Abstract
ClpC is an ATP-dependent Hsp100/Clp chaperone involved in protein quality control in low-GC Gram-positive bacteria. Previously, we found that ClpC affected the expression of a large number of genes, including capsule genes in Staphylococcus aureus. Here we constructed a His-tagged ClpC variant (ClpCtrap) with mutations within the Walker B motifs to identify the direct substrates of ClpC by copurification with ClpCtrap followed by gel electrophoresis combined with liquid chromatography-tandem mass spectrometry proteomics. We identified a total of 103 proteins that are potential substrates of ClpC in strain Newman. The direct protein-protein interaction of ClpC with a subset of the captured proteins was verified in a bacterial two-hybrid system. The captured proteins could be grouped into various functional categories, but most were related to proteins involved in the stress response. Several known ClpC substrates were captured, including ClpP, TrfA/MecA, ClpB, DnaK, DnaJ, GroL, RecA, and CodY, supporting the validity of our approach. Our results also revealed many new ClpC substrates, including AgrA, CcpA, RsbW, MurG, FtsA, SrtA, Rex, Atl, ClfA, and SbcC. Analysis of capsule production showed that three of the captured proteins, which were not previously known to be transcriptional regulators, did affect capsule production.
INTRODUCTION
Staphylococcus aureus is a major cause of bacterial infections, capable of causing a wide range of diseases ranging from simple skin infections to life-threatening diseases, such as endocarditis or pneumonia. Infections due to S. aureus were once limited to hospital settings and could be effectively treated with antibiotics. However, methicillin-resistant S. aureus (MRSA) with resistance to multiple antibiotics has become widespread, and highly virulent strains have now spread to the community, causing infections in normally healthy individuals. This rapid emergence of highly virulent MRSA strains in the community setting is considered one of the most surprising events in infectious diseases in recent years (1). It has been estimated that the number of deaths caused by MRSA in the United States has surpassed those caused by HIV/AIDS (2, 3).
The ability of S. aureus to cause a wide range of diseases stems from the fact that it can produce an abundance of virulence factors, including secreted toxins, enzymes, and cell surface molecules (4). The capsule is an important virulence factor in S. aureus, and it has been used for vaccine development (5, 6) and is of particular interest to our laboratory, as we have used it as a target to understand virulence gene regulation. Previously, our laboratory identified ClpC as a factor that affects capsule gene transcription, based on our screening of a transposon library (7). The fact that ClpC plays a role in transcriptional regulation is interesting because it is not a typical regulator that regulates through direct DNA binding. ClpC, which is conserved in all low-GC Gram-positive bacteria, is an ATP-dependent Hsp100/Clp chaperone of the AAA+ superfamily involved in protein quality control (8). ClpC and another Clp ATPase, ClpX, can associate with the ClpP protease to form proteolytic complexes that can affect many cellular functions, including gene regulation (8, 9). In S. aureus, very few substrates of ClpC/XP have been identified, although ClpCP has been implicated in degradation of antitoxins (10). Cohn et al. (11) showed that ClpXP, and to a lesser extent ClpCP, are involved in regulating the SOS response and thus affect expression of a subset of SOS regulon genes by degrading the LexA N-terminal domain after autocleavage of LexA.
Recently, Feng et al. (12) used a proteolytically inactive ClpP (ClpPtrap) and identified about 70 ClpP substrates in S. aureus. In addition, they used the ClpPtrap construct in clpC or clpX mutants to capture ClpXP or ClpCP substrates, respectively. In S. aureus, ClpC has been shown to affect a large number of genes and proteins based on microarray and proteomic analyses (13, 14), but how ClpC affects gene expression or protein production is largely unknown. In this study, we aimed to identify proteins that directly interact with ClpC, including those that are not destined for ClpCP proteolysis. We were especially interested in transcriptional regulators, as these could lead to a further understanding of gene regulation by ClpC. To this end, we developed a trapping method to identify ClpC substrates in S. aureus.
MATERIALS AND METHODS
Bacterial strains, culture media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Staphylococci were cultured in tryptic soy broth (TSB; Difco Laboratories, Detroit, MI). Escherichia coli was cultivated in Luria-Bertani broth or agar (Difco). MacConkey agar (Difco) plates containing 1% maltose were used for the bacterial two-hybrid assays. Antibiotics were added to culture media, as appropriate, at final concentrations of 10 μg/ml chloramphenicol, 3 μg/ml tetracycline, 10 μg/ml erythromycin, 50 μg/ml spectinomycin, and 100 μg/ml penicillin. Phages 52A and 80α were used for plasmid and chromosomal DNA transduction between S. aureus strains.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| S. aureus strains | ||
| Newman | Wild-type CP5 strain | T. Foster |
| CYL6841 | Newman ΔclpC::cat | 14 |
| CYL12447 | Newman ΔclpC::cat(pJG4017) | This study |
| CYL12448 | Newman ΔclpC::cat(pLL31) | This study |
| CYL12683 | Newman ΔclpC::cat(pJG4080) | This study |
| CYL6620 | Newman ΔsbcC | 15 |
| IK184 | Newman ΔrsbUVWsigB | 16 |
| NE1922 | USA300 nusG::bursa | NARSA |
| NE1495 | USA300 murA::bursa | NARSA |
| NE460 | USA300 atl::bursa | NARSA |
| E. coli strains | ||
| DH5α | Host strain for plasmids | Invitrogen |
| XL1 Blue | Host strain for plasmids | Stratagene |
| BTH101 | Recipient strain for two-hybrid system | 17 |
| Plasmids | ||
| pLL31 | E. coli-S. aureus shuttle vector with Pspac | 14 |
| pJG4017 | pLL31-clpCtrap-His6 | This study |
| pJG4080 | pLL31-clpC-His6 | This study |
| pKT25 | Bacterial two-hybrid system vector | 17 |
| pUT18C | Bacterial two-hybrid system vector | 17 |
Plasmid and strain construction.
To construct a clpC variant suitable for trapping ClpC substrates, we replaced the conserved Glu residue in each of the two Walker B domains with an Ala residue. The clpC(E280A/E618A) mutation, referred to as clpCtrap, was constructed via overlapping PCR in two steps, using the primers listed in Table 2. In the first step, one primer set (clpCtrap1 to -4) was used to construct the E280A mutation, whereas another primer set (clpCtrap5, clpCtrap9, clpCtrap10, and clpCtrap8) was used to construct the E618A mutation. In the second step, the two PCR fragments from the first step were used to construct the entire clpCtrap gene, using primers clpCtrap1 and clpCtrap8. The resulting PCR fragment, which also incorporated the His6 tag sequence, was cloned into pLL31 (14) carrying a Tobacco etch virus protease (TEV)-Myc tag sequence (GSGGENLYFQGAYTSGEQKLISEEDLNGE) with a TTA stop codon, resulting in pJG4017, which contains the clpCtrap gene with the His6-TEV-Myc sequence at the 3′ ends. A control plasmid, pJG4080, carrying the wild-type clpC gene and the His6-myc tag sequence at the 3′ end, was also constructed using primers clpCtrap1 and clpCtrap11. The clones were verified by DNA sequencing. The resulting plasmids were transduced into the clpC deletion strain CYL6841 (i.e., Newman ΔclpC::cat). To construct plasmids for the two-hybrid assay, the inserts were amplified using the primers listed in Table 2 and cloned into either pUT18C or pKT25 (17). The inserts were verified by sequencing. The transposon insertion mutants used in capsule assays were constructed by phage transduction of defined bursa aurealis transposon mutations in the Nebraska transposon library obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA). Transposon insertions were confirmed by PCR.
Table 2.
Primers used in this study
| Primer use and name | Sequencea |
|---|---|
| Primers for ClpCtrap construction | |
| ClpCtrap1 | 5′-GAATTCTGGAAGAGGCTTCTTATAGAT-3′ |
| ClpCtrap2 | 5′-AGTATGCAACGCATCAATAAATAG-3′ |
| ClpCtrap3 | 5′-CTATTTATTGATGCGTTGCATACT-3′ |
| ClpCtrap4 | 5′-GGTGTCGTATGACTCTTAAGTCTTAC-3′ |
| ClpCtrap5 | 5′-GTAAGACTTAAGAGTCATACGACACC-3′ |
| ClpCtrap9 | 5′-TCAATTGCATCAAATAAAATTACAGAATATGGTTTAC-3′ |
| ClpCtrap10 | 5′-CATATTCTGTAATTTTATTTGATGCAATTGAAAAAGCTCAT-3′ |
| ClpCtrap8 | 5′-GGATCCatggtgatggtgatgatgTCCACCTGCTTGCGATGGTGTTTTAGTTTC-3′ |
| ClpCtrap11 | 5′-GGATCCatggtgatggtgatgatgTCCCCATGCTTGCGATGGTGTTTTAGTTTC-3′ |
| Primers for two-hybrid test | |
| 2h clpC F | 5′-CTGCAGTCATTATTTATGTTATTTGGTAGATT-3′ |
| 2h clpC R | 5′-GGATCCTGCTTGCGATGGTGTTTTAGTTTC-3′ |
| 2h ctsR F | 5′-CTGCAGGTGATATACATGCACAATATGTCT-3′ |
| 2h ctsR R | 5′-GGATCCGTAATAATTTATAACTGGTAACAA-3′ |
| 2h mecA F | 5′-CTGCAGTGAGATGATATGAGAATAGAA-3′ |
| 2h mecA R | 5′-GGATCCTTCAGTTGTCTCTGGAAAATA-3′ |
| 2h codY Fnew | 5′-CTGCAGGAAAAATTCATGAGCTTATTATCT-3′ |
| 2h codY Rnew | 5′-GGATCCTTTACTTTTTTCTAATTCATCTAA-3′ |
| 2h rsbW Fnew | 5′-CTGCAGTCGAATAACATGCAATCTAAAGAA-3′ |
| 2h rsbW Rnew | 5′-GGATCCGCTGATTTCGACTCTTTCGCCATT-3′ |
Underlined sequences denote restriction sites. Nucleotides shown in bold are mutations made for clpCTrap construction. Lowercase letters denote the His6 sequence.
In vivo trapping of ClpC substrates and MS analysis.
Overnight S. aureus cultures were diluted in 200 ml of TSB with tetracycline to an optical density at 660 nm (OD660) of 0.05 and incubated to an OD660 of 0.3 to 0.5 (∼2 h) at 37°C with shaking at 225 rpm. Cultures were then induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and incubated for 3 h to an OD660 of 2.9 (ranging from 2.7 to 3.1). The cultures were then centrifuged at 8,000 × g for 10 min and washed with 40 ml of cold phosphate-buffered saline (PBS; pH 7.4). The pellets were resuspended in 8 ml of PBS with 1× protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Cell lysis was achieved by physical disruption of 1-ml portions of cell suspensions by using 0.1-mm zirconia-silica beads (Biospec, Bartlesville, OK) in a FastPrep instrument (Qbiogene, Carlsbad, CA) with six 40-s pulses at 6 m/s and 5-min incubations on ice in between pulses. The lysed cells were centrifuged at 8,000 × g for 15 min at 4°C, and supernatants were saved. The pellet was extracted twice, first with 800 μl and then with 500 μl of PBS containing 1× protease inhibitor cocktail, using the FastPrep instrument as described above, but with four 40-s pulses. The supernatants were combined after centrifugation. The pooled supernatants were then centrifuged at 18,000 × g at 4°C for 20 min and adjusted to contain 50 mM Na-phosphate, 300 mM NaCl, and 5 mM imidazole (pH 7.4). Separate HisPur cobalt resin columns (Thermo Scientific, Hudson, NH) were used for control experiments and for isolation of the His-tagged ClpCtrap-substrate complexes according to the manufacturer's instructions. The column was washed until the absorbance at 280 nm approached baseline. The column-bound proteins were eluted with 50 mM Na-phosphate and 300 mM NaCl (pH 7.4) buffer containing 150 mM imidazole. Proteins in the eluted fractions were then analyzed by using 4-to-12% SDS-PAGE gradient gels. Each selected lane of the SDS-PAGE gel was equally divided into 20 slices and subjected to in-gel trypsin digestion and mass spectrometry (MS) analysis as described previously (18). Proteins were identified by Mascot searches using thresholds of 95% protein probability, 95% peptide probability, and a minimum of two peptides per protein sequence. The spectral counts for each protein were normalized to the total counts to account for between-sample variation. The normalized spectral counts were compared using Student's t test (P ≤ 0.05) to identify proteins that were differentially enriched by ClpCtrap.
Other methods.
Bacterial two-hybrid experiments were done using the bacterial adenylate cyclase two-hybrid system (BACTH) as described previously (17). Capsule assays were performed using cultures grown in TSB without glucose, essentially as described previously (14). Western analyses were carried out using anti-His antibody (Abcam, Cambridge, MA), anti-RecA antibody (Abcam), and anti-CodY antibody (generated by 21st Century Biochemicals, Marlboro, MA). Protein stability tests for CodY and RecA were carried out as described previously (12).
RESULTS AND DISCUSSION
Construction of ClpCtrap in S. aureus.
The Clp ATPases of the AAA+ superfamily are closely related chaperones with one or two highly conserved AAA domains that consist of Walker A and Walker B motifs, which are involved in nucleotide binding and hydrolysis, respectively (19). To capture the ClpC substrates, we constructed a ClpCtrap variant based on the methods used in the study reported by Weibezahn et al. (20), in which an E. coli ClpB variant, with mutations in the Walker B motifs of both AAA domains, was able to form stable complexes with its substrates. ClpB and ClpC are both members of the class 1 HSP100/Clp family of ATPases, with two AAA domains (21). A comparison of the Walker B motifs of E. coli ClpB with those of S. aureus ClpB and ClpC showed highly conserved residues, including the Glu residues involved in ATP hydrolysis (Fig. 1). To construct a ClpCtrap variant, the Glu residues in the Walker B motifs of both AAA domains in ClpC were changed to Ala (E280A/E618A). We also engineered a His6 tag at the C-terminal end for purification purposes. The clpCtrap construct was expressed in the clpC-null background of S. aureus Newman under the control of an IPTG-inducible Pspac promoter. The expression and binding of the His-tagged ClpCtrap protein to cobalt affinity columns were validated by Western blotting using anti-His antibody (data not shown). A similar plasmid carrying a His-tagged, wild-type ClpC protein was also constructed. The plasmid was able to complement a clpC mutant strain to the wild-type level of capsule production, indicating that the tag did not alter the activity of ClpC (data not shown).
Fig 1.

Amino acid sequence alignment of E. coli ClpB and S. aureus ClpB and ClpC proteins. The conserved sequences within the Walker B motifs are shown in bold. The conserved active site glutamic acid residues involved in ATP hydrolysis were changed to alanine residues in ClpCtrap (arrows).
In vivo trapping of ClpC substrates.
To carry out in vivo trapping, we performed two independent trapping experiments using strains CYL12447 carrying ClpCtrap-His and CYL12448 carrying the empty vector. Protein extracts were obtained and loaded onto cobalt affinity columns as described in Materials and Methods. The bound proteins were eluted with buffer containing 150 mM imidazole. This concentration of imidazole was chosen based on the results of a pilot study, wherein we eluted proteins using a step gradient of an increasing imidazole concentration (data not shown). The eluted proteins were analyzed in 4-to-12% SDS-PAGE gradient gels (Fig. 2). As expected, considerably more protein bands were found in the ClpCtrap strain than the strain with the empty vector. However, some protein bands were also found in the strain with the empty vector, suggesting that these proteins interact with the cobalt resin nonspecifically. The gel lanes were subjected to gel electrophoresis combined with liquid chromatography-tandem MS (GeLC-MS/MS) analysis, and the proteins were identified by Mascot searches as described in Materials and Methods. A total of 465 and 634 proteins were identified from the two ClpCtrap samples, respectively, and 382 and 521 were identified from the two control samples (see Table S1 in the supplemental material). After comparing the means of the normalized spectral counts between the ClpCtrap and the control samples via Student's t test, we identified a total of 91 proteins for which there was at least a 2.0-fold difference in counts between the two samples. However, we also found that 8 proteins were preferentially captured in the empty vector samples. It is unclear why some of the proteins were preferentially captured in the vector control samples, where there was no ClpCtrap-His. One possibility is that the unbound resin may allow some native proteins, for example, those with a metal binding capability, to bind better in the absence of a His-tagged protein (22). The proteins that were significantly enriched by ClpCtrap by at least 2-fold are shown in Table 3. Table 3 also includes an additional 12 proteins (indicated by asterisks) that were found in both samples of ClpCtrap but were absent in both control vector samples, implying that they specifically interact with ClpC. Because these proteins were exclusively captured by ClpCtrap, we considered these proteins to be potential ClpC substrates, despite the fact that their statistical P values were above 0.05.
Fig 2.
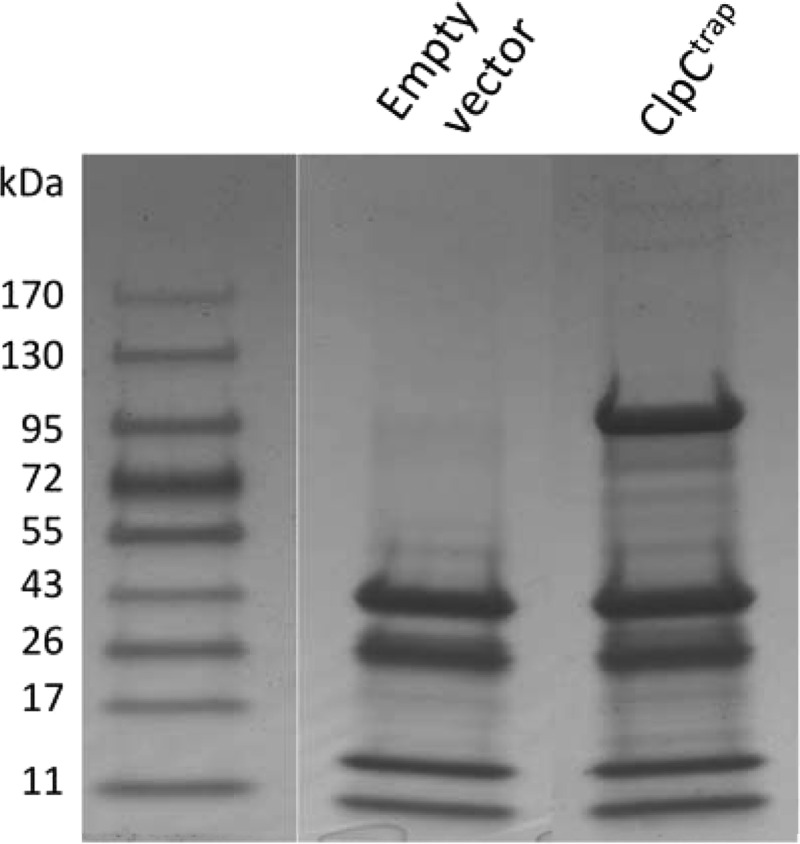
Proteins trapped by ClpCtrap. Proteins eluted from cobalt affinity columns were separated on 4-to-12% SDS-PAGE gradient gels and stained with Coomassie blue. Gel lanes were sliced and subjected to in-gel trypsin digestion, and proteins were identified by GeLC-MS/MS analyses. The left-most lane shows molecular mass standards, with sizes indicated in kDa.
Table 3.
Proteins that interact with ClpCtrap identified by GeLC-MS/MS
| Functional group and gene | ORFa | Identified protein | Mass (kDa) | IDb | Normalized count (expt 1/2)c |
P value | Fold change | |
|---|---|---|---|---|---|---|---|---|
| ClpCtrap | Vector | |||||||
| Regulatory functions | ||||||||
| ccpA | 1629 | Catabolite control protein A | 36 | A6QHR9 | 8.0/8.7 | 0.00/1.5 | 0.006 | 11.3 |
| codY | 1165 | GTP-sensing transcriptional pleiotropic repressor CodY | 29 | A6QGF5 | 19.0/28.3 | 11.0/7.9 | 0.050 | 2.5 |
| agrA | 1946 | Staphylococcal accessory gene regulator A | 28 | A6QIN6 | 31.0/31.8 | 10.0/14.2 | 0.006 | 2.6 |
| rsbW | 1971 | Serine-protein kinase RsbW | 18 | A6QIR1 | 27.0/23.1 | 11.0/8.3 | 0.011 | 2.6 |
| rex | 1953 | Redox-sensing transcriptional repressor Rex | 24 | A6QIP3 | 5.0/4.6 | 0.0/1.0 | 0.007 | 9.8 |
| hprK | 0728 | HPr kinase/phosphorylase | 34 | A6QF68 | 3.0/4.0 | 0.0/0.0 | 0.011 | infd |
| Protein fate | ||||||||
| clpC | 0487 | Clp protease, ATP binding subunit ClpC | 91 | A6QEH7 | 5028.0/3263.6 | 49.0/0.0 | 0.021 | 169.2 |
| clpB | 0845 | Clp protease, ATP binding subunit ClpB | 98 | A6QFI5 | 363.0/266.8 | 112.0/177.1 | 0.050 | 2.2 |
| clpP | 0736 | ATP-dependent Clp protease proteolytic subunit | 22 | A6QF76 | 29.0/19.6 | 10.0/5.4 | 0.043 | 3.2 |
| dnaK | 1483 | Chaperone protein DnaK | 66 | A6QHC3 | 158.0/181.9 | 33.0/29.0 | 0.004 | 5.5 |
| dnaJ | 1482 | Chaperone protein DnaJ | 42 | A6QHC2 | 34.0/46.2 | 6.0/3.9 | 0.015 | 8.1 |
| groL | 1937 | 60-kDa chaperonin | 58 | A6QIM7 | 50.0/60.1 | 11.0/16.2 | 0.009 | 4.0 |
| trfA | 0868* | Adaptor protein MecA/TrfA | 28 | A6QFK8 | 1.0/4.6 | 0.0/0.0 | 0.130 | inf |
| —e | 0495* | Conserved hypothetical protein | 20 | A6QEI5 | 3.0/0.6 | 0.0/0.0 | 0.139 | inf |
| — | 1187* | Conserved hypothetical protein | 49 | A6QGH7 | 3.0/1.2 | 0.0/0.0 | 0.077 | inf |
| Biosynthesis of cofactors, prosthetic groups, and carriers | ||||||||
| lipA | 0796 | Lipoyl synthase | 35 | A6QFD6 | 21.0/16.2 | 8.0/6.9 | 0.023 | 2.5 |
| ispD1 | 0185 | 2-C-methyl-d-erythritol 4-phosphate cytidylyltransferase 1 | 27 | A6QDM5 | 8.0/6.4 | 0.0/0.0 | 0.006 | inf |
| sufC | 0785 | FeS assembly ATPase SufC | 28 | A6QFC5 | 37.0/43.9 | 8.0/8.3 | 0.006 | 3.6 |
| sufD | 0786 | FeS assembly protein SufD | 49 | A6QFC6 | 15.0/18.5 | 4.0/7.9 | 0.027 | 2.5 |
| folD | 0932 | Bifunctional protein FolD | 31 | A6QFS2 | 6.0/7.5 | 0.0/0.5 | 0.007 | 27.5 |
| hemE | 1725 | Uroporphyrinogen decarboxylase | 39 | A6QI15 | 2.0/2.9 | 0.0/1.0 | 0.049 | 5.0 |
| hemL | 1756 | Glutamate-1-semialdehyde 2,1-aminomutase 2 | 47 | A6QI46 | 2.0/3.5 | 0.0/0.0 | 0.032 | inf |
| thiD | 0543 | Phosphomethylpyrimidine kinase | 30 | A6QEN3 | 10.0/15.6 | 0.0/3.9 | 0.043 | 6.5 |
| Amino acid metabolism | ||||||||
| sbnB | 0061 | Ornithine cyclodeaminase | 38 | A6QDA1 | 4.0/3.5 | 0.0/1.5 | 0.031 | 5.1 |
| — | 1421 | 2-Oxoisovalerate dehydrogenase, E2 component | 47 | A6QH61 | 7.0/6.9 | 4.0/1.5 | 0.039 | 2.5 |
| — | 1422 | 2-Oxoisovalerate dehydrogenase, E1 component | 36 | A6QH62 | 6.0/7.5 | 0.0/0.5 | 0.007 | 27.5 |
| — | 0475 | Cysteine synthase | 33 | A6QEG5 | 6.0/8.1 | 0.0/0.5 | 0.012 | 28.7 |
| — | 1871 | Aspartate transaminase | 48 | A6QIG1 | 3.0/2.3 | 0.0/0.5 | 0.015 | 10.8 |
| Cell envelope | ||||||||
| atl | 0922 | Bifunctional autolysin | 137 | A6QFR2 | 117.0/106.8 | 55.0/59.9 | 0.005 | 2.0 |
| murG | 2028 | UDP-glucose diacylglycerol glucosyltransferase | 45 | A6QGX0 | 6.0/10.4 | 0.0/1.0 | 0.038 | 16.7 |
| femB | 1287* | Methicillin resistance expression factor FemB | 50 | A6QGS7 | 3.0/6.4 | 0.0/0.0 | 0.054 | inf |
| srtA | 2426 | Sortase A, peptide LPXTG peptidoglycan transferase | 24 | A6QK16 | 3.0/2.9 | 1.0/1.0 | 0.000 | 3.0 |
| clfA | 0756 | Clumping factor A | 97 | Q53653 | 10.0/9.8 | 0.0/4.9 | 0.047 | 4.0 |
| — | 0369 | Putative lipoprotein | 24 | A6QE59 | 4.0/4.6 | 1.0/2.0 | 0.019 | 2.9 |
| — | 2356 | Putative lipoprotein | 17 | A6QJU6 | 8.0/5.8 | 2.0/1.5 | 0.023 | 4.0 |
| Cellular processes | ||||||||
| ahpF | 0371 | Alkyl hydroperoxide reductase subunit F | 55 | A6QE61 | 8.0/11.6 | 0.0/1.5 | 0.021 | 13.3 |
| — | 1639 | Propeptide, PepSY, and peptidase M4 | 12 | A6QHS9 | 3.0/2.3 | 0.0/0.0 | 0.008 | inf |
| Cell division | ||||||||
| ftsA | 1095 | Cell division protein FtsA | 53 | A6QG85 | 59.0/51.4 | 22.0/28.5 | 0.013 | 2.2 |
| divIVA | 1102* | Putative uncharacterized protein | 24 | A6QG92 | 8.0/3.5 | 0.0/0.0 | 0.064 | inf |
| DNA metabolism | ||||||||
| dnaN | 0002 | DNA polymerase III subunit beta | 42 | A6QD42 | 40.0/25.4 | 4.0/9.3 | 0.039 | 4.9 |
| recA | 1194 | Protein RecA | 38 | A6QGI4 | 58.0/86.6 | 16.0/12.8 | 0.028 | 5.0 |
| recN | 1425 | DNA repair protein RecN | 64 | A6QH65 | 7.0/6.4 | 0.0/2.0 | 0.016 | 6.8 |
| sbcC | 1258 | Nuclease SbcCD subunit C | 117 | A6QGP8 | 9.0/8.7 | 0.0/3.4 | 0.027 | 5.1 |
| mutS | 1204 | DNA mismatch repair protein MutS | 100 | A6QGJ4 | 8.0/7.5 | 2.0/4.4 | 0.033 | 2.4 |
| Central intermediary metabolism | ||||||||
| glmS | 2056 | Glucosamine-fructose-6-phosphate aminotransferase, isomerizing | 66 | A6QIZ6 | 84.0/56.0 | 17.0/20.1 | 0.034 | 3.8 |
| — | 0584 | Hydrolase | 31 | A6QES4 | 7.0/4.0 | 0.0/0.0 | 0.032 | inf |
| — | 0973 | Inositol-1-monophosphatase family protein | 30 | A6QFW3 | 6.0/6.4 | 0.0/2.0 | 0.017 | 6.3 |
| — | 2375 | NAD-dependent epimerase/dehydratase | 25 | A6QJW5 | 2.0/1.2 | 0.0/0.0 | 0.032 | inf |
| — | 2434 | Conserved hypothetical protein | 37 | A6QK24 | 2.0/3.5 | 0.0/0.5 | 0.042 | 11.1 |
| Energy metabolism | ||||||||
| citC | 1587 | Isocitrate dehydrogenase [NADP] | 46 | A6QHM7 | 5.0/5.8 | 0.0/0.0 | 0.003 | inf |
| pycA | 0979 | Pyruvate carboxylase | 129 | A6QFW9 | 5.0/4.6 | 0.0/0.0 | 0.001 | inf |
| gapA | 0741 | Glyceraldehyde 3-phosphate dehydrogenase 1 | 36 | A6QF81 | 17.0/22.5 | 0.0/0.0 | 0.009 | inf |
| sucB | 1325 | Dihydrolipoamide succinyltransferase E2 component of 2-oxoglutarate dehydrogenase complex | 47 | A6QGW5 | 2.0/4.0 | 0.0/0.0 | 0.049 | inf |
| sucC | 1155 | Succinyl coenzyme A ligase (ADP-forming) subunit beta | 42 | A6QGE5 | 9.0/12.7 | 0.0/0.0 | 0.014 | inf |
| zwf | 1412 | Glucose-6-phosphate 1-dehydrogenase | 57 | A6QH52 | 15.0/9.2 | 3.0/2.0 | 0.041 | 4.9 |
| glk | 1451 | Glucokinase | 35 | A6QH91 | 5.0/4.0 | 0.0/0.0 | 0.006 | inf |
| pgk | 0742 | Phosphoglycerate kinase | 43 | A6QF82 | 8.0/6.9 | 3.0/0.0 | 0.032 | 5.0 |
| ackA | 1605 | Acetate kinase | 44 | A6QHP5 | 46.0/38.7 | 14.0/10.3 | 0.009 | 3.5 |
| fbaA | 2029 | Fructose-bisphosphate aldolase | 31 | A6QIW9 | 1.0/1.7 | 0.0/0.0 | 0.032 | inf |
| — | 2210 | Formate dehydrogenase homolog | 111 | A6QJF0 | 7.0/6.9 | 4.0/1.5 | 0.039 | 2.5 |
| — | 1672* | Transaldolase | 26 | A6QHW2 | 3.0/0.6 | 0.0/0.0 | 0.139 | inf |
| Fatty acid and phospholipid metabolism | ||||||||
| accC | 1431 | Biotin carboxylase subunit of acetyl-CoA carboxylase | 50 | A6QH71 | 9.0/6.4 | 0.0/2.0 | 0.028 | 7.8 |
| gpsA | 1383 | Glycerol-3-phosphate dehydrogenase | 36 | A6QH23 | 3.0/4.6 | 0.0/0.0 | 0.021 | inf |
| Protein synthesis | ||||||||
| rsmH | 1089 | rRNA small subunit methyltransferase H | 36 | A6QG79 | 5.0/4.0 | 0.0/1.5 | 0.025 | 6.1 |
| rplA | 0500 | 50S ribosomal protein L1 | 25 | A6QEJ0 | 18.0/18.5 | 7.0/6.4 | 0.001 | 2.7 |
| rpsA | 1385 | 30S ribosomal protein S1 | 43 | A6QH25 | 29.0/20.8 | 13.0/9.8 | 0.046 | 2.2 |
| rpsP | 1148 | 30S ribosomal protein S16 | 10 | A6QGD8 | 8.0/6.4 | 4.0/2.0 | 0.043 | 2.4 |
| rpsD | 1613 | 30S ribosomal protein S4 | 23 | A6QHQ3 | 65.0/50.2 | 22.0/33.9 | 0.044 | 2.1 |
| gatA | 1838 | Glutamyl-tRNA(Gln) amidotransferase subunit A | 53 | A6QIC8 | 40.0/65.3 | 6.0/16.2 | 0.046 | 4.7 |
| gatB | 1837 | Aspartyl/glutamyl-tRNA(Asn/Gln) amidotransferase subunit B | 54 | A6QIC7 | 42.0/27.1 | 4.0/11.3 | 0.041 | 4.5 |
| tyrS | 1622 | Tyrosine-tRNA ligase | 48 | A6QHR2 | 2.0/1.7 | 0.0/0.5 | 0.014 | 7.6 |
| ileS | 1103* | Isoleucyl-tRNA ligase | 48 | A6QG93 | 1.0/3.5 | 0.0/0.0 | 0.106 | inf |
| — | 0721 | Sigma 54 modulation protein | 22 | A6QF61 | 31.0/26.6 | 15.0/13.7 | 0.012 | 2.0 |
| — | 1408 | SpoU rRNA methylase family protein | 27 | A6QG38 | 3.0/3.5 | 0.0/1.0 | 0.019 | 6.6 |
| Purine, pyrimidine, nucleoside, nucleotides, purine ribonucleotide synthesis | ||||||||
| guaB | 0380 | Inosine-5′-monophosphate dehydrogenase | 53 | A6QE70 | 42.0/27.1 | 4.0/11.3 | 0.041 | 4.5 |
| prs | 0463 | Ribose-phosphate pyrophosphokinase | 35 | A6QEF3 | 44.0/40.4 | 4.0/15.2 | 0.016 | 4.4 |
| pyrH | 1168 | Uridylate kinase | 26 | A6QGF8 | 7.0/5.8 | 1.0/1.0 | 0.006 | 6.4 |
| pyrR | 1109 | Bifunctional protein PyrR | 20 | A6QG99 | 15.0/25.4 | 0.0/4.9 | 0.045 | 8.2 |
| nrdE | 0700 | Ribonucleoside-diphosphate reductase | 82 | A6QF40 | 55.0/56.0 | 27.0/28.5 | <0.001 | 2.0 |
| — | 0284 | Conserved hypothetical protein | 21 | A6QDX4 | 3.0/2.9 | 0.0/0.0 | <0.001 | inf |
| Transcription | ||||||||
| sigA | 1464 | RNA polymerase sigma factor | 42 | A6QHA4 | 14.0/9.8 | 0.0/2.0 | 0.021 | 12.1 |
| rpoA | 2126 | DNA-directed RNA polymerase subunit alpha | 35 | A6QJ66 | 13.0/7.5 | 0.0/0.0 | 0.032 | inf |
| nusA | 1176 | Transcription termination-antitermination factor | 44 | A6QGG6 | 14.0/12.1 | 2.0/7.4 | 0.049 | 2.8 |
| nusG | 0498 | Transcription antitermination protein NusG | 21 | P0C1S3 | 9.0/6.9 | 4.0/1.5 | 0.043 | 2.9 |
| Transport and binding proteins | ||||||||
| — | 0581 | Iron compound ABC transporter, iron compound binding protein | 33 | A6QES1 | 2.0/2.3 | 0.0/0.0 | 0.003 | inf |
| — | 2312 | Amino acid ABC transporter, permease protein | 26 | A6QJQ2 | 2.0/2.3 | 0.0/0.0 | 0.003 | inf |
| — | 0705 | Ferrichrome ABC transporter lipoprotein | 38 | A6QF45 | 10.0/9.8 | 4.0/3.9 | <0.001 | 2.5 |
| — | 0954 | Conserved hypothetical protein | 24 | A6QFU4 | 3.0/2.3 | 0.0/0.0 | 0.008 | 3.00 |
| Unknown | ||||||||
| — | 0272 | Putative uncharacterized protein | 21 | A6QDW2 | 6.0/4.6 | 0.0/0.0 | 0.008 | inf |
| — | 0632 | Putative uncharacterized protein | 24 | A6QEX2 | 7.0/6.4 | 1.0/2.9 | 0.022 | 3.4 |
| — | 0737 | Putative uncharacterized protein | 34 | A6QF77 | 4.0/3.5 | 0.0/1.5 | 0.031 | 5.1 |
| — | 0976 | Putative uncharacterized protein | 19 | A6QFW6 | 7.0/4.6 | 1.0/1.5 | 0.032 | 4.7 |
| — | 1265* | Putative uncharacterized protein | 11 | A6QGQ5 | 3.0/1.2 | 0.0/0.0 | 0.077 | inf |
| — | 1381 | Putative uncharacterized protein | 22 | A6QH21 | 2.0/1.7 | 0.0/0.0 | 0.003 | inf |
| — | 1730 | Putative uncharacterized protein | 13 | A6QI20 | 7.0/5.8 | 1.0/2.0 | 0.012 | 4.3 |
| — | 1820* | Putative uncharacterized protein | 10 | A6QIB0 | 5.0/1.2 | 0.0/0.0 | 0.125 | inf |
| — | 2067 | ATP binding Mrp/Nbp35 family protein | 38 | A6QJ07 | 3.0/5.8 | 0.0/0.0 | 0.044 | inf |
| — | 2002* | Putative uncharacterized protein | 19 | A6QIU2 | 1.0/2.9 | 0.0/0.0 | 0.088 | inf |
| — | 2201* | Dehydrogenase family protein | 41 | A6QJE1 | 25.0/6.4 | 0.0/0.0 | 0.117 | inf |
| — | 2209 | Putative uncharacterized protein | 17 | A6QJE9 | 2.0/2.3 | 0.0/0.0 | 0.003 | inf |
| — | 2405 | Putative uncharacterized protein | 27 | A6QJZ5 | 1.0/1.2 | 0.0/0.0 | 0.003 | inf |
| — | 2468 | Acetyltransferase, GNAT family protein | 19 | A6QK58 | 3.0/2.3 | 0.0/0.0 | 0.008 | inf |
| — | 2511* | Putative uncharacterized protein | 22 | A6QKA1 | 4.0/1.7 | 0.0/0.0 | 0.064 | inf |
ORF numbers are based on strain Newman. ORF numbers followed by an asterisk indicate proteins with a P value of >0.05 but that were specifically captured by ClpCtrap.
The ID number is the UniProt accession number.
Two biological replicates were used to identify substrates of ClpC. The spectral counts were normalized based on total counts.
inf, the fold change could not be accurately estimated due to no detection in the negative-control samples.
—, a gene name has not yet been designated.
Potential ClpC substrates.
As shown in Table 3, proteins in various functional categories were captured by ClpCtrap, suggesting that ClpC is involved in many different cellular processes. Collectively, proteins involved in determining protein fate, including ClpB, DnaK, DnaJ, GroL, and ClpP, are among the most abundant proteins trapped by ClpCtrap. The trapping of ClpP was expected, since ClpC can form a proteolytic complex with ClpP to degrade misfolded proteins, especially under stress conditions. ClpB, which does not associate with a protease, has been shown to associate with Hsp70 chaperones (DnaK, DnaJ, and GrpE [referred to as KJE]) to disaggregate or refold aggregated proteins under stress or nonstress conditions (23, 24). GroEL, which also does not partner with a protease either, does interact with GroES to properly fold certain proteins (25). Capture of these chaperones in large amounts by ClpC suggests that ClpC interacts with these chaperones in S. aureus. Since ClpC has not been shown to associate with ClpB/KJE or GroESL for protein folding activities, we speculate that ClpC may escort these chaperones for ClpCP degradation when in excess. Indeed, ClpB, DnaK, and DnaJ have been identified as the ClpCP substrates (12). The E. coli ClpA protein, an equivalent to S. aureus ClpC, has been shown to participate in self-degradation by ClpAP (26, 27). Thus, it is possible that ClpCP could be responsible for the turnover of other chaperones in S. aureus.
The Bacillus subtilis ClpC has a low intrinsic ATPase activity and appears to rely on adaptor proteins for chaperone activity (9). Three adaptors have been shown to interact with ClpC and modulate its function. These adaptors are also substrates for degradation by ClpCP when not involved in substrate delivery. Homologs of two of these adaptors were also found in S. aureus. MecA is the first characterized adaptor protein found in B. subtilis, and it enables the recognition of specific substrates by ClpC, including ComK, as well as misfolded or aggregated proteins (28, 29). ComK, which is involved in competence regulation, is normally bound by the MecA-ClpC complex and destined for degradation by ClpCP. Upon competence induction, ComS is produced and displaces ComK in the ComK-MecA-ClpC ternary complex, which allows ComK to activate the transcription of competence genes (30). We found TrfA, the MecA homolog in S. aureus (based on the terminology used in reference 12), was present in the ClpCtrap samples but absent in the controls (Table 3), suggesting that ClpC interacts with this adaptor in S. aureus. Another well-characterized ClpC adaptor in B. subtilis is McsB, whose kinase activity is inhibited by interaction with ClpC (31, 32). McsB is released from the McsB/ClpC complex by unfolded proteins, which allows McsB to autophosphorylate in the presence of McsA. Phosphorylated McsB then interacts with CtsR and acts as an adaptor of ClpC, leading to degradation of CtsR by the ClpCP proteolytic complex. Degradation of CtsR results in upregulation of genes involved in stress tolerance, including clpC, clpB, and clpE (32). In this study, we found that McsB was enriched 3.2-fold by ClpCtrap and CtsR was enriched by 4.0-fold. However, the statistical significance of enrichment in either case was slightly above the cutoff level (P = 0.064 and 0.107 for McsB and CtsR, respectively). Nonetheless, based on the conservation of these proteins between B. subtilis and S. aureus and the high level of enrichment by ClpCtrap found in this study, we suggest that McsB and CtsR interact with ClpC in S. aureus similarly as in B. subtilis.
Several proteins with regulatory function (CcpA, CodY, AgrA, RsbW, Rex, and HprK) were identified in this study, including those that are directly involved in transcriptional regulation, suggesting that ClpC can exert its regulatory function by modulating these regulators. In particular, AgrA (by affecting expression of the Agr quorum-sensing effector, RNAIII), RsbW (by controlling the activity of sigma factor SigB), and CodY affect a large number of genes, including virulence genes (33–35). Interaction of ClpC with these regulators may account for previous findings in which deletion of clpC affected transcription of many genes, including those involved in virulence (13, 14). It is interesting that all of these regulators identified here are involved in some form of stress-related response, although to different extents and in response to different forms of stress. Specifically, CcpA, CodY, and HprK are involved in nutrition sensing (36–39), AgrA is involved in cell density quorum sensing (40), Rex is involved in redox stress sensing (41), and RsbW (by modulating SigB) is involved in stationary-phase gene expression (42). Thus, ClpC may regulate different stress responses by interacting with these regulators and modulating their activities.
Enzymes involved in cell wall synthesis, cell division, and DNA repair are important targets for regulation when bacteria encounter stress. MurAA, which is involved in the first step of peptidoglycan synthesis, has been shown to be a substrate of ClpCP in B. subtilis (43). FtsZ, a cell division protein, has been shown to be a target of ClpXP protease in E. coli (44, 45). In S. aureus, FtsZ and RecA were both identified as potential substrates of ClpCP (12). Here, we found several proteins that were enriched by ClpCtrap and that are involved in cell wall metabolism (MurG and FemB), cell division (FtsA and DivIVA), and DNA repair (DnaN, RecA, RecN, SbcC, and MutS). In addition, we found MurA (a homolog of MurAA) was enriched by 5.9-fold (P = 0.052). The fact that MurAA is a substrate of ClpCP in B. subtilis suggests that MurA is a likely substrate of ClpC in S. aureus. MurA was not captured by ClpPtrap of S. aureus, however (12). Similarly, FtsZ was enriched by 2.1-fold (P = 0.146), suggesting that FtsZ may also interact with ClpC. In support of this, Feng et al. (12) reported that FtsZ was a potential substrate of ClpCP in strain 8325-4. In the same study, however, FtsZ was not captured by ClpPtrap in strain Newman. Together, these results suggest that ClpC may interact with FtsZ and MurA, but further study is required to conclusively demonstrate their interactions.
Many proteins involved in energy metabolisms were captured, including several enzymes involved in the tricarboxylic acid (TCA) cycle (PycA, CitC, SucB, and SucC) and glycolysis (GapA, Glk, Pgk, and FbaA). Numerous other proteins involved in various metabolic pathways were also identified as ClpC substrates. These include those involved in protein translation and synthesis, nucleic acid synthesis, transcription, transport, fatty acid synthesis, cofactor synthesis, and central metabolism (Table 3). These results are consistent with previous reports that ClpC affects cellular metabolism, including the TCA cycle, nucleotide metabolism, and the stringent response (13, 46). Not surprisingly, most of the identified proteins can be related to the stress response, consistent with the general biological role of ClpC. Interestingly, we noted that two cell surface proteins, ClfA and Atl, were also captured in our assay. The interaction of ClpC with surface proteins has not been previously reported and was unanticipated. However, it seems likely that ClpC may interact with the precursors of these proteins in the cytoplasm, prior to their export to the cell surface. It is also of interest that SrtA, the sortase protein responsible for anchoring most surface proteins on cell wall, including ClfA, was also captured.
Interaction of ClpC and its substrates.
To verify that the trapped proteins identified in the GeLC-MS/MS analysis interact with ClpC in vivo, we employed a bacterial two-hybrid system based on the reconstitution of Bordetella pertussis adenylate cyclase activity in E. coli (17). We chose four proteins, MecA, CtsR, CodY, and RsbW, to test for direct interactions with ClpC. As indicated above, both MecA and CtsR have been shown to interact with ClpC in B. subtilis. As expected, both proteins were found to interact with ClpC (Fig. 3). These results confirmed that ClpC interacts with MecA and CtsR in S. aureus, similarly to B. subtilis. It is interesting that although we found enrichment of CtsR by ClpCtrap, the data were not statistically significant compared to the empty vector control.
Fig 3.
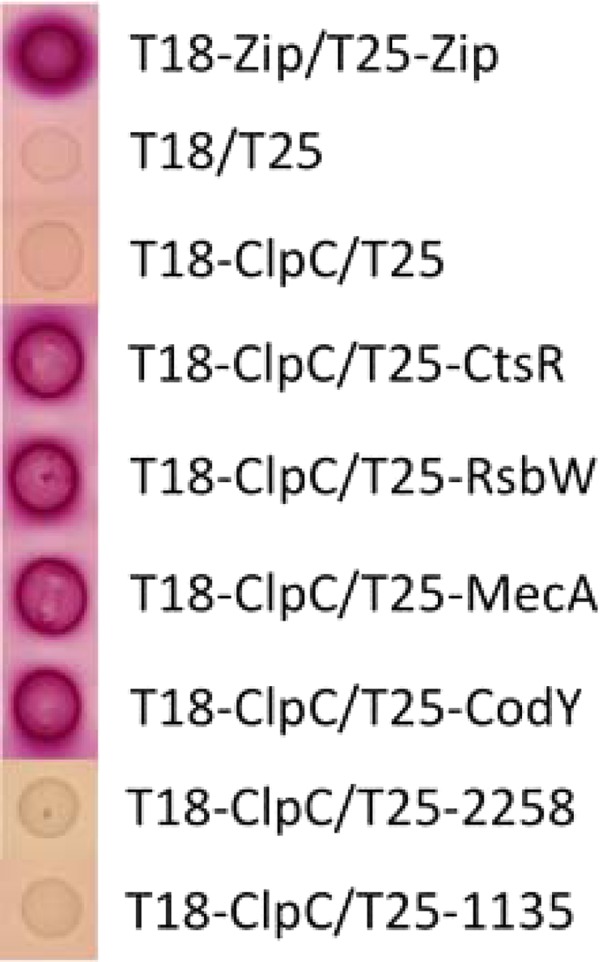
Demonstration of protein-protein interactions, by use of an E. coli bacterial two-hybrid system. Red appearance of the colonies on MacConkey agar supplemented with 1% maltose indicates a positive interaction of proteins fused to T18 and T25 fragments of Bordetella pertussis adenylate cyclase, respectively. ClpC was expressed from pUT18C (T18-ClpC), whereas putative ClpC substrate proteins were expressed from pKT25 (T25-CtsR, T25-RsbW, T25-MecA, and T25-CodY). A positive control with leucine zipper domains (T18-Zip/T25-Zip) and negative controls (T18/T25 and T18-ClpC/T25) are also shown. Two proteins (NWMN_2258 and NWMN_1135 with 0.97- and 1.07-fold changes, respectively) were also included to show lack of interaction of ClpC with proteins that were not specifically trapped.
CodY, which is highly conserved in low-GC Gram-positive bacteria, is a transcriptional regulator involved in metabolic stress (37). Here, we found that CodY was enriched by ClpCtrap by 2.5-fold. Using the two-hybrid system, we showed that CodY could indeed interact with ClpC (Fig. 3). Interestingly, CodY was also trapped by ClpPtrap in both the clpC and clpX mutant background, indicating that it is likely a substrate of ClpCP and/or ClpXP. The stability of CodY, however, was not affected by clpP deletion, suggesting that although CodY is bound, it is not degraded by ClpCP or ClpXP (12). To test whether ClpC is involved in the stability of CodY, we performed Western blot analyses with uninduced and mupirocin-induced cultures (mupirocin induces the stringent response that releases CodY from DNA binding [47]). Our results, which are consistent with those of Feng et al. (12), showed that ClpC, similar to ClpP, did not affect CodY stability either with or without mupirocin induction (Fig. 4A). Taken together, these results indicate that CodY is not regulated by ClpCP proteolysis, nor is its stability affected by ClpC. What role then does ClpC play by binding to CodY? One possibility is that ClpC modulates CodY activity without affecting the stability of the protein. For example, ClpC binding to CodY could affect CodY binding to branched-chain amino acids or to DNA. Interestingly, we previously reported that ClpC repressed CodY at the transcriptional level (14). Thus, ClpC appears to affect CodY at both the transcriptional and posttranscriptional levels. CodY has been shown to be negatively autoregulated in some low-GC bacteria (48, 49). It is therefore possible that the interaction of ClpC and CodY could result in transcriptional autorepression of codY. However, no CodY binding site has been found upstream of the codY gene in S. aureus (35), suggesting that CodY is not autoregulated. Thus, more complicated mechanisms than those described above are likely involved, and further in-depth studies are needed to understand how ClpC affects CodY.
Fig 4.
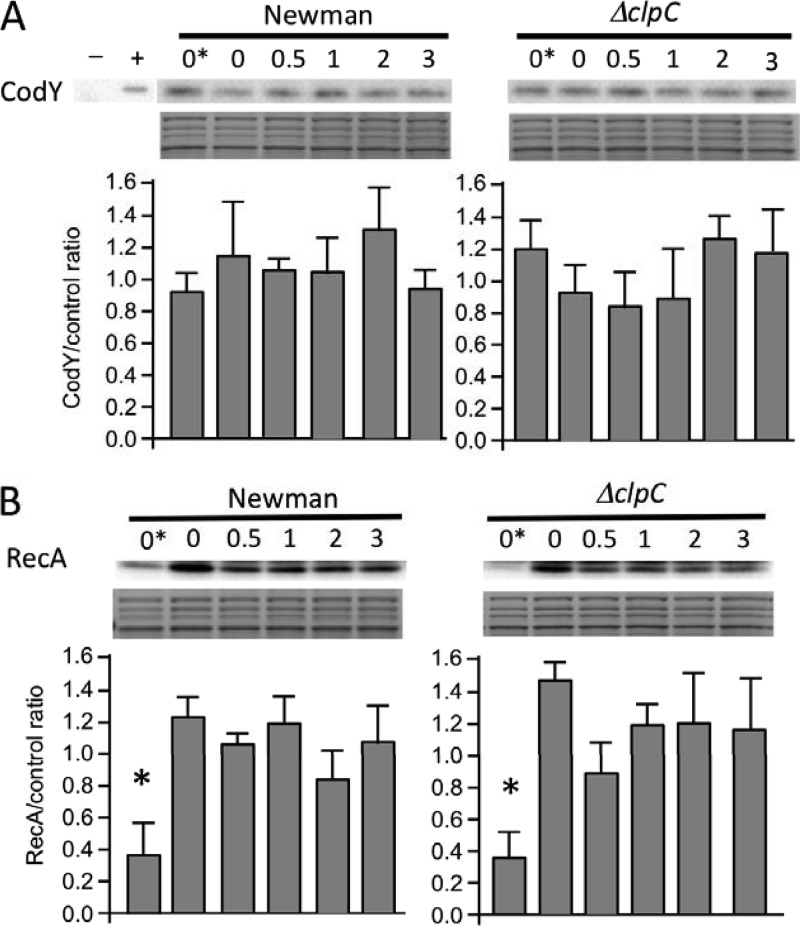
Protein stability test of CodY and RecA in Newman and Newman ΔclpC strains by Western blotting. A portion of the gel stained with Coomassie blue was used as a loading control below each blot. Western blots (n = 3) were quantified by densitometry, normalized to one of the stained protein bands, and analyzed by a one-way analysis of variance. (A) CodY stability was tested after inducing the stringent response by adding mupirocin to mid-log-phase cultures at time zero (T = 0*). Chloramphenicol was added 30 min after mupirocin addition. Cells were harvested immediately after chloramphenicol addition (labeled as 0) and 0.5, 1, 2, and 3 h thereafter. A codY-deleted strain and a codY-complemented strain, denoted − and +, respectively, were also included as controls. There was no significant difference between results at the different time points. (B) RecA stability was tested after SOS induction by adding mitomycin C at T = 0*. Chloramphenicol was added 15 min after mitomycin C addition. Cells were harvested as described for panel A. The RecA level was significantly lower (*, P < 0.05) in preinduced samples (T = 0*) than at other time points for both strains.
RsbW is an anti-sigma factor that binds to and inhibits the activity of SigB under nonstressed conditions. Upon stress induction, RsbU dephosphorylates RsbV, leading to the formation of an RsbVW complex and thereby relieving inhibition of SigB by RsbW (50). In this study, we found that RsbW was trapped by ClpCtrap (Table 3), suggesting that ClpC could affect SigB activity by binding to RsbW, thereby increasing transcription of SigB-dependent genes. As shown in Fig. 3, we validated the RsbW-ClpC interaction in the two-hybrid system. Interestingly, neither SigB nor any of its regulatory proteins were captured by ClpPtrap (12). These results suggest that ClpC can affect SigB activity independently from ClpP, supporting the contention that ClpC may exert its regulatory function by binding and sequestering target proteins rather than by ClpCP-directed proteolysis.
RecA is another protein identified by the ClpPtrap method as a potential ClpCP substrate. It was further shown that RecA was very stable, but that ClpP-mediated proteolysis of RecA could be observed 3 h after SOS induction. Although this effect was slight, the results argued that ClpCP plays a role in poststress rebalancing of RecA (12). Because we also captured RecA with our ClpCtrap method, we expected that ClpC would have an effect on RecA stability following SOS induction. Therefore, we carried out experiments similar to those described by Feng et al. (12) to determine whether ClpC is involved in RecA degradation. We did not detect any apparent difference in RecA stability between the wild-type and clpC mutant strains despite repeated efforts (Fig. 4B). The apparent discrepancy between the two studies may be due to strain differences; however, it is possible that RecA could be degraded by ClpXP rather than ClpCP.
Effect of ClpC substrates on the capsule.
Among the transcriptional regulators identified by ClpCtrap in this study, AgrA and RsbW/SigB have been shown to activate capsule gene expression, whereas CodY and CcpA have been shown to repress capsule gene expression (14, 36, 51, 52). These regulators could therefore function downstream of ClpC to regulate the capsule genes. In addition, SbcC, which is likely involved in DNA repair and has been shown to repress capsule genes (15), was captured by ClpCtrap, indicating that ClpC could also affect the capsule through SbcC. As expected, deletion of sbcC in strain Newman increased capsule production, whereas deletion of the rsbUVWsigB operon reduced capsule production (Fig. 5). To determine whether any new ClpC substrates identified in this study had an effect on capsule production, we selected three substrates, Atl, MurA, and NusG, that do not have a known effect on the capsule and inactivated the genes encoding these proteins in strain Newman. We found all three genes had an effect on capsule production (Fig. 5). Because murA is located upstream of a closely linked gene, we also performed a complementation experiment to rule out potential polar effects. Our results confirmed that MurA was involved in capsule production (data not shown). A complementation experiment was not done with an atl or nusG mutant, since neither gene is likely to affect its downstream genes, based on genetic organization. These results were surprising, as these genes are not likely to be directly involved in capsule synthesis. Atl is a murein hydrolase that is involved in cell separation following cell division (53). MurA is a primary enzyme involved in catalyzing the first committed step in peptidoglycan biosynthesis (54). Because the capsule is anchored to the cell wall (55), it is conceivable that genes involved in cell wall synthesis, like murA and atl, could affect capsule production. NusG is a general transcription factor that binds to and affects transcription by RNA polymerase, primarily by affecting transcript elongation (56). The finding here that the nusG::bursa mutation reduced the capsule suggests that NusG may be required for transcription of the full-length cap operon. In some bacteria, NusG paralogs (termed NusGSP [57]) can selectively promote antitermination of operons, including capsule operons (58, 59). Because S. aureus capsule genes are transcribed as a long (∼17-kb) transcript (60), it seems likely that NusG may promote production of full-length transcripts through its antitermination activity. However, further studies are needed to test this possibility. Taken together, our results suggest that ClpC cannot only regulate capsule through transcriptional regulators like AgrA, CodY, and SigB at the transcriptional level but also can affect capsule production through nonregulatory proteins, like MurA and Atl, most probably at the posttranscriptional level. Our findings therefore highlight the complexity of capsule regulation and regulation of other virulence factors in S. aureus.
Fig 5.
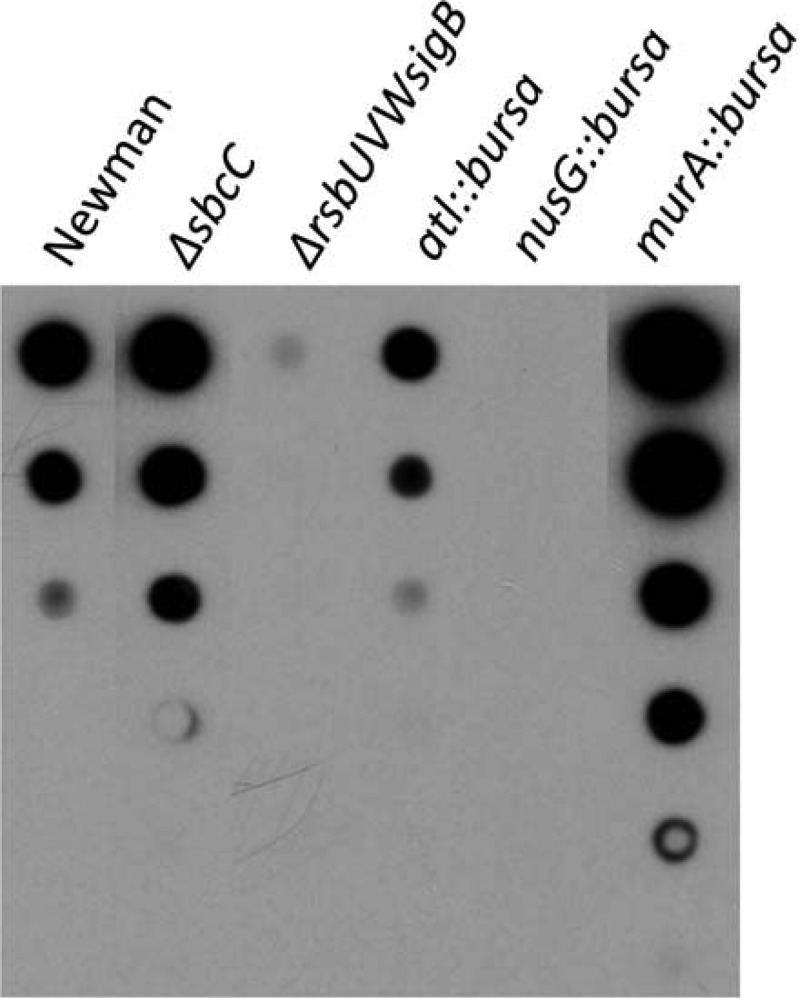
Effect of bursa aurealis transposon and deletion mutations on capsule production. Capsules isolated were serially diluted (3-fold) and analyzed by immunoblotting.
Conclusion.
In this study, we constructed a ClpCtrap variant to capture substrates that interact with ClpC and identified more than 100 potential substrates. The captured substrates included those that have previously been shown to directly interact with ClpC in low-GC Gram-positive bacteria as well as many novel ClpC substrates. To further verify the proteomic results, we employed a bacterial two-hybrid system and demonstrated that four of the captured proteins were capable of interacting with ClpC in E. coli. Recently, Feng et al. (12) employed the ClpPtrap method in a clpX mutant of strain 8325-4 to identify ClpCP substrates that are targeted for proteolysis. Of the 31 proteins identified by them, 10 were also identified in our study, including Prs, GuaB, NrdE, RecA, CodY, DnaJ, DnaK, ClpB, ClpC, and GlmS. Our results are in good agreement with their report, although a number of proteins were not captured by ClpCtrap in our study. These discrepancies could be due to differences in the strains used or to differences in growth conditions between the studies. However, since we employed a more sensitive GeLC-MS/MS method than the matrix-assisted laser desorption ionization–time of flight method employed by Feng et al., it is possible that some of the substrates identified in this study are proteolytic substrates of ClpCP that were not detected by Feng et al. Moreover, it seems more likely that many proteins that we identified may interact with ClpC without being delivered to the ClpCP protease complex for degradation, suggesting that ClpC could have a biological impact independent of ClpP. Little is known regarding how ClpC, ClpA, and ClpX affect gene expression, other than by association with their proteolytic partner, ClpP. This is true even in highly developed model bacteria, such as E. coli and B. subtilis (8, 9). In S. aureus, ClpX has been shown to affect protein A independently of ClpP, but the mechanism is unknown (61, 62). In our laboratory, we found that ClpC could affect capsule and other virulence factors by a ClpP-independent mechanism (unpublished data). Thus, unraveling how the ClpC ATPase affects its substrates independently of ClpP will undoubtedly lead to further understanding of the regulatory function of ClpC and its role in pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank David Cue and Ravi Gupta for critical reading of the manuscript. We thank Ralph Bertram for help with the bacterial two-hybrid experiments.
This work is supported by grant AI037027 from the National Institute of Allergy and Infectious Diseases. We also acknowledge the UAMS Proteomics Core, which is supported in part by NIH grants P20GM103429, P30GM103450, and P20GM103625, for proteomic analyses.
Footnotes
Published ahead of print 2 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00758-13.
REFERENCES
- 1.DeLeo FR, Chambers HF. 2009. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J. Clin. Invest. 119:2464–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 3.Bancroft EA. 2007. Antimicrobial resistance: it's not just for hospitals. JAMA 298:1803–1804 [DOI] [PubMed] [Google Scholar]
- 4.Foster TJ. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3:948–958 [DOI] [PubMed] [Google Scholar]
- 5.O'Riordan K, Lee JC. 2004. Staphylococcus aureus Capsular polysaccharides. Clin. Microbiol. Rev. 17:218–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CY, Lee JC. 2006. Staphylococcal capsules, p 456–463 In Fischetti VA, Novick RP, Ferretti JJ, Portnoy DA, Rood J. (ed), Gram-positive pathogens, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 7.Luong TT, Lee CY. 2006. The arl locus positively regulates Staphylococcus aureus type 5 capsule via an mgrA-dependent pathway. Microbiology 152:3123–3131 [DOI] [PubMed] [Google Scholar]
- 8.Frees D, Savijoki K, Varmanen P, Ingmer H. 2007. Clp ATPases and ClpP proteolytic complexes regulate vital biological processes in low GC, Gram-positive bacteria. Mol. Microbiol. 63:1285–1295 [DOI] [PubMed] [Google Scholar]
- 9.Kirstein J, Moliere N, Dougan DA, Turgay K. 2009. Adapting the machine: adaptor proteins for Hsp100/Clp and AAA+ proteases. Nat. Rev. Microbiol. 7:589–599 [DOI] [PubMed] [Google Scholar]
- 10.Donegan NP, Thompson ET, Fu Z, Cheung AL. 2010. Proteolytic regulation of toxin-antitoxin systems by ClpPC in Staphylococcus aureus. J. Bacteriol. 192:1416–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn MT, Kjelgaard P, Frees D, Penadés JR, Ingmer H. 2011. Clp-dependent proteolysis of the LexA N-terminal domain in Staphylococcus aureus. Microbiology 157:677–684 [DOI] [PubMed] [Google Scholar]
- 12.Feng J, Michalik S, Varming AN, Andersen JH, Albrecht D, Jelsbak L, Krieger S, Ohlsen K, Hecker M, Gerth U, Ingmer H, Frees D. 2013. Trapping and proteomic identification of cellular substrates of the ClpP protease in Staphylococcus aureus. J. Proteome Res. 12:547–558 [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee I, Schmitt S, Batzilla CF, Engelmann S, Keller A, Ring MW, Kautenburger R, Ziebuhr W, Hecker M, Preissner KT, Bischoff M, Proctor RA, Beck HP, Lenhof HP, Somerville GA, Herrmann M. 2009. Staphylococcus aureus ClpC ATPase is a late growth phase effector of metabolism and persistence. Proteomics 9:1152–1176 [DOI] [PubMed] [Google Scholar]
- 14.Luong TT, Sau K, Roux C, Sau S, Dunman PM, Lee CY. 2011. Staphylococcus aureus ClpC divergently regulates capsule via sae and codY in strain newman but activates capsule via codY in strain UAMS-1 and in strain Newman with repaired saeS. J. Bacteriol. 193:686–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Luong TT, Lee CY. 2007. The sbcDC locus mediates repression of type 5 capsule production as part of the SOS response in Staphylococcus aureus. J. Bacteriol. 189:7343–7350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kullik I, Giachino P, Fuchs T. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814–4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karimova G, Pidoux J, Ullmann A, Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 95:5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zielinska AK, Beenken KE, Mrak LN, Spencer HJ, Post GR, Skinner RA, Tackett AJ, Horswill AR, Smeltzer MS. 2012. sarA-mediated repression of protease production plays a key role in the pathogenesis of Staphylococcus aureus USA300 isolates. Mol. Microbiol. 86:1183–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogura T, Wilkinson AJ. 2001. AAA+ superfamily ATPases: common structure—diverse function. Genes Cells 6:575–597 [DOI] [PubMed] [Google Scholar]
- 20.Weibezahn J, Schlieker C, Bukau B, Mogk A. 2003. Characterization of a trap mutant of the AAA+ chaperone ClpB. J. Biol. Chem. 278:32608–32617 [DOI] [PubMed] [Google Scholar]
- 21.Schirmer EC, Glover JR, Singer MA, Lindquist S. 1996. HSP100/Clp proteins: a common mechanism explains diverse functions. Trends Biochem. Sci. 21:289–296 [PubMed] [Google Scholar]
- 22.Bolanos-Garcia VM, Davies OR. 2006. Structural analysis and classification of native proteins from E. coli commonly co-purified by immobilised metal affinity chromatography. Biochim. Biophys. Acta 1760:1304–1313 [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Sowa ME, Choi JM, Tsai FT. 2004. The ClpB/Hsp104 molecular chaperone-a protein disaggregating machine. J. Struct. Biol. 146:99–105 [DOI] [PubMed] [Google Scholar]
- 24.Doyle SM, Wickner S. 2009. Hsp104 and ClpB: protein disaggregating machines. Trends Biochem. Sci. 34:40–48 [DOI] [PubMed] [Google Scholar]
- 25.Walter S. 2002. Structure and function of the GroE chaperone. Cell. Mol. Life Sci. 59:1589–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maglica Z, Striebel F, Weber-Ban E. 2008. An intrinsic degradation tag on the ClpA C-terminus regulates the balance of ClpAP complexes with different substrate specificity. J. Mol. Biol. 384:503–511 [DOI] [PubMed] [Google Scholar]
- 27.Gottesman S, Clark WP, Maurizi MR. 1990. The ATP-dependent Clp protease of Escherichia coli. Sequence of clpA and identification of a Clp-specific substrate. J. Biol. Chem. 265:7886–7893 [PubMed] [Google Scholar]
- 28.Turgay K, Hahn J, Burghoorn J, Dubnau D. 1998. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 17:6730–6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlothauer T, Mogk A, Dougan DA, Bukau B, Turgay K. 2003. MecA, an adaptor protein necessary for ClpC chaperone activity Proc. Natl. Acad. Sci. U. S. A. 100:2306–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubnau D, Losick R. 2006. Bistability in bacteria. Mol. Microbiol. 61:564–572 [DOI] [PubMed] [Google Scholar]
- 31.Kirstein J, Zühlke D, Gerth U, Turgay K, Hecker M. 2005. A tyrosine kinase and its activator control the activity of the CtsR heat shock repressor in B. subtilis. EMBO J. 24:3435–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirstein J, Dougan DA, Gerth U, Hecker M, Turgay K. 2007. The tyrosine kinase McsB is a regulated adaptor protein for ClpCP. EMBO J. 26:2061–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunman PM, Murphy E, Haney S, Palacios D, Tucker-Kellogg G, Wu S, Brown EL, Zagursky RJ, Shlaes D, Projan SJ. 2001. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 183:7341–7353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bischoff M, Dunman P, Kormanec J, Macapagal D, Murphy E, Mounts W, Berger-Bächi B, Projan S. 2004. Microarray-based analysis of the Staphylococcus aureus σB regulon. J. Bacteriol. 186:4085–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majerczyk CD, Dunman PM, Luong TT, Lee CY, Sadykov MR, Somerville GA, Bodi K, Sonenshein AL. 2010. Direct targets of CodY in Staphylococcus aureus. J. Bacteriol. 192:2861–2877 (Erratum, 192:4258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pohl K, Francois P, Stenz L, Schlink F, Geiger T, Herbert S, Goerke C, Schrenzel J, Wolz C. 2009. CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. J. Bacteriol. 191:2953–2963 (Erratum, 191:4695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seidl K, Stucki M, Ruegg M, Goerke C, Wolz C, Harris L, Berger-Bächi B, Bischoff M. 2006. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 50:1183–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonenshein AL. 2005. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 8:203–207 [DOI] [PubMed] [Google Scholar]
- 39.Deutscher J, Francke C, Postma PW. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70:939–1031 (Erratum, 72:555, 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thoendel M, Kavanaugh JS, Flack CE, Horswill AR. 2011. Peptide signaling in the staphylococci. Chem. Rev. 111:117–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somerville GA, Proctor RA. 2009. At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiol. Mol. Biol. Rev. 73:233–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hecker M, Pané-Farré J, Völker U. 2007. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 61:215–236 [DOI] [PubMed] [Google Scholar]
- 43.Kock H, Gerth U, Hecker M. 2004. MurAA, catalysing the first committed step in peptidoglycan biosynthesis, is a target of Clp-dependent proteolysis in Bacillus subtilis. Mol. Microbiol. 51:1087–1102 [DOI] [PubMed] [Google Scholar]
- 44.Camberg JL, Hoskins JR, Wickner S. 2009. ClpXP protease degrades the cytoskeletal protein, FtsZ, and modulates FtsZ polymer dynamics. Proc. Natl. Acad. Sci. U. S. A. 106:10614–10619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camberg JL, Hoskins JR, Wickner S. 2011. The interplay of ClpXP with the cell division machinery in Escherichia coli. J. Bacteriol. 193:1911–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chatterjee I, Becker P, Grundmeier M, Bischoff M, Somerville GA, Peters G, Sinha B, Harraghy N, Proctor RA, Herrmann M. 2005. Staphylococcus aureus ClpC is required for stress resistance, aconitase activity, growth recovery, and death. J. Bacteriol. 187:4488–4496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reiss S, Pané-Farré J, Fuchs S, François P, Liebeke M, Schrenzel J, Lindequist U, Lalk M, Wolz C, Hecker M, Engelmann S. 2012. Global analysis of the Staphylococcus aureus response to mupirocin. Antimicrob. Agents Chemother. 56:787–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.den Hengst CD, van Hijum SA, Geurts JM, Nauta A, Kok J, Kuipers OP. 2005. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J. Biol. Chem. 280:34332–34342 [DOI] [PubMed] [Google Scholar]
- 49.Malke H, Ferretti JJ. 2007. CodY-affected transcriptional gene expression of Streptococcus pyogenes during growth in human blood. J. Med. Microbiol. 56:707–714 [DOI] [PubMed] [Google Scholar]
- 50.Senn MM, Giachino P, Homerova D, Steinhuber A, Strassner J, Kormanec J, Flückiger U, Berger-Bächi B, Bischoff M. 2005. Molecular analysis and organization of the σB operon in Staphylococcus aureus. J. Bacteriol. 187:8006–8019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luong T, Sau S, Gomez M, Lee JC, Lee CY. 2002. Regulation of Staphylococcus aureus capsular polysaccharide expression by agr and sarA. Infect. Immun. 70:444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meier S, Goerke C, Wolz C, Seidl K, Homerova D, Schulthess B, Kormanec J, Berger-Bächi B, Bischoff M. 2007. σB and the σB-dependent arlRS and yabJ-spoVG loci affect capsule formation in Staphylococcus aureus. Infect. Immun. 75:4562–4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oshida T, Sugai M, Komatsuzawa H, Hong YM, Suginaka H, Tomasz A. 1995. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-l-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc. Natl. Acad. Sci. U. S. A. 92:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blake KL, O'Neill AJ, Mengin-Lecreulx D, Henderson PJ, Bostock JM, Dunsmore CJ, Simmons KJ, Fishwick CW, Leeds JA, Chopra I. 2009. The nature of Staphylococcus aureus MurA and MurZ and approaches for detection of peptidoglycan biosynthesis inhibitors. Mol. Microbiol. 72:335–343 [DOI] [PubMed] [Google Scholar]
- 55.Gründling A, Schneewind O. 2006. Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus. J. Bacteriol. 188:2463–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGary K, Nudler E. 2013. RNA polymerase and the ribosome: the close relationship. Curr. Opin. Microbiol. 16:112–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Belogurov GA, Mooney RA, Svetlov V, Landick R, Artsimovitch I. 2009. Functional specialization of transcription elongation factors. EMBO J. 28:112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Artsimovitch I, Landick R. 2002. The transcriptional regulator RfaH stimulates RNA chain synthesis after recruitment to elongation complexes by the exposed nontemplate DNA strand. Cell 109:193–203 (Erratum, 110:801, 2002) [DOI] [PubMed] [Google Scholar]
- 59.Chatzidaki-Livanis M, Coyne MJ, Comstock LE. 2009. A family of transcriptional antitermination factors necessary for synthesis of the capsular polysaccharides of Bacteroides fragilis. J. Bacteriol. 191:7288–7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sau S, Sun J, Lee CY. 1997. Molecular characterization and transcriptional analysis of type 8 capsule genes in Staphylococcus aureus. J. Bacteriol. 179:1614–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frees D, Sørensen K, Ingmer H. 2005. Global virulence regulation in Staphylococcus aureus: pinpointing the roles of ClpP and ClpX in the sar/agr regulatory network. Infect. Immun. 73:8100–8108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frees D, Qazi SN, Hill PJ, Ingmer H. 2003. Alternative roles of ClpX and ClpP in Staphylococcus aureus stress tolerance and virulence. Mol. Microbiol. 48:1565–1578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.