Abstract
Energy homeostasis is maintained by balancing energy intake and expenditure. Many signals regulating energy intake are conserved between the human and teleost. However, before this work, there was no sensitive high-throughput system to monitor energy expenditure in the teleost. We exploit the nonfluorescent and fluorescent properties of resazurin and its reduced form resorufin (alamarBlue®) to monitor energy expenditure responses to drug application and genetic manipulation. We show that leptin, insulin, and alpha-melanocyte-stimulating hormone (α-MSH) increase energy expenditure dose dependently in the larval zebrafish. As previously established in the mouse, etomoxir, a carnitine palmitoyl transferase I inhibitor, blocks leptin-induced energy expenditure in the zebrafish. Metformin, the most commonly prescribed insulin sensitizer, increases the insulin-induced metabolic rate. Using genetic knockdown, we observed that α-MSH treatment increases the metabolic rate, as does knockdown of the melanocortin antagonist, agouti-related protein. The agouti-related protein and multiple melanocortin receptors are shown to be involved in these effects. These studies confirm that aspects of hormonal regulation of energy expenditure are conserved in the teleost, and suggest that this assay may provide a unique tool to perform in vivo screens for drugs or genes that affect the metabolic rate, including insulin or leptin sensitizers.
Introduction
Rodents have served as the primary model organism for studying the control of energy balance. However, zebrafish have many inherent advantages as a model organism for the study of energy balance.1 First, zebrafish are small in size allowing them to be placed in a 96-well plate for rapid screening. Additionally, genetic knockdown using morpholino technology allows for rapid analysis of gene function. Moreover, because of the large number of offspring and ease of chemical mutagenesis, zebrafish are ideally suited for forward genetic screens. Finally, 90% of mammalian genes are found in zebrafish; thus, one can expect findings in the zebrafish to frequently translate directly to mammalian species, including humans.
Many of the hormonal signals that regulate energy intake and energy expenditure in the human have been shown to also control energy intake in the teleost. Leptin, alpha-melanocyte-stimulating hormone (α-MSH), and insulin all decrease food intake in rodents,2–4 and have been shown to elicit similar effects in goldfish5,6 and rainbow trout.7 Furthermore, transgenic overexpression of the melanocortin inverse agonist/antagonist, agouti-related protein (AgRP), has been shown to increase body weight in both mice8 and zebrafish.9 Finally, fasting increases the AgRP signal in both mice10 and zebrafish.11 Thus, aspects of hormonal regulation of food intake are conserved from teleosts to mammals.
We designed an assay to monitor the hormonal regulation of energy expenditure in the teleost. Energy expenditure changes were mediated by drug application and genetic manipulation, and monitored by measuring the conversion of the nonfluorescent resazurin to its reduced, fluorescent form resorufin (AlamarBlue®; TREK Diagnostic Systems, Inc., Cleveland, OH). Similar to the energy intake findings described above, aspects of hormonal regulation of energy expenditure are conserved from teleosts to mammals.
Materials and Methods
Animal care and maintenance
The wild-type Tab 14 and AB strain zebrafish were raised and bred at 26°C–28°C, with a 14-h light–10-h dark cycle. The larval stage was determined according to Kimmel et al. (1995).12 Fish aged from 5–10 days postfertilization (dpf) were fed four times a day with baby powder, fish from 10 dpf to 15 dpf were fed baby power supplemented with uncapsulated brine shrimp, and fish from 15 dpf to 1 month or older were fed with uncapsulated brine shrimp. For adult fish, food was prepared by mixing four parts of tropical flakes (Aquatic Eco-systems, Inc., Apopka, FL) and one part of brine shrimp (Brine Shrimp Direct, Ogden, UT) in distilled water. Melanocortin-4 receptor (MC4R) null zebrafish (allele: sa0149) were originally generated and provided by the Wellcome Trust Sanger Institute. MC4R mutants were identified by genotyping as previously described,13 and reared identically to wild-type fish. MC4R −/−, +/−, and +/+ embryos were created by +/−X +/−breedings. For studies using −/−, +/−, and +/+MC4R embryos derived from heterozygous mating, genotyping was performed after the metabolic rate assay was performed. For experiments that used only −/−embryos, MC4R −/−males were bred to MC4R −/−females. All studies were conducted according to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the animal care and use committee of Vanderbilt University.
BD oxygen biosensor metabolic rate assay
Zebrafish were maintained in 10-cm Petri dishes at 28°C from 0–4 dpf. Egg water was replaced daily. At day 4, fish were rinsed with autoclaved egg water and pipetted into a BD Biosystems 96-well Oxygen biosensor plate (BD, Franklin Lakes, NJ). One, three, or five fish were put into each well in a volume of 200 μL. Fluorescence of the plate was immediately read on a SpectraMax® M5 multidetection reader (Molecular Devices, Sunnyvale, CA) with excitation at 485 and emission monitored at 630. The SpectraMax M5 multidetection reader was set to monitor fluorescence six times/well per reading. Subsequent readings were taken at 0.5, 1, 2, 3, and 4 h. The change in fluorescence from time 0 was calculated. Controls were set to 1 and all data are expressed relative to the control value.
Alamar Blue metabolic rate assay
Zebrafish were maintained in 10-cm Petri dishes at 28°C from 0–4 dpf. Egg water was replaced daily. At day 4, fish were rinsed with autoclaved egg water and pipetted into a modified Millicell cell culture insert plate (1 or 3 zebrafish/well; Millipore, Inc., Billerica, MA). The receiver tray was loaded with the assay buffer. The assay buffer is egg water supplemented to contain 0.1% dimethyl sulfoxide (DMSO; Sigma-Aldrich, Corp., St. Louis, MO), 1% (10×) Alamar Blue, and 4 mM sodium bicarbonate. All chemicals tested for their effects on metabolic rate were added to the assay buffer. Sodium chloride, bovine serum albumin (BSA), 3-isobutyl-1-methylxanthine (IBMX), isoproterenol hydrochloride, ascorbic acid, imperatorin, rolipram, metformin hydrochloride, etomoxir sodium salt hydrate, and insulin (human) were all purchased from Sigma-Aldrich (Sigma-Aldrich Corp.). For studies on the effect of β-adrenergic receptor agonism, 0.1% ascorbate was used to prevent oxidation of isoproterenol. Human MSH trifluoroacetate salt was purchased from Bachem (Bachem Americas, Inc., Torrance, CA) and human leptin was purchased from the National Hormone and Peptide Program (Torrance, CA). The fish-loaded insert plate was tamped dry on paper towels and transferred into the buffer-loaded 96-well receiver tray. Fluorescence of the plate was immediately read on a SpectraMax M5 multidetection reader (Molecular Devices, Sunnyvale, CA) with excitation at 530 nm and emission monitored at 590 nm. The plate was incubated in the dark at 28°C for 24 h and read again. The SpectraMax M5 multidetection reader was set to monitor fluorescence six times/well per reading. Any wells that had fish die during the assay were excluded from analyses. The change in fluorescence from time 0 to 24 h was calculated. Data were corrected by setting the average of the no drug wild-type control to 1. The insert plate and receiver plates were washed with water and 100% ethanol between uses and the nylon membrane of the insert plate was photobleached under a fluorescent light for 24 h before the next use.
Morpholino oligonucleotides
Antisense morpholino oligonucleotide (MO) against the ATG translation initiation site of zebrafish genes were designed and synthesized by GeneTools, LLC (Table 1; GeneTools LLC, Philomath, OR). MOs were dissolved in nuclease-free water and stored at −20°C as 1 mM stock. Serial dilutions were made using nuclease-free water to the 0.1, 0.2, 0.3, and 0.4 mM working solution with 20% Phenol Red (Sigma, St. Louis, MO. 0.5% in Dulbecco's phosphate buffered saline (DPBS), sterile filtered, endotoxin tested). Before the injection, MOs were denatured at 65°C for 5 min and quickly spun to avoid the formation of aggregates. About 3–5 uL was loaded in a microinjection machine and embryos at one or two cell stages were injected with 1–2 nL of a solution containing antisense targeting morpholino or standard control oligo. Each MO injection was repeated at least three times and doses were adjusted to optimize the phenotype-to-toxicity ratio. Following MO injections, embryos were raised in egg water, changed daily under the standard light/dark cycle to 5 days postfertilization. Dead embryos were excluded at 1dpf. Gene expression was analyzed by quantitative reverse transcription polymerase chain reaction (qRT-PCR) at 4–5 dpf.
Table 1.
List of Morpholino Oligonucleotides Employed to Knockdown AgRP, MC3R, and MC5Rb and Their Sequences
| Gene and location | Morpholino oligonucleotide sequence |
|---|---|
| agrp ATG | 5′ACTGTGTTCAGCATCATAATCACTC3′ |
| mc3r ATG | 5′GAAACTGCAGATGTGAGTCGTTCAT3′ |
| mc5rb ATG | 5′GTGTGGGCCACTCCGAAGAGTTCAT3′ |
| Zebrafish nontargeted standard control | 5′CCTCTTACCTCAGTTACAATTTATA3′ |
Cell culture
HEK293 cells were transiently cotransfected using LipoD293™ (Ver. II; SignaGen Laboratories, Rockville, MD) with a cre-luciferase plasmid kindly provided by Dr. George Holz14 and either the zmc3r or hmc3r (pcDNA3.1 + vector, Invitrogen, Carlsbad, CA) with 0.12 μg total plasmid in each well of a 96-well plate. The zmc3r plasmid has been previously employed for studies in our laboratory.15,16 The hmc3r plasmid was made by cloning in 1172 bp cDNA, including the coding region for hmc3r (Gene Band accession number NM_019888.3; 972 bp) into pcDNA3.1+at HindII (5′) and Xbal (3′). Transfection efficiency was visually observed by transfecting a plasmid coding for green fluorescent protein (GFP; kindly provided by Patricia M. Hinkle) and assays were only run when transfection efficiency of the GFP plasmid exceeded 90%.
To conduct the luciferase assay transfection, media were removed and replaced with 90 μL of the Dulbecco's modified eagle medium (DMEM)/F-12 (1×) 1:1 (Life Technologies, Grand Island, NY). About 10 μL of test compound was added to each well and cells were incubated at 37°C for 4 h. Media were aspirated off the cells and 50 μL of the NF-Luc-Reagent was added (Nano Light Technology, Pinetop, AZ). Following a 10-min room temperature incubation, luminescence was measured on a SpectraMax M5 multidetection plate reader (Molecular Devices, LLC, Sunnyvale, CA). All data were corrected to a maximal response to forskolin (20 μM).
RNA extraction, cDNA synthesis, and real-time quantitative PCR
Embryos were homogenized in the lysis buffer with a sonic dismembrator (model 100, Fisher Scientific, Pittsburgh, PA). Total RNA was extracted using an RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. To remove genomic DNA, On-column DNase Digestion was performed using an RNase free DNase Set (Qiagen, Valencia, CA). About 1 μg of purified total RNA was reverse transcribed with iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative PCR (Q-PCR) primers were designed using Beacon Designer 7.0 (Table 2; Premier Biosoft International, Palo Alto, CA) to minimize primer self-dimerization. All gene expression was normalized to the house-keeping gene, ef1α (Elongation Factor 1α). Q-PCR was performed using 2 μL of cDNA (20 ng) as template, 5 pmol of each of the forward and reverse primers, 2×Power SYBR PCR mix (Applied Biosystems, Carlsbad, CA) with nuclease-free water (Promega, Madison, WI) to result in 20 μL/reaction volume (Bioexpress, Kaysville, UT). Q-PCRs were performed using an Mx3000PTM (Stratagene, Santa Clara, CA). The PCR cycle was performed according to the manufacturer's instructions with initial denaturation at 95°C for 10 min, followed by 45 cycles of 95°C 20 s, 60°C 60 s. Melting curves of the products were verified for the specificity of PCR products. A standard curve with serial dilutions of the cDNA sample was performed on each plate. All qPCR reactions were performed in duplicate.
Table 2.
Quantitative PCR Primers
| Gene | Forward primer 5′→3′ | Reverse primer 5′→3′ |
|---|---|---|
| agrp (agouti-related protein) | GGTGAATGTTGTGGTGATGG | GCGTGTGCCTCTTCTCTG |
| pomca (proopiomelanocortin a) | TCTTGGCTCTGGCTGTTC | TCGGAGGGAGGCTGTAG |
| pomcb (proopiomelanocortin b) | GCTCGGGTTTGATAGACTGC | ACTCTGCTCCTCTACCTGTTC |
| mc1r (melanocortin receptor 1) | ATCTTGGTGGTGTGGCTTGC | CCGTGATGCGTCTTGAGTGG |
| mc3r (melanocortin receptor 3) | TTCTTCTCGCCAGACTTCAC | TGAGGACAGGACACCAGTAG |
| mc4r (melanocortin receptor 4) | AACCTGACCAACCGTGAGAG | AGCGTAGAAGATTGTGATGTAGC |
| mc5ra (melanocortin receptor 5a) | TCCTGAACGCCACTGAGACC | GACTGACGATGCCAAGGATGAG |
| mc5rb (melanocortin receptor 5b) | TCAGCGATGAGTCAAGTAGG | ACATTGGTGAGTGGAGGTTC |
| ef1α (elongation factor 1α) | CTGGAGGCCAGCTCAAACAT | ATCAAGAAGAGTAGTACCGCTAGCATTAC |
Statistics
All metabolic rate data are presented as mean±SEM. Data were analyzed by ANOVA using Proc Mixed in SAS (SAS Institute, Cary, NC). For assays that were run on multiple dates, the date was used as a covariate. Differences between means were determined using the pdiff function and the Bonferroni correction for multiple comparisons. Gene expression and cell culture data were analyzed using GraphPad Prism Version 5.0 for Windows® (GraphPad Software, San Diego, CA).
Results
Assay development
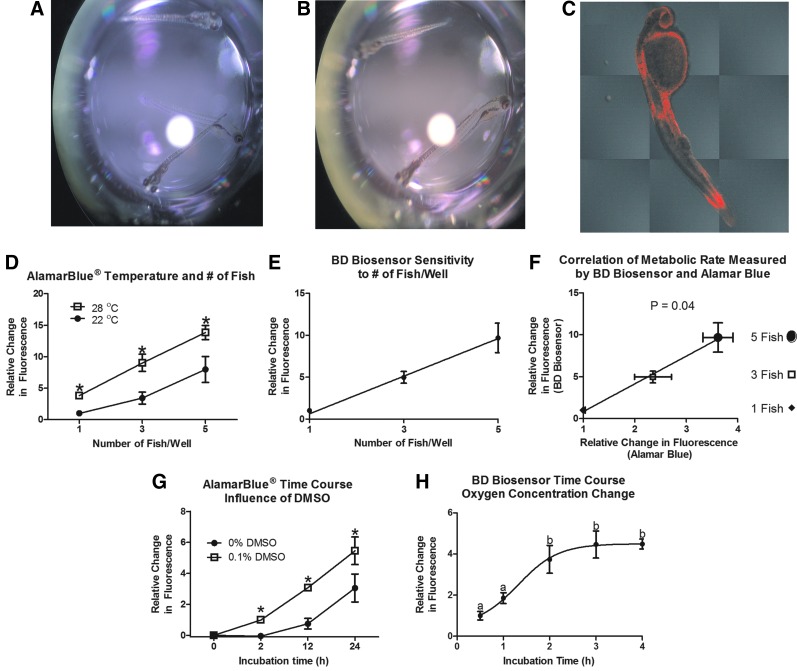
We first established that Alamar Blue would both enter and exit the fish to affect a change in the reduction state and fluorescent emission of the media. Figure 1A and B show the color change of Alamar Blue immediately following addition of the media and 24 h after, respectively. Figure 1C is a confocal image of a fish exposed for 1 h to Alamar Blue providing direct evidence that Alamar Blue enters the zebrafish tissue. The restricted distribution and lack of fluorescence in muscle tissue may be associated with the short duration of Alamar Blue treatment (1 h). When fish were exposed to Alamar Blue for 24 h, the signal was too intense to see any variation in the distribution of Alamar Blue (data not shown).
FIG. 1.
Assay development and validation. Image of zebrafish in wells of a 96-well plate at (A) 0 h and (B) 24 h of incubation in Alamar Blue®. (C) A confocal microscopy image of Alamar Blue within a zebrafish after 1 h of exposure to an assay solution. (D) Alamar Blue assay results varying the number of fish/well and the temperature of incubation (n=7–8, *p<0.05, relative to 22°C). (E) BD Oxygen Biosensor assay results varying the number of fish/well (n=5). (F) Correlation of relative change in fluorescence measured by Alamar Blue assays and BD Oxygen Biosensor assays conducted with 1, 3, and 5 fish. (G) Alamar Blue assay results with incubation time (0–24 h) and the addition of 0.1% dimethyl sulfoxide (DMSO) to an assay medium (n=8, *p<0.05, relative to no DMSO). (H) BD Oxygen Biosensor assay results as a function of incubation time (0.5–4 h,a,b indicate data points that differ significantly [p<0.05]).
To compare the Alamar Blue assay to an oxygen consumption assay (BD Biosensor), we varied the number of fish/well (1, 3, or 5; Fig. 1D, E). As the number of fish increased in the well, the relative change in fluorescence as measured by either assay increased. In fact, the results from either measure were highly correlated (p=0.04; Fig. 1F). We also compared the time course of response in both the Alamar Blue and BD Biosensor assays. Both assays showed that the signal increased with the incubation time (Fig. 1G, H). However, the signal monitored with the BD Biosensor plate, reached maximal within 2 h, when oxygen consumed by the fish was equal to the oxygen transfer from air to water. The signal measured by the Alamar Blue assay did not plateau even at 24 h. The increase in time sensitivity makes the Alamar Blue assay more amenable to studying the effect of drugs that may take time to induce an effect.
In the zebrafish, the metabolic rate is directly proportional to the temperature of the environment. To confirm that the Alamar Blue assay can be used to measure metabolic rate changes associated with changes in an ambient temperature, we incubated fish at 22°C or 28°C. Independent of the number of fish/well, fish maintained at 28°C had a higher metabolic rate than fish maintained at 22°C (p<0.05; Fig. 1D). Since many drugs are easily dissolvable in DMSO, we tested the effect of DMSO on the Alamar Blue signal. As expected, DMSO increased the signal at 2, 12, and 24 h (Fig. 1G). Through the use of confocal microscopy, we determined that DMSO enhanced the ability of Alamar Blue to enter the fish tissues, where it is reduced by NADH2 (data not shown).
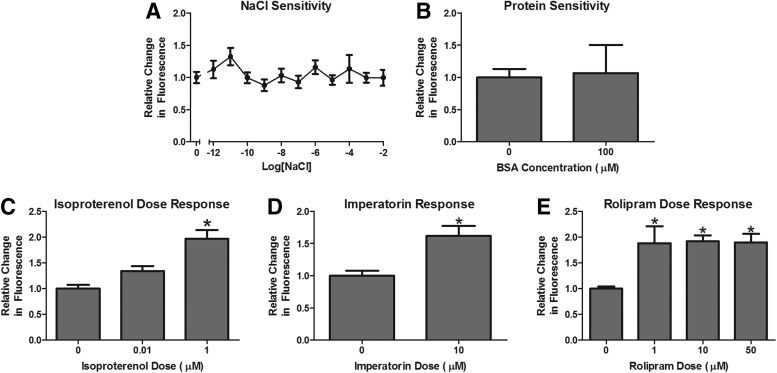
Many drugs are provided as salts; thus, we tested the sensitivity of the assay to a range of NaCl concentrations up to 10 mM (Fig. 2A). The relative change in fluorescence was not affected by the NaCl concentration. Furthermore, because we wanted to examine the effect of peptide hormones on energy expenditure, we tested and confirmed that there was no nonspecific response to proteins, specifically 100 μM BSA (Fig. 2B).
FIG. 2.
Validation for use in drug screening. Three fish/well were incubated in 4 mM sodium bicarbonate with 1% Alamar Blue (24 h). The relative change in fluorescence does not vary with (A) NaCl concentrations up to 10 mM (n=7–8) or (B) bovine serum albumin (BSA) concentrations of 100 μM (n=7). Stimulation of cAMP by (C) the adrenergic agonist isoproterenol (n=8) and phosphodiesterase blockers (D) imperatorin (n=8) or (E) rolipram (n=8) increases the relative change in fluorescence of the Alamar Blue media. *Significantly different from no drug treatment (p<0.05).
β-adrenergic receptor stimulation with isoproterenol increases cAMP and the metabolic rate.17 We show here that isoproterenol can increase metabolic rate of zebrafish (Fig. 2C). CyclicAMP is degraded by phophodiesterases within the cell. Phosphodiesterase inhibitors will result in increased intracellular cAMP by blocking cAMP degradation. Imperatorin and rolipram, phosphodiesterase inhibitors, also increase the metabolic rate of zebrafish (Fig. 2D, E).
Assay application
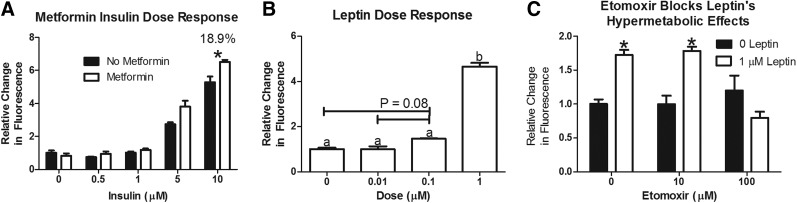
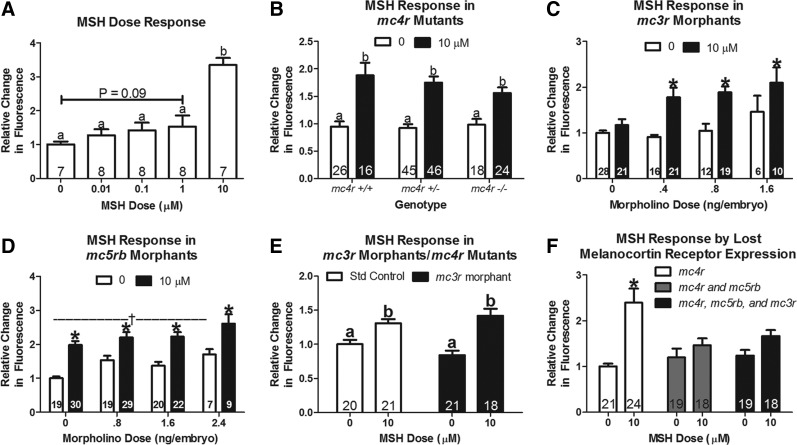
Both insulin and leptin increase the metabolic rate in a dose-dependent manner (Fig. 3A, B). A 24-h pretreatment with metformin increased the response to 10 μM insulin by 18.9% (Fig. 3A). Inhibition of fatty acid oxidation with treatment of 100 μM Etomoxir blocked the increase in the metabolic rate associated with leptin treatment (1 μM; Fig. 3C). α-MSH also dose dependently stimulated the metabolic rate of zebrafish (Fig. 4A). In order to test the role of the different melanocortin receptors in this response we utilized the MC4R knockout strain, and injected morpholino oligonucleotides to knock down gene expression of MC3R and/or MC5R. The response to α-MSH was not affected by knockout of MC4R or knockdown of MC3R or MC5R alone (Fig. 4B–D). Knockdown of MC3R in MC4R knockout zebrafish did not affect the response to 10 μM α-MSH (Fig. 4E). However, knockdown of MC5R in MC4R knockouts completely eliminated the response to α-MSH (Fig. 4F). MC3R knockdown in these fish lacking MC5R and MC4R had no additional effect. Thus, both MC4R and MC5R are responsible for α-MSH-induced increases in the metabolic rate in the zebrafish.
FIG. 3.
Effects of insulin and leptin in the Alamar Blue assay. Three fish/well were incubated in 4 mM sodium bicarbonate with 1% Alamar Blue. Fluorescence of the solution was measured at time 0 (4 days postfertilization; dpf) and 24 h later (5 dpf). Data are reported as the relative change in fluorescence (mean±SEM). (A) Insulin increases the metabolic rate in a dose-dependent manner. Metformin further increases the response to high-dose insulin (n=7–8). (B) Leptin increases the metabolic rate in a dose-dependent manner (n=7–8). (C) Leptin's hypermetabolic effects are blocked by 100 μM etomoxir (n=10–16). (A) *Indicates difference from no metformin (p<0.05). (B) a,bColumns that do not share a superscript differ significantly (p<0.05). (C) *Indicates difference from 0 leptin treatment at same etomoxir dose (p<0.05).
FIG. 4.
Response to alpha-melanocyte-stimulating hormone (α-MSH) in the Alamar Blue assay. One (B) or three (A, C–F) fish/well were incubated in 4 mM sodium bicarbonate with 1% Alamar Blue. Fluorescence of the solution was measured at time 0 (4 days postfertilization; dpf) and 24 h later (5 dpf). Data are reported as the relative change in fluorescence (mean±SEM). (A) α-MSH response in wild-type AB fish. B–F show that α-MSH (10 μM) induced a metabolic rate increase in fish lacking 1 or multiple melanocortin receptors. (B) α-MSH response in melanocortin 4-receptor (mc4r), wild-type (+/+), heterozygous (+/−), and knockout (−/−) fish. (C) α-MSH response in fish treated with 0.4, 0.8, or 1.6 ng/embryo of a morpholino targeted to knockdown melanocortin 3-receptor (mc3r). (D) α-MSH response in fish treated with 0.8, 1.6, or 2.4 ng/embryo of a morpholino targeted to knockdown melanocortin 5-receptor b (mc5rb). (E) α-MSH response in mc4r −/− fish treated with 1.6 ng/embryo of a morpholino targeted to knockdown mc3r. (F) α-MSH response in mc4r −/− fish treated with 3.2 ng/embryo standard control morpholino, 1.6 ng/embryo mc5rb morpholino+1.6 ng/embryo control morpholino, or 1.6 ng/embryo mc5rb morpholino and 1.6 ng/embryo mc3r morpholino. Numbers within each bar indicate the number of samples (n). a,bColumns with differing superscripts differ significantly (p<0.05). *Indicates difference from 0 α-MSH dose (p<0.05). †Indicates difference from no morpholino injection (p<0.05).
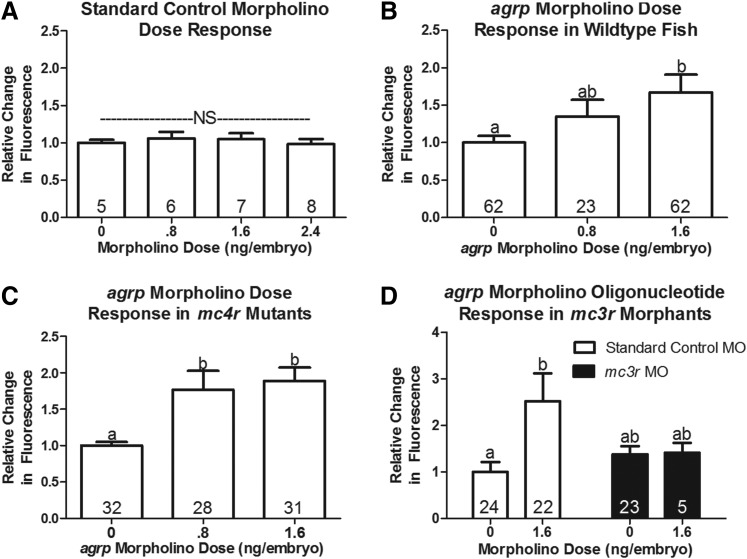
Injection of a nontargeted standard control morpholino did not affect the metabolic rate (Fig. 5A). However, a morpholino targeted to suppress AgRP increased the metabolic rate dose dependently (Fig. 5B). Suppression of endogenous AgRP, an inverse agonist, would be expected to both increase the activity of endogenous α-MSH as well as allow constitutive receptor activity to occur. To identify the receptor involved, we tested the AgRP morpholino response in MC4R knockout fish and MC3R morpholino knockdown fish (Fig. 5C, D). AgRP morpholino injection increased the metabolic rate in MC4R mutants, but not in MC3R morphants. This suggests that MC3R is responsible for the enhanced metabolic rate associated with AgRP knockdown.
FIG. 5.
Response to knockdown of agrp in the Alamar Blue assay. Three fish/well were incubated in 4 mM sodium bicarbonate with 1% Alamar Blue. Fluorescence of the solution was measured at time 0 (4 days postfertilization; dpf) and 24 h later (5 dpf). Data are reported as the relative change in fluorescence (mean±SEM). Numbers within the bar indicate number of wells (n). (A) Response to injection of a standard control morpholino used as the control (0) treatment in the agrp morpholino studies, (B) agrp morpholino dose response in wild-type fish. (C) agrp morpholino dose response in mc4r −/− fish. (D) Response to 1.6 ng/embryo of agrp morpholino in fish injected with 1.6 ng/embryo standard control morpholino (clear) or 1.6 ng/embryo mc3r morpholino (black). Numbers within each bar indicate the number of samples (n). a,bBars that do not share a superscript differ significantly (p<0.05).
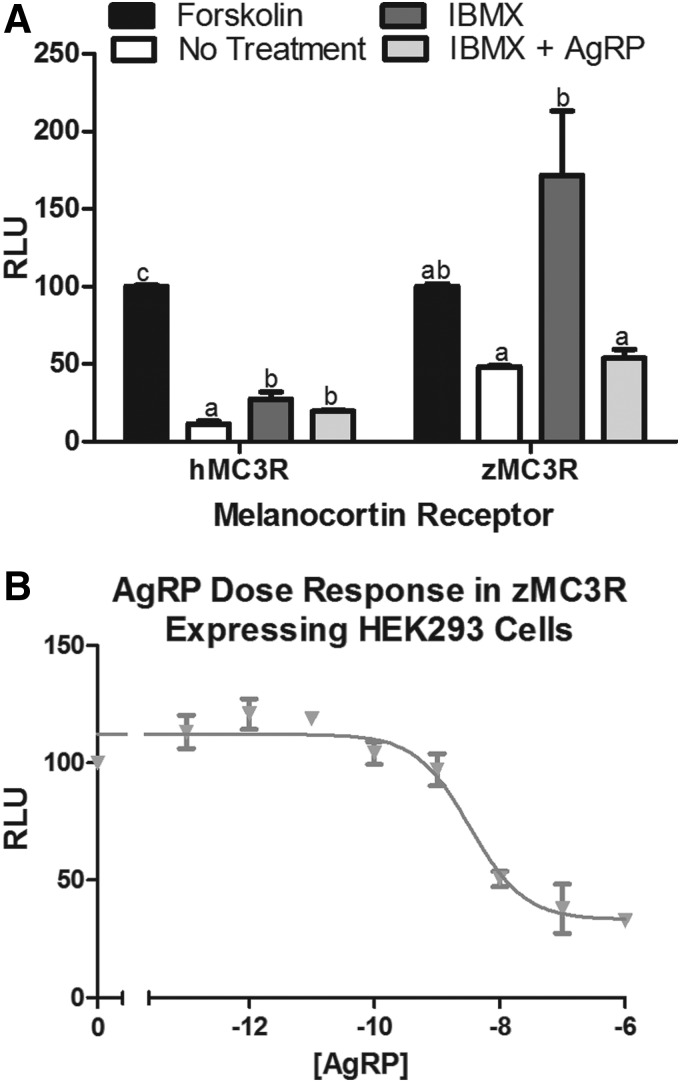
Since MC3R knockdown did not appear to alter the increase in metabolic rate in response to α-MSH, we decided to test for the constitutive activity of the zebrafish MC3R, previously well documented for the human and zebrafish MC4R. In HEK293 cells transiently cotransfected to express one melancortin receptor (hMC3R or zMC3R) and a cAMP-responsive luciferase, we tested the constitutive activity of these receptors by blocking phosphodiesterase breakdown of cAMP with IBMX (Fig. 6A). IBMX increased the relative luminescence units (RLU) measured from cells expressing zMC3R. AgRP decreased the IBMX response in these cells. As expected, IBMX had only a mild effect in cells expressing the nonconstitutively active hMC3R and AgRP did not suppress the RLU response to IBMX. An AgRP dose response in cells exposed to IBMX show that AgRP dose dependently decreases RLU in cells that transiently express zMC3R (EC50-3.325×10−9M; Fig. 6B).
FIG. 6.
Relative luminescence units (RLU) of human embryonic kidney (HEK293) cells expressing human (h) or zebrafish (z) melanocortin 3 receptors (MC3R). (A) When RLU are corrected for responsiveness to Forskolin, HEK293 cells that express zMC3R display high levels of constitutive activity (IBMX treatment) that is blocked by agouti-related protein (AgRP) treatment. (B) AgRP dose dependently decreases RLU in IBMX-treated cells that express zMC3R. a,b,cColumns with different superscripts differ significantly from other columns that signify treatment of cells expressing the same receptor (p<0.05. n=4).
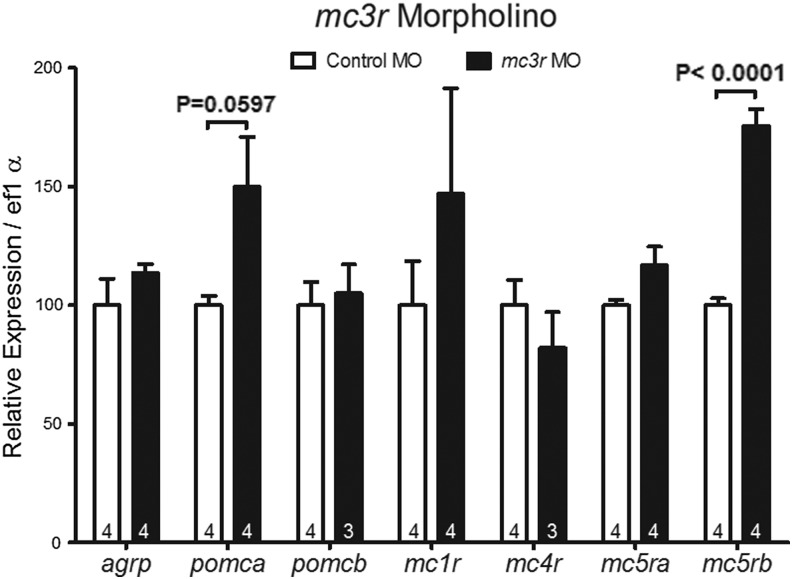
Finally, we examined mRNA expression of melanocortin proteins in zebrafish lacking MC3R. MC3R knockdown tended to increase pomca (p=0.0597; Fig. 7) and increased mc5rb expression, which may explain the apparent increase in MSH sensitivity in MC3R morphants.
FIG. 7.
mRNA expression of the melanocortin system proteins in zebrafish at 4 dpf treated with a morpholino targeting knockdown of MC3R. Numbers within each bar indicate the number of samples (n). p>0.05 unless labeled.
Discussion
Zebrafish hold promise as a useful model organism for studying energy balance. Zebrafish are small and ideally suited for assays in 96-well plates, and morpholinos can be employed to quickly assess the effect of genetic knockdown. Furthermore, aspects of the control of food intake are highly conserved between fish and mammals. The melanocortin system has been studied in both teleosts and mammals. Central α-MSH administration decreases the food intake in the rat and goldfish.4,6 Similarly, overexpression of the inverse agonist of the MC4R, AgRP, increased body weight in both mice and zebrafish.8,9 In the goldfish, administration of an MC4R antagonist increases food intake.18 Furthermore, the melanocortin system responds to nutritional restriction similarly in rats and zebrafish by increasing expression of AgRP to drive food consumption.11,18,19
The conservation of the regulation of food intake is not limited to the melanocortin system, but extends to other hormones and neuropeptides, including neuropeptide Y (NPY), leptin, and insulin. NPY injections increase the food intake in mouse, goldfish, trout, and catfish.20–23 Restricted food intake has been shown to increase brain NPY in mice, rats, and salmonids.19,23,24 Leptin, an adipose tissue-derived hormone, has been shown to decrease food intake in mice, chickens, and goldfish,2,5,25,26 with chronic leptin suppressing the body weight in both mice and goldfish.5,27 Transgenic overexpression of leptin suppresses body weight in rainbow trout.28 Insulin is known to suppress food intake in both mice and rainbow trout.3,7 Thus, the control of food intake appears to be conserved between mammals and teleost species.
The hormonal signals that control food intake also have robust effects on energy expenditure. Hormonal signals that increase food intake signal a state of food deprivation and often decrease energy expenditure, while hormonal signals that induce satiety, signal food abundance and tend to increase energy expenditure. In the rodent, leptin, insulin, and α-MSH suppress food intake and increase metabolic rate.29–31 Given that the hormones that regulate food intake are conserved from the mouse to the teleost, and that these same hormones affect energy expenditure in the rodent, we expected that these hormones would affect energy expenditure in the zebrafish.
Measurement of the metabolic rate in teleosts has required a closed system to measure oxygen consumption. This closed system does not allow for the rapid screening of many specimens. The use of an assay to monitor the metabolic rate of zebrafish in a 96-well plate was first implemented by Makky et al. (2008).32 They employed the common cell culture reagent phenol red to measure the acidification of water induced by accumulation of CO2. However, because gas readily exchanges between water and air, this assay can only detect very robust changes in the metabolic rate. The authors attempted to limit gas exchange by covering the water with mineral oil. Yet, mineral oil is known to allow for rapid exchange of gases. We avoid this limitation associated with gas exchange by instead measuring NADH2 production using the commercially available Alamar Blue. Therefore, we can monitor NADH2 production, which is directly linked with the metabolic rate, to identify drug-, hormone-, and gene-induced changes in the metabolic rate.
After validating this assay for use in the zebrafish, we were able to show that, similar to the mouse,31,33 insulin and leptin increase the metabolic rate in the zebrafish (Fig. 3A, B).29,31 We were further able to show that the metabolic rate response to insulin was increased when fish were pretreated with the insulin sensitizer, metformin (Fig. 3A). This suggests that this assay may be employed to screen for insulin-sensitizing drugs. In the mouse, the hypermetabolic effects of leptin on skeletal muscle explants are dependent on increased β-oxidation and can be blocked using the carnitine palmitoyl transferase I inhibitor, etomoxir.33 Using our assay, we were able to show that etomoxir blocks the ability of leptin to stimulate energy expenditure in vivo (Fig. 3C). Thus, this assay may be a valuable tool to screen for both leptin inhibitors and sensitizers in vivo.
To show that genetic manipulation could affect the hormone response, we studied the response to administered α-MSH, and to AgRP knockdown in fish with knockout of MC4R or morpholino knockdown of MC3R and MC5R. Peripheral α-MSH increases energy expenditure in the mouse.30 We show that it has a similar effect in the zebrafish (Fig. 4A). We further show that the increased energy expenditure in response to α-MSH in the zebrafish appears to result from effects at both MC4R and MC5R (Fig. 4F). Intraperitoneal injection of MTII, a melanocortin agonist, into wild-type and MC4R −/− mice shows that the increased energy expenditure in response to MTII is MC4R dependent. However, this study does not eliminate a role for MC5R in regulation of energy expenditure as MTII has a sevenfold higher affinity for MC4R than for MC5R.34 In fact, multiple lines of evidence suggest a role for MC5R in regulation of energy expenditure. The Quebec Family Study identified variants in MC5R that were associated with BMI, fat mass, and resting metabolic rate.35 Studies to identify the mechanism by which MC5R may affect energy metabolism have shown that MC5R is expressed in skeletal muscle and knockout of MC5R prevents binding of 125I-NDP MSH to skeletal muscle plasma membranes.36 Furthermore, α-MSH dose dependently stimulates carnitine palmitoyl transferase I and fatty acid oxidation in mouse skeletal muscle in vivo.37 In fact, serum α-MSH has been shown to increase following a meal and induce a thermogenic response in skeletal muscle.38 These results in the mouse suggest that MC5R in skeletal muscle may increase energy expenditure. Thus, our findings, which suggest that MC5R may be partly responsible for increased energy expenditure in response to α-MSH in the fish, are consistent with the literature in mammals. These findings show that this assay is sufficiently sensitive to measure a blunted hormonal response induced by knockout or knockdown of genes that mediate the hormonal effect.
AgRP serves as an antagonist for both MC3R and MC4R, and an inverse agonist of both the mammalian and teleost MC4R. In the mouse, AgRP affects energy expenditure through actions at MC4R that stimulate brown adipose tissue (BAT) thermogenesis and thyrotropine-releasing hormone (TRH) release.39 However, it should be noted that AgRP did affect T4 in MC4R knockouts, suggesting that MC3R may play a role in AgRP-mediated effects on thermogenesis. Our group has previously shown that AgRP knockdown in the zebrafish results in decreased body size, dependent on effects at the MC4R.13 We show here that AgRP knockdown increases energy expenditure in both wild-type (WT) and MC4R mutant zebrafish. We further show that MC3R morpholino eliminates the response to AgRP knockdown. In HEK293 cells expressing human (h) and zebrafish (z) MC3R, we show that the zMC3R, but not hMC3R, has constitutive activity.40 Furthermore, we show that AgRP appears to act as an inverse agonist at the zMC3R. Thus, it appears that AgRP may be acting to affect the metabolic rate through inverse agonism at MC3R. Thus, taken together, these data suggest that activation of MC3R, MC4R, and MC5R all increase metabolism in zebrafish. Most importantly, these studies show that combining this assay with genetic manipulation allows one to dissect the pathways that manipulate energy expenditure in the zebrafish.
We have developed an assay that can very effectively monitor changes in NADH2 production in zebrafish to measure energy expenditure. This assay can be used to understand the role of hormonal systems in the fish and to compare the hormonal regulation of energy expenditure of teleosts to that of mammals. The relative ease of genetic manipulation in the zebrafish also makes them ideal for studies investigating the genetic mechanisms underlying a physiologic response. Furthermore, we have shown that this assay can be used to monitor the effects of an insulin sensitizer in vivo. Finally, we were able to show that leptin's effects on metabolic rate can be mitigated by blocking fatty acid metabolism, as previously seen in the rodent. Together, these studies suggest that this assay may provide a unique tool to perform in vivo screens for drugs that affect metabolic rate, including insulin or leptin sensitizers.
Acknowledgments
The authors wish to thank Dr. Julien Sebag for his guidance and advice in the cell pharmacology experiments, Dr. Jacques Pantel for his aid in development of the hMC3R plasmid, and the staff of the Vanderbilt University Zebrafish Aquatic Facility (ZCORE) for their maintenance of the zebrafish colonies. This work was supported by NIH RO1 DK075721 (RDC) and NIH 1F32DK082167-01 (BJR).
Author Disclosure Statement
The authors have no competing financial interests.
References
- 1.Lieschke GJ. Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 2.Barrachina MD. Martinez V. Wei JY. Tache Y. Leptin-induced decrease in food intake is not associated with changes in gastric emptying in lean mice. Am J Physiol. 1997;272:R1007–R1011. doi: 10.1152/ajpregu.1997.272.3.R1007. [DOI] [PubMed] [Google Scholar]
- 3.Brown LM. Clegg DJ. Benoit SC. Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–691. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 4.McMinn JE. Wilkinson CW. Havel PJ. Woods SC. Schwartz MW. Effect of intracerebroventricular alpha-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R695–R703. doi: 10.1152/ajpregu.2000.279.2.R695. [DOI] [PubMed] [Google Scholar]
- 5.de Pedro N. Martinez-Alvarez R. Delgado MJ. Acute and chronic leptin reduces food intake and body weight in goldfish (Carassius auratus) J Endocrinol. 2006;188:513–520. doi: 10.1677/joe.1.06349. [DOI] [PubMed] [Google Scholar]
- 6.Cerda-Reverter JM. Schioth HB. Peter RE. The central melanocortin system regulates food intake in goldfish. Regul Pept. 2003;115:101–113. doi: 10.1016/s0167-0115(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 7.Soengas JL. Aldegunde M. Brain glucose and insulin effects on food intake and brain biogenic amines of rainbow trout. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:641–649. doi: 10.1007/s00359-004-0524-5. [DOI] [PubMed] [Google Scholar]
- 8.Ollmann MM. Wilson BD. Yang YK. Kerns JA. Chen Y. Gantz I, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 9.Song Y. Cone RD. Creation of a genetic model of obesity in a teleost. FASEB J. 2007;21:2042–2049. doi: 10.1096/fj.06-7503com. [DOI] [PubMed] [Google Scholar]
- 10.Hahn TM. Breininger JF. Baskin DG. Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 11.Song Y. Golling G. Thacker TL. Cone RD. Agouti-related protein (AGRP) is conserved and regulated by metabolic state in the zebrafish, Danio rerio. Endocrine. 2003;22:257–265. doi: 10.1385/ENDO:22:3:257. [DOI] [PubMed] [Google Scholar]
- 12.Kimmel CB. Ballard WW. Kimmel SR. Ullmann B. Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C. Forlano PM. Cone RD. AgRP and POMC neurons are hypophysiotropic and coordinately regulate multiple endocrine axes in a larval teleost. Cell Metab. 2012;15:256–264. doi: 10.1016/j.cmet.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chepurny OG. Holz GG. A novel cyclic adenosine monophosphate responsive luciferase reporter incorporating a nonpalindromic cyclic adenosine monophosphate response element provides optimal performance for use in G protein coupled receptor drug discovery efforts. J Biomol Screen. 2007;12:740–746. doi: 10.1177/1087057107301856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantel J. Williams SY. Mi D. Sebag J. Corbin JD. Weaver CD, et al. Development of a high throughput screen for allosteric modulators of melanocortin-4 receptor signaling using a real time cAMP assay. Eur J Pharmacol. 2011;660:139–147. doi: 10.1016/j.ejphar.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C. Song Y. Thompson DA. Madonna MA. Millhauser GL. Toro S, et al. Pineal-specific agouti protein regulates teleost background adaptation. Proc Natl Acad Sci U S A. 2010;107:20164–20171. doi: 10.1073/pnas.1014941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owicki JC. Parce JW. Kercso KM. Sigal GB. Muir VC. Venter JC, et al. Continuous monitoring of receptor-mediated changes in the metabolic rates of living cells. Proc Natl Acad Sci U S A. 1990;87:4007–4011. doi: 10.1073/pnas.87.10.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerda-Reverter JM. Peter RE. Endogenous melanocortin antagonist in fish: structure, brain mapping, and regulation by fasting of the goldfish agouti-related protein gene. Endocrinology. 2003;144:4552–4561. doi: 10.1210/en.2003-0453. [DOI] [PubMed] [Google Scholar]
- 19.Korner J. Savontaus E. Chua SC., Jr. Leibel RL. Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol. 2001;13:959–966. doi: 10.1046/j.1365-2826.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- 20.Aldegunde M. Mancebo M. Effects of neuropeptide Y on food intake and brain biogenic amines in the rainbow trout (Oncorhynchus mykiss) Peptides. 2006;27:719–727. doi: 10.1016/j.peptides.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Marsh DJ. Hollopeter G. Kafer KE. Palmiter RD. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat Med. 1998;4:718–721. doi: 10.1038/nm0698-718. [DOI] [PubMed] [Google Scholar]
- 22.Narnaware YK. Peyon PP. Lin X. Peter RE. Regulation of food intake by neuropeptide Y in goldfish. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1025–R1034. doi: 10.1152/ajpregu.2000.279.3.R1025. [DOI] [PubMed] [Google Scholar]
- 23.Silverstein JT. Plisetskaya EM. The effects of NPY and insulin on food intake regulation in fish. Am Zool. 2000;40:296–308. [Google Scholar]
- 24.Sahu A. Kalra PS. Kalra SP. Food deprivation and ingestion induce reciprocal changes in neuropeptide Y concentrations in the paraventricular nucleus. Peptides. 1988;9:83–86. doi: 10.1016/0196-9781(88)90013-7. [DOI] [PubMed] [Google Scholar]
- 25.Denbow DM. Meade S. Robertson A. McMurtry JP. Richards M. Ashwell C. Leptin-induced decrease in food intake in chickens. Physiol Behav. 2000;69:359–362. doi: 10.1016/s0031-9384(99)00258-9. [DOI] [PubMed] [Google Scholar]
- 26.Volkoff H. Eykelbosh AJ. Peter RE. Role of leptin in the control of feeding of goldfish Carassius auratus: interactions with cholecystokinin, neuropeptide Y and orexin A, and modulation by fasting. Brain Res. 2003;972:90–109. doi: 10.1016/s0006-8993(03)02507-1. [DOI] [PubMed] [Google Scholar]
- 27.Keung W. Palaniyappan A. Lopaschuk GD. Chronic central leptin decreases food intake and improves glucose tolerance in diet-induced obese mice independent of hypothalamic malonyl CoA levels and skeletal muscle insulin sensitivity. Endocrinology. 2011;152:4127–4137. doi: 10.1210/en.2011-1254. [DOI] [PubMed] [Google Scholar]
- 28.Murashita K. Uji S. Yamamoto T. Ronnestad I. Kurokawa T. Production of recombinant leptin and its effects on food intake in rainbow trout (Oncorhynchus mykiss) Comp Biochem Physiol B Biochem Mol Biol. 2008;150:377–384. doi: 10.1016/j.cbpb.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Doring H. Schwarzer K. Nuesslein-Hildesheim B. Schmidt I. Leptin selectively increases energy expenditure of food-restricted lean mice. Int J Obes Relat Metab Disord. 1998;22:83–88. doi: 10.1038/sj.ijo.0800547. [DOI] [PubMed] [Google Scholar]
- 30.Hoggard N. Rayner DV. Johnston SL. Speakman JR. Peripherally administered [Nle4,D-Phe7]-alpha-melanocyte stimulating hormone increases resting metabolic rate, while peripheral agouti-related protein has no effect, in wild type C57BL/6 and ob/ob mice. J Mol Endocrinol. 2004;33:693–703. doi: 10.1677/jme.1.01632. [DOI] [PubMed] [Google Scholar]
- 31.Menendez JA. Atrens DM. Insulin increases energy expenditure and respiratory quotient in the rat. Pharmacol Biochem Behav. 1989;34:765–768. doi: 10.1016/0091-3057(89)90272-4. [DOI] [PubMed] [Google Scholar]
- 32.Makky K. Duvnjak P. Pramanik K. Ramchandran R. Mayer AN. A whole-animal microplate assay for metabolic rate using zebrafish. J Biomol Screen. 2008;13:960–967. doi: 10.1177/1087057108326080. [DOI] [PubMed] [Google Scholar]
- 33.Solinas G. Summermatter S. Mainieri D. Gubler M. Pirola L. Wymann MP, et al. The direct effect of leptin on skeletal muscle thermogenesis is mediated by substrate cycling between de novo lipogenesis and lipid oxidation. FEBS Lett. 2004;577:539–544. doi: 10.1016/j.febslet.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 34.Grieco P. Cai M. Han G. Trivedi D. Campiglia P. Novellino E, et al. Further structure-activity studies of lactam derivatives of MT-H and SHU-9119: their activity and selectivity at human melanocortin receptors 3, 4, and 5. Peptides. 2007;28:1191–1196. doi: 10.1016/j.peptides.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chagnon YC. Chen WJ. Perusse L. Chagnon M. Nadeau A. Wilkison WO, et al. Linkage and association studies between the melanocortin receptors 4 and 5 genes and obesity-related phenotypes in the Quebec Family Study. Mol Med. 1997;3:663–673. [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W. Kelly MA. Opitz-Araya X. Thomas RE. Low MJ. Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 37.An JJ. Rhee Y. Kim SH. Kim DM. Han DH. Hwang JH, et al. Peripheral effect of alpha-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle. J Biol Chem. 2007;282:2862–2870. doi: 10.1074/jbc.M603454200. [DOI] [PubMed] [Google Scholar]
- 38.Enriori PJ. Evans AE. Williams SM. Grayson BE. Garcia-Rudaz MC. Roth CL, et al. Peripheral alpha-melanocortin stimulating hormone participate in glucose homeostasis enhancing glucose uptake by skeletal muscle. Keystone Symposium: Neuronal Control of Appetite, Metabolism and Weight; Keystone, CO. 2010. [Google Scholar]
- 39.Fekete C. Marks DL. Sarkar S. Emerson CH. Rand WM. Cone RD, et al. Effect of agouti-related protein in regulation of the hypothalamic-pituitary-thyroid axis in the melanocortin 4 receptor knockout mouse. Endocrinology. 2004;145:4816–4821. doi: 10.1210/en.2004-0476. [DOI] [PubMed] [Google Scholar]
- 40.Tao YX. Huang H. Wang ZQ. Yang F. Williams JN. Nikiforovich GV. Constitutive activity of neural melanocortin receptors. Methods Enzymol. 2010;484:267–279. doi: 10.1016/B978-0-12-381298-8.00014-9. [DOI] [PubMed] [Google Scholar]