Abstract
Circulating fibroblast growth factor 21 (FGF21) levels are elevated in diabetic subjects and correlate directly with abnormal glucose metabolism, while pharmacologically administered FGF21 can ameliorate hyperglycemia. The pancreatic islet is an FGF21 target, yet the actions of FGF21 in the islet under normal and diabetic conditions are not fully understood. This study investigated the effects of high glucose on islet FGF21 actions in a diabetic mouse model by investigating db/db mouse islet responses to exogenous FGF21, the direct effects of glucose on FGF21 signaling, and the involvement of peroxisome proliferator–activated receptor γ (PPARγ) in FGF21 pathway activation. Results showed that both adult db/db mouse islets and normal islets treated with high glucose ex vivo displayed reduced β-klotho expression, resistance to FGF21, and decreased PPARγ expression. Rosiglitazone, an antidiabetic PPARγ ligand, ameliorated these effects. Our data indicate that hyperglycemia in type 2 diabetes mellitus may lead to FGF21 resistance in pancreatic islets, probably through reduction of PPARγ expression, which provides a novel mechanism for glucose-mediated islet dysfunction.
Type 2 diabetes mellitus (T2DM), a chronic debilitating disease, results when insulin resistance develops in association with dysregulated insulin secretion and loss of β-cell mass (1). However, emerging physiologic and genetic data suggest that dysfunction of the pancreatic β-cell is the key determinant of whether an insulin-resistant individual will progress to frank hyperglycemia and diabetes (2–4). Previous studies have identified fibroblast growth factor 21 (FGF21) as a potent metabolic regulator; it is a distinctive member of the FGF family that acts through a canonical FGF receptor (FGFR) with four isoforms in the presence of the cofactor β-klotho (5–7). Binding of FGFs to FGFRs leads to receptor dimerization and autophosphorylation, which phosphorylate the tyrosine residues of FGF receptor substrate 2 (FRS2) by tyrosine kinase. Phosphorylated FRS2 acts as a docking protein forming a complex with Grb2/Sos, which in turn activates the extracellular signal–regulated kinase (ERK) pathway (6,8,9). Nuclear translocation of phosphorylated ERK1/2 triggers rapid transcription of immediate early genes such as Egr1 and cFos (10,11). Indeed, restricted expression of β-klotho in metabolically potent organs such as liver, adipose tissue, and pancreas (7,12) provides a mechanistic basis for FGF21’s tissue-specific influence on glucose and lipid homeostasis, suggesting important roles for β-klotho and FGF21 signaling in these tissues.
Growing evidence points to FGF21 as a potential therapeutic agent for treatment of T2DM, obesity, and their complications since pharmacological doses of FGF21 reduce plasma glucose and triglycerides to near normal levels and improve glucose clearance and insulin sensitivity in both ob/ob and db/db mice; transgenic mice overexpressing FGF21 exhibit similar effects and are resistant to diet-induced weight gain and fat accumulation (13,14). Furthermore, treatment of nonhuman primates with pharmacologic doses of FGF21 leads to improvements in lipoprotein profiles and levels of circulating cardiovascular risk markers (15). In high-fat diet–induced obese mice, FGF21 treatment reverses hepatic steatosis (16,17), and consistent with its actions on lipid oxidation in liver and lipolysis in white adipose tissue, mice lacking FGF21 develop mild obesity and have increased hepatic fat content when fed a ketogenic diet (18). Notably, FGF21 has also been reported to improve pancreatic β-cell function and preserve islet and β-cell mass (19); most prior studies of this factor have focused on the benefits of treatment of T2DM and obesity with pharmacologic doses of FGF21; however, as a metabolic modulator, the actions of FGF21 in target tissues under normal and diabetic conditions, and in the pathogenesis of T2DM, are not fully understood. Clinical studies have shown that circulating FGF21 levels correlate with abnormalities of glucose metabolism and with insulin resistance (20–22). FGF21 expression in the liver and white adipose tissue is increased in diabetic rodents (23), but these increases occur in the context of impaired glucose tolerance and increased hepatic lipid content, suggesting that the ability of endogenous FGF21 to exert beneficial effects on glucose homeostasis and lipid oxidation is impaired in the diabetic state (i.e., T2DM may be a state of FGF21 resistance) (10).
Given that pancreatic islet dysfunction is the central factor determining the progression of T2DM and that the pancreatic islet is an FGF21 target, we hypothesized that FGF21 action is altered in pancreatic islets under diabetic as compared with normal conditions. To test this hypothesis, the action of FGF21 on pancreatic islets throughout progression to T2DM in diabetic db/db and lean mice was examined. We examined the direct effects of glucose on FGF21 actions in islets, including involvement of peroxisome proliferator–activated receptor γ (PPARγ).
RESEARCH DESIGN AND METHODS
Animal models.
Male genetically diabetic C57BL/KSJ db/db mice, their age-matched, nondiabetic C57BL/KSJ m+/db littermates, and C57BL/KSJ mice were obtained from the Laboratory Animal Services Center of the Chinese University of Hong Kong. The experimental procedures were approved by the Animal Experimentation Ethics Committee of the Chinese University of Hong Kong (reference number 10/059/GRF-4).
Pancreatic islet isolation, primary culture, and treatments.
Intact pancreatic islets were isolated from mice as previously described (24). Islets were cultured overnight in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% (volume for volume) FBS (Gibco Laboratories, Grand Island, NY), 1% (volume for volume) penicillin, and streptomycin (Invitrogen). Isolated islets were treated with 5.6 or 28 mmol/L d-glucose (Sigma-Aldrich, St. Louis, MO), 20 μmol/L rosiglitazone, or 20 μmol/L GW9662 (Sigma-Aldrich) for the indicated periods of time.
Analysis of FGF21 signaling.
For analyses of acute FGF21-induced signaling events in the pancreatic islet, isolated islets were exposed to endotoxin-free tagless recombinant FGF21 (Antibody and Immunoassay Services, University of Hong Kong) (25); briefly, isolated islets were treated with FGF21 (0–100 nmol/L) for 10 min or 1 h and then subjected to quantitative protein or mRNA analysis, respectively.
Western blotting.
Total protein per standardized number of islets was extracted using the CytoBuster Protein Extraction Reagent (Novagen, Madison, WI). Proteins were separated by SDS-PAGE, transferred to nitrocellulose membrane (Bio-Rad, Munich, Germany), and probed with antibodies against the following proteins: β-klotho, β-actin, total FRS2, total PPARγ (Santa Cruz Biotechnology Inc., Santa Cruz, CA), and phospho-FRS2 (Y196; Cell Signaling Technology, Danvers, MA). The Western blot bands were quantitated with ImageJ software (National Institutes of Health).
Quantitative RT-PCR.
Total RNA from islets or flash-frozen liver tissue samples was extracted using TRIzol reagent (Invitrogen) and subjected to reverse transcription using the iScript Select cDNA Synthesis Kit (Bio-Rad). Relative gene expression was quantified by real-time PCR using iQ SYBR Green Supermix (Bio-Rad). The reactions were performed using an i-Cycler Thermal Cycler (version 3.1; Bio-Rad). Relative gene expression was analyzed using the 2(−ΔΔCt) method (26) and normalized relative to glyceraldehyde 3-phosphate dehydrogenase. The sequences of the primers used are listed in Supplementary Table 1.
Plasma FGF21 concentrations.
Blood samples were collected on ice and spun at 4°C. Plasma FGF21 concentrations were determined by a specific mouse ELISA kit (Antibody and Immunoassay Services, University of Hong Kong), as previously described (27).
Statistical analysis.
Data are displayed as means ± SEs. Comparisons between groups were analyzed by two-tailed Student t test, or one-way ANOVA, followed by Tukey post hoc test, in which P < 0.05 was considered statistically significant.
RESULTS
Hepatic FGF21 mRNA and circulating levels of FGF21 are elevated in diabetic db/db mice in an age-dependent manner.
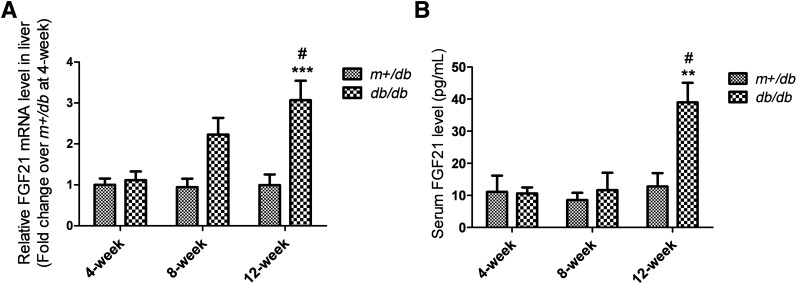
Hepatic FGF21 mRNA levels in db/db diabetic mice and m+/db lean mice were similar at 4 weeks, but were approximately twofold and threefold greater in db/db mice than m+/db mice at 8 and 12 weeks, respectively (Fig. 1A). Similar results were obtained for circulating FGF21 levels; the levels did not differ significantly between db/db and m+/db mice at 4 and 8 weeks, but were elevated approximately threefold in db/db mice relative to m+/db mice at 12 weeks, thereby demonstrating an age-dependent change (Fig. 1B; 4 weeks: m+/db, 11.10 ± 5.04 pg/mL and db/db, 10.62 ± 1.87 pg/mL; 8 weeks: m+/db, 8.58 ± 2.22 pg/mL and db/db, 11.65 ± 5.41 pg/mL; 12 weeks: m+/db, 12.76 ± 4.53 pg/mL and db/db, 39.00 ± 6.08 pg/mL).
FIG. 1.
Hepatic FGF21 mRNA and circulating levels of FGF21 are increased in db/db mice by 12 weeks of age. A: mRNA expression as quantified by standard quantitative RT-PCR. B: Circulating FGF21 levels as determined by ELISA of serum from a terminal bleed. Data are means ± SEs. **P < 0.01; ***P < 0.001 vs. age-matched m+/db group; #P < 0.05 vs. 4-week db/db group (n = 5 to 6).
β-Klotho is downregulated in pancreatic islets from db/db mice in an age-dependent manner.
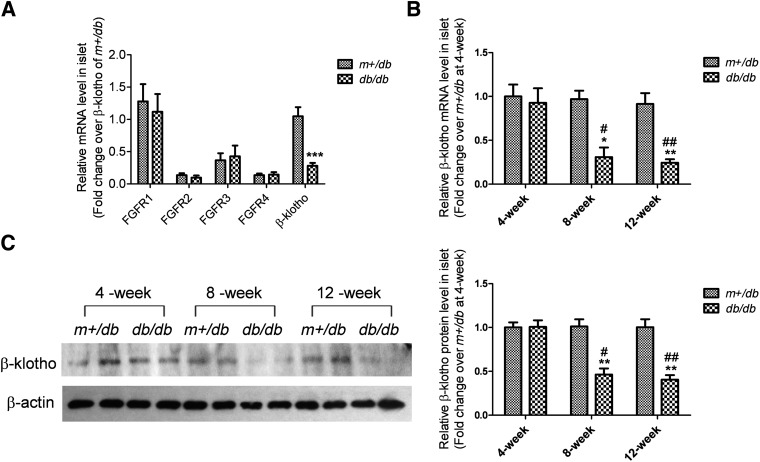
To study the action of FGF21 in islets under diabetic conditions, the expression of FGFRs and the cofactor β-klotho in islets of db/db and m+/db mice was examined. In 12-week-old mice, there was no apparent effect of diabetes on islet FGFRs expression since the mRNA levels of all FGFRs were unchanged in db/db mouse islets relative to levels in m+/db mouse islets. However, mRNA levels of the FGF21 cofactor β-klotho were reduced by 72% in db/db mouse islets compared with levels observed in m+/db mouse islets (Fig. 2A).
FIG. 2.
β-Klotho is downregulated in islets of adult db/db mice. mRNA levels of FGFRs and β-klotho (A) were determined in 12-week-old m+/db and db/db mice. mRNA (B) and protein (C) levels of β-klotho in islets were analyzed in 4-, 8-, and 12-week-old mice. For the Western blot, β-actin was used as a loading control. Densitometry is shown and was calculated as β-klotho/β-actin. Data are means ± SEs. *P < 0.05; **P < 0.01; ***P < 0.001 vs. age-matched m+/db group; #P < 0.05; ##P < 0.01 vs. 4-week db/db group (n = 4 to 5).
A progressive reduction in β-klotho mRNA levels was seen with increasing age (Fig. 2B). At 4 weeks of age, β-klotho mRNA levels were similar in db/db islets versus m+/db islets. However, β-klotho mRNA levels were 69 and 72% lower in db/db islets versus m+/db islets at 8 and 12 weeks of age, respectively. There was a corresponding decrease in β-klotho protein levels in db/db mouse islets that became apparent at 8 and 12 weeks of age (Fig. 2C).
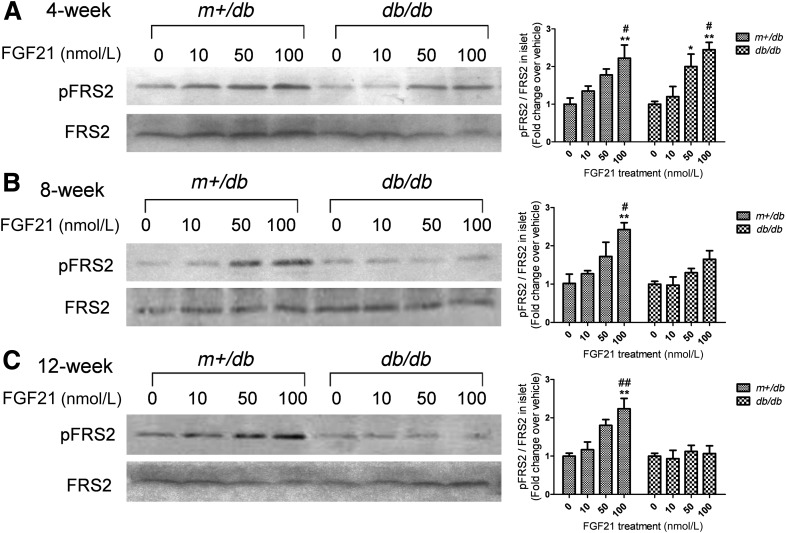
FGF21-induced phosphorylation of FRS2 is attenuated in islets from adult db/db mice.
To determine FGF21 signaling in islets, FRS2 phosphorylation was used as a reporter. In isolated islets treated with FGF21 (0, 10, 50, or 100 nmol/L) for 10 min, FGF21 dose-dependently induced FRS2 phosphorylation in islets from both 4-week-old lean and diabetic mice (Fig. 3A). In contrast, in 8-week-old db/db mice, FGF21-induced phosphorylation was decreased with profound impairment noted in islets isolated from 12-week-old db/db mice (Fig. 3B and C).
FIG. 3.
FGF21-induced FRS2 phosphorylation is attenuated in islets from adult db/db mice relative to m+/db mice. Islets were isolated from the mice at 4 (A), 8 (B), and 12 weeks (C) of age and treated with FGF21 (at the indicated concentrations) for 10 min. Phosphorylated (pFRS2) and total FRS2 expression were quantitated in Western blots. Densitometry is shown and calculated as pFRS2/total FRS2. Data are means ± SEs. *P < 0.05; **P < 0.01 vs. 0 nmol/L group; #P < 0.05; ##P < 0.01 vs. 10 nmol/L group (n = 4).
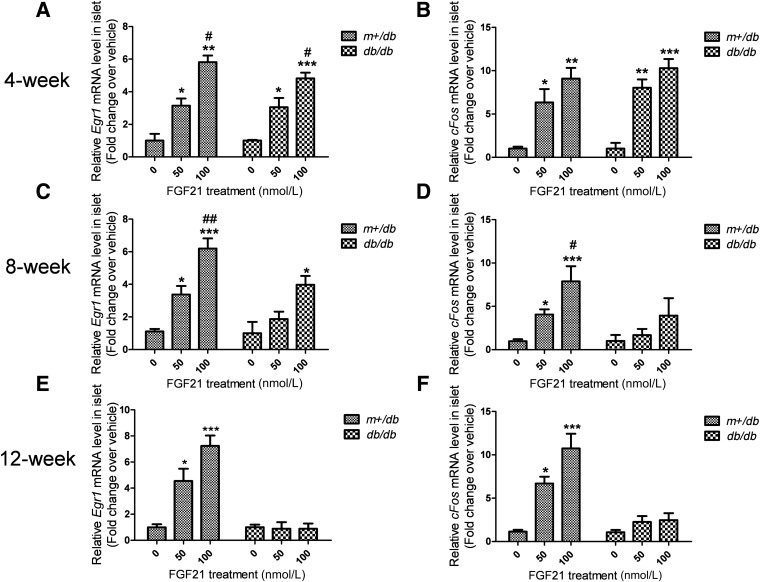
FGF21-induced expression of immediate early genes is impaired in islets from adult db/db mice.
To confirm FGF21 resistance, FGF21-induced immediate early genes expression was used as a secondary readout for FGF21 action in islets. As shown in Fig. 4A and B, both Egr1 and cFos mRNA expression was induced in islets from 4-week-old m+/db and db/db mice after stimulation with FGF21 (0, 50, or 100 nmol/L) for 1 h. However, FGF21 induction of these immediate early genes was attenuated in db/db mice, relative to m+/db mice, at 8 weeks of age (Fig. 4C and D) and profoundly impaired in islets from 12-week-old db/db mice (Fig. 4E and F). Thus, the aforementioned reduced induction of FRS2 phosphorylation in db/db islets was accompanied by attenuated induction of expression of immediate early genes in diabetic mouse islets in an age-dependent manner.
FIG. 4.
FGF21-induced expression of immediate early genes is attenuated in islets of adult db/db mice relative to m+/db mice. Islets were isolated from the mice at 4 (A and B), 8 (C and D), and 12 weeks (E and F) of age and treated with FGF21 (at the indicated concentrations) for 1 h. Egr1 (A, C, and E) and cFos (B, D, and F) mRNA levels were analyzed. Data are means ± SEs. *P < 0.05; **P < 0.01; ***P < 0.001 vs. 0 nmol/L group; #P < 0.05; ##P < 0.01 vs. 50 nmol/L group (n = 5 to 6).
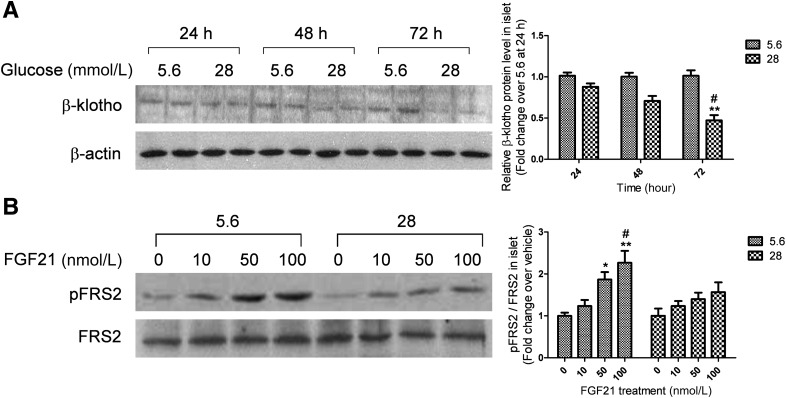
High glucose treatment reduces the expression of β-klotho and attenuates FGF21 signaling in isolated islets.
To investigate the effects of isolated hyperglycemia on FGF21 action, islets isolated from normal C57BL/KSJ mice were treated with 28 mmol/L glucose and FGF21 signaling was examined. As shown in Fig. 5A, a time-course analysis showed that high glucose (28 mmol/L) reduced β-klotho protein expression with maximal inhibition occurring after 72 h of glucose treatment (55% reduction), while the FGFRs mRNA expression remained unchanged (Supplementary Fig. 1). Exposure to 22.4 mmol/L L-glucose together with 5.6 mmol/L d-glucose for 72 h (to control for potential nonspecific effects of high sugar osmolarity) did not affect β-klotho expression. Palmitic acid (400 μmol/L) also did not exert any effect (Supplementary Fig. 2A and B). To determine whether downregulation of β-klotho by high glucose was associated with alterations in FGF21 action, we evaluated the ability of FGF21 to induce FRS2 phosphorylation in islets after glucose treatment. Islets that were pretreated with high glucose for 72 h displayed markedly weakened FGF21-induced FRS2 phosphorylation compared with those pretreated with normal glucose (5.6 mmol/L) (Fig. 5B).
FIG. 5.
High glucose reduces β-klotho expression and impairs FGF21-induced FRS2 phosphorylation in islets. A: Islets were isolated from lean mice and treated with 5.6 or 28 mmol/L glucose for 24, 48, or 72 h. **P < 0.01 vs. time-matched 5.6 mmol/L group; #P < 0.05 vs. 24-h 28 mmol/L group (n = 4). B: Islets were isolated from lean mice and pretreated with 5.6 mmol/L or 28 mmol/L glucose for 72 h, then were treated with FGF21 (at the indicated concentrations) for 10 min. *P < 0.05; **P < 0.01 vs. 0 nmol/L group; #P < 0.05 vs. 10 nmol/L group (n = 4). Data are means ± SEs. pFRS2, phosphorylated FRS2.
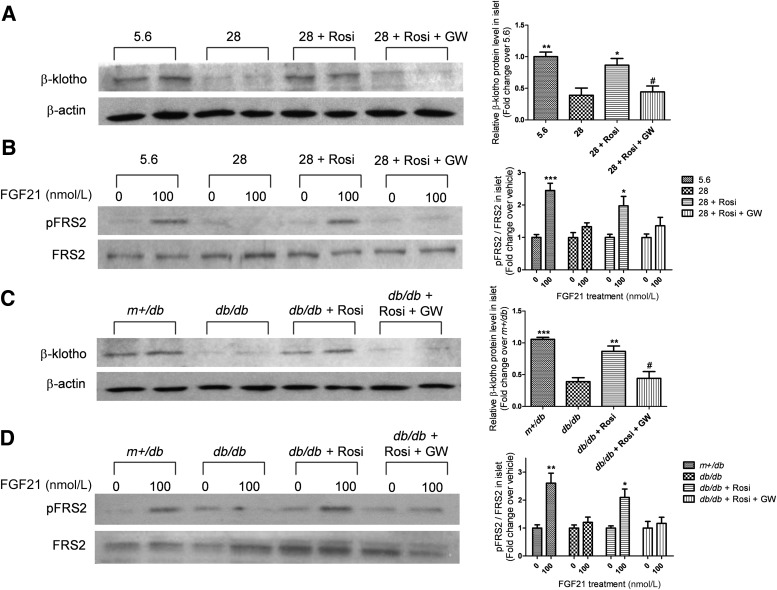
PPARγ activation prevents downregulation of β-klotho and rescues impairment of FGF21 signaling in high glucose–treated islets or db/db mouse islets.
Since elevated glucose reduced β-klotho expression and FRS2 phosphorylation in pancreatic islets, we tested whether the PPARγ ligand rosiglitazone, an agent that protects islets against glucotoxicity, might prevent β-klotho repression. As shown in Fig. 6A, for islets treated with 28 mmol/L glucose for 72 h, cotreatment with rosiglitazone restored β-klotho protein expression to levels not significantly different from levels observed in islets maintained in normal conditions. Overnight pretreatment with the PPARγ antagonist GW9662 blocked the effect of rosiglitazone.
FIG. 6.
Rosiglitazone reverses the downregulation of β-klotho and impairment of FGF21-induced FRS2 phosphorylation in islets subjected to high glucose treatment and in adult db/db mouse islets, and these effects are blocked by the PPARγ antagonist GW9662. A: Islets were isolated from lean mice and treated with 5.6 or 28 mmol/L glucose, 20 μmol/L rosiglitazone (Rosi), with or without 20 μmol/L GW9662 (GW) for 72 h. *P < 0.05; **P < 0.01 vs. 28 mmol/L group; #P < 0.05 vs. 28 mmol/L + Rosi group (n = 4). B: Pretreated islets were exposed to 0 or 100 nmol/L FGF21 for 10 min for signaling analyses. *P < 0.05; ***P < 0.001 vs. 0 nmol/L group (n = 4). C: Isolated islets of adult m+/db and db/db mice were treated with 20 μmol/L Rosi, with or without 20 μmol/L GW, for 72 h. **P < 0.01; ***P < 0.001 vs. db/db group; #P < 0.05 vs. db/db + Rosi group (n = 4). D: Pretreated islets were exposed to 0 or 100 nmol/L FGF21 for 10 min for signaling analyses. *P < 0.05; **P < 0.01 vs. 0 nmol/L group (n = 4). Data are means ± SEs. pFRS2, phosphorylated FRS2.
The alterations of β-klotho expression by rosiglitazone and GW9662 were accompanied by corresponding alterations in FGF21-induced FRS2 phosphorylation. FRS2 phosphorylation impaired by high glucose was restored by rosiglitazone, and this restoration could then be counteracted by GW9662 (Fig. 6B). Similarly, rosiglitazone rescued β-klotho repression and impaired FRS2 phosphorylation in db/db mouse islets, while GW9662 again cancelled the effects of rosiglitazone (Fig. 6C and D).
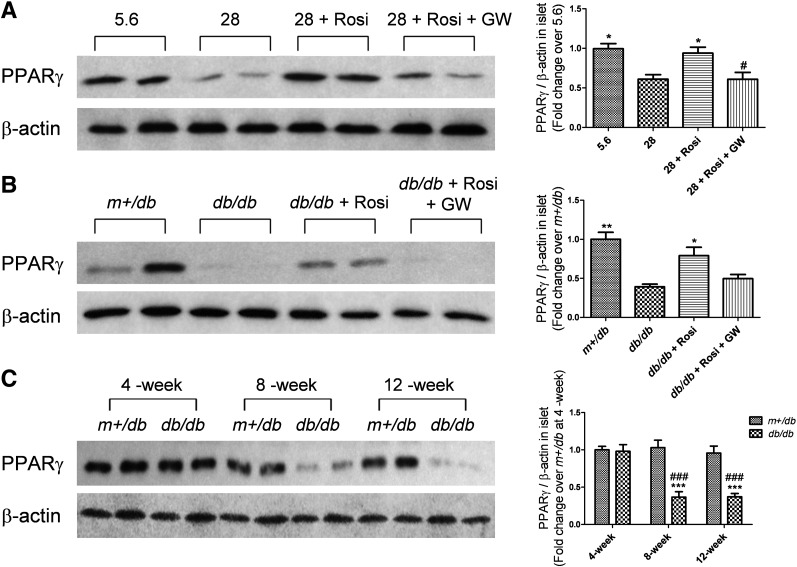
PPARγ expression is reduced in high glucose–treated islets and db/db mouse islets, but is improved by rosiglitazone.
To elucidate the interactions between glucose and PPARγ in pancreatic islets, PPARγ expression was evaluated in high glucose–treated islets and db/db mouse islets. As shown in Fig. 7A, 72-h high glucose treatment decreased PPARγ expression. Rosiglitazone inhibited such change, while GW9662 cancelled rosiglitazone’s effect. Relative to islets from m+/db mice, islets from 12-week-old db/db mice showed a significant decrease in PPARγ level. Again, rosiglitazone reversed such an effect, and the rosiglitazone’s effect was blocked by GW9662 (Fig. 7B). A progressive age-related decrease in PPARγ expression was observed in db/db mouse islets at 8 and 12 weeks of age, while no significant difference was detected at 4 weeks versus m+/db mice (Fig. 7C). Consistent with the aforementioned alterations in β-klotho expression, age-related changes in PPARγ expression were observed in high glucose–treated islets and db/db mouse islets.
FIG. 7.
PPARγ expression is reduced in high glucose–treated islets and adult db/db mouse islets, which is reversed by rosiglitazone. A: Isolated islets from lean mice were treated with 5.6 or 28 mmol/L glucose, 20 μmol/L rosiglitazone (Rosi), with or without 20 μmol/L GW9662 (GW), for 72 h. *P < 0.05 vs. 28 mmol/L group; #P < 0.05 vs. 28 mmol/L + Rosi group (n = 6). B: Isolated islets from 12-week-old m+/db and db/db mice were treated with 20 μmol/L Rosi with or without 20 μmol/L GW for 72 h. *P < 0.05; **P < 0.01 vs. db/db group (n = 6). C: Islet PPARγ expression was examined in m+/db and db/db mice at 4, 8, and 12 weeks of age. ***P < 0.001 vs. age-matched m+/db group; ###P < 0.001 vs. 4-week db/db group (n = 4). β-Actin was used as a loading control. Densitometry is shown and calculated as PPARγ/β-actin. Data are means ± SEs.
DISCUSSION
The present data show for the first time that high glucose, a pivotal component of the type 2 diabetic milieu, induces FGF21 resistance in pancreatic islets, likely through a reduction in PPARγ expression. The concept of FGF21 resistance originated from studies showing that serum concentrations of FGF21 were increased in db/db mice and human patients with T2DM (23,28). Clinical studies have revealed that serum FGF21 levels correlate with severity of glucose intolerance and insulin resistance (20,21). In this study, we confirmed that increased hepatic expression and serum levels of FGF21 were associated with the diabetic state and showed that these increases were age-dependent and, as with progressive hyperglycemia, appeared only in adult db/db mice with overt T2DM.
Paradoxically, despite high endogenous levels of FGF21 in diabetic adult db/db mice, pharmacological doses of exogenous FGF21 have been shown to improve metabolic parameters (13). The fact that high endogenous FGF21 levels fail to produce beneficial effects while high pharmacological doses induce the expected results suggests a state of FGF21 resistance existing in overt T2DM. We postulated that FGF21 actions in pancreatic islets might be abnormal in T2DM (19). Our results indicated that, under diabetic conditions, pancreatic islets were FGF21-resistant, largely due to downregulation of β-klotho, an essential cofactor for FGF21 (5,7). That was associated with a reduction in the capacity of FGF21 to induce signaling in db/db mouse islets, as shown by marked reductions in the induction of FRS2 phosphorylation, the essential step linking FGFRs to the ERK1/2 signaling pathway (9); and the reduced expression of immediate early genes (Egr1 and cFos) regulated by the ERK1/2 pathway (10,11). These effects were not apparent until the db/db mice reached adulthood (after 8 weeks of age), a finding matching previous work showing that mice lacking β-klotho are unresponsive to FGF21 stimulation (29).
The natural history of db/db mice follows a distinct pattern: they have normal glycemia at 4 weeks of age due to compensatory increases in circulating insulin; hyperglycemia develops once insulin secretion can no longer compensate for the increased insulin resistance, from ∼7 weeks of age, and blood glucose continues to increase with age (30). We found that the patterns of β-klotho expression and FGF21’s actions in db/db mouse islets followed these changes during the progression toward overt diabetes, suggesting that glucose itself is a potential mediator of islet FGF21 resistance. Our ex vivo experiments further demonstrated that prolonged high glucose exposure can dramatically reduce β-klotho expression and impair FGF21 signaling in pancreatic islets. High glucose concentrations in culture media mimicked diabetic hyperglycemia, supporting the possibility that β-klotho repression and blunted FGF21 activity in diabetic mouse islets were, at least in part, due to hyperglycemia. Furthermore, in both high glucose–treated and db/db mouse islets, β-klotho, but not the FGFRs, was downregulated, and, since β-klotho has tissue-restricted expression as opposed to the widespread localization of FGFRs (31), this suggests different roles for β-klotho and FGF21 signaling in modulating glucose-induced toxicity in islets in T2DM.
We hypothesized that antihyperglycemic drugs (e.g., thiazolidinediones) exert some of their beneficial effects through improvement in FGF21 responsivity in islets. The thiazolidinediones rosiglitazone and pioglitazone are strong synthetic agonists of PPARγ and have potent antidiabetic effects (32,33). PPARγ is expressed in pancreatic islets (34) and PPAR-responsive elements have been identified in the promoters of genes involved in islet function, such as Glut2 and Pdx1 (35–37). Moreover, PPARγ activation has a number of direct prosurvival and profunction effects on pancreatic islet cells (38,39). In our study, we found that rosiglitazone reversed the downregulation of β-klotho in both db/db mouse islets and high glucose–treated islets, leading to restoration of FGF21 signaling. GW9662, a PPARγ antagonist (40), completely blocked the effects of rosiglitazone, implying that direct binding of the ligand was required for PPARγ's actions. These findings are consistent with prior reports showing that rosiglitazone promoted β-klotho expression and enhanced FGF21 actions on adipocytes (41,42). Our data illustrate that β-klotho expression and FGF21 activity in pancreatic islets can be regulated by PPARγ modulation. Therefore, we suggest that glucose may regulate islet FGF21 actions through modulation of PPARγ expression and/or activity, a signaling that can be interrupted by rosiglitazone.
PPARγ expression may be altered by environmental changes or stress. Chronic high glucose exposure can decrease PPARγ mRNA levels in mouse islets (43). Consistently, in both high glucose–treated islets and db/db mouse islets, PPARγ expression was found to be reduced that could be reversed with rosiglitazone. The timeline of these effects is worthwhile noting; the age-associated development of the diabetic phenotype: db/db mice developing high blood glucose as they reach adulthood was associated with reductions in PPARγ and β-klotho expression. These data suggest that hyperglycemia reduces PPARγ expression in db/db mouse islets. The fact that rosiglitazone-induced PPARγ activation rescues β-klotho repression makes it likely that repression of β-klotho by high glucose is the result of glucose-mediated downregulation of PPARγ and provides clues as to why rosiglitazone-mediated PPARγ activation favors FGF21 effects (42). PPARγ has been characterized as a master regulator of genes transcription; in islets, pharmacologic PPARγ activation regulates various genes for which products mediate key aspects of β-cell function. Transcription of klotho, the homolog of β-klotho, was also shown to be directly regulated by PPARγ (44,45), suggesting that the whole klotho family would be regulated by PPARγ. However, whether PPARγ regulates β-klotho expression by direct activation of gene transcription or through modulation of other signaling pathways is still unclear, and further investigation is needed to delineate PPARγ and β-klotho interactions.
Chronic hyperglycemia contributes to the progressive loss of islet function and survival through mechanisms involving activation of oxidative stress and/or fatty acid toxicity (46). In this study, we present a novel pathway for glucotoxicity in islets through hyperglycemia-induced FGF21 resistance, probably through downregulation of PPARγ.
In conclusion, our results highlight the involvement of PPARγ in the negative regulation of islet β-klotho expression and FGF21 action by high glucose. Thus, we suggest that FGF21 could prove to have therapeutic value, especially in combination with an antihyperglycemic agent that itself promotes FGF21 actions in T2DM.
ACKNOWLEDGMENTS
This work was supported by grants from The Research Grants Council of the Hong Kong Special Administrative Region, China (CUHK468912 and HKU/CRF/09), awarded to P.S.L. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
No potential conflicts of interest relevant to this article were reported.
W.Y.S. designed and performed experiments, analyzed and interpreted data, and drafted the manuscript. Q.C. and L.C. performed experiments and reviewed the manuscript. C.E.-M. reviewed the manuscript. A.X. and K.S.L.L. reviewed and edited the manuscript. P.S.L. designed the experiments, analyzed and interpreted data, and edited and revised the manuscript. P.S.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0645/-/DC1.
REFERENCES
- 1.Kahn SE. The importance of the beta-cell in the pathogenesis of type 2 diabetes mellitus. Am J Med 2000;108(Suppl. 6a):2S–8S [DOI] [PubMed] [Google Scholar]
- 2.Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature 2001;414:788–791 [DOI] [PubMed] [Google Scholar]
- 3.Del Prato S, Marchetti P, Bonadonna RC. Phasic insulin release and metabolic regulation in type 2 diabetes. Diabetes 2002;51(Suppl. 1):S109–S116 [DOI] [PubMed] [Google Scholar]
- 4.Gerich JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev 1998;19:491–503 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki M, Uehara Y, Motomura-Matsuzaka K, et al. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol 2008;22:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kharitonenkov A, Dunbar JD, Bina HA, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol 2008;215:1–7 [DOI] [PubMed] [Google Scholar]
- 7.Ogawa Y, Kurosu H, Yamamoto M, et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A 2007;104:7432–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yie J, Hecht R, Patel J, et al. FGF21 N- and C-termini play different roles in receptor interaction and activation. FEBS Lett 2009;583:19–24 [DOI] [PubMed] [Google Scholar]
- 9.Kouhara H, Hadari YR, Spivak-Kroizman T, et al. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 1997;89:693–702 [DOI] [PubMed] [Google Scholar]
- 10.Fisher FM, Chui PC, Antonellis PJ, et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010;59:2781–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chai J, Tarnawski AS. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J Physiol Pharmacol 2002;53:147–157 [PubMed] [Google Scholar]
- 12.Ito S, Kinoshita S, Shiraishi N, et al. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev 2000;98:115–119 [DOI] [PubMed] [Google Scholar]
- 13.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005;115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharitonenkov A, Shanafelt AB. Fibroblast growth factor-21 as a therapeutic agent for metabolic diseases. BioDrugs 2008;22:37–44 [DOI] [PubMed] [Google Scholar]
- 15.Kharitonenkov A, Wroblewski VJ, Koester A, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 2007;148:774–781 [DOI] [PubMed] [Google Scholar]
- 16.Coskun T, Bina HA, Schneider MA, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008;149:6018–6027 [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Lloyd DJ, Hale C, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009;58:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 2009;150:4931–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wente W, Efanov AM, Brenner M, et al. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 2006;55:2470–2478 [DOI] [PubMed] [Google Scholar]
- 20.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009;32:1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semba RD, Sun K, Egan JM, Crasto C, Carlson OD, Ferrucci L. Relationship of serum fibroblast growth factor 21 with abnormal glucose metabolism and insulin resistance: the Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2012;97:1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hojman P, Pedersen M, Nielsen AR, et al. Fibroblast growth factor-21 is induced in human skeletal muscles by hyperinsulinemia. Diabetes 2009;58:2797–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008;57:1246–1253 [DOI] [PubMed] [Google Scholar]
- 24.Chu KY, Cheng Q, Chen C, et al. Angiotensin II exerts glucose-dependent effects on Kv currents in mouse pancreatic beta-cells via angiotensin II type 2 receptors. Am J Physiol Cell Physiol 2010;298:C313–C323 [DOI] [PubMed] [Google Scholar]
- 25.Ge X, Chen C, Hui X, Wang Y, Lam KS, Xu A. Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/Ets-like protein-1 in adipocytes. J Biol Chem 2011;286:34533–34541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu KY, Lau T, Carlsson PO, Leung PS. Angiotensin II type 1 receptor blockade improves beta-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes 2006;55:367–374 [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Hoo RL, Konishi M, et al. Growth hormone induces hepatic production of fibroblast growth factor 21 through a mechanism dependent on lipolysis in adipocytes. J Biol Chem 2011;286:34559–34566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mraz M, Bartlova M, Lacinova Z, et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 2009;71:369–375 [DOI] [PubMed] [Google Scholar]
- 29.Ding X, Boney-Montoya J, Owen BM, et al. βKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab 2012;16:387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wyse BM, Dulin WE. The influence of age and dietary conditions on diabetes in the db mouse. Diabetologia 1970;6:268–273 [DOI] [PubMed] [Google Scholar]
- 31.Kharitonenkov A, Larsen P. FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrinol Metab 2011;22:81–86 [DOI] [PubMed] [Google Scholar]
- 32.Balfour JA, Plosker GL. Rosiglitazone. Drugs 1999;57:921–930; discussion 931–932 [DOI] [PubMed]
- 33.Campbell IW. Long-term glycaemic control with pioglitazone in patients with type 2 diabetes. Int J Clin Pract 2004;58:192–200 [DOI] [PubMed] [Google Scholar]
- 34.Dubois M, Pattou F, Kerr-Conte J, et al. Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in normal human pancreatic islet cells. Diabetologia 2000;43:1165–1169 [DOI] [PubMed] [Google Scholar]
- 35.Gupta D, Jetton TL, Mortensen RM, Duan SZ, Peshavaria M, Leahy JL. In vivo and in vitro studies of a functional peroxisome proliferator-activated receptor gamma response element in the mouse pdx-1 promoter. J Biol Chem 2008;283:32462–32470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Im SS, Kim JW, Kim TH, et al. Identification and characterization of peroxisome proliferator response element in the mouse GLUT2 promoter. Exp Mol Med 2005;37:101–110 [DOI] [PubMed] [Google Scholar]
- 37.Kim HI, Ahn YH. Role of peroxisome proliferator-activated receptor-gamma in the glucose-sensing apparatus of liver and beta-cells. Diabetes 2004;53(Suppl. 1):S60–S65 [DOI] [PubMed] [Google Scholar]
- 38.Gupta D, Kono T, Evans-Molina C. The role of peroxisome proliferator-activated receptor γ in pancreatic β cell function and survival: therapeutic implications for the treatment of type 2 diabetes mellitus. Diabetes Obes Metab 2010;12:1036–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell IW, Mariz S. Beta-cell preservation with thiazolidinediones. Diabetes Res Clin Pract 2007;76:163–176 [DOI] [PubMed] [Google Scholar]
- 40.Leesnitzer LM, Parks DJ, Bledsoe RK, et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 2002;41:6640–6650 [DOI] [PubMed] [Google Scholar]
- 41.Díaz-Delfín J, Hondares E, Iglesias R, Giralt M, Caelles C, Villarroya F. TNF-α represses β-Klotho expression and impairs FGF21 action in adipose cells: involvement of JNK1 in the FGF21 pathway. Endocrinology 2012;153:4238–4245 [DOI] [PubMed] [Google Scholar]
- 42.Moyers JS, Shiyanova TL, Mehrbod F, et al. Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARgamma signaling. J Cell Physiol 2007;210:1–6 [DOI] [PubMed] [Google Scholar]
- 43.Chuang JC, Cha JY, Garmey JC, Mirmira RG, Repa JJ. Research resource: nuclear hormone receptor expression in the endocrine pancreas. Mol Endocrinol 2008;22:2353–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Li Y, Fan Y, et al. Klotho is a target gene of PPAR-gamma. Kidney Int 2008;74:732–739 [DOI] [PubMed] [Google Scholar]
- 45.Yang HC, Deleuze S, Zuo Y, Potthoff SA, Ma LJ, Fogo AB. The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol 2009;20:2380–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Prato S. Role of glucotoxicity and lipotoxicity in the pathophysiology of Type 2 diabetes mellitus and emerging treatment strategies. Diabet Med 2009;26:1185–1192 [DOI] [PubMed] [Google Scholar]