Abstract
Neutralizing antibodies may have critical importance in immunity against human immunodeficiency virus type 1 (HIV-1) infection. However, the amount of protective antibody needed at mucosal surfaces has not been fully established. Here, we evaluated systemic and mucosal pharmacokinetics (PK) and pharmacodynamics (PD) of 2F5 IgG and 2F5 Fab fragments with respect to protection against vaginal challenge with simian-human immunodeficiency virus-BaL in macaques. Antibody assessment demonstrated that 2F5 IgG was more potent than polymeric forms (IgM and IgA) across a range of cellular and tissue models. Vaginal challenge studies demonstrated a dose-dependent protection for 2F5 IgG and no protection with 2F5 Fab despite higher vaginal Fab levels at the time of challenge. Animals receiving 50 or 25 mg/kg of body weight 2F5 IgG were completely protected, while 3/5 animals receiving 5 mg/kg were protected. In the control animals, infection was established by a minimum of 1 to 4 transmitted/founder (T/F) variants, similar to natural human infection by this mucosal route; in the two infected animals that had received 5 mg 2F5 IgG, infection was established by a single T/F variant. Serum levels of 2F5 IgG were more predictive of sterilizing protection than measured vaginal levels. Fc-mediated antiviral activity did not appear to influence infection of primary target cells in cervical explants. However, PK studies highlighted the importance of the Fc portion in tissue biodistribution. Data presented in this study may be important in modeling serum levels of neutralizing antibodies that need to be achieved by either vaccination or passive infusion to prevent mucosal acquisition of HIV-1 infection in humans.
INTRODUCTION
Neutralizing antibodies are thought to have critical importance in protective immunity against human immunodeficiency virus type 1 (HIV-1) infection and may be particularly effective if present at mucosal portals of infection (1). This is supported by a growing number of in vivo studies demonstrating that passively infused human anti-HIV-1 neutralizing antibodies are able to protect nonhuman primates (NHPs) from intravenous or mucosal simian HIV (SHIV) challenge infection (2–7). Furthermore, additional studies demonstrate that topical application of neutralizing monoclonal antibodies is sufficient to provide protection against vaginal SHIV challenge (8–10). However, the amount of antibody following passive infusion or vaccination needed at mucosal surfaces to prevent infection has not been fully established. The growing number of increasingly potent, broadly neutralizing monoclonal antibodies isolated from serum of a small percentage of HIV-1-infected individuals is driving interest in their potential prophylactic use, either systemically or topically. To date, most isolated neutralizing antibodies are of the monomeric IgG isotype (11–15). However, this might not fully represent antibodies at mucosal surfaces where polymeric secretory IgA (sIgA) has also been associated with virus neutralization (16). Furthermore, the observation that the modest protective efficacy of the Thai RV-144 vaccine trial (31%) (17) did not correlate with neutralizing responses suggests that mechanisms other than neutralization contribute to mucosal protection (18).
The 2F5 monoclonal antibody was originally isolated as an IgG3 isotype and subsequently class switched to IgG1 to facilitate production (19). 2F5 IgG recognizes an epitope on the membrane-proximal external region (MPER) of gp41, neutralizing >60% of viral isolates (14, 20). Unlike many neutralizing antibodies that bind directly to gp120, 2F5 is unable to target the untriggered prefusion state of the functional envelope trimer, as its known epitope within the MPER is either poorly exposed or inaccessible (21). Thus, a two-step model for 2F5 binding has been proposed (22) where 2F5 initially attaches to the viral membrane through low-affinity, reversible hydrophobic interaction via its long CDR H3 loops. Following CD4 and coreceptor engagement, the HIV envelope then undergoes a cascade of structural rearrangements, triggering the prehairpin intermediate form of gp41 that allows insertion of the fusion peptide into the target cell membrane and facilitating membrane fusion. In this two-step model, the 2F5 epitope becomes accessible only on exposure of the prehairpin intermediate. Prepositioning of 2F5 IgG on the viral membrane through initial hydrophobic interaction is thought to potentiate subsequent binding to its epitope in the prehairpin intermediate, preventing or destabilizing further structural rearrangements required for fusion and thereby delivering effective neutralization (22–24).
However, 2F5 IgG expresses a number of antiviral functions beyond classical neutralization that might contribute to mucosal protection. Previous studies have demonstrated that 2F5 IgG can provide potent Fc-mediated inhibition of HIV infection of antigen-presenting cells prevalent in mucosal tissues, including macrophages, dendritic cells (DCs), and Langerhan's cells. Although initially thought to be mediated by phagocytosis and degradation of opsonized viral particles (25–27), more recent data have suggested that binding to FcγRI provides a kinetic advantage for accessing partially cryptic epitopes, such as that recognized by 2F5 that is independent of phagocytosis (28). Additional Fc-mediated activity includes antibody-dependent cellular cytotoxicity (ADCC) (29) and antibody-dependent cellular viral inhibition (ADCVI) (8), potentially leading to killing of infected cells. Here, the efficiency of recognition is likely enhanced by the accessibility of the 2F5 epitope on noncleaved immature envelope as expressed on budding particles (30). In addition, both monomeric 2F5 IgG and polymeric 2F5 IgA have been shown to interfere with HIV-1 epithelial transcytosis in vitro, a proposed mechanism of HIV-1 penetration across mucosal epithelium in vivo (31, 32).
Here, we initially assessed the antiviral activity of 2F5 in three different isotype forms, monomeric IgG, dimeric IgA, and polymeric IgM, in a range of in vitro cell and ex vivo tissue models designed to mimic different aspects of mucosal transmission. Based on these studies, 2F5 IgG was selected for passive infusion studies in a rhesus macaque vaginal challenge study. To study the importance of the Fc portion of IgG in retention and distribution at mucosal surfaces, we compared the systemic and mucosal pharmacokinetics (PK) of infused 2F5 IgG and Fab following intravenous infusion. Subsequently, we investigated the relative protective role of 2F5 IgG and Fab against vaginal SHIV-BaL challenge.
MATERIALS AND METHODS
Antibodies and tissue.
Human monoclonal antibody 2F5, in its three different isotype forms (IgG, IgA, and IgM), and the Fab fragment of 2F5, used for initial in vitro studies, were kindly provided by Polymun Scientific, Austria. As non-HIV-1-specific antibody controls, commercially available purified human IgA, human IgG, human IgM (Sigma, United Kingdom), and human IgG Fab (Abcam, United Kingdom) were used. Human cervical tissue was obtained from consenting patients undergoing therapeutic hysterectomy at St. George's or Kingston Hospital (London, United Kingdom) according to guidelines of the local Research Ethics Committee.
Production of recombinant monoclonal antibody 2F5 IgG and Fab antibodies for passive infusion.
Monoclonal antibody 2F5 expression constructs and recombinant monoclonal 2F5 IgG1 and Fab antibodies were generated using the methods described by Liao and Nicely (33, 34). Briefly, DNA sequences encoding the variable region of the heavy and light chains of monoclonal antibody 2F5 (35) were reconstructed using amino acid sequences from the Protein Data Bank (PDB; code 1TJG, molecules H and L) and the published DNA sequence (36) and were de novo synthesized (Blue Heron, Bothell, WA) (33). Full-length and Fab IgG1 heavy-chain gene and kappa-chain gene expression constructs were generated and cloned into pCDNA3.1+/Hygro (Invitrogen, Carlsbad, CA) (33, 34) for production of recombinant monoclonal antibody 2F5 IgG and Fab antibodies. Recombinant monoclonal antibody 2F5 IgG and Fab antibodies were produced in CHO cells transduced using the same 2F5 IgG and Fab constructs expressed in recombinant retrovirus (Catalent, Somerset, NJ) and purified by using a protein A column.
Cell and virus culture.
PM-1 T cells (kindly provided by the NIH AIDS Research & Reference Reagent Program) cultured in complete RPMI (Sigma, United Kingdom) were used to grow HIV-1BaL. The 50% tissue culture infectious dose (TCID50) of the virus was determined as described previously (37). Peripheral blood mononuclear cells (PBMCs) for infectivity assay were obtained from leukocyte cones (NHS Blood and Transplant, Wakefield, United Kingdom). Leukocytes were separated by Histopaque (Sigma, United Kingdom) gradient centrifugation. Before HIV-1 infection, PBMCs were activated with 5 μg/ml phytohemagglutinin (PHA) (Sigma, United Kingdom) in complete RPMI. After 2 days the culture medium was replaced with fresh medium supplemented with 5 μg/ml PHA and 200 U/ml interleukin-2 (IL-2; Novartis, United Kingdom) and cultured for a further 3 days. To culture monocyte-derived macrophages (MDM), recovered and washed mononuclear cells were diluted to 3 × 106 cells/ml in serum-free AIM-V medium (Invitrogen, United Kingdom) containing 20 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems, United Kingdom). One hundred μl of the cell suspension was plated in flat-bottomed high-binding 96-well plates (Corning Life Sciences, Netherlands) and incubated at 37°C. After 3 days, culture medium was replaced with serum-free AIM-V medium supplemented with 20 ng/ml GM-CSF. MDM were used for neutralization assays on day 5 of culture. To generate monocyte-derived dendritic cells (MDDCs), CD14+ mononuclear cells were enriched by CD14 magnetic bead isolation using an AutoMacs separation system according to the manufacturers' instructions. Briefly, 20 μl of CD14 MicroBeads (Miltenyi Biotec, United Kingdom) per 107 cells was incubated for 20 min at 4°C. The cells were washed once with AutoMacs running buffer (Miltenyi Biotec, United Kingdom) and centrifuged for 10 min at 300 × g. The cell pellet was resuspended in 2 ml of AutoMacs running buffer for magnetic separation. After separation, the supernatant containing CD14+ cells was centrifuged for 10 min at 400 × g. Pelleted cells were resuspended in 40 ml RPMI 1640 complete medium supplemented with 30 ng/ml IL-4 and 25 ng/ml GM-CSF (R&D Systems, United Kingdom) at a concentration of 5 × 105 cells/ml and cultured in a tissue culture flask at 37°C. After 3 days in culture, the medium was changed and replaced with fresh RPMI complete medium supplemented with 30 ng/ml IL-4 and 25 ng/ml GM-CSF and incubated for another 3 days.
Inhibition assays against HIV infection of primary cells and cervical tissue.
For PBMCs, 50 μg/ml of 2F5 IgG (333 nM), IgA (125 nM), IgM (51.5 nM), and 2F5 Fab (1 μM) were serially diluted 1:10 in complete RPMI before being preincubated with cell-free HIV-1BaL (104 TCID50) for 1 h at 37°C. PBMCs (5 × 104) that were PHA activated in complete RPMI supplemented with 200 U/ml IL-2 (Novartis, United Kingdom) were added to 100 μl of the virus antibody suspension in round-bottomed 96-well plates and incubated for 2 h at 37°C. PBMCs were washed 3 times with phosphate-buffered saline (PBS) with media and antibodies replenished. Plates were then cultured for 7 days at 37°C and the supernatant harvested for assessment of p24. For macrophages, HIV-1BaL (104 TCID50) was incubated with antibodies starting at 50 μg/ml, followed by 100-fold dilutions for 2F5 IgG and 10-fold dilutions for 2F5 IgA, IgM, and Fab for 1 h at 37°C. Subsequently, 100 μl of the virus was added to 5-day-old MDM cultures seeded in flat-bottomed high-binding 96-well plates and incubated for 2 h at 37°C. Cultures were washed 3 times with PBS, and then antibodies were added at the same concentration as before in 200 μl AIM-V medium supplemented with 20 ng/ml GM-CSF and cultured for 7 days at 37°C before assessment of p24. For MDDCs, antibodies starting at 50 μg/ml followed by 10-fold dilutions were preincubated with cell-free HIV-1BaL (104 TCID50) for 1 h at 37°C. MDDCs (4 × 104) in complete RPMI were added to 100 μl of antibody-treated HIV-1BaL in round-bottomed tissue culture plates and incubated for 2 h at 37°C. Following washing 3 times with PBS, antibodies were readded at the same concentration in 200 μl complete RPMI. The plates were cultured for a further 7 days at 37°C and supernatant was harvested for the assessment of p24. To assess trans-infection of HIV-1 by DCs to PM-1 T cells, 2 × 104 MDDCs were plated into round-bottomed tissue culture plates and exposed to 104 TCID50 of HIV-1BaL at 37°C. For FcγR blocking studies, MDDCs were exposed for 30 min to 20 μl of Fc-blocking reagent (Miltenyi Biotec, United Kingdom) per 107 cells prior to addition of HIV-1BaL. After 1 h, the MDDCs were washed thoroughly 3 times with PBS before addition of antibodies, starting at 50 μg/ml and followed by 10-fold dilutions and 4 × 104 PM-1 T cells per well in complete RPMI (supplemented with Fc-blocking reagent as described above for blocking studies) for an additional 7 days before assessment of p24. For human cervical explant tissue culture and HIV infection studies, cervical explant culture was performed as previously described (38–41). Briefly, cervical tissue, including both epithelium and stroma, was cut into 3-mm3 explants and cultured in complete RPMI 1640 medium. Antibodies at 50 μg/ml and 8-fold dilutions were preincubated with cell-free HIV-1BaL (5 × 104 TCID50) for 1 h at 37°C. The 3-mm3 dissected cervical tissue explants were then exposed to virus and antibody for 2 h at 37°C. Following viral incubation, explants were washed three times with PBS, placed into 96-well tissue culture plates, and cultured with fresh media containing antibody at the same concentration. The next day, the tissue explants were transferred into a new 96-well tissue culture plate and washed twice with PBS. Tissue explants were subsequently cultured for 14 days in 200 μl of supplemented medium. On days 4, 7, 11, and 14 postinfection, 100 μl of supernatant was harvested and replaced with 100 μl of fresh antibody solution. To assess migratory cells present in the overnight culture of explanted tissue, cells were washed twice with PBS and cocultured with 4 × 104 PM-1 indicator T cells in 200 μl of medium containing antibodies. The supernatant was collected on days 4, 7, 11, and 14 postinfection and replaced with medium supplemented with antibodies. All assays were performed in triplicate unless otherwise stated, and controls included virus only, medium only, and antibody isotype controls titrated in the same manner as the test antibodies. p24 content in culture supernatant was measured using an enzyme-linked immunosorbent assay (ELISA) from NCI (SAIC, Frederick, MD) or by a high-sensitivity INNOTEST p24 ELISA kit (Innogenetics, Belgium), where lower levels of p24 were produced. Ninety and 50% inhibitory concentration (IC90 and IC50, respectively) values were calculated according to linear regression of the antibody titration using GraphPad Prism4.
Passive antibody infusion and virus challenge.
A total of 28 female rhesus macaques (Macaca mulatta) were used in these studies. All animals were housed at the Tulane National Primate Research Center in accordance with the recommendations of the National Institutes of Health (42). All research was reviewed and approved by the Institutional Animal Care and Use Committee of Tulane University. Macaques were pretreated with a 30-mg intramuscular (i.m.) injection of depomedroxyprogesterone acetate (Depo-provera) (Pharmacia & Upjohn) 28 to 34 days before the start of experiments to synchronize menstrual cycles and to thin the vaginal mucosa (43). Macaques were sedated with ketamine for subsequent inoculations and sample collections. Macaques were infused with antibodies into the saphenous vein by slow (5-min duration) intravenous (i.v.) push at the dose specified. Where indicated, 6 h after antibody infusion animals were challenged atraumatically by insertion of a soft catheter into the vagina and injection of 1 ml (300 TCID50) of SHIV-BaL (kindly provided by Norman Letvin).
Pharmacokinetic analysis of infused antibody.
For PK studies, 4 macaques were infused with 25 mg/kg of body weight 2F5 IgG, and 2 macaques were infused with 25 mg/kg of intact 2F5 Fab fragment. For the vaginal challenge study, 5 macaques were infused with either 50, 25, or 5 mg/kg 2F5 IgG, and a further 5 macaques received no antibodies as a control group. In the 2F5 Fab challenge study, 4 animals received a dose of 25 mg/kg. For the PK studies, blood and mucosal samples were taken at 0, 4, 6, 12, 24, 48, and 72 h and 6 days after infusion. In the challenge studies, blood samples were collected at day −14, 6 h after infusion (day 0), and at 7, 14, and 21 days postinfusion.
Additional aliquots of plasma were taken before and 6 h after antibody infusion just prior to challenge and on weeks 1, 2, 3, 4, 6, 9, 12, 16, and 20 after challenge for later analysis of viral RNA loads. Plasma viral loads were analyzed using the quantitative branched DNA (bDNA) signal amplification assay, performed by Siemens Inc. For challenge studies, mucosal secretion samples were collected 6 h after antibody infusion just prior to challenge. Prior to sample collection, premoistened (100 μl PBS) Weck-cel surgical spears (Medtronic) were preweighed in spin-X centrifuge tubes (Costar). Mucosal samples were then collected by placement of a premoistened Weck-cel surgical spear into the vagina, rectum, or mouth for 5 min to allow soaking up of secretions. Subsequently, sponges were withdrawn and returned to the top chamber of the sterile spin-X centrifuge tube filter (0.22 μm) and weighed again before addition of 300 μl extraction buffer containing protease cocktail I and 10% sodium azide solution, followed by centrifugation for 15 min at 13,000 × g at 4°C to extract the Weck-cel sample from the top chamber. Samples were stored at −80°C until analysis.
Quantitative determination of antibodies.
All plasma samples were heat inactivated at 56°C for half an hour prior to analysis. 2F5 antibody concentrations in plasma and mucosal samples were determined by a direct 2F5-specific ELISA. High-binding 96-well ELISA plates (Nunc, United Kingdom) were coated with 100 μl per well of 1 μg/ml of the 2F5 specific epitope GGGLELDKWASL (Polymun, Austria) in PBS overnight at 4°C. Plates were washed 4 times with PBS containing 0.05% Tween (PBS-T). Plates were blocked with PBS-T containing 1% bovine serum albumin (BSA) (Sigma, United Kingdom) for 1 h at 37°C. Plates were washed 4 times with PBS-T. 2F5 IgG (Polymun, Austria) and 2F5 Fab (Polymun, Austria) at a starting concentration of 600 ng/ml followed by one in three serial dilutions were added to the plates as standards. Fifty-μl aliquots of sample dilutions were added to triplicates and incubated for 1 h at 37°C. 2F5 IgG and 2F5 Fab were detected with horseradish peroxidase (HRP)-conjugated goat anti-human IgG (Fc specific) (1:10,000) (Sigma, United Kingdom) or goat anti-human IgG (Fab specific) (1:5,000) (Sigma, United Kingdom), respectively, and incubated for 1 h at 37°C. After incubation, TMB developing reagent (SureBlue TMB 1-component peroxidase substrate; KPL) was added and incubated for 5 min at room temperature in the dark. The reaction was stopped by addition of 1N H2SO4. The plates were read at 450 nm with a FLUOstar Omega microplate reader (BMG Labtech, United Kingdom). Antibody concentrations were calculated using MARS data analysis software, which was provided with the microplate reader, and compared to the corresponding antibody standard curve. The antibody concentrations of mucosal samples were adjusted for dilution based on the volume of the Weck-cel according to weight. Antibody half-life (t1/2) rates were calculated by linear regression of log-transformed sample concentrations, as determined by ELISA, in relation to the number of days since assessed peak concentrations using GraphPad Prism4.
Enumeration of T/F variants.
Transmitted founder (T/F) viral sequences were inferred by single-genome sequencing as described by Keele et al. (44). Viral RNA was purified from the first viral RNA-positive plasma sample from each animal by a Qiagen QiaAmp viral RNA minikit and subjected to cDNA synthesis using 1× reaction buffer, 0.5 mM each deoxynucleoside triphosphate (dNTP), 5 mM dithiothreitol (DTT), 2 U/ml RNaseOUT, 10 U/ml of SuperScript III reverse transcription mix (Invitrogen), and 0.25 mM antisense primer SHIVBalEnvR1 (5′-CTG TAA TAA ATC CCT TCC AGT CC-3′; nucleotides [nt] 9458 to 9480 in SIVsmm239). The resulting cDNA was endpoint diluted in 96-well plates (Applied Biosystems, Inc.) and PCR amplified using high-fidelity platinum Taq DNA polymerase (Invitrogen) so that ≤30% of reactions were positive in order to maximize the likelihood of amplification from a single genome. A second round of PCR amplification was conducted using 1 μl of the first-round products as the template. SHIVBalEnvR1 and SIVsm/macEnvF1 (5′-CCT CCC CCT CCA GGA CTA GC-3′; nt 6127 to 6146 in SIVsmm239) were used in the first-round PCR amplification step, followed by a second round with primers envB5-in (5′-TTA GGC ATC TCC TAT GGC AGG AAG AAG-3′; nt 5960 to 5983 in HXB2) and BKSIVsm/macEnvR261 (5′-ATG AGA CAT RTC TAT TGC CAA TTT GTA-3′; nt 9413 to 9436 in SIVsmm239). PCR was carried out using 1× buffer, 2 mM MgSO4, 0.2 mM each dNTP, 0.2 μM each primer, and 0.025 U/μl platinum Taq high-fidelity polymerase (Invitrogen) in a 20-μl reaction mix. Round 1 amplification conditions were 1 cycle of 94°C for 2 min; 35 cycles of 94°C for 15 s, 58°C for 30 s, and 68°C for 4 min; and 1 cycle of 68°C for 10 min. Round 2 conditions were one cycle of 94°C for 2 min; 45 cycles of 94°C for 15 s, 58°C for 30 s, and 68°C for 4 min; and 1 cycle of 68°C for 10 min. Round 2 PCR amplicons were visualized by agarose gel electrophoresis and directly sequenced using an ABI 3730xl genetic analyzer (Applied Biosystems). Partially overlapping sequences from each amplicon were assembled and edited using Sequencher (Gene Codes, Inc.). Sequences with ≥2 double peaks, indicating amplification from multiple templates, were discarded. Sequences with one double peak were retained, as this most likely represents a Taq polymerase error in an early round of PCR rather than multiple-template amplification; such sequence ambiguities were read as the consensus nucleotide. Sequence alignments and phylogenetic trees were constructed using ClustalW, and Highlighter plots were created using the tool at http://www.lanl.gov.
RESULTS
Neutralization of HIV-1BaL in primary cells.
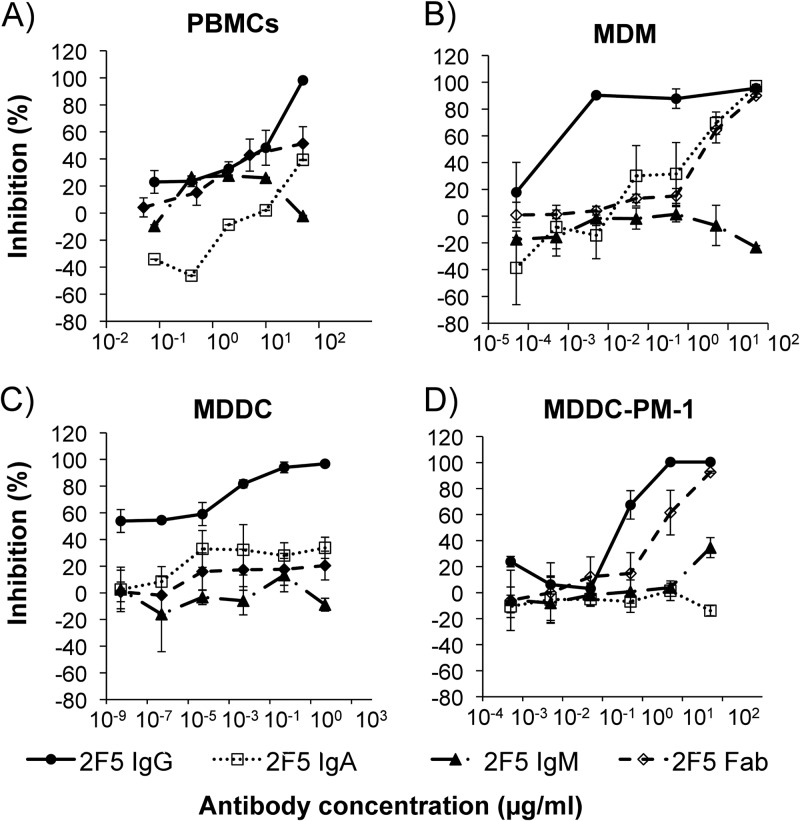
Activated CD4+ CCR5+ T cells are thought to be the primary targets of HIV infection in cervicovaginal tissue. The activity of 2F5 IgG, IgA, and IgM and the Fab fragment against infection of primary CD4+ T cells with HIV-1BaL was determined using PHA-stimulated PBMCs. 2F5 IgG showed potent inhibition, with 90 and 50% inhibitory concentrations (IC90 and IC50, respectively) of 41.6 and 10.52 μg/ml, respectively. 2F5 Fab presented an IC90 of >50 μg/ml and IC50 of 27.7 μg/ml. 2F5 IgA and IgM failed to display any inhibitory activity against HIV infection in human PBMCs (Fig. 1A and Table 1). HIV-1BaL was chosen for these studies to allow comparative assessment of the mucosal SHIV-BaL NHP challenge model. We assessed the inhibitory activity of 2F5 against both HIV-1BaL and SHIV-BaL in a TZM-bl neutralization assay. 2F5 showed similar levels of neutralization, with IC50 values of 17.3 ± 2.5 and 20.6 ± 0.3 μg/ml for HIV-1BaL and SHIV-BaL, respectively (data not shown).
Fig 1.
Inhibition of HIV-1BaL by 2F5 IgG, IgA, IgM, and Fab in human primary cells. The inhibitory activities of 2F5 IgG, IgA, IgM, and Fab were tested in PHA-stimulated PBMCs, macrophages, and MDDCs. 2F5 IgG, IgA, IgM, or Fab dilutions were incubated with HIV-1BaL for 1 h at 37°C prior to addition of 5 × 104 PBMCs (A), macrophages (B), or 4 × 104 MDDCs (C). After 2 h of incubation at 37°C, the cells were washed and incubated with medium in the presence of the antibodies for 7 days. (D) To determine inhibition of HIV-1 trans-infection by DCs to CD4+ T cells, 2 × 104 MDDCs were incubated with HIV-1BaL for 1 h at 37°C. After the incubation, the cells were washed and 2F5 IgG, IgA, IgM, or Fab dilutions together with 4 × 104 PM-1 T cells were added and incubated for 7 days. HIV-1 replication was assessed by measurement of p24 release into culture supernatant. Reduction in p24 levels was expressed as percent inhibition ± standard errors of the means (SEM) in relation to the corresponding isotype control. Data are represented as the means from three independent experiments, where each dilution was tested in triplicate.
Table 1.
Summary of the IC90 and IC50s of 2F5 IgG, IgA, IgM, and the 2F5 Fab fragment against HIV-1BaL infection in all in vitro cellular and ex vivo cervical tissue explant assays tested
| Sample | IC90 (μg/ml) |
IC50 (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|---|
| IgG | IgA | IgM | Fab | IgG | IgA | IgM | Fab | |
| PBMC | 41.7 ± 7.3 | >50 | >50 | >50 | 10.5 ± 4.9 | >50 | >50 | 27.7 ± 0.03 |
| MDM | 0.004 ± 1.9 | 28.2 ± 4.0 | >50 | 46.5 ± 8.5 | 0.0006 ± 0.04 | 1.2 ± 2.2 | >50 | 2.8 ± 2.3 |
| MDDCs | 1.4 ± 1.9 | >50 | >50 | >50 | <0.005 | >50 | >50 | >50 |
| MDDC-PM1 | 0.7 ± 0.05 | >50 | >50 | 32.4 ± 3.8 | 0.4 ± 0.05 | >50 | >50 | 3.1 ± 0.9 |
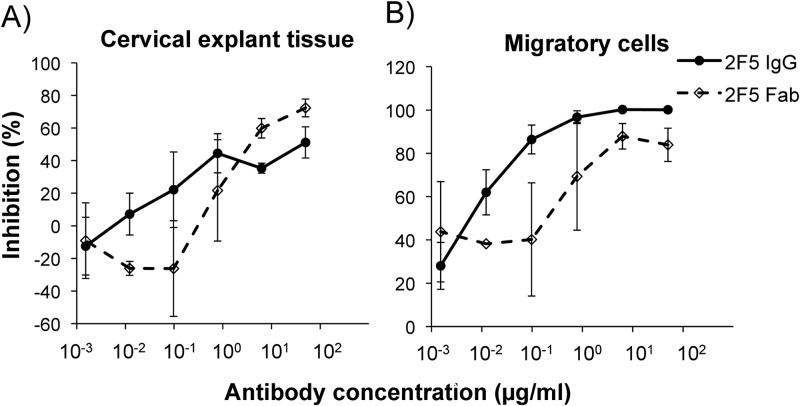
| Cervical tissue | >50 | >50 | >50 | >50 | 1.9 ± 0.4 | NDa | ND | 1.1 ± 2.8 |
| Migratory cells | 0.2 ± 0.2 | >50 | >50 | >50 | 0.006 ± 0.004 | ND | ND | 0.8 ± 0.0 |
ND, not done.
Macrophages are an important line of defense against pathogens. These cells are abundant in the cervicovaginal submucosa and are among the first cells exposed to HIV-1 infection. We found 2F5 IgG to potently inhibit HIV-1BaL infection of MDM, with IC90 and IC50s of 0.05 and 0.0001 μg/ml, respectively. 2F5 IgA and 2F5 Fab were able to inhibit infection of MDM; however, the 90 and 50% inhibitory concentrations of 2F5 IgA (28.17 and 1.24 μg/ml, respectively) and 2F5 Fab (46.53 and 2.75 μg/ml) were considerably higher than that of 2F5 IgG. No inhibitory activity could be observed when 2F5 IgM was used in the macrophage assay (Fig. 1B).
DCs are professional antigen-presenting cells (APCs) that reside at the sites of pathogen entry and can be directly infected by HIV-1, resulting in viral replication and long-term transmission to CD4+ T cells. Therefore, we assessed the inhibitory activity of 2F5 IgG, IgA, IgM, and Fab in MDDCs. Here, we show 2F5 IgG to significantly reduce infection, with an IC90 of 1.36 μg/ml and 96.7% inhibition at the highest concentration tested (50 μg/ml). No specific inhibition could be determined for 2F5 IgA, IgM, or Fab (Fig. 1C and Table 1). Another potential pathway of HIV-1 transmission is through DC-mediated trans-infection of CD4 T cells. Here, the C-type lectin receptor DC-SIGN and the sialic acid-binding Ig-like lectin 1 (Siglec-1) contribute to HIV-1 capture in immature DCs (45, 46), while in mature DCs capture of virus is independent of DC-SIGN but requires Siglec-1 (46, 47). After antigen uptake, DCs become activated and are thought to migrate from submucosal tissue to the draining lymph nodes, where they present virus in trans to CD4+ T cells through virological synapses (45). Moreover, in vitro HIV-1 T/F variants are captured by MDDCs and transferred to CD4+ T cells with higher efficiency than control viruses from chronic subjects (48). We assessed the ability of the different forms of 2F5 to prevent DC-mediated trans-infection of CD4 T cells. 2F5 IgG antibody was able to inhibit HIV-1 trans-infection by 100% at the highest concentration tested. Ninety and 50% inhibitory concentrations were 0.7 and 0.42 μg/ml, respectively. 2F5 Fab was able to inhibit HIV DC-mediated trans-infection of PM-1 T cells by 92.8% with an IC90 and IC50 of 32.4 and 3.14 μg/ml, respectively. 2F5 IgA and 2F5 IgM did not show any inhibitory activity against virus transmission to T cells (Fig. 1D). The observation that the inhibitory activity of 2F5 Fab was only 1/10 that of 2F5 IgG suggested that the Fc domain of 2F5 contributed substantially to the mechanism of inhibition in the DC-T cell cocultures. To test this possibility, we performed parallel studies with 2F5 IgG in the presence of blocking antibodies to the Fc-gamma receptors (Fcγ-Rs). Blocking the binding of 2F5 IgG to the Fcγ-Rs present on the cell surface of the DCs reduced the inhibitory activity of 2F5 to 1/10, matching the activity of 2F5 Fab (data not shown).
Activity of 2F5 IgG and Fab against HIV-1BaL infection of human ectocervical tissue explants.
To assess the ability of 2F5 IgG and 2F5 Fab to inhibit HIV-1BaL infection of the female reproductive tract tissue, a nonpolarized ectocervical tissue explant model was used (38–41). Both 2F5 IgG and 2F5 Fab showed inhibition at the highest concentration tested of 51 and 72% (Fig. 2A), with IC50s of 1.88 and 1.1 μg/ml, respectively, which were not significantly different (Table 1). Following overnight culture of cervical explant tissues, a proportion of DCs, able to bind HIV via lectin receptors and/or CD4, emigrate from explant tissue into the culture medium (41). These migratory cells were harvested from overnight cultures and cocultured with CD4+ PM-1 T cells in the presence of antibody (2F5 IgG or Fab). One hundred percent inhibition of HIV-1BaL transmission by migratory cells to PM-1 T cells was achieved with 2F5 IgG and 83% with 2F5 Fab at the highest concentration tested (50 μg/ml) (Fig. 2B). Ninety and 50% inhibitory concentrations were 0.16 and 0.006 μg/ml, respectively, for 2F5 IgG and were >50 (IC90) and 0.8 (IC50) μg/ml for Fab (Table 1).
Fig 2.
Ability of 2F5 to inhibit HIV-1 infection of cervical tissue and transmission by cells migrating out from cervical tissue explants. (A) 2F5 IgG and 2F5 Fab were preincubated with HIV-1BaL for 1 h at 37°C prior to being added to cervical tissue explants for 2 h. After washing, the explants were cultured in the presence of the antibodies for a further 14 days. (B) Twenty-four h after the initiation of explant culture, migratory cells that emigrate out of the tissue were recovered and cocultured with 4 × 104 PM-1 T cells in the presence of the antibody. HIV-1 infection was determined by p24 ELISA. Reduction in p24 levels was expressed as percent inhibition ± SEM in relation to the virus control. Data are represented as the means from three independent donors, where each dilution was tested in triplicate.
Pharmacokinetics study of infused monoclonal antibody 2F5 IgG in plasma and mucosal secretions of rhesus macaques.
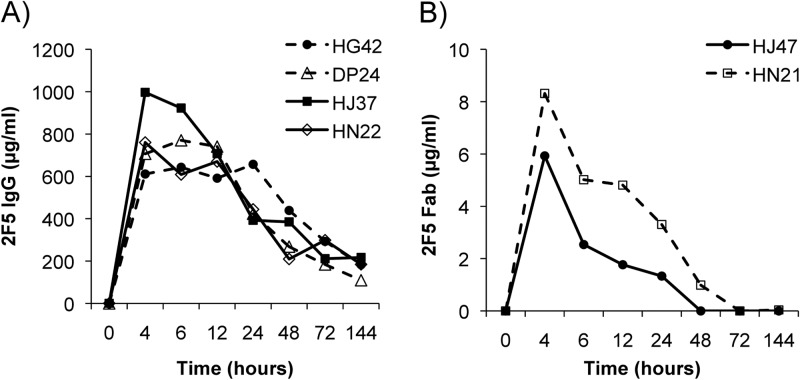
Prior to challenge studies, PK analysis was performed in female rhesus macaques after i.v. infusion of 2F5 IgG or 2F5 Fab in order to assess peak levels of the antibody in plasma and mucosal secretions and to determine the optimal time point for subsequent vaginal challenge. Four macaques were infused i.v. with 25 mg/kg of 2F5 IgG. Plasma samples were collected and 2F5 IgG concentrations determined. A peak systemic concentration of 2F5 IgG was observed 4 to 6 h after infusion, with mean concentrations of 768.8 (range, 611.3 to 997.0 μg/ml) and 736.2 μg/ml (range, 608.9 to 922.5 μg/ml), respectively (Fig. 3A). The mean systemic t1/2 for 2F5 IgG was determined to be 34.5 h (range, 20.1 to 64.1 h).
Fig 3.
Pharmacokinetics of infused monoclonal antibody 2F5 IgG or 2F5 Fab in plasma. Female Indian rhesus macaques were i.v. infused with 25 mg/kg of 2F5 IgG (A) or 2F5 Fab (B). Plasma samples were taken prior to infusion and 4, 6, 12, 24, 48, 72, and 144 h after infusion. The concentration of antibody in plasma (in μg/ml) was determined by direct ELISA and compared to a corresponding standard curve. Each sample was done in triplicate. The symbols in the key identify different macaques.
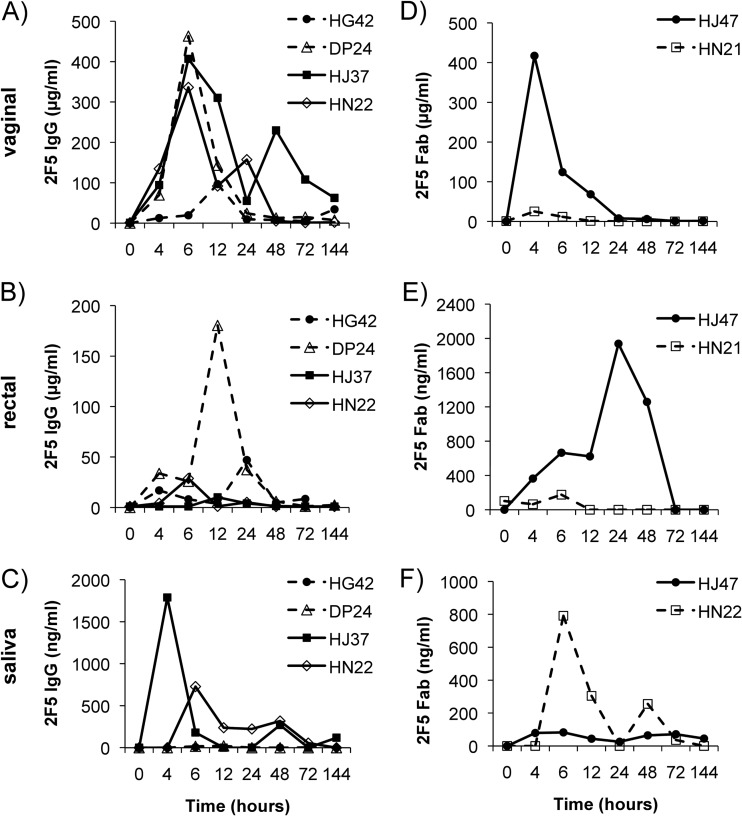
To determine the distribution of 2F5 IgG after passive infusion to different mucosal surfaces, vaginal and rectal secretions as well as saliva samples were collected from the same animals and analyzed for their 2F5 IgG concentrations. Four h after infusion, 2F5 IgG could be observed in vaginal secretions. However, peak concentrations were detected 6 h after infusion with a mean concentration of 306 μg/ml (range, 19.4 to 463.2 μg/ml) in three out of four macaques. The IgG levels for the fourth animal peaked 12 h after administration at lower levels of 96.6 μg/ml (Fig. 4A). In rectal secretions, low levels of 2F5 IgG with a peak concentration of 28.8 μg/ml 6 h after infusion for animal HN22, 10.2 and 180.4 μg/ml 12 h after administration for animals HJ37 and DP24, respectively, and a peak concentration of 47 μg/ml 24 h after infusion for animal HG42 (Fig. 4B) were found. 2F5 IgG could be detected in only 2 out of 4 animals in saliva, with a peak for one animal (HJ37) 4 h (1,789.2 ng/ml) and for the other (HN22) 6 h (728.3 ng/ml) after infusion (Fig. 4C).
Fig 4.
Pharmacokinetics of 2F5 IgG and 2F5 Fab in vaginal and rectal secretions and saliva following intravenous administration. Female Indian rhesus macaques were i.v. infused with 25 mg/kg of 2F5 IgG or 2F5 Fab. Mucosal secretions were collected by Weck-cel sponges prior to infusion and 4, 6, 12, 24, 48, 72, and 144 h after infusion. The concentrations (in μg/ml) of 2F5 IgG in vaginal secretions (A), rectal secretions (B), and saliva (C) and of 2F5 Fab in vaginal secretions (D), rectal secretions (E), and saliva (F) were determined by direct ELISA (each sample in triplicate) and compared to a corresponding standard curve. The symbols in the key identify different macaques.
Systemic and mucosal pharmacokinetics of Fab fragments of 2F5 IgG.
To compare the systemic and mucosal PK of Fab fragments to those of intact IgG 2F5, two female Indian rhesus macaques were infused with 25 mg/kg 2F5 Fab. A mean peak concentration of 7.1 μg/ml (range, 5.9 to 8.3 μg/ml) 2F5 Fab in plasma was observed at 4 h after infusion (Fig. 3B). The mean systemic t1/2 for 2F5 Fab was assessed to be 9.5 h (6.5 and 12.5 h). A peak of 2F5 Fab (417 μg/ml) could be detected in macaque HJ47 in vaginal secretions 4 h after infusion, whereas in the second animal (HN21) only very low levels of vaginal 2F5 Fab could be detected, with a peak at 4 h after infusion (Fig. 4D). In rectal samples, animal HJ47 had a peak concentration of 2F5 Fab (1,937.8 ng/ml) 24 h after infusion, while in animal HN21 2F5 Fab peaked 6 h after infusion, with a very low concentration of 175.8 ng/ml (Fig. 4E). In saliva, 2F5 Fab could be observed in only one monkey (animal HN21), with a peak at 6 h after infusion (791.6 ng/ml) (Fig. 4F).
Correlation of 2F5 IgG concentrations in plasma and mucosal secretions of rhesus macaques against protection following vaginal challenge with SHIV-BaL.
To correlate protection against vaginal SHIV-BaL challenge to the 2F5 antibody levels after infusion, 5 macaques each were infused with 50, 25, 5, or 0 mg/kg of 2F5 IgG 6 h prior to challenge. Plasma was taken at various time points and analyzed for viral RNA levels and 2F5 IgG concentration. Vaginal, rectal, and saliva samples were taken only at the time of challenge.
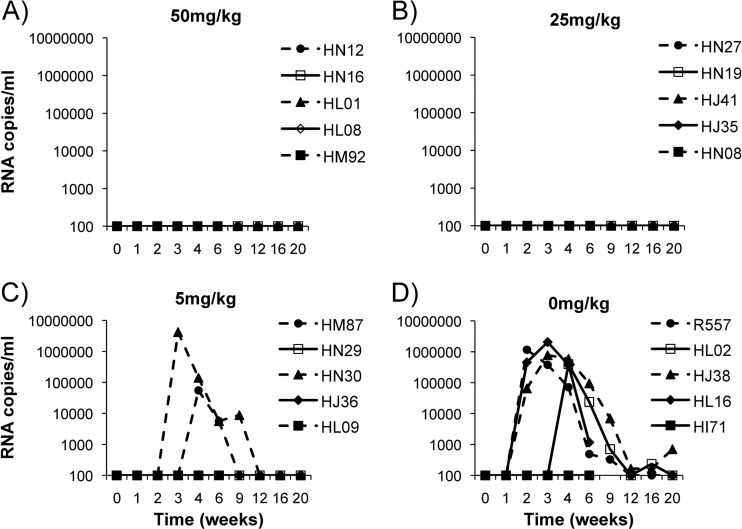
In the group receiving 50 mg/kg 2F5 IgG, all five macaques were completely protected from SHIV-BaL challenge, as were animals receiving 25 mg/kg 2F5 IgG (Fig. 5A and B). Three out of five animals were protected in the group infused with 5 mg/kg 2F5 IgG. Two animals became infected: one animal (HN30) displayed peak viral loads 21 days after challenge that were cleared by 63 days, while the second animal (HM87) displayed a peak viral load 28 days after challenge, which cleared by day 84 (Fig. 5C). The peak viral load for animal HM87 was 1 log lower than that of infected control animals. In the control group receiving no antibody, four out of five animals became infected, while in one animal (HI71) all samples were negative for viral RNA (Fig. 5D).
Fig 5.
Plasma viral loads after vaginal SHIV-BaL challenge. Female rhesus macaques were i.v. infused with 50 mg/kg (A), 25 mg/kg (B), 5 mg/kg (C), and 0 mg/kg (D) of 2F5 IgG. Six h after antibody infusion, macaques were vaginally challenged with SHIV-BaL. Serum samples were taken at week 0 (6 h after infusion and just prior to challenge) and 1, 2, 3, 4, 6, 9, 12, 16, and 20 weeks postchallenge. Plasma was analyzed for viral RNA levels.
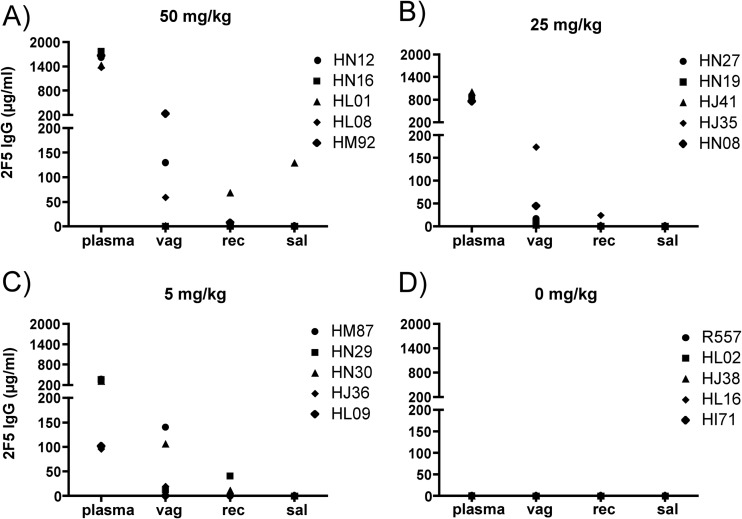
A dose-dependent PK of 2F5 IgG in plasma could be observed between the groups with peak antibody levels at 6 h after infusion (Fig. 6). Here, the groups infused with 50, 25, or 5 mg/kg of 2F5 IgG showed mean plasma peak levels of 1,573 μg/ml (range, 1,373.9 to 1,774 μg/ml), 858.4 μg/ml (range, 756 to 1,003.8 μg/ml), and 249.6 μg/ml (range, 96.3 to 370.8 μg/ml), respectively (Fig. 6A, B, and C). The mean 2F5 levels in vaginal secretions at the time of challenge for animals infused with 50, 25, or 5 mg/kg were 84.6 μg/ml (range, 0.5 to 232.4 μg/ml), 49.7 μg/ml (range, 3.5 to 174.3 μg/ml), and 55.9 μg/ml (range, 0.2 to 140.8 μg/ml), respectively (Fig. 7A, B, and C). At the time of challenge, 2F5 IgG levels in rectal secretions were very low, with mean levels of 16.3 μg/ml (range, 0.3 to 68.4 μg/ml), 5.5 μg/ml (range, 0 to 24.5 μg/ml), and 11 μg/ml (range, 0 to 41.1 μg/ml) for macaques infused with 50, 25, or 5 mg/kg of 2F5 IgG, respectively (Fig. 7A, B, and C). 2F5 IgG levels in saliva were very low, with 0.3 μg/ml in two macaques infused with 50 mg/kg and 0.1 μg/ml in one animal infused with 5 mg/kg, while no 2F5 IgG could be detected in the group infused with 25 mg/kg (Fig. 7A, B, and C).
Fig 6.
Pharmacokinetics of infused 2F5 IgG after SHIV-BaL challenge. Each group consists of five female Indian rhesus macaques. Animals were i.v. infused with 50 (A), 25 (B), or 5 (C) mg/kg 2F5 IgG or received no antibodies as a negative-control group (D). Six h after infusion, all macaques were challenged intravaginally with SHIV-BaL (day 0 indicates the time point 6 h after infusion). Plasma samples were taken prior to infusion, 6 h after infusion (time point of intravaginal SHIV-BaL challenge), and 7, 14, and 21 days after infusion. The concentration of antibody in plasma (in μg/ml) was determined by direct ELISA and compared to a corresponding standard curve. Each sample was analyzed in triplicate. The symbols in the key identify different macaques.
Fig 7.
Comparison of 2F5 IgG levels in plasma and mucosal secretions at time of challenge. Each group consists of five female Indian rhesus macaques. Animals were i.v. infused with 50 (A), 25 (B), or 5 (C) mg/kg 2F5 IgG or received no antibodies as a negative-control group (E). Plasma, vaginal (vag) and rectal (rec) secretion, and saliva (sal) samples were taken 6 h after infusion and just prior to intravaginal challenge with SHIV-BaL. The concentration of antibody in plasma (in μg/ml) was determined by direct ELISA and compared to a corresponding standard curve. Each sample was done in triplicate. The symbols in the key identify different macaques.
2F5 Fab fails to protect rhesus macaques from vaginal challenge with SHIV-BaL.
To determine the efficacy of the 2F5 Fab fragment to protect from vaginal challenge, macaques were infused with 25 mg/kg 6 h prior to SHIV-BaL challenge. Plasma, vaginal, rectal, and saliva samples were analyzed for 2F5 Fab concentrations and for plasma viral loads.
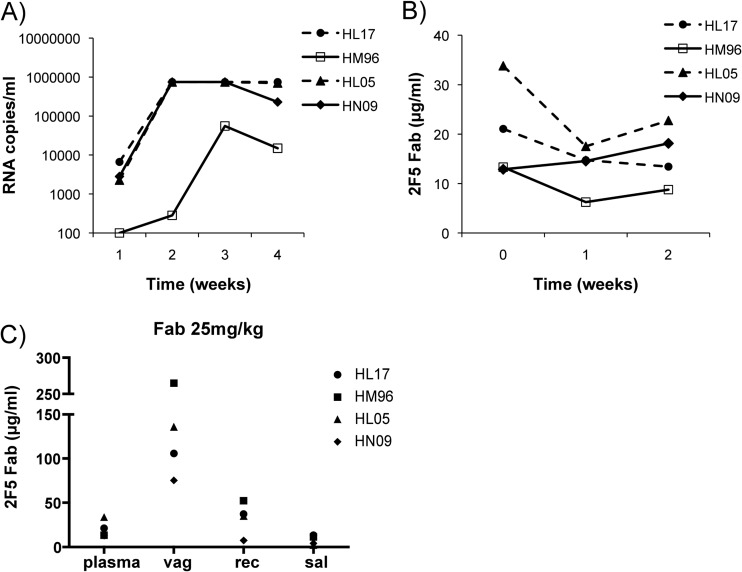
In macaques infused with 25 mg/kg 2F5 Fab, three out of four animals were infected and displayed detectable viral loads by 7 days, with peaks by day 14. A fourth animal had detectable viral RNA by 14 days, with a peak by 21 days (Fig. 8A). In this respect, there appeared to be no major difference in the kinetics or magnitude of viremia relative to those of untreated controls.
Fig 8.
Correlation of 2F5 Fab concentrations and protection following vaginal challenge with SHIV-BaL. Four female rhesus macaques were i.v. infused with 25 mg/kg of 2F5 Fab. Six h after antibody infusion, macaques were vaginally challenged with SHIV-BaL. Serum samples were taken prior to infusion, 6 h after infusion (time point of intravaginal SHIV-BaL challenge), and 7, 14, and 21 days after infusion and analyzed for viral RNA levels (A) and for 2F5 Fab concentration (B). (C) Comparison of 2F5 Fab levels in plasma, vaginal and rectal secretion, and saliva samples at the time of challenge. Antibody levels (in μg/ml) were determined by direct ELISA and compared to a corresponding standard curve. Each sample was tested in triplicate. The symbols in the key identify different macaques.
Low 2F5 Fab antibody levels were detected in the plasma of all animals (Fig. 8B). Six h after infusion, high levels of 2F5 Fab (145.5 μg/ml; range, 75.4 to 265 μg/ml) could be observed in vaginal secretions (Fig. 8C). Only low mean levels of 2F5 Fab were detected in rectal secretions (33.1 μg/ml; range, 7.5 to 52.4 μg/ml) and in saliva (7.7 μg/ml; range, 2.1 to 13.4 μg/ml) 6 h after infusion (Fig. 8C).
Identification and enumeration of T/F viruses in control and 2F5 IgG-infused rhesus macaques.
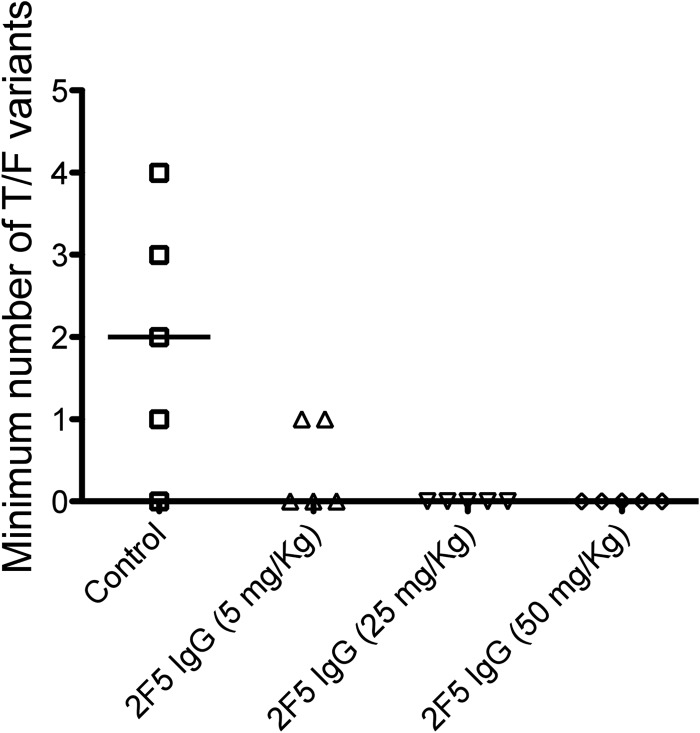
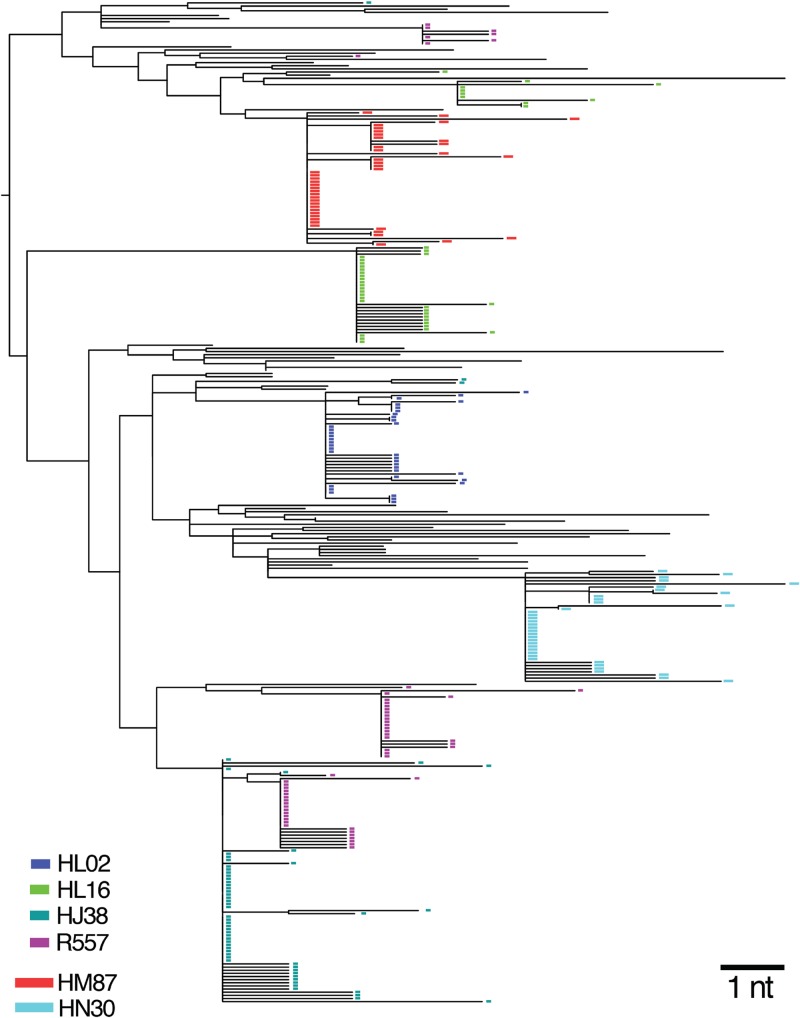
Mucosal HIV-1 infection in humans typically results from transmission and productive replication by 1 to as many as 6 or more T/F viruses (median, 1) (49–52). To establish how closely the SHIV-BaL rhesus macaque vaginal inoculation model recapitulates the human transmission bottleneck, we estimated the number of T/F variants that caused infection in control and 2F5-treated animals. A total of 279 full-length env sequences (total of 3,221 nucleotides) were generated by single-genome sequencing from the earliest viral RNA+ time point in the 6 infected animals. In each animal, low-diversity sequence lineages were observed, reflecting virus diversification from discrete T/F genomes, as previously described for SIVsmmE660- and SIVmac251-inoculated rhesus macaques (44). Maximum, median, and mean percent sequence diversity for each T/F lineage was 0.16, 0.01, and 0.03, which was significantly lower than corresponding values for the SHIV-BaL inoculum stock (0.6, 0.3, and 0.3; P < 0.004 for each). Clustered sequences followed a Poisson distribution of infrequent, independent, and essentially stochastic events, again as described for SIVsmmE660, SIVmac251, and HIV-1 mucosal infections (44, 49, 51–53). Figure 9 summarizes the T/F analyses, and Fig. 10 shows a neighbor-joining phylogenetic tree with relationships between SHIV-BaL inoculum sequences and variants that infected each rhesus macaque. Infections were initiated in control animals by a minimum of 1 to 4 T/F variants, with an overall median of 2. A single T/F variant initiated productive infection in each of the two animals treated with 5 mg/kg 2F5 IgG. Antibody treatment was significantly correlated with a reduction in numbers of T/F viruses when all animals in all groups were considered (P = 0.01393 by Fisher's exact test).
Fig 9.
Multiplicity of SHIV-BaL infection in rhesus macaques. Numbers of T/F viruses were estimated from analysis of the first plasma viral RNA-positive plasma sample from each infected rhesus macaque by single-genome sequencing. In untreated animals, numbers of T/F viruses ranged from 0 to 4 with a median of 2. In 2F5 IgG-treated animals, there was a significant dose-related reduction in numbers of T/F viruses establishing productive clinical infection (P = 0.01393 by Fisher's exact test).
Fig 10.
Neighbor-joining phylogenetic tree of SHIV-BaL sequences from the inoculum and from each of 6 infected animals. The tree reveals discrete low-diversity sequence lineages corresponding to the progeny of T/F viral genomes (colored rectangles) interspersed among diverse sequences from the virus inoculum represented by black lines. Sequences with G-A hypermutation were excluded from the tree.
DISCUSSION
Neutralizing antibodies may provide significant levels of protection against HIV-1 acquisition if present in mucosal secretions. Indeed, we and others have previously shown that topical administration of neutralizing antibodies can protect against vaginal challenge in a dose-dependent manner (9, 10). These data demonstrate that the presence of neutralizing antibodies at the mucosal portals of viral entry is sufficient to prevent viral acquisition. However, it is unclear how topical application recapitulates the normal distribution of serum-derived antibodies at mucosal surfaces. Here, we evaluated the systemic and mucosal pharmacokinetics (PK) and pharmacodynamics (PD), with respect to protection against vaginal challenge with SHIV-BaL, of 2F5 IgG and 2F5 Fab fragments in an infused macaque model.
In vitro neutralization studies demonstrated that 2F5 IgG was more potent against HIV-1BaL than polymeric IgM or IgA in all culture models. Indeed, 2F5 IgM had no inhibitory activity, while 2F5 IgA was only active in macrophage cultures. These results confirm and expand previous studies demonstrating that the activity of 2F5 against different primary HIV-1 isolates in PBMCs was reduced when class switched to polymeric IgM or IgA (54). The poor performance of the polymeric forms of 2F5 likely reflects structural constraints imposed by their multimeric form that prevent access to the 2F5 epitope in the prehairpin intermediate (22). Indeed, recent data have shown that an IgA2 monomeric version of 2F5 displays neutralizing activity comparable to that of IgG (55). It is likely that the more compact structure of IgA2 than that of IgA1 preserves the ability to protrude into the MPER and bind the 2F5 epitope.
2F5 IgG was most potent against infection of MDM, although this was reduced in the presence of serum, as previously described (56). Comparison of 2F5 IgG and Fab highlighted the important influence of Fc-mediated effects in the different in vitro models. Here, the most pronounced differences in activity were with MDM and MDDC cultures and for DC-mediated trans-infection, whether assessing activity against MDDC-T cell cultures or migratory cells from cervical explants. Blocking experiments confirmed the importance of Fcγ-R engagement by 2F5 in inhibiting HIV replication in MDDC-T cell cocultures. These data are in agreement with previous studies showing 2F5 IgG as highly active in preventing HIV-1 infection of immature MDM and MDDCs relative to 2F5 Fab (25–27, 57) and the importance of Fcγ-R engagement in reducing DC-mediated trans-infection of CD4 T cells (58). Interestingly, the inhibitory activities (IC50) of 2F5 IgG and Fab were similar in cervical explant cultures despite such tissue containing a high density of macrophages and dendritic cells. This likely reflects previous observations that CD4 T cells represent the primary targets of infection in cervical tissue (59).
Initial PK studies indicated that peak systemic 2F5 IgG concentrations were achieved at 4 to 6 h postinfusion, with a half-life of 34 h, and were still detectable after 144 h (Fig. 3A). Serum levels of 2F5 Fab were a hundred-fold lower than those for 2F5 IgG, with a half-life of 9.5 h, and were undetectable 72 h postinfusion (Fig. 3B). The observed differences likely are due to a number of factors. Fab fragments in murine models have been shown to have a half-life of 3 to 5 h (60, 61) and are known to be distributed more rapidly and to be cleared from the body significantly faster than whole IgG1 (60, 62). This most likely reflects binding of the Fc portion of infused IgG to endothelial FcRn, facilitating its recycling and ultimately protecting it from degradation and removal by hepatic first-pass metabolism (63–67). Indeed, systemic clearance of IgG in FcRn knockout mice is significantly increased, suggesting that the serum half-life of IgG is dependent on binding affinity to FcRn (63, 65, 66, 68). Thus, it is likely that in the absence of any Fc domain, circulating Fab fragments were cleared more efficiently by the renal system, reducing systemic residency time and overall protective effects. We have not assessed the interaction of human 2F5 IgG with macaque FcRn; however, the reported serum half-life of 2F5 IgG in infused humans is calculated to be 3.2 days (69), significantly longer than that observed here in macaques. Differences in binding between human and macaque FcRn offers one explanation for the differences in the serum half-lives of 2F5 IgG in these different species.
Originally, the function of FcRn was described in the neonatal intestine of mice and rats (70); however, FcRn is also expressed in adult human intestinal epithelial cells (64). More recently it has been shown that human uterine and vaginal tissue cells express FcRn (71) and can facilitate bidirectional IgG transcytosis across female genital epithelial cells. Thus, FcRn may have contributed to the mucosal PK of 2F5 IgG but not to that of 2F5 Fab. Nevertheless, both 2F5 IgG and Fab were detectable at several mucosal surfaces (vaginal, rectal, and oral) (Fig. 4). This is in keeping with previous studies showing that infused monoclonal antibodies are distributed to a range of mucosal compartments after passive infusion (5).
Vaginal challenge studies demonstrated a dose-dependent protection for 2F5 IgG. Animals receiving 50 or 25 mg/kg IgG were completely protected, with 3/5 of animals that received 5 mg/kg being protected. In the four control animals, infection was established with a minimum of 1 to 4 T/F variants; however, infection was initiated with only a single T/F variant in the two infected animals that had received 5 mg 2F5 IgG. This suggests that while 2F5 IgG levels were below the threshold required for sterilizing protection in these two animals, levels may have been sufficient to affect the number of transmitted isolates. Similar dose-dependent protection has been reported previously for b12 IgG (6) when challenged vaginally with SHIVSF162P4. These results are in accord with studies looking at sexual transmission of HIV-1, where at least 20% of infections are established by two or more viruses, analogous to our control animals (51, 72), while in 80% of cases infection is established with a single T/F virus (50, 52). These data suggest that while the challenge dose chosen for these experiments is at the higher end of the range observed in humans, it is physiologically relevant.
Importantly, we observed that 25 mg/kg 2F5 Fab provided no protection despite higher vaginal levels at the time of challenge than were observed in protected animals infused with 25 and 5 mg/kg 2F5 IgG. In contrast, systemic levels of 2F5 Fab at the time of challenge were far lower than those in protected animals receiving 25 and 5 mg/kg 2F5 IgG. Interestingly, the three protected animals infused with 5 mg/kg 2F5 IgG had lower vaginal antibody levels at the time of challenge (0.2 to 19 μg/ml) than the two infected animals (107 and 141 μg/ml). These data together suggest that luminal concentrations of 2F5 are a poor predictor of protective efficacy. Importantly, in a viral capture assay, 2F5 was unable to bind a detectable fraction of virions as determined by p27 antigen or infectivity. These data fit with the proposed model where the 2F5 neutralizing epitope is occluded on functional envelope spikes prior to CD4 binding (22–24). Previous studies have suggested that the 2F5 epitope is critical to the binding and transcytosis of virions across mucosal epithelia (31). Lack of interaction between 2F5 IgG and most, if not all, infectious virions suggests that 2F5 IgG would be highly unlikely to affect putative transcytosis of infectious SHIV-BaL. Indeed, previous studies have shown that the BaL envelope does not facilitate transcytosis (73). Therefore, these data suggest that transcytosis is unlikely to be a prerequisite for effective mucosal transmission in this model.
As initial interaction between virus and susceptible target cells is predicted to occur within mucosal tissue, our results suggest that tissue antibody levels are a more important biomarker of protective efficacy. The mechanism governing partitioning of plasma antibody into lower genital tract tissue has not been defined. Convection and diffusion are thought to be the predominant mechanism for transvascular transport of IgG in many tissues, while murine studies suggest that active transport by FcRn plays a more predominant role in tissues such as skin and muscle (74). While IgG subclass distribution for many mucosal secretions reflects a purely transudative origin in humans, there appears to be an active accumulation of total IgG (IgG1, IgG2, and IgG4) in the lower female genital tract, resulting in its known predominance over total IgA (75). The extent to which FcRn regulates partitioning of plasma antibody into lower genital tract tissue merits further investigation. Indeed, decreased protection has previously been observed for b12 IgG when Fc receptor binding activity was mutated (76). It is unclear whether these mutations impaired FcRn binding. While our data confirm the importance of the IgG Fc portion in protection against infectious challenge, Fc-mediated inhibition did not appear to play a significant role in the inhibitory activity of 2F5 within cervical explants despite a predominance of DC and MDM populations within these tissues (77). These data suggest that Fc-mediated antiviral activity is unlikely to have affected initial infection of CD4 T cells. This confirms previous studies showing that other anti-gp41 monoclonal antibodies with potent Fc-inhibitory activity had no impact on acquisition of infection (9) despite evidence for interaction between human IgG and macaque Fc gamma receptors (1, 9, 78). However, the Fc portion of 2F5 IgG did affect dissemination of virus by migratory cells emigrating from tissue explants. This reflects earlier observations that binding of neutralizing antibodies to Fcγ-Rs on DC significantly augments the inhibition of HIV replication in DC-CD4 T cell clusters (58). This mechanism might affect viral dissemination in vivo and have an influence on peak viral load or subsequent viral set point. Although the two infected animals treated with 5 mg/kg 2F5 appeared to be infected with a single T/F virus, as opposed to 1 to 4 T/F viruses in the controls, more animals would be needed to draw firm conclusions about viral control. Interestingly, serum levels of 2F5 in fully protected animals infused with 25 mg/kg were 20 times the in vitro IC90 in PBMC assays at the time of challenge, while vaginal levels where at or just above the IC90. These systemic levels are remarkably similar to those reported for 2F5 IgG as being protective against rectal challenge with the same virus (26 times the IC90) (8), suggesting that systemic levels are predictive of protection in the different mucosal compartments.
In conclusion, data from the described passive infusion studies demonstrate that serum levels of the monoclonal 2F5 IgG antibody were predictive of sterilizing protection in primates in a dose-dependent manner. Fc-mediated antiviral activity did not appear to influence infection of the primary target cells in cervical explants. However, PK studies highlighted the importance of the Fc portion of IgG in tissue biodistribution, reflecting the known predominance of total IgG over IgA in the lower female genital tract. Furthermore, the rapid decay in systemic 2F5 levels suggests that a constant level of antibody is required to maintain protective levels against viral infection. Here, the shorter half-life of 2F5 IgG in macaques than in humans, potentially influenced by differences in binding to macaque FcRn, is likely to have caused an underestimation of its protective effects. Furthermore, additional gains in protective efficacy are likely to be provided with the more potent, recently described 10E8 MPER antibody that can neutralize >98% of tested viruses (79). Data presented in this study may be important in modeling serum levels of neutralizing IgG that need to be achieved by either vaccination or passive infusion to prevent mucosal acquisition of HIV-1 infection in humans.
ACKNOWLEDGMENTS
Research for this publication was supported by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Division of AIDS (DAIDS), U.S. Department of Health and Human Services (HHS), Center for HIV/AIDS Vaccine Immunology (CHAVI) U19 AI067854-05, and the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery, grant number UM1-AI100645-01. We gratefully acknowledge an equipment grant from Dormeur Investment Service Ltd. that provided funding to purchase equipment used in these studies.
The content is solely our responsibility and does not necessarily represent the official views of the National Institutes of Health.
We also acknowledge Asna Siddiqui, who acted as project manager for this study. We are grateful for the generous contribution of 2F5 IgA and IgM by Dietmar Katinger of Polymun.
Footnotes
Published ahead of print 21 August 2013
REFERENCES
- 1.Haase AT. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5:783–792 [DOI] [PubMed] [Google Scholar]
- 2.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 [DOI] [PubMed] [Google Scholar]
- 3.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5:e1000433. 10.1371/journal.ppat.1000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210 [DOI] [PubMed] [Google Scholar]
- 6.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu W, Hofmann-Lehmann R, McClure HM, Ruprecht RM. 2002. Passive immunization with human neutralizing monoclonal antibodies: correlates of protective immunity against HIV. Vaccine 20:1956–1960 [DOI] [PubMed] [Google Scholar]
- 8.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci. U. S. A. 108:11181–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moog C, Dereuddre-Bosquet N, Teillaud JL, Biedma ME, Holl V, VanHam G, Heyndrickx L, Van Dorsselaer V, Katinger D, Vcelar B, Zolla-Pazner S, Mangeot I, Kelly C, Shattock RJ, Le Grand R. 2013. Protective effect of vaginal application of neutralizing and non-neutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. [Epub ahead of print.] 10.1038/mi.2013.23 [DOI] [PubMed] [Google Scholar]
- 10.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 9:343–346 [DOI] [PubMed] [Google Scholar]
- 11.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, et al. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 10:359–369 [DOI] [PubMed] [Google Scholar]
- 12.Burton DR, Pyati J, Koduri R, Sharp SJ, Thornton GB, Parren PW, Sawyer LS, Hendry RM, Dunlop N, Nara PL, Lamacchia M, Garratty E, Stiehm ER, Bryson YJ, Cao Y, Moore JP, Ho DD, Barbas CF., III 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027 [DOI] [PubMed] [Google Scholar]
- 13.Stiegler G, Kunert R, Purtscher M, Wolbank S, Voglauer R, Steindl F, Katinger H. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 17:1757–1765 [DOI] [PubMed] [Google Scholar]
- 14.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazanec MB, Nedrud JG, Kaetzel CS, Lamm ME. 1993. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today 14:430–435 [DOI] [PubMed] [Google Scholar]
- 17.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 18.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunert R, Steinfellner W, Purtscher M, Assadian A, Katinger H. 2000. Stable recombinant expression of the anti HIV-1 monoclonal antibody 2F5 after IgG3/IgG1 subclass switch in CHO cells. Biotechnol. Bioeng. 67:97–103 [DOI] [PubMed] [Google Scholar]
- 20.Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232–13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakrabarti BK, Walker LM, Guenaga JF, Ghobbeh A, Poignard P, Burton DR, Wyatt RT. 2011. Direct antibody access to the HIV-1 membrane-proximal external region positively correlates with neutralization sensitivity. J. Virol. 85:8217–8226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, Rits-Volloch S, Sun L, Harrison SC, Haynes BF, Chen B. 2009. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U. S. A. 106:20234–20239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frey G, Peng H, Rits-Volloch S, Morelli M, Cheng Y, Chen B. 2008. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U. S. A. 105:3739–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guenaga J, Wyatt RT. 2012. Structure-guided alterations of the gp41-directed HIV-1 broadly neutralizing antibody 2F5 reveal new properties regarding its neutralizing function. PLoS Pathog. 8:e1002806. 10.1371/journal.ppat.1002806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holl V, Hemmerter S, Burrer R, Schmidt S, Bohbot A, Aubertin AM, Moog C. 2004. Involvement of Fc gamma RI (CD64) in the mechanism of HIV-1 inhibition by polyclonal IgG purified from infected patients in cultured monocyte-derived macrophages. J. Immunol. 173:6274–6283 [DOI] [PubMed] [Google Scholar]
- 26.Holl V, Peressin M, Decoville T, Schmidt S, Zolla-Pazner S, Aubertin AM, Moog C. 2006. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J. Virol. 80:6177–6181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holl V, Peressin M, Schmidt S, Decoville T, Zolla-Pazner S, Aubertin AM, Moog C. 2006. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood 107:4466–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez LG, Zolla-Pazner S, Montefiori DC. 2013. Antibody-dependent, FcgammaRI-mediated neutralization of HIV-1 in TZM-bl cells occurs independently of phagocytosis. J. Virol. 87:5287–5290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tudor D, Bomsel M. 2011. The broadly neutralizing HIV-1 IgG 2F5 elicits gp41-specific antibody-dependent cell cytotoxicity in a FcgammaRI-dependent manner. AIDS 25:751–759 [DOI] [PubMed] [Google Scholar]
- 30.Chakrabarti BK, Pancera M, Phogat S, O'Dell S, McKee K, Guenaga J, Robinson J, Mascola J, Wyatt RT. 2011. HIV type 1 Env precursor cleavage state affects recognition by both neutralizing and nonneutralizing gp41 antibodies. AIDS Res. Hum. Retrovir. 27:877–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bomsel M, Heyman M, Hocini H, Lagaye S, Belec L, Dupont C, Desgranges C. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9:277–287 [DOI] [PubMed] [Google Scholar]
- 32.Shen R, Drelichman ER, Bimczok D, Ochsenbauer C, Kappes JC, Cannon JA, Tudor D, Bomsel M, Smythies LE, Smith PD. 2010. GP41-specific antibody blocks cell-free HIV type 1 transcytosis through human rectal mucosa and model colonic epithelium. J. Immunol. 184:3648–3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao HX, Levesque MC, Nagel A, Dixon A, Zhang R, Walter E, Parks R, Whitesides J, Marshall DJ, Hwang KK, Yang Y, Chen X, Gao F, Munshaw S, Kepler TB, Denny T, Moody MA, Haynes BF. 2009. High-throughput isolation of immunoglobulin genes from single human B cells and expression as monoclonal antibodies. J. Virol. Methods 158:171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicely NI, Dennison SM, Spicer L, Scearce RM, Kelsoe G, Ueda Y, Chen H, Liao HX, Alam SM, Haynes BF. 2010. Crystal structure of a non-neutralizing antibody to the HIV-1 gp41 membrane-proximal external region. Nat. Struct. Mol. Biol. 17:1492–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. 2004. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J. Virol. 78:10724–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunert R, Ruker F, Katinger H. 1998. Molecular characterization of five neutralizing anti-HIV type 1 antibodies: identification of nonconventional D segments in the human monoclonal antibodies 2G12 and 2F5. AIDS Res. Hum. Retrovir. 14:1115–1128 [DOI] [PubMed] [Google Scholar]
- 37.Johnson V, Byington R. 1990. Quantitative assay for virus infectivity: infectivity assay (virus yield assay), p 71–76 In Aldovini A, Walker BD. (ed), Techniques in HIV research. Stockton Press, New York, NY [Google Scholar]
- 38.Balzarini J, Van Laethem K, Hatse S, Froeyen M, Peumans W, Van Damme E, Schols D. 2005. Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV GP120: a new therapeutic concept to hit the Achilles heel of HIV. J. Biol. Chem. 280:41005–41014 [DOI] [PubMed] [Google Scholar]
- 39.Fletcher P, Kiselyeva Y, Wallace G, Romano J, Griffin G, Margolis L, Shattock R. 2005. The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. J. Virol. 79:11179–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 74:5577–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu Q, Frank I, Williams V, Santos JJ, Watts P, Griffin GE, Moore JP, Pope M, Shattock RJ. 2004. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J. Exp. Med. 199:1065–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 43.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084–1089 [DOI] [PubMed] [Google Scholar]
- 44.Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, Mascola JR, Nabel GJ, Haynes BF, Bhattacharya T, Perelson AS, Korber BT, Hahn BH, Shaw GM. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206:1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597 [DOI] [PubMed] [Google Scholar]
- 46.Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, Pino M, Erkizia I, Glass B, Clotet B, Keppler OT, Telenti A, Krausslich HG, Martinez-Picado J. 2012. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 10:e1001448. 10.1371/journal.pbio.1001448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu X, Reinhard BM, Gummuluru S. 2013. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog. 9:e1003291. 10.1371/journal.ppat.1003291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, Hora B, Berg A, Cai F, Hopper J, Denny TN, Ding H, Ochsenbauer C, Kappes JC, Galimidi RP, West AP, Jr, Bjorkman PJ, Wilen CB, Doms RW, O'Brien M, Bhardwaj N, Borrow P, Haynes BF, Muldoon M, Theiler JP, Korber B, Shaw GM, Hahn BH. 2013. Phenotypic properties of transmitted founder HIV-1. Proc. Natl. Acad. Sci. U. S. A. 110:6626–6633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abrahams MR, Anderson JA, Giorgi EE, Seoighe C, Mlisana K, Ping LH, Athreya GS, Treurnicht FK, Keele BF, Wood N, Salazar-Gonzalez JF, Bhattacharya T, Chu H, Hoffman I, Galvin S, Mapanje C, Kazembe P, Thebus R, Fiscus S, Hide W, Cohen MS, Karim SA, Haynes BF, Shaw GM, Hahn BH, Korber BT, Swanstrom R, Williamson C. 2009. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J. Virol. 83:3556–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haaland RE, Hawkins PA, Salazar-Gonzalez J, Johnson A, Tichacek A, Karita E, Manigart O, Mulenga J, Keele BF, Shaw GM, Hahn BH, Allen SA, Derdeyn CA, Hunter E. 2009. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 5:e1000274. 10.1371/journal.ppat.1000274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH, Shaw GM. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 206:1273–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee HY, Giorgi EE, Keele BF, Gaschen B, Athreya GS, Salazar-Gonzalez JF, Pham KT, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Hahn BH, Shaw GM, Korber BT, Bhattacharya T, Perelson AS. 2009. Modeling sequence evolution in acute HIV-1 infection. J. Theor. Biol. 261:341–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolbank S, Kunert R, Stiegler G, Katinger H. 2003. Characterization of human class-switched polymeric (immunoglobulin M [IgM] and IgA) anti-human immunodeficiency virus type 1 antibodies 2F5 and 2G12. J. Virol. 77:4095–4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tudor D, Yu H, Maupetit J, Drillet AS, Bouceba T, Schwartz-Cornil I, Lopalco L, Tuffery P, Bomsel M. 2012. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc. Natl. Acad. Sci. U. S. A. 109:12680–12685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez LG, Costa MR, Todd CA, Haynes BF, Montefiori DC. 2009. Utilization of immunoglobulin G Fc receptors by human immunodeficiency virus type 1: a specific role for antibodies against the membrane-proximal external region of gp41. J. Virol. 83:7397–7410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peressin M, Holl V, Schmidt S, Decoville T, Mirisky D, Lederle A, Delaporte M, Xu K, Aubertin AM, Moog C. 2011. HIV-1 replication in Langerhans and interstitial dendritic cells is inhibited by neutralizing and Fc-mediated inhibitory antibodies. J. Virol. 85:1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su B, Xu K, Lederle A, Peressin M, Biedma ME, Laumond G, Schmidt S, Decoville T, Proust A, Lambotin M, Holl V, Moog C. 2012. Neutralizing antibodies inhibit HIV-1 transfer from primary dendritic cells to autologous CD4 T lymphocytes. Blood 120:3708–3717 [DOI] [PubMed] [Google Scholar]
- 59.Merbah M, Arakelyan A, Edmonds T, Ochsenbauer C, Kappes JC, Shattock RJ, Grivel JC, Margolis LB. 2012. HIV-1 expressing the envelopes of transmitted/founder or control/reference viruses have similar infection patterns of CD4 T-cells in human cervical tissue ex vivo. PLoS One 7:e50839. 10.1371/journal.pone.0050839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bazin-Redureau MI, Renard CB, Scherrmann JM. 1997. Pharmacokinetics of heterologous and homologous immunoglobulin G, F(ab′)2 and Fab after intravenous administration in the rat. J. Pharm. Pharmacol. 49:277–281 [DOI] [PubMed] [Google Scholar]
- 61.Sedlacek HH, Gronski P, Hofstaetter T, Kanzy EJ, Schorlemmer HU, Seiler FR. 1983. The biological properties of immunoglobulin G and its split products [F(ab′)2 and Fab]. Klin. Wochenschr. 61:723–736 [DOI] [PubMed] [Google Scholar]
- 62.Covell DG, Barbet J, Holton OD, Black CD, Parker RJ, Weinstein JN. 1986. Pharmacokinetics of monoclonal immunoglobulin G1, F(ab′)2, and Fab′ in mice. Cancer Res. 46:3969–3978 [PubMed] [Google Scholar]
- 63.Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. 1996. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur. J. Immunol. 26:690–696 [DOI] [PubMed] [Google Scholar]
- 64.Israel EJ, Taylor S, Wu Z, Mizoguchi E, Blumberg RS, Bhan A, Simister NE. 1997. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology 92:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. 1996. Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology 89:573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Junghans RP, Anderson CL. 1996. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. U. S. A. 93:5512–5516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu X, Meng G, Dickinson BL, Li X, Mizoguchi E, Miao L, Wang Y, Robert C, Wu B, Smith PD, Lencer WI, Blumberg RS. 2001. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J. Immunol. 166:3266–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dall'Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. 2002. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J. Immunol. 169:5171–5180 [DOI] [PubMed] [Google Scholar]
- 69.Armbruster C, Stiegler GM, Vcelar BA, Jager W, Koller U, Jilch R, Ammann CG, Pruenster M, Stoiber H, Katinger HW. 2004. Passive immunization with the anti-HIV-1 human monoclonal antibody (hMAb) 4E10 and the hMAb combination 4E10/2F5/2G12. J. Antimicrob. Chemother. 54:915–920 [DOI] [PubMed] [Google Scholar]
- 70.Junghans RP. 1997. Finally! The Brambell receptor (FcRB). Mediator of transmission of immunity and protection from catabolism for IgG. Immunol. Res. 16:29–57 [DOI] [PubMed] [Google Scholar]
- 71.Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. 2011. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. U. S. A. 108:4388–4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. 2004. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303:2019–2022 [DOI] [PubMed] [Google Scholar]
- 73.Chomont N, Hocini H, Gody JC, Bouhlal H, Becquart P, Krief-Bouillet C, Kazatchkine M, Belec L. 2008. Neutralizing monoclonal antibodies to human immunodeficiency virus type 1 do not inhibit viral transcytosis through mucosal epithelial cells. Virology 370:246–254 [DOI] [PubMed] [Google Scholar]
- 74.Garg A, Balthasar JP. 2007. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J. Pharmacokinet. Pharmacodyn. 34:687–709 [DOI] [PubMed] [Google Scholar]
- 75.Raux M, Finkielsztejn L, Salmon-Ceron D, Bouchez H, Excler JL, Dulioust E, Grouin JM, Sicard D, Blondeau C. 2000. IgG subclass distribution in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Res. Hum. Retrovir. 16:583–594 [DOI] [PubMed] [Google Scholar]
- 76.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104 [DOI] [PubMed] [Google Scholar]
- 77.Pudney J, Quayle AJ, Anderson DJ. 2005. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol. Reprod. 73:1253–1263 [DOI] [PubMed] [Google Scholar]
- 78.Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, Dugast AS, Parren PW, Nimmerjahn F, Evans DT, Alter G, Forthal DN, Schmitz JE, Iida S, Poignard P, Watkins DI, Hessell AJ, Burton DR. 2012. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcgammaRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J. Virol. 86:6189–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]