Abstract
Background/Objectives
The purpose of this study is to compare surgically resected intraductal papillary mucinous neoplasms (IPMNs) in patients with and without a family history of pancreatic cancer to gain insight into differences that may suggest the need for differential management.
Methods
A retrospective review of patients who underwent resection of an IPMN at the Massachusetts General Hospital (1990–2011) was conducted. Three hundred and twenty-four patients of whom 45 (13.9%) had a family history of pancreatic cancer were identified. Patients with (PFH) and without (NFH) a family history of pancreatic cancer were compared.
Results
There were no differences in demographic characteristics between groups. Extra-pancreatic malignancies diagnosed prior to the IPMN were more common in those with a PFH (35.6% vs 20.1%, p = 0.03). There were no differences in IPMN characteristics between groups including no difference in the presence of invasive disease (p = 0.55). Concurrent pancreatic ductal adenocarcinomas were more common in those with a PFH (11.1% vs 2.9%, p = 0.02). The survival in the PFH group was marginally lower than the NFH group, a difference found to be attributable to the higher prevalence of extra-pancreatic malignancies.
Conclusion
Characteristics of surgically resected IPMNs are not different between patients with and without a family history of pancreatic cancer. Most importantly, the incidence of invasive disease is not different, suggesting that these lesions may not be more aggressive when they occur in the presence of a family history of pancreatic cancer.
Keywords: Intraductal papillary mucinous neoplasm, Family history, Pancreatic cancer
1. Introduction
Intraductal papillary mucinous neoplasms (IPMNs) are cystic neoplasms of the pancreas characterized by papillary projections of duct epithelium, mucin-production and dilation of the pancreatic duct, with an associated risk of malignant transformation [1]. Most IPMNs are currently identified as incidental findings on cross-sectional imaging, and because their natural history is mostly unknown, it is unclear what proportion of these lesions will undergo malignant transformation and when this progression will occur.
An association between IPMN and a family history of pancreatic cancer has been recently suggested. Canto et al. [2] reported that the screening of high-risk individuals with a substantive family history of pancreatic cancer resulted in the detection of an IPMN in 10% of patients. Additionally, Shi et al. [3] demonstrated associated IPMN lesions in 18% of pancreatic cancer specimens in patients with a family history of pancreatic cancer. However, to date, there is no data in the literature to provide guidance regarding whether IPMN lesions diagnosed in patients with a family history of pancreatic cancer are more aggressive, and therefore should be managed differently from those occurring in patients without a positive family history.
The purpose of this study is to compare IPMN patients with and without a family history of pancreatic cancer from our institutional experience with surgically resected IPMNs. Our goal is to gain insight into any important differences that may suggest that IPMNs occurring in patients with a family history of pancreatic cancer should be managed differently from those that occur in the absence of such family history.
2. Methods
After Institutional Review Board approval we identified 324 patients who underwent surgical resection of an IPMN of the pancreas at the Massachusetts General Hospital between August 1990 and February 2011. Patients were identified with an institutional database that is maintained to monitor all patients who undergo surgical resection for a cystic neoplasm of the pancreas, and the IPMN diagnosis was confirmed by an expert pathologist (as described below). All patients had a known cystic neoplasm of the pancreas prior to surgical resection and in no incidence was the cystic pancreatic lesion discovered during operative intervention for another indication. A retrospective chart review was performed using only those patients in whom the diagnosis of an IPMN was confirmed based on review of the resection specimen.
2.1. Data collection
Demographic characteristics including patient age at diagnosis, sex and ethnicity as well as the existence of a smoking history, diabetes, symptoms (acute pancreatitis, abdominal pain, jaundice, steatorrhea, weight loss) related to the pancreatic lesion and the presence of other malignancies both prior to and following the diagnosis of IPMN were recorded. The existence of one or more family members with pancreatic cancer and the relation of this relative to the patient were noted. CA 19-9 level at the time of diagnosis and radiologic findings regarding tumor size, tumor location (head/neck/uncinate, body, tail, 2 involved regions, diffuse) and cyst character (multi-cystic, single-cyst, no cyst) in addition to type of operative intervention performed were recorded.
All pathologic specimens from the operative resection were individually reviewed by an expert pathologist in the field (M.M.K.) and were confirmed to be an IPMN, regardless of classification on the initial pathology report. Only those patients with specimens that satisfied the diagnostic criteria for IPMNs as defined by the World Health Organization as a neoplasm with tall, columnar, mucin-containing epithelium with or without papillary proliferations and involving the main pancreatic ducts and/or major side branches [4] were included. The presence of ovarian-like stroma was an exclusion criterion. Pathologically confirmed IPMNs were further classified based on the IPMN type (main-duct, branch-duct or combined variant), epithelial type (gastric, intestinal, pancreaticobiliary, oncocytic or mixed) and the degree of cytologic and architectural dysplasia (low-, intermediate- or high-grade). All lesions were assessed for the presence of invasion, defined as an IPMN with an associated infiltrating carcinoma. Invasive lesions were further assessed for degree of the invasive component (superficial, macroscopic) and invasive type (colloid, tubular, oncocytic, mixed). The presence of a pancreatic intraepithelial neoplasia (PanIN) in the resected specimen and the grade of the lesion were recorded, as was data regarding other malignancies incidentally diagnosed at the time of operative intervention for the IPMN. Pancreatic ductal adenocarcinomas (PDACs) that were noted in the pancreas specimen were considered a concurrent PDAC only in those cases in which invasive components were physically (anatomically) distant from the IPMN lesion and the IPMN background had only low- to (at most) intermediate-grade epithelium with no high-grade dysplasia.
Follow-up information including status at the most recent follow-up, recurrence of disease, death and cause of death were collected. This information was obtained by a combination of chart review and direct contact with the patient or family, as well as from the social security index. Cause of death could not be accurately obtained for all patients; thus, the survival statistics represent overall and not disease-specific survival.
Following data collection, all 324 patients with a pathologically confirmed IPMN were categorized into two groups; those with a positive family history of pancreatic cancer (PFH) and those with a negative family history of pancreatic cancer (NFH), and comparisons regarding each of the collected variables were made between the groups.
2.2. Statistical analysis
All continuous data are presented as mean ± standard error of the mean (SEM). Continuous variables were evaluated using the unpaired t-test with Welch’s correction with the Mann–Whitney test used in cases where there was a significant difference in variance. Categorical variables were evaluated using the Fisher’s exact test or χ2-test as appropriate. Survival estimates were calculated using the method of Kaplan and Meier and survival curves were compared using the log-rank test. The Cox regression model was used to assess the simultaneous impact of several variables on survival. Significance in all cases was assessed using two-sided 5% level tests.
3. Results
Three hundred and twenty-four patients underwent surgical resection of a pancreatic lesion confirmed to be an IPMN on pathological analysis over a 21 year period (August 1990–February 2011) at the Massachusetts General Hospital. Forty-five (13.9%) patients had a PFH with at least one affected first or second degree relative. Of these 45 patients with a PFH, 34 (75.6%) had at least one affected first degree relative and the remaining 11 (24.4%) had at least one affected second degree relative. Eleven patients (24.4%) had more than one affected first or second degree relative. Six (13.3%) had two or more affected first degree relatives and thus satisfy criteria for familial pancreatic cancer [5].
3.1. Demographics
The average age of all patients who underwent surgical resection of a pathologically confirmed IPMN at our institution was 66.8 ± 0.6 years. There was an equal sex distribution and the vast majority of the patients (93.5%) were Caucasian or Asian. There were no significant differences in any demographic characteristics between the PFH and NFH groups (Table 1).
Table 1.
Demographic and Baseline Patient Characteristics.a
| All patients | PFH | NFH | ||
|---|---|---|---|---|
| (n = 324) | (n = 45) | (n = 279) | P | |
| Age | 66.8 ± 0.6 | 66.3 ± 1.5 | 66.9 ± 0.7 | 0.70 |
| Sex | 0.34 | |||
| Male | 159 (49.1) | 19 (42.2) | 140 (50.2) | |
| Female | 165 (50.9) | 26 (57.8) | 139 (49.8) | |
| Ethnicity | 0.38 | |||
| Caucasian/Asian | 303 (93.5) | 44 (97.8) | 259 (92.8) | |
| Black/Hispanic | 10 (3.1) | 0 (0) | 10 (3.6) | |
| Other | 11 (3.4) | 1 (0.2) | 10 (3.6) | |
| Smoker | 155 (47.8) | 21(46.7) | 134 (48.0) | 0.87 |
| PPY | 31.4 ± 2.3 | 23.4 ± 4.5 | 32.7 ± 2.5 | 0.08 |
| Diabetes mellitus | 60 (18.5) | 8 (17.8) | 52 (18.6) | 0.94 |
| Presence of symptoms | 176 (54.2) | 20 (44.4) | 156 (55.9) | 0.20 |
| Duration of symptomsb | 0.07 | |||
| Weeks (≤4) | 21 (11.9) | 6 (30.0) | 15 (9.6) | |
| Months (1–12) | 81 (46.0) | 7 (35.0) | 74 (47.4) | |
| Years (>1) | 54 (30.7) | 5 (25.0) | 49 (31.4) | |
| Unknown | 20 (11.4) | 2 (10.0) | 18 (11.5) | |
| Type of symptomsb | 0.59 | |||
| Pancreatitis | 71 (40.3) | 9 (45.0) | 62 (39.7) | |
| Abdominal pain | 139 (79.0) | 13 (65.0) | 126 (80.8) | |
| Jaundice | 28 9 (15.9) | 4 (20.0) | 24 (15.4) | |
| Steatorrhea | 11 (6.3) | 0 (0) | 11 (7.1) | |
| Weight loss | 86 (48.9) | 12 (60.0) | 74 (47.4) | |
| Other malignancy | ||||
| Overall | 91 (28.1) | 18 (40.0) | 73 (26.2) | 0.07 |
| Prior to IPMN | 72 (22.2) | 16 (35.6) | 56 (20.1) | 0.03 |
| Concurrent with IPMN | 12 (3.7) | 2 (4.4) | 10 (3.6) | 0.68 |
| Following IPMN | 14 (4.3) | 3 (6.7) | 11 (3.9) | 0.42 |
| CA 19-9 at diagnosis | 128.6 ± 47.0 | 97.5 ± 43.2 | 133.7 ± 54.2 | 0.93 |
Data presented as either mean ± SEM or number of patients (percentage) as appropriate and P-values represent a comparison of the PFH and NFH groups.
Percentages based on number of symptomatic patients in each category.
3.2. Baseline patient characteristics
Approximately half of the patients (47.8%) had a smoking history with an average consumption of 31.4 ± 2.3 packs-per-year (PPY). There was no statistically significant difference in the incidence of smoking or the PPY consumed between groups, although there was a trend towards higher cigarette consumption amongst patients with a NFH (32.7 ± 2.5 vs. 23.4 ± 4.5, p = 0.08). Sixty patients (18.5%) carried a diagnosis of diabetes mellitus prior to being diagnosed with an IPMN. Overall 176 patients (54.2%) had symptoms related to the pancreatic lesion with the remaining 148 (45.8%) patients being asymptomatic at the time of operative intervention. There were no statistically significant differences between PFH and NFH groups with regards to the presence (p = 0.20) or the nature of the symptoms (p = 0.59), however there was a trend towards a shorter duration of symptoms prior to surgical intervention in the PFH group (p = 0.07) (Table 1).
Overall, 91 patients (28.1%) had another malignancy that was diagnosed either prior to the diagnosis of the IPMN, concurrent with the IPMN or during the follow-up period. Patients with a PFH had a marginally statistically higher incidence of another malignancy than those with a NFH (40.0% vs. 26.2%, p = 0.07). The majority of these other malignancies were diagnosed prior to the IPMN diagnosis, and there was a significantly higher prevalence of a pre-IPMN malignancy in patients with a PFH (35.6% vs. 20.1%, p = 0.03). Only a minority of the other malignancies was diagnosed concurrent with or following the diagnosis of the IPMN, with no difference in either of these between the two groups (Table 1).
The most commonly occurring malignancies amongst all patients were breast and prostate cancer, which together accounted for 39.0% of all pre-IPMN malignancies (Table 2). Interestingly, despite an equal sex distribution, breast cancers were more common in those with a PFH while prostate cancers were more common in those with a NFH.
Table 2.
Pre-IPMN Extra-pancreatic Malignancy Type and Frequency.a
| All patients (72 pts, 77 tumors) |
PFH (16 pts, 17 tumors) |
NFH (56 pts, 60 tumors) |
|
|---|---|---|---|
| Breast | 16 (20.8) | 6 (35.3) | 10 (16.7) |
| Prostate | 14 (18.2) | 1 (5.9) | 13 (21.7) |
| Gynecologic | 9 (11.7) | 2 (11.8) | 7 (11.7) |
| Skin | 7 (9.1) | 3 (17.6) | 4 (6.7) |
| Bladder | 5 (6.5) | 0 (0) | 5 (8.3) |
| Colorectal | 5 (6.5) | 2 (11.8) | 3 (5.0) |
| Lymphoma/Leukemia | 5 (6.5) | 1 (5.9) | 4 (6.7) |
| Lung | 4 (5.2) | 1 (5.9) | 3 (5.0) |
| Thyroid | 2 (2.6) | 0 (0) | 2 (3.3) |
| Renal | 2 (2.6) | 1 (5.9) | 1 (1.7) |
| Other | 8 (10.4) | 0 (0) | 8 (13.3) |
Data presented as number of patients (percentage).
The most commonly occurring concurrent malignancy was a pancreatic neuroendocrine tumor (PNET), accounting for 7 (58.3%) of the 12 concurrently diagnosed malignancies. Only one of the seven patients was symptomatic from the lesion and was suspected to have a PNET prior to operative intervention, the remaining six patients had small lesions incidentally diagnosed in the resection specimen. Other concurrent malignancies included: duodenal adenocarcinoma (16.7%), gastrointestinal stromal tumor (16.7%) and mucosa-associated lymphoid tissue lymphoma (8.3%). PDACs noted in the resected pathologic specimen were not counted as a concurrent malignancy to allow for the independent assessment of this important lesion.
There was no difference in the prevalence of other malignancies diagnosed following surgical resection of the IPMN. The overall prevalence of these malignancies over a median follow-up duration of 4.7 ± 0.2 years was 4.3% (Table 1).
3.3. Tumor characteristics
The average IPMN size was 3.04 ± 0.13 cm. The majority of the tumors (61.4%) were located in the head, neck or uncinate process of the pancreas with fewer located in the body (12.3%) and tail (13.0%). Diffuse tumors extending through the entire pancreas accounted for 2.8% of all tumors. There were no differences in any radiographic tumor characteristics between the PFH and NFH groups (Table 3).
Table 3.
Radiographic Tumor Characteristics.a
| All patients (n = 324) |
PFH (n = 45) |
NFH (n = 279) |
P | |
|---|---|---|---|---|
| Largest diameter (cm) | 3.04 ± 0.13 | 3.42 ± 0.47 | 2.98 ± 0.14 | 0.79 |
| Location | 0.66 | |||
| Head/Neck/Uncinate | 199 (61.4) | 24 (53.3) | 175 (62.7) | |
| Body | 40 (12.3) | 6 (13.3) | 34 (12.2) | |
| Tail | 42 (13.0) | 6 (13.3) | 36 (12.9) | |
| Two involved regions | 34 (10.5) | 7 (15.6) | 27 (9.7) | |
| Diffuse (entire pancreas) | 9 (2.8) | 2 (4.4) | 7 (2.5) | |
| Cyst character | 0.31 | |||
| Multi-cystic | 161 (49.7) | 18 (40.0) | 143 (51.3) | |
| Single cyst | 107 (33.0) | 19 (42.2) | 88 (31.5) | |
| No cyst | 56 (17.3) | 8 (17.8) | 48 (17.2) |
Data presented as either mean ± SEM or number of patients (percentage) as appropriate. P-values represent a comparison of the PFH and NFH groups.
On review of the pathology, 146 (45.1%) tumors were of the branch-duct variant, 153 (47.2%) were of the combined variant and 25 (7.7%) were of the main duct variant. The most common type of epithelium seen on pathologic evaluation was gastric (59.0%) with the intestinal type being the second most common (29.9%) and tumors of the oncocytic (4.0%), pancreatobiliary (0.9%) and mixed (6.2%) types being less frequent. All lesions were evaluated for highest grade: 59 (18.2%) were low grade; 123 (38.0%) were intermediate grade; and 142 (43.8%) were high grade. A similar distribution was seen in the PFH and NFH groups with regards to IPMN type, epithelial type and highest grade of the lesion. Of all 324 IPMN lesions, 65 (20.1%) had an invasive component with a prevalence of 15.6% in those with a PFH and 20.8% in those with a NFH (p = 0.55). Of the 65 invasive tumors, 39 (60.0%) were tubular,19 (29.2%) of the colloid type, and the remaining 7 (10.8%) of the oncocytic type. Invasive lesions were further assessed for degree of the invasive component (superficial, macroscopic): 42 (64.6%) had a macroscopic invasive component and the remaining 23 (35.4%) had only a superficial invasive component. Invasive tumors in the PFH and NFH groups were alike with regards to both invasive type and size. PanIN lesions were present in 143 (44.1%) surgical specimens and there was no difference between groups in the incidence of PanIN lesions or the grade of these lesions. A minority of IPMN lesions (4.0%) was associated with a concurrent PDAC, defined as ductal adenocarcinoma occurring at an anatomically distant site from the IPMN. The prevalence of a concurrent PDAC was significantly higher in patients with a PFH (11.1% vs. 2.9%, p = 0.02) (Table 4).
Table 4.
Pathologic Tumor Characteristics.a
| All patients (n = 324) | PFH (n =45) | NFH (n = 279) | P | |
|---|---|---|---|---|
| IPMN Type | 0.64 | |||
| Main-duct | 25 (7.7) | 2 (4.4) | 23 (8.2) | |
| Branch-duct | 146 (45.1) | 20 (44.4) | 126 (45.2) | |
| Combined | 153 (47.2) | 23 (51.1) | 130 (46.6) | |
| Epithelial Type | 0.12 | |||
| Gastric | 191 (59.0) | 24 (53.3) | 167 (59.9) | |
| Intestinal | 97 (29.9) | 12 (26.7) | 85 (30.5) | |
| Oncocyticc | 13 (4.0) | 1 (2.2) | 12 (4.3) | |
| Pancreatobiliaryc | 3 (0.9) | 1 (2.2) | 2 (0.7) | |
| Mixedc | 20 (6.2) | 7 (15.6) | 13 (4.7) | |
| Highest Grade | 0.62 | |||
| Low | 59 (18.2) | 7 (15.6) | 52 (18.6) | |
| Intermediate | 123 (38.0) | 20 (44.4) | 103 (36.9) | |
| High | 142 (43.8) | 18 (40.0) | 124 (44.4) | |
| Invasive | 65 (20.1) | 7 (15.6) | 58 (20.8) | 0.55 |
| Invasive Typeb | 0.50 | |||
| Tubular | 39 (60.0) | 4 (57.1) | 35 (60.3) | |
| Colloid | 19 (29.2) | 2 (28.6) | 17 (29.3) | |
| Oncocytic | 7 (10.8) | 1 (14.3) | 6 (10.3) | |
| Invasive Sizeb | 0.23 | |||
| Superficial | 23 (35.4) | 4 (57.1) | 19 (32.8) | |
| Macroscopic | 42 (64.6) | 3 (42.9) | 39 (67.2) | |
| Concurrent PDAC | 13 (4.0) | 5 (11.1) | 8 (2.9) | 0.02 |
| PanIN lesion | 143 (44.1) | 23 (51.1) | 120 (43.0) | 0.33 |
| PanIN lesion gradeb | 0.68 | |||
| 1 | 101 (70.6) | 17 (73.9) | 84 (70.0) | |
| 2 | 33 (23.1) | 4 (17.4) | 29 (24.2) | |
| 3 | 9 (6.3) | 2 (8.7) | 7 (5.8) |
Data presented as number of patients (percentage). P-values represent a comparison of the PFH and NFH groups.
Percentages based on number of invasive tumors or PanIN lesions.
Pancreaticobiliary, oncocytic and mixed groups combined for chi-square analysis due to the low incidence of these epithelial types.
3.4. Surgical intervention
Two hundred of the 324 patients (61.7%) underwent a pancreaticoduodenectomy (Whipple) procedure. The other surgical resections performed included 76 (23.1%) distal pancreatectomies, 27 (8.3%) middle pancreatectomies and 21 (6.5%) total pancreatectomies. There was no difference in the type of surgical resection performed between PFH and NFH groups (p = 0.18).
3.5. Survival
The median duration of follow-up for all patients with an IPMN was 4.7 ± 0.2 years. The median duration of follow-up was not different between patients with a PFH and NFH (5.0 vs. 4.5years, p = 0.40). At the time of analysis, 252 (77.8%) of the 324 patients with an IPMN were alive. These 252 patients included 31 (68.9%) of the 45 with a PFH and 221 (79.2%) of the 279 with a NFH.
Sixty-two of the 72 patients who died during the follow-up period had a known cause of death. Of these 62 patients, 31 (50.0%) died of metastatic/recurrent cancer and 31 (50.0%) died of other causes. Of the 11 patients with a PFH who died of a known cause, 8 (72.7%) died of metastatic/recurrent PC as compared to 23 (45.1%) of the 51 patients with NFH who died of a known cause (p = 0.18). Three of the 8 patients (37.5%) with a PFH who died of metastatic/recurrent cancer had a concurrent PDAC at the time of surgery for the IPMN whereas only 2 of the 23 patients (8.7%) with a NFH had a concurrent PDAC. Additionally, another 3 (37.5%) of the 8 patients with a PFH and 16 (69.6%) of those with a NFH had an IPMN with an invasive component. Based on the data available it is unclear whether the deaths that occurred as a result of metastatic/recurrent cancer were the result of an invasive IPMN or a concurrent PDAC.
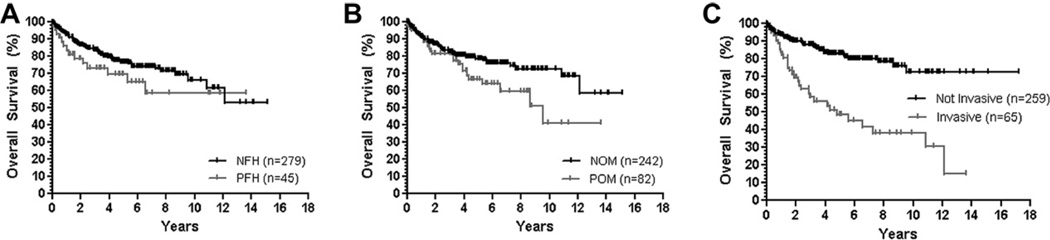
Overall, patients with an IPMN had 6-month, 1-year, and 5-year actuarial survival rates of 95.7%, 91.6%, and 75.8%. When comparing survival rates based on family history of pancreatic cancer, the survival of patients with a PFH was lower (but not statistically significantly so) than that seen for patients with a NFH (Table 5). After taking into account other factors that may affect the survival of these patients, the presence of a pre-IPMN or concurrent malignancy was found to have a significant impact on survival. The prevalence of a pre-IPMN or concurrent malignancy was significantly higher in patients with a PFH (37.8% vs. 23.3%, p = 0.04), and this factor appeared to be responsible for the survival difference seen between the PFH and NFH groups. When the presence of another malignancy was corrected for, there was no difference in survival between the two groups (Table 5, Fig. 1). The only other variable that was found to have a significant impact on patient survival was the presence of invasive carcinoma at the time of diagnosis (Fig. 1) although, given the equal incidence of invasive carcinoma in the PFH and NFH groups there was no impact of this variable on the survival curves between these two groups.
Table 5.
Survival at 6-months, 1-year and 5-years.a
| 6-month | 1-year | 5-year | P | |
|---|---|---|---|---|
| Overall | 95.7 | 91.6 | 75.8 | NA |
| NFH | 94.7 | 93.2 | 80.0 | 0.27 |
| PFH | 95.3 | 87.3 | 73.3 | |
| NOMb | 95.9 | 92.6 | 79.7 | 0.04 |
| POMb | 95.2 | 89.0 | 64.6 |
Data represented as percentages.
NOM = no other pre-IPMN or concurrent malignancy, POM = presence of other pre-IPMN or concurrent malignancy.
Figure 1. Survival curves.
A) Survival by FH of PC. B) Survival by personal history of another malignancy (prior to/concurrent with IPMN). NOM = no other pre-IPMN/concurrent malignancy, POM = presence of other pre-IPMN/concurrent malignancy. C) Survival by presence of invasive carcinoma within the IPMN.
3.6. Subset of patients with familial pancreatic cancer
Six patients met the definition of familial pancreatic cancer based on the presence of two or more first degree relatives with pancreatic cancer. The average age of this cohort was 77.7 ± 7.3 years with 2/6 (33%) being symptomatic at the time of operative intervention. Overall, 4/6 patients (67%) had a total of five other malignancies with three diagnosed prior (two skin, one colorectal) and two concurrent (1 MALT, 1 PNET) with the IPMN. The average tumor size was 5.11 ± 1.52 cm at operative intervention with 1/6 (17%) low-grade, 3/6 (50%) intermediate-grade and 2/6 (33%) high-grade. PanIN lesions were identified in 3/6 (50%) surgical specimens. No patient had invasive disease or a concurrent PDAC. Over an average follow-up of 3.3 ± 0.8years, two patients died with one dying of recurrent or metastatic disease.
4. Discussion
Individuals with a family history of pancreatic cancer have an increased incidence of pancreatic cancer, and it is estimated that 5–10% of pancreatic cancer cases have a hereditary association [6–8]. In addition, recent reports screening high-risk individuals with a strong family history of pancreatic cancer resulted in the detection of IPMNs in 10–18% of patients [3,9–11]. IPMNs, due to their slow growing nature but clear malignant potential, are unique pancreatic neoplasms that, if diagnosed and treated early, are associated with a favorable prognosis [12]. We sought to compare IPMN lesions diagnosed in patients with and without a family history of pancreatic cancer to gain insight into any important differences that may suggest the need for differential management. To this end we did not focus on patients with familial pancreatic cancer, defined by the presence of two or more first degree relatives with pancreatic cancer, but rather sought to evaluate the larger cohort of patients with at least one affected first or second degree relative as this was felt more accurately encompass the cohort of patients likely to be seen by the average practitioner.
Our data does not suggest that surgically resected IPMN lesions in patients with a family history of pancreatic cancer are more advanced or aggressive at the time of operative intervention compared to lesions in patients without family history of pancreatic cancer. Specifically, there was no difference in any radiographic or pathologic tumor characteristics. Most importantly, there was no difference in the incidence of invasive IPMN cancer between the two groups, which prior studies have suggested is the single strongest adverse predictor of survival [12].
Patients with an IPMN and a family history of pancreatic cancer did have a nearly four-fold higher prevalence of a concurrent PDAC (11.1% vs. 2.9%, p = 0.023), strictly defined by the presence of a PDAC anatomically distinct from the IPMN and occurring in the absence of high-grade dysplasia surrounding the IPMN. Prior studies estimate that individuals with one first-degree relative with pancreatic cancer have a 4.5-fold increased risk of developing pancreatic cancer whereas those with two and three affected first-degree relatives have a 6.4 and 32-fold increased risk, respectively [5]. In contrast, a second degree relative with pancreatic cancer increases the risk by only 1.3-fold [13]. In our cohort of patients with a PFH (n = 45), 62.2% had one, 11.1% had two and 2.2% had three affected first-degree relatives whereas 24.4% had one or more affected second-degree relative. Given this distribution of affected family members, the 4-fold higher risk of PDAC among those with a family history of pancreatic cancer who were diagnosed with an IPMN is consistent with previously published reports of the effect of a family history of pancreatic cancer alone on an individual’s risk of developing pancreatic cancer. This suggests that the presence of the IPMN has no additional bearing on the incidence of PDAC in patients with a family history of pancreatic cancer.
The prevalence of other malignancies in our cohort of 324 IPMN patients was28.1%. This is consistent with prior studies reporting that 28–36% of patients diagnosed with an IPMN will also be diagnosed with an extra-pancreatic malignancy before, at, or after the diagnosis of an IPMN [14–17]. Our data also corroborate the previously described [18–21] association between IPMNs and PNETs with 2.1% of patients in our cohort of surgically resected IPMNs having a concomitant PNET. Interestingly, in our series, patients with a family history of pancreatic cancer had a higher prevalence of other malignancies as compared to those without a family history of pancreatic cancer (40.0% vs. 26.2%, p = 0.07). Of note, the most notable difference in the prevalence of other malignancies was seen in those malignancies that occurred prior to the diagnosis of the IPMN: the prevalence was 35.1% in those with a PFH of pancreatic cancer compared to 20.1% in those with a NFH of pancreatic cancer (p = 0.03). Since the most prominent of the extra-pancreatic malignancies seen in the IPMN patients with a family history of pancreatic cancer was breast cancer, there may have been BRCA-1, BRCA-2 or STK11/LKB1 mutations in this cohort. The high incidence of other malignancies in patients with IPMNs needs to be kept in mind by physicians taking care of these patients in order to diagnose and manage these co-existing malignancies in a timely fashion.
In this series, the overall survival for all patients who underwent surgical resection of an IPMN was 91.6% at 1-year and 75.8% at 5-years. Initially patients with a family history of pancreatic cancer appear to have a marginally lower survival than those without a family history of pancreatic cancer at 6-months, 1-year and 5-years (Fig. 1A). However, this effect is abolished after correcting for the higher prevalence of other malignancies (not including concurrent PDAC) seen in patients with a family history of pancreatic cancer, suggesting that the survival disadvantage seen in patients with a family history of pancreatic cancer is simply secondary to the higher frequency of other malignancies that occur, and not because of an inherent difference in the behavior of the IPMNs seen in these patients. Certainly, the higher prevalence of potentially lethal PDAC in the patients with a PFH may also negatively impact the survival of this group of patients, however, this is difficult to truly determine as these lesions were infrequent and were likely resected early due the incidental nature of their discovery in this cohort.
An analysis of the cause of death is limited in this study due to the lack of known cause in 10 of the 72 patients (13.9%) who expired during the follow-up period. However, analyzing those patients with a known cause of death, it seems that a higher percentage of patients with a family history of pancreatic cancer died of metastatic or recurrent pancreatic cancer (72.7% vs. 45.1%) although, this difference does not reach statistical significance (p = 0.18). Unfortunately it remains unclear in all of these cases whether the patients died of a metastatic/recurrent invasive IPMN or the concurrent PDAC. The prevalence of a concurrent PDAC was higher in those with a family history of pancreatic cancer who expired during the follow-up period as compared to those without a family history of pancreatic cancer (28.6% vs. 8.6%, p=0.06) whereas, the incidence of an invasive carcinoma associated with the IPMN was not different between the groups (28.6% vs. 48.3%, p=0.23). Although, the data in these subsets of patients is limited, it does suggest that the IPMN itself is not responsible for the lower survival seen in patients but rather that the higher prevalence of other extra-pancreatic malignancies and concurrent PDAC are more likely the culprit.
This study caries several limitations including its retrospective nature and the fact that the determination of a family history of pancreatic cancer was made by the patient’s report, thus raising the possibility of potential errors in classification. The subset of patients with a family history of pancreatic cancer was also small (n = 45) relative to the group without a family history of pancreatic cancer (n = 279). Of note, all patients with any first or second-degree relative with a family history of pancreatic cancer were classified as having a positive family history and this was not restricted to only at those with familial pancreatic cancer as defined by the presence of two or more first-degree relatives with pancreatic cancer. For this reason, it must be noted that the results of this study can not be generalized to particularly high-risk patients, such as those with familial pancreatic cancer or syndromic pancreatic cancer, and further studies will be necessary to truly address the behavior or cystic lesions of the pancreas in these subsets of patients. Additionally, this study looks only at pathologically confirmed IPMNs, which is important to ensure that all patients do in fact have a true IPMN, but at the same time inherently causes problems with application of the current findings to lesions that are radiographically suspicious for an IPMN but do not meet criteria for resection. The indication for operative intervention was to the best of our knowledge not different between patients with and without a family history of pancreatic cancer, however, the potential for bias towards resecting lesions in those with a concerning family history earlier certainly exists and should be recognized. Indicators that such a bias did not play a large role in this study include the similar tumor sizes and the lack of a significant difference between groups with regards to the presence, character and duration of symptoms at the time of operative intervention. Despite these limitations and given the strengths of the study (conducted at a center with expertise in the management of pancreatic lesions, large cohort of patients, long-term follow-up) we feel that this study represents an important initial step at defining the behavior of IPMNs in high-risk patients such as those with a family history of pancreatic cancer.
In conclusion, our results suggest that surgically resected IPMNs do not differ with regard to any important tumor characteristics in patients with and without a family history of pancreatic cancer. Most importantly, the incidence of invasive disease was not different between groups, suggesting that these lesions may not be more aggressive when they occur in the presence of a family history of pancreatic cancer. Patients with a family history of pancreatic cancer do appear to be prone to the development of PDAC and all patients with IPMNs have a very high incidence of extra-pancreatic malignancies supporting lifelong monitoring and surveillance strategies for these patients. Future prospective studies are necessary to better characterize the natural history of IPMNs in patients with a family history of pancreatic cancer and particularly in those very high-risk cohorts of patients with familial pancreatic cancer and syndromic pancreatic cancer.
Acknowledgments
Grant support
None.
Abbreviations
- IPMN
Intraductal papillary mucinous neoplasm
- NFH
negative family history of pancreatic cancer
- PDAC
pancreatic ductal adenocarcinoma
- PFH
positive family history of pancreatic cancer
- PNET
pancreatic neuroendocrine tumor
- SEM
standard error of the mean
Footnotes
Disclosures/conflict of interest
No conflict of interest exist.
Writing assistance
None.
References
- 1.Augustin T, Vandermeer TJ. Intraductal papillary mucinous neoplasm: a clinicopathologic review. The Surgical Clinics of North America. 2010;90:377–398. doi: 10.1016/j.suc.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–781. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 3.Shi C, Klein AP, Goggins M, Maitra A, Canto M, Ali S, et al. Increased prevalence of precursor lesions in familial pancreatic cancer patients. Clinical Cancer Research: an Official Journal of the American Association for Cancer Research. 2009;15:7737–7743. doi: 10.1158/1078-0432.CCR-09-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. World health organization international classification of tumors. Berlin: Springer; 1996. Histological typing of tumors of the exocrine pancreas; pp. 11–20. [Google Scholar]
- 5.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Research. 2004;64:2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 6.Bartsch DK. Familial pancreatic cancer. Br J Surg. 2003;90:386–387. doi: 10.1002/bjs.4127. [DOI] [PubMed] [Google Scholar]
- 7.Ghadirian P, Liu G, Gallinger S, Schmocker B, Paradis AJ, Lal G, et al. Risk of pancreatic cancer among individuals with a family history of cancer of the pancreas. Int J Cancer. 2002;97:807–810. doi: 10.1002/ijc.10123. [DOI] [PubMed] [Google Scholar]
- 8.Lynch HT, Fitzsimmons ML, Smyrk TC, Lanspa SJ, Watson P, McClellan J, et al. Familial pancreatic cancer: clinicopathologic study of 18 nuclear families. The American Journal of Gastroenterology. 1990;85:54–60. [PubMed] [Google Scholar]
- 9.Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clinical Gastroenterology and Hepatology: the Official Clinical Practice Journal of the American Gastroenterological Association. 2006;4:766–781. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 10.Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–621. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 11.Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. The American Journal of Gastroenterology. 2009;104:2175–2181. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 12.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Annals of Surgery. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. [discussion 797–789]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amundadottir LT, Thorvaldsson S, Gudbjartsson DF, Sulem P, Kristjansson K, Arnason S, et al. Cancer as a complex phenotype: pattern of cancer distribution within and beyond the nuclear family. PLoS Medicine. 2004;1:e65. doi: 10.1371/journal.pmed.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi MG, Kim SW, Han SS, Jang JY, Park YH. High incidence of extrapancreatic neoplasms in patients with intraductal papillary mucinous neoplasms. Arch Surg. 2006;141:51–56. doi: 10.1001/archsurg.141.1.51. [discussion 56]. [DOI] [PubMed] [Google Scholar]
- 15.Eguchi H, Ishikawa O, Ohigashi H, Tomimaru Y, Sasaki Y, Yamada T, et al. Patients with pancreatic intraductal papillary mucinous neoplasms are at high risk of colorectal cancer development. Surgery. 2006;139:749–754. doi: 10.1016/j.surg.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Kamisawa T, Tu Y, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Malignancies associated with intraductal papillary mucinous neoplasm of the pancreas. World J Gastroenterol. 2005;11:5688–5690. doi: 10.3748/wjg.v11.i36.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riall TS, Stager VM, Nealon WH, Townsend CM, Jr, Kuo YF, Goodwin JS, et al. Incidence of additional primary cancers in patients with invasive intraductal papillary mucinous neoplasms and sporadic pancreatic adenocarcinomas. J Am Coll Surg. 2007;204:803–813. doi: 10.1016/j.jamcollsurg.2007.01.015. [discussion 813–804]. [DOI] [PubMed] [Google Scholar]
- 18.Gill KR, Scimeca D, Stauffer J, Krishna M, Woodward TA, Jamil LH, et al. Pancreatic neuroendocrine tumors among patients with intraductal papillary mucinous neoplasms: real association or just a coincidence? JOP. 2009;10:515–517. [PubMed] [Google Scholar]
- 19.Goh BK, Tan YM, Yap WM, Cheow PC, Chow PK, Chung YF, et al. Pancreatic serous oligocystic adenomas: clinicopathologic features and a comparison with serous microcystic adenomas and mucinous cystic neoplasms. World J Surg. 2006;30:1553–1559. doi: 10.1007/s00268-005-0749-7. [DOI] [PubMed] [Google Scholar]
- 20.Ishida M, Egawa S, Kawaguchi K, Aoki T, Sakata N, Mikami Y, et al. Synchronous and metachronous extrapancreatic malignant neoplasms in patients with intraductal papillary-mucinous neoplasm of the pancreas. Pancreatology: Official Journal of the International Association of Pancreatology. 2008;8:577–582. doi: 10.1159/000159844. [DOI] [PubMed] [Google Scholar]
- 21.Larghi A, Stobinski M, Galasso D, Lecca PG, Costamagna G. Concomitant intraductal papillary mucinous neoplasm and pancreatic endocrine tumour: report of two cases and review of the literature. Dig Liver Dis. 2009;41:759–761. doi: 10.1016/j.dld.2009.01.005. [DOI] [PubMed] [Google Scholar]