Abstract
The ability to achieve energy saving in architectures and optimal solar energy utilisation affects the sustainable development of the human race. Traditional smart windows and solar cells cannot be combined into one device for energy saving and electricity generation. A VO2 film can respond to the environmental temperature to intelligently regulate infrared transmittance while maintaining visible transparency, and can be applied as a thermochromic smart window. Herein, we report for the first time a novel VO2-based smart window that partially utilises light scattering to solar cells around the glass panel for electricity generation. This smart window combines energy-saving and generation in one device, and offers potential to intelligently regulate and utilise solar radiation in an efficient manner.
Most efforts to efficiently utilise solar energy have been focused on improving the efficiency in the conversion and storage using solar cells1,2,3,4,5,6,7 and large capacity batteries8, respectively. However, these cells, which have been used on housetops and wall periphery, could not be integrated into windows that require the material to be transparent. Traditionally designed energy-saving windows, such as electrochromic thermochromic, and gasochromic, typically function by exterior stimuli involving either an electric field, heat stimulus or a gas9. It is not possible to alter the optical performance, which involves intelligently passing or blocking solar energy in response to environmental changes, and simultaneously generate electricity10,11. Herein, a novel smart window was designed such that the VO2 films or particles regulate solar infrared radiation and scatter partial light to a solar cell for electricity generation.
It is known that VO2 undergoes a fully reversible metal-semiconductor transition (MST) at a critical temperature (Tc) of 68°C12. Below Tc (T<Tc), VO2 is a monoclinic crystalline structure, which is insulating and transparent to infrared light, but it becomes a tetragonal crystalline structure that is metallic and reflective to infrared light above Tc (T>Tc)13,14. This phase transition property makes VO2 an attractive material for smart windows15. Current research efforts have been primarily focused on enhancing the optical properties of VO2 films (e.g., the improvement of the solar energy modulation ability and visible transmittance of VO2 using multi-layered structures by designing high-reflective-index dielectric top or under layers (i.e., SiO2/VO216,21, TiO2/VO217 and In2O3:Sn/VO2/In2O3:Sn18), forming nanoparticle composite foils (i.e., SiO2/VO2 core-shell19,VO2/ATO20) or enhancing the visible transmittance by doping (i.e., F-doped VO221 and Mg-doped VO222). The focal points of the above-mentioned studies involve changing the transmittance, absorption and reflection characteristics of VO2. However, the scattering interaction between the material and light has been ignored, resulting in a loss of the energy associated with scattering.
Results
Three devices that can save energy and simultaneously generate electricity were designed
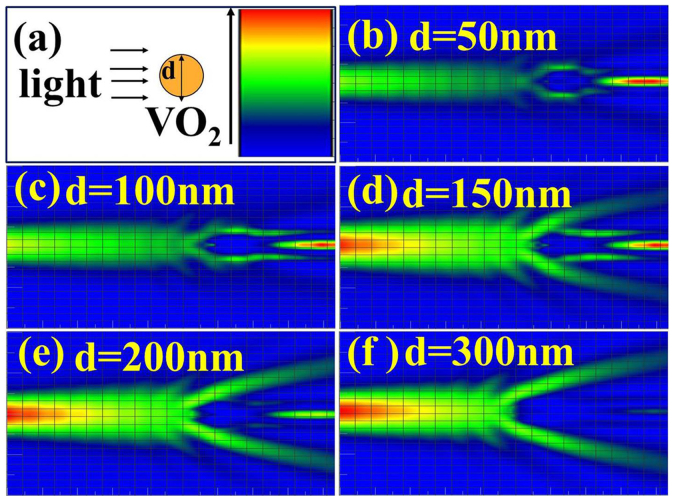
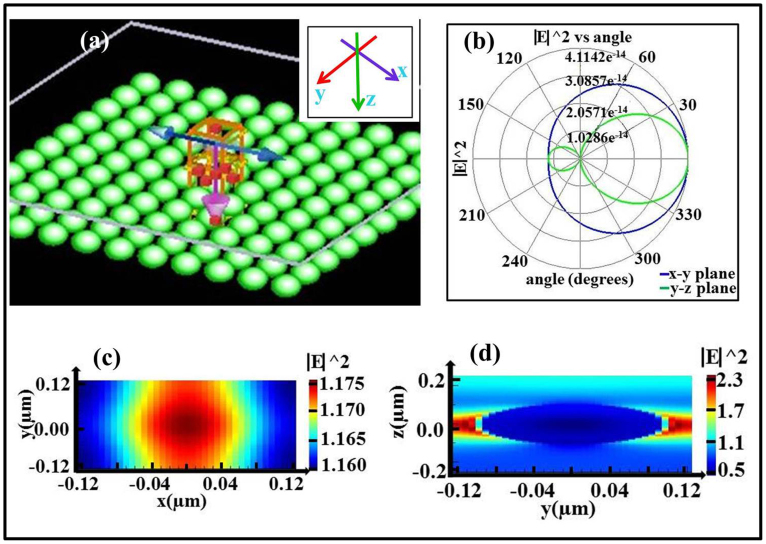
Scattering can be defined as Tyndall, Mie or Rayleigh types based on the interactional relationship between wavelength (λ) of light and size of material, which implies the possibility to size-dependent control the strength of scattering23. A finite-difference-time-domain (FDTD) algorithm is useful for designing and investigating a varieties of devices and applications involving the propagation of electromagnetic radiation through complicated media, and was employed to investigate the interaction between VO2 particles and light (Figure 1a). The electric field distribution of different particle sizes with light24 (Figures 1b through 1f) indicates that light bypasses the particle propagation and that the scattering is so weak at a VO2 particle size of less than 50 nm. However, the scattering is significantly enhanced with an increase in the particle size (i.e., 100 nm, 150 nm, 200 nm and 300 nm). The scattering behaviour between VO2 particle arrays and light was further simulated by FDTD to illustrate the potential use of scattered light for electricity generation (Figure 2). In this simulation, both the radius of the VO2 particle and the distance between two particles are 100 nm. The scattering of normal incident light in a wavelength range of 350–780 nm along the z-direction and polarised along the y/x-direction was simulated25 (Figure 2a). The far field angular scattering shown in Figure 2b suggests that the scattering field intensity extended to a far zone, which means the scattering field is obvious and large. The scattering energy distribution in the y-z plane is larger than that in the x-y plane, which changed the transmittance of the film in x-y plane.
Figure 1. The scatter electric field distribution of the interaction between a single VO2 particle and light was simulated by FDTD.
(a), The interaction of a single VO2 particle with light, where the colour represents the incident light. The particle size of VO2 is 50, 100, 150, 200 and 300 nm for (b, c, d, e and f), respectively. The scattering was enhanced as the particle size increased.
Figure 2. The scattering of a VO2 particle arrays film and the electric field intensity profiles of scattered light were simulated.
(a), The simulation scheme (inset shows coordinate directions). The normal incident light was along the z-direction and polarised along the y/x-direction. (b), Far field angular scattering, which shows the behaviour of the scattered field in the far zone in the x-y and y-z planes. The vertical direction numbers represent the relative intensity. (c and d), The electric field intensity scattered by the VO2-based particle film is indicated by a colour scale in the x-y and y-z planes.
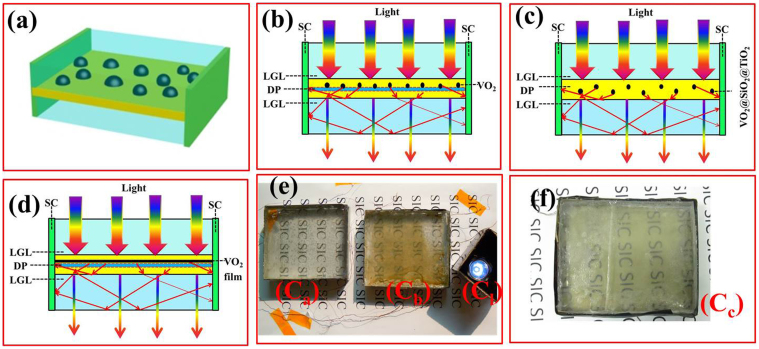
Based on this model, a solar cell module possessing energy-saving characteristics was designed and is shown in Figures 3a–3d. The structure consisted of three sections, including a low reflective index medium (e.g., VO2-based particle or film), light guider layers and solar cells. To collect scattered light using this structure, the light guider layers should have a high reflective index associated with the reflective material, which enables the scattering light to propagate between the light guider layers and be reflected to the solar cells. The total internal reflection occurs when light propagates into an optically thinner medium from a denser medium with an incident angle larger than the total internal reflection angle c. The VO2-based particle can scatter some of light to solar cells for generation.
Figure 3. The work principle scheme and the structure description of the VO2-based thermochromic/generating smart window.
(a), Three-dimensional structure of the prepared VO2-based smart window. The solar cells are assembled in a manner that surrounds the module. Here, 3 sides are shown. (b), c and d are the cross-sectional views of a with different DP. (b), A VO2 film on quartz that served as a scattering medium. The scattering medium in c is a composite created by the dispersion of VO2@SiO2@TiO2 core-shell-shell particles in PU. This composite is cast in PDMS. The scattering medium in d is a smooth VO2 thin film. SC, LGL and DP refer to the solar cell, light guider layer and low reflective index medium, respectively. (b, c and d) show the principle scheme of the devices Ca, Cb (Figure 3e) and Cc (Figure 3f), respectively. When light interacts with the VO2 particle, partial light was scattered and reflected to the solar cell to generate electricity. Cl is a 1.5-V lamp, which was employed to demonstrate whether the smart window works for generation. The two devices in series in Figure 3e could light a 1.5 V lamp.
In Figure 3e–3f, the three devices (i.e., Ca, Cb and Cc) have been designed, a polycarbonate plate (PC plate, refractive index: 1.59) is employed as the light guider layer to gather scattering light to polysilicon solar cells. In Figure 3e-Ca, the low reflective index medium for the device Ca (Figure 3b) and Cb (Figure 3c) was a VO2 particle film on a quartz plate and VO2-based power arrays, respectively. The latter was designed as a core-shell-shell structure (i.e., VO2@SiO2@TiO2) to decrease the absorption of VO2 while maintaining the overall size of the particles to fulfil the scattering conditions26. In Figure 3f, the low reflective index medium for the device Cc (Figure 3d) was a smooth VO2 thin film. The thickness of the PC is about 4 mm and thickness of the low reflective index medium is about 200 nm in these devices.
Discussion
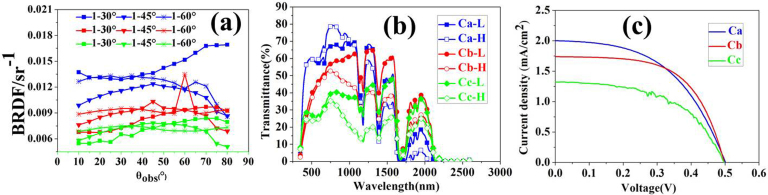
Figure 4 shows the optical scattering spectra, the transmittance spectra and the I-V curves of devices Ca, Cb and Cc. The scattering measured by the bidirectional reflectance distribution function (BRDF) method27 indicated that the scattering of Ca is larger than Cb and Cc (Figure 4a) because the absorption of the film cast in PDMS for the Cb is enhanced. The scattering of Cc that used a smooth thin film as medium decreased, and the corresponding value of the BRDF for Cc is lower than Ca and Cb. The BRDF curve is consistent with the simulation of FDTD, which indicates that the scattering is large enough for generation. Figure 4b shows the optical transmittance spectra of Ca, Cb and Cc before and after the MST. Consistently, the transmittance in the wavelength range of 300–1,000 nm for the device Ca at a high temperature (i.e., 90°C) is higher than that at a low temperature (i.e., 25°C), which is due to the enhanced scattering while the absorption is weakened. In addition, the transmittance of Ca is higher than that of Cb due to the structural difference.
Figure 4. The results of the BRDF curve, transmittance spectra of three windows and I-V curves of the three devices.
In Figure 4a, the BRDF curve under the measurement conditions has a polarisation incidence with an incident wavelength of 635 nm. Ca, Cb and Cc represent the curve of the Ca, Cb and Cc devices in Figure 3e and 3f, respectively. The incident angle was fixed at 30°, 45° and 60°, and the intensity of the reflective light was measured from 10° to 80°. The results indicate that the scattering intensity of Ca is larger than Cb and Cc. In the Figures 4b and 4c, the optical transmittance spectra at low and high temperatures and the I-V curves under AM 1,5 illumination of the three devices in Figure 3e (Ca, and Cb) and 3f-Cc are shown.
To investigate the optical properties of the devices, the solar modulation efficiency (ΔTsol) was calculated to characterise the thermochromic smart window properties of the devices. Tvis denotes the integral visible transmittance of the devices. The calculated results of the three devices are shown in Table 1. These results indicate that the ΔTsol of the Ca is only 2.0%, and the Tvis values at high and low temperatures are 65.6% and 61.7%, respectively. The ΔTsol of Cb is 7.5%, which is much higher than Ca, and the Tvis values at high and low temperatures are 45.6% and 42.7%, respectively. The results suggest that the VO2-based core-shell-shell structure is beneficial for energy saving. For the Cc with a smooth VO2 thin film, the solar energy modulation ability of VO2 was maintained.
Table 1. The visible transmittance, solar energy transmittance and solar modulation ability of the windows in Figure 3e and 3f.
| Visible transmittance Tvis (%) | Solar Transmittance Tsol (%) | ||||
|---|---|---|---|---|---|
| Sample | 25°C | 90°C | 25°C | 90°C | Solar modulation ability (%) |
| Ca | 61.7 | 65.6 | 56.2 | 58.2 | 2.0 |
| Cb | 45.6 | 42.7 | 46.9 | 39.3 | 7.5 |
| Cc | 31.5 | 28.0 | 32.1 | 24.1 | 8.0 |
The I-V curves are shown in Figure 4c, and the results are summarised in Table 2. The Ca and Cb devices show efficiencies of 0.50% and 0.52%, Voc values of 0. 501 V and 0.498 V, FF values of 50.18% and 59.68% and Jsc values of 2.0 mA/cm2 and 1.74 mA/cm2 for Ca and Cb, respectively. The efficiency and FF values of Cb are higher than those of Ca, which shows that the Cb structure is more efficient compared to that of Ca in terms of gathering scattering energy. Note that the area of Ca is large than Cb, so the scattering light in Ca attenuated bigger than that in Cb. The properties of the device Cc are worse than Ca and Cb, probably because of decreased scattering.
Table 2. The efficiency, open-circuit voltage Voc, fill factor FF and short-circuit current density Jsc under AM 1.5 illumination for three devices.
| cell | Voc (V) | Jsc (mA/cm2) | FF (%) | Eff (%) | Area (cm2) |
|---|---|---|---|---|---|
| Ca | 0.501 | 2.00 | 50.18 | 0.50 | 76 |
| Cb | 0.498 | 1.74 | 59.68 | 0.52 | 60 |
| Cc | 0.497 | 1.32 | 52.06 | 0.34 | 70 |
Buildings and other man-made structures consume 30–40% of the primary energy for heating, cooling, ventilation and lighting. This phenomenon will increase with the growth of the population and the associated energy consumption28. The ability to reduce energy consumption has become an urgent priority. The successful design and preparation of a novel smart window that combines energy-saving and electricity generation achieves comprehensive utilisation of solar energy, which supports an important new insight into resolving the energy consumption.
Methods
Preparation of VO2 films using a solution-based process
The thermochromic VO2 films were prepared according to a previously reported protocol29. Vanadium pentoxide (V2O5, analytically pure), diamide hydrochloride (N2H4·HCl, analytically pure) and PVP (K90, average molecular weight: 1,300,000) were used as starting materials to prepare vanadium precursors. Quartz glass was used as the substrate and was subsequently cleaned in H2O2, HCl, and NH3·H2O. Precursor films were prepared by shin-coating at 3,000 rpm for 40 s. Then, the films were annealed at 520 in an N2 (100%) and N2-O2 flow (N2: 99.5%, O2 0.5%) to prepare the smooth VO2 thin film and VO2 particle film, respectively.
Preparation of the VO2@SiO2@TiO2/PU composite film
Preparation of VO2 nanoparticles
The VO2 nanoparticles were synthesised using the hydrothermal method according to a previously reported protocol30. In a typical procedure, vanadium pentoxide (V2O5, analytically pure) powder was added to an oxalic acid dehydrate aqueous solution to form a yellowish slurry. Then, the slurry was transferred to a 50 mL teflon-lined stainless-steel autoclave, which was maintained at 240°C for 24 h, and was then air-cooled to room temperature.
Preparation of VO2@SiO2 nanoparticles
First, VO2 nanoparticles were pretreated with poly(vinylpyrrolidone) (PVP) K-30. Then, the VO2@SiO2 core-shell structure was prepared by the hydrolysis of TEOS, which is known as the modified Stöber growth method. Finally, the prepared sample was collected by centrifugation and washed with deionised water and ethanol several times19.
Preparation of VO2@SiO2 @TiO2 nanoparticles
First, the as-prepared VO2@SiO2 nanoparticles were dispersed in an ethanol solution under strong stirring for 30 min. Then, the required amount of tetrabutyl titanate (TBOT) was quickly added. Finally, an ethanol solution containing 0.03 mL of NH3·H2O was added drop-wise to the solution over 5 min. The reaction was maintained at 45°C for 24 h. When the reaction was complete, the sample was collected by centrifugation, washed with deionised water and ethanol several times, and dried at 50°C for 6 h31.
Please see the reference for further information on the preparation of VO2 nanoparticles and VO2@SiO2 nanoparticles19.
Preparation of VO2@SiO2@TiO2/PU composite film
The as-prepared particles were ultrasonically dispersed in deionised water and an appropriate amount of the saline coupler KH-570 was added under stirring. To this solution, the organic matrix material, polyurethane (PU, DISPERCOLLU 54, Bayer), was added with stirring for 10 min. Finally, the mixture was uniformly cast onto a PC substrate using an automatic coating machine and then dried to prepare the VO2 solar cell.
Preparation of VO2-based solar cells
The VO2 smart window solar cells were assembled according to the structure in Figure 3. The PC plate was used as the light guider layer. The low reflective index medium, VO2-based film or nanoparticles with PDMS were adhered on the PC plate, and another PC plate was adhered on the low reflective index medium. After that, the silicon solar cells were adhered with PDMS. All of the cells were connected in parallel and the device was installed.
Characterization
The morphology of the VO2 film was determined by field emission scanning electron microscopy (FESEM, JEOL Corp., Model JSM-6700F). The VO2 nanoparticles were observed using transmission electron microscopy (TEM) and energy dispersive spectrometry (EDS, JEM2010F, JEOL, Japan), and the VO2 film was observed using transmission electron microscopy (TEM). The phase identification was obtained via X-ray diffraction (XRD, Model D/Max 2550 V, Rigaku, Japan, Cu Kα, λ = 0.15406 nm). The transmittance spectra at normal incidence from 240 nm to 2600 nm were measured using a Hitachi U-4100 spectrometer. The scattering interaction between the VO2 particles and light was simulated by the finite difference time domain solution (FDTD). The scattering of the VO2-based smart windows was measured using the method of the bidirectional reflectance distribution function.
Author Contributions
J.Z., Z.Z. and Y.G. designed the experiment. J.Z. performed synthesis experiments and characterization. Z.Z. and J.Z. performed simulation and analysis. J.Z. and Y.G. wrote the paper. J.Z., Z.Z., Y.G., H.L., C.C., Z.C., L.D. and X.L. contributed to analysis the experimental data.
Supplementary Material
SI
Acknowledgments
This study was supported in part by funds from MOST (2009CB939904, 2012AA030605, 2012BAA10B03) and NSFC (Contract No: 51172265, 51032008).
References
- Chu S. & Majumdar A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012). [DOI] [PubMed] [Google Scholar]
- Tomabechi K. Energy Resources in the Future. Energies 3, 686–695 (2010). [Google Scholar]
- Peng K. Q. & Lee S. T. Silicon Nanowires for Photovoltaic Solar Energy Conversion. Adv. Mater. 23, 198–215 (2011). [DOI] [PubMed] [Google Scholar]
- Chopra K. L., Paulson P. D. & Dutta V. Thin-film solar cells: An overview. Prog. Photovoltaics 12, 69–92 (2004). [Google Scholar]
- Shah A., Torres P., Tscharner R., Wyrsch N. & Keppner H. Photovoltaic technology: The case for thin-film solar cells. Science 285, 692–698 (1999). [DOI] [PubMed] [Google Scholar]
- Chung I., Lee B., He J. Q., Chang R. P. H. & Kanatzidis M. G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 485, 486–494 (2012). [DOI] [PubMed] [Google Scholar]
- Graetzel M., Janssen R. A. J., Mitzi D. B. & Sargent E. H. Materials interface engineering for solution-processed photovoltaics. Nature 488, 304–312 (2012). [DOI] [PubMed] [Google Scholar]
- Blom P. W. M., Mihailetchi V. D., Koster L. J. A. & Markov D. E. Device physics of polymer: fullerene bulk heterojunction solar cells. Adv. Mater. 19, 1551–1566 (2007). [Google Scholar]
- Baetens R., Jelle B. P. & Gustavsen A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: A state-of-the-art review. Sol. Energy. Mater. Sol. Cells 94, 87–105 (2010). [Google Scholar]
- Cheng F. Y., Liang J., Tao Z. L. & Chen J. Functional Materials for Rechargeable Batteries. Adv. Mater. 23, 1695–1715 (2011). [DOI] [PubMed] [Google Scholar]
- Li S. Y., Niklasson G. A. & Granqvist C. G. Thermochromic fenestration with VO2-based materials: Three challenges and how they can be met. Thin. Solid. Films 520, 3823–3828 (2012). [Google Scholar]
- Qazilbash M. M. et al. Mott transition in VO2 revealed by infrared spectroscopy and nano-imaging. Science 318, 1750–1753 (2007). [DOI] [PubMed] [Google Scholar]
- Liu M. K. et al. Terahertz-field-induced insulator-to-metal transition in vanadium dioxide metamaterial. Nature 487, 345–348 (2012). [DOI] [PubMed] [Google Scholar]
- Nakano M. et al. Collective bulk carrier delocalization driven by electrostatic surface charge accumulation. Nature 487, 459–462 (2012). [DOI] [PubMed] [Google Scholar]
- Lee M. H. Thermochromic glazing of windows with better luminous solar transmittance. Sol. Energy. Mater. Sol. Cells 71, 537–540 (2002). [Google Scholar]
- Chen Z. et al. VO2-based double-layered films for smart windows: Optical design, all-solution preparation and improved properties. Sol. Energy. Mater. Sol. Cells 95, 2677–2684 (2011). [Google Scholar]
- Jin P., Xu G., Tazawa M. & Yoshimura K. A VO2-based multifunctional window with highly improved luminous transmittance. Jpn. J. Appl. Phys. 41, L278–L280 (2002). [Google Scholar]
- Heinilehto S. T., Lappalainen J. H., Jantunen H. M. & Lantto V. IR-wavelength optical shutter based on ITO/VO2/ITO thin film stack. J. Electroceram. 27, 7–12 (2011). [Google Scholar]
- Gao Y. F. et al. Enhanced chemical stability of VO2 nanoparticles by the formation of SiO2/VO2 core/shell structures and the application to transparent and flexible VO2-based composite foils with excellent thermochromic properties for solar heat control. Energ. Environ. Sci. 5, 9947–9947 (2012). [Google Scholar]
- Gao Y. F. et al. VO2-Sb:SnO2 composite thermochromic smart glass foil. Energ. Environ. Sci. 5, 8234–8237 (2012). [Google Scholar]
- Burkhardt W. et al. W- and F-doped VO2 films studied by photoelectron spectrometry. Thin. Solid. Films 345, 229–235 (1999). [Google Scholar]
- Hu S. L. et al. Optical properties of Mg-doped VO2: Absorption measurements and hybrid functional calculations. Appl. Phys. Lett. 101, 201902 (2012). [Google Scholar]
- Hodkinso Jr Particle Sizing by Means of Forward Scattering Lobe. Appl. Optics. 5, 839–844 (1966). [DOI] [PubMed] [Google Scholar]
- Goodenou J.b 2 Components of crystallographic transition in VO2. J. Solid State. Chem. 3, 490–& (1971). [Google Scholar]
- Zhao J. et al. Methods for Describing the Electromagnetic Properties of Silverand Gold Nanoparticles. Acc. Chem. Res. 41, 1710–1720 (2008). [DOI] [PubMed] [Google Scholar]
- Li S. Y., Niklasson G. A. & Granqvist C. G. Nanothermochromics: Calculations for VO2 nanoparticles in dielectric hosts show much improved luminous transmittance and solar energy transmittance modulation. J. Appl. Phys. 108, (2010). [Google Scholar]
- Schaaf C. B. et al. First operational BRDF, albedo nadir reflectance products from MODIS. Remote. Sens. Environ. 83, 135–148 (2002). [Google Scholar]
- Rotzetter A. C. C. et al. Thermoresponsive Polymer Induced Sweating Surfaces as an Efficient Way to Passively Cool Buildings. Adv. Mater. 24, 5352–5356 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang Z. et al. Thermochromic VO2 Thin Films: Solution-Based Processing, Improved Optical Properties, and Lowered Phase Transformation Temperature. Langmuir 26, 10738–10744 (2010). [DOI] [PubMed] [Google Scholar]
- Cao C. X., Gao Y. F. & Luo H. J. Pure Single-Crystal Rutile Vanadium Dioxide Powders: Synthesis, Mechanism and Phase-Transformation Property. J. Phys. Chem. C 112, 18810–18814 (2008). [Google Scholar]
- Li W. et al. A Versatile Kinetics-Controlled Coating Method To Construct Uniform Porous TiO2 Shells for Multifunctional Core-Shell Structures. J. Am. Chem. Soc. 134, 11864–11867 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SI