Abstract
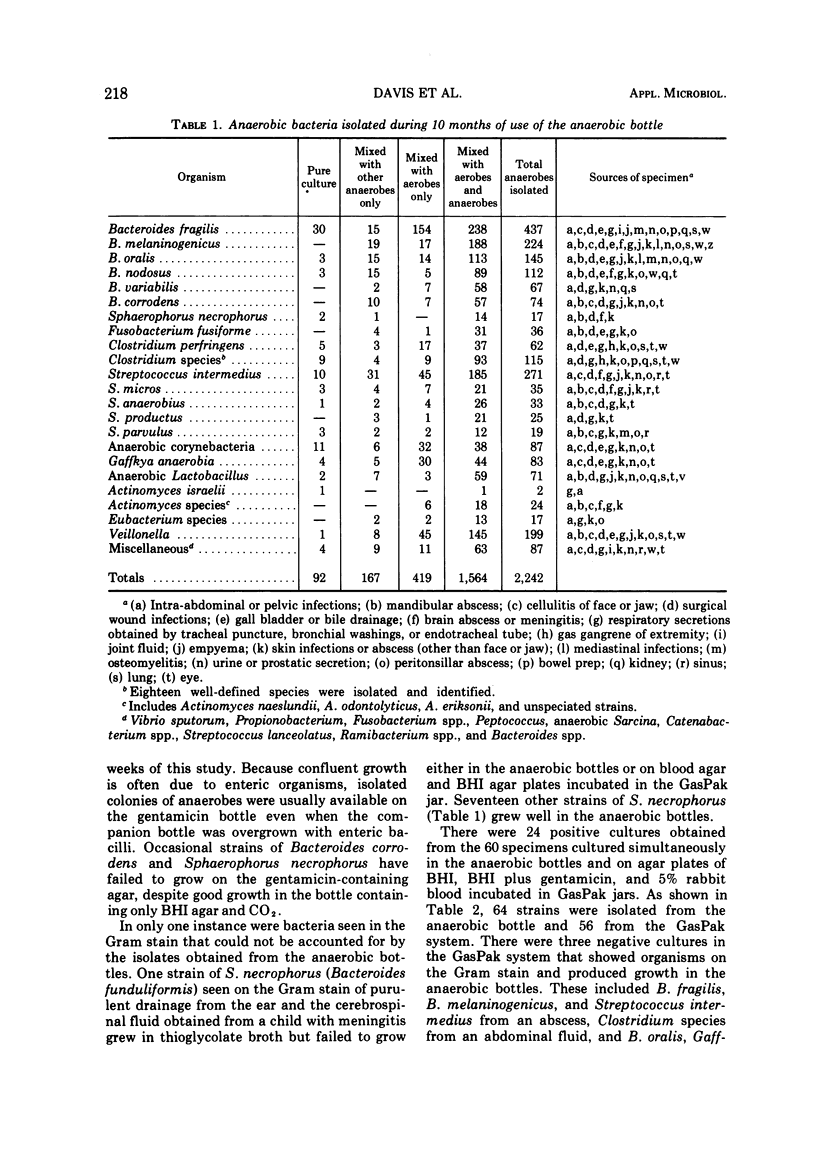
A simple, effective method is needed for growing obligate anaerobes in the clinical laboratory. This report describes a pre-reduced anaerobic bottle that can be taken to the bedside for direct inoculation, provides a flat agar surface for evaluation of number and morphology of colonies, and can be incubated in conventional bacteriological incubators. Each anaerobic culture set consisted of two bottles containing brain heart infusion agar and CO2. Gentamicin sulfate (50 μg/ml) was added to one of these to inhibit facultative enteric bacilli. Comparison of the anaerobic bottles with an identical aerobic bottle which was also routinely inoculated permitted early identification of anaerobic colonies. Representative species of most anaerobic genera of proven pathogenicity for man have been isolated from this system during 10 months of routine use.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attebery H. R., Finegold S. M. Photomicrography of bacterial colonies in rool tubes. J Biol Photogr Assoc. 1969 Oct;37(4):199–208. [PubMed] [Google Scholar]
- BARRY V. C., CONALTY M. L., DENNENY J. M., WINDER F. Peroxide formation in bacteriological media. Nature. 1956 Sep 15;178(4533):596–597. doi: 10.1038/178596a0. [DOI] [PubMed] [Google Scholar]
- Drasar B. S. Cultivation of anaerobic intestinal bacteria. J Pathol Bacteriol. 1967 Oct;94(2):417–427. doi: 10.1002/path.1700940223. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E., BRYANT M. P., MAH R. A. THE RUMEN BACTERIA AND PROTOZOA. Annu Rev Microbiol. 1964;18:131–166. doi: 10.1146/annurev.mi.18.100164.001023. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROOM H., WOIWOD A. J., BARNES J. M., ORBELL W. G. A growth-inhibitory effect on Shigella dysenteriae which occurs with some batches of nutrient agar and is associated with the production of peroxide. J Gen Microbiol. 1950 May;4(2):270–276. doi: 10.1099/00221287-4-2-270. [DOI] [PubMed] [Google Scholar]
- ROSEBURY T., REYNOLDS J. B. CONTINUOUS ANAEROBIOSIS FOR CULTIVATION OF SPIROCHETES. Proc Soc Exp Biol Med. 1964 Dec;117:813–815. doi: 10.3181/00379727-117-29706. [DOI] [PubMed] [Google Scholar]
- SOCRANSKY S., MACDONALD J. B., SAWYER S. The cultivation of Treponema microdentium as surface colonies. Arch Oral Biol. 1959 Oct;1:171–172. doi: 10.1016/0003-9969(59)90009-3. [DOI] [PubMed] [Google Scholar]
- Spears R. W., Freter R. Improved isolation of anaerobic bacteria from the mouse cecum by maintaining continuous strict anaerobiosis. Proc Soc Exp Biol Med. 1967 Mar;124(3):903–909. doi: 10.3181/00379727-124-31882. [DOI] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J. Studies of the cultivable flora of normal human feces. Antonie Van Leeuwenhoek. 1966;32(2):212–222. doi: 10.1007/BF02097463. [DOI] [PubMed] [Google Scholar]
- Zabransky R. J. Isolation of anaerobic bacteria from clinical specimens. Mayo Clin Proc. 1970 Apr;45(4):256–264. [PubMed] [Google Scholar]