1R, a plasmid encoding a short hairpin RNA (shRNA) that targets human IGF-1R at a 19-bp sequence (ACGCCAATAAGTTCGTCCA) beginning at nt3425 of its mRNA. LNCaP cells were transfected with pRNAU6.1/Neo-shIGF-1R or the empty vector as a control. Stably transfected pools (LNCaP-vec vs LNCaP-shIGF-1R) were selected by growth in medium containing G418 (1 mg/mL).
Generation of recombinant adenoviruses
Adenoviruses encoding the catalytically-inactive, dominant-negative mutant PTP-1B-C215S (Ad-PTP-1B-DN [33, 34]) and the catalytically inactive, substrate-trapping mutant PTP-1B-D181A (Ad-PTP-1B-D/A [35, 36]) were generated as previously described for other adenoviruses (19). In brief, appropriate cDNAs were subcloned into the pAdTrack shuttle vector, which was linearized with PacI and cotransformed with pAdEASY (a helper plasmid) into Escherichia coli BJ5138 cells. Colonies harboring recombinants were selected by virtue of kanamycin resistance. Linearized recombinant plasmid was transfected into human embryonic kidney-293 cells and high-titer viral stock was obtained. Adenovirus-constitutive photomorphogenesis 1 (COP1) (encoding wild-type COP1) and Ad-E-COP1 (encoding an E3 ligase deficient COP1) have been described (37).
Adenovirus preparation and infection
Adenoviruses were amplified by infecting human embryonic kidney-293 cells (19, 38) Cells were harvested when cytopathic effects became apparent. After lysis by five freeze/thaw cycles, cell debris was pelleted by centrifugation and subject to further cesium chloride purification procedures. Concentration of purified virus was calculated by measuring the value of OD260. For deletion of the IGF-1R, osteoblasts containing floxed IGF-1R alleles were cultured to 70% confluence and then, in the absence of serum, were infected with an adenovirus encoding Cre recombinase (Ad-Cre) or, as a control, an adenovirus encoding green fluorescent protein (Ad-GFP), at 800 multiplicity of infection (MOI), unless otherwise noted (19, 32). After 1 hour, culture medium containing 10% fetal bovine serum was added, and the cells were allowed to recover for 48 hours prior to stimulation. Coinfection with other adenoviruses in osteoblasts was at an MOI of 400 and accomplished as previously described (19). Adenoviral infection of LNCaP cells was at an MOI of 400.
Cell starvation, cell stimulation, and protein extraction
As previously described (19), serum starvation of primary osteoblasts (∼1 × 106 cells per 6 cm2 dish/sample) was accomplished by substitution of 0.5% (wt/vol) bovine serum albumin (fraction V; Roche Molecular Biochemicals) for serum in their respective culture media for 16–20 hours prior to experiments. Stimulations were performed at 37°C. The cells were stimulated in starvation medium and stimulations were terminated by washing the cells once and then harvesting by scraping in ice-cold PBS in the presence of 0.4 mM sodium orthovanadate. Pelleted cells were collected by brief centrifugation and solubilized for 30 minutes at 4°C in fusion lysis buffer (1% [vol/vol] Triton X-100, 150 mM NaCl, 10% [vol/vol] glycerol, 50 mM Tris-HCl [pH 8.0], 100 mM NaF, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 10 mM benzamidine, 10 μg/mL aprotinin). After centrifugation at 15 000 × g for 15 minutes at 4°C, the detergent extracts were electrophoresed under reducing conditions or subjected to immunoprecipitations, as indicated.
Immunoprecipitation, electrophoresis, and immunoblotting
Immunoprecipitation of proteins from detergent cell extracts in osteoblasts and LNCaP cells was accomplished as previously described (19, 39). Eluates were resolved by SDS-PAGE and immunoblotted, as below. For analysis without immunoprecipitation, proteins resolved by SDS-PAGE were transferred to Hybond ECL nitrocellulose membranes (Amersham Biosciences). The membranes were blocked with a buffer of 20 mM Tris-HCl (pH 7.6), 150 mM NaCl, and 0.1% (vol/vol) Tween 20 containing 2% (wt/vol) BSA and incubated with primary antibodies (0.5–1 μg/mL) as specified in each experiment. After three washes with a buffer of 20 mM Tris-HCl (pH 7.6), 150 mM NaCl, and 0.1% (vol/vol) Tween 20, the membranes were incubated with appropriate secondary antibodies (1:7500 dilution) and washed. The bound antibodies were detected with SuperSignal chemiluminescent substrate (Pierce Chemical Co). Membrane stripping was performed according to the manufacturer's suggestions (Amersham Biosciences).
Figure presentation and densitometric analysis
Immunoblots shown are in all instances representative of at least three experiments, as indicated in the figure legends. In some figures, irrelevant intervening lanes from original immunoblots have been cropped for clarity of presentation. In these cases, a space is maintained in which intervening lanes were cropped. In all cases, only data from the same original blots are incorporated in figures with consistent brightness/contrast adjustment made across each blot. Immunoblots were obtained digitally using a Biospectrum 500 imaging system (UVP, LLC). Densitometric quantitation of immunoblots was performed using the ImageJ 1.30 program (developed by W. S. Rasband, Research Services Branch, National Institute of Mental Health). Pooled data from several experiments are displayed as mean ± SE. In each experiment, the maximum signal was considered as 100%. The significance of differences (P value) of pooled results was estimated using paired t tests.
Results and Discussion
IGF-1R deletion in osteoblasts results in reduced GH-induced STAT5 tyrosine phosphorylation that is reversed by the general tyrosine phosphatase inhibitor, sodium orthovanadate
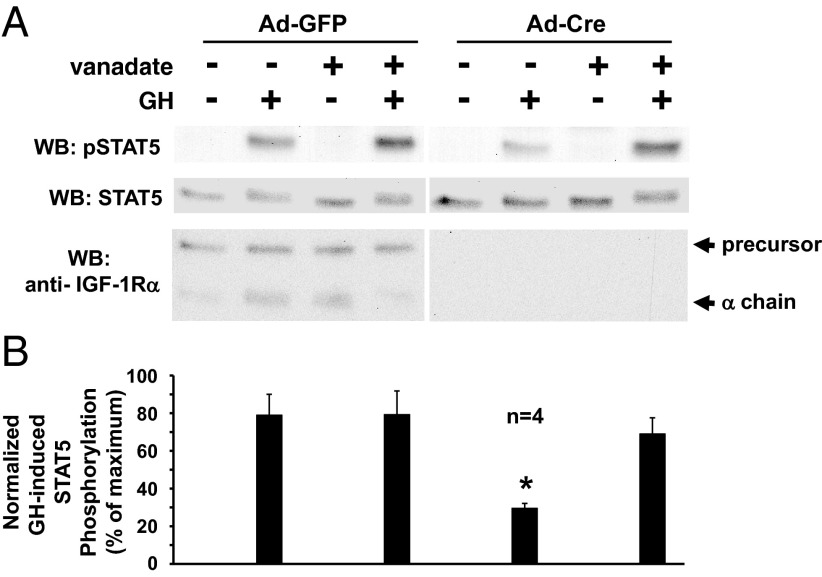
We previously demonstrated that the deletion of IGF-1R in osteoblasts causes dramatic desensitization to GH for acute STAT5 activation and downstream STAT5-dependent gene activation (19, 32). For these studies, we have used mice that harbor lox-P sites flanking both IGF-1R alleles and procedures for harvesting calvarial osteoblasts from newborn pups and exposing these primary osteoblasts in vitro to infection with an adenovirus driving the expression of the Cre recombinase (Ad-Cre) to delete the IGF-1R gene (19, 40). When infected with a control adenovirus that drives the expression of green fluorescent protein (Ad-GFP) instead of Cre, serum-starved osteoblasts responded robustly to GH (10 min; 250 ng/mL) (Figure 1A). When infected with Ad-Cre, the level of IGF-1R protein, as assessed by immunoblotting of the IGF-1Rα chain, was dramatically reduced and, consistent with our previous findings, GH-induced STAT5 phosphorylation was markedly diminished compared with the Ad-GFP-infected cells (Figure 1A). (We note that Ad-Cre infection of nonfloxed osteoblasts has no significant effect on GH signaling [19] and that Ad-GFP-infected IGF-1R-floxed osteoblasts behave no differently from the noninfected osteoblasts in terms of GH response [Gan et al, submitted for publication]).
Figure 1.
IGF-1R deletion in osteoblasts results in reduced GH-induced STAT5 tyrosine phosphorylation that is reversed by the general tyrosine phosphatase inhibitor, sodium orthovanadate. A and B, Primary osteoblasts were harvested from newborn mice bearing lox-P sites flanking both IGF-1R alleles. Osteoblasts were infected with Ad-Cre vs Ad-GFP, as indicated, as in Materials and Methods. Serum-starved cells were treated with (+) or without (−) sodium orthovanadate (200 μM; 1 hour) and then with GH (+; 250 ng/mL) or vehicle (−) for 10 minutes. Detergent cell extracts were resolved by SDS-PAGE and serially immunoblotted with anti-pSTAT5, anti-STAT5, and anti-IGF-1Rα. A, Representative immunoblots. WB, Western blotting. B, Densitometric quantitation of pSTAT5/STAT5 signals from GH-treated samples from four independent experiments (including that shown in A). In each experiment, the maximum signal was considered 100%. Data are plotted as mean ± SE. *, P < .03 for comparison of Ad-Cre-infected, nonvanadate-treated, GH-treated group to any of the other GH-treated groups. Arrows indicate the positions of the IGF-1R precursor and α-chain.
PTP activity has been demonstrated to have a role in GH action in several experimental systems (20–29). To investigate potential PTP influence in our osteoblast GH signaling system, we first examined the effects of treatment with sodium orthovanadate (vanadate), a general PTP inhibitor. Treatment with vanadate for 60 minutes prior to stimulation with GH or vehicle had no significant effect on the levels of either basal or GH-induced STAT5 tyrosine phosphorylation in osteoblasts infected with the control Ad-GFP (Figure 1, A and B). Similarly, vanadate had no effect on basal STAT5 phosphorylation in Ad-Cre-infected osteoblasts. However, in contrast to Ad-GFP-infected cells, in osteoblasts in which IGF-1R was deleted by Ad-Cre infection, vanadate treatment resulted in rescue of GH-induced STAT5 phosphorylation, bringing the level back to nearly that achieved in the Ad-GFP-infected (IGF-1R replete) cells. Thus, the diminished GH-induced STAT5 phosphorylation found upon deletion of IGF-1R was restored when vanadate was present. This suggests that at least part of the negative effect of the IGF-1R deletion on the GH-induced STAT5 signaling might be PTP activity dependent.
A PTP-1B inhibitor, but not an SHP1/2 inhibitor, reverses the diminished GH-induced STAT5 phosphorylation resulting from IGF-1R deletion in osteoblasts
Among the phosphatases implicated in GH action, several nontransmembrane PTPs have emerged as likely quite important. These include SHP-1 (24, 25), SHP-2 (26–29), PTP-H1 (20, 23), and PTP-1B (20–22). SHP-1 and SHP-2 are structurally related dual-SH2 domain-containing molecules that are important in the activation and desensitization of various cytokine and growth factor signaling pathways (41, 42). To determine whether SHP-1 or SHP-2 might be involved in the rescue effect of PTPs implied by the results with vanadate in Figure 1, we used the SHP-1/2 inhibitor, NSC-87877, which can potently inhibit these related phosphatases (43). Unlike vanadate treatment, however, incubation with the SHP-1/2 inhibitor (up to 50 μM) had no effect on the reduction in GH-induced STAT5 phosphorylation observed in Ad-Cre-infected osteoblasts (data not shown).
PTP-1B, like SHP-1 and SHP-2, is also an intracellular PTP; however, PTP-1B contains no SH2 domains and has a C-terminal portion of the protein that targets it to the cytoplasmic face of the endoplasmic reticulum (41, 44, 45). PTP-1B is an important modulator of growth factor signaling (41, 45); most notably, global deletion of PTP-1B in mice results in enhanced insulin sensitivity and resistance to high-fat diet-induced obesity (46). Among other effects, PTP-1B may regulate growth factor and, potentially cytokine, signaling by affecting the rate and degree of activated receptor down-regulation (47, 48).
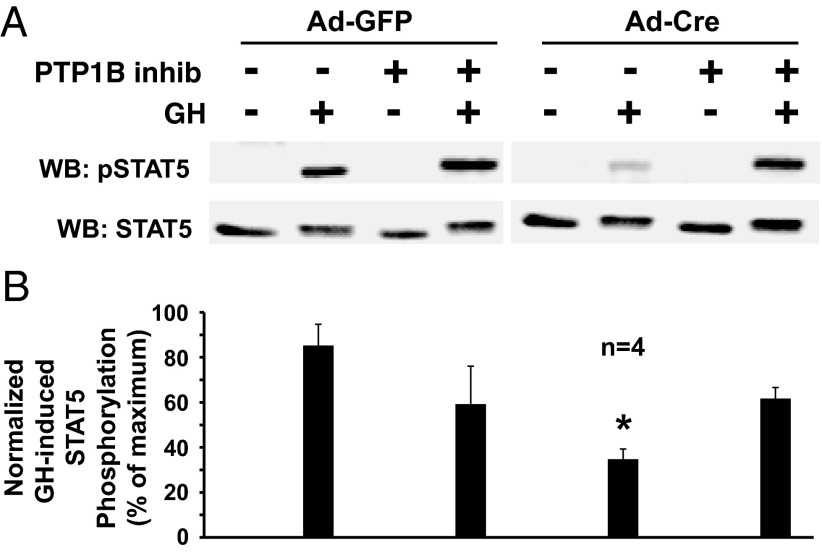
Given PTP-1B's implied roles in GH signaling (20–22), we first examined the effects of chemical inhibition of PTP-1B on GH-induced STAT5 phosphorylation in osteoblasts with intact vs deleted IGF-1R, using a selective, reversible, noncompetitive PTP-1B inhibitor, 3-(3,5-dibromo-4-hydroxy-benzoyl)-2-ethyl-benzofuran-6-sulfonicacid-(4-(thiazol-2-ylsulfamyl)-phenyl)-amide (49, 50) (Figure 2). As expected, treatment of the osteoblasts with Ad-Cre to delete the IGF-1R dramatically reduced GH-induced STAT5 phosphorylation, as demonstrated in the representative immunoblot in Figure 2A and the densitometric quantitation of four experiments (Figure 2B). Notably, similar to the results with vanadate, treatment with the PTP-1B inhibitor resulted in a substantial rescue of the GH-induced STAT5 phosphorylation in the Ad-Cre-treated cells. These data with a chemical inhibitor of PTP-1B suggest that this phosphatase known to be relevant for GH signaling is particularly able to lessen GH-induced STAT5 activation in the setting of IGF-1R deficiency.
Figure 2.
A PTP-1B inhibitor reverses the diminished GH-induced STAT5 phosphorylation resulting from IGF-1R deletion in osteoblasts. A and B, Primary osteoblasts were infected with Ad-Cre vs Ad-GFP, as indicated, as in Figure 1. Serum-starved cells were treated with (+) or without (−) the PTP-1B inhibitor, 3-(3,5-dibromo-4-hydroxy-benzoyl)-2-ethyl-benzofuran-6-sulfonicacid-(4-(thiazol-2-ylsulfamyl)-phenyl)-amide (10 μM; 1 hour) and then with GH (+; 250 ng/mL) or vehicle (−) for 10 minutes. Detergent cell extracts were resolved by SDS-PAGE and immunoblotted with anti-pSTAT5 and anti-STAT5. A, Representative immunoblots. WB, Western blotting. B, Densitometric quantitation of pSTAT5/STAT5 signals from GH-treated samples from four independent experiments (including that shown in A). In each experiment, the maximum signal was considered 100%. Data are plotted as mean ± SE. *, P < .01 for comparison of Ad-Cre-infected, non-PTP-1B inhibitor-treated, GH-treated group to either the Ad-GFP-infected, non-PTP-1B inhibitor-treated, GH-treated group or the Ad-Cre-infected, PTP-1B inhibitor-treated, GH-treated group.
shRNA-mediated silencing of IGF-1R in human LNCaP prostate cancer cells reduces GH-induced STAT5 phosphorylation in a PTP-1B inhibitor-sensitive fashion
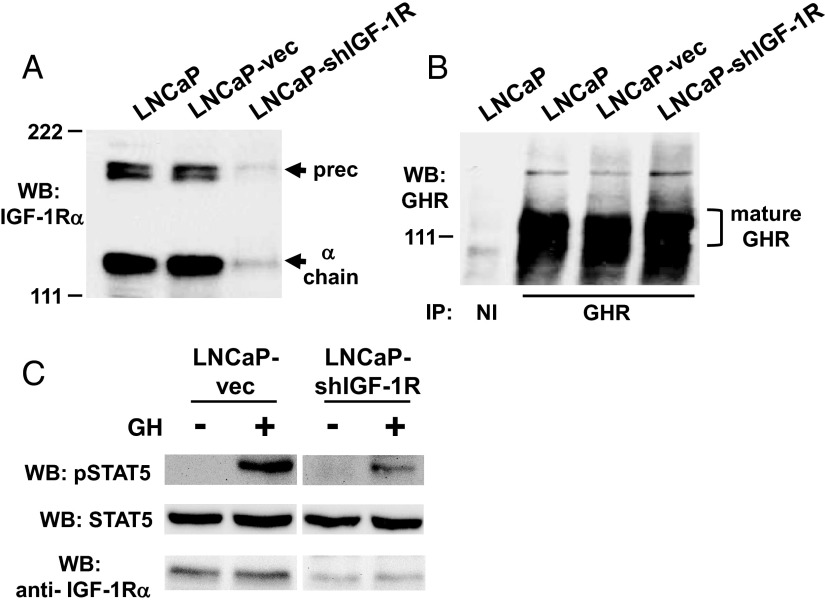
Our observations concerning the effects of the presence of IGF-1R on the strength of acute GH signaling have to date been limited to primary mouse osteoblasts harboring loxP-flanked IGF-1R genes (Reference 19, 32 and this manuscript) and cultured mouse islet β-cells (51). To pursue this further, we targeted a GH-responsive human cell line that bears endogenous GHR and IGF-1R to test the effect of IGF-1R silencing on GH signaling. For these studies, we used the human prostate cancer cell line, LNCaP, in which we previously demonstrated that GH acutely promotes endogenous GHR-mediated STAT5 phosphorylation (39). We transfected LNCaP cells with a plasmid-expressed shRNA targeting IGF-1R or, as a control, the plasmid vector alone and selected stable pools of transfected cells using the same selectable marker for each. As assessed by anti-IGF-1Rα chain immunoblotting, the IGF-1R abundance in LNCaP-vec (vector only transfected) cells was similar to that in the parental LNCaP cells, whereas LNCaP-shIGF-1R cells (shRNA transfected) manifested a markedly reduced IGF-1R level (Figure 3A). Although IGF-1R was substantially silenced in LNCaP-shIGF-1R cells, GHR abundance was similar in parental LNCaP, LNCaP-vec, and LNCaP-shIGF-1R cells (Figure 3B). Likewise, STAT5 (Figure 3C) and JAK2 (not shown) levels were unchanged in the cells in which the shIGF-1R was expressed. Notably, however, acute GH-induced STAT5 phosphorylation was substantially lessened in LNCaP-shIGF-1R cells compared with LNCaP-vec cells (∼50% reduction in GH induced STAT5 phosphorylation observed in LNCaP-shIGF-1R cells compared with LNCaP-vec cells [see Figure 4B below]). Thus, this RNA interference-based method of reducing IGF-1R content in human cancer cells resulted in desensitization to GH similar to that observed in the mouse osteoblast model, in which genetic (Cre-lox) methods were used to deplete IGF-1R.
Figure 3.
shRNA-mediated silencing of IGF-1R in LNCaP cells results in reduced GH-induced STAT5 phosphorylation. A–C, Characterization of pools of LNCaP human prostate cancer cells stably transfected with either vector only (LNCaP-vec) or a vector driving expression of an shRNA directed at IGF-1R (LNCaP-shIGF-1R). A, Equal amounts of protein from detergent cell extracts of parental LNCaP, LNCaP-vec, and LNCaP-shIGF-1R cells were resolved by SDS-PAGE and immunoblotted with anti-IGF-1Rα antibody. Arrows indicate the positions of the IGF-1R precursor and α-chain. Note the similarity of abundance of IGF-1R in LNCaP and LNCaP-vec cells but marked reduction in IGF-1R in LNCaP-shIGF-1R cells. WB, Western blotting. B, Equal amounts of protein from detergent cell extracts of parental LNCaP, LNCaP-vec, and LNCaP-shIGF-1R cells were immunoprecipitated with anti-GHR cytoplasmic domain antiserum or nonimmune (NI) serum. Eluates were resolved by SDS-PAGE and immunoblotted with anti-GHR. Bracket indicates the position of the mature GHR. Note similarity of GHR abundance among the three cell lines. C, Serum-starved LNCaP-vec and LNCaP-shIGF-1R cells were treated with GH (+; 250 ng/ml) or vehicle (−) for 15 minutes. Detergent cell extracts were resolved by SDS-PAGE and serially immunoblotted with anti-pSTAT5, anti-STAT5, and anti-IGF-1Rα. Note marked reduction in GH-stimulated STAT5 phosphorylation in LNCaP-shIGF-1R cells compared with LNCaP-vec cells.
Figure 4.
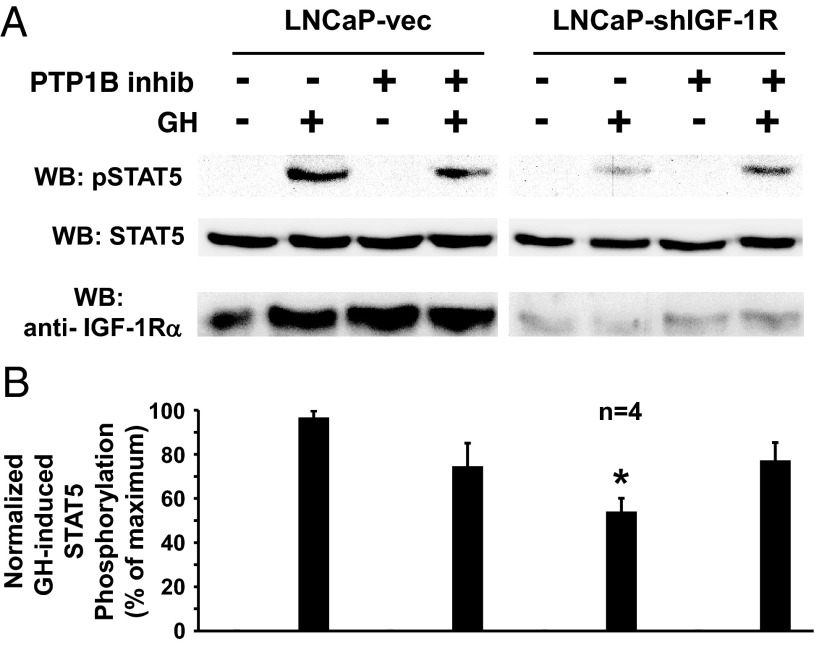
A PTP-1B inhibitor reverses the diminished GH-induced STAT5 phosphorylation resulting from IGF-1R silencing in LNCaP cells. A and B, Serum-starved LNCaP-vec and LNCaP-shIGF-1R cells were treated with (+) or without (−) the PTP-1B inhibitor, 3-(3,5-dibromo-4-hydroxy-benzoyl)-2-ethyl-benzofuran-6-sulfonicacid-(4-(thiazol-2-ylsulfamyl)-phenyl)-amide (10 μM; 1 hour) and then with GH (+; 250 ng/mL) or vehicle (−) for 10 minutes. Detergent cell extracts were resolved by SDS-PAGE and serially immunoblotted with anti-pSTAT5, anti-STAT5, and anti-IGF-1Rα. A, Representative immunoblots. WB, Western blotting. B, Densitometric quantitation of pSTAT5/STAT5 signals from GH-treated samples from four independent experiments (including that shown in A). In each experiment, the maximum signal was considered 100%. Data are plotted as mean ± SE. *, P < .01 for comparison of LNCaP-shIGF-1R, non-PTP-1B inhibitor-treated, GH-treated group with either the LNCaP-vec, non-PTP-1B inhibitor-treated, GH-treated group or the LNCaP-shIGF-1R, PTP-1B inhibitor-treated, GH-treated group.
We also examined the effect of PTP inhibitors on acute GH-induced STAT5 phosphorylation in LNCaP-vec vs LNCaP-shIGF-1R cells. As in the osteoblast system, the reduced GH-induced STAT5 phosphorylation in LNCaP-shIGF-1R cells was not affected by treatment with the SHP-1/2 inhibitor (not shown). However, treatment with the PTP-1B inhibitor largely reversed the reduced GH-induced STAT5 phosphorylation in LNCaP-shIGF-1R cells (Figure 4, A and B). These data strongly suggest that in both systems, the reduction in acute GH-induced STAT5 phosphorylation resulting from silencing or elimination of IGF-1R is related to enhanced PTP-1B regulatory activity.
A dominant-negative mutant PTP-1B rescues the diminished GH-induced STAT5 phosphorylation resulting from IGF-1R depletion
The data in Figures 2 and 4 with the chemical PTP-1B inhibitor suggest that this phosphatase activity may negatively regulate GH-induced activation of STAT5 in the setting of either relative or absolute deficiency of IGF-1R. Indeed, the most compelling demonstrations of the role of PTP-1B in regulation of GH signaling have related to GH action in cells and tissues, such as liver, that express relatively low levels of IGF-1R (21). To further investigate PTP-1B's role in modulating GH signaling in the setting of IGF-1R deficiency, we pursued an independent method of PTP-1B inhibition. Ad-DN-PTP-1B is an adenovirus programmed to direct expression of a mutated form of PTP-1B that harbors a change of residue 215 from cysteine to serine (C215S), which renders the mutant catalytically inactive and confers dominant negativity for PTP-1B function (33, 34). Thus, Ad-DN-PTP-1B expression specifically reduces endogenous PTP-1B activity in the infected cells.
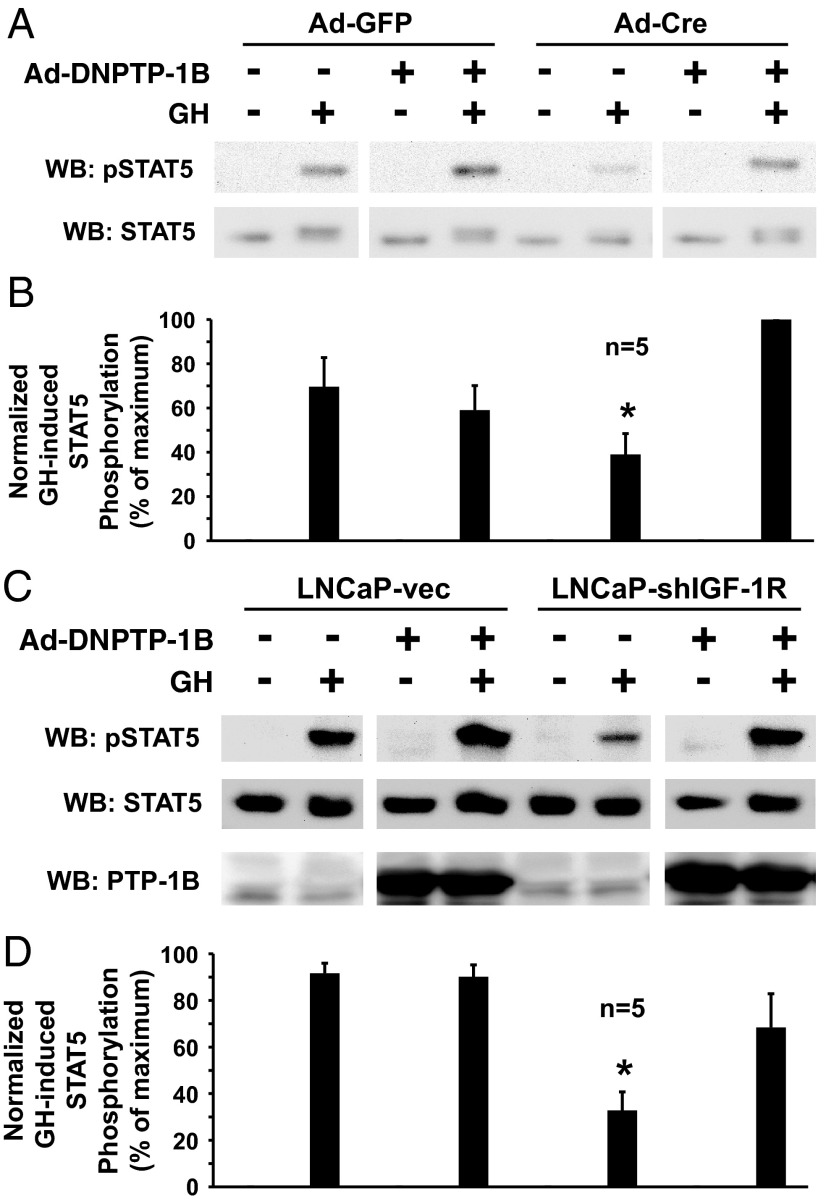
IGF-1R-floxed osteoblasts were infected with Ad-GFP (control) or Ad-Cre (to eliminate IGF-1R) and then infected with Ad-DN-PTP-1B vs control (Ad-GFP) (Figure 5, A and B). Notably, expression of DN-PTP-1B in Ad-GFP-infected (IGF-1R-replete) control cells did not, on average, affect GH-induced STAT5 phosphorylation, consistent with the results in Figure 2 with the chemical inhibitor of PTP-1B in control cells. In contrast, however, the diminished GH-induced STAT5 phosphorylation in Ad-Cre-infected osteoblasts was dramatically restored by coinfection with Ad-DN-PTP-1B. Thus, like chemical inhibition of PTP-1B, suppression of PTP-1B action by DN-PTP-1B expression selectively affected acute GH signaling in osteoblasts that lack IGF-1R.
Figure 5.
A dominant-negative mutant PTP-1B rescues the diminished GH-induced STAT5 phosphorylation resulting from IGF-1R depletion. A and B, Primary osteoblasts were infected with Ad-Cre vs Ad-GFP, as indicated, and Ad-DNPTP-1B (+) or Ad-GFP (−), as indicated. Serum-starved cells were treated with GH (+; 250 ng/mL) or vehicle (−) for 10 minutes. Detergent cell extracts were resolved by SDS-PAGE and immunoblotted with anti-pSTAT5, anti-STAT5, and anti-PTP-1B. A, Representative immunoblots. WB, Western blotting. B, Densitometric quantitation of pSTAT5/STAT5 signals from GH-treated samples from five independent experiments (including that shown in A). In each experiment, the maximum signal was considered 100%. Data are plotted as mean ± SE. *, P < .04 for comparison of Ad-Cre-infected, non-Ad-DNPTP-1B-infected, GH-treated group with either the Ad-GFP-infected, non-Ad-DNPTP-1B-infected, GH-treated group or the Ad-Cre-infected, Ad-DNPTP-1B-infected, GH-treated group. C and D, LNCaP-vec and LNCaP-shIGF-1R cells were infected with Ad-DNPTP-1B (+) or Ad-GFP (−), as indicated. Serum-starved cells were treated with GH (+; 250 ng/mL) or vehicle (−) for 10 minutes. Detergent cell extracts were resolved by SDS-PAGE and serially immunoblotted with anti-pSTAT5 and anti-STAT5. C, Representative immunoblots. D, Densitometric quantitation of pSTAT5/STAT5 signals from GH-treated samples from five independent experiments is displayed. In each experiment, the maximum signal was considered 100%. Data are plotted as mean ± SE. *, P < .03 for comparison of the LNCaP-shIGF-1R, non-Ad-DNPTP1B-infected group with any of the other GH-treated groups.
To further confirm this important finding, we pursued DN-PTP-1B expression in our separate experimental system of IGF-1R silencing, the LNCaP-shIGF-1R cells compared to LNCaP-vec cells (Figure 5, C and D). Again, expression of Ad-DN-PTP-1B in LNCaP-vec cells had no effect on the degree of GH-induced STAT5 activation. However, DN-PTP-1B expression in LNCaP-shIGF-1R cells, in which IGF-1R expression is markedly reduced and GH-induced STAT5 phosphorylation is similarly lessened, nearly completely rescued the reduced STAT5 phosphorylation. Collectively, the dominant-negative PTP-1B expression experiments in osteoblast and LNCaP cells suggest that loss of IGF-1R blunts GH signaling at least in part by allowing PTP-1B negative regulatory access to either activated GHR or GHR-associated STAT5-activating molecule(s) that is not accessible when IGF-1R is present.
Effects of COP1 expression on GH-induced STAT5 activation in the setting of IGF-1R deficiency
COP1 is a protein with multiple functions across species, including modulation of transcription factor stability and regulation of light signaling in plants and effects on stability of several proteins in mammals that are important in metabolism and growth (52, 53). COP1 harbors E3 ubiquitin ligase activity but has some functions that are apparently independent of this activity. Importantly, recent studies indicate that COP1 or a COP1 mutant devoid of E3 ligase activity physically interacts with PTP-1B and functions as a suppressor of PTP-1B activity to enhance hepatic insulin action (54). Thus, COP1 can be considered a specific negative regulator of PTP-1B.
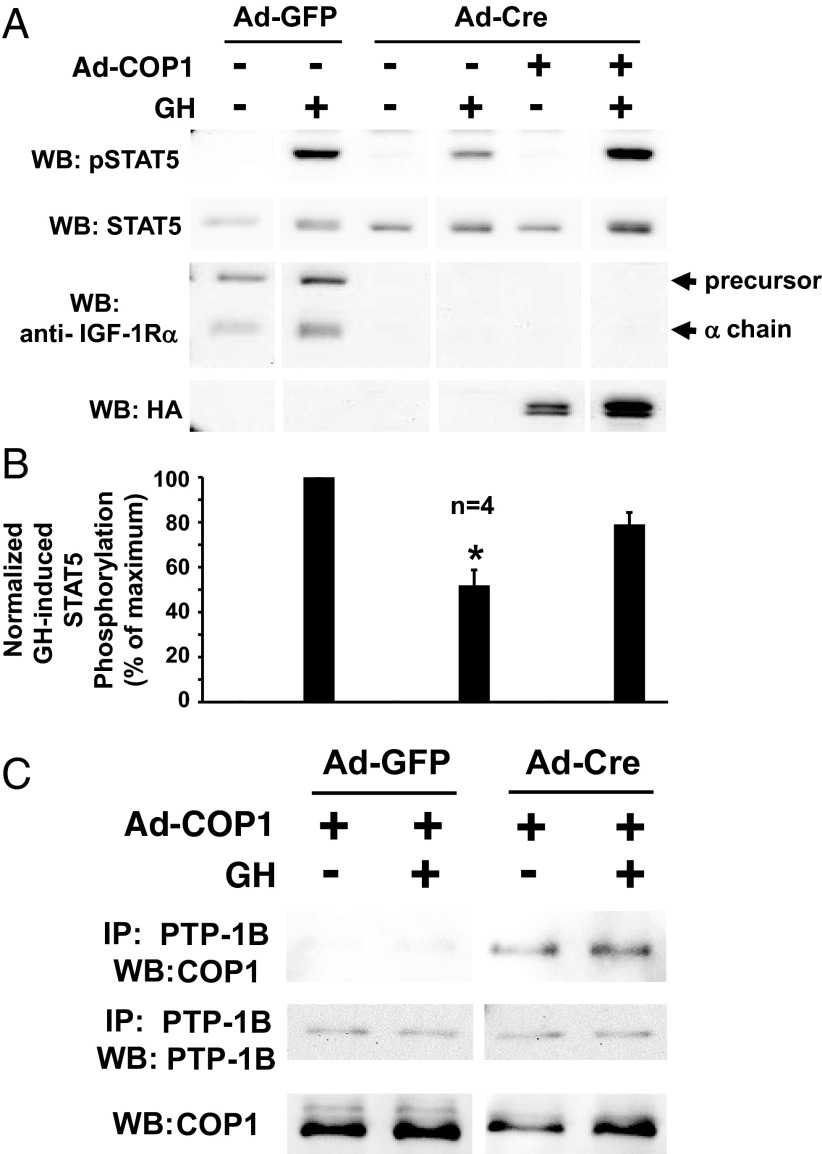
We hypothesized that, like the chemical PTP-1B inhibitor and expression of DN-PTP-1B, COP1 might rescue the diminished GH-induced STAT5 phosphorylation conferred by silencing of IGF-1R. To test this, we prepared an adenovirus that drives expression of human COP1 (37). In osteoblasts infected with Ad-Cre to delete the IGF-1R, we observed the expected reduction in the degree of acute GH-induced acute STAT5 phosphorylation; notably, coinfection of Ad-COP1 in Ad-Cre-infected cells resulted in restoration of nearly 80% of the loss of GH-induced STAT5 phosphorylation (Figure 6, A and B). Furthermore, infection with Ad-E-COP1, which drives expression of an E3 ligase-defective mutant COP1, also facilitated restoration of GH-induced STAT5 phosphorylation in the setting of Cre-mediated IGF-1R gene deletion (not shown). We also tested whether the presence or absence of IGF-1R affected COP1 interaction with PTP-1B. Notably, we found that adenovirally expressed COP1 was coimmunoprecipitated with PTP1B to a much greater extent in Ad-Cre-infected compared with Ad-GFP-infected osteoblasts (Figure 6C). These data with expression of COP1 and the E3 ligase-deficient form of COP1 strongly bolster that view that PTP-1B activity plays a role in the dampening of GH-induced acute STAT5 signaling in the setting of IGF-1R deficiency and complement the findings with the chemical PTP-1B inhibitor and with expression of the dominant-negative form of PTP-1B.
Figure 6.
COP1 rescues the diminished GH-induced STAT5 phosphorylation resulting from IGF-1R depletion and COP1-PTP-1B interaction is enhanced in IGF-1R-depleted osteoblasts. A and B, Primary osteoblasts were infected with Ad-Cre vs Ad-GFP, as indicated, and Ad-COP1 (+) or Ad-GFP (−), as indicated. Serum-starved cells were treated with GH (+; 250 ng/mL) or vehicle (−) for 10 minutes. Detergent cell extracts were resolved by SDS-PAGE and serially immunoblotted with anti-pSTAT5, anti-STAT5, anti-IGF1Rα, and anti-HA (to detect HA tagged COP1 proteins). A, Representative immunoblots. WB, Western blotting. B, Densitometric quantitation of pSTAT5/STAT5 signals from GH-treated samples from four independent experiments (including that shown in A). In each experiment, the maximum signal was considered 100%. Data are plotted as mean ± SE. *, P < .02 for comparison of Ad-Cre-infected, non-Ad-COP1-infected, GH-treated group with either the Ad-GFP-infected, non-Ad-COP1-infected, GH-treated group or the Ad-Cre-infected, Ad-COP1-infected, GH-treated group. C, Coimmunoprecipitation of COP1 with PTP-1B. Primary osteoblasts were infected with Ad-Cre vs Ad-GFP, as indicated, and Ad-COP1 (+), as indicated. Serum-starved cells were treated with GH (+; 250 ng/mL) or vehicle (−) for 10 minutes. Detergent cell extracts were either immunoprecipitated with anti-PTP-1B or not immunoprecipitated. Eluates or extracts were resolved by SDS-PAGE and immunoblotted with anti-COP1 or anti-PTP-1B, as indicated. IP, immunoprecipitation; WB, Western blotting.
JAK2 is a substrate of PTP-1B in cells that lack IGF-1R
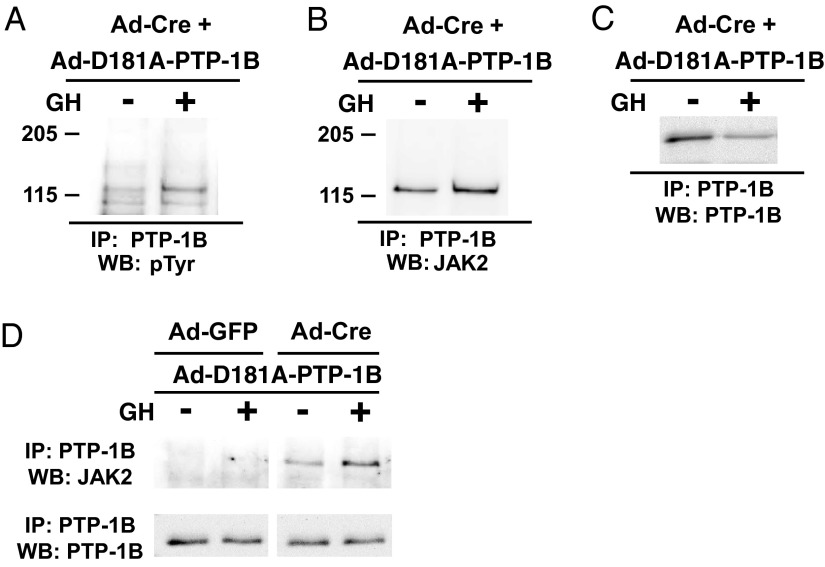
In systems in which PTP-1B exerts a negative regulatory role on GH-induced signaling, tyrosine-phosphorylated JAK2 has been identified as a substrate (21, 22). PTP-1B-D181A (called D/A) is a site-directed mutant form of PTP-1B that is catalytically impaired but retains the ability to bind and form stable complexes with tyrosine phosphorylated substrates; thus, PTP-1B D/A is a substrate-trapping mutant (35) and has thus been used to identify PTP-1B substrates in several studies (eg, Reference 36). We prepared an adenovirus (Ad-PTP-1B-D/A) that directs expression of this substrate-trapping PTP-1B mutant. Osteoblasts infected with either Ad-GFP (IGF-1R replete) or Ad-Cre (IGF-1R deficient) were coinfected with Ad-PTP-1B-D/A and stimulated with either vehicle or GH. Immunoprecipitation of PTP-1B-D/A and immunoblotting with anti-pY revealed coprecipitation of a tyrosine phosphoprotein of Mr approximately 120 kDa selectively in Ad-Cre-infected cells, the signal of which was augmented after GH treatment (Figure 7A). Reprobing indicated that this coprecipitated tyrosine phosphoprotein was immunoreactive with anti-JAK2 serum, strongly suggesting that the substrate-trapping PTP-1B mutant formed a GH-enhanced complex with tyrosine phosphorylated JAK2 in cells that lack IGF-1R (Figure 7B). This GH-induced augmented coprecipitation of tyrosine-phosphorylated JAK2 with PTP-1B-D/A could not be explained by enhanced direct PTP-1B precipitation (Figure 7C). Notably, JAK2 was not coprecipitated with PTP-1B-D/A in cells infected with Ad-GFP (Figure 7D). Collectively, these data support the notion that PTP-1B has enhanced physical and/or functional access to the most proximal element(s) of GH-activated signaling, including at least JAK2, when IGF-1R is not present and that this enhanced phosphatase access may explain the desensitization to GH for STAT5 activation under IGF-1R-deficient circumstances.
Figure 7.
JAK2 is a substrate for PTP-1B preferentially in cells in which IGF-1R is depleted. A–D, Primary osteoblasts were infected with Ad-Cre or Ad-GFP, as indicated, along with Ad-D181A-PTP-1B, which drives the expression of a substrate-trapping mutant PTP-1B. Cells were treated with GH (+; 250 ng/mL) or vehicle (−) for 10 minutes, as indicated. Ad-Cre + Ad-D181A-PTP-1B-infected cells were solubilized and detergent extracts immunoprecipitated with anti-PTP-1B and eluates were resolved and immunoblotted sequentially with antiphosphotyrosine antibody (pTyr; A), anti-JAK2 (B), and anti-PTP1B (C). In D, cells infected with Ad-GFP or Ad-Cre and coinfected with Ad-D181A-PTP-1B were treated with or without GH, and anti-PTP-1B-immunopreciptated proteins were immunoblotted sequentially with anti-JAK2 and anti-PTP-1B, as indicated. Note the coprecipitation of tyrosine phosphorylated JAK2 in the Ad-Cre-infected but not Ad-GFP-infected cells. IP, immunoprecipitation; WB, Western blotting.
Conclusions
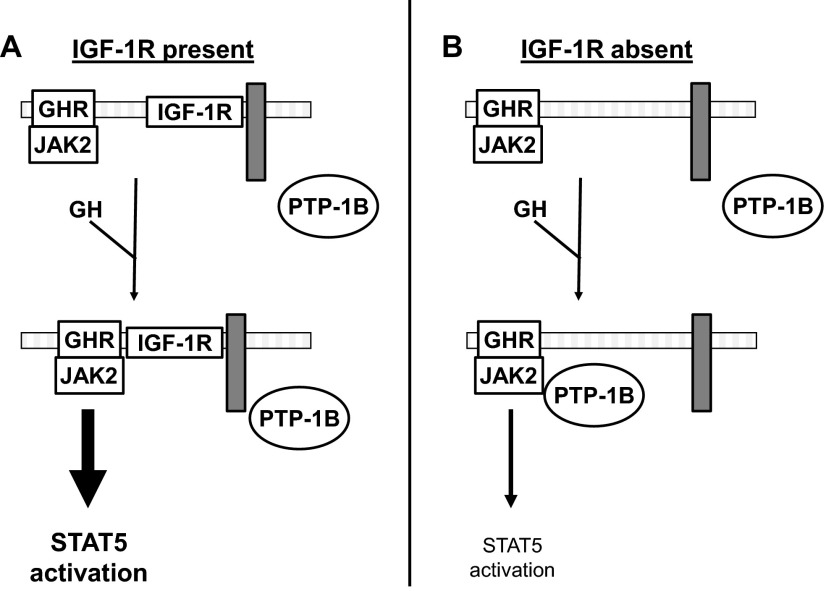
We previously found that deletion of IGF-1R in murine osteoblasts and reduction of IGF-1R abundance in islet β-cells markedly desensitizes the cells to GH, reducing acute GH-induced STAT5 phosphorylation. Herein we extended these findings to another model system, the GH-responsive LNCaP human prostate cancer cell line; again, shRNA-mediated silencing of IGF-1R resulted in diminished GH-induced acute STAT5 phosphorylation. We probed potential PTP involvement in this desensitization and found that in both osteoblasts and LNCaP cells the general PTP inhibitor, orthovanadate, had no effect on GH-induced STAT5 phosphorylation unless IGF-1R was either absent or relatively silenced, in which case the ability of GH to promote STAT5 phosphorylation was restored. Chemical inhibitor experiments implicated PTP-1B, but not SHP-1 or SHP-2, as mediating this loss of GH sensitivity upon IGF-1R reduction. Further experiments in which either a dominant-negative PTP-1B or a recently defined PTP-1B-interacting inhibitory protein, COP1, were expressed further pointed to PTP-1B as the relevant PTP. In addition, the use of a substrate-trapping PTP suggested that GH-induced tyrosine phosphorylated JAK2 is targeted more avidly by PTP-1B in cells in which IGF-1R abundance is diminished. These results allow us to hypothesize that, in addition to functioning in a GH-to-IGF-1-to-IGF-1R fashion (the somatomedin hypothesis of GH action), GH might use IGF-1R to facilitate or strengthen GH action by preventing access to PTP-1B, a negative regulator of GH signaling (Figure 8). Future studies will probe this hypothesis by further identifying other associated protein(s) or features of IGF-1R's structure that allow IGF-1R to function in this fashion and determining whether such a role for IGF-1R relates to the varying biological effects of GH among target cells and tissues.
Figure 8.
IGF-1R as a regulator of access of PTP-1B to GH-activated JAK2. A and B, Diagram highlighting the current findings. A, GH-induced STAT5 activation is enhanced in the presence of IGF-1R. Our findings suggest that IGF-1R, likely by inducibly associating with the GHR-JAK2 complex, prevents access of PTP-1B to its substrate, JAK2, and thereby potentiates GH-induced STAT5 phosphorylation. This blockade of access of PTP-1B may be afforded by IGF-1R itself or by a yet-unidentified IGF-1R-associated protein, here diagrammed as a gray rectangle. B, GH-induced STAT5 activation is diminished in the absence of IGF-1R. When IGF-1R is absent (or dramatically reduced in abundance), it can no longer interact with GHR-JAK2 (or possibly the IGF-1R associated gray rectangle protein); therefore, PTP-1B gains better access to GH-activated JAK2 to dephosphorylate it and diminish acute GH-activated STAT5 signaling.
Acknowledgments
We appreciate the helpful conversations with Drs Y. Huang, J. Messina, L. Deng, J. Xu, and Y. Liu and the generous provision of reagents by those named in the text.
This work was supported by National Institutes of Health Grants DK46395 (to S.J.F.) and CA69202 (to Z.-Y.Z.).
Parts of this work were presented at the 93rd Annual Meeting of The Endocrine Society, Boston, Massachusetts, 2011, and the 94th Annual Meeting of The Endocrine Society, Houston, Texas, 2012.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ad-Cre
- adenovirus encoding Cre recombinase
- Ad-GFP
- adenovirus encoding green fluorescent protein
- COP1
- constitutive photomorphogenesis 1
- GHR
- GH receptor
- IGF-1R
- IGF receptor
- JAK
- Janus kinase
- MOI
- multiplicity of infection
- PTP-1B
- protein tyrosine phosphatase-1B
- SHP
- src homology region 2 domain-containing phosphatace
- shRNA
- short hairpin RNA
- STAT
- signal transducer and activator of transcription.
References
- 1. Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30:152–177 [DOI] [PubMed] [Google Scholar]
- 2. Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol. 2010;6:515–525 [DOI] [PubMed] [Google Scholar]
- 3. Herrington J, Carter-Su C. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol Metab. 2001;12:252–257 [DOI] [PubMed] [Google Scholar]
- 4. Frank SJ, Messina JL. 2002 Growth hormone receptor. In: Oppenheim JJ, Feldman M, eds. Cytokine Reference On-Line. London: Academic Press, Harcourt; 1–21 [Google Scholar]
- 5. Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology. 2001;142:3836–3841 [DOI] [PubMed] [Google Scholar]
- 6. Woelfle J, Billiard J, Rotwein P. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J Biol Chem. 2003;278:22696–22702 [DOI] [PubMed] [Google Scholar]
- 7. Ullrich A, Gray A, Tam AW, et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LeRoith D. Insulin-like growth factor I receptor signaling—overlapping or redundant pathways? Endocrinology. 2000;141:1287–1288 [DOI] [PubMed] [Google Scholar]
- 9. Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev. 2001;22:818–835 [DOI] [PubMed] [Google Scholar]
- 10. Salmon WD, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957;49:825–836 [PubMed] [Google Scholar]
- 11. Daughaday WH. Growth hormone axis overview—somatomedin hypothesis. Pediatr Nephrol. 2000;14:537–540 [DOI] [PubMed] [Google Scholar]
- 12. Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74 [DOI] [PubMed] [Google Scholar]
- 13. Fan Y, Menon RK, Cohen P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem. 2009;284:19937–19944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162 [DOI] [PubMed] [Google Scholar]
- 15. Frank SJ. Growth hormone, insulin-like growth factor I, and growth: local knowledge. Endocrinology. 2007;148:1486–1488 [DOI] [PubMed] [Google Scholar]
- 16. Ashcom G, Gurland G, Schwartz J. Growth hormone synergizes with serum growth factors in inducing c-fos transcription in 3T3-F442A cells. Endocrinology. 1992;131:1915–1921 [DOI] [PubMed] [Google Scholar]
- 17. Edmondson SR, Russo VC, McFarlane AC, Wraight CJ, Werther GA. Interactions between growth hormone, insulin-like growth factor I, and basic fibroblast growth factor in melanocyte growth. J Clin Endocrinol Metab. 1999;84:1638–1644 [DOI] [PubMed] [Google Scholar]
- 18. Huang Y, Kim S-O, Yang N, Jiang J, Frank SJ. Physical and functional interaction of GH and IGF-I signaling elements. Mol Endocrinol. 2004;18:1471–1485 [DOI] [PubMed] [Google Scholar]
- 19. Gan Y, Zhang Y, Digirolamo DJ, et al. Deletion of IGF-1 receptor (IGF-IR) in primary osteoblasts reduces GH-induced STAT5 signaling. Mol Endocrinol. 2010;24:644–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pasquali C, Curchod ML, Walchli S, et al. Identification of protein tyrosine phosphatases with specificity for the ligand-activated growth hormone receptor. Mol Endocrinol. 2003;17:2228–2239 [DOI] [PubMed] [Google Scholar]
- 21. Gu F, Dube N, Kim JW, et al. Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling. Mol Cell Biol. 2003;23:3753–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi JH, Kim HS, Kim SH, et al. Phospholipase Cγ1 negatively regulates growth hormone signalling by forming a ternary complex with Jak2 and protein tyrosine phosphatase-1B. Nat Cell Biol. 2006;8:1389–1397 [DOI] [PubMed] [Google Scholar]
- 23. Pilecka I, Patrignani C, Pescini R, et al. Protein-tyrosine phosphatase H1 controls growth hormone receptor signaling and systemic growth. J Biol Chem. 2007;282:35405–35415 [DOI] [PubMed] [Google Scholar]
- 24. Ram PA, Waxman DJ. Interaction of growth hormone-activated STATs with SH2-containing phosphotyrosine phosphatase SHP-1 and nuclear JAK2 tyrosine kinase. J Biol Chem. 1997;272:17694–17702 [DOI] [PubMed] [Google Scholar]
- 25. Hackett RH, Wang YD, Sweitzer S, Feldman G, Wood WI, Larner AC. Mapping of a cytoplasmic domain of the human growth hormone receptor that regulates rates of inactivation of Jak2 and Stat proteins. J Biol Chem. 1997;272:11128–11132 [DOI] [PubMed] [Google Scholar]
- 26. Kim SO, Jiang J, Yi W, Feng GS, Frank SJ. Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem. 1998;273:2344–2354 [DOI] [PubMed] [Google Scholar]
- 27. Stofega MR, Herrington J, Billestrup N, Carter-Su C. Mutation of the SHP-2 binding site in growth hormone (GH) receptor prolongs GH-promoted tyrosyl phosphorylation of GH receptor, JAK2, and STAT5B. Mol Endocrinol. 2000;14:1338–1350 [DOI] [PubMed] [Google Scholar]
- 28. Stofega MR, Wang H, Ullrich A, Carter-Su C. Growth hormone regulation of SIRP and SHP-2 tyrosyl phosphorylation and association. J Biol Chem. 1998;273:7112–7117 [DOI] [PubMed] [Google Scholar]
- 29. De Rocca Serra-Nedelec A, Edouard T, Treguer K, et al. Noonan syndrome-causing SHP2 mutants inhibit insulin-like growth factor 1 release via growth hormone-induced ERK hyperactivation, which contributes to short stature. Proc Natl Acad Sci USA. 2012;109:4257–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Guan R, Jiang J, et al. Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis. J Biol Chem. 2001;276:24565–24573 [DOI] [PubMed] [Google Scholar]
- 31. Jiang J, Liang L, Kim SO, Zhang Y, Mandler R, Frank SJ. Growth hormone-dependent tyrosine phosphorylation of a GH receptor-associated high molecular weight protein immunologically related to JAK2. Biochem Biophys Res Commun. 1998;253:774–779 [DOI] [PubMed] [Google Scholar]
- 32. DiGirolamo DJ, Mukherjee A, Fulzele K, et al. Mode of growth hormone action in osteoblasts. J Biol Chem. 2007;282:31666–31674 [DOI] [PubMed] [Google Scholar]
- 33. Chang Y, Ceacareanu B, Zhuang D, et al. Counter-regulatory function of protein tyrosine phosphatase 1B in platelet-derived growth factor- or fibroblast growth factor-induced motility and proliferation of cultured smooth muscle cells and in neointima formation. Arterioscler Thromb Vasc Biol. 2006;26:501–507 [DOI] [PubMed] [Google Scholar]
- 34. Sreejayan N, Lin Y, Hassid A. NO attenuates insulin signaling and motility in aortic smooth muscle cells via protein tyrosine phosphatase 1B-mediated mechanism. Arterioscler Thromb Vasc Biol. 2002;22:1086–1092 [DOI] [PubMed] [Google Scholar]
- 35. Flint AJ, Tiganis T, Barford D, Tonks NK. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci USA. 1997;94:1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bettaieb A, Bakke J, Nagata N, et al. Protein-tyrosine phosphatase 1B regulates pyruvate kinase M2 tyrosine phosphorylation. J Biol Chem. 2013;288(24):17360–17371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kato S, Ding J, Pisck E, Jhala US, Du K. COP1 functions as a FoxO1 ubiquitin E3 ligase to regulate FoxO1-mediated gene expression. J Biol Chem. 2008;283:35464–35473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frank SJ, Wang X, He K, et al. In vivo imaging of hepatic growth hormone signaling. Mol Endocrinol. 2006;20:2819–2830 [DOI] [PubMed] [Google Scholar]
- 39. Xu J, Zhang Y, Berry PA, et al. Growth hormone signaling in human T47D breast cancer cells: potential role for a growth hormone receptor-prolactin receptor complex. Mol Endocrinol. 2011;25:597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang M, Xuan S, Bouxsein ML, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012 [DOI] [PubMed] [Google Scholar]
- 41. Tiganis T, Bennett AM. Protein tyrosine phosphatase function: the substrate perspective. Biochem J. 2007;402:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol. 2000;12:361–378 [DOI] [PubMed] [Google Scholar]
- 43. Carver KC, Piazza TM, Schuler LA. Prolactin enhances insulin-like growth factor I receptor phosphorylation by decreasing its association with the tyrosine phosphatase SHP-2 in MCF-7 breast cancer cells. J Biol Chem. 285:8003–8012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Frangioni JV, Beahm PH, Shifrin V, Jost CA, Neel BG. The nontransmembrane tyrosine phosphatase PTP-1B localizes to the endoplasmic reticulum via its 35 amino acid C-terminal sequence. Cell. 1992;68:545–560 [DOI] [PubMed] [Google Scholar]
- 45. Tonks NK. PTP1B: from the sidelines to the front lines!. FEBS Lett. 2003;546:140–148 [DOI] [PubMed] [Google Scholar]
- 46. Elchebly M, Payette P, Michaliszyn E, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548 [DOI] [PubMed] [Google Scholar]
- 47. Sangwan V, Abella J, Lai A, et al. Protein-tyrosine phosphatase 1B modulates early endosome fusion and trafficking of Met and epidermal growth factor receptors. J Biol Chem. 2011;286:45000–45013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carbone CJ, Zheng H, Bhattacharya S, et al. Protein tyrosine phosphatase 1B is a key regulator of IFNAR1 endocytosis and a target for antiviral therapies. Proc Natl Acad Sci USA. 2012;109:19226–19231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wiesmann C, Barr KJ, Kung J, et al. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat Struct Mol Biol. 2004;11:730–737 [DOI] [PubMed] [Google Scholar]
- 50. Sun T, Ye F, Ding H, Chen K, Jiang H, Shen X. Protein tyrosine phosphatase 1B regulates TGFβ1-induced Smad2 activation through PI3 kinase-dependent pathway. Cytokine. 2006;35:88–94 [DOI] [PubMed] [Google Scholar]
- 51. Ma F, Wei Z, Shi C, et al. Signaling cross talk between growth hormone (GH) and insulin-like growth factor-I (IGF-I) in pancreatic islet beta-cells. Mol Endocrinol. 2011;25:2119–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yi C, Deng XW. COP1—from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005;15:618–625 [DOI] [PubMed] [Google Scholar]
- 53. Marine JC. Spotlight on the role of COP1 in tumorigenesis. Nat Rev Cancer. 2012;12:455–464 [DOI] [PubMed] [Google Scholar]
- 54. Ren W, Sun Y, Cheema S, Du K. Interaction of constitutive photomorphogenesis 1 protein with protein-tyrosine phosphatase 1B suppresses protein-tyrosine phosphatase 1B activity and enhances insulin signaling. J Biol Chem. 2013;288:10902–10913 [DOI] [PMC free article] [PubMed] [Google Scholar]