Abstract
Properties of the cell-material interface are determining factors in the successful function of cells for cartilage tissue engineering. Currently, cell adhesion is commonly promoted through the use of polypeptides; however, due to their lack of complementary or modulatory domains, polypeptides must be modified to improve their ability to promote adhesion. In this study, we utilized the principle of matrix-based biomimetic modification and a recombinant protein, which spans fragments 7–10 of fibronectin module III (heterophilic motif ) and extracellular domains 1–2 of cadherin-11 (rFN/Cad-11) (homophilic motif ), to modify the interface of collagen type II (Col II) sponges. We showed that the designed material was able to stimulate cell proliferation and promote better chondrogenic differentiation of rabbit mesenchymal stem cells (MSCs) in vitro than both the FN modified surfaces and the negative control. Further, the Col II/rFN/Cad-11-MSCs composite stimulated cartilage formation in vivo; the chondrogenic effect of Col II alone was much less significant. These results suggested that the rFN/Cad-11-modified collagen type II biomimetic interface has dual biological functions of promoting adhesion and stimulating chondrogenic differentiation. This substance, thus, may serve as an ideal scaffold material for cartilage tissue engineering, enhancing repair of injured cartilage in vivo.
Introduction
Properties of the cell-material interface are decisive factors in influencing the function of cells for cartilage tissue engineering because the interface provides the microenvironment for cell migration, adhesion, proliferation, differentiation, and matrix mineralization.1 The process by which cells adhere to the material surface can be divided into four consecutive and overlapping phases: cell attachment, spreading, cytoskeletal organization, and the formation of focal adhesion plaques.2,3 Cell-material adhesion is a complex, multifactorial process. Studies have demonstrated that a number of physical, chemical, and biological factors can all regulate and influence cell adhesion and chondrogenic differentiation. These factors include the roughness of the material interface, hydrophilicity, stress distribution, surface charge distribution, the density and strength of biological ligands, and the local microstructure.4,5
The biology of chondrocytes is highly influenced by their interactions with specific extracellular matrix (ECM) molecules.6 In recent years, the principle of surface modification has been utilized to mimic the interaction between ECM proteins and their surrounding cells. In this way, the adhesion, proliferation, and differentiation of seeded cells on the surfaces of materials for cartilage tissue engineering can be specifically targeted and promoted. Adhesive proteins or peptides are attached to the scaffold materials to provide them with biological recognition sequences and to thereby improve the attachment of seeded cells. Among these adhesive peptides, the most widely used ones are polypeptides containing an arginine-glycine-aspartic acid (RGD) sequence, which can be grafted to a wide variety of polymers to improve the efficiency of cell adhesion.
Polypeptides are more controllable than full-length proteins. However, compared with intact natural ligands, the activity of polypeptides is limited due to the lack of complementary or modulatory domains. For example, the α5β1 integrin needs to interact with the PHSRN (proline-histidine-serine-arginine-asparagine) motif and the RGD motif in the ninth type III repeat of fibronectin (FN). Although the binding affinity for each isolated motif is relatively weak, the combined interaction yields significant biological effects and can provide stable adhesion.7 In addition, linear RGD specifically binds to integrin receptors.8 Yamato et al. and Jeschke et al. demonstrated that synthetic RGD peptides often failed to achieve the ideal promotion of adhesion because they were restricted by their density and conformational specificity and had issues with desorption in particular force fields.9,10 Synthetic RGD peptides are deprived of the surrounding protein conformation, leading to greatly reduced binding affinity between the ligand and receptor.11 Further, studies in some laboratories suggested that scaffolds functionalized with RGD peptides inhibit mesenchymal stem cells' (MSCs) chondrogenesis.12,13 Therefore, it is necessary to consider the possibility of grafting motifs with adhesive functions to other molecules with known three-dimensional structures, adhesive properties, and chondrogenic functions to achieve maximum adhesion.
Integrin receptors expressed on the surface of chondrocytes, including α5β1, α1β1, α2β1, α10β1, α6β1, and αVβ3, can bind to FN, collagen type II and type VI, laminin, osteopontin, and other ECM components.14–16 As an important integrin ligand in the ECM, FN can connect and stabilize a variety of matrix components, such as collagen and proteoglycans.17–19 FN-integrin binding facilitates cell adhesion, spreading, cytoskeletal organization, and the formation of adhesion plaques. In addition, FN activates a series of signaling molecules, including focal adhesion kinase (FAK), paxillin, and Src, thus regulating cell growth and differentiation.20,21 The superior ability of FN to promote adhesion has been confirmed and has been applied to interface modification. The application of FN to the surface of nonglycolide polymers has been successful in a large number of experiments.22,23 Cadherin-11 (Cad-11) is a type II cadherin, which is a single-chain transmembrane glycoprotein that mediates calcium-dependent cell–cell adhesion. The characteristic structure of Cad-11 contains 5 extracellular domains (EC1-EC5). Extracellular domains 1 and 2 of Cad-11 (Cad-11 EC1-2) determine adhesive interactions or recognition specificity and provide important interfaces for cadherin monomers that mediate cell adhesion.24 Cad-11 plays an important role in mesenchymal condensation during bone formation due to homophilic binding specificity and in a calcium-dependent manner.25,26 Kii et al. confirmed that Cad-11 could promote the differentiation of MSCs into chondrocytes.27 Matsusaki et al. found that Cad-11 was expressed in growth plate chondrocytes.28 Others groups had identified the expression of Cad-11 in the synovial lining of mice, indicating that Cad-11 played an important role in limb and joint development.29,30
Thus, FN can assume the functional role of enhancing cell adhesion through heterophilic interactions, whereas Cad-11 can assume the functional role of enhancing chondrogenic differentiation through homophilic interactions. In our preliminary studies, we constructed a novel recombinant fragment of FN7-10/Cad-11 EC1-2 (rFN/Cad-11).31 The surface of the biphasic calcium phosphate (BCP) ceramic was functionalized with this recombinant protein using a dimethyl-3,3′-dithiobispropionimidate cross-linking method. The rFN/Cad-11-BCP surface possessed an improved capacity for adhesion. The in vitro study of the novel material demonstrated that the cell proliferation rate, adhesion, and ossification were significantly improved as compared to pure BCP and the FN- and Cad-11-biofunctionalized surfaces. Collagen type II had been used in porous scaffolds in cartilage tissue engineering.32,33 It is crucial to modify this scaffold such that it is suitable to cells. Considering the different characteristics and contributions of FN and Cad-11 to adhesion and differentiation, we proposed that a collagen type II surface modified with rFN7-10/Cad-11 EC1-2 would result in the cooperative promotion of cell adhesion and chondrogenic differentiation. Allogenous MSCs from newborn rabbits were used in this study. We observed the in vivo reconstruction of ectopic cartilage tissue in nude mice using a collagen type II (Col II) surface modified with rFN/Cad-11. A rabbit model of an articular cartilage defect was then used to analyze the capacity for repair.
Materials and Methods
Extraction of collagen type II and preparation of collagen sponges
Collagen type II was extracted from the articular cartilage of bovine calf legs, which were provided by the laboratory animal center of the Third Military Medical University with pepsin treatment and salt precipitation, as previously described.34,35 The Southwest Institutional Animal Care and Use Committee at the Third Military Medical University approved all animal protocols. The purification of the solubilized collagen was performed using sodium chloride fractionation and fibril assembly. The collagen solution was lyophilized on a Savant ModulyoD-115 system (Thermo Electron) at −40°C and stored at −20°C. The collagen type II solution (5 mg/mL) was neutralized using 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer and 1 M sodium hydroxide. A 2-mL volume of the collagen solution was poured into the wells of a 24-well culture plate (BD Falcon) and kept still at 4°C for 24 h to eliminate the bubbles possibly formed during stirring. The solution was then frozen at −20°C for 24 h and lyophilized at −50°C for 48 h to form collagen sponges, and the sponges (5 cm in width ×5 cm in length ×2 mm in depth) were cross-linked by an overnight treatment with glutaraldehyde vapor at room temperature. After the cross-linking, the sponges were treated with a 0.1 M glycine solution for 12 h at 4°C to block unreacted aldehyde groups and then rinsed extensively with water and lyophilized for the following experiments.
Design and preparation of the FN/Cad-11 recombinant protein
Preparation of rFN/Cad-11, a recombinant protein spanning the FNIII 7–10 and Cad-11 EC 1–2 fragments, was described in a previous study.36 In brief, the protein was designed using a homology modeling strategy based on simulations performed by Silicon Graphics Workstation (Silicon Graphics International Corp.). Both of the gene fragments were amplified by PCR and ligated between the SalI and NotI sites of the pET-22b backbone fragment. After transformation into competent Rosetta-gami(DE3) cells, the recombinant clones were screened with ampicillin (100 μg/mL), kanamycin (15 μg/mL), chloramphenicol (34 μg/mL), and tetracycline (12.5 μg/mL). The recombinant rFN/Cad-11 was expressed following induction with IPTG for 12 h, and the soluble rFN/Cad-11 was purified from the supernatant by Ni-NTA affinity chromatography. The molecular weight of rFN/Cad-11 was approximately 75 kDa, as confirmed by 15% SDS-PAGE. A protein (∼50 kDa) consisting of domains 7–10 of FNIII was also expressed by the above protocol as the positive control for this study.
Surface modification for type II collagen sponges
The collagen sponges were cut into sheets (5×5×1 mm) for cell seeding. The homobifunctional amine cross-linker glutaraldehyde, employed in a variety of applications in which the maintenance of the structural rigidity of proteins is important, was used according to the manufacturer's instructions. Briefly, the collagen sponges were incubated with 200 μL of a freshly prepared 1% glutaraldehyde solution (pH 7.5) for 20 min at 37°C and then rinsed twice with distilled water. The sponge sheets were then immersed in 70% ethanol for 20 min and rinsed thrice with phosphate-buffered saline (PBS). Next, the sponges were immersed in rFN/Cad-11 solution (10 μg/mL) overnight to allow protein coating and then they were lyophilized. The single FNIII7-10 fusion protein was offered as a positive control, whereas unmodified collagen sponges were used as a negative control. Before seeding cells onto the scaffolds, the sponges were immersed in culture medium overnight to make equilibrium swelling and then placed into the wells of a 24-well culture plate.
Surface characterization
The obtained biomimetic surfaces functionalized with rFN/Cad-11 were dried at temperature of 25°C for 48 h and then under vacuum for 24 h. The surface was characterized by scanning electron microscopy (SEM) along with the following methods as described. For contact angle analysis, the static contact angles of the sample surfaces were investigated with a contact angle-measuring device (Magicdroplet Model 200; Sindatek) using the sessile drop method. For the characterization of the crystal structure, thin-film X-ray diffraction (TF-XRD) was performed on an X-ray diffractometer (XD2/3, BPGI Co., Ltd). The tested samples were collagen sponge, collagen sponge modified by FNIII7-10, and collagen sponge modified by rFN/Cad-11. The samples were prepared according to the instrument manufacturers' instructions. The samples were irradiated with a monochromatized Cu Kα (1.54056 Å) X-ray source with a step size (2θ) of 0.01° and scan step time (s) of 1.0. The operating voltage and the current applied were 36 kV and 30 mA, respectively, and the scanning range was 5–70° (2θ).
Isolation, culture, and expansion of rabbit MSCs
The isolation, culture, and expansion of rabbit bone marrow MSCs were described in a previous study.37 In brief, MSCs were isolated from the femurs of 4-week-old Japanese white rabbits (1.5–2.0 kg; Animal Research Center, Third Military Medical University). After anesthesia, rabbit bone marrow was collected into a 10-mL syringe that contained 5000 U of heparin. The marrow was resuspended in a 0.84% NH4Cl solution. After 5 min, approximately 2.0×106 marrow cells were plated in a 25-cm2 plastic flask in Dulbecco's modified Eagle medium (DMEM), which was supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories), 2 mM l-glutamine, 100 U/mL penicillin, and 0.1 mg/mL streptomycin. For expansion, the cells were cultured in the growth medium at 37°C in a humidified atmosphere with 5% CO2. The medium was changed every 3 days. After 10–14 days of primary culture, the cells reached confluence. The cells were passaged and plated again at a dilution of 1:3. Cells from the second through fourth passages were implemented for the experiments described in this study.
Immunohistochemistry staining
The details of the immunohistochemical staining of MSCs have been described previously.37 Cells were digested by 0.25% trypsin solution and plated on cell culture coverslips (Cosmobrand). On the second day, cells were fixed for 15 min at room temperature with 4% paraformaldehyde in PBS and then washed twice with PBS. The endogenous peroxidase activity was inactivated by incubating the cells for 30 min in 1% H2O2 in PBS. The cells were then washed thrice with PBS and blocked with blocking solution containing 10% FBS and 0.1% Triton X-100 in PBS for 30 min. The blocked cells were incubated with primary antibodies against the integrin subunits α5 (mouse anti-human CD49e; Santa Cruz Biotechnology), β1 (goat anti-human CD29; Santa Cruz Biotechnology), and Cad-11(goat polyclonal IgG; Santa Cruz Biotechnology). Cy3- or FITC-conjugated secondary antibodies were used. The cells were then stained with Hoechst 33342 (Sigma-Aldrich).
Cell adhesion assay
The effect of the Col II/rFN/Cad-11 surface on MSC adhesion was performed by the centrifugal cell adhesion assay as previously described.11 The MSCs were pelleted by centrifugation, and the supernatant was removed by aspiration. The cells were then gently resuspended in prewarmed PBS containing 10 μM carboxyfluorescein diacetate succinimidyl ester (CFDA SE; Invitrogen), a cell membrane-permeable fluorescent dye (492 nm/517 nm), in PBS and incubated for 15 min at 37°C. The cells were pelleted again by centrifugation and resuspended in fresh prewarmed medium. The labeled MSCs were then seeded at 10,000 cells/well onto surfaces with ligand density gradients in 96-well cell culture plates (BD Biosciences) and centrifuged at 500 g for 30 min (Beckman Allegra X-22R; Beckman Coulter, Inc.). For the initial fluorescence readings, the plates were sealed, inverted, and centrifuged at 500 g for 5 min. Postspin fluorescence readings were used to calculate the density of the adherent cells. In addition, the cells were photographed with a Nikon TE-300 fluorescence microscope at a magnification of 100×. For the adhesion blocking studies, the cells were incubated for 15 min with 0.5 μg/mL β1 integrin-specific antibodies or 0.1 mg/mL Cad-11 antibodies under gentle agitation prior to being seeded onto the Col II/rFN/Cad-11 surfaces. The cells were seeded for 1 h following the antibody preliminary incubation, and the adhesion assay was then performed as described above.
Cell migration assay
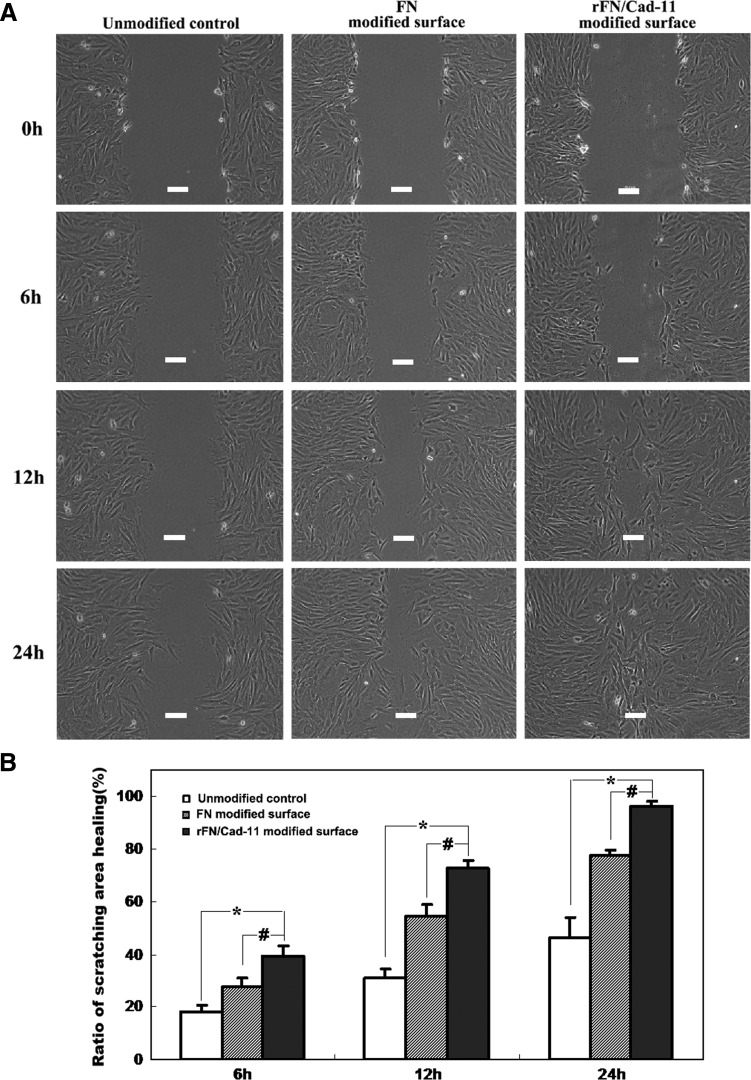
The migration of cells on the functionalized surface was analyzed by a cell-scratching assay. Briefly, MSCs were seeded for 12 h in six-well cell culture plates coated with rFN/Cad-11 or control; then an area on each plate was cleared using a 20-μL pipette tip. The cells were cultured in low serum medium (1% FBS) at 37°C with 5% CO2 and 95% air. The width of the scratched areas is about 320–360 μm. The migration was recorded with microscopy (Olympus BX50) with approximately the same fields and photographed with a digital microscope camera (FX1520; Diagnostic Instruments, Inc.) at 0, 6, 12, and 24 h after scratching. The cell migration was determined by measuring the difference in the cleared area before and after migration (when the area was restored). The results were analyzed by measuring the confluence area of the cells that migrated to the scratching area using Image-Pro Plus 6.0 software. The representative results from three independent experiments were shown.
Chondrogenesis assay
The chondrogenic capacity of MSCs seeded in 24-well cell culture plates coated either with rFN/Cad-11 or controls was investigated in chondrogenic culture using a chemically defined medium, which consisted of high-glucose DMEM containing 100 μg/mL sodium pyruvate (Gibco), 10 ng/mL TGF-β3 (R&D Systems), 100 nM dexamethasone, 1× ITS+1 premix, 40 μg/mL proline, 50 μg/mL ascorbate-2-phosphate (Sigma-Aldrich), and 10% FBS.38 The MSCs of passage 2–3 were trypsinized with 0.25% trypsin and a total of 1×106 cells were seeded in 24-well plates. After 24 h, the culture medium was changed to chondrogenic medium and cells were cultured for 14 days at 37°C/5% CO2 and 95% air. To demonstrate the deposition of cartilage matrix proteoglycans, representative cultures were collected at 14 days following induction, and the sulfated cartilage glycosaminoglycans (GAGs) were measured by Alcian blue staining. Prior to Alcian blue staining, the cells were fixed with 4% formaldehyde in PBS for 20 min, washed twice with 0.01 M PBS, and then stained with 0.5% Alcian blue 8GX (Sigma Chemical Co.) for 20 min.
Cell seeding experiment
The manufactured scaffolds were assigned into three experimental groups that were seeded with cultured MSCs: (1) collagen type II sponge with rFN/Cad-11 (Col II/rFN/Cad-11 group), (2) collagen type II sponge with FN (Col II/FN group), and (3) unmodified collagen type II sponge (Col II group). When the MSCs of passage 2–3 reached 80 to 90% confluence, they were trypsinized with 0.25% trypsin/EDTA and counted with a hemocytometer. After centrifugation, the cell pellets were resuspended into DMEM and about 5.0×106 cells were seeded into the collagen sponges (5×5×1 mm) with or without rFN/Cad-11 (final concentration of 10 μg/mL). The cell suspension was gently poured into the pores of scaffold sponges in a 12-well plate. After incubation for 1 h at 37°C, 2-mL DMEM with 10% FBS was added to each well, and the plates were cultured in a 37°C/5% CO2 and 95% air incubator. After 24 h, the medium was altered as mentioned above to induce the chondrogenic differentiation of the MSCs. All of the constructs were cultured for 2 weeks in vitro prior to implantation in vivo.
Scanning electron microscopy
After the cells were cultured in the scaffolds for 7 days, the morphology and internal structure of the constructs were examined using a scanning electron microscope (Hitachi S-3400N II). The collagen sponges with or without the rFN/Cad-11 modification were frozen in liquid nitrogen and immediately fractured. The samples were lyophilized and sputter-coated with gold for 40 s with an Emitech K575×sputter coater (EM Technologies Ltd). The samples were viewed with an accelerating voltage of 15 kV and a working distance of 18–20 mm.
Real-time qPCR for gene expression analysis
To determine the expression levels of Col2a1 (Collagen, type II, alpha 1), Sox9 (SRY-related high mobility group-box gene 9), Aggrecan, COMP (Cartilage oligomeric matrix protein), ColX (Collagen type X), Runx2, and vascular endothelial growth factor (VEGF), total RNA was performed RT-PCR using the Rever TraAce-a -First Strand cDNA Synthesis Kit (Toyobo) followed by real-time quantitative PCR with SYBR Green. The RNA samples were quantified using a spectrophotometer (Nanodrop Technologies ND-1000, NanoDrop Technologies, Inc.). Real-time PCR analysis performed in an Applied Biosystem 7500 Fast detector system using the SYBR green PCR method according to manufacturer's instruction (Applied Biosystems). The mean cycle threshold value (Ct) from triplicate samples was used to calculate gene expression, and PCR products were normalized to β-actin levels for each reaction. The data were evaluated relative to a calibrator according to the 2−ΔΔCt method. β-actin acted as an internal control. Each value in this work represented the mean±standard deviation (SD) of at least three independent samples obtained under the same conditions. The rabbit primers used for qPCR were shown in Table 1.
Table 1.
Primers Used for Real-Time Reverse Transcriptase–Polymerase Chain Reaction Experiments
| Genes | Forward primer | Reverse primer | Accession number | Product length (bp) |
|---|---|---|---|---|
| β-actin | AGTGCGACGTGGACATCCG | TGGCTCTAACAGTCCGCCTAG | NM001101683 | 295 |
| Col2a1 | CTGGTGGAGCAGCAAGAGC | TTGGCAGTGTTGGGAGGC | NM001195671 | 111 |
| Sox9 | AGTACCCGCACCTGCACAAC | CGCTTCTCGCTCTCGTTCAG | AY598935 | 79 |
| Aggrecan | TCTCCAAGGACAAGGAGGTG | AGGCTCTGGATCTCCAAGGT | L38480 | 123 |
| COMP | TGGTGCTCAATCAGGGAA | ATCAGTGGCGGTGTTTACAT | NM016685 | 125 |
| ColX | CCCTTCTGCTGCTAGTGTC | GTCTTGGTGTTGGGTTGTG | XM002714724 | 103 |
| Runx2 | GACCAGCAGCACTCCATATCTC | TCAGCGTCAACACCATCATTC | XM002714704 | 177 |
| VEGF | CTCCGCCTCCTACTCCAAGC | CGATGGCAGGACCTCAAACA | XM002714697 | 191 |
Transplantation of constructs into subcutaneous dorsal pockets in nude mice
Athymic nude mice were anesthetized for surgery. Minor skin incisions were made at the back of nude mice. Four nude mice (BALB-C nu/nu), for which the approval was obtained from the local animal ethical committee, were used to prepare the subcutaneous pockets. Each mouse was implanted with six constructs: two Col II/rFN/Cad-11 constructs (Col II/rFN/Cad-11 group), two Col II/rFN constructs (Col II/FN group), and two control collagen type II sponge scaffolds (Col II group). The constructs were treated with the same procedure used for in vitro experiment “cell seeding experiment.” The wounds were carefully rinsed with a 0.9% saline solution and closed with sutures. After 8 weeks, the mice were sacrificed by cervical dislocation, and all constructs were harvested.
Repair of articular cartilage defects in rabbits
Twelve young female rabbits (Japanese white rabbits, 3 months of age) were used for this experiment. The operative procedure and the care of the rabbits were performed under the regulation of the Experimental Animal Center, Third Military Medical University. The rabbits were acclimated for 1 week before operation and monitored for their general appearance, activity, excretion, and weight. All operations were performed under general anesthesia by intravenous injection of a mixture of ketamine hydrochloride (40 mg/kg) and xylazine (10 mg/kg). To generate a cartilage defect on both of the rabbit's legs of the trochlear grooves of the distal femurs, longitudinal skin incisions were made at the lateral of knee joint, and the patellar grooves were exposed. The defect was created by the dental micromotor (204-H35SP1; Xinghua Dental Equipment Co., Ltd). The drilling hole was 3 mm in diameter and 1 mm in depth. The rabbits were then randomly divided into three groups: the Col II/rFN/Cad-11 group (4 rabbits, 8 legs, n=8), the Col II/FN group (4 rabbits, 8 legs, n=8), and the control Col II group (4 rabbits, 8 legs, n=8). The knee joint was then sutured closed. All rabbits received ampicillin for two consecutive postoperative days. After 12 weeks postoperation, all animals were euthanized by an overdose of anesthesia, and the femoral articular cartilage of the knee was taken for gross evaluation by International Cartilage Repair Society (ICRS) recommended guidelines on histological scores (ICRS-II).39–41
Histological analysis
The skin sample harvested from nude mice was fixed in 4% neutral buffered formalin overnight and embedded in paraffin after dehydration in 70% ethanol. The paraffin sections were cut at a thickness of 5 μm and were stained with Safranin O. The distal femurs with articular cartilage defects were removed from the rabbits and fixed in 4% neutral buffered formalin. The distal ends of the femurs with defects were then decalcified in 10% EDTA solution until all calcium was removed. Longitudinal sections were cut at a thickness of 6 μm that were then stained with hematoxylin and eosin, Masson's Trichrome, Safranin O, and Sirius red. The slides were analyzed under a light microscope (Olympus BX50). Among them, different types of collagen could be analyzed by Sirius red staining and the polarization method.
Statistical analysis
Triplicate samples were used for the chemical and biochemical assays and duplicate samples for the microscopy analysis. The mRNA expressing level and migration assay from different groups were compared respectively using one-way ANOVA test by SPSS Statistics 15.0 software (SPSS, Inc.). Normality of data was tested by use of the Kolmogorov–Smirnov test while equality of variances was tested with the Levene test. The level of significance was set to p<0.05.
Results
Expression of integrin α5β1 and Cad-11 in MSCs
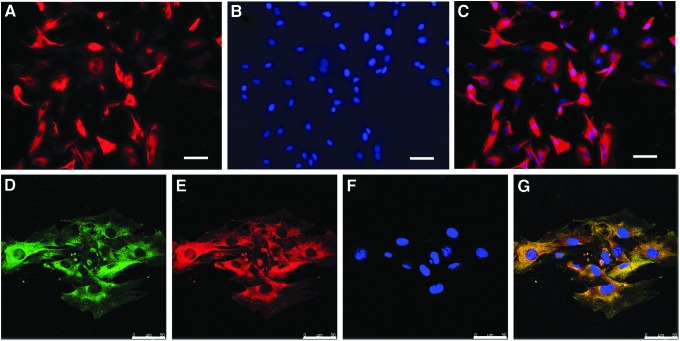
To detect the potential effects of the fusion proteins rFN/Cad-11 on MSCs, we examined the expression of integrin α5β1 and Cad-11 in MSCs. α5β1 and Cad-11 were both transmembrane proteins that were expressed at the plasma membrane. By immunofluorescence staining, we detected strong positive fluorescence signals on the plasma membranes of MSCs and found that MSCs expressed Cad-11 (Fig. 1A–C) and α5β1 (Fig. 1D–G). Among these proteins, α5 (Fig. 1D) and β1 (Fig. 1E) were co-expressed on the plasma membrane, indicating that the two subunits had similar localization (Fig. 1G). The results suggested that the fusion protein designed in this study has the cellular and structural basis to interact with integrin and Cad-11 proteins.
FIG. 1.
The expression of integrin α5β1 and Cad-11 in MSCs detected by immunofluorescence staining. Cad-11 was expressed at the plasma membrane (A and C). α5 (D) and β1 (E) were co-expressed on the plasma membrane. The merge figure indicated that α5 and β1 subunits had similar localization (G). The cells were stained with Hoechst 33342 to label nuclear DNA (B and F). Bar: 50 μm. MSCs, mesenchymal stem cells; Cad-11, cadherin-11.
Fabrication of the rFN/Cad-11-functionalized collagen surface
Scanning electron microscopy
The micromorphologies of the collagen sponge interface before and after biomimetic modification with rFN/Cad-11 are shown in Figure 2. Panel A and Panel B present the microstructure of the unmodified interface and modified interface under 200× magnification, respectively. The data revealed that the modified and unmodified collagen interfaces possessed a grid-like irregular morphology with a staggered distribution of pores. Judging from the ruler, the grid diameter was in the 50–100 μm range. The microscopic morphology of the collagen microfibrils seemed to be well preserved after the biomimetic modification (Fig. 2).
FIG. 2.

SEM images of collagen type II sponge interface before and after biomimetic modification with rFN/Cad-11. The microstructure of the unmodified collagen type II sponge interface (A), Col II/FN interface (B), and Col II/rFN/Cad-11 interface (C) under 200× magnification. The collagen microfibrils were well preserved after the biomimetic modification. Bar: 200 μm. Col II, collagen II; SEM, scanning electron microscopy.
Contact angle results
The hydrophilicity of the smooth surfaces modified with rFN/Cad-11 was measured by a contact angle analyzer and statistically analyzed. Our results showed that the contact angle of the pure collagen interface was 64.3±4.2°, indicating strong hydrophobicity, and that the contact angle of the interface modified by rFN alone was 54.6±2.2°. In contrast, the contact angle of the interface bionically modified with rFN/Cad-11 was 40.5±1.8° (Table 2), indicating that its hydrophilicity was greatly and significantly improved over that of the other two groups (p<0.01).
Table 2.
Contact Angle for Unmodified and Modified Collagen Sponge
| T (°C) | Col II sponge (deg) | Col II modified FN (deg) | Col II modified rFN/Cad-11 (deg) |
|---|---|---|---|
| 25 | 64.3±4.2° | 54.6±2.2° a | 40.5±1.8° b,c |
p<0.05, significant against Col II sponge.
p<0.01, significant against Col II sponge.
p<0.01, significant against the interface bionically modified with FN (Col II modified FN).
FN, fibronectin; Col II, collagen II.
X-ray diffraction
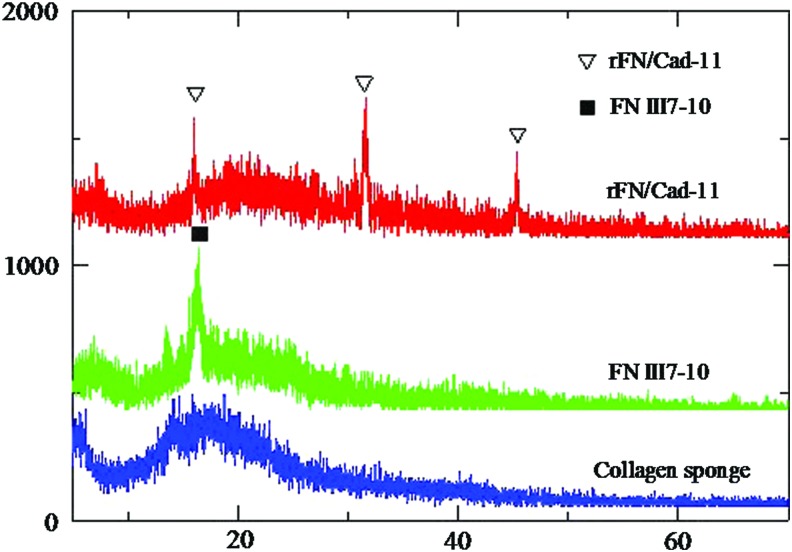
Figure 3 illustrated the X-ray diffraction patterns of the unmodified collagen sponge, the collagen sponge modified with FNIII7-10, and the collagen sponge modified with rFN/Cad-11. The main reflection peaks of the above scaffolds were at 2θ=17.6°, 15.4°, and 14.9°, respectively. The intermolecular lateral packing (peak 1) increased to a greater extent when the FNIII7-10 or rFN/Cad-11 protein interacted with collagen, indicating that the collagen packing order increased. That increase may be due to the introduction of cross-links between collagen molecules and the protein, leading to an increased order of collagen organization. In addition, new peaks at 32.5° and 46.3° were visible in the collagen sponge modified with rFN/Cad-11 (Fig. 3). These results suggested that the proteins were successfully cross-linked to the surfaces of the scaffold materials, allowing subsequent biological examinations.
FIG. 3.
X-ray diffraction patterns of the three collagen sponge. The unmodified collagen sponge (blue), the collagen sponge modified with FNIII7-10 (green), and the collagen sponge modified with rFN/Cad-11 (red). Standard peak positions are indicated with triangle and black square symbols. Color images available online at www.liebertpub.com/tea
Effects of Col II/rFN/Cad-11 on MSC growth and morphology
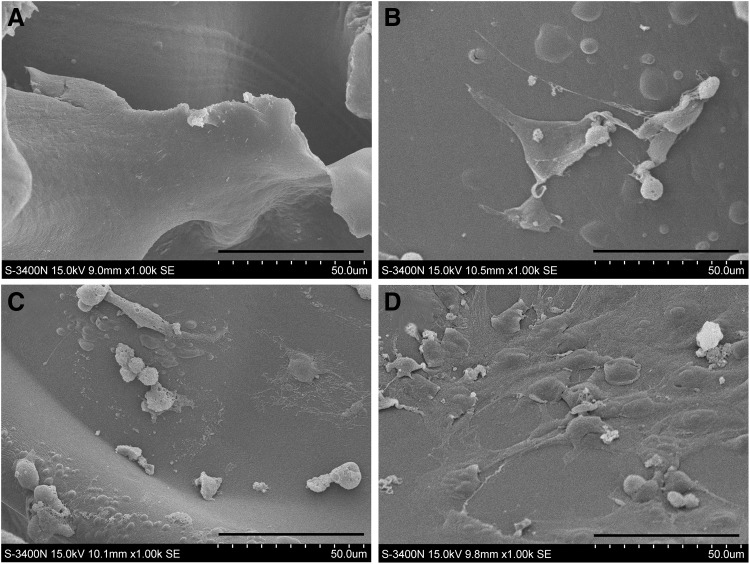
The MSCs were cultured continuously for 7 days with the Col II/rFN/Cad-11 interface. The growth of the cells on the surfaces was observed by SEM. Our results showed that the MSCs poorly spread on the pure Col II interface, exhibiting cord-like morphologies. In addition, a large number of pseudopodia-like structures projected deep into the pores of the material, suggesting climbing-like growth. On the Col II/rFN/Cad-11 surface, the MSCs appeared to be well spread. The cells appeared to be polygonal or disc-shaped with reduced pseudopodia-like structures and formed a dense lamellar-like structure covering the surface (Fig. 4). On the Col II/FN surface, the cell morphology and status grew between the others surfaces mentioned above. These results suggested that the rFN/Cad-11-modified Col II interface was more conductive to the growth of adherent cells and could significantly improve the biocompatibility of the material.
FIG. 4.
SEM images of MSCs attached to the scaffold surfaces. (A) collagen type II sponge unseeded cells. Bar: 1 mm. (B) collagen type II sponge. Bar: 100 μm. (C) Col II/FN interface. (D) Col II/rFN/Cad-11 interface.
rFN/Cad-11 protein promotes cell adhesion
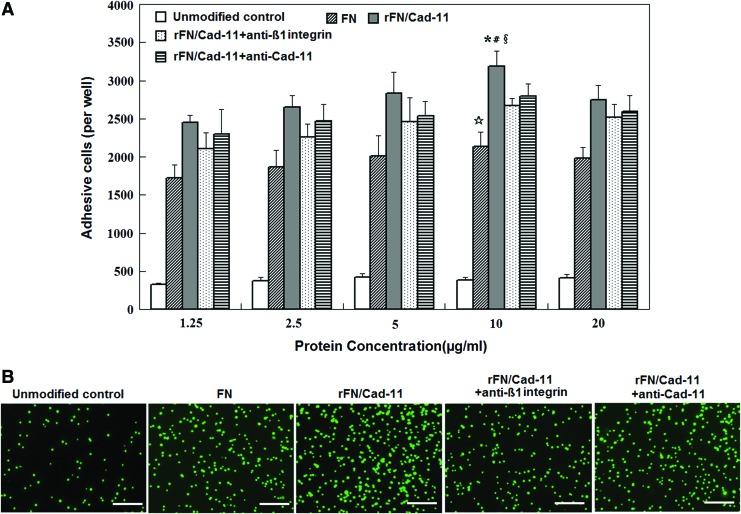
To observe the effects of the Col II/rFN/Cad-11 biomimetic interface on MSC adhesion, we performed a centrifugal cell adhesion assay to further analyze the relationship between the density of biological ligands on the interface and cell adhesion. The results showed that the number of MSCs adhered to the Col II/rFN/Cad-11 surface positively correlated with the density of the biological ligands on the interface. The number of adhered cells gradually increased with increasing rFN/Cad-11 densities. When comparing the numbers of cells adhered to the various surfaces, the Col II/rFN/Cad-11 surface was significantly superior to the control Col II/FN group. The Col II/rFN/Cad-11 surface had 1.5 times (p<0.05) and 8.2 times (p<0.01) more adhered cells than the Col II/FN and unmodified Col II surfaces, respectively, and performed particularly well below the protein concentration of 10 μg/mL (Fig. 5A).
FIG. 5.
Adhesion capacity of rFN/Cad-11 detected by cell centrifugal adhesive assay. (A) The density–concentration curve showing increased MSCs on rFN/Cad-11 surface. ( p<0.01, rFN/Cad-11 surface compared with unmodified control. #p<0.05, rFN/Cad-11 surface compared with FN modified surface. §p<0.05, rFN/Cad-11 surface compared with rFN/Cad-11+anti-β1 integrin.
p<0.01, rFN/Cad-11 surface compared with unmodified control. #p<0.05, rFN/Cad-11 surface compared with FN modified surface. §p<0.05, rFN/Cad-11 surface compared with rFN/Cad-11+anti-β1 integrin.  p<0.01, FN modified surface compared with unmodified control.) (B) Fluorescent graphs of MSCs labeled by CFDA SE on the surfaces of unmodified control, FN (10 μg/mL), rFN/Cad-11 (10 μg/mL), rFN/Cad-11+ anti-β1 integrin, rFN/Cad-11+ anti-Cad-11. Scale bars in B: 200 μm. Color images available online at www.liebertpub.com/tea
p<0.01, FN modified surface compared with unmodified control.) (B) Fluorescent graphs of MSCs labeled by CFDA SE on the surfaces of unmodified control, FN (10 μg/mL), rFN/Cad-11 (10 μg/mL), rFN/Cad-11+ anti-β1 integrin, rFN/Cad-11+ anti-Cad-11. Scale bars in B: 200 μm. Color images available online at www.liebertpub.com/tea
In addition, when using specific antibodies to block β1 integrin, we found that the number of MSCs adhered to the Col II/rFN/Cad-11 surface decreased approximately 15% under the protein concentration of 10 μg/mL. This result suggests that β1 integrin is of great significance for MSCs adhesion to Col II/rFN/Cad-11. In addition, the adhesion of MSCs to the differently modified Col II surfaces was monitored by fluorescence microscopy, providing visual evidence for the above results (Fig. 5B).
Further, we also focused on the impact of the rFN/Cad-11 protein on MSCs migration. MSCs were seeded onto the rFN/Cad-11 modified surface. Utilizing a cell-scratching assay, we found that the ability of the cells to migrate was significantly enhanced compared with the unmodified control and FN surfaces and exhibited a greater ability to close the gap created in the assay (p<0.05) (Fig. 6).
FIG. 6.
The effect of the rFN/Cad-11 protein on MSCs migration. (A) Scratched areas of the MSCs layers on the rFN/Cad-11 modified surface, FN modified surface and unmodified control at different times (Bar: 100 μm); (B) The ratio of cell confluence area that migrated to the scratch area. Data are expressed as mean±SD. *p< 0.01, significantly different from unmodified control. #p<0.05, significantly different from FN modified surface.
Col II/rFN/Cad-11 scaffolds can promote chondrogenic differentiation in MSCs
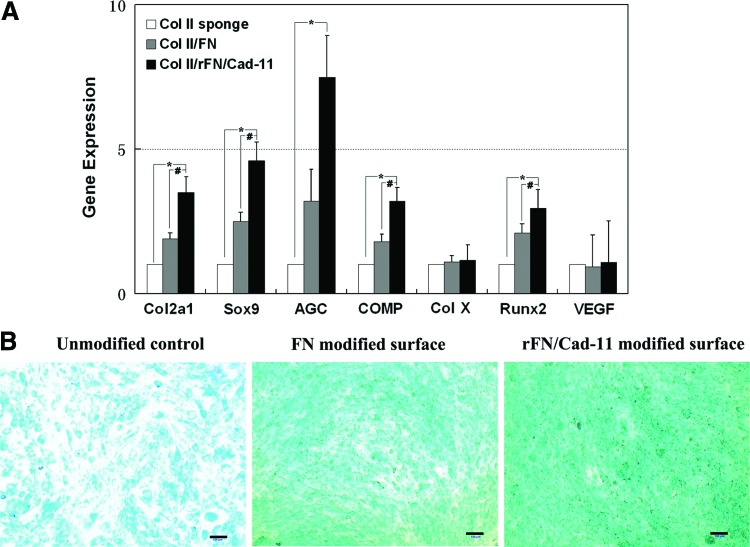
To detect the effects of the rFN/Cad-11 protein on the chondrogenic differentiation of MSCs, we performed cytological staining and real-time PCR. The real-time PCR analysis showed that MSCs seeded onto the Col II/rFN/Cad-11 surface exhibited significantly increased mRNA expression levels of the chondrogenesis markers Col2a1, Sox9, Aggrecan, and cartilage oligomeric matrix protein (COMP) when compared with the control cells seeded onto Col II or Col II/FN 24 h after induction (Fig. 7A). Additionally, we detected the expression of some hypertrophic marker genes. The expression change of ColX and VEGF was not obvious between the two groups, while the expression of Runx2 increased compared with the control group. This result suggests that the modification of the surface with rFN/Cad-11 has an important function in the early stages of chondrogenic differentiation. Alcian blue staining showed that, compared with the control group, the rFN/Cad-11 protein significantly stimulated cells to secrete cartilage-specific ECM GAGs (Fig. 7B).
FIG. 7.
The expression of chondrocyte-related genes in MSCs on rFN/Cad-11 modified surface and control surfaces. The cells on rFN/Cad-11 modified surface expressed markedly higher levels of Col2a1, Sox9, AGC, and COMP. The hypertrophic marker genes, ColX and VEGF, showed no significant change, while the expression of Runx2 was increased (A). Alcian blue staining showed that, compared with the control group, the rFN/Cad-11 protein significantly promoted cells to secrete GAGs (B). (*p<0.05 with respect to Col II sponge control. #p<0.05 with respect to Col II/FN control. Note that the gene expression levels were normalized with respect to their own controls). Bar: 100 μm. VEGF, vascular endothelial growth factor. Color images available online at www.liebertpub.com/tea
Ectopic cartilage tissue formation revealed good chondrogenesis in Col II/rFN/Cad-11
To observe ectopic cartilage tissue formation, we subcutaneously implanted scaffolds seeded with cells in nude mice. For the Col II/rFN/Cad-11 group, the ectopic formation of cartilage tissue could be observed in the subcutaneous tissues of nude mice after 8 weeks of in vivo culture. Gross observation showed that the implanted scaffold was wrapped in a capsule, appeared to be translucent porcelain white, and showed a certain degree of pliancy upon contact and pressure. The implant was coated with a small amount of fibroblasts and fibrous tissue and that lymphocytes were presented but not immersed into the deep layer. This examination also revealed that the implants mostly formed cartilage-like tissue with the typical cartilage lacuna-like structures. Many round chondrocytes were present in the lacuna. The cartilage was surrounded by a homogeneously stained hyaline matrix. Safranin O staining revealed the secretion of proteoglycan and GAG deposition around the cells (Fig. 8). The control Col II/FN group implants formed less cartilage and the Col II group implants formed the smallest amount of cartilage-like tissue.
FIG. 8.
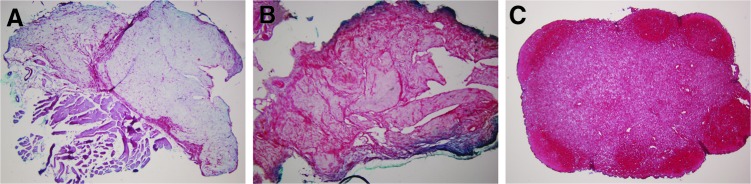
Cross-sections of harvested tissues in ectopic cartilage tissue formation after 8 weeks. A much less intense extracellular matrix (ECM) was stained for constructs in collagen type II sponge group (A) and Col II/FN group (B). Deep red color of ECM shown using Safranin O staining indicated intense glycosaminoglycan deposition around cells for constructs of Col II/rFN/Cad-11 and showed that new tissue contained islets of cartilage-like tissue (C). (Original magnification ×40). Color images available online at www.liebertpub.com/tea
In vivo repair experiments demonstrated a satisfactory repair capacity for Col II/rFN/Cad-11
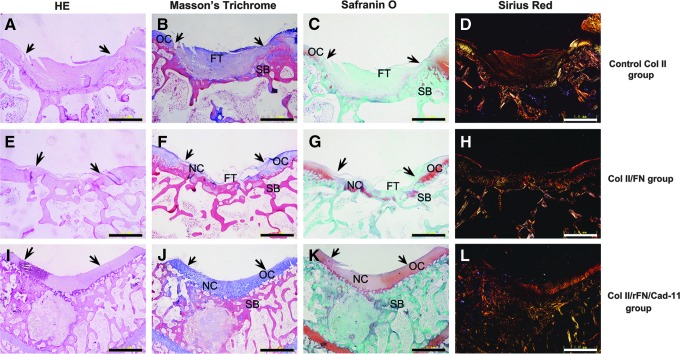
The knee joints of the experimental animals showed no adhesions, no loose bodies, and no significant synovial proliferation or hyperemia. After 12 weeks of implantation, gross observation showed that the defected areas in the three groups appeared mostly flat. The Col II/rFN/Cad-11 group had a smooth repair surface and was paler than normal tissue because it contained more moisture. The repaired tissue was closely integrated to the surrounding cartilage and had a similar thickness. The histological observation revealed that isogenous groups were visible in the repair zone (Fig. 9I). Cartilage-like tissue formation could be seen in the experimental group with strong Masson's Trichrome and Safranin O staining, indicating massive content of proteoglycan (Fig. 9J, K). There were more immature chondrocytes that mostly accumulated on the surface. The cells were smaller, mostly long and spindle-shaped, and were present in large numbers. The cells in the deep layer were larger, arranged irregularly, and showed typical cartilage lacuna structure. The arrangement of collagen type II in the repair tissue were similar to that in normal cartilage tissue (Fig. 9L). For the Col II/FN group, a general observation revealed relatively good repair of the defects, and the repair surface was not smooth. The histological observation showed the partial degradation of the scaffolds and the invasion of long spindle fibroblast-like cells. In the Col II/FN group, cartilage tissue and fibrous tissue were clearly mixed to repair the defects (Fig. 9E–H). The ECM and collagen type II were reduced compared with normal tissue and were clearly separated from the surrounding tissues in histology and morphology. In the Col II group, large amounts of fibrous tissue filled the defects, and fewer regenerated cartilage appeared (Fig. 9A–D).
FIG. 9.
Hematoxylin and eosin (HE), Masson's Trichrome, Safranin O, and Sirius red staining of rabbit cartilage defects repaired using the constructs of Col II/rFN/Cad-11 after 12 weeks. Much fibrous tissue was found in the defect, and the boundary was loose and separated in the collagen type II sponge control group (A–D). In the Col II/FN group, cartilage tissue and fibrous tissue were clearly mixed to repair the defects (E–H). In Col II/rFN/Cad-11 group, the neocartilage tissue was closely integrated to the original cartilage, as indicated by arrows, and had a similar thickness. The cell morphology and arrangement in the repair tissue were similar to that in normal cartilage tissue (I–L). (original magnification ×40). The arrows showed the edges of the defects. Scale bars: 1.0 mm. OC, original cartilage; NC, neocartilage; SB, subchondral bone; FT, fibrous tissue.
The repair conditions of the cartilage defects in each group were assessed by the ICRS recommended guidelines. The mean histological scores were 14.21±1.15 (SD) 12 weeks postimplantation in the Col II/rFN/Cad-11 group, 8.41±2.26 (SD) in Col II/FN group, and 4.93±1.12 (SD) in Col II group. The differences between the 3 groups were statistically significant (p<0.05, Col II/rFN/Cad-11 group versus Col II/FN group and Col II/FN group versus Col II group, p<0.01, Col II/rFN/Cad-11 group versus Col II group) (Table 3).
Table 3.
Histological Scores of Experimental and Control Groups
| Group | Col II group | Col II/FN group | Col II/rFN/Cad-11 group |
|---|---|---|---|
| ICRS score | 4.93±1.12a,b | 8.41±2.26c | 14.21±1.15 |
p<0.05, versus Col II/FN group.
p<0.01, versus Col II/rFN/Cad-11 group.
p<0.05, versus Col II/rFN/Cad-11 group.
ICRS, International Cartilage Repair Society.
Discussion
Interactions between cells and ECM provide important cues for the differentiation and development of many tissues, including cartilage. In recent years, the surface modification of cartilage scaffold materials is mostly based on biomolecular biomimetics, that is, the simulation of the interactions between ECM proteins (such as FN, collagen type I, and laminin) and the surrounding cells. To avoid the limitations of natural proteins, including high cost, immunogenesis, facile digestion by enzymes, and decreased binding specificities of short peptide fragments, many current cartilage scaffold modifications use polypeptides or mimetic peptides. Polypeptides, such as FNIII7-10,5 take into account the roles of both the core RGD sequence and the auxiliary PHSRN motif. Mimetic peptides, such as those containing the Gly-Phe-hydroxyproline-Gly-Glu-Arg (GFOGER) and Asp-Gly-Glu-Ala (DGEA) motifs of collagen, mediate the functions of integrin subtypes in the non-RGD pathway.42 Although polypeptides and mimetic peptides both reasonably promote the adhesion of cells onto material surfaces, they have limited function in the chondrogenic differentiation of seed cells. In this study, we used a heterologous chimeric fusion protein (rFN/Cad-11) that was designed to cover FNIII7-10 and the extracellular fragments of Cad-11 for surface modification. The seeding density and the strength of the cell–cell interactions of the seeded cells on the material interface were enhanced by the heterophilic interactions mediated by FNIII7-10 and the homophilic interactions mediated by Cad-11. The cells were thus cross-linked to the more hydrophilic surfaces of the collagen sponges. Cell adhesion and chondrogenic differentiation experiments showed that collagen sponges modified with this fusion protein were significantly able to absorb MSCs and promote chondrogenic differentiation than the sponges modified with a FNIII7-10 protein and the unmodified control sponges. This result shows that linking motifs with binding functions to three-dimensionally structured molecules with known adhesive and chondrogenic functions can have a significant synergistic effect.
Being mediated by integrin, cells can adhere to and spread on the ECM. Integrin exhibits dot-like distributions in the adhesion sites on the cell surface. Cytoskeletal proteins are centered on the dots and form adhesion plaques in the cytoplasmic surfaces of cell membranes. Thus, integrin binds the ECM proteins with the cytoskeleton inside cells, which mediates both cell–cell and cell–matrix adhesion. The focal complex affects late cellular behavior, such as cell migration, adhesion, and phagocytosis.43 In this experiment, we found the expression of α5β1 integrin in MSCs, thus providing the structural basis for the binding between integrin and FN fragment in this project design. During the adhesion assay in vitro, the cell adhesion significantly decreased after we treated the interfaces with a β1-integrin antibody (p<0.05), suggesting that integrin-mediated adhesion plays an important role in MSCs adhesion.
Adhesion occurs during the early stages of the interactions between cells and the material surface. Cells, therefore, bind to material surfaces, the ECM, or other cells through certain adhesion molecules (such as selectins, integrins, FN, etc.). At the cell-material level, adhesion is closely related to cell migration, proliferation, differentiation, matrix deposition, vascular bud generation, and material degradation. Therefore, the adhesive property of cells on the material surface is an important indicator of the biocompatibility of materials.44,45 In the present study, we applied the centrifugal cell adhesion assay that was used by Garcia and coworkers to measure the adhesion of MSCs onto the Col II/rFN/Cad-11 interface. Compared to the traditional method of seeding/incubating/washing/counting/staining, this method employs appropriate centrifugal force to promote the adsorption and desorption between cells and materials and applies fluorescent labeling to count the effectively adhered cells, providing a simple, accurate, and highly controllable assay with a low false positive rate. The results from the centrifugal cell adhesion assay in this study showed that the number of MSCs effectively adhered to the Col II interface positively correlated with the density of the rFN/Cad-11 ligand. In addition, the proportion of the effectively adherent cells accounted for approximately 25%–30% of the total number of inoculated cells, which was significantly higher than that found with the unmodified Col II and the FN-modified Col II interfaces. Our experimental results demonstrated that rFN/Cad-11 achieved the superposition of the adhesive functions of FN and Cad-11 to a certain extent and that the adhesion-promoting capacity of the fusion protein was substantially increased. These results confirmed that Col II/rFN/Cad-11 was a biological interface with highly efficient adhesive properties. Because the cell density on the material surface determines the capacity for subsequent cell differentiation, the positive results in the adhesion assay in our study suggested a valuable potential for high chondrogenic differentiation ability in subsequent in vitro and in vivo studies.
Currently, cell proliferation and vitality are commonly used as indicators to evaluate the impact of biomaterial scaffolds on cell damage, growth, and metabolism. From a morphological point of view, this project used SEM techniques to analyze the morphological status of MSCs on the Col II/rFN/Cad-11 surface. The purpose of this project is to investigate whether the biomimetic modifications could affect the properties of cells. By SEM, we observed that the MSCs on the Col II/rFN/Cad-11 surface were fully spread and presented overall as a fully integrated lamellar-like structure. Our previous results demonstrated that the proliferation and viability of MSCs on the rFN/Cad-11 surface were significantly better than those on the unmodified materials, as indicated by the significant increase in MTT metabolites. As a preliminary result, we conclude that the Col II/FN/Cad-11 biomimetic modification is of great significance for improving the ability of scaffold material surfaces to promote cell adherence. This result effectively proved that the Col II/rFN/Cad-11 interface is an ideal biological interface that is suitable for the growth of MSCs.
Bone marrow MSCs are an attractive cell source for tissue engineering and cell-based therapies given their multilineage differentiation potential and ability to be expanded in vitro. Through specific procedures, MSCs can be reliably induced toward osteogenic, chondrogenic, and adipogenic differentiation lineages.46,47 Due to the limited capacity for cartilage regeneration and the rapid dedifferentiation of chondrocytes during monolayer expansion, MSCs are well suited for application in the repair of damaged articular cartilage. To evaluate the chondrogenic differentiation of MSCs on the Col II/rFN/Cad-11 interface, we examined the expression of the chondrogenic differentiation marker genes Col2a1, Aggrecan, Sox9, and COMP, which were used in combination with Alcian blue staining as indicators to evaluate the levels of GAGs. The nuclear transcription factor Sox9, one of the early markers expressed in cells undergoing condensation, is required for the expression of the type II collagen gene (Col2a1) and certain other cartilage-specific matrix proteins. COMP can interact with cell adhesion molecules to activate intracellular signaling pathways involving FAK and paxillin, thereby initiating the transition from chondroprogenitor cells to fully committed chondrocytes. In this study, TGF-β3 was used to induce MSCs for 14 days, they were then examined for their differentiation ability. The results from the real-time PCR showed that the Sox9 levels in MSCs on the Col II/rFN/Cad-11 interface were significantly increased and were 4.6 and 2.6-folds higher than those of the unmodified Col II and of the FN-modified Col II (p<0.05), respectively. Additionally, the level of COMP was 3.2 and 1.8-folds that of the unmodified Col II and of the FN-modified Col II (p<0.05), respectively. These results indicated that, following 14 days of induction, the MSCs had differentiated into fully committed chondrocytes.
As the major components of cartilage matrix, we believe collagen type II may provide the microenvironment for stem cells in differentiating to chondrocytes. rFN/Cad-11 promotes the differentiative capacity in this process. Also, we tested the expression of hypertrophic marker genes, ColX, Runx2, and VEGF. The results showed no obvious change in ColX and VEGF while Runx2 is increased. However, Runx2 is the indicator of both osteogenic and hypertrophic differentiation. Together with the increased expression of Sox9 in this experiment, we believe that rFN/Cad-11 promotes chondrogenic differentiation instead of hypertrophic differentiation. Additional experiments, including the staining of GAGs with Alcian blue and in vivo experiments with nude mice and with the repair of cartilage defects in the femoral trochlea of rabbits, also proved that the chondrogenic activity of the Col II/rFN/Cad-11 group was significantly higher than that of the control group. Based on the above results, rFN/Cad-11 can promote chondrogenic differentiation of MSCs in vitro and chondrogenesis in vivo.
The specific adhesion of cells to the scaffold surface is mainly determined by the layer of adsorbed protein molecules. After implantation in vivo, scaffolds can select and take up certain protein components (such as FN, laminin, vitronectin, etc.) from the tissue fluid surrounding the cells in the tissue. The Vroman effect, which describes the final state of the competitive adsorption and desorption of a variety of proteins on the material interface, determines the material surface properties and the biological behaviors of MSCs.48
Based on fully recognizing the function of the ECM in cartilage development, maintenance, and repair, i.e., and the importance of the dialogue and communication between the ECM microenvironment, stem cells, and chondrocytes, this study used a material modification method according to the principle of matrix-based biomimetic modification.49 We employed a fusion protein containing elements of FN and Cad-11 for interface modification and cross-linked it to the surface of Col II sponges that were then characterized with XRD and SEM. We demonstrated the reconstruction of a microenvironment with a certain degree of the three-dimensional structure and molecular signals naturally present at the interface between the matrix and material. Therefore, the novel interface served as a niche to increase cell seeding density and adhesion efficiency. Additionally, this constructed microenvironment specifically promoted chondrogenic differentiation of MSCs.
Conclusion
In this study, Col II sponges with the rFN/Cad-11 biomimetic modification can promote cell adhesion and chondrogenic differentiation of MSCs in cartilage tissue engineering, which can be observed in the enhanced expression of cartilage-related genes and proteins. The ectopic cartilage formation and cartilage defect repair experiments also confirmed that this biomimetic interface scaffold material has a high capacity for cartilage repair in vivo. Therefore, rFN/Cad-11 has the dual biological functions of promoting adhesion and promoting chondrogenic differentiation and can be used in tissue-engineering scaffold design and biomimetic modeling.
Acknowledgments
This study was supported by grants from the Nature Science Foundation of China (81271980, 31070864), the National Basic Research Program (2011CB964701), and the National Key Technology Research and Development Program of China (2012BAI42G01).
Disclosure Statement
No competing financial interests exist.
References
- 1.Kambe Y. Takeda Y. Yamamoto K. Kojima K. Tamada Y. Tomita N. Effect of RGDS-expressing fibroin dose on initial adhesive force of a single chondrocyte. Biomed Mater Eng. 2010;20:309. doi: 10.3233/BME-2010-0644. [DOI] [PubMed] [Google Scholar]
- 2.Stockton R.A. Jacobson B.S. Modulation of cell-substrate adhesion by arachidonic acid: lipoxygenase regulates cell spreading and ERK1/2-inducible cyclooxygenase regulates cell migration in NIH-3T3 fibroblasts. Mol Biol Cell. 2001;12:1937. doi: 10.1091/mbc.12.7.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavalcanti-Adam E.A. Volberg T. Micoulet A. Kessler H. Geiger B. Spatz J.P. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys J. 2007;92:2964. doi: 10.1529/biophysj.106.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamath S. Bhattacharyya D. Padukudru C. Timmons R.B. Tang L. Surface chemistry influences implant-mediated host tissue responses. J Biomed Mater Res A. 2008;86:617. doi: 10.1002/jbm.a.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrie T.A. Raynor J.E. Reyes C.D. Burns K.L. Collard D.M. García A.J. The effect of integrin-specific bioactive coatings on tissue healing and implant osseointegration. Biomaterials. 2008;29:2849. doi: 10.1016/j.biomaterials.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonwil D. Schuler M. Barbero A. Ströbel S. Wendt D. Textor M. Aebi U. Martin I. An RGD-restricted substrate interface is sufficient for the adhesion, growth and cartilage forming capacity of human chondrocytes. Eur Cell Mater. 2010;20:316. doi: 10.22203/ecm.v020a26. [DOI] [PubMed] [Google Scholar]
- 7.García A.J. Schwarzbauer J.E. Boettiger D. Distinct activation states of alpha5beta1 integrin show differential binding to RGD and synergy domains of fibronectin. Biochemistry. 2002;41:9063. doi: 10.1021/bi025752f. [DOI] [PubMed] [Google Scholar]
- 8.García A.J. Reyes C.D. Bio-adhesive surfaces to promote osteoblast differentiation and bone formation. J Dent Res. 2005;84:407. doi: 10.1177/154405910508400502. [DOI] [PubMed] [Google Scholar]
- 9.Yamato M. Konno C. Utsumi M. Kikuchi A. Okano T. Thermally responsive polymer-grafted surfaces facilitate patterned cell seeding and co-culture. Biomaterials. 2002;23:561. doi: 10.1016/s0142-9612(01)00138-7. [DOI] [PubMed] [Google Scholar]
- 10.Jeschke B. Meyer J. Jonczyk A. Kessler H. Adamietz P. Meenen N.M. Kantlehner M. Goepfert C. Nies B. RGD-peptides for tissue engineering of articular cartilage. Biomaterials. 2002;23:3455. doi: 10.1016/s0142-9612(02)00052-2. [DOI] [PubMed] [Google Scholar]
- 11.Petrie T.A. Capadona J.R. Garcia A.J. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27:5459. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Connelly J.T. Garcia A.J. Levenston M.E. Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials. 2007;28:1071. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Connelly J.T. Garcia A.J. Levenston M.E. Interactions between integrin ligand density and cytoskeletal integrity regulate BMSC chondrogenesis. J Cell Physiol. 2008;217:145. doi: 10.1002/jcp.21484. [DOI] [PubMed] [Google Scholar]
- 14.Takagi J. Structural basis for ligand recognition by RGD (Arg-Gly-Asp)-dependent integrins. Biochem Soc Trans. 2004;32:403. doi: 10.1042/BST0320403. [DOI] [PubMed] [Google Scholar]
- 15.Loeser R.F. Chondrocyte integrin expression and function. Biorheology. 2000;37:109. [PubMed] [Google Scholar]
- 16.Camper L. Hellman U. Lundgren-Akerlund E. Isolation, cloning, and sequence analysis of the integrin subunit alpha10, a beta1-associated collagen binding integrin expressed on chondrocytes. J Biol Chem. 1998;273:20383. doi: 10.1074/jbc.273.32.20383. [DOI] [PubMed] [Google Scholar]
- 17.Fukui N. Ikeda Y. Tanaka N. Wake M. Yamaguchi T. Mitomi H. Ishida S. Furukawa H. Hamada Y. Miyamoto Y. Sawabe M. Tashiro T. Katsuragawa Y. Tohma S. αvβ5 integrin promotes dedifferentiation of monolayer-cultured articular chondrocytes. Arthritis Rheum. 2011;63:1938. doi: 10.1002/art.30351. [DOI] [PubMed] [Google Scholar]
- 18.Loeser R.F. Integrins and cell signaling in chondrocytes. Biorheology. 2002;39:119. [PubMed] [Google Scholar]
- 19.Tanaka K. Yokosaki Y. Higashikawa F. Saito Y. Eboshida A. Ochi M. The integrin alpha5beta1 regulates chondrocyte hypertrophic differentiation induced by GTP-bound transglutaminase 2. Matrix Biol. 2007;26:409. doi: 10.1016/j.matbio.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Balanis N. Yoshigi M. Wendt M.K. Schiemann W.P. Carlin C.R. β3 integrin-EGF receptor cross-talk activates p190RhoGAP in mouse mammary gland epithelial cells. Mol Biol Cell. 2011;22:4288. doi: 10.1091/mbc.E10-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J.H. Reiske H. Guan J.L. Regulation of the cell cycle by focal adhesion kinase. J Cell Biol. 1998;143:1997. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhati R.S. Mukherjee D.P. McCarthy K.J. Rogers S.H. Smith D.F. Shalaby S.W. The growth of chondrocytes into a fibronectin-coated biodegradable scaffold. J Biomed Mater Res. 2001;56:74. doi: 10.1002/1097-4636(200107)56:1<74::aid-jbm1070>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Jiao Y.P. Cui F.Z. Surface modification of polyester biomaterials for tissue engineering. Biomed Mater. 2007;2:R24. doi: 10.1088/1748-6041/2/4/R02. [DOI] [PubMed] [Google Scholar]
- 24.Patel S.D. Ciatto C. Chen C.P. Bahna F. Rajebhosale M. Arkus N. Schieren I. Jessell T.M. Honig B. Price S.R. Shapiro L. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell. 2006;124:1255. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 25.Okazaki M. Takeshita S. Kawai S. Kikuno R. Tsujimura A. Kudo A., et al. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J Biol Chem. 1994;269:12092. [PubMed] [Google Scholar]
- 26.Kawaguchi J. Kii I. Sugiyama Y. Takeshita S. Kudo A. The transition of cadherin expression in osteoblast differentiation from mesenchymal cells: consistent expression of cadherin-11 in osteoblast lineage. J Bone Miner Res. 2001;16:260. doi: 10.1359/jbmr.2001.16.2.260. [DOI] [PubMed] [Google Scholar]
- 27.Kii I. Amizuka N. Shimomura J. Saga Y. Kudo A. Cell-cell interaction mediated by cadherin-11 directly regulates the differentiation of mesenchymal cells into the cells of the osteo-lineage and the chondro-lineage. J Bone Miner Res. 2004;19:1840. doi: 10.1359/JBMR.040812. [DOI] [PubMed] [Google Scholar]
- 28.Matsusaki T. Aoyama T. Nishijo K. Okamoto T. Nakayama T. Nakamura T., et al. Expression of the cadherin-11 gene is a discriminative factor between articular and growth plate chondrocytes. Osteoarthritis Cartilage. 2006;14:353. doi: 10.1016/j.joca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal S.K. Lee D.M. Kiener H.P. Brenner M.B. Coexpression of two mesenchymal cadherins, cadherin 11 and N-cadherin, on murine fibroblast-like synoviocytes. Arthritis Rheum. 2008;58:1044. doi: 10.1002/art.23369. [DOI] [PubMed] [Google Scholar]
- 30.Lee D.M. Kiener H.P. Agarwal S.K. Noss E.H. Watts G.F. Chisaka O., et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y. Xiang Q. Dong S. Li C. Zhou Y. Fabrication and characterization of a recombinant fibronectin/cadherin bio-inspired ceramic surface and its influence on adhesion and ossification in vitro. Acta Biomater. 2010;6:776. doi: 10.1016/j.actbio.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Chang K.Y. Hung L.H. Chu I.M. Ko C.S. Lee Y.D. The application of type II collagen and chondroitin sulfate grafted PCL porous scaffold in cartilage tissue engineering. J Biomed Mater Res A. 2010;92:712. doi: 10.1002/jbm.a.32198. [DOI] [PubMed] [Google Scholar]
- 33.Francioli S.E. Candrian C. Martin K. Heberer M. Martin I. Barbero A. Effect of three-dimensional expansion and cell seeding density on the cartilage-forming capacity of human articular chondrocytes in type II collagen sponges. J Biomed Mater Res A. 2010;95:924. doi: 10.1002/jbm.a.32917. [DOI] [PubMed] [Google Scholar]
- 34.Calderon L. Collin E. Velasco-Bayon D. Murphy M. O'Halloran D. Pandit A. Type II collagen-hyaluronan hydrogel—a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater. 2010;20:134. doi: 10.22203/ecm.v020a12. [DOI] [PubMed] [Google Scholar]
- 35.Barnes C.P. Pemble C.W. Brand D.D. Simpson D.G. Bowlin G.L. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007;13:1593. doi: 10.1089/ten.2006.0292. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y. Zhou Y. Zhu J. Dong S. Li C. Xiang Q. Effect of a novel recombinant protein of fibronectinIII7-10/cadherin 11 EC1-2 on osteoblastic adhesion and differentiation. Biosci Biotechnol Biochem. 2009;73:1999. doi: 10.1271/bbb.90187. [DOI] [PubMed] [Google Scholar]
- 37.Dong S.W. Ying D.J. Duan X.J. Xie Z. Yu Z.J. Zhu C.H., et al. Bone regeneration using an acellular extracellular matrix and bone marrow mesenchymal stem cells expressing Cbfa1. Biosci Biotechnol Biochem. 2009;73:2226. doi: 10.1271/bbb.90329. [DOI] [PubMed] [Google Scholar]
- 38.Yang B. Guo H. Zhang Y. Chen L. Ying D. Dong S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS One. 2011;6:e21679. doi: 10.1371/journal.pone.0021679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutgers M. van Pelt M.J. Dhert W.J. Creemers L.B. Saris D.B. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010;18:12. doi: 10.1016/j.joca.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Hoemann C.D. Kandel R.A. Roberts K. Saris D.B. Creemers L.B. Mainil-Varlet P., et al. International Cartilage Repair Society (ICRS) recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage. 2011;2:153. doi: 10.1177/1947603510397535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mainil-Varlet P. Van Damme B. Nesic D. Knutsen G. Kandel R. Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38:880. doi: 10.1177/0363546509359068. [DOI] [PubMed] [Google Scholar]
- 42.Hennessy K.M. Pollot B.E. Clem W.C. Phipps M.C. Sawyer A.A. Culpepper B.K., et al. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials. 2009;30:1898. doi: 10.1016/j.biomaterials.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brakebusch C. Fassler R. Beta 1 integrin function in vivo: adhesion, migrate and more. Cancer Metastasis Rev. 2005;24:403. doi: 10.1007/s10555-005-5132-5. [DOI] [PubMed] [Google Scholar]
- 44.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 45.Bigerelle M. Anselme K. A kinetic approach to osteoblast adhesion on biomaterial surface. J Biomed Mater Res A. 2005;75:530. doi: 10.1002/jbm.a.30473. [DOI] [PubMed] [Google Scholar]
- 46.Mikami Y. Ishii Y. Watanabe N. Shirakawa T. Suzuki S. Irie S., et al. CD271/p75(NTR) inhibits the differentiation of mesenchymal stem cells into osteogenic, adipogenic, chondrogenic, and myogenic lineages. Stem Cells Dev. 2011;20:901. doi: 10.1089/scd.2010.0299. [DOI] [PubMed] [Google Scholar]
- 47.Yang C. Frei H. Rossi F.M. Burt H.M. The differential in vitro and in vivo responses of bone marrow stromal cells on novel porous gelatin-alginate scaffolds. J Tissue Eng Regen Med. 2009;3:601. doi: 10.1002/term.201. [DOI] [PubMed] [Google Scholar]
- 48.Noh H. Vogler E.A. Volumetric interpretation of protein adsorption: competition from mixtures and the Vroman effect. Biomaterials. 2007;28:405. doi: 10.1016/j.biomaterials.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoshiba T. Yamada T. Lu H. Kawazoe N. Chen G. Maintenance of cartilaginous gene expression on extracellular matrix derived from serially passaged chondrocytes during in vitro chondrocyte expansion. J Biomed Mater Res A. 2012;100:694. doi: 10.1002/jbm.a.34003. [DOI] [PubMed] [Google Scholar]