Abstract
Spinal cord injury results from an insult inflicted on the spinal cord that usually encompasses its 4 major functions (motor, sensory, autonomic, and reflex). The type of deficits resulting from spinal cord injury arise from primary insult, but their long-term severity is due to a multitude of pathophysiological processes during the secondary phase of injury. The failure of the mammalian spinal cord to regenerate and repair is often attributed to the very feature that makes the central nervous system special—it becomes so highly specialized to perform higher functions that it cannot effectively reactivate developmental programs to re-build novel circuitry to restore function after injury. Added to this is an extensive gliotic and immune response that is essential for clearance of cellular debris, but also lays down many obstacles that are detrimental to regeneration. Here, we discuss how the mature chromatin state of different central nervous system cells (neural, glial, and immune) may contribute to secondary pathophysiology, and how restoring silenced developmental gene expression by altering histone acetylation could stall secondary damage and contribute to novel approaches to stimulate endogenous repair.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0228-z) contains supplementary material, which is available to authorized users.
Keywords: Inflammation, Regeneration, Neuroprotection, Myelin repair, Neuroglial progenitors
Physical, Medical, and Cellular Sequelae After Spinal Cord Injury
Spinal cord injury (SCI) results from a physical insult that encompasses—either completely or incompletely—4 major functions (motor, sensory, autonomic, and reflex). The complex variety of motor, sensory, and autonomic problems that arise from SCI are a direct result of the level of their lesion, and the extent of spinal cord disruption following the development of secondary pathology. The most common injury mechanisms are 1) contusion of the spinal cord by external force injury [1]; 2) impact alone with more transient compression; 3) laceration and transection (most common in thin, thoracic segments); or 4) distraction—forcible stretching of the spinal column in the axial plane (reviewed in [2] and [3]).
The pathophysiological processes that lay the groundwork for the long-term deficits due to SCI comprise both primary and secondary phases of injury [4, 5]. Primary injury produces direct immediate, mechanical disruption of spinal cord function, but the more widespread mechanisms of secondary damage ultimately determine the final chronic extent of neurological deficits (summarized in Fig. 1). Even if axons were made capable of regenerating along multiple tracts after SCI, they are faced with a formidable barrier of secondary glial and inflammatory pathophysiology [6, 7]. Because of this, a clear understanding of pathophysiological mechanisms that produce primary and secondary damage after SCI is critical in order to facilitate targeted therapeutic intervention aimed at ameliorating the specific pathology of an individual injury [8].
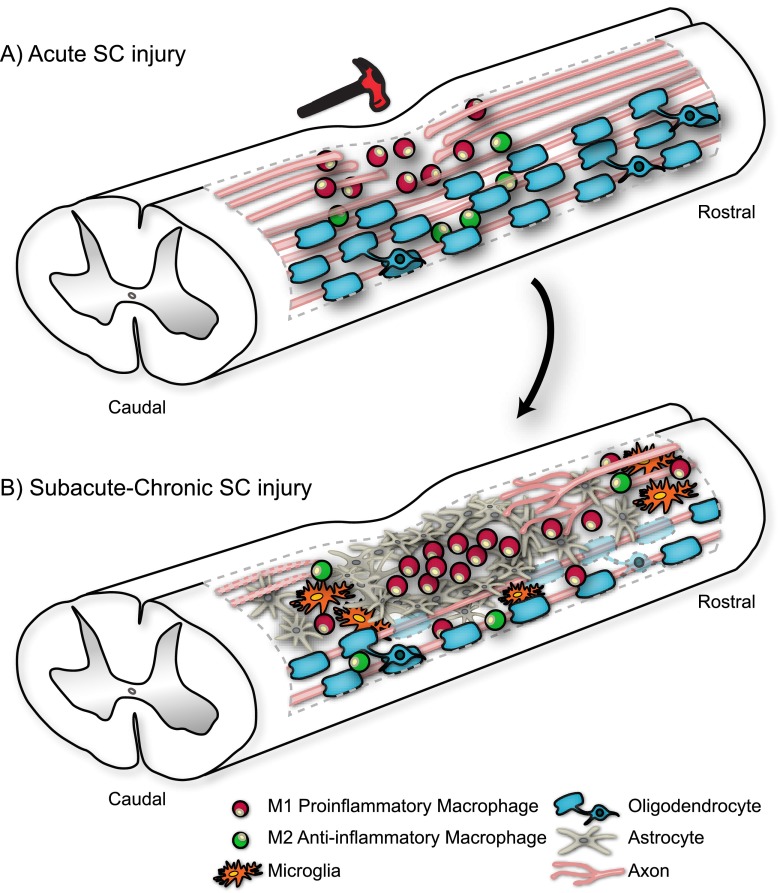
Fig. 1.
Pathophysiological progression of spinal cord (SC) injury from (a) acute to (b) subacute/chronic stage involves shifts in state and distribution of i) resident microglia and invading inflammatory cells (neutrophils, monocyte-derived macrophages, and T-cells); ii) macrophages which can be polarized to either pro-inflammatory (M1) or anti-inflammatory (M2) state; iii) astrocytes, which first need to contain the lesion, but later interact with glial progenitors, fibroblasts, meningeal cells, microglia, and macrophages to form the physical and molecular barrier of the glial scar; iv) oligodendrocytes, which first undergo apoptosis when they dislodge from damaged axons, and later need to recreate myelin on spared axons; and v) many ascending and descending axons that are broken and need to either regenerate over long distances or sprout to form alternative circuitry
Secondary Damage Produces Multiple Therapeutic Challenges at Different Stages of the Repair Process
The immediate damage following SCI is to neurons located within, and passing through, the injury site that become physically damaged and sever axons from their targets. The spinal cord rapidly develops hemorrhage at the site of impact, disrupting local blood flow and producing a microenvironment of hypoxia and ischemia, which creates a further block to neural circuit function [9–11]. Spinal gray matter is believed to be irreversibly damaged within the first 1–3 h after injury, with white matter damage more extensive by 72 h after injury [12]. These time windows vary depending on the extent and level of lesion, and can be influenced by age and health status at injury. Given that early intervention that minimizes secondary damage may be the best way to enhance repair, it is important to establish how and when damage is propagated mechanistically, and understand how this varies between individuals. This is perhaps even more important in light of the fact that both primary and secondary pathophysiology in SCI shares common cellular and molecular mechanisms with other types of central nervous system (CNS) injury, such as traumatic brain injury (TBI), cerebral ischemia, and subarachnoid hemorrhage [13–15].
The mechanical injury site is not only the nidus of primary clinical symptoms, but also the core from which the post-injury inflammatory response orchestrates extensive secondary damage after SCI via a series of complex cellular and molecular interactions [16] (Fig. 1). In addition to the immediate activation of local microglia in the injury core, the blood–spinal–cord barrier disruption from hemorrhage and local inflammation [17] causes peripheral inflammatory cells to invade the lesion site with increased production of chemokines and cytokines of the IL-1 family [18–20]. Although initial immune responses are essential for clearing primary lesion damage, the recruitment and persistent activation of both CNS resident inflammatory microglia and peripheral infiltrates (including macrophages, monocytes, T lymphocytes, and neutrophils) increases the severity of secondary damage following SCI [21] (reviewed in [3]).
The secondary phase of SCI has 3 distinct phases—acute, subacute, and chronic—which each carry a different sequence of secondary damage cascades [1, 7, 22]. Progression from one stage to the next is driven by the shifting activation state of cells in and around the injury site, with each phase yielding distinct biochemical and cellular signatures (summarized in Fig. 1) [23]. Although this cascade of secondary damage clearly expands the injury size and increases cell and axonal loss, individuals can have broadly different primary and secondary inflammatory responses to very similar lesions, with vastly different clinical outcomes [19]. Similar variations are seen in multiple rodent models where the same lesion tested across different laboratories—even in genetically homogenous strains of rats or mice—can produce different functional outcomes, suggesting an epigenetic–immune contribution may underlie the extent of secondary damage. Some of these variations could also be attributed to the health or nutritional status of individuals at the time of, or following, injury, and also to their health in the postoperative care environment [24].
Given emerging evidence of the complex interplay between immune system activation, nutritional well-being and epigenetic variation, here we consider if the intrinsic epigenetic status of cells participating in SCI repair may contribute to the variation seen in the inflammatory response. If epigenetic mechanisms do regulate the plasticity and activation state of multiple cells involved in SCI pathophysiology, understanding their role in the development of individual lineages and using this to guide the critical timing of plasticity-promoting approaches will be essential to temporally target epigenetic plasticity after SCI. Here, we review whether enhancing the epigenetic plasticity of key cells participating in repair at different stages of SCI recovery might allow us to harness their developmental capacity to drive repair after injury. Key to this is understanding how different chromatin remodeling factors work together to ensure successful development.
Epigenetic Regulation Mechanisms Important for Development
All cells in a multicellular organism have an essentially identical genotype, and yet produce a wide range of differentiated cell types with very different gene expression profiles that drive divergent functions. This progressive restriction in gene expression in development results from a combination of state-dependent (stage- and lineage-specific) transcriptional activation that interact with epigenetic gene silencing mechanisms to restrict gene expression (reviewed in [25]). Epigenetic mechanisms thus provide the bridge that connects how the environment affects how each cell uses its genome [26–28]. Understanding mechanistic modifications that have occurred at the environment–genome interface during life could hold critical clues as to why different individuals display experience-dependent variations in learning, personality disorders, plasticity, and long-term responses to neurological disease or injury.
Chromatin Structure and Gene Expression
Chromatin has a highly malleable structure that provides the substrate for transcriptional processes that establish distinct cellular identities and states. Although the term “epigenetic” now also encompasses the activities of a myriad of noncoding RNAs for the purposes of this review we will focus largely on histone–DNA interactions [29]. The majority of covalent histone modifications occur at their N- terminals (which are highly conserved), which act as substrates for several different types of post-translational modifications that alter chromatin structure, including acetylation, methylation, adenosine diphosphate ribosylation, ubiquitylation, and phosphorylation. These modifications can be correlated with various nuclear functions, including replication, chromatin assembly, and transcription, as well as gene silencing. Distinct patterns of histone modifications act in concert with DNA methylation, noncoding RNAs, and transcription factors to generate “histone–epigenetic codes” that are read by effector proteins to modulate chromatin function and transcriptional output [30–34].
DNA Methylation
The covalent addition of a methyl group at the pyrimidine ring of cytosine residues is catalyzed by DNA methyltransferases (DNMTs) [35], and occurs in vertebrates almost exclusively at cytosine–phosphate–guanine dinucleotides [36–38]. DNA methylation is the primary impetus for recruitment of histone deacetylases (HDACs), and is essential for mammalian development, evidenced by the ultimate developmental lethality of DNMT knockout mice [39, 40]. A methylated cytosine can repress transcription either directly by precluding transcriptional activators or indirectly by promoting recruitment of inhibitory regulatory proteins [37]. The indirect mode of repression requires the specific binding of methyl–cytosine–phosphate–guanine–binding domain proteins to methylated DNA, mediating transcription normally through the recruitment of repressor complexes, usually containing HDACs (reviewed in [37, 41]). How DNA methylation contributes to the stability of gene expression state is reviewed elsewhere [33].
Histone Acetylation and Deacetylation
Acetylation at several different lysine residues in the N-terminal tails of histone proteins generally delineates zones of open chromatin and gene activation. Acetylation opposes and neutralizes the positive charge of histone proteins, decreasing the attraction between histones and negatively-charged DNA [29]. This relaxed chromatin structure then allows for the recruitment of the transcriptional machinery. This delicate balance of acetylation and deacetylation is reversibly catalyzed by multiple classes of histone acetyl transferases (HATs), which add acetyl groups to histone lysine residues, and HDACs, which can remove these modifications [42–45].
HDAC Inhibitors
A wide range of naturally occurring and synthetic compounds have been found to inhibit the activity of class I and class II HDACs, some of which are now Food and Drug Administration-approved and in use for their effectiveness as anticancer or neuropsychiatric agents [46, 47]. Inhibitors of the class I and II HDACs are categorized into several different classes, based on their chemical structure [47]. HDAC inhibitors (HDACi) can be divided into structural classes, including 1) small-molecule hydroxamates, such as Trichostatin A (TSA), suberoylanilide hydroxamic acid (SAHA), scriptaid, and oxamflatin; 2) short-chain fatty acids, such as sodium butyrate, sodium phenylbutyrate, and valproic acid (VPA); 3) cyclic tetrapeptides, such as apicidin, trapaxin, and the depsipeptide FK-228; and 4) benzamides, such as MS-275 and Cl-994 (for a review see [48]).
Young and Old Animals and Cells are Very Different in Their Response to Lesion
Some of the most promising strategies to promote motor function following brain injury, stroke, and SCI in humans and experimental animals involves targeted training and rehabilitation to enhance residual plasticity. Such approaches are a constantly evolving “state of the art” treatment approach, but successful rehabilitation outcomes vary significantly and depend on several variables, including the health and emotional motivation of the individual. Perhaps the variable producing the greatest shift in rehabilitation-based recovery is age [49–51], which is also a critical feature of likelihood of morbidity within the first year after SCI [52]. Whether in patients or animal models, the state of developmental plasticity of the nervous system and its accompanying immune responses clearly underscores its fundamental capacity to drive functional recovery [53–55]. Age at injury is a prognostic indicator in animal models regardless of whether locomotor recovery is spontaneous [56] or stimulated by training [57, 58]. During recovery from lesion, do younger rats and humans remain more plastic because they are less restricted (fixed) epigenetically? If so, understanding the epigenetic basis underlying the differential plasticity of “young” and “old” cells could open avenues that allow us to specifically target chromatin-based plasticity at key stages of the recovery process to enhance rehabilitation-stimulated functional recovery.
Although chromatin-remodeling activities are integral to the proper development of the nervous system, the potential effect of altering such activities in adult brain repair is only just beginning to be explored, and its success will rely on understanding the chromatin remodeling factors that are specific to distinct developmental time windows in the cells that need to be most manipulated following SCI.
Epigenetic Regulation of Nervous System Developmental Plasticity
Epigenetic mechanisms can alter distinct cell gene expression profiles in vivo without directly affecting DNA sequence, and thus influence transcription in a malleable manner with far-reaching implications for understanding individual human diversity, and variability in injury and disease responses [59–61]. No other tissue or organ rivals the brain’s ability to diversify transcriptional programs, enabling complex patterns of postmitotic gene regulation [27]. The progressive differentiation of individual neurons and glial cells is driven by a fine balance of transcriptional activation, and the epigenetic regulation of chromatin structure, with histones, in particular [62], increasingly implicated in modifying neuronal learning, synaptic plasticity, and cognition [26]. Our understanding of how some chromatin remodeling factors function in the brain has been illuminated by the multiple neurological syndromes in which they are mutated. This includes Rett syndrome, (Immunodeficiency–centromeric instability–facial anomalies) ICF, fragile-X syndrome, ATRX (Alpha-Thalassemia X-Linked Intellectual Disability), Rubinstein–Taybi, and Angelman syndrome (reviewed in [59]), and complex psychiatric disorders such as schizophrenia [63–65]. Similarly, a large number of neural-related phenotypes are beginning to emerge from genetic studies of chromatin remodeling proteins in animal models (see [66] for a review).
It is not hard to imagine that holding in place the remarkably complex patterning of the brain, whilst supporting the stimulus-dependent plasticity that allows the brain to “learn”, may require higher-order gene regulation mechanisms in order to distinguish billions of otherwise genomically-identical neurons from each other. In accord, DNA methylation of distinct genomic sites in the human brain increases with activity and neuronal learning [67], producing a distinct “methylation signature” in different adult human brain regions [68]. Many questions remain, however, concerning how life experience affects other chromatin remodeling factors in individual cells of the adult CNS. A large variety of environmental stimuli (e.g., stress, nutrition, nurturing, exposure to drugs of abuse, social interactions, sensory learning, etc.) can alter the expression and activity of DNA methylation and histone-modifying enzymes throughout the neuro-genome [66, 69, 70]. Epigenetic regulation also provides a coordinated system for regulating gene expression at each stage of neurogenesis—where each neuron assumes a distinct chromatin profile as a result of environmental stimulation over the lifespan [25, 71, 72]. Collectively, less mature (“plastic”) cells should be able to more rapidly change state and respond to the needs of an injured environment better than older, more specialized, or senescent cells. Following CNS injury, it may thus be advantageous to help cells revert to a more plastic (“open chromatin”) genomic state after injury, so they can adapt as they once did developmentally, to promote the restoration of function. This may be even more critical to consider in approaches that incorporate neural stem cell-based approaches to promote regeneration.
Could Altering Cellular Epigenetic Changes in State Enhance SCI Repair?
So, epigenetic mechanisms are clearly essential for normal brain development, and chromatin remodeling is now linked to neural plasticity at the molecular, cellular, and organismal level, and multiple forms of behavioral learning [61, 73–75]. However, promoting long-term neurological recovery—in SCI and TBI in particular—will not only require us to promote learning and plasticity in neurons, but also manipulate the orchestrated responses of glia, de novo angiogenesis, and central and peripheral immune cells. The next frontier of using epigenetics to enhance SCI repair will depend on understanding how distinct chromatin modifications drive function in individual cells that contribute to SCI pathophysiology (Fig. 1). Understanding the correlation between chromatin state and immature (plastic, developing) or differentiated (committed) function in non-neuronal cells will be an essential part of minimizing long-term damage and supporting functional recovery following SCI. Below, we first introduce how environmental factors (like nutrition) may create epigenetic changes and review known pathways activated after SCI that have recently been shown to affect chromatin remodeling directly. We next discuss how histone acetylation status (which is reversible) is mediated by different enzymes in different cell types, and how this could be potentially targeted to enhance endogenous repair of the spinal cord.
Some Established Pathways Activated After SCI Alter Chromatin Remodeling
Interestingly, some of the major signaling cascades that are differentially activated in the CNS in development and after injury have recently been implicated in controlling histone acetylation and chromatin structure. First, the mitogen-activated protein kinase (MAPK) superfamily is central to plasticity signaling in the CNS. Its prototype is the extracellular signal-regulated kinase (ERK)/mitogen- and stress-activated kinase (MSK)/cyclic-adenosine monophosphate regulatory element binding protein (CREB) pathway, where ERK activates its downstream target, MSK, to phosphorylate CREB [76–79]. This phosphorylation and activation of CREB recruits CREB binding protein, a HAT that regulates local chromatin structure as part of CREB-dependent activation of nuclear gene transcription [80]. Second, the nuclear factor kappa B (NFκB) signaling pathway appears to control histone acetylation and chromatin structure in the CNS by mechanisms that are still being elucidated [81, 82]. In the immune system and CNS, NFκB is controlled by its upstream regulator, inhibitor of kappa B kinase, which itself is a target of multiple upstream regulatory signaling cascades affected by chromatin remodeling factors. Thus, both the ERK/MSK/CREB pathway and the inhibitor of kappa B kinase/NFκB pathway can dynamically regulate chromatin structure in the mature CNS. In addition, some class II HDACs can act on nonhistone proteins like the transcriptional co-repressor YY1, the neuronal protein alpha-tubulin [83], and NFκB itself [84–87] to alter their function. Finally, peroxisome proliferator-activated receptor gamma (PPARγ) function is dependent on the availability of co-regulator proteins that modify chromatin states. As a result, chromatin modifying factors could differentially regulate the transcriptional activities of PPARγ and its target genes. Many known co-activators and co-repressors of PPARγ and other nuclear receptors have intrinsic histone modifying activities [88].
Nutritional Changes Can Affect Chromatin Structure and SCI Recovery
Changes in prenatal and perinatal nutrition can modify epigenetic state, thereby altering gene expression, neurogenesis, and behavior in offspring (reviewed in [89–91]). The nutrients that appear to have the greatest effects on brain plasticity include protein, folate, iron, selenium, zinc, iodine, vitamin A, and docosahexaenoic acid. Mechanistically, protein-restricted diets can inhibit DNMTs and cause hypomethylation of specific gene promoters [92], an upstream event of HDAC recruitment and activation. In addition, folate and vitamin B12 are essential cofactors for the methylation cycle; thus, deficiencies in these vitamins also inhibit DNMTs and DNA methylation, which can cause oxidative stress and neuronal cell death [93–96]. Whereas nutrition and health status have long been debated for their contribution to SCI recovery, nutrition and metabolism are now implicated bi-directionally in determining epigenetic status—with different potential effects across individuals [97]. This might explain, in part, studies where manipulating timing and type of caloric intake can enhance some parameters of recovery following SCI [98, 99].
Cells Regulated by HDACs That Could be Targeted to Alter the Course of Secondary Damage in SCI
Chromatin maintenance in dividing and nondividing cells relies on the action of replication-independent histone variants. Even simply understanding variability in distribution of histone variants could greatly affect our understanding of the plasticity of individual cells, or even reveal mechanisms underlying diverse repair responses between individuals. A failure to regenerate after SCI has 3 major cell groups that could each serve as independent targets for histone manipulation to enhance endogenous repair: 1) neurons with different intrinsic capacities to initiate a regeneration or plasticity response; 2) a hostile glial and immune response that is inhibitory or detrimental to repair; and 3) endogenous progenitors that could be activated to replace lost cells or promote remyelination.
Chromatin and Neuronal Regeneration
Chromatin-remodeling has now emerged as a core mechanism mediating neuronal differentiation and activity-dependent transcription, a process important for plasticity in both development and adulthood (see [66, 100, 101]). However, in order to regenerate, a neuron must first survive, and maintaining the balance between the activity of HATs and HDACs also appears to be pivotal for neuronal survival, even in the absence of pathology [102, 103]. Direct effects of HDACi (and other alterations in chromatin structure) on neuronal survival are covered in more detail elsewhere in this issue. Here, we focus on how chromatin modification may enhance neuronal outgrowth following injury—an essential step in rebuilding linear or alternative circuitry in a superhighway like the spinal cord.
Developing a functional circuit requires 2 major components—the structural components that build axons and dendrites and their transport systems, and the molecular pathways that direct axons and match them to their appropriate targets [104]. During CNS development, oriented axonal growth results from combinations of factors that attract or repulse the movement of neuronal processes [105, 106] (reviewed in [104, 105, 107, 108]). Forces driving neurite growth are intrinsic to developing neurons (motor machinery, cytoskeleton, metabolism, surface receptors), whereas guidance cues are programmed by the environment. Once targets have been reached, both of these sets of machinery are generally suppressed (some by chromatin-mediated silencing) in order to allow that neuron to mature in response to stimulation. Although many of these events may need to be reinitiated to promote regeneration, most are no longer available in the complex milieu of the lesioned, mature nervous system [109–112].
Chromatin remodeling factors are now emerging as regulators of chromatin remodeling that may gate access to genes essential for axon and dendrite growth and regeneration. Neurological disorders involving chromatin modification suggest that epigenetic silencing of transcription for outgrowth in mammalian CNS neurons may help to explain the developmental loss of intrinsic neurite growth and targeting capacity upon maturation [113]. For example, GAP43—a prototypical “regeneration-associated gene” is directly silenced by MeCP2, a methyl–DNA binding protein induced in terminally differentiating neurons that recruits HDAC2 to silence developmental genes [114]. When mutated, MeCP2 results in Rett syndrome, where neurons retain a prolonged immature state [115]. In accord with this, histone acetylation levels (histone 3 (H3) K9/14) increase in cortical neurons and cerebellar granule neurons as they mature [116]. The expression of epigenetic regulators also appears to tip the balance of attenuating transcription following stroke in cortical neurons, where neurons that do not sprout up-regulate HDAC4 and down-regulate p300 (HAT) compared with their axon-sprouting counterparts [117]. Furthermore, TSA can increase neurite growth in postnatal day 7 cerebellar granule neurons on permissive and inhibitory substrate, and enhance GAP43 expression and H3K9/14 hyper-acetylation [116].
However, TSA can also stimulate neurite growth beyond the nucleus by enhancing acetylation of transcription factors or proteins like α-tubulin that are substrates for axonal elongation [118–121]. HDAC6 also functions largely outside the nucleus, with a number of cytosolic and axonal targets that could affect endogenous outgrowth (reviewed in [122]). HDAC1 can also be shuttled out of the nucleus and retained more highly in the cytoplasm in damaged neurons in response to inflammatory stimuli. Once in the cytosol, it is directed to axons where it can impair mitochondrial transport and induce neurite beading in response to excitatory amino acids and cytokines [120]. Cytosolic HDAC1 has been detected in damaged axons in brains of multiple sclerosis patients, in animal models of demyelination (cuprizone), and in cultured neurons exposed to excitatory amino acids (glutamate) and cytokines [tumor necrosis factor (TNF)-α]. Although cytoplasmic acetylation targets (like tubulin) could alter neurite outgrowth, a number of lines of evidence in cortical neurons [117], cerebellar granule neurons, and retinal ganglion cells [116] indicate that HATs, CREB binding protein/p300, and P/CAF (P300/CBP-associated factor) are required for their hyperacetylation-induced increase in neurite growth on inhibitory substrates. However, p300 and HDAC inhibition may act on other independent aspects of neuronal outgrowth (survival, then outgrowth signaling) and may need to be considered independently for the more critical activity following a severe injury like SCI, especially when considering the complexity of the injury environment compared to an in vitro assay. Therefore, if HDAC inhibition is to be used to promote neuronal survival and regeneration following SCI in vivo, it could be important to target its use to the most beneficial acute time window, provided the glial-immune environment can support regrowth.
Can HDAC Manipulation Help to Break Down Glial–immune Barriers?
Following focal stroke, TBI, and SCI, a series of immediate changes in inflammatory signaling that directly result from the extent and type of impact initiate the second, prolonged phase of subacute inflammatory responses [123] (Fig. 1). Macrophages play a major role in both the acute and sustained SCI response, with 2 major macrophage phenotypes (M1 and M2) emerging as playing different roles that shift the balance of neurotoxicity and regeneration in the injured mouse spinal cord [124]. M1 macrophages, stimulated by interferon-gamma (IFNγ), are the “classical” pro-inflammatory macrophage subtype. They express CD86, inducible nitric oxide synthase (iNOS), CD16/32, and major histocompatibility complex II, and produce high levels of oxidative metabolites and pro-inflammatory cytokines. Although essential for host defense and tumor cell killing, they can cause collateral damage to healthy cells/tissue, especially in a “closed” system like the CNS. M2 macrophages express CD206 and Arginase 1 in presence of interleukin (IL)-4 or IL-13, and can enhance angiogenesis, suppress destructive immunity, decrease lesion size, and also promote long-distance axon growth of dorsal root ganglion neurons—even in the presence of growth inhibitory substrates [124]. M1 and M2 are both activated following SCI, but M2 declines and M1 persists (after the first week), resulting in prolonged inflammation. The M2 state can be maintained by PPARγ agonists, which are neuroprotective in SCI. Epigenetic status appears key in regulating the phenotype of macrophages and T-cells during their responses to injury, and HDAC inhibition could promote repair, whilst minimizing inflammatory damage [125]. IFNγ (from activated T-cells) and Toll-like receptor activation can enhance histone acetylation in macrophages, and promotes the M1 phenotype. HDAC3 is a direct regulator of transcription factors promoting M1 in the alternative activation of M1 and M2 states in response to cytokines like IL-4. When HDAC3 is deleted, it allows for the accumulation of M2 macrophages, which produce a pro-repair, anti-inflammatory environment [126]. This balance of reactions involves both PPARγ and Jmjd3m, a demethylase that is critical for M2 polarization in response to concurrent activation by NFκB and Toll-like receptor [127].
Following SCI, microglia also undergo a distinct series of morphological and biochemical changes in state that can be distinct from those of microglia in the brain following physical injury, and can directly lead to acute and prolonged neuropathic pain [128]. Following injury, spinal cord microglia rapidly up-regulate TNF-α, IL-1β, IL-6, nitric oxide (NO), Bradykinin (BK), matrix metalloproteinases, and cathepsin S, all of which initiate and maintain neuropathic pain. In particular, microglial cathepsin S contributes to acute pain following injury via the p38 MAPK pathway—a reaction that can be inhibited by the broad-spectrum HDACi, VPA. Some of these same signaling changes occur in microglia after TBI, where microglia and macrophages become hypomethylated concomitant with a shift in activation state [129]. DNA methylation and histone deactylation changes may thus determine the strength and extent of the immune response following CNS lesion. A different HDAC-specific inhibitor, MS-275 (which preferentially targets HDAC1 and 2), applied 10 days after the initiation of rat experimental autoimmune neuritis suppressed influx of T-cells, B-cells, and macrophages, and facilitated recovery, at the same time as minimizing long-term damage in the rat. MS-275 can also enhance the production of M2 macrophages over M1 [130].
Astrocytes undergo reactive astrogliosis in response to all forms of CNS insults—infection, trauma, ischemia, and neurodegenerative disease—by undergoing hypertrophy and changing gene expression. In rodent models of SCI, astrocytes can be either beneficial or detrimental after injury, depending on the type of lesion and the immediate inflammatory environment. Many of the cytokines released after SCI stimulate reactive astrogliosis, glial scar formation, and neurotoxicity, and can impede endogenous repair (reviewed elsewhere [131–133]). During reactive gliosis there are increases in glial fibrillary acidic protein (GFAP) and cyclooxygenase (COX)-2 in astrocytes, and increases in iNOS, NO, IL-6, and TNF-α in both astrocytes and microglia (reviewed in [122]). Each of these genes can be regulated by HDAC inhibition—usually to attenuate the inflammatory response— in different lesion scenarios. In addition, reactive astrocytes up-regulate glycosaminoglycans, like chondroitin sulfate proteoglycan, and some glycosaminoglycans (e.g., heparin) can themselves act as HAT inhibitors and oppose potential increases in acetylation levels in other cells [134]. It is thus challenging to analyze the effect of HDAC and HAT activation independently on astrocytes following SCI because their activity is intrinsically related to the proximity and state of local microglia and macrophages, the cytokines of which can alter astrocyte acetylation.
Multiple lines of evidence indicate that HDACs play a core role in regulating astrocytic gene expression. Differentiation of neural stem cells into astrocytes appears to require the recruitment of the co-activator complex members, including STAT (Signal Transducer and Activator of Transcription) 1/3, HATs and Smad1 to specific regions of the Gfap promoter, where GFAP is the “prototypic” astrocyte gene [135]. Treatment with HDACi increases the expression of S100 and Gfap in cultured oligodendrocyte cells (OPCs) in neonatal rats [136]. Furthermore, Gfap is actively repressed by complexes containing HDAC, the nuclear receptor co-repressor and adaptor proteins [137], where nuclear receptor co-repressor knockout mice are characterized by precocious expression of Gfap [137, 138].
HDACi and Astrocytes
The responses of astrocytes and microglia to HDAC inhibition is highly context-dependent, and can change with species, HDACi used, and assay system employed. In human microglia and astrocytes in vitro, for example, HDAC inhibition with TSA or VPA can suppress cytokine and chemokine gene expression, with different effects on different groups of cytokines in each cell type [139]. An alternative HDACi, SAHA, also suppresses IFNγ-induced neurotoxicity of human astrocytes through inhibition of the STAT3 signaling pathway. Sodium butyrate and TSA both induce COX-1 (but not COX-2) in a normal astrocyte cell line [140], even though COX-2 expression can be regulated by HDAC inhibition in other non-neural cells (see [141]). Similarly, in mixed cultures of mouse astrocytes and microglia, suppression of HDAC activity using SAHA and ITF2357 (a SAHA analog), inhibited the inflammatory response to lipopolysaccharide (LPS) by direct impairment of transcriptional machinery, with a dramatic inhibition of iNOS and COX-2 induction [142]. In mouse cells, sodium butyrate also inhibits hypoxia-induced iNOS protein and down-regulation of TNF-α messenger RNA, thus reducing the inflammatory response [143]. Sodium butyrate can also induce an adaptive response to LPS-stimulated microglial activation by attenuating NO, IL-6, and TNF secretion—all classic microglial inflammatory responses [144]. However, this anti-inflammatory activity may be specific to short-chain fatty acid HDACi, as the hydroxamate HDACi, TSA and SAHA, strongly potentiate the LPS-induced inflammatory response in primary microglial, neural co-cultures, and hippocampal slices [145]. Activated microglia-conditioned medium can induce HDAC activity in astrocytes (through p38 MAPK or glycogen synthase kinase 3 beta (GSK3β) activation), decreasing H3 acetylation, down-regulating Nrf2 (astroglial nuclear factor involved in anti-oxidant defense), thus decreasing protection. Inhibitors of HDACs, p38 MAPK, and GSK3β, can ameliorate this response in vitro, thus setting the stage for assisting in neuroprotective mechanisms in vivo [146].
Through either direct modulation of transcriptional activity or inhibition of inflammatory signaling in microglia and astrocytes, HDACi’s could, ostensibly, decrease chemokine and cytokine release, decrease neurotoxicity, enhance protection against anti-oxidative damage, and promote functional recovery in rat models of SCI. Thus, modulation of HAT and HDAC function through targeted inhibition could powerfully modulate astrocyte responses, enhance protection, and reduce barriers to regeneration after lesion [133].
Histone Modifications Drive Transitions from Embryonic Stem Cells to Multipotent Neural Stem Cells to CNS Precursors and Differentiated Cells
Chromatin remodeling plays a distinct role during progressive restriction of cell lineage in CNS differentiation at 3 stages (reviewed in [25]): 1) the transition from pluripotent embryonic stem (ES) cells to multipotent neural precursors—where the ES cell chromatin state is comparatively “open” in order to generate all the cell types required in an organism, and suppress expression of genes for lineage commitment and differentiation, whilst retaining DNA in a conformation that is poised for transcription [147]; 2) the restriction of multipotent neural precursors to lineage committed neural or glial precursors—characterized by the stable silencing of genes involved in other lineages (i.e., endoderm and mesoderm) and expression of neural precursor genes; 3) terminal differentiation of specialized cells (i.e., neurons, astrocytes, and oligodendrocytes) from precursors [148]. In considering how to manipulate chromatin to better stimulate adult neural stem cells (NSC) to participate in endogenous repair, or to control stem cells prior to or after SCI transplantation, it is important to understand how the chromatin state of NSCs in adult differs from those in the embryo [149]. This may be particularly important in the spinal cord, where our understanding of adult progenitor subtypes lags far behind that in the adult brain [150].
Histone Acetylation Alters Neural Stem Cell Function
HDACs 1 and 2 are clearly critical for CNS development as their ablation results in disorganization of brain structures and postnatal lethality [151]. In accord, during NSC differentiation, HAT p300 is recruited to GFAP during astrogliogenesis, controlling its expression [152, 153]. Removal of HDAC complexes from neurogenic transcription factors such as NeuroD is also necessary for neuronal differentiation to proceed [154]. The action of HDACi on different classes of CNS progenitors is context- and age-dependent. For example, both VPA and TSA can revert committed oligodendrocyte precursors to multipotent neural progenitors [136, 155], and HDAC inhibition can favor neuronal differentiation at the expense of glial differentiation in both embryonic and adult progenitors in vitro [154, 156, 157]. Furthermore, astrocytes and oligodendrocytes differentiated in vitro from adult hippocampal progenitors have lower levels of acetylated histones H3 and H4 than undifferentiated progenitors or neurons, suggesting that there is more deacetylation during the differentiation of glial lineages [154].
VPA may thus increase neuronal differentiation from adult neural progenitor cells whilst inhibiting astrocyte and oligodendrocyte differentiation [154]. Treatment of rats with VPA during the first 2 weeks postnatally induces a stalling of oligodendrocyte differentiation in the developing rat corpus callosum, which is reversible if VPA is removed for 2 days [158]. We have also recently shown that postnatal Subventricular zone neural stem cell activity and inhibitory neurogenesis, in particular, is also profoundly altered in vivo and in vitro by short-term treatment with both VPA and TSA [72]. In contrast, treatment with VPA after the third week postnatally, when differentiation is largely complete, has little effect on the expression of oligodendrocyte stage-specific proteins, or myelination, indicating a critical time window and transitional function for histone deacetylation in the differentiation of oligodendrocytes [158]. Neuronal differentiation may therefore be a default pathway in the absence of specific HDAC activity, which serves to permit glial differentiation in key developmental time windows.
Remyelination: Epigenetic Regulation of Oligodendrocytes
Oligodendrocytes play 2 opposing roles in a potential repair scenario following SCI. First, their myelin components play a profoundly inhibitory role to axonal regeneration [159]. Even lesioned spinal axons that do have the capacity to initiate a regeneration program may be stopped in their tracks by mature oligodendrocytes and their myelin proteins [160]. Holding back the differentiation of oligodendrocytes, whilst clearing myelin debris, will likely enhance endogenous sprouting and regeneration in spinal pathways. Furthermore, spared axons in the peri-lesion areas become rapidly demyelinated after lesion, and must be re-myelinated in order to restore function. Understanding HDAC action within OPCs mechanistically will be a critical component of identifying time windows after SCI in which to first inhibit OPC differentiation during a period of neurite outgrowth, then allow HDAC action in order to restore remyelination programs. A series of elegant studies has clearly demonstrated that histone deacetylation is critical for oligodendrocyte differentiation (reviewed in [161]), and should be carefully considered in the manipulation of chromatin plasticity in a SCI scenario. Coupled with the profound effect that HDAC inhibition could have on producing myelin to support excitatory neurotransmission [162], HDAC inhibition during repair could result in long-term beneficial shifts in the excitatory/inhibitory balance in different neuronal circuits, depending on the critical period of treatment [163].
HDACi in SCI Repair—The Story So Far
HDAC inhibition is also now being explored clinically to treat neuropsychiatric disease and neurodegenerative disorders [164], but only limited studies have directly tested HDAC inhibition in SCI models. When delivered by minipump in vivo, VPA can attenuate microgliosis in lesioned spinal cord, and purinergic P2X4R expression in activated microglia, which is associated with neuropathic pain [165]. VPA treatment in vitro appears to decrease microglial activation, whereas, in contrast, TSA and sodium butyrate appear to enhance activation. VPA treatment can also enhance neuronal protection and improves open-field behavioral assays following SCI [165]. In an alternative SCI model, VPA also reduced cavitation and gliosis, enhanced neuronal sprouting, and increased the endogenous production of brain- and glial-derived neurotrophic factors around the lesion site [166]. These behavioral or pathological improvements are suggested to be due, in part, to changes in H3 and H4 histone acetylation as early as 1 day after treatment [167]. In addition, VPA concurrent with transplantation of NSCs also enhanced their survival, integration and migration, and improved their neuronal differentiation, promoting some functional recovery [168]. Despite variations in these findings—perhaps owing to varying methods of administration (intravenous, intrathecally, or by mini-pump)—VPA could be effective in SCI if given early after injury, but has many off-target effects that may make it less desirable. Finally, drugs that target class II (but not class I) HDACs have recently emerged as potential tools for inhibiting the development of inflammatory hyperalgesia [169], a common problem after SCI. Thus, cell-specific action of distinct HDACs appears to be the beginning of being able to specifically target the cells and symptoms that accompany different phases of SCI (summarized in Fig. 2).
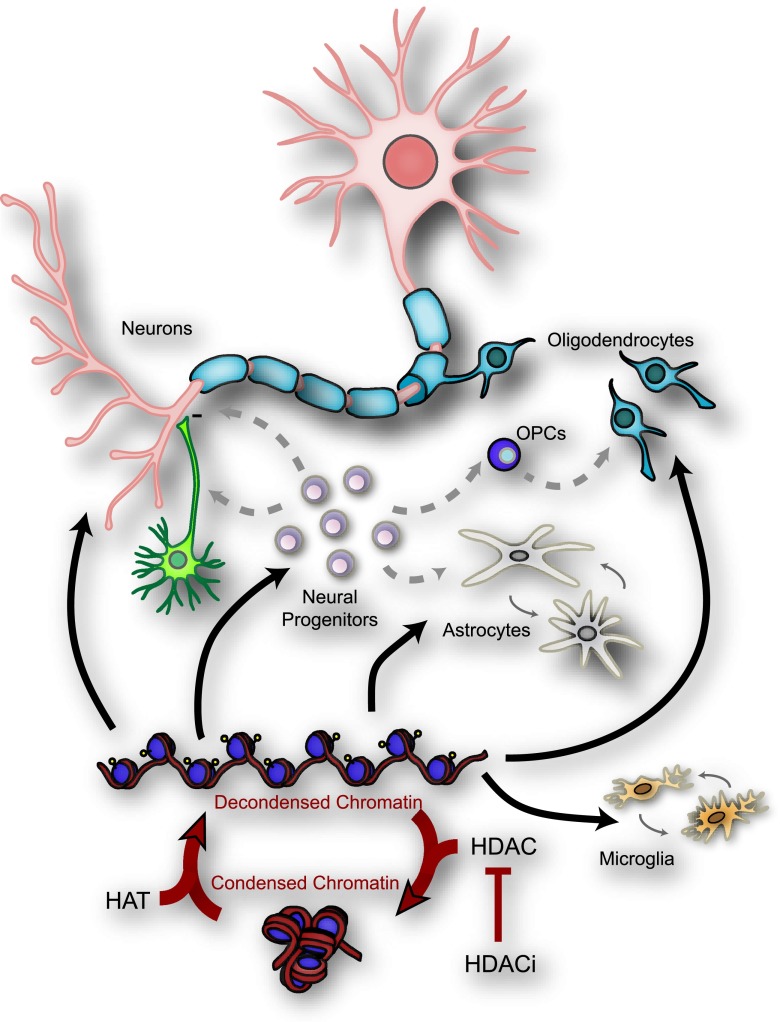
Fig. 2.
Epigenetic regulation of histone acetylation in different cells participating in spinal cord injury repair. Acetylation of lysine residues in histone tails constitutes the major post-translational histone modification. Histone Acetyl Transferase (HAT) and histone deacetylase (HDAC) can affect many cell functions through chromatin remodeling or post-translational modifications of transcription factors and cytoplasmic proteins. HDAC inhibitors (HDACi) act on many cell types in the central nervous system: neurons, astrocytes, oligodendrocytes, microglia, and neural progenitors. OPC = oligodendrocyte precursor cells
Moving Towards Targeted Chromatin-Based Therapy for SCI
Unlocking the activity of pro-regenerative transcription factors and allowing greater access—and recruitment [30]—to promoters of pro-regenerative genes could be a powerful approach to drive neuronal regeneration. Equally important, manipulating chromatin to block access to gene loci in glia and inflammatory cells that currently inhibit regeneration could transiently remove blocks to repair. In an ideal SCI repair scenario, you would want—in defined time windows—to 1) minimize neuroinflammation and promote protection; 2) promote regeneration of spared neurons; 3) harness progenitor activity to best respond to the needs of the environment; and 4) promote re-myelination of spared and regenerated tracts. Each of these processes can be differentially impacted by HDAC inhibition—depending on the experimental paradigm used. Timing truly is everything if we are to consider targeting individual cell types at different phases of SCI pathology to promote repair, at the same time as minimizing side-effects (Figs. 1 and 2).
A number of HDACi’s are already in use as anticancer agents because they are able to increase genes involved in growth arrest and promote apoptosis of cancer cells, but new-generation and highly-specific HDACi are emerging almost weekly. Use of HDACi for therapeutic purposes within the CNS is, however, still a topic of intensive debate, as their mechanisms of action in different CNS cells are only beginning to be understood, and histone manipulation yields conflicting results at different ages, even in the same model [170]. We are approaching a time when targeting HDAC inhibition in time and space and carefully monitoring its activity will be possible. New inhibitors aimed at individual HDACs enriched in distinct cells, coupled with assays of acetylation and methylation, and high-throughput genomic analysis of chromatin modification in small numbers of peripheral and CNS cells will allow us to test the specificity of response. Luciferase reporter mice allow us to observe the efficacy of intervention during the lesion response in vivo. Biomarkers analysis from serum and cerebrospinal fluid that change with glial-immune activation will also help to better monitor the effectiveness of this kind of pharmacological intervention.
With clinical data already highlighting a broad range of pathological and functional outcomes in human SCI patients with the same lesions, we should begin to consider the effect that an individual’s life experience has had on their epigenome, and better assess how to build upon this to enhance and design their own profile of recovery. At the brink of a revolution in personalized medicine, fully understanding a patient’s epigenetic status at the time of injury may not only help us to understand disparities in outcome, but also help to define the best course of action in manipulating their own epigenetic status to facilitate optimal functional recovery.
Electronic supplementary material
(PDF 1224 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 2.Dumont RJ, Okonkwo DO, Verma S, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254–264. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. Biomed Res Int. 2013;2013:786475. doi: 10.1155/2013/786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5:407–413. doi: 10.1111/j.1750-3639.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 5.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 6.Yip PK, Malaspina A. Spinal cord trauma and the molecular point of no return. Mol Neurodegener. 2012;7:6. doi: 10.1186/1750-1326-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 8.Su H, Wu Y, Yuan Q, Guo J, Zhang W, Wu W. Optimal time point for neuronal generation of transplanted neural progenitor cells in injured spinal cord following root avulsion. Cell Transplant. 2011;20:167–176. doi: 10.3727/096368910X522090. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DK HE. Pathophysiology of spinal cord trauma. Ann Emerg Med 1993;22:987–992. [DOI] [PubMed]
- 10.Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury--a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med. 1991;324:1829–1838. doi: 10.1056/NEJM199106273242601. [DOI] [PubMed] [Google Scholar]
- 11.Lapchak PA, Araujo DM, Song D, Zivin JA. Neuroprotection by the selective cyclooxygenase-2 inhibitor SC-236 results in improvements in behavioral deficits induced by reversible spinal cord ischemia. Stroke. 2001;32:1220–1225. doi: 10.1161/01.STR.32.5.1220. [DOI] [PubMed] [Google Scholar]
- 12.Blight AR, Young W. Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J Neurol Sci. 1989;91:15–34. doi: 10.1016/0022-510X(89)90073-7. [DOI] [PubMed] [Google Scholar]
- 13.McIntosh TK, Juhler M, Wieloch T. Novel pharmacologic strategies in the treatment of experimental traumatic brain injury: 1998. J Neurotrauma. 1998;15:731–69. doi: 10.1089/neu.1998.15.731. [DOI] [PubMed] [Google Scholar]
- 14.Rhoney DH, Luer MS, Hughes M, Hatton J. New pharmacologic approaches to acute spinal cord injury. Pharmacotherapy. 1996;16:382–392. [PubMed] [Google Scholar]
- 15.Tator CH. Pathophysiology and pathology of spinal cord injury. In Neurosurgery, Wilkins RH RS, editor. Baltimore: Williams & Wilkins, 1996, p. 2847–2859.
- 16.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 17.Zhang N, Yin Y, Xu SJ, Wu YP, Chen WS. Inflammation & apoptosis in spinal cord injury. Indian J Med Res. 2012;135:287–296. [PMC free article] [PubMed] [Google Scholar]
- 18.Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol. 2011;24:577–583. doi: 10.1097/WCO.0b013e32834c208d. [DOI] [PubMed] [Google Scholar]
- 19.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 20.Campbell SJ, Perry VH, Pitossi FJ, et al. Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol. 2005;166:1487–1497. doi: 10.1016/S0002-9440(10)62365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaerve A, Muller HW. Chemokines in CNS injury and repair. Cell Tissue Res. 2012;349:229–248. doi: 10.1007/s00441-012-1427-3. [DOI] [PubMed] [Google Scholar]
- 22.Kakulas BA. A review of the neuropathology of human spinal cord injury with emphasis on special features. J Spinal Cord Med. 1999;22:119–1124. doi: 10.1080/10790268.1999.11719557. [DOI] [PubMed] [Google Scholar]
- 23.Deumens R, Koopmans GC, Honig WM, et al. Chronically injured corticospinal axons do not cross large spinal lesion gaps after a multifactorial transplantation strategy using olfactory ensheathing cell/olfactory nerve fibroblast-biomatrix bridges. J Neurosci Res. 2006;83:811–820. doi: 10.1002/jnr.20768. [DOI] [PubMed] [Google Scholar]
- 24.Failli V, Kopp MA, Gericke C, et al. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain. 2012;135:3238–3250. doi: 10.1093/brain/aws267. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald JL, Roskams AJ. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog Neurobiol. 2009;88:170–183. doi: 10.1016/j.pneurobio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Day JJ, Sweatt JD. Epigenetic mechanisms in cognition. Neuron. 2011;70:813–829. doi: 10.1016/j.neuron.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maze I, Noh KM, Allis CD. Histone regulation in the CNS: basic principles of epigenetic plasticity. Neuropsychopharmacology. 2013;38:3–22. doi: 10.1038/npp.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 31.Turner BM. Histone acetylation and an epigenetic code. Bioessays. 2000;22:836–845. doi: 10.1002/1521-1878(200009)22:9<836::AID-BIES9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 32.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 33.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 34.Spitale RC, Tsai MC, Chang HY. RNA templating the epigenome: long noncoding RNAs as molecular scaffolds. Epigenetics. 2011;6:539–543. doi: 10.4161/epi.6.5.15221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bird A. The essentials of DNA methylation. Cell. 1992;70:5–8. doi: 10.1016/0092-8674(92)90526-I. [DOI] [PubMed] [Google Scholar]
- 36.Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 37.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 38.Ramsahoye BH, Biniszkiewicz D, Lyko F, Clark V, Bird AP, Jaenisch R. Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a. Proc Natl Acad Sci U S A. 2000;97:5237–5242. doi: 10.1073/pnas.97.10.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-F. [DOI] [PubMed] [Google Scholar]
- 40.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 41.Nan X, Cross S, Bird A. Gene silencing by methyl-CpG-binding proteins. Novartis Found Symp. 1998;214:6–16. doi: 10.1002/9780470515501.ch2. [DOI] [PubMed] [Google Scholar]
- 42.Tanner KG, Trievel RC, Kuo MH, et al. Catalytic mechanism and function of invariant glutamic acid 173 from the histone acetyltransferase GCN5 transcriptional coactivator. J Biol Chem. 1999;274:18157–18160. doi: 10.1074/jbc.274.26.18157. [DOI] [PubMed] [Google Scholar]
- 43.Tanner KG, Langer MR, Kim Y, Denu JM. Kinetic mechanism of the histone acetyltransferase GCN5 from yeast. J Biol Chem. 2000;275:22048–22055. doi: 10.1074/jbc.M002893200. [DOI] [PubMed] [Google Scholar]
- 44.Lau OD, Courtney AD, Vassilev A, et al. p300/CBP-associated factor histone acetyltransferase processing of a peptide substrate. Kinetic analysis of the catalytic mechanism. J Biol Chem. 2000;275:21953–21959. doi: 10.1074/jbc.M003219200. [DOI] [PubMed] [Google Scholar]
- 45.Tanner KG, Langer MR, Denu JM. Kinetic mechanism of human histone acetyltransferase P/CAF. Biochemistry. 2000;39:15652. doi: 10.1021/bi005121q. [DOI] [PubMed] [Google Scholar]
- 46.Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol. 2001;13:477–483. doi: 10.1097/00001622-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Villar-Garea A, Esteller M. Histone deacetylase inhibitors: understanding a new wave of anticancer agents. Int J Cancer. 2004;112:171–178. doi: 10.1002/ijc.20372. [DOI] [PubMed] [Google Scholar]
- 48.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai LL, Courtine G, Fong AJ, Burdick JW, Roy RR, Edgerton VR. Plasticity of functional connectivity in the adult spinal cord. Philos Trans R Soc Lond B Biol Sci. 2006;361:1635–1646. doi: 10.1098/rstb.2006.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edgerton VR, Roy RR. A new age for rehabilitation. Eur J Phys Rehabil Med. 2012;48:99–109. [PubMed] [Google Scholar]
- 51.Lorenz DJ, Datta S, Harkema SJ. Longitudinal patterns of functional recovery in patients with incomplete spinal cord injury receiving activity-based rehabilitation. Arch Phys Med Rehabil. 2012;93:1541–1552. doi: 10.1016/j.apmr.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 52.Furlan JC, Fehlings MG. The impact of age on mortality, impairment, and disability among adults with acute traumatic spinal cord injury. J Neurotrauma. 2009;26:1707–1717. doi: 10.1089/neu.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeVivo MJ, Kartus PL, Stover SL, Fine PR. Benefits of early admission to an organised spinal cord injury care system. Paraplegia. 1990;28:545–555. doi: 10.1038/sc.1990.74. [DOI] [PubMed] [Google Scholar]
- 54.Krause JS, Crewe NM. Chronologic age, time since injury, and time of measurement: effect on adjustment after spinal cord injury. Arch Phys Med Rehabil. 1991;72:91–100. [PubMed] [Google Scholar]
- 55.Scivoletto G, Mancini M, Fiorelli E, Morganti B, Molinari M. A prototype of an adjustable advanced reciprocating gait orthosis (ARGO) for spinal cord injury (SCI) Spinal Cord. 2003;41:187–191. doi: 10.1038/sj.sc.3101417. [DOI] [PubMed] [Google Scholar]
- 56.Leung PY, Wrathall JR. Local and distal responses to injury in the rapid functional recovery from spinal cord contusion in rat pups. Exp Neurol. 2006;202:225–237. doi: 10.1016/j.expneurol.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 57.Siegenthaler MM, Berchtold NC, Cotman CW, Keirstead HS. Voluntary running attenuates age-related deficits following SCI. Exp Neurol. 2008;210:207–216. doi: 10.1016/j.expneurol.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gwak YS, Hains BC, Johnson KM, Hulsebosch CE. Locomotor recovery and mechanical hyperalgesia following spinal cord injury depend on age at time of injury in rat. Neurosci Lett. 2004;362:232–235. doi: 10.1016/j.neulet.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 59.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 60.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 61.Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma RP, Grayson DR, Guidotti A, Costa E. Chromatin, DNA methylation and neuron gene regulation—the purpose of the package. J Psychiatry Neurosci. 2005;30:257–263. [PMC free article] [PubMed] [Google Scholar]
- 63.Grayson DR, Jia X, Chen Y, et al. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veldic M, Caruncho HJ, Liu WS, et al. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoo AS, Crabtree GR. ATP-dependent chromatin remodeling in neural development. Curr Opin Neurobiol. 2009;19:120–126. doi: 10.1016/j.conb.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siegmund KD, Connor CM, Campan M, et al. DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS One. 2007;2:e895. doi: 10.1371/journal.pone.0000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ladd-Acosta C, Pevsner J, Sabunciyan S, et al. DNA methylation signatures within the human brain. Am J Hum Genet. 2007;81:1304–1315. doi: 10.1086/524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sng J, Meaney MJ. Environmental regulation of the neural epigenome. Epigenomics. 2009;1:131–151. doi: 10.2217/epi.09.21. [DOI] [PubMed] [Google Scholar]
- 71.Hsieh J, Gage FH. Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol. 2005;17:664–671. doi: 10.1016/j.ceb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Foti SB, Chou A, Moll AD, Roskams AJ. HDAC inhibitors dysregulate neural stem cell activity in the postnatal mouse brain. Int J Dev Neurosci 2013;31(6):437–447. [DOI] [PubMed]
- 73.Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- 74.Dulac C. Brain function and chromatin plasticity. Nature. 2010;465:728–735. doi: 10.1038/nature09231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maze I, Nestler EJ. The epigenetic landscape of addiction. Ann N Y Acad Sci. 2011;1216:99–113. doi: 10.1111/j.1749-6632.2010.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swank MW, Sweatt JD. Increased histone acetyltransferase and lysine acetyltransferase activity and biphasic activation of the ERK/RSK cascade in insular cortex during novel taste learning. J Neurosci. 2001;21:3383–3391. doi: 10.1523/JNEUROSCI.21-10-03383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chwang WB, O'Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brami-Cherrier K, Valjent E, Herve D, et al. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J Neurosci. 2007;27:12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghasemlou N, Lopez-Vales R, Lachance C, et al. Mitogen-activated protein kinase-activated protein kinase 2 (MK2) contributes to secondary damage after spinal cord injury. J Neurosci. 2010;30:13750–13759. doi: 10.1523/JNEUROSCI.2998-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lubin FD, Sweatt JD. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 2007;55:942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yeh SH, Lin CH, Gean PW. Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol. 2004;65:1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]
- 83.Jeong JW, Bae MK, Ahn MY, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/S0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- 84.Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 85.Oliveira AM, Wood MA, McDonough CB, Abel T. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn Mem. 2007;14:564–572. doi: 10.1101/lm.656907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim SY, Levenson JM, Korsmeyer S, Sweatt JD, Schumacher A. Developmental regulation of Eed complex composition governs a switch in global histone modification in brain. J Biol Chem. 2007;282:9962–9972. doi: 10.1074/jbc.M608722200. [DOI] [PubMed] [Google Scholar]
- 87.Chandramohan Y, Droste SK, Reul JM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-D-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J Neurochem. 2007;101:815–828. doi: 10.1111/j.1471-4159.2006.04396.x. [DOI] [PubMed] [Google Scholar]
- 88.Wu SC, Zhang Y. Minireview: role of protein methylation and demethylation in nuclear hormone signaling. Mol Endocrinol. 2009;23:1323–1334. doi: 10.1210/me.2009-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85:614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 90.Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog Neurobiol. 2011;93:182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 91.Stangl D, Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr. 2009;4:271–282. doi: 10.1007/s12263-009-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson's disease. J Neurochem. 2002;80:101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 94.Kruman II, Kumaravel TS, Lohani A, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer's disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 96.Shea TB, Lyons-Weiler J, Rogers E. Homocysteine, folate deprivation and Alzheimer neuropathology. J Alzheimers Dis. 2002;4:261–267. doi: 10.3233/jad-2002-4401. [DOI] [PubMed] [Google Scholar]
- 97.Kaelin WG, Jr, McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153:56–69. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jeong MA, Plunet W, Streijger F, et al. Intermittent fasting improves functional recovery after rat thoracic contusion spinal cord injury. J Neurotrauma. 2011;28:479–492. doi: 10.1089/neu.2010.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Streijger F, Plunet WT, Plemel JR, Lam CK, Liu J, Tetzlaff W. Intermittent fasting in mice does not improve hindlimb motor performance after spinal cord injury. J Neurotrauma. 2011;28:1051–1061. doi: 10.1089/neu.2010.1715. [DOI] [PubMed] [Google Scholar]
- 100.Guan JS, Haggarty SJ, Giacometti E, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vogel-Ciernia A, Matheos DP, Barrett RM, et al. The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nat Neurosci. 2013;16:552–561. doi: 10.1038/nn.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rouaux C, Jokic N, Mbebi C, Boutillier S, Loeffler JP, Boutillier AL. Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. EMBO J. 2003;22:6537–4659. doi: 10.1093/emboj/cdg615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boutillier AL, Trinh E, Loeffler JP. Selective E2F-dependent gene transcription is controlled by histone deacetylase activity during neuronal apoptosis. J Neurochem. 2003;84:814–828. doi: 10.1046/j.1471-4159.2003.01581.x. [DOI] [PubMed] [Google Scholar]
- 104.Hou ST, Jiang SX, Smith RA. Permissive and repulsive cues and signalling pathways of axonal outgrowth and regeneration. Int Rev Cell Mol Biol. 2008;267:125–181. doi: 10.1016/S1937-6448(08)00603-5. [DOI] [PubMed] [Google Scholar]
- 105.Doucet G, Petit A. Seeking axon guidance molecules in the adult rat CNS. Prog Brain Res. 2002;137:453–465. doi: 10.1016/S0079-6123(02)37036-5. [DOI] [PubMed] [Google Scholar]
- 106.Gaillard S, Nasarre C, Gonthier B, Bagnard D. [The cellular and molecular basis of axonal growth]. Rev Neurol (Paris) 2005;161:153–172. [DOI] [PubMed]
- 107.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 108.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 109.DeBellard ME, Tang S, Mukhopadhyay G, Shen YJ, Filbin MT. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol Cell Neurosci. 1996;7:89–101. doi: 10.1006/mcne.1996.0007. [DOI] [PubMed] [Google Scholar]
- 110.Moore DL, Goldberg JL. Multiple transcription factor families regulate axon growth and regeneration. Dev Neurobiol 2011;71:1186–1211. [DOI] [PMC free article] [PubMed]
- 111.Muramatsu R, Ueno M, Yamashita T. Intrinsic regenerative mechanisms of central nervous system neurons. Biosci Trends. 2009;3:179–183. [PubMed] [Google Scholar]
- 112.Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci 2011;34:131–152. [DOI] [PubMed]
- 113.Trakhtenberg EF, Goldberg JL. Epigenetic regulation of axon and dendrite growth. Front Mol Neurosci 2012;5:24. [DOI] [PMC free article] [PubMed]
- 114.Macdonald JL, Verster A, Berndt A, Roskams AJ. MBD2 and MeCP2 regulate distinct transitions in the stage-specific differentiation of olfactory receptor neurons. Mol Cell Neurosci. 2010;44:55–67. doi: 10.1016/j.mcn.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 115.Smrt RD, Eaves-Egenes J, Barkho BZ, et al. Mecp2 deficiency leads to delayed maturation and altered gene expression in hippocampal neurons. Neurobiol Dis. 2007;27:77–89. doi: 10.1016/j.nbd.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gaub P, Tedeschi A, Puttagunta R, Nguyen T, Schmandke A, Di Giovanni S. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 2010;17:1392–1408. doi: 10.1038/cdd.2009.216. [DOI] [PubMed] [Google Scholar]
- 117.Li S, Overman JJ, Katsman D, et al. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci. 2010;13:1496–1504. doi: 10.1038/nn.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hubbert C, Guardiola A, Shao R, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 119.Rivieccio MA, Brochier C, Willis DE, et al. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc Natl Acad Sci U S A. 2009;106:19599–19604. doi: 10.1073/pnas.0907935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim JY, Shen S, Dietz K, et al. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat Neurosci. 2010;13:180–189. doi: 10.1038/nn.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tapia M, Wandosell F, Garrido JJ. Impaired function of HDAC6 slows down axonal growth and interferes with axon initial segment development. PLoS One. 2010;5:e12908. doi: 10.1371/journal.pone.0012908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Langley B, Gensert JM, Beal MF, Ratan RR. Remodeling chromatin and stress resistance in the central nervous system: histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr Drug Targets CNS Neurol Disord. 2005;4:41–50. doi: 10.2174/1568007053005091. [DOI] [PubMed] [Google Scholar]
- 123.Carmichael ST. Gene expression changes after focal stroke, traumatic brain and spinal cord injuries. Curr Opin Neurol. 2003;16:699–704. doi: 10.1097/00019052-200312000-00009. [DOI] [PubMed] [Google Scholar]
- 124.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Halili MA, Andrews MR, Sweet MJ, Fairlie DP. Histone deacetylase inhibitors in inflammatory disease. Curr Top Med Chem. 2009;9:309–319. doi: 10.2174/156802609788085250. [DOI] [PubMed] [Google Scholar]
- 126.Mullican SE, Gaddis CA, Alenghat T, et al. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 2011;25:2480–2488. doi: 10.1101/gad.175950.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takeuch O, Akira S. Epigenetic control of macrophage polarization. Eur J Immunol. 2011;41:2490–2493. doi: 10.1002/eji.201141792. [DOI] [PubMed] [Google Scholar]
- 128.Schomberg D, Olson JK. Immune responses of microglia in the spinal cord: contribution to pain states. Exp Neurol. 2012;234:262–270. doi: 10.1016/j.expneurol.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 129.Zhang ZY, Zhang Z, Fauser U, Schluesener HJ. Global hypomethylation defines a sub-population of reactive microglia/macrophages in experimental traumatic brain injury. Neurosci Lett. 2007;429:1–6. doi: 10.1016/j.neulet.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 130.Zhang ZY, Zhang Z, Schluesener HJ. MS-275, an histone deacetylase inhibitor, reduces the inflammatory reaction in rat experimental autoimmune neuritis. Neuroscience. 2010;169:370–377. doi: 10.1016/j.neuroscience.2010.04.074. [DOI] [PubMed] [Google Scholar]
- 131.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 132.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 133.Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics. 2010;7:494–506. doi: 10.1016/j.nurt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Buczek-Thomas JA, Hsia E, Rich CB, Foster JA, Nugent MA. Inhibition of histone acetyltransferase by glycosaminoglycans. J Cell Biochem. 2008;105:108–120. doi: 10.1002/jcb.21803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nakashima K, Yanagisawa M, Arakawa H, et al. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 136.Liu A, Han YR, Li J, et al. The glial or neuronal fate choice of oligodendrocyte progenitors is modulated by their ability to acquire an epigenetic memory. J Neurosci. 2007;27:7339–7343. doi: 10.1523/JNEUROSCI.1226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 138.Hermanson O, Jepsen K, Rosenfeld MG. N-CoR controls differentiation of neural stem cells into astrocytes. Nature. 2002;419:934–939. doi: 10.1038/nature01156. [DOI] [PubMed] [Google Scholar]
- 139.Suh HS, Choi S, Khattar P, Choi N, Lee SC. Histone deacetylase inhibitors suppress the expression of inflammatory and innate immune response genes in human microglia and astrocytes. J Neuroimmune Pharmacol. 2010;5:521–532. doi: 10.1007/s11481-010-9192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taniura S, Kamitani H, Watanabe T, Eling TE. Transcriptional regulation of cyclooxygenase-1 by histone deacetylase inhibitors in normal human astrocyte cells. J Biol Chem. 2002;277:16823–16830. doi: 10.1074/jbc.M200527200. [DOI] [PubMed] [Google Scholar]
- 141.Tong X, Yin L, Giardina C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem Biophys Res Commun. 2004;317:463–471. doi: 10.1016/j.bbrc.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 142.Faraco G, Pittelli M, Cavone L, et al. Histone deacetylase (HDAC) inhibitors reduce the glial inflammatory response in vitro and in vivo. Neurobiol Dis. 2009;36:269–279. doi: 10.1016/j.nbd.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 143.Qi X, Hosoi T, Okuma Y, Kaneko M, Nomura Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol Pharmacol. 2004;66:899–908. doi: 10.1124/mol.104.001339. [DOI] [PubMed] [Google Scholar]
- 144.Huuskonen J, Suuronen T, Nuutinen T, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol. 2004;141:874–880. doi: 10.1038/sj.bjp.0705682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suuronen T, Huuskonen J, Pihlaja R, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by histone deacetylase inhibitors. J Neurochem. 2003;87:407–416. doi: 10.1046/j.1471-4159.2003.02004.x. [DOI] [PubMed] [Google Scholar]
- 146.Correa F, Mallard C, Nilsson M, Sandberg M. Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: restoring effects of inhibitors of HDACs, p38 MAPK and GSK3beta. Neurobiol Dis. 2011;44:142–151. doi: 10.1016/j.nbd.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meshorer E. Chromatin in embryonic stem cell neuronal differentiation. Histol Histopathol. 2007;22:311–319. doi: 10.14670/HH-22.311. [DOI] [PubMed] [Google Scholar]
- 148.Lee S, Lee SK. Crucial roles of histone-modifying enzymes in mediating neural cell-type specification. Curr Opin Neurobiol. 2010;20:29–36. doi: 10.1016/j.conb.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Juliandi B, Abematsu M, Nakashima K. Epigenetic regulation in neural stem cell differentiation. Dev Growth Differ 2010;52:493–504. [DOI] [PubMed]
- 150.Petit A, Sanders AD, Kennedy TE, et al. Adult spinal cord radial glia display a unique progenitor phenotype. PLoS One. 2011;6:e24538. doi: 10.1371/journal.pone.0024538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Montgomery RL, Hsieh J, Barbosa AC, Richardson JA, Olson EN. Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proc Natl Acad Sci U S A. 2009;106:7876–7881. doi: 10.1073/pnas.0902750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Asklund T, Appelskog IB, Ammerpohl O, Ekstrom TJ, Almqvist PM. Histone deacetylase inhibitor 4-phenylbutyrate modulates glial fibrillary acidic protein and connexin 43 expression, and enhances gap-junction communication, in human glioblastoma cells. Eur J Cancer. 2004;40:1073–1081. doi: 10.1016/j.ejca.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 153.Song MR, Ghosh A. FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat Neurosci. 2004;7:229–235. doi: 10.1038/nn1192. [DOI] [PubMed] [Google Scholar]
- 154.Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci U S A. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lyssiotis CA, Walker J, Wu C, Kondo T, Schultz PG, Wu X. Inhibition of histone deacetylase activity induces developmental plasticity in oligodendrocyte precursor cells. Proc Natl Acad Sci U S A. 2007;104:14982–14987. doi: 10.1073/pnas.0707044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Laeng P, Pitts RL, Lemire AL, et al. The mood stabilizer valproic acid stimulates GABA neurogenesis from rat forebrain stem cells. J Neurochem. 2004;91:238–251. doi: 10.1111/j.1471-4159.2004.02725.x. [DOI] [PubMed] [Google Scholar]
- 157.Siebzehnrubl FA, Buslei R, Eyupoglu IY, Seufert S, Hahnen E, Blumcke I. Histone deacetylase inhibitors increase neuronal differentiation in adult forebrain precursor cells. Exp Brain Res. 2007;176:672–678. doi: 10.1007/s00221-006-0831-x. [DOI] [PubMed] [Google Scholar]
- 158.Shen S, Li J, Casaccia-Bonnefil P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J Cell Biol. 2005;169:577–589. doi: 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chaudhry N, Filbin MT. Myelin-associated inhibitory signaling and strategies to overcome inhibition. J Cereb Blood Flow Metab. 2007;27:1096–1107. doi: 10.1038/sj.jcbfm.9600407. [DOI] [PubMed] [Google Scholar]
- 160.Giger RJ, Venkatesh K, Chivatakarn O, et al. Mechanisms of CNS myelin inhibition: evidence for distinct and neuronal cell type specific receptor systems. Restor Neurol Neurosci. 2008;26:97–115. [PMC free article] [PubMed] [Google Scholar]
- 161.Liu J, Casaccia P. Epigenetic regulation of oligodendrocyte identity. Trends Neurosci. 2010;33:193–201. doi: 10.1016/j.tins.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rosenzweig I, Vukadinovic Z, Turner AJ, Catani M. Neuroconnectivity and valproic acid: the myelin hypothesis. Neurosci Biobehav Rev. 2012;36:1848–1856. doi: 10.1016/j.neubiorev.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 163.Roth TL, Sweatt JD. Annual research review: Epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. J Child Psychol Psychiatry. 2011;52:398–408. doi: 10.1111/j.1469-7610.2010.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lu WH, Wang CY, Chen PS, et al. Valproic acid attenuates microgliosis in injured spinal cord and purinergic P2X4 receptor expression in activated microglia. J Neurosci Res. 2013;91:694–705. doi: 10.1002/jnr.23200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abdanipour A, Schluesener HJ, Tiraihi T. Effects of valproic acid, a histone deacetylase inhibitor, on improvement of locomotor function in rat spinal cord injury based on epigenetic science. Iran Biomed J. 2012;16:90–100. doi: 10.6091/ibj.1060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lv L, Sun Y, Han X, Xu CC, Tang YP, Dong Q. Valproic acid improves outcome after rodent spinal cord injury: potential roles of histone deacetylase inhibition. Brain Res. 2011;1396:60–68. doi: 10.1016/j.brainres.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 168.Abematsu M, Tsujimura K, Yamano M, et al. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest. 2010;120:3255–3266. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bai G, Wei D, Zou S, Ren K, Dubner R. Inhibition of class II histone deacetylases in the spinal cord attenuates inflammatory hyperalgesia. Mol Pain. 2010;6:51. doi: 10.1186/1744-8069-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shen S, Sandoval J, Swiss VA, et al. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)