Abstract
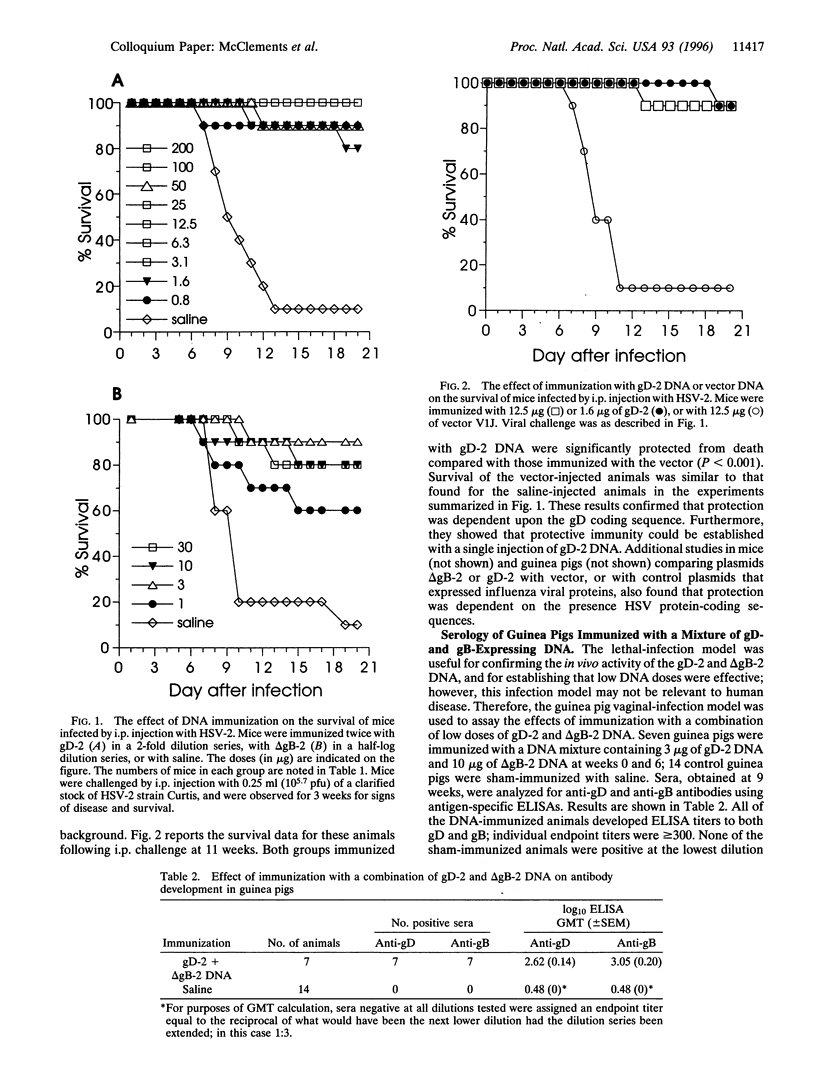
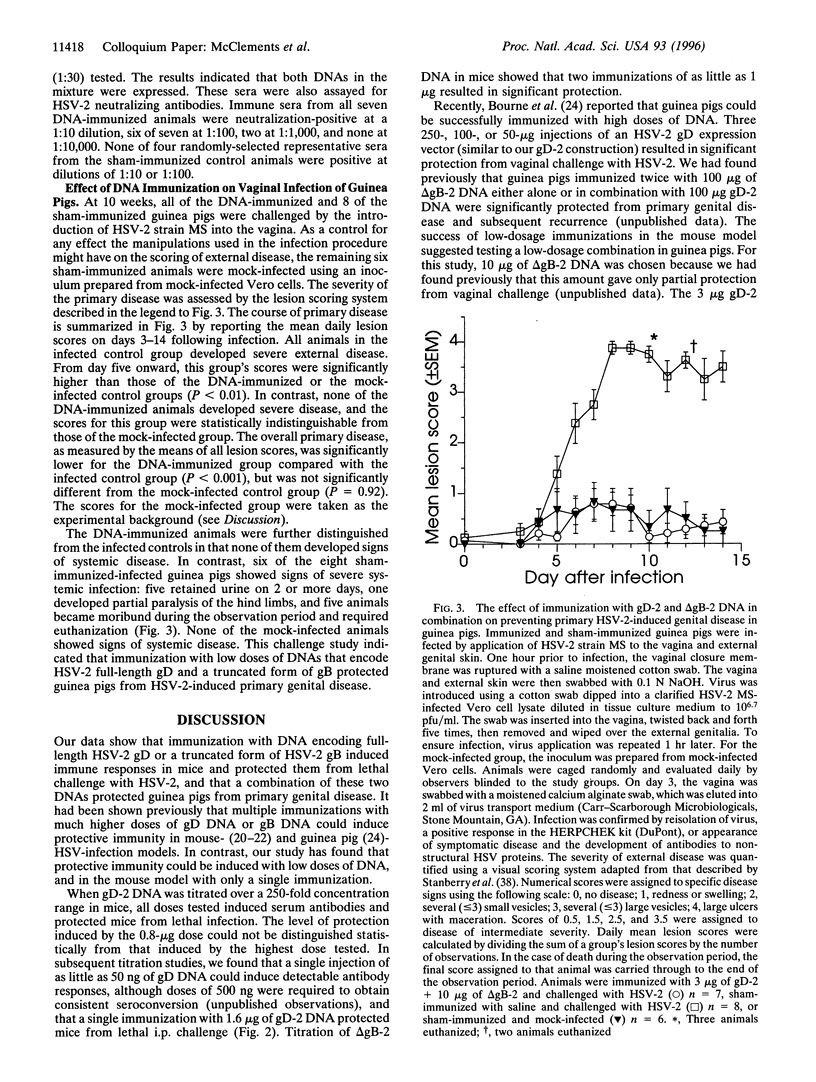
DNA vaccines expressing herpes simplex virus type 2 (HSV-2) full-length glycoprotein D (gD), or a truncated form of HSV-2 glycoprotein B (gB) were evaluated for protective efficacy in two experimental models of HSV-2 infection. Intramuscular (i.m.) injection of mice showed that each construction induced neutralizing serum antibodies and protected the mice from lethal HSV-2 infection. Dose-titration studies showed that low doses (< or = 1 microgram) of either DNA construction induced protective immunity, and that a single immunization with the gD construction was effective. The two DNAs were then tested in a low-dosage combination in guinea pigs. Immune sera from DNA-injected animals had antibodies to both gD and gB, and virus neutralizing activity. When challenged by vaginal infection with HSV-2, the DNA-immunized animals were significantly protected from primary genital disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurelian L., Smith C. C., Wachsman M., Paoletti E. Immune responses to herpes simplex virus in guinea pigs (footpad model) and mice immunized with vaccinia virus recombinants containing herpes simplex virus glycoprotein D. Rev Infect Dis. 1991 Nov-Dec;13 (Suppl 11):S924–S934. doi: 10.1093/clind/13.supplement_11.s924. [DOI] [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry M. A., Lai W. C., Johnston S. A. Protection against mycoplasma infection using expression-library immunization. Nature. 1995 Oct 19;377(6550):632–635. doi: 10.1038/377632a0. [DOI] [PubMed] [Google Scholar]
- Bourne N., Stanberry L. R., Bernstein D. I., Lew D. DNA immunization against experimental genital herpes simplex virus infection. J Infect Dis. 1996 Apr;173(4):800–807. doi: 10.1093/infdis/173.4.800. [DOI] [PubMed] [Google Scholar]
- Bravo F. J., Stanberry L. R., Kier A. B., Vogt P. E., Kern E. R. Evaluation of HPMPC therapy for primary and recurrent genital herpes in mice and guinea pigs. Antiviral Res. 1993 May;21(1):59–72. doi: 10.1016/0166-3542(93)90067-s. [DOI] [PubMed] [Google Scholar]
- Burke R. L. Development of a herpes simplex virus subunit glycoprotein vaccine for prophylactic and therapeutic use. Rev Infect Dis. 1991 Nov-Dec;13 (Suppl 11):S906–S911. doi: 10.1093/clind/13.supplement_11.s906. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Debroy C., Fox B. A., Pederson N. E., Person S. The nucleotide sequence of the gB glycoprotein gene of HSV-2 and comparison with the corresponding gene of HSV-1. Virology. 1986 Dec;155(2):322–333. doi: 10.1016/0042-6822(86)90196-0. [DOI] [PubMed] [Google Scholar]
- Cantin E. M., Eberle R., Baldick J. L., Moss B., Willey D. E., Notkins A. L., Openshaw H. Expression of herpes simplex virus 1 glycoprotein B by a recombinant vaccinia virus and protection of mice against lethal herpes simplex virus 1 infection. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5908–5912. doi: 10.1073/pnas.84.16.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney E. L., Collier A. C., Greenberg P. D., Coombs R. W., Zarling J., Arditti D. E., Hoffman M. C., Hu S. L., Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991 Mar 9;337(8741):567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- Cox G. J., Zamb T. J., Babiuk L. A. Bovine herpesvirus 1: immune responses in mice and cattle injected with plasmid DNA. J Virol. 1993 Sep;67(9):5664–5667. doi: 10.1128/jvi.67.9.5664-5667.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denniston K. J., Madden M. J., Enquist L. W., Vande Woude G. Characterization of coliphage lambda hybrids carrying DNA fragments from Herpes simplex virus type 1 defective interfering particles. Gene. 1981 Dec;15(4):365–378. doi: 10.1016/0378-1119(81)90180-3. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly J. J., Friedman A., Martinez D., Montgomery D. L., Shiver J. W., Motzel S. L., Ulmer J. B., Liu M. A. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med. 1995 Jun;1(6):583–587. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- Donnelly J. J., Martinez D., Jansen K. U., Ellis R. W., Montgomery D. L., Liu M. A. Protection against papillomavirus with a polynucleotide vaccine. J Infect Dis. 1996 Feb;173(2):314–320. doi: 10.1093/infdis/173.2.314. [DOI] [PubMed] [Google Scholar]
- Farrell H. E., McLean C. S., Harley C., Efstathiou S., Inglis S., Minson A. C. Vaccine potential of a herpes simplex virus type 1 mutant with an essential glycoprotein deleted. J Virol. 1994 Feb;68(2):927–932. doi: 10.1128/jvi.68.2.927-932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fynan E. F., Webster R. G., Fuller D. H., Haynes J. R., Santoro J. C., Robinson H. L. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallichan W. S., Johnson D. C., Graham F. L., Rosenthal K. L. Mucosal immunity and protection after intranasal immunization with recombinant adenovirus expressing herpes simplex virus glycoprotein B. J Infect Dis. 1993 Sep;168(3):622–629. doi: 10.1093/infdis/168.3.622. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Cai S., Slanina S., Nesburn A. B., Wechsler S. L. Vaccination of mice with herpes simplex virus type 1 glycoprotein D DNA produces low levels of protection against lethal HSV-1 challenge. Antiviral Res. 1995 Oct;28(2):147–157. doi: 10.1016/0166-3542(95)00045-n. [DOI] [PubMed] [Google Scholar]
- Heineman T. C., Connelly B. L., Bourne N., Stanberry L. R., Cohen J. Immunization with recombinant varicella-zoster virus expressing herpes simplex virus type 2 glycoprotein D reduces the severity of genital herpes in guinea pigs. J Virol. 1995 Dec;69(12):8109–8113. doi: 10.1128/jvi.69.12.8109-8113.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriesel J. D., Spruance S. L., Daynes R. A., Araneo B. A. Nucleic acid vaccine encoding gD2 protects mice from herpes simplex virus type 2 disease. J Infect Dis. 1996 Mar;173(3):536–541. doi: 10.1093/infdis/173.3.536. [DOI] [PubMed] [Google Scholar]
- Lane J. M., Ruben F. L., Neff J. M., Millar J. D. Complications of smallpox vaccination, 1968. N Engl J Med. 1969 Nov 27;281(22):1201–1208. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Dowbenko D. J. DNA sequence analysis of the type-common glycoprotein-D genes of herpes simplex virus types 1 and 2. DNA. 1984;3(1):23–29. doi: 10.1089/dna.1.1984.3.23. [DOI] [PubMed] [Google Scholar]
- Manickan E., Rouse R. J., Yu Z., Wire W. S., Rouse B. T. Genetic immunization against herpes simplex virus. Protection is mediated by CD4+ T lymphocytes. J Immunol. 1995 Jul 1;155(1):259–265. [PubMed] [Google Scholar]
- Manickan E., Yu Z., Rouse R. J., Wire W. S., Rouse B. T. Induction of protective immunity against herpes simplex virus with DNA encoding the immediate early protein ICP 27. Viral Immunol. 1995;8(2):53–61. doi: 10.1089/vim.1995.8.53. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Graham F. L., Hanke T., Johnson D. C. Protection of mice against lethal challenge with herpes simplex virus by vaccination with an adenovirus vector expressing HSV glycoprotein B. Virology. 1989 Mar;169(1):244–247. doi: 10.1016/0042-6822(89)90064-0. [DOI] [PubMed] [Google Scholar]
- McDermott M. R., Smiley J. R., Leslie P., Brais J., Rudzroga H. E., Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984 Sep;51(3):747–753. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meignier B., Longnecker R., Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis. 1988 Sep;158(3):602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- Montgomery D. L., Shiver J. W., Leander K. R., Perry H. C., Friedman A., Martinez D., Ulmer J. B., Donnelly J. J., Liu M. A. Heterologous and homologous protection against influenza A by DNA vaccination: optimization of DNA vectors. DNA Cell Biol. 1993 Nov;12(9):777–783. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- Pachl C., Burke R. L., Stuve L. L., Sanchez-Pescador L., Van Nest G., Masiarz F., Dina D. Expression of cell-associated and secreted forms of herpes simplex virus type 1 glycoprotein gB in mammalian cells. J Virol. 1987 Feb;61(2):315–325. doi: 10.1128/jvi.61.2.315-325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B. Introduction: objectives of herpes simplex virus vaccines seen from a historical perspective. Rev Infect Dis. 1991 Nov-Dec;13 (Suppl 11):S892–S894. doi: 10.1093/clind/13.supplement_11.s892. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador L., Burke R. L., Ott G., Van Nest G. The effect of adjuvants on the efficacy of a recombinant herpes simplex virus glycoprotein vaccine. J Immunol. 1988 Sep 1;141(5):1720–1727. [PubMed] [Google Scholar]
- Sedegah M., Hedstrom R., Hobart P., Hoffman S. L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry L. R., Bernstein D. I., Burke R. L., Pachl C., Myers M. G. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1987 May;155(5):914–920. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- Stanberry L. R., Kern E. R., Richards J. T., Abbott T. M., Overall J. C., Jr Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J Infect Dis. 1982 Sep;146(3):397–404. doi: 10.1093/infdis/146.3.397. [DOI] [PubMed] [Google Scholar]
- Stanberry L. R., Myers M. G., Stephanopoulos D. E., Burke R. L. Preinfection prophylaxis with herpes simplex virus glycoprotein immunogens: factors influencing efficacy. J Gen Virol. 1989 Dec;70(Pt 12):3177–3185. doi: 10.1099/0022-1317-70-12-3177. [DOI] [PubMed] [Google Scholar]
- Stuve L. L., Brown-Shimer S., Pachl C., Najarian R., Dina D., Burke R. L. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J Virol. 1987 Feb;61(2):326–335. doi: 10.1128/jvi.61.2.326-335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer J. B., Donnelly J. J., Parker S. E., Rhodes G. H., Felgner P. L., Dwarki V. J., Gromkowski S. H., Deck R. R., DeWitt C. M., Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993 Mar 19;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- Wachsman M., Aurelian L., Smith C. C., Lipinskas B. R., Perkus M. E., Paoletti E. Protection of guinea pigs from primary and recurrent herpes simplex virus (HSV) type 2 cutaneous disease with vaccinia virus recombinants expressing HSV glycoprotein D. J Infect Dis. 1987 Jun;155(6):1188–1197. doi: 10.1093/infdis/155.6.1188. [DOI] [PubMed] [Google Scholar]
- Wang B., Ugen K. E., Srikantan V., Agadjanyan M. G., Dang K., Refaeli Y., Sato A. I., Boyer J., Williams W. V., Weiner D. B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley R. J., Kern E. R., Chatterjee S., Chou J., Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus gamma 1 34.5 deletion mutants in rodent models. J Clin Invest. 1993 Jun;91(6):2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Xiang Z. Q., Spitalnik S., Tran M., Wunner W. H., Cheng J., Ertl H. C. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994 Feb 15;199(1):132–140. doi: 10.1006/viro.1994.1105. [DOI] [PubMed] [Google Scholar]
- Xu D., Liew F. Y. Genetic vaccination against leishmaniasis. Vaccine. 1994 Dec;12(16):1534–1536. doi: 10.1016/0264-410x(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Zhang J., Whitton J. L. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995 Apr;69(4):2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]



