Abstract
Bone tissue healing is a dynamic, orchestrated process that relies on multiple growth factors and cell types. Platelet-derived growth factor-BB (PDGF-BB) is released from platelets at wound sites and induces cellular migration and proliferation necessary for bone regeneration in the early healing process. Bone morphogenetic protein-2 (BMP-2), the most potent osteogenic differentiation inducer, directs new bone formation at the sites of bone defects. This study evaluated a combinatorial treatment protocol of PDGF-BB and BMP-2 on bone healing in a critical-sized defect model. To mimic the bone tissue healing process, a dual delivery approach was designed to deliver the rhPDGF-BB protein transiently during the early healing phase, whereas BMP-2 was supplied by rat bone marrow stromal cells (BMSCs) transfected with an adenoviral vector containing the BMP2 gene (AdBMP2) for prolonged release throughout the healing process. In in vitro experiments, the dual delivery of rhPDGF-BB and BMP2 significantly enhanced cell proliferation. However, the osteogenic differentiation of BMSCs was significantly suppressed even though the amount of BMP-2 secreted by the AdBMP2-transfected BMSCs was not significantly affected by the rhPDGF-BB treatment. In addition, dual delivery inhibited the mRNA expression of BMP receptor type II and Noggin in BMSCs. In in vivo experiments, critical-sized calvarial defects in rats showed enhanced bone regeneration by dual delivery of autologous AdBMP2-transfected BMSCs and rhPDGF-BB in both the amount of new bone formed and the bone mineral density. These enhancements in bone regeneration were greater than those observed in the group treated with AdBMP2-transfected BMSCs alone. In conclusion, the dual delivery of rhPDGF-BB and AdBMP2-transfected BMSCs improved the quality of the regenerated bone, possibly due to the modulation of PDGF-BB on BMP-2-induced osteogenesis.
Introduction
Growth factors are known to mediate wound healing and to regulate critical cellular activities, such as cellular recruitment, proliferation and differentiation of cell processes necessary for tissue regeneration.1–3 The platelet-derived growth factor (PDGF) is released from aggregated platelets during the early healing phase at the wound site and exerts chemotactic and mitogenic effects on inflammatory cells and undifferentiated mesenchymal cells.4 Although the osteogenic effects of PDGF in vitro are still controversial, regenerative therapy using rhPDGF-BB in preclinical and clinical studies has been reported to enhance bone regeneration, particularly in periodontal tissues.5–8 Bone morphogenetic proteins (BMPs) regulate differentiation, chemotaxis, growth and apoptosis of osteogenic cells and induce significant bone regeneration both orthotopically and ectopically.9–10 Among them, BMP-2 is one of the most potent osteoinductive proteins affecting osteoblast differentiation.11 Therefore, many studies have investigated bone regeneration in craniofacial and periodontal defects through the in vivo application of rhBMP-2 or the BMP2 gene.12–15
Bone formation is achieved through a sequential cascade of events relying on chemotaxis and mitosis of mesenchymal cells and differentiation of mesenchymal cells into osteoblasts.16 This process is directed by the coordinated expression of growth factors, including BMPs and PDGF, to regulate osteogenic differentiation in the proper sequence and time.17 PDGF-BB has a strong chemotactic effect on osteoblasts and acts to recruit mesenchymal cells into the wound site during bone formation.18,19 BMP-2 can direct these cells to undergo osteogenic differentiation into osteoblasts and to form bone nodules.20 Bone tissue engineering studies have also demonstrated that the combined therapy with PDGF-BB and BMP-2 induced more bone regeneration than either factor alone.21–23 However, the control over their release is one of the major concerns in growth factor delivery because each growth factor has distinct actions in bone formation.
In this study, we hypothesized that the dual delivery of PDGF-BB and BMP-2 could enhance bone regeneration and better simulate the bone healing process; we further hypothesized that this delivery would increase the number of cells capable of differentiating into osteoblasts and subsequently differentiate these cells into osteoblasts. This delivery strategy was accomplished using rhPDGF-BB protein delivery for its transient actions in the early healing phase and the BMP2 gene delivery to promote prolonged, sustained action. Therefore, rat bone marrow stromal cells (BMSCs) were transfected with adenoviral human BMP2 and delivered with rhPDGF-BB into a critical-sized defect in a rat calvarium. Before their in vivo application, the effects of the dual delivery of rhPDGF-BB and BMP2 on BMSCs were examined in vitro.
Materials and Methods
In vitro experiments
Cell isolation and culture
Rat BMSCs were harvested from both tibias of rats under general anesthesia of ketamine (90 mg/kg; Yuhan Co.) and xylazine (10 mg/kg; Bayer). Briefly, blood was collected from the tibial bone marrow.24 BMSCs were then isolated by centrifugation and suspended in the α-minimum essential medium (MEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Gibco) and a 1% penicillin–streptomycin solution (Gibco). The cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. The medium was changed twice per week. Cells from passages two to three were used for transfection.
BMP2 gene transfection and rhPDGF-BB treatment
The construction of the adenovirus encoding human BMP2 (AdBMP2) has been previously described.25 BMSCs were plated at a density of 1×104 cells/well on a 24-well plate (for the alkaline phosphatase [ALP] activity assay, BMP-2 expression quantitation assay and the mineralization assay) and at 5×103 cells/well on a 96-well plate (for the cell proliferation assay). BMSCs were treated with AdBMP2 at a multiplicity of infection of 100 pfu for 4 h. The cells were serum-starved in a medium containing 0.5% FBS overnight and were subsequently treated with rhPDGF-BB (30 ng/mL26; R&D System) for 6, 12, 24, or 48 h.
Cell proliferation assay
Cell proliferation was assessed with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (Sigma). The MTT solution was added to each cultured sample on a different day, and the solution was further incubated for 4 h to promote MTT formazan formation. The formazan was solubilized in dimethyl sulfoxide (DMSO; Sigma), and the absorbance of the solution was measured at 540 nm with a microplate reader (Molecular Devices). Triplicate readings were taken for each sample.
Quantitation of BMP-2 expression
The culture medium from the BMSCs in each well of the 24-well plates was sampled at 3, 7, 10, 14, 16, and 21 days after the rhPDGF-BB treatment and frozen at −80°C for later analysis. The amount of BMP-2 in the collected medium on each examination day was measured using a commercial enzyme immunoassay kit (R&D System). All of the experiments were performed in triplicate.
ALP activity assay
The ALP activity was measured at 7 and 14 days after rhPDGF-BB treatment according to the manufacturer's instructions (Anaspec Co.). The cell lysates were transferred to 96-well plates and incubated with the ALP substrate at 37°C for 30 min; the reaction was then halted by the addition of a stop buffer. The p-nitrophenol (pNP) product formed by the enzymatic hydrolysis of p-nitrophenylphosphate (pNPP) was measured at 405 nm using an absorbance microplate reader. The protein concentrations of the samples were measured using a protein assay kit (iNtRON Biotechnology). The ALP activity was expressed as the concentration of pNP transformed per microgram of protein.
Mineralization assay
For the mineralization assay, both AdBMP2-transfected BMSCs and control BMSCs were treated with rhPDGF-BB (according to the treatment time protocol) and cultured in osteogenic media (α-MEM containing 15% FBS supplemented with 0.2 mM ascorbic acid [Sigma] and 10 mM β-glycerolphosphate [Sigma]) for 28 days. The cells were then fixed in 95% cold ethanol and stained with a 1% Alizarin Red S solution (Wako Chemicals) for 5 min. Mineralization was examined and imaged. Finally, quantitation was performed using an eluting procedure with cetylpyridinium chloride in 10 mM sodium phosphate.
Real-time PCR assay
BMSCs were plated in a 92-mm-diameter dish at a density of 1×106 cells and transfected with AdBMP2. As described above, rhPDGF-BB was applied to the AdBMP2-transfected BMSCs. Total RNA was isolated from the cells at 0, 6, 12, 24, and 48 h after rhPDGF-BB treatment using the TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. To remove any residual genomic DNA in the RNA solutions, an RNase-free DNase (Qiagen) treatment was performed. The complementary DNA (cDNA) that was synthesized from 5 μg of RNA using a Superscript III First-Strand Synthesis System (Invitrogen) was used as a polymerase chain reaction (PCR) template. Real-time PCR (qPCR) was performed using a real-time PCR system (Applied Biosystems); the reactions contained cDNA, primers (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/tea) and SYBR Premix Ex Taq II (Takara Bio Co.). The thermal cycling conditions were as follows: one cycle at 95°C for 15 s and 40 cycles at 95°C for 15 s, 60°C for 15 s, and 72°C for 33 s. Post-PCR melting curves confirmed the specificity of single-target amplification, and the fold change of the gene of interest relative to Gapdh was determined. All of the reactions were performed in triplicate.
In vivo experiments
Animals
Seventy-two male Sprague-Dawley rats (8 weeks old) were used in this study. The care and treatment of the animals were conducted in accordance with guidelines established by the Seoul National University Institutional Animal Care and Use Committee. This study conformed to Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines for preclinical studies. The animal research protocol was approved by the Institute of Laboratory Animal Resources, Seoul National University (SNU-090410-1).
Preparation of autologous AdBMP2-transfected BMSCs and rhPDGF-BB delivery
To implant autologous BMSCs into defect sites, BMSCs were first harvested from both tibias of each animal under general anesthesia 3 weeks before calvarial surgery. The harvested BMSCs were cultured in the α-MEM supplemented with 10% FBS and an antibiotic–antimycotic solution. The cells from passages two and three were seeded at a density of 1×106 cells/dish and transfected with AdBMP2 according to their designated experimental group. BMP-2 expression was confirmed by ELISA before surgery.
Cells were labeled with chloromethylbenzamido-1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate (CM-DiI; Invitrogen) to track the transplanted cells according to the manufacturer's instructions.25 Briefly, cells were incubated with 5 μM CM-DiI for 5 min at 37°C and additionally for 15 min at 4°C.
BMSCs and 10 μg of rhPDGF-BB were delivered using 200 μL of 1% collagen hydrogel, which was prepared from purified porcine skin-derived type I atelocollagen, sterile lyophilized collagen (Matrixen-PSP; Bioland), 0.001 N HCl, 26 mM NaHCO3, 20 mM HEPES, and 0.025 N NaOH at pH 7.4.
Surgical procedure
The surgical procedure was performed under general anesthesia. A midline incision was made over the calvarium, and a full-thickness flap was elevated. An 8-mm critical-sized calvarial defect was created using a trephine bur (3i Implant Innovation) under sterile saline irrigation. The animals were divided into the following six experimental groups: (1) negative control group—empty; (2) vehicle group—collagen hydrogel alone; (3) BMSC group—collagen hydrogel containing autologous BMSCs; (4) rhPDGF-BB group—collagen hydrogel mixed with rhPDGF-BB; (5) BMSC/BMP2 group—collagen hydrogel containing autologous AdBMP2-transfected BMSCs; and (6) BMSC/BMP2/rhPDGF-BB group—collagen hydrogel containing both rhPDGF-BB and autologous AdBMP2-transfected BMSCs.
The incisions were sutured in layers with 5-0 chromic gut and 4-0 silk. All of the animals received a single intramuscular injection of cefazolin (30 mg/kg) 2 days after surgery. Six rats from each group were sacrificed 2 weeks after surgery, and the remaining six rats from each group were sacrificed 4 weeks after surgery.
Microcomputed tomography analysis
Tissues, including the surgical sites, were harvested and fixed in 10% neutralized buffered formalin, and microcomputed tomography (micro-CT) images were taken using a SkyScan 1172 (SkyScan) scanner. The percentage of mineralized bone volume of the defect tissue volume (BV/TV) was measured using a computer program (CT-analyzer; SkyScan) with a lower gray threshold level of 65.27 The bone mineral density (BMD; g/cm3) of the regenerated bone in the defect site was measured with the same program.
Histological and histomorphometric analyses
The specimens were decalcified with a 10% EDTA solution for 2 weeks before they were dehydrated through a series of ethanol solutions of increasing concentrations and embedded in paraffin. Five-micrometer-thick coronal sections through the center of the circular defects were obtained and stained with hematoxylin and eosin. The prepared specimens were examined by light microscopy.
After microscopic examination, a photograph of each slide was taken using a digital camera, and the resulting images were saved to a computer for histomorphometric analysis. The newly formed bone area (mm2) within the defect was measured using an automated image analysis system (Tomoro Scope Eye 3.5 Image Analyzer; Techsan Digital Imaging). Defect closure (percentage) was measured as the ratio of the area of newly formed bone divided by the area of the entire defect.
Statistical analysis
Triplicate results were analyzed for each in vitro experiment using two-way analysis of variance (ANOVA) with Tukey's post hoc tests. ANOVA with Tukey's post hoc tests was performed to evaluate the differences among the experimental groups at each interval in the in vivo experiment. A p-value less than 0.05 was considered statistically significant.
Results
Results of the in vitro experiment
The effects of the dual delivery of rhPDGF-BB and BMP2 on cell proliferation and osteogenic differentiation of BMSCs
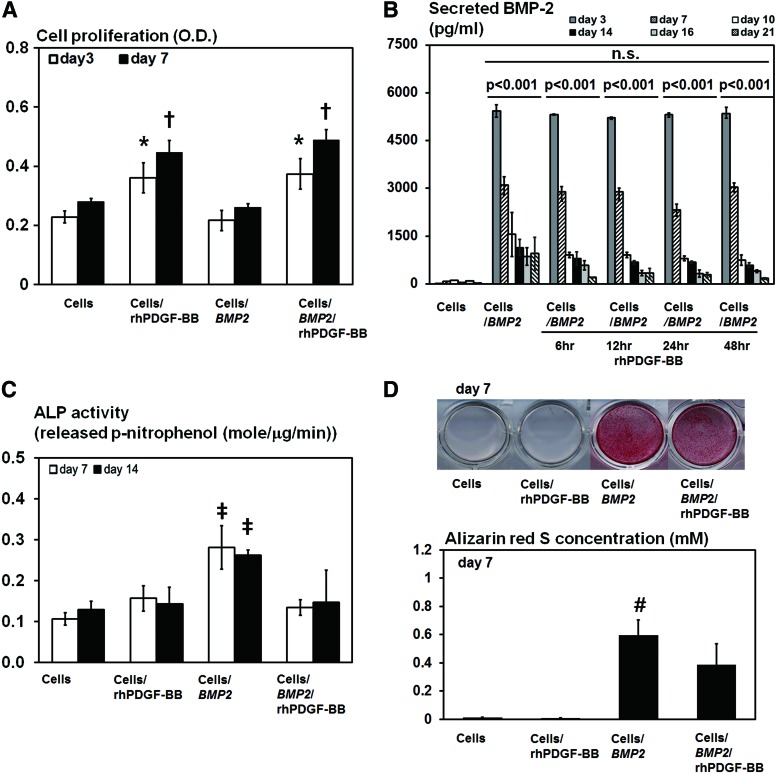
In the proliferation assay, rhPDGF-BB significantly enhanced cell proliferation of both BMSCs and AdBMP2-transfected BMSCs at days 3 and 7 (p<0.001 and p<0.001, respectively; Fig. 1A). The AdBMP2-transfected BMSCs significantly induced BMP-2 overexpression during a 21-day period, which was not affected by the duration of the rhPDGF-BB treatment (Fig. 1B). However, osteogenic differentiation of the AdBMP2-transfected BMSCs was significantly suppressed by the rhPDGF-BB treatment. The rhPDGF-BB treatment of the AdBMP2-transfected BMSCs significantly decreased their ALP activity at both days 7 and 14 (p<0.001 and p<0.001, respectively; Fig. 1C), which was similar to other groups in which BMP2 was not delivered. In addition, mineralized nodule formation was negatively affected by the rhPDGF-BB treatment of the AdBMP2-transfected BMSCs at day 7 (p=0.001; Fig. 1D).
FIG. 1.
The in vitro effects of rhPDGF-BB on AdBMP2-transfected BMSCs. (A) Effects of rhPDGF-BB treatment for 48 h on the proliferation of AdBMP2-transfected BMSCs (5×103 cells/well). The cell proliferation of both nontransfected BMSCs and AdBMP2-transfected BMSCs was significantly increased by the rhPDGF-BB treatment at days 3 and 7 (ANOVA, p<0.001 and p<0.001, respectively). (B) The amount of BMP-2 produced by AdBMP2-transfected BMSCs (1×104 cells/well), as determined by ELISA. Although BMP-2 was overexpressed over a 21-day period in AdBMP2-transfected BMSCs, BMP-2 expression was significantly decreased after 21 days compared to the initial levels (ANOVA, p<0.001). Furthermore, the rhPDGF-BB treatment did not significantly influence BMP-2 expression at each interval. (C) Effects of rhPDGF-BB on ALP (1×104 cells/well). The ALP activity was significantly increased by BMP2 gene delivery, which was suppressed by the rhPDGF-BB treatment for 48 h after 7 and 14 days (ANOVA, p<0.001 and p<0.001, respectively). (D) Effects of rhPDGF-BB on the mineralization of AdBMP2-transfected BMSCs (1×104 cells/well). AdBMP2-transfected BMSCs formed mineralization nodules at day 7, which was significantly suppressed by the rhPDGF-BB treatment for 48 h (ANOVA, p=0.001). *Statistically significant difference from the BMSCs and AdBMP-2-trasnfected BMSCs at day 3. †Statistically significant difference from the BMSCs and AdBMP-2-trasnfected BMSCs at day 7. ‡Statistically significant difference from the other groups. #Statistically significant difference from the other groups. Values represent mean±SD of three samples. OD, optical density. BMP-2, bone morphogenetic protein-2; BMSC, bone marrow stromal cell; ALP, alkaline phosphatase; ANOVA, analysis of variance; PDGF-BB, platelet-derived growth factor-BB; AdBMP-2, adenoviral vector containing the BMP2 gene. Color images available online at www.liebertpub.com/tea
Gene expression patterns of AdBMP2-transfected BMSCs following the rhPDGF-BB treatment
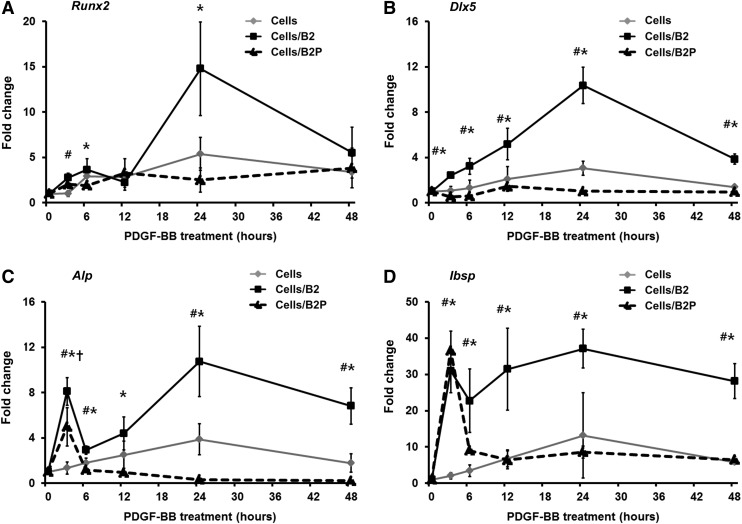
The rhPDGF-BB treatment significantly suppressed the mRNA expression of osteogenic factors in AdBMP2-transfected BMSCs; the mRNA expression of runt-related transcription factor 2 (Runx2), distal-less homeobox 5 (Dlx5), ALP, and integrin-binding sialoprotein (Ibsp) of AdBMP2-transfected BMSCs were significantly suppressed by rhPDGF-BB over 48 h; the suppression levels were the highest at 24 h (Fig. 2A–D).
FIG. 2.
Gene expression patterns of osteogenic markers in AdBMP2-transfected BMSCs following rhPDGF-BB treatment. The rhPDGF-BB treatment significantly suppressed the mRNA expression of (A) Runx2, (B) Dlx5, (C) Alp, and (D) Ibsp induced by the AdBMP2-transfected BMSCs, especially at 24 h. The mRNA expression of each osteogenic factor was normalized against Gapdh. *Statistically significant difference between AdBMP2-transfected BMSCs and AdBMP2-transfected BMSCs/rhPDGF-BB (ANOVA, p<0.05). #Statistically significant difference between nontransfected BMSCs and AdBMP2-transfected BMSCs (ANOVA, p<0.05). †Statistically significant difference between nontransfected BMSCs and AdBMP2-transfected BMSCs/rhPDGF-BB (ANOVA, p<0.05). Cell, nontransfected BMSCs; cells/B2, AdBMP2-transfected BMSCs; cells/B2P, AdBMP2-transfected BMSCs/rhPDGF-BB. Values represent mean±SD of three samples.
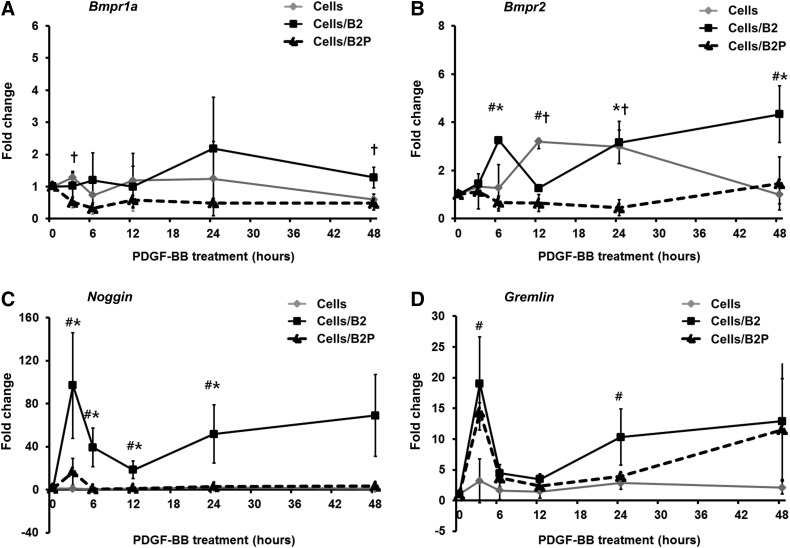
The mRNA expression of BMP receptor type II (BMPRII), a major receptor of BMP-2, was also significantly inhibited by the rhPDGF-BB treatment in the AdBMP2-transfected BMSCs, while the mRNA levels of BMP receptor type Ia (BMPRIa) were not significantly affected (Fig. 3A, B). In addition, the mRNA expression of Noggin, an inhibitory molecule of BMP-2 signal transduction, was significantly suppressed by the rhPDGF-BB treatment of the AdBMP2-transfected BMSCs compared to the AdBMP2-transfected BMSCs (Fig. 3C). The AdBMP2-transfected BMSCs highly overexpressed Noggin mRNA over the course of 48 h, whereas the dual delivery of BMP2 and rhPDGF-BB maintained Noggin expression at low levels. Gremlin, another inhibitory molecule, was also slightly decreased in the rhPDGF-BB-treated AdBMP2-transfected BMSCs; however, the decrease was not statistically significant (Fig. 3D).
FIG. 3.
Gene expression patterns of BMP-2 related factors Bmpr1a (A), Bmpr2 (B), Noggin (C), and Gremlin (D) in AdBMP2-transfected BMSCs following the rhPDGF-BB treatment. The mRNA expression of both BMPRII and Noggin in the AdBMP2-transfected BMSCs was significantly decreased by the rhPDGF-BB treatment. The mRNA expression of each factor was normalized against Gapdh. *Statistically significant difference between AdBMP2-transfected BMSCs and AdBMP2-transfected BMSCs/rhPDGF-BB (ANOVA, p<0.05). #Statistically significant difference between nontransfected BMSCs and AdBMP2-transfected BMSCs (ANOVA, p<0.05). †Statistically significant difference between nontransfected BMSCs and AdBMP2-transfected BMSCs/rhPDGF-BB (ANOVA, p<0.05). Cell, nontransfected BMSCs; cells/B2, AdBMP2-transfected BMSCs; cells/B2P, AdBMP2-transfected BMSCs/rhPDGF-BB. Bmpr1a, BMP receptor type Ia; Bmpr2, BMP receptor type II. Values represent mean±SD of three samples.
Results of the in vivo experiment
Histological findings and histomorphometric analysis
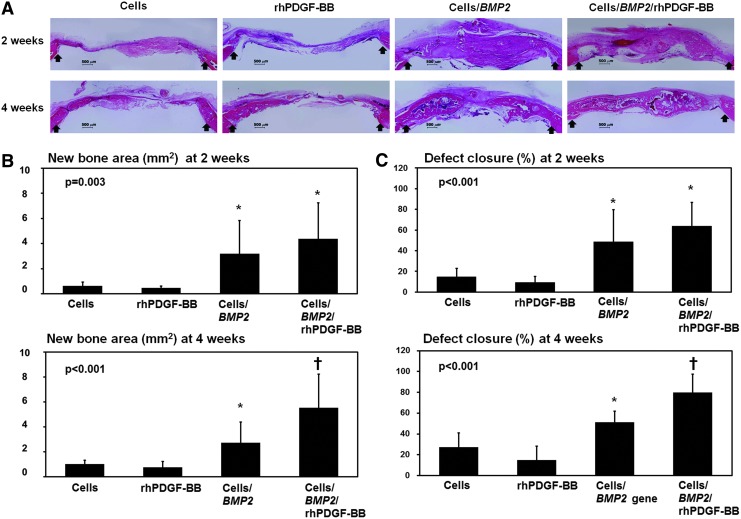
The implanted cells on the calvarial defects were detected in the regenerative bone tissue on histological sections at 2 and 4 weeks after surgery (Supplementary Fig. S1). When nontransfected BMSCs alone or rhPDGF-BB alone was applied into a critical-sized defect, a limited amount of bone regeneration was observed along the margin of the defects at 2 and 4 weeks after surgery (Fig. 4A). However, both the BMSC/BMP2 and the BMSC/BMP2/rhPDGF-BB groups showed extensive immature woven bone formation at 2 weeks; these formations were replaced with rich bone marrow at 4 weeks. Moreover, it was found that bone bridging connected the central region of the defect in the BMSC/BMP2/rhPDGF-BB group. The negative and the vehicle groups showed minimal bone regeneration (Supplementary Fig. S2 and Supplementary Table S2).
FIG. 4.
In vivo histology and histomorphometric analysis. (A) Representative histology at 2 and 4 weeks postsurgery (decalcified; H&E stain; original magnification 2×). Both the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups showed extensive new bone formation at 2 and 4 weeks. In the BMSC/BMP2/rhPDGF-BB group, bone bridging that covered the central region of the defect area was observed. Arrow: margin of defect. (B) New bone area (mm2) at the defect site. Both the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups showed significantly higher amounts of new bone area at 2 weeks after surgery (ANOVA, p=0.003). However, there was no significant difference between the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups. At 4 weeks, the BMSC/BMP2/rhPDGF-BB group had significantly higher levels of new bone area compared to the other groups (ANOVA, p<0.001). (C) Defect closure (%) within the defects. Both the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups produced a significantly higher percentage of defect closure at 2 weeks (ANOVA, p<0.001). However, there was no significant difference between the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups. At 4 weeks, the defect closure of the BMSC/BMP2/rhPDGF-BB group was significantly higher than the other groups (ANOVA, p<0.001). Defect closure was measured as the ratio of the area of newly formed bone divided by the area of the whole defect. *Statistically different from the BMSCs and rhPDGF-BB groups. †Statistically different from the other groups. Values represent mean±SD of six samples.
In the histomorphometric analysis, the most new bone area at 2 weeks was observed in the BMSC/BMP2/rhPDGF-BB group (Fig. 4B). Both the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups showed a significantly greater new bone area than the other groups at 2 weeks after surgery (p=0.003). However, no significant difference was observed between the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups. At 4 weeks, the BMSC/BMP2/rhPDGF-BB group showed significantly more new bone area than other groups (p<0.001).
The defect closures of the critical-sized calvarial defects in both the BMSC/BMP2 and the BMSC/BMP2/rhPDGF-BB groups (48.60%±30.90% and 63.75%±22.94%, respectively), were significantly greater than those of the other groups at 2 weeks (p<0.001; Fig. 4C). However, no significant difference was observed between the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups. At 4 weeks, the BMSC/BMP2/rhPDGF-BB group showed significantly greater defect closure rates (79.68%±17.64%) than the other groups (p<0.001).
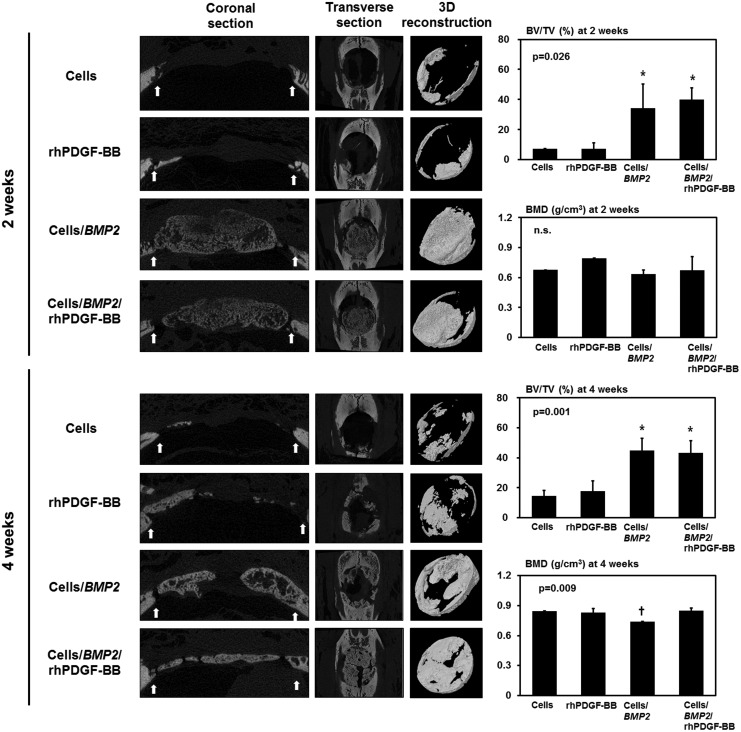
Micro-CT findings and BMD
The newly formed BV at each defect site was evaluated using three-dimensional reconstructed micro-CT images (Fig. 5). Both the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups showed extensive new bone formation, although the newly formed bone had low radiopacity at 2 weeks after surgery. At 4 weeks, the radiopacity of the newly formed bone was enhanced in both the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups compared to the 2-week samples.
FIG. 5.
Micro-CT analysis and quantitative measurements of calvarial defects at 2 and 4 weeks. Both the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups showed extensive new bone formation, in which the new bone had low radiopacity at 2 weeks. The new bone at 4 weeks was more mature and had enhanced radiopacity compared to the bone at 2 weeks. Both BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups showed significantly higher levels of mineralized BV percentage to TV of defect at 2 and 4 weeks (ANOVA, p=0.026 and p=0.001, respectively). However, there was no significant difference between BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups. In terms of BMD, there was no statistical significance at 2 weeks. At 4 weeks, the BMSC/BMP2 group showed significantly less BMD of newly formed bone than other groups, including BMSC/BMP2/rhPDGF-BB groups (ANOVA, p=0.009). *Statistically different from the BMSCs and rhPDGF-BB groups. †Statistically different from the other groups. Micro-CT images: left, coronal section view; middle, transverse view; right, 3D reconstruction of newly formed bone in the defect site; arrow, margin of defect; BV/TV, the percentage of mineralized bone volume of the defect tissue volume; BMD, bone mineral density. Values represent mean±SD of six samples. micro-CT, microcomputed tomography.
For quantitative analysis, the ratio of mineralized BV/TV was measured using micro-CT images. Both the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups showed significantly higher BV/TV values compared to the other groups at 2 weeks (p=0.026). However, no statistical significance was observed between the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups. The BMD of the newly formed bone at 2 weeks was similar in all the groups.
At 4 weeks, BV/TV was significantly greater in the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups (p=0.001). However, there was no statistical significance between the BMSC/BMP2 and BMSC/BMP2/rhPDGF-BB groups. The BMD of all of the experimental groups was higher at 4 weeks compared to 2 weeks except for the BMSC/BMP2 group. In addition, the BMD of the BMSC/BMP2 group was significantly less than the other groups (p=0.009).
Discussion
Bone healing is a highly coordinated process that is orchestrated by various growth factors and cells. Tissue engineering mimics these healing process using exogenous or endogenous growth factors, the required cells and scaffolds. Many studies have described the effects of single and multifactor application on bone healing. Our previous work showed that the transplantation of autologous BMSCs and PDL cells with HA/TCP was more effective than cell-free HA/TCP in a canine model.28 We also demonstrated that the combined therapy of BMP2 with a collagen sponge or BMP2 with bone graft materials promoted bone regeneration in animal studies.25,29 Although combined therapies of BMP-2 and BMSC or carrier enhanced bone healing, these therapies have distinct mechanisms in bone formation. This study designed a system of combined and concerted delivery of BMP-2 and PDGF-BB using BMSCs and collagen gel to mimic the concomitant interactions among the various factors involved in bone regeneration; PDGF-BB was delivered in protein form to act transiently during early healing, whereas BMP-2 was produced from BMSCs transfected with BMP2 to provide BMP-2 during the entire wound-healing period, thereby mimicking natural healing events. Therefore, the present study demonstrated that the dual delivery of rhPDGF-BB and BMP2-transfected BMSCs improved bone formation in terms of bone quantity and quality.
When BMP2-transfected BMSCs were applied alone, new bone formation increased after 2 weeks at levels similar to a dual-delivery group. However, the BMD did not improve after 4 weeks when compared to the dual-delivery groups. The BMD of the newly formed bone is one of the critical parameters of bone quality because BMD indicates bone strength and maturation.30,31 Similar to this study, it has been reported that the single application of BMP-2 as a protein or gene does not lead to bone healing in some critical-sized bone defects.32–36 Virk et al.37 proposed that the long-term application of BMP-2, such as more than 8 weeks of BMP-2 using a lentivirus, was required to stimulate mechanically superior bone formation. However, lentiviruses may be inserted into the host genome, whereas adenoviruses can only be inherited by daughter cells of previously transfected cells. Accordingly, additional signal transduction is needed to facilitate the maturation of bone in BMP2-transfected animals, and PDGF-BB may represent one of these signaling molecules. In fact, some studies have reported superior biomechanical properties in bone treated with rhPDGF-BB. In the ovariectomized osteoporotic rat model, the systemic administration of PDGF-BB prevented bone loss and increased bone density and skeletal strength.38 Moreover, in geriatric osteoporotic rat models, a PDGF-BB treatment accelerated fracture healing; the rhPDGF-BB-treated tibias had equivalent torsional strengths compared to the contralateral nonoperated site.39 The PDGF-BB treatment in a rabbit tibial osteotomy site increased the callus density and volume and bone strength; the resulting values were similar to nonoperated contralateral bones.40 Tokunaga et al.41 also reported that PDGF receptor-β-depleted mice showed an increased immature woven bone ratio even though PDGF receptor-β signaling inhibited the osteogenic differentiation of mesenchymal stem cells. Similar to previous studies, rhPDGF-BB application in the current study also resulted in higher BMD increases compared to the BMP2 treatment alone.
However, some inconsistencies were apparent between the in vitro and in vivo studies. The in vitro results demonstrated that rhPDGF-BB suppresses osteogenic differentiation, while the in vivo results demonstrated that rhPDGF-BB cannot affect the amount of bone formation. The mitogenic effects of rhPDGF-BB were not hindered by BMP2 delivery, and rhPDGF-BB did not interfere with BMP-2 secretion in AdBMP2-transfected BMSCs. However, osteogenic differentiation with BMP-2 was affected by rhPDGF-BB. This result may be attributed to the reduced gene expression of BMP-2 signaling-related proteins in the present study. The mRNA expression levels of Runx2 and Dlx5, a mediator between BMP-2 and Runx2,42 were significantly suppressed by rhPDGF-BB. In addition, BMPRII mRNA expression was suppressed by rhPDGF-BB. Consequently, it seems that rhPDGF-BB interferes with BMP-2-dependent osteogenic differentiation in vitro via the suppression of BMPRII, which is required to initiate BMP-2 signaling, resulting in the downregulation of Runx2 and Dlx5.43–45 The suppressive effect of PDGF-BB on osteogenic differentiation has also been reported in previous studies.46,47 Gruber et al.48 also reported that PDGF decreased osteogenic differentiation of mesenchymal cells, but increased cellular migration and proliferation. Recently, Gharibi et al.49 suggested that PDGF-BB regulates proliferation and differentiation in relation to self-renewal in mesenchymal cells, thereby maintaining their multipotency through PDGFRβ-induced Akt and Erk pathways. This result may help to explain the synergistic or additive effects of rhPDGF-BB and AdBMP2-transfected BMSCs in this in vivo study. In conclusion, rhPDGF-BB may modulate differentiation in vivo and suppress osteogenic differentiation in vitro. Kratchmarova et al.50 also reported that PDGF increased new bone formation in vivo, although PDGF minimally influenced osteogenic differentiation in vitro.
Some authors have reported that Noggin can negatively affect bone formation. Gazzerro et al.51 reported that Noggin overexpression arrested stromal cell differentiation and prevented cellular maturation. Additionally, murine stromal cells, which overexpressed Noggin, showed a delayed appearance of mineralized nodules and an absence of osteocalcin in their study. The overexpression of Noggin has also been reported to decrease trabecular BV and impair osteoblastic function, leading to osteopenia and fractures.52,53 In fact, significant increases of Noggin mRNA have been reported in nonunion human samples compared with healing bone tissue, and this imbalanced Noggin expression was proposed to be the reason for nonunion fracture healing.54–56 Furthermore, Jin et al.57 reported that periodontal bone repair was significantly inhibited by adenoviral Noggin gene delivery. The present study demonstrated that rhPDGF-BB suppressed Noggin expression (Fig. 3C). Noggin expression was significantly increased in AdBMP2-transfected BMSCs. However, Noggin expression was not increased following the rhPDGF-BB treatment in the AdBMP2-transfected BMSCS. The inhibitory effect of PDGF-BB on Noggin expression may also positively affect bone maturation of newly formed bone induced by BMP-2.
Taken together, BMP-2 expression through ex vivo adenoviral BMP2 gene delivery was maintained for a 21-day period and was not influenced by rhPDGF-BB. rhPDGF-BB increased the number of cells capable of differentiating into various cell types, including osteoblasts needed for bone tissue healing. rhPDGF-BB demonstrated a modulatory effect on BMP-2-induced osteogenesis through the inhibition of the expression of both BmprII and Noggin. Although rhPDGF-BB suppressed the BMP-2-induced osteogenic differentiation of BMSCs in vitro, the dual delivery of rhPDGF-BB and AdBMP2-transfected BMSCs induced excellent new bone formation and significantly increased BMD compared to the BMP2/BMSC group.
Supplementary Material
Acknowledgments
The authors would like to appreciate the careful reading and helpful advice of Professor W.V. Giannobile (Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, USA; Michigan Center of Oral Health Research, Ann Arbor, USA). The authors thank Ms. Kwak, Eun-Hye for her technical support. This study was supported by grant no 03-2009-0018 from the SNUDH Research Fund.
Disclosure Statement
The authors have no competing financial interests.
References
- 1.Giannobile W.V. Periodontal tissue engineering by growth factors. Bone. 1996;19:23S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaigler D. Cirelli J.A. Giannobile W.V. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin Drug Deliv. 2006;3:647. doi: 10.1517/17425247.3.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen F.M. Zhang M. Wu Z.F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 4.Canalis E. Varghese S. McCarthy T.L. Centrella M. Role of platelet derived growth factor in bone cell function. Growth Regul. 1992;2:151. [PubMed] [Google Scholar]
- 5.Nevins M. Kao R.T. McGuire M.K. McClain P.K. Hinrichs J.E. McAllister B.S., et al. PDGF promotes periodontal regeneration in localized osseous defects: 36 month extension results from a randomized, controlled, double-masked clinical trial. J Periodontol. 2013;84:456. doi: 10.1902/jop.2012.120141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke J.W. Sarment D.P. Whitesman L.A. Miller S.E. Jin Q. Lynch S.E., et al. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng. 2006;12:1441. doi: 10.1089/ten.2006.12.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollinger J.O. Hart C.E. Hirsch S.N. Lynch S. Friedlaender G.E. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90(Suppl 1):48. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- 8.Hock J.M. Canalis E. Platelet-derived growth factor enhances bone cell replication, but not differentiated function of osteoblasts. Endocrinology. 1994;134:1423. doi: 10.1210/endo.134.3.8119182. [DOI] [PubMed] [Google Scholar]
- 9.Reddi A.H. Bone morphogenetic proteins: from basic science to clinical applications. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S1. doi: 10.2106/00004623-200100001-00001. [DOI] [PubMed] [Google Scholar]
- 10.Urist M.R. Bone: formation by autoinduction. Science. 1965;150:893. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 11.Groeneveld E.H. Burger E.H. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol. 2000;142:9. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.H. Kim C.S. Choi K.H. Jung U.W. Yun J.H. Choi S.H., et al. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials. 2010;31:3512. doi: 10.1016/j.biomaterials.2010.01.075. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y.L. Chen P.K. Jeng L.B. Huang C.S. Yang L.C. Chung H.Y., et al. Periodontal regeneration using ex vivo autologous stem cells engineered to express the BMP-2 gene: an alternative to alveolaplasty. Gene Ther. 2008;15:1469. doi: 10.1038/gt.2008.131. [DOI] [PubMed] [Google Scholar]
- 14.Seol Y.J. Park Y.J. Lee S.C. Kim K.H. Lee J.Y. Kim T.I., et al. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. J Biomed Mater Res A. 2006;77:599. doi: 10.1002/jbm.a.30639. [DOI] [PubMed] [Google Scholar]
- 15.Chang S.C. Chuang H. Chen Y.R. Yang L.C. Chen J.K. Mardini S., et al. Cranial repair using BMP-2 gene engineered bone marrow stromal cells. J Surg Res. 2004;119:85. doi: 10.1016/j.jss.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Jones E. Yang X. Mesenchymal stem cells and bone regeneration: current status. Injury. 2011;42:562. doi: 10.1016/j.injury.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Hughes F.J. Turner W. Belibasakis G. Martuscelli G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontol 2000. 2006;41:48. doi: 10.1111/j.1600-0757.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 18.Heldin C.H. Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 19.Park Y.J. Lee Y.M. Lee J.Y. Seol Y.J. Chung C.P. Lee S.J. Controlled release of platelet-derived growth factor-BB from chondroitin sulfate-chitosan sponge for guided bone regeneration. J Control Release. 2000;67:385. doi: 10.1016/s0168-3659(00)00232-7. [DOI] [PubMed] [Google Scholar]
- 20.Hughes F.J. Collyer J. Stanfield M. Goodman S.A. The effects of bone morphogenetic protein-2, -4, and -6 on differentiation of rat osteoblast cells in vitro. Endocrinology. 1995;136:2671. doi: 10.1210/endo.136.6.7750491. [DOI] [PubMed] [Google Scholar]
- 21.Hsiong S.X. Mooney D.J. Regeneration of vascularized bone. Periodontol 2000. 2006;41:109. doi: 10.1111/j.1600-0757.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y. Cheng N. Miron R. Shi B. Cheng X. Delivery of PDGF-B and BMP-7 by mesoporous bioglass/silk fibrin scaffolds for the repair of osteoporotic defects. Biomaterials. 2012;33:6698. doi: 10.1016/j.biomaterials.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y.C. Kaigler D. Rice K.G. Krebsbach P.H. Mooney D.J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 24.Maniatopoulos C. Sodek J. Melcher A.H. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- 25.Shin J.H. Kim K.H. Kim S.H. Koo K.T. Kim T.I. Seol Y.J., et al. Ex vivo bone morphogenetic protein-2 gene delivery using gingival fibroblasts promotes bone regeneration in rats. J Clin Periodontol. 2010;37:305. doi: 10.1111/j.1600-051X.2009.01522.x. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhary L.R. Avioli L.V. Extracellular-signal regulated kinase signaling pathway mediates downregulation of type I procollagen gene expression by FGF-2, PDGF-BB, and okadaic acid in osteoblastic cells. J Cell Biochem. 2000;76:354. [PubMed] [Google Scholar]
- 27.Park S.Y. Kim K.H. Koo K.T. Lee K.W. Lee Y.M. Chung C.P., et al. The evaluation of the correlation between histomorphometric analysis and micro-computed tomography analysis in AdBMP-2 induced bone regeneration in rat calvarial defects. J Periodontal Implant Sci. 2011;41:218. doi: 10.5051/jpis.2011.41.5.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S.H. Kim K.H. Seo B.M. Koo K.T. Kim T.I. Seol Y.J., et al. Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: a pilot study. J Periodontol. 2009;80:1815. doi: 10.1902/jop.2009.090249. [DOI] [PubMed] [Google Scholar]
- 29.Jhin M.J. Kim K.H. Kim S.H. Kim Y.S. Kim S.T. Koo K.T., et al. Ex vivo bone morphogenetic protein-2 gene delivery using bone marrow stem cells in rabbit maxillary sinus augmentation in conjunction with implant placement. J Periodontol. 2013;84:985. doi: 10.1902/jop.2012.120221. [DOI] [PubMed] [Google Scholar]
- 30.Lam T.P. Hung V.W. Yeung H.Y. Tse Y.K. Chu W.C. Ng B.K., et al. Abnormal bone quality in adolescent idiopathic scoliosis: a case-control study on 635 subjects and 269 normal controls with bone densitometry and quantitative ultrasound. Spine (Phila Pa 1976) 2011;36:1211. doi: 10.1097/BRS.0b013e3181ebab39. [DOI] [PubMed] [Google Scholar]
- 31.Misra M. Katzman D. Miller K.K. Mendes N. Snelgrove D. Russell M., et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26:2430. doi: 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wikesjo U.M.E. Qahash M. Polimeni G. Susin C. Shanaman R.H. Rohrer M.D., et al. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: histologic observations. J Clin Periodontol. 2008;35:1001. doi: 10.1111/j.1600-051X.2008.01321.x. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S. Wan C. Ramaswamy G. Clemens T.L. Ponnazhagan S. Mesencymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18:1026. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao C. Zhou H. Liu G. Zhang P. Fu Y. Gu P., et al. Bone marrow stromal cells with a combined expression of BMP-2 and VEGF-165 enhanced bone regeneration. Biomed Mater. 2011;16:015013. doi: 10.1088/1748-6041/6/1/015013. [DOI] [PubMed] [Google Scholar]
- 35.Menendez M.I. Clark D.J. Carlton M. Flanigan D.C. Jia G. Sammet S., et al. Direct delayed human adenoviral BMP-2 or BMP-6 gene therapy for bone and cartilage regeneration in a pony osteochondral model. Osteoarthritis Cartilage. 2011;19:1066. doi: 10.1016/j.joca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Lieberman J.R. Daluiski A. Stevenson S. Wu L. McAllister P. Lee Y.P., et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Virk M.S. Conduah A. Park S.H. Liu N. Sugiyama O. Cuomo A., et al. Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model. Bone. 2008;42:921. doi: 10.1016/j.bone.2007.12.216. [DOI] [PubMed] [Google Scholar]
- 38.Mitlak B.H. Finkelman R.D. Hill E.L. Li J. Martin B. Smith T., et al. The effect of systemically administered PDGF-BB on the rodent skeleton. J Bone Miner Res. 1996;11:238. doi: 10.1002/jbmr.5650110213. [DOI] [PubMed] [Google Scholar]
- 39.Hollinger J.O. Onikepe A.O. MacKrell J. Einhorn T. Bradica G. Lynch S., et al. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet-derived growth factor-BB and an injectable beta-tricalcium phosphate/collagen matrix. J Orthop Res. 2008;26:83. doi: 10.1002/jor.20453. [DOI] [PubMed] [Google Scholar]
- 40.Nash T.J. Howlett C.R. Martin C. Steele J. Johnson K.A. Hicklin D.J. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15:203. doi: 10.1016/8756-3282(94)90709-9. [DOI] [PubMed] [Google Scholar]
- 41.Tokunaga A. Oya T. Ishii Y. Motomura H. Nakamura C. Ishizawa S., et al. PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res. 2008;23:1519. doi: 10.1359/jbmr.080409. [DOI] [PubMed] [Google Scholar]
- 42.Lee M.H. Kim Y.J. Kim H.J. Park H.D. Kang A.R. Kyung H.M., et al. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem. 2003;278:34387. doi: 10.1074/jbc.M211386200. [DOI] [PubMed] [Google Scholar]
- 43.Yu P.B. Beppu H. Kawai N. Li E. Bloch K.D. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J Biol Chem. 2005;280:24443. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- 44.Jin W. Yun C. Kim H.S. Kim S.J. TrkC binds to the bone morphogenetic protein type II receptor to suppress bone morphogenetic protein signaling. Cancer Res. 2007;67:9869. doi: 10.1158/0008-5472.CAN-07-0436. [DOI] [PubMed] [Google Scholar]
- 45.Liu H. Zhang R. Chen D. Oyajobi B.O. Zhao M. Functional redundancy of type II BMP receptor and type IIB activin receptor in BMP2-induced osteoblast differentiation. J Cell Physiol. 2012;227:952. doi: 10.1002/jcp.22802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsieh S.C. Graves D.T. Pulse application of platelet-derived growth factor enhances formation of a mineralizing matrix while continuous application is inhibitory. J Cell Biochem. 1998;69:169. doi: 10.1002/(sici)1097-4644(19980501)69:2<169::aid-jcb7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 47.Yu X. Hsieh S.C. Bao W. Graves D.T. Temporal expression of PDGF receptors and PDGF regulatory effects on osteoblastic cells in mineralizing cultures. Am J Physiol. 1997;272:C1709. doi: 10.1152/ajpcell.1997.272.5.C1709. [DOI] [PubMed] [Google Scholar]
- 48.Gruber R. Karreth F. Kandler B. Fuerst G. Rot A. Fischer M.B., et al. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets. 2004;15:29. doi: 10.1080/09537100310001643999. [DOI] [PubMed] [Google Scholar]
- 49.Gharibi B. Ghuman M.S. Hughes F.J. Akt and Erk-mediated regulation of proliferation and differentiation during PDGFRbeta-induced MSC self-renewal. J Cell Mol Med. 2012;16:2789. doi: 10.1111/j.1582-4934.2012.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kratchmarova I. Blagoev B. Haack-Sorensen M. Kassem M. Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308:1472. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 51.Gazzerro E. Du Z. Devlin R.D. Rydziel S. Priest L. Economides A.N., et al. Noggin arrests stromal cell differentiation in vitro. Bone. 2003;32:111. doi: 10.1016/s8756-3282(02)00948-1. [DOI] [PubMed] [Google Scholar]
- 52.Devlin R.D. Du Z. Pereira R.C. Kimble R.B. Economides A.N. Jorgetti V., et al. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144:1972. doi: 10.1210/en.2002-220918. [DOI] [PubMed] [Google Scholar]
- 53.Aspenberg P. Jeppsson C. Economides A.N. The bone morphogenetic proteins antagonist Noggin inhibits membranous ossification. J Bone Miner Res. 2001;16:497. doi: 10.1359/jbmr.2001.16.3.497. [DOI] [PubMed] [Google Scholar]
- 54.Kloen P. Lauzier D. Hamdy R.C. Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone. 2012;51:59. doi: 10.1016/j.bone.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 55.Niikura T. Hak D.J. Reddi A.H. Global gene profiling reveals a downregulation of BMP gene expression in experimental atrophic nonunions compared to standard healing fractures. J Orthop Res. 2006;24:1463. doi: 10.1002/jor.20182. [DOI] [PubMed] [Google Scholar]
- 56.Fajardo M. Liu C.J. Egol K. Levels of expression for BMP-7 and several BMP antagonists may play an integral role in a fracture nonunion: a pilot study. Clin Orthop Relat Res. 2009;467:3071. doi: 10.1007/s11999-009-0981-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin Q.M. Anusaksathien O. Webb S.A. Rutherford R.B. Giannobile W.V. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol. 2003;74:202. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.