Abstract
Background and Aims
The juvenile to adult transition (JAT) in higher plants is required for them to reach reproductive competence. However, it is a poorly understood process in woody plants, where only a few genes have been definitely identified as being involved in this transition. This work aims at increasing our understanding of the mechanisms regulating the JAT in citrus.
Methods
Juvenile and adult plants from Pineapple sweet orange (Citrus sinensis) and Rough lemon (C. jambhiri) were used to screen for differentially expressed transcription factors (TFs) using a 1·15K microarray developed on the basis of the CitrusTF database. Murcott tangor (C. reticulata × C. sinensis) and Duncan grapefruit (C. paradisi) were incorporated into the quantitative real-time reverse transcription–PCR validation in order to select those genes whose phase-specific regulation was common to the four species.
Key Results
A browsable web database has been created with information about the structural and functional annotation related to 1152 unigenes of putative citrus TFs (CTFs). This database constitutes a valuable resource for research on transcriptional regulation and comparative genomics. Moreover, a microarray has been developed and used that contains these putative CTFs, in order to identify eight genes that showed differential expression in juvenile and adult meristems of four different species of citrus. Those genes have been characterized, and their expression pattern in vegetative and reproductive tissues has been analysed. Four of them are MADS-box genes, a family of TFs involved in developmental processes, whereas another one resembles MADS-box genes but lacks the MADS box itself. The other three showed high partial sequence similarity restricted to specific Arabidopsis protein domains but negligible outside those domains.
Conclusions
The work presented here indicates that the JAT in citrus could be controlled by mechanisms that are in part common to those of Arabidopsis, but also somehow different, since specific factors without Arabidopsis orthologues have also been characterized. The potential involvement of the genes in the JAT is discussed.
Keywords: Citrus sinensis, C. jambhiri, C. reticulata, C. paradisi, database, differential gene expression, juvenile to adult transition, juvenility, transcription factor, JAT
INTRODUCTION
Development of higher plants has two distinct phases, juvenile and adult. In general, the juvenile phase is characterized by the inability to initiate floral development in response to floral-inductive cues (Zimmerman et al., 1985).
Phase change in Arabidopsis and other herbaceous species involves the activation of new gene expression programmes. Genetic analyses have identified many genes that allow us to better understand how reproductive competence is regulated. Four genetic pathways exist that mediate the effect of hormones, photoperiod, light quality, temperature and other environmental factors in a complex way that involves an intricate network of signalling pathways (Amasino and Michaels, 2010).
One well-known class of regulators involved in floral transition is the MADS-box gene family (Becker and Theissen, 2003). Among them, FLOWERING LOCUS C (FLC), one of the central flowering regulators, and a small clade of closely related genes called MADS AFFECTING FLOWERING (MAF genes), encode repressors of flowering and regulate the autonomous and vernalization pathways (Michaels and Amasino, 1999; Ratcliffe et al., 2003). FLC expression is repressed by vernalization treatments, thereby promoting flowering after winter has passed (Michaels and Amasino, 1999). On the other hand, the second central regulator of the transition to reproductive phase, CONSTANS (CO), mediates the response to long-day photoperiod (Suarez-Lopez et al., 2001). FLC and CO regulate the expression of genes responsible for the integration of the signals from the multiple flowering pathways (Simpson and Dean, 2002). One of these genes is FLOWERING LOCUS T (FT), a mobile flowering signal that acts as a floral promoter. FT activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1), a MADS-box floral activator (Simpson and Dean, 2002), leading to induction of LEAFY (LFY), another MADS-box gene. FT is also responsible for the activation of the MADS-box gene APETALA 1 (AP1), which is necessary to establish and maintain flower meristem identity. Once established, LFY and AP1 lead to flower formation according to the ABCE model (Ditta et al., 2004; for a review, see Krizek and Fletcher, 2005). SOC1 is also activated by an age-dependent mechanism in which SQUAMOSA BINDING FACTOR-LIKE 9 (SPL9) and microRNA156 (miR156) are involved (J. W. Wang et al., 2009). MiR172 and other SPL and APETALA2 (AP2)-like genes have been described as regulators of shoot maturation and the developmental timing (Schwartz et al., 2009; Wu et al., 2009).
In woody species, the relevant molecular mechanisms do not seem to match exactly those of Arabidopsis (Rottman et al., 2000; Tan and Swain, 2006), although they seem to share some similarities. Only a few genes have been clearly linked to the juvenile to adult transition (JAT). In spruce, the transcription factor DAL1 (a MADS-box gene) is involved in the transition to adulthood (Carlsbecker et al., 2004). Ectopic expression of DAL1 in Arabidopsis shortens the juvenile phase and induces flowering (Carlsbecker et al., 2004). In olive tree, the JAT gene, which is highly expressed in juvenile phases but repressed in adult phases, seems to be involved in the control of phase transition (Fernandez-Ocaña et al., 2010). Van der Linden et al. (2002) suggested that the MADS-box gene MdMADS12 plays a role in phase transition. MdMADS12 was isolated from vegetative apple tissues and falls into the AP1 clade of genes involved in floral transition and meristem development (Van der Linden et al., 2002). In kiwifruit, nine MADS-box genes have been characterized and the phenotypes of the corresponding transgenic Arabidopsis lines as well as their expression patterns suggest their involvement in phase change, flowering time and flower development (Varkonyi-Gasic et al., 2011). Likewise, overexpression of the gene CsTFL of Citrus sinensis, which encodes a regulatory protein of the Raf-1/MEK transduction pathway, is able to rescue the TFL mutation of Arabidopsis and appears to be involved in the process of juvenility in citrus (Pillitteri et al., 2004). Conversely, overexpression of the Arabidopsis genes LFY and AP1 in citrus causes a drastic reduction in the juvenile phase (Peña et al., 2001). In poplar and Poncirus (a close relative of Citrus) trees, the ectopic expression of FT or its homologues accelerated the transition from vegetative shoot apical meristems into inflorescence meristems (Endo et al., 2005; Böhlenius et al., 2006), and in apple, constitutive expression of the FUL-like BpMADS4 gene from Betula pendula resulted in flowering during in vitro culture of some shoots after transformation (Flachowsky et al., 2007). Recently, Wang et al. (2011) overexpressed the Arabidopsis miR156 in transgenic Populus × canadiensis, resulting in a drastic prolongation of its juvenile phase, suggesting a conserved regulatory role for miR156 in woody species. However, there is still much work ahead to determine the gene expression programmes that are taking place in the JAT in woody plants.
In citrus, flowering and fruit production are the main characters related to the adult phase (Navarro, 1990). However, although the loss of juvenility and the capacity to flower are associated, juvenility presents some other features apart from flowering, such as thorniness and vigorous growth (Cameron and Frost, 1968). Some species such as limes and lemons have juvenile phases shorter than 4 years when growing in tropical climates, whereas mandarins, grapefruits and sweet oranges may spend up to 10 years in the juvenile phase when grown from seeds. The long juvenile phase, in citrus in particular but in woody plants in general, constitutes a drawback for studies of reproductive biology and genetics, as well as a serious constraint for molecular and conventional breeding. Due to that, research on flowering in woody plants has historically been done for the purposes of accelerating progeny testing and speeding up the production of genetically improved seeds.
Citrus is an ideal model to approach studies of phase change due to the phenomenon of apomixis that allows asexual reproduction from seeds. This enables the production of juvenile plants by seed that have exactly the same genotype as the seed source plant, which may be hundreds of years old. Clonal propagation of juvenile and adult plants allows plants with the same genotype and similar size to be produced that still maintain their different juvenile or adult characteristics, and that can be grown under the same conditions. In addition, a recently propagated citrus adult plant initially produces only vegetative growth, and only starts flowering when it reaches a certain size. Thus, these recently propagated adult plants are competent to flower (but do not flower) and allow differentiation of the stages of competence and floral induction.
In this work, with the aim of exploring further and understanding the mechanisms regulating the JAT in citrus, we have created a web-browsable database with information about the structural and functional annotation concerning 1152 unigenes of putative citrus transcription factors (CTFs). We have developed a CTF microarray containing these putative unigenes and we have used it to screen for TFs differentially expressed between meristems from juvenile and adult plants from four citrus species, Pineapple sweet orange, Murcott tangor, Duncan grapefruit and Rough lemon, according to the experimental design described in Fig. 1.
Fig. 1.
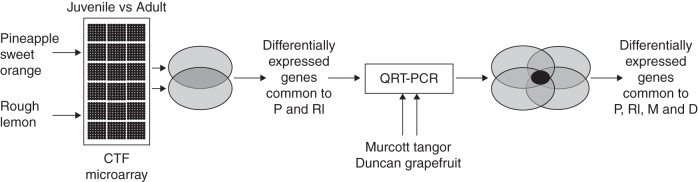
RNAs from meristems from juvenile and adult plants from Pineapple sweet orange and Rough lemon were labelled for microarray hybridization. A number of transcription factors were identified as differentially expressed. Those common to both lists of genes were selected and further validated by quantitative real-time RT–PCR. At this point, Murcott tangor and Duncan meristem RNAs were incorporated into the quantitative real-time RT–PCR validation in order to select those genes whose phase-specific regulation was common to the four varieties.
MATERIALS AND METHODS
Plant material
Plant material used for this study came from the Citrus Germplasm Bank of pathogen-free plants of the Instituto Valenciano de Investigaciones Agrarias (IVIA) (Navarro et al., 2002). Juvenile and adult buds of Pineapple sweet orange [Citrus sinensis (L.) Osb.], Murcott tangor (C. reticulata × C. sinensis), Duncan grapefruit (C. paradisi Macf.) and Rough lemon (C. jambhiri Lush) were grafted on sour orange (C. aurantium L.) and grown for 5 months under the same greenhouse conditions. The juvenile buds came from 3-month-old seedlings. Adult buds came directly from adult trees. Meristems were dissected under a binocular microscope and immediately frozen in liquid nitrogen. Some plants were used for the microarray experiments and the remaining plants were left as a control to examine their flowering time.
Leaves, bark and flowers were collected during the full flowering season, and different floral organs were dissected by hand using tweezers and a scalpel, and immediately frozen in liquid nitrogen.
Putative transcription factor identification and annotation
All GenBank citrus mRNA sequences (n = 148 813), including expressed sequence tags (ESTs), were downloaded from NCBI (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov/entrez) and assembled with 85 965 ESTs generated by the Citrus Functional Genomics Project (http://www.ibmcp.upv.es/genomics/cfgpDB), using CAP3 (Huang and Madan, 1999). A BLASTX (Altschul et al., 1997) search was then performed against the UniRef90 non-redundant protein database (http://www.ebi.ac.uk/uniref) to identify putative TFs in the citrus unigene set. Citrus unigenes were tagged as putative TFs if a BLAST hit annotated the sequence as a TF with an E-value lower than 1E-05.
Microarray design and printing
Unigenes with a sequence overlap >300 bp, with an identity >90 % and covering >75 % of the length of one of them, were assumed to represent the same gene, and only one of them was selected for oligo design. In these cases, the longest unigene with a clementine EST was selected. Searching for a specific oligonucleotide probe for each of the citrus unigenes selected was carried out using ‘YODA’ (http://pathport.vbi.vt.edu/YODA). For each unigene, 70-mer oligonucleotides were designed.
Every citrus clone was spotted twice. All samples were printed onto UltraGAPS aminosilane Corning slides, using a MicroGrid II arrayer (Genomic Solutions) in a 16-block format and 12 × 12 spots per block. Relative humidity was kept to 45 %. After printing, the slides were UV cross-linked at 150 mJ.
RNA extraction and labelling
Total RNA was extracted from meristems and individual floral organs using an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) and from leaf and bark tissues according to Ancillo et al. (2007). RNA was then used in an amplification reaction with the AminoAllyl Message Amp™ aRNA Amplification kit (Ambion, Inc., Austin, TX, USA) following the manufacturer's instructions. UTP-aminoallyl-amplified RNA (7·5 mg) was labelled using Cy3 or Cy5 dye (GE Healthcare, Bio-Sciences AB, Uppsala, Sweden).
Microarray hybridization
Microarray hybridization and washing were performed as previously described by Bassene et al. (2010). Four biological replicates were made, with two of them reverse-labelled (dye switch) with respect to the other two, to correct for dye bias. Afterwards, arrays were scanned at 532 nm for the Cy3 dye and at 635 nm for the Cy5 dye, with a GenePix 4000B scanner (Axon Molecular Devices, Sunnyvale, CA, USA), at a 10 nm resolution and 100 % laser power. Spot intensities were quantified using GenePix Pro 6·0 (Axon Molecular Devices). Spots with a net intensity in both channels <2-fold the mean background intensity were considered low-signal spots and removed.
Microarray data analysis and sequence analysis
Data were global median normalized using GenePix Pro 6·0 (Axon Molecular Devices), and LOWESS correction was applied using the Acuity 4·0 software (Axon Molecular Devices). To detect differentially expressed genes, data were analysed with the Significance Analysis of Microarray (SAM) package (Tusher et al., 2001) using two-class unpaired comparison.
For fine homology studies of the selected genes, BLASTX against the NCBI and TAIR9 (www.arabidopsis.org) databases was carried out using default parameters and an arbitrary non-stringent threshold of 1E-05 for the E-value.
Quantitative real-time reverse transcription–PCR (RT–PCR)
Prior to cDNA synthesis, total RNA was treated with DNase I according to Bassene et al. (2009). Then, 2 µg of DNase-treated RNA was reverse transcribed with oligo(dT)23 primer in a 20 µL reaction mixture using RevertAid M-MuLV reverse transcriptase (Fermentas). After heat inactivation, 1 µl of the 3-fold diluted cDNA was used as a template to perform real-time quantitative PCR in a total volume of 20 µl. All quantitative PCR analyses were performed with the LightCycler 480system (Roche Applied Science, Indianapolis, IN, USA) and PerfeCTa® SYBR® Green FastMix® (Quanta Bioscience, Gaithersburg, MD, USA). The PCR conditions were as follows: 95 °C for 3 min and 40 cycles of PCR (95 °C for 15 s and 60 °C for 1 min). Primer pairs for each gene are shown in Table 1. Two pooled biological replicates with three technical replicates were performed. Data were normalized against the citrus ACT11 gene by using the primers 5′-CAGTGTTTGGATTGGAGGATCA-3′ and 5′-TCGCCCTTTGAGATCCACAT-3′.
Table 1.
Changes in gene expression estimated by microarray hybridization and by quantitative real-time RT–PCR
| Fold change average in microarray* |
Fold change average in qRT–PCR† |
||||||
|---|---|---|---|---|---|---|---|
| Unigene ID | Primers | Rough lemon | Pineapple seet orange | Duncan grapefruit | Rough lemon | Murcott tangor | Pineapple sweet orange |
| CTF0607 | 5′-AAGCCATGGCAGCAATCG-3′ | 4·70 | 3·98 | 6·41 | 7·40 | 6·94 | 6·29 |
| 5′-GATGTCTGAACTCTGGCCACTT-3′ | |||||||
| CTF0899 | 5′-AACTGCTATGACATGGAAAACATCA-3′ | 2·17 | 1·85 | 1·58 | 1·62 | 1·68 | 1·30 |
| 5′-CCTGAACCCAGTTGTTGAAGTCT-3′ | |||||||
| CTF0931 | 5′-GGACATCTGAATGAAATTGATGCT-3′ | 7·00 | 1·48 | 2·90 | 2·80 | 6·12 | 1·93 |
| 5′-CATGCTGTTTCAATCTCAGGTTCT-3′ | |||||||
| CTF0516 | 5′-AAGTTCCTCCTCATCGACTAGCA-3′ | 2·47 | 3·14 | 1·93 | 1·89 | 2·70 | 2·03 |
| 5′-TGATTGAACTGGGTAGCGATTG-3′ | |||||||
| CTF0558 | 5′-CGAATGAGGTCTCGAGAAGCA-3′ | 1·57 | 2·05 | 5·39 | 4·83 | 6·67 | 1·94 |
| 5′-AATCTTGATCCCTTCAAAATCGAA-3′ | |||||||
| CTF0173 | 5′-AAAGAAGGTAAAAGAAAAGGAGAAGCT-3′ | 6·33 | 13·05 | 10·02 | 7·00 | 3·79 | 14·25 |
| 5′-GGATGAGTTCCAGTCATGATTTAGC-3′ | |||||||
| CTF0316 | 5′-CGCTACCAGAGTCGCATTACAG-3′ | 2·87 | 3·17 | 7·22 | 5·27 | 3·10 | 1·63 |
| 5′-AGTTGCAGGCTCGAATATCCA-3′ | |||||||
| CTF0397 | 5′-CGATCCCTGCGGTATCAAGT-3′ | 2·37 | 1·41 | 3·90 | 2·55 | 3·58 | 3·32 |
| 5′-GCTCATCTGAACCAGAGTTTGAGTT-3′ | |||||||
*Average fold change of the mean of expression values of four replicates in the microarray in adult samples with respect to juvenile samples.
†Average fold change of three independent RT–PCRs for three independent samples for each variety.
RESULTS
Database construction and development of an oligonucleotide microarray of CTFs
In order to obtain a comprehensive non-redundant set of citrus unique sequences, all available citrus mRNA sequences, including ESTs, were downloaded and assembled, resulting in a total of 49 155 non-redundant unigenes (see the Materials and Methods). Of these, 1834 were identified as putative TFs, based on their similarity to a UniRef90 protein annotated as a TF. To reduce further the sequence redundancy in the putative CTFs found, very similar unigenes were assumed to represent the same gene, and only the longest unigene with clementine ESTs was selected, resulting in 1306 non-redundant putative CTFs. When searching for a specific oligonucleotide probe for each of these selected citrus unigenes, a total of 154 unigenes did not yield an oligonucleotide specific enough to be used as a probe, resulting in a specific oligonucleotide set for putative CTFs of 1152 sequences. An oligo-based microarray was constructed using 70-mer specific oligonucleotides representing these 1152 putative CTFs.
A web-browsable CTF database was created containing information about the 1152 identified putative CTFs. The database is available at http://www.ibmcp.upv.es/genomics/citrusTF. The web interface to the CitrusTF database is not just a simple query to search by using sequence identifiers or keywords, or a collection of mere data tables. It can also be used with any combination of almost every different functional and structural annotation criterion in the queries. In addition, bulk queries using a file with a list of unigene names are implemented. The unigenes obtained as query results can be inspected individually, but also bulk downloads of the sequences, names or orthologues are allowed. The individual unigene web page view contains graphical and textual summaries of the assembly and annotation processes, and hyperlinks to the first hits of the external databases searched with BLAST are provided, as well as their descriptions and E-values. The full BLAST results can also be retrieved. Gene Ontology annotation results are also shown in a table with links to the GO term description pages, using the AmiGO tool (http://amigo.geneontology.org).
Identification of TFs differentially expressed in meristems from juvenile and adult plants
In order to identify TFs differentially expressed in meristems from juvenile and adult plants, four citrus species showing significant phenotypic differences in both developmental phases were chosen. Thus, juvenile and adult plants from Pineapple sweet orange, Murcott tangor, Duncan grapefruit and Rough lemon were grown as described in the Materials and Methods. Some of the plants were collected 5 months after grafting and used to dissect their meristems. Some others were used as control plants to examine their flowering time in order to ensure that, in our experimental conditions, adult plants were competent to flower but did not undergo floral induction. Control plants did not flower during the first spring following harvest of the experimental samples and only flowered during the second spring, thus ensuring that plants did not undergo floral induction at the moment of harvesting the material. RNA was extracted from meristems and, according to the experimental design shown in Fig. 1, that of Pineapple sweet orange and Rough lemon was hybridized to the oligonucleotide microarray. Four replicates were analysed for each species, comparing juvenile vs. adult expression using a dye switch experimental design (GEO accession numbers GSE39798 and GSE39799). After data normalization, expression levels were analysed with the SAM package (Tusher et al., 2001) using two-class unpaired comparison. Several TFs were identified as differentially expressed between adult and juvenile plants for both species. The SAM output for Rough lemon identified 53 genes as differentially expressed between adult and juvenile plants, with a false discovery rate of 4·9 %. Forty of them were overexpressed in juvenile plants and 13 in adult plants (Supplementary Data Table S1). In the case of Pineapple sweet orange, 46 genes were identified as differentially expressed. Twelve of them were overexpressed in juvenile plants and 34 in adult plants (see Supplementary Data Table S1). The two SAM outputs were then compared and 12 genes were common to both lists of differentially expressed genes (Table 2). These genes were selected and considered for further analysis. In order to identify the genes whose phase-specific regulation was recurrent not only in the two species analysed in the microarray approach but also in two other Citrus species, i.e. Duncan grapefruit and Murcott tangor, quantitative real-time RT–PCR was accomplished and RNA from the four species was included in the analysis. Eight out of 12 genes showed significant expression level differences between the two phases in the four species studied (Table 1). All eight genes common to the four species showed higher expression in adult samples than in juvenile samples.
Table 2.
List of differentially expressed genes common to Rough lemon and Pineapple sweet orange
| Rough lemon |
Pineapple sweet orange |
||||
|---|---|---|---|---|---|
| Unigene ID | Annotation | M-value* | FDR (%)† | M-value* | FDR (%)† |
| CTF0607 | MADS-box protein PTM5 | –2·232 | 0 | –1·993 | 0 |
| CTF0899 | MYB transcription factor MYB84 | –1·120 | 0 | –0·891 | 0 |
| CTF0931 | SVP-like floral repressor | –2·807 | 0 | –0·568 | 0 |
| CTF0516 | MADS-box protein 2 | –1·305 | 4·9 | –1·650 | 0 |
| CTF0558 | APETALA1 | –0·648 | 5 | –1·037 | 0 |
| CTF0173 | MADS5 protein | –2·663 | 0 | –3·707 | 0 |
| CTF0316 | MADS-box protein 15 | –1·523 | 4·9 | –1·663 | 0 |
| CTF0397 | At3g49760 | –1·244 | 4·9 | –0·498 | 1·9 |
| CTF0515 | AtMYB2 | –0·377 | 4·9 | –1·038 | 0 |
| CTF0588 | Putative homeodomain protein | –0·348 | 4·9 | –0·278 | 2·87 |
| CTF0170 | Ethylene response factor 5 | 0·655 | 4·1 | 14·405 | 5·1 |
| CTF0723 | Pathogenesis-related transcription factor and ERF | 2·337 | 0 | 0·368 | 5·1 |
Genes highlighted in bold were upregulated in the juvenile phase; the other genes were overexpressed in the adult phase.
*The M-value is the base two logarithm of the ratio between the background-substracted foreground intensity measured in the red and the green channels.
†Negative values mean higher expression in adult samples and positive ones higher expression in juvenile samples. FDR is the false discovery rate estimated for each gene by SAM (Significance Analysis of Microarrays) as the percentage of genes falsely identified as differentially expressed.
Expression pattern of the identified genes in young and adult vegetative tissues
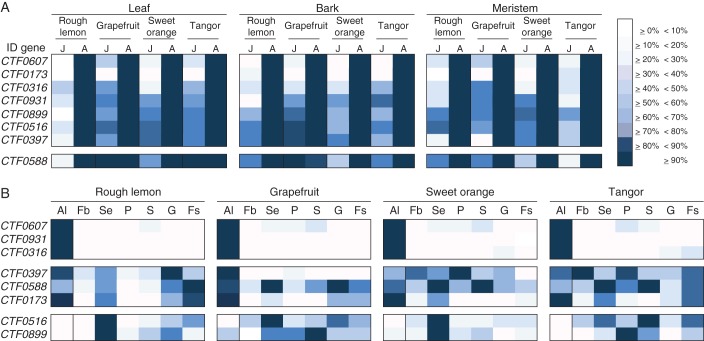
Expression analysis of the eight selected genes in meristems, leaf and bark tissues from young and adult plants was accomplished by quantitative real-time RT–PCR. According to the experimental design and quantitative validation, all CTFs analysed showed a marked difference in expression between meristems from juvenile and adult plants, being significantly more expressed in adult plant meristems than in juvenile meristems (Fig. 2A; Supplementary Data Table S2A). When the differences in CTF expression between leaves and bark from young and adult plants were analysed, most of the genes (CTF0607, CTF0931, CTF0173, CTF0899, CTF0516, CTF0397 and CTF0316) were in the same range as that present in meristems (Fig. 2A; Supplementary Data Table S2A), showing no expression in juvenile tissues or a much higher expression in adult than in juvenile tissues. CTF0588 showed a broader expression pattern. Differences between juvenile and adult leaf could be observed in Rough lemon and sweet orange, but not in grapefruit or tangor, where transcripts were detected in both juvenile and adult leaf and bark tissues (Fig. 2A; Supplementary Data Table S2A). In bark, differences could be observed in Rough lemon, sweet orange and tangor, but not in grapefruit.
Fig. 2.
Expression patterns of selected CTF genes in juvenile and adult plant tissues (A) and in different floral organs (B) in citrus plants. The analysis was performed in four Citrus species (Rough lemon, Duncan grapefruit, Pineapple sweet orange and Murcott tangor). The figure illustrates relative expression levels of each gene measured by quantitative real-time RT–PCR given as heat maps in blue-scale, where darker colours represent stronger expression. For each gene, all gene level profiles were normalized as follows. The highest signal intensity, for each comparison, is given a value of 100 % (shown in darkest blue), whereas absence of signal in given a value of 0 % (shown in white). Normalization was assessed by analysis of the citrus ACTIN 11 transcript in the same samples. J, juvenile; A, adult; Al, adult leaf; Fb, flower bud; Se, sepals; P, petals; S, stamens; G, gynoecium; and Fs, fruit set.
Expression pattern of the identified genes in reproductive organs
When gene expression was analysed by quantitative real-time RT–PCR in floral organs, two groups of genes could be distinguished according to their different pattern of expression (Fig. 2B; Supplementary Data Table S2B). CTF0607, CTF0931 and CTF0316 showed no expression or very low levels of expression in all reproductive organs (Fig. 2B; Supplementary Data Table S2B). The second, more heterogeneous group, consisted of CTF0516, CTF0588, CTF0899, CTF0397 and CTF0173, which showed considerable levels of expression in specific reproductive organs (Fig. 2B; Supplementary Data Table S2B). CTF0397, CTF0588 and CTF0173 were mainly expressed in sepals, carpels and fruit primordia (Fig. 2B; Supplementary Data Table S2B), whereas CTF0899 and CTF0516 appeared to be overexpressed in sepals and to a lesser extent in carpels and fruit (Fig. 2B; Supplementary Data Table S2B).
Sequence and bioinformatic analysis of CTF genes
Selected genes were re-checked by BLASTX searches against the TAIR9 (www.arabidopsis.org) database. Results (shown in Table 3) revealed that five of them showed high similarity to Arabidopsis MADS-box factors, although one lacks the MADS domain and could not strictly be considered a bona fide MADS-box TF. CTF0173 showed high similarity to the Arabidopsis gene FRUITFULL (AtFUL), showing 99, 81 and 34 % similarity at the amino acid level in the MADS domain, K-box domain and in the rest of the protein, respectively (Fig. 3). The best blast hit for CTF0516 was SEPALLATA1 (AtSEP1), for CTF0607 it was AGAMOUS-LIKE 42 (AGL42) and for CTF0316 it was FLC. As for CTF0173, the percentage similarity in the case of the amino acid sequence of CTF0516, CTF0607 and CTF0316 with their respective best blast hits was very high in the MADS domain (ranging from 84 to 96 %), also high but somewhat lower in the K-box domain (ranging from 64 to 81 %), and lower in the rest of the protein (ranging from 45 to 62 %) (Fig. 3). CTF0931 lacks the MADS domain but showed 82 % similarity to the K-box domain of SHORT VEGETETIVE PHASE (AtSVP) and 68 % in the rest of the protein.
Table 3.
Sequence analysis of the CTF genes
| Unigene ID | Protein length | Homologous locus* | Locus description/function | BLASTX E-value | Query coverage† | Domains‡ |
|---|---|---|---|---|---|---|
| CTF0173 | 244 | At5g60910·1 | FRUITFULL, AGAMOUS-LIKE 8 | 1·00E-63 | 98 % | MADS and K-box |
| CTF0516 | 249 | At5g15800·1 | SEPALLATA1, AGAMOUS-LIKE 2 | 2·00E-77 | 100 % | MADS and K-box |
| CTF0607 | 212 | At5g62165·3 | FOREVER YOUNG FLOWER, AGAMOUS-LIKE 42 | 1·00E-54 | 97 % | MADS and K-box |
| CTF0316 | 188 | At5g10140·2 | FLOWERING LOCUS C, AGAMOUS-LIKE 25 | 1·00E-33 | 100 % | MADS and K-box |
| CTF0931 | 152 | At2g22540·2 | SHORT VEGETATIVE PHASE, AGAMOUS-LIKE 22 | 2·00E-23 | 75 % | K-box |
| CTF0588 | 642 | At5g02030·1 | BEL1-LIKE HOMEODOMAIN 9 | 1·00E-107 | 80 % | POX and homeodomain |
| CTF0397 | 185 | At3g49760·1 | BASIC LEUCINE-ZIPPER 5 | 2·00E-16 | 87 % | bZIP1 |
| CTF0899 | 324 | At1g68320·1 | MYB DOMAIN PROTEIN 62 | 4·00E-62 | 44 % | SANT1 and SANT2 |
The deduced protein sequences of citrus cDNAs were searched against the Arabidopsis Information Resource (TAIR) database.
*The results refer to the best hit when using the BLASTX algorithm to query the non-redundant protein sequence data set of TAIR at www.arabidopsis.org.
†Extension of the successful alignment between citrus and Arabidopsis proteins using the NCBI BLAST 2 sequences software (http://www.ncbi.nlm.nih.gov/blast/bl2seq/bl2.html).
‡The conserved domains in each citrus deduced protein were predicted with the Conserved Domain Database (CDD) from the NCBI (http://www.ncbi.nlm.nih.gov/cdd).
Fig. 3.
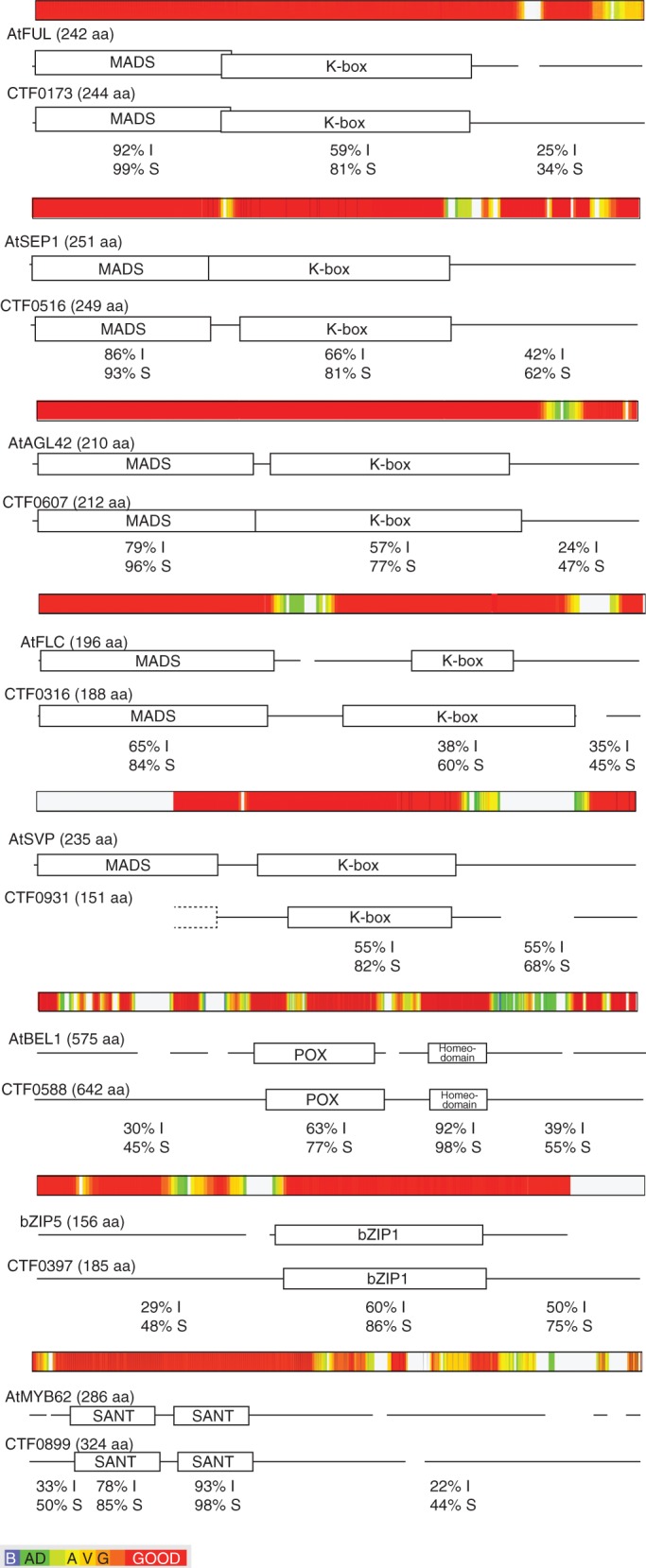
For each protein, the boxes represent the conserved domains described in the Conserved Domain Database (CDD). The colour-coded alignments between citrus deduced protein and the corresponding Arabidopsis protein showing the best BLAST hit were done by Expresso Alignment of the T-Coffee software (http://www.tcoffee.org). The graphic coloured output indicates the level of consistency between the final alignment and the library used by T-Coffee (Di Tomaso et al., 2011). The red colour corresponds to alignment portions with a strong consistency, and blue colour corresponds to poorly supported alignments.
The other three CTF genes showed high partial sequence similarity restricted to specific domains but negligible outside those domains. CTF0588 contains a POX domain and a homeodomain that shares 77 and 98 % similarity, respectively. CTF0397 bears a bZIP1 domain with a similarity percentage of 86 %. Finally, CTF0899 is a MYB-like protein that contains two SANT domains with 85 and 98 % similarity to those of the MYB62 protein of Arabidopsis.
DISCUSSION
Genomics and microarray technology constitute a powerful tool to accomplish analysis of large numbers of genes at the same time, enabling a comprehensive knowledge of the processes under study to be gained. Genome-wide identification of CTFs and the subsequent construction of a database of these TFs are of major importance to explore in greater depth the biological function of TFs and the mechanisms of transcriptional regulation. The CitrusTF database constitutes a valuable resource for research on transcriptional regulation and comparative genomics. Together with DPTF from poplar (http://dptf.cbi.pku.edu.cn/), CitrusTF is the only existing database of TFs from woody plants. The citrus genome has been predicted to have 29 445 protein-coding genes (Xu et al., 2013) and Arabidopsis 27 416 (TAIR, www.arabidopsis.org). The Arabidopsis database RARTF (http://rarge.psc.riken.jp/rartf/), developed by Iida et al. (2005), contains 1978 TFs. On the other hand, Zhu et al. (2007) have been able to identify 1922 TFs in poplar, and the DPTF database currently has 2576 putative TFs. We have been able to identify 1152 CTFs, a relatively high number of TFs that may reflect a significantly high percentage of the total CTFs.
By using the CitrusTF database resource, a microarray of 1·152K was generated, that has been used as a tool for the identification of TFs involved in the JAT in citrus. We have identified a number of TFs differentially expressed in juvenile and adult phases in four citrus species. Since we are interested in general regulatory mechanisms in citrus, we selected those common to the four analysed species and characterized eight TFs putatively involved in this process. These genes exhibited a strong increase at the mRNA level in adult plant meristem samples with respect to juvenile samples and, in addition, some of them are expressed in reproductive tissues, suggesting that they may be involved in the JAT in citrus.
In citrus it is well known that meristems from juvenile plants only produce vegetative growth for several years after seed germination (Cameron and Frost, 1968). In contrast, meristems from adult or mature plants produce both vegetative growth and flowering. A recently propagated citrus adult or mature plant initially produces only vegetative growth, and only starts flowering when it reaches a certain size. Under greenhouse conditions, where the experiments presented here were carried out, 5-month-old plants of the four species studied are still too small to start flowering. Thus, juvenile plants and adult plants competent to flower (but not flowering) were used in this experimental design. Consequently, neither the juvenile nor the adult propagated plants produced flowers during the course of the study. In fact, control plants grown in parallel did not flower until the second spring. In citrus, grafting does not affect the maturity status at all (Navarro, 1990) and this is the basis for commercial propagation by grafting of hundreds of millions of mature plants at the nurseries.
Blast sequence analyses of CTF genes against the TAIR9 protein data set revealed that five of them are highly similar to MADS-box proteins of Arabidopsis (i.e. CTF0607, CTF0931, CTF0516, CTF0173 and CTF0316). CTF0173 showed high similarity to AtFUL, which controls development of the dehiscent valves of the Arabidopsis seed case as well as flower development (Gu et al., 1998). Endo et al. (2006) isolated and characterized a MADS-box gene from Citrus unshiu Marc. (CitMADS5) which, on the basis of its similarity and its expression pattern, was assumed to represent the citrus FUL orthologue. The CitMADS5 sequence is 100 % identical to CTF0173, and in a similar manner as occurs in Arabidopsis, both CTF0173 and CitMADS5 were detected in both adult vegetative and reproductive tissues, with only small differences that can be attributed to differences in the Citrus species analysed, in the growing conditions or in the harvesting time of the samples. The mRNA of CTF0516, which has high similarity to AtSEP1, accumulated abundantly in reproductive tissues, especially in sepals, gynoecium and the fruit primordium, and it was also abundant in juvenile tissues of leaves and bark. Similarly, in Arabidopsis, SEP1 is expressed in early flower development and in developing seed (Ma et al., 1991; Pelaz et al., 2000). SEP1 encodes a MADS-box TF involved in flower and ovule development, which is functionally redundant with SEP2, SEP3 and SEP4. Nishikawa et al. (2009) analysed the expression profile of two SEP homologues (CuSEP1 and CuSEP3) from C. unshiu. The similarity of CTF0516 to these genes is 76 and 66 %, respectively. As the SEP subfamily in Arabidopsis is formed by four SEP genes (Malcomber and Kellogg, 2005), it is quite probable that several SEP genes also exist in citrus. Whether these members are functionally redundant or not is a question to be elucidated. The best blast hit for CTF0607 in Arabidopsis is AGL42, which is a SOC1-like gene involved in the vegetative to floral transition in Arabidopsis (Dorca-Fornell et al., 2011). The expression of SOC1 increases according to developmental age (Moon et al., 2003) in the same way as CTF0607. Tan and Swain (2007) functionally characterized two SOC1-like genes from C. sinensis (CsSL1 and CsSL2). Overexpression of these genes in Arabidopsis shortened the time taken to flower, performing the endogenous function of Arabidopsis SOC1. The amino acid sequence of CsSL2 showed 100 % identity to that of CTF0607. Thus, CTF0607 may function as the SOC1 orthologue in citrus.
The CTF0316 mRNA is expressed only in vegetative tissues, showing higher accumulation in adult than in juvenile stages. CTF0316 shares close amino acid identity with Arabidopsis FLC. In Arabidopsis, FLC acts as a repressor of SOC1 by sensing seasonal changes. In summer/autumn, FLC levels are high to prevent flowering by repressing floral integrators (CO/FT). In winter, vernalization leads to repression of FLC, and the level of expression decreases until finally it is repressed when the spring season arrives. Therefore, relieved from repression, floral integrators activate flowering. In perennials such as Arabis alpina, the regulation of the response to vernalization appears to be similarly preserved. PEP1, the A. alpina orthologue of FLC, is downregulated during prolonged exposure to cold temperatures (coinciding with the initiation of flowering) and is upregulated after vernalization to repress flowering (R.H. Wang et al., 2009). In Poncirus trifoliata (a deciduous relative of Citrus which is an evergreen species), five amplicons produced by alternative splicing were characterized for FLC (Zhang et al., 2009). Furthermore, in Citrus clementina hort. ex Tanaka, Muñoz-Fambuena et al. (2011) reported the effect of fruits on the expression of a citrus FLC homologue among other genes involved in alternate bearing. However, whether these genes are indeed FLC orthologues has been questioned due to the methodology used for their identification (Samach, 2013). CTF0316 was 100 % identical in amino acid sequence to the variant 5 characterized by these authors (PtFLC5). In a similar way to CTF0316, PtFLC5 expression was only detectable in the adult period; however, their expression patterns in P. trifoliata differ from citrus in reproductive organs, since PtFLC5 was detected in flowers. However, Nishikawa et al. (2009) found that some flowering-related genes that were analysed in parallel in P. trifoliata and C. unshiu showed different expression patterns, indicating that the contribution of some flowering genes might differ between evergreen and deciduous species.
CTF0931 shares high similarity with the MADS-box genes SVP and PtSVP (an SVP homologue from P. trifoliata; Li et al., 2010) in the I and K domains and to a lesser extent in the C region, but lacks the MADS domain, the most highly conserved region of the MADS proteins (Riechmann et al., 1996; Hartmann et al., 2000). The MADS domain is the major determinant of DNA binding, but it also performs dimerization and accessory factor binding functions (Shore and Sharrocks, 1995). Although several SVP-like genes have been characterized in different plant species (Brill and Watson, 2004; Bielenberg et al., 2008; Diaz-Riquelme et al., 2009; Li et al., 2010; Wu et al., 2011) and an alternative splicing has been reported for SVP (Severing et al., 2012), to our knowledge no SVP-like protein lacking the MADS domain has been reported. Therefore, CTF0931 may constitute a specific TF of citrus. The lack of the MADS domain would prevent CTF0931 binding to the CArG-box sequence motif in the DNA, but whether an alternative DNA-binding motif not yet described is present in CTF0931 is something that still remains to be discovered. Apart from this, CTF0931 still retains the dimerization and transactivation domains, so it may act by binding to other TFs and modulating their functions. Autoregulatory loops are a common phenomenon in the regulation of MADS-box genes in plants (Honma and Goto, 2001; Gomez-Mena et al., 2005; Zhu and Perry, 2005). Severing et al. (2012) demonstrated that the deletion of a short fragment with a predicted interaction motif in the sequence of SVP by alternative splicing resulted in different interaction specificities and therefore different functionalities. In fact, SVP has been predicted to have different dimerization partners (Van Dijk et al., 2010), and different SVP-like genes have been proven to perform similar but distinct functions (Hartmann et al., 2000; Fornara et al., 2008; Wu et al. 2011).
As in SVP, CTF0931 also contains the EAR repression motif LxLxL. Proteins containing these motifs play key roles in diverse biological functions by negatively regulating genes involved in developmental, hormonal and stress signalling pathways (Kagale et al., 2010). One of these proteins is AGAMOUS-LIKE15 (AGL15), a MADS-box TF that, along with AGL18, is known to act as a repressor of floral transition in Arabidopsis (Adamczyk et al., 2007). Whether CTF0931 functions as a repressor of the phase transition in citrus is something that will require additional experiments.
CTF0588 carries a POX domain and a homeodomain that confers high similarity to the BELL1 (BEL1) Arabidopsis gene. BEL1 participates in ovule development and morphogenesis, and the specification of outer and inner integuments (Reiser et al., 1995) but, besides this, numerous BEL1-like homeobox genes have been identified ubiquitously in the plant kingdom that regulate numerous developmental processes as diverse as tuber formation in potato (Chen et al., 2003) or the leaf shape in Arabidopsis (Kumar et al., 2007). Overexpression of barley BEL1 in tobacco plants caused dwarfism and leaf and flower malformation (Müller et al., 2001). On the other hand, MDH1, a BEL1-like homeodomain gene of apple, is involved in growth, fertility, development of carpels and fruit shape (Dong et al., 2000). Interestingly, two Arabidopsis null mutants of the BEL1-like genes PENNYWISE and POUND-FOOLISH disrupt the transition from vegetative to floral development (Smith et al., 2004). Both the expression pattern of CTF0588 and its homology with other genes that are involved in the transition from vegetative to reproductive phase in Arabidopsis suggest that CTF0588 also plays a role in this phase transition in citrus, although further analysis will be required to elucidate its specific function.
Sequence analysis showed that CTF0397 and CTF0899, despite their high percentage of similarity in specific domains (bZIP1 and SANT, respectively), are novel genes that have not been functionally characterized in other plants, so it was not possible to predict their function, although some bZIP and MYB proteins involved in flowering development have been identified in Arabidopsis (Chuan et al., 1999; Abe et al., 2005; Tominaga et al., 2008). Overexpression in Arabidopsis will be an important clue to elucidate their specific roles in phase transition.
The results presented here indicate that the molecular mechanisms operating in the JAT in citrus plants could be controlled by genetic and/or environmental pathways that are in part common to those of Arabidopsis, but also somehow different since specific factors without Arabidopsis orthologues have been characterized. The approach used in this study has allowed us to identify both TFs with a high similarity to those of Arabidopsis thaliana (some of them already identified just because of this high similarity) and also TFs very distant from those of A. thaliana in terms of homology that may be involved in those pathways that are not common. How the regulation of the transition from juvenile to adult phase takes place in woody plants and how these specific TFs relate to this regulation are the most interesting questions to be investigated in the future. Additional work is still required to answer these questions, but our preliminary results could help to decipher the molecular mechanisms involved in the JAT in citrus plants as well as to reduce the generation time for genetic studies and breeding programmes and offer the prospect of rapid transfer of information to other woody plants.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
The Bioinformatics Core Service of the IBMCP (UPV-CSIC) is acknowledged for its help in bioinformatic analyses. This work was supported by Conselleria de Agricultura, Pesca y Alimentación of Generalitat Valenciana [Proy_IVIA09/03] to G.A., and by the Ministry of ‘Economia y Competividad’- Fondo Europeo de Desarrollo Regional (FEDER) [AGL2011-26490] and the Ministry of ‘Ciencia e Innovación’ [AGL2008-01491] and Prometeo II 2013/008.
LITERATURE CITED
- Abe M, Kobayashi Y, Yamamoto S, et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- Adamczyk B, Lehti-Shiu M, Fernandez D. The MADS-domain factors AGL15 and AGL18 act redundantly as repressors of the floral transition in Arabidopsis. The Plant Journal. 2007;50:1007–1019. doi: 10.1111/j.1365-313X.2007.03105.x. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Reseach. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasino RM, Michaels SD. The timing of flowering. Plant Physiology. 2010;154:516–520. doi: 10.1104/pp.110.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancillo G, Gadea J, Forment J, Guerri J, Navarro L. Class prediction of closely related plant varieties using gene expression profiling. Journal of Experimental Botany. 2007;58:1927–1933. doi: 10.1093/jxb/erm054. [DOI] [PubMed] [Google Scholar]
- Bassene JB, Froelicher Y, Dhuique-Mayer, et al. Non-additive phenotypic and transcriptomic inheritance in a citrus allotetraploid somatic hybrid between C. reticulata and C. limon: the case of pulp carotenoid biosynthesis pathway. Plant Cell Reports. 2009;28:1689–1697. doi: 10.1007/s00299-009-0768-1. [DOI] [PubMed] [Google Scholar]
- Bassene JB, Froelicher Y, Ferrer R, Navarro L, Ollitrault P, Ancillo G. Non additive gene regulation in a citrus allotetraploid somatic hybrid between C. reticulata Blanco and C. limon (L.) Burm. Heredity. 2010;105:299–308. doi: 10.1038/hdy.2009.162. [DOI] [PubMed] [Google Scholar]
- Becker A, Theissen G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Molecular Phylogenetics and Evolution. 2003;29:464–489. doi: 10.1016/s1055-7903(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Bielenberg DG, Wang Y, Li ZG, et al. Sequencing and annotation of the evergrowing locus in peach [Prunus persica (L.) Batsch] reveals a cluster of six MADS-box transcription factors as candidate genes for regulation of terminal bud formation. Tree Genetics and Genomes. 2008;4:495–507. [Google Scholar]
- Böhlenius H, Huang T, Charbonnel-Campaa L, et al. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science. 2006;312:1040–1043. doi: 10.1126/science.1126038. [DOI] [PubMed] [Google Scholar]
- Brill EM, Watson JM. Ectopic expression of a Eucalyptus grandis SVP orthologue alters the flowering time of Arabidopsis thaliana. Functional Plant Biology. 2004;31:217–224. doi: 10.1071/FP03180. [DOI] [PubMed] [Google Scholar]
- Carlsbecker A, Tandre K, Johanson U, Englund M, Engstrom P. The MADS-box gene DAL1 is a potential mediator of the juvenile to adult transition in Norway spruce (Picea abies) The Plant Journal. 2004;40:546–557. doi: 10.1111/j.1365-313X.2004.02226.x. [DOI] [PubMed] [Google Scholar]
- Cameron JW, Frost HF. Genetics, breeding and nucellar embryony. In: Reuther W, Batchelor LD, Webber HJ, editors. The citrus industry. University of California Press; 1968. pp. 325–370. [Google Scholar]
- Chen H, Rosin FM, Prat S, Hannapel DJ. Interacting transcription factors from the TALE superclass regulate tuber formation. Plant Physiology. 2003;132:1391–1404. doi: 10.1104/pp.103.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CF, Running MP, Williams RW, Meyerowitz EM. The PERIANTHIA gene encodes a bZIP protein involved in the determination of floral organ number in Arabidopsis thaliana. Genes and Development. 1999;13:334–44. doi: 10.1101/gad.13.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Riquelme J, Lijavetzky D, Martinez-Zapater JM, Carmona MJ. Genome-wide analysis of MIKCC-type MADS box genes in grapevine. Plant Physiology. 2009;149:354–369. doi: 10.1104/pp.108.131052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso P, Moretti S, Xenarios I, et al. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Research. 2011;39:W13–W17. doi: 10.1093/nar/gkr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF. The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Current Biology. 2004;14:1935–1940. doi: 10.1016/j.cub.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Dong Y-H, Yao J-L, Atkinson RG, Putterill JJ, Morris BA, Gardner RC. MDH1: an apple homeobox gene belonging to the BEL1 family. Plant Molecular Biology. 2000;42:623–633. doi: 10.1023/a:1006301224125. [DOI] [PubMed] [Google Scholar]
- Dorca-Fornell C, Gregis V, Grandi V, Coupland G, Colombo L, Kater MM. The Arabidopsis SOC1-like genes AGL42, AGL71 and AGL72 promote flowering in the shoot apical and axillary meristems. The Plant Journal. 2011;67:1006–1017. doi: 10.1111/j.1365-313X.2011.04653.x. [DOI] [PubMed] [Google Scholar]
- Endo T, Shimada T, Fujii H, Kobayashi Y, Araki T, Omura M. Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.) Transgenic Research. 2005;14:703–712. doi: 10.1007/s11248-005-6632-3. [DOI] [PubMed] [Google Scholar]
- Endo T, Shimada T, Fujii H, Omura M. Cloning and characterization of 5 MADS-box cDNAs isolated from citrus fruit tissue. Scientia Horticulturae. 2006;109:315–321. [Google Scholar]
- Fernández-Ocaña A, García-López MC, Jiménez-Ruiz J, et al. Identification of a gene involved in the juvenile-to-adult transition (JAT) in cultivated olive trees. Tree Genetics and Genome. 2010;6:891–903. [Google Scholar]
- Flachowsky H, Peil A, Sopanen T, Elo A, Hanke V. Overexpression of BpMADS4 from silver birch (Betula pendula Roth.) induces early-flowering in apple (Malus×domestica Borkh.) Plant Breeding. 2007;126:137–145. [Google Scholar]
- Fornara F, Gregis V, Pelucchi N, Colombo L, Kater M. The rice StMADS11-like genes OsMADS22 and OsMADS47 cause floral reversions in Arabidopsis without complementing the svp and agl24 mutants. Journal of Experimental Botany. 2008;59:2181–2190. doi: 10.1093/jxb/ern083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Mena C, de Folter S, Costa MMR, Angenent GC, Sablowski R. Transcriptional program controlled by the floral homeotic gene AGAMOUS during early organogenesis. Development. 2005;132:429–438. doi: 10.1242/dev.01600. [DOI] [PubMed] [Google Scholar]
- Gu Q, Ferrandiz C, Yanofsky MF, Martienssen R. The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development. 1998;125:1509–1517. doi: 10.1242/dev.125.8.1509. [DOI] [PubMed] [Google Scholar]
- Hartmann U, Höhmann S, Nettesheim K, Wisman E, Saedler H, Huijser P. Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. The Plant Journal. 2000;21:352–360. doi: 10.1046/j.1365-313x.2000.00682.x. [DOI] [PubMed] [Google Scholar]
- Honma T, Goto K. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature. 2001;409:525–529. doi: 10.1038/35054083. [DOI] [PubMed] [Google Scholar]
- Huang X, Madan A. CAP3: a DNA sequence assembly program. Genome Research. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K, Seki M, Sakurai T, et al. RARTF: database and tools for complete sets of Arabidopsis transcription factors. DNA Research. 2005;12:247–256. doi: 10.1093/dnares/dsi011. [DOI] [PubMed] [Google Scholar]
- Kagale S, Links MG, Rozwadowski K. Genome-wide analysis of ethylene-responsive element binding factor-associated amphiphilic repression motif-containing transcriptional regulators in Arabidopsis. Plant Physiology. 2010;152:1109–1134. doi: 10.1104/pp.109.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA, Fletcher JC. Molecular mechanism of flower development: an armchair guide. Nature Reviews Genetics. 2005;6:688–698. doi: 10.1038/nrg1675. [DOI] [PubMed] [Google Scholar]
- Kumar R, Kushalappa K, Godt D, et al. The Arabidopsis BEL1-LIKE HOMEODOMAIN proteins SAW1 and SAW2 act redundantly to regulate KNOX expression spatially in leaf margins. The Plant Cell. 2007;19:2719–2735. doi: 10.1105/tpc.106.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZM, Zhang JZ, Mei L, Deng XX, Hu CG, Yao JL. PtSVP, an SVP homolog from trifoliate orange (Poncirus trifoliata L. Raf.), shows seasonal periodicity of meristem determination and affects flower development in transgenic Arabidopsis and tobacco plants. Plant Molecular Biology. 2010;74:129–142. doi: 10.1007/s11103-010-9660-1. [DOI] [PubMed] [Google Scholar]
- Ma H, Yanofsky MF, Meyerowitz EM. AGL1–AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factor genes. Genes and Development. 1991;5:484–495. doi: 10.1101/gad.5.3.484. [DOI] [PubMed] [Google Scholar]
- Malcomber ST, Kellogg EA. SEPALLATA gene diversification: brave new whorls. Trends in Plant Science. 2005;10:428–435. doi: 10.1016/j.tplants.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. The Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Suh SS, Lee H, et al. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. The Plant Journal. 2003;35:613–623. doi: 10.1046/j.1365-313x.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Müller J, Wang Y, Franzen R, Santi L, Salamini F, Rohde W. In vitro interactions between barley TALE homeodomain proteins suggest a role for protein–protein associations in the regulation of Knox gene function. The Plant Journal. 2001;27:13–23. doi: 10.1046/j.1365-313x.2001.01064.x. [DOI] [PubMed] [Google Scholar]
- Munoz-Fambuena N, Mesejo C, Gonzalez-Mas MC, Primo-Millo E, Agusti M, Iglesias DJ. Fruit regulates seasonal expression of flowering genes in alternate-bearing ‘Moncada’ mandarin. Annals of Botany. 2011;108:511–519. doi: 10.1093/aob/mcr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L. Shoot-tip grafting in vitro of woody species and its influence on plant age. In: Rodríguez R, Sánchez Tamés R, Durzan DJ, editors. Plant aging. Vol. 186. New York: Plenum Press; 1990. pp. 117–123. NATO ASI Series. [Google Scholar]
- Navarro L, Pina JA, Juarez J, et al. The citrus variety improvement program in Spain in the period 1975–2001. Proceedings of the 15th Conference of the International Organization of Citrus Virologists. 2002 IOCV, Riverside, 306–316. [Google Scholar]
- Nishikawa F, Endo T, Shimada T, et al. Differences in seasonal expression of flowering genes between deciduous trifoliate orange and evergreen Satsuma mandarin. Tree Physiology. 2009;29:921–926. doi: 10.1093/treephys/tpp021. [DOI] [PubMed] [Google Scholar]
- Peña L, Martín-Trillo M, Juárez J, Pina JA, Navarro L, Martínez-Zapater JM. Constitutive expression of Arabidopsis LEAFY or APETALA1 genes in citrus reduces their generation time. Nature Biotechnology. 2001;19:263–267. doi: 10.1038/85719. [DOI] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature. 2000;405:200–203. doi: 10.1038/35012103. [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Lovatt CJ, Walling LL. Isolation and characterization of a TERMINAL FLOWER homolog and its correlation with juvenility in citrus. Plant Physiology. 2004;3:1540–1551. doi: 10.1104/pp.103.036178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe OJ, Kumimoto RW, Wong BJ, Riechmann JL. Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. The Plant Cell. 2003;15:1159–1169. doi: 10.1105/tpc.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser L, Modrusan Z, Margossian L, et al. The BELL1 gene encodes a homeodomain protein involved in pattern formation in the Arabidopsis ovule primordium. Cell. 1995;83:735–742. doi: 10.1016/0092-8674(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Riechmann JL, Krizek BA, Meyerowitz EM. DNA-binding properties of Arabidopsis MADS domain homeotic proteins APETALA1, APETALA3, PISTILLATA and AGAMOUS. Proceedings of the National Academy of Sciences, USA. 1996;93:4793–4798. doi: 10.1073/pnas.93.10.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottman WH, Meilan R, Sheppard LA, et al. Diverse effects of overexpression of LEAFY and PTLF, a poplar (Populus) homolog of LEAFY/FLORICAULA, in transgenic poplar and Arabidopsis. The Plant Journal. 2000;22:235–245. doi: 10.1046/j.1365-313x.2000.00734.x. [DOI] [PubMed] [Google Scholar]
- Samach A. Congratulations, you have been carefully chosen to represent an important developmental regulator! Annals of Botany. 2013;111:329–333. doi: 10.1093/aob/mcs161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Grande AV, Bujdoso N, Saedler H, Huijser P. The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis. Plant Molecular Biology. 2009;67:183–195. doi: 10.1007/s11103-008-9310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severing EI, van Dijk ADJ, Morabito G, et al. Predicting the impact of alternative splicing on plant MADS domain protein function. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030524. pe30524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore P, Sharrocks AD. The MADS-box family of transcription factors. European Journal of Biochemistry. 1995;229:1–13. doi: 10.1111/j.1432-1033.1995.tb20430.x. [DOI] [PubMed] [Google Scholar]
- Simpson GG, Dean C. Arabidopsis, the Rosetta stone of flowering time? Science. 2002;296:285–289. doi: 10.1126/science.296.5566.285. [DOI] [PubMed] [Google Scholar]
- Smith HM, Campbell BC, Hake S. Competence to respond to floral inductive signals requires the homeobox genes PENNYWISE and POUND-FOOLISH. Current Biology. 2004;14:812–817. doi: 10.1016/j.cub.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- Tan F, Swain SF. Genetics of flower initiation and development in annual and perennial plants. Physiologia Plantarum. 2006;128:8–17. [Google Scholar]
- Tan F, Swain SF. Functional characterization of AP3, SOC1 and WUS homologues from citrus (Citrus sinensis) Physiologia Plantarum. 2007;131:481–495. doi: 10.1111/j.1399-3054.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- Tominaga R, Iwata M, Sano R, Inoue K, Okada K, Wada T. Arabidopsis CAPRICE-LIKE MYB 3 (CPL3) controls endoreduplication and flowering development in addition to trichome and root hair formation. Development. 2008;135:1335–1345. doi: 10.1242/dev.017947. [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proceedings of the National Academy of Sciences, USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linden CG, Vosman B, Smulders MJ. Cloning and characterization of four apple MADS box genes isolated from vegetative tissue. Journal of Experimental Botany. 2002;53:1025–1036. doi: 10.1093/jexbot/53.371.1025. [DOI] [PubMed] [Google Scholar]
- Van Dijk AD, Morabito G, Fiers M, Van Ham RC, Angenent GC, Immink RG. Sequence motifs in MADS transcription factors responsible for specificity and diversification of protein–protein interaction. PLoS Computational Biology. 2010;6 doi: 10.1371/journal.pcbi.1001017. pe1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkonyi-Gasic E, Moss SM, Voogd C, et al. Identification and characterization of flowering genes in kiwifruit: sequence conservation and role in kiwifruit flower development. BMC Plant Biology. 2011;11:72–86. doi: 10.1186/1471-2229-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Czech B, Weigel D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell. 2009;138:738–749. doi: 10.1016/j.cell.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Wang J-W, Park MY, Wang L-J, et al. MiRNA control of vegetative phase change in trees. PLoS Genetics. 2011;7 doi: 10.1371/journal.pgen.1002012. e1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RH, Farrona S, Vincent C, et al. PEP1 regulates perennial flowering in Arabis alpina. Nature. 2009;459:423–427. doi: 10.1038/nature07988. [DOI] [PubMed] [Google Scholar]
- Wu G, Park MY, Conway SR, et al. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell. 2009;138:750–759. doi: 10.1016/j.cell.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RM, Walton EF, Richardson AC, Wood M, Hellens RP, Varkonyi-Gasic E. Conservation and divergence of four kiwifruit SVP-like MADS-box genes suggest distinct roles in kiwifruit bud dormancy and flowering. Journal of Experimental Botany. 2011;63:797–807. doi: 10.1093/jxb/err304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Chen L-L, Ruan X, et al. The draft genome of sweet orange (Citrus sinensis) Nature Genetics. 2013;45:59–66. doi: 10.1038/ng.2472. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Li ZM, Mei L, Yao JL, Hu CG. PtFLC homolog from trifoliate orange (Poncirus trifoliata) is regulated by alternative splicing and experiences seasonal fluctuation in expression level. Planta. 2009;229:847–859. doi: 10.1007/s00425-008-0885-z. [DOI] [PubMed] [Google Scholar]
- Zimmerman RH, Hackett WP, Pharis RP. Hormonal aspects of phase-change and precocious flowering. In: Pharis RP, Reid DM, editors. Encyclopedia of plant physiology (NS) Vol. II. Berlin: Springer-Verlag; 1985. pp. 79–115. [Google Scholar]
- Zhu C, Perry SE. Control of expression and autoregulation of AGL15, a member of the MADS-box family. The Plant Journal. 2005;41:583–594. doi: 10.1111/j.1365-313X.2004.02320.x. [DOI] [PubMed] [Google Scholar]
- Zhu QH, Guo AY, Gao G, et al. DPTF: a database of poplar transcription factors. Bioinformatics. 2007;23:1307–1308. doi: 10.1093/bioinformatics/btm113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.