Abstract
Rewards have been shown to improve behavior and cognitive processes implicated in ADHD, but the information processing mechanisms by which these improvements occur remain unclear. We examined the effect of performance-based rewards on ERPs related to processing of the primary task stimuli, errors, and feedback in children with ADHD and typically developing controls. Participants completed a flanker task containing blocks with and without performance-based rewards. Children with ADHD showed reduced amplitude of ERPs associated with processing of the flanker stimuli (P3) and errors (ERN, Pe), but did not differ in feedback-processing (FRN). Rewards enhanced flanker-related P3 amplitude similarly across groups and error-related Pe amplitude differentially for children with ADHD. These findings suggest that rewards may improve cognitive deficits in children with ADHD through enhanced processing of relevant stimuli and increased error evaluation.
Attention-deficit hyperactivity disorder (ADHD) is one of the most commonly diagnosed childhood disorders (Polanczyk, de Lima, Horta, Biederman, & Rohde, 2007), characterized by persistent and impairing developmentally inappropriate levels of inattentive and/or hyperactive and impulsive behavior (DSM-IV; American Psychiatric Association, 2000). The heterogeneity of ADHD and the variability of symptom presentation in particular settings as well as the instability of the subtypes of ADHD (Lahey, Pelham, Loney, Lee, & Willcutt, 2005) pose a significant challenge for etiological theories attempting to account for the disorder. Atypical motivation is central to many theories of ADHD, with a particular emphasis on the contribution of altered reinforcement processes to symptoms of hyperactivity and impulsivity (see review by Luman, Tripp, & Scheres, 2010). Specifically, hypothesized alterations in reward processes in children with clinically significant levels of hyperactivity and impulsivity may be due to an elevated reward threshold (Haenlein & Caul, 1987), greater discounting of delayed consequences (Luman et al., 2010; Shiels et al., 2009; Sonuga-Barke, 2003), or a steepened delay of reinforcement gradient (Sagvolden, Johansen, Aase, & Russell, 2005). Sagvolden and colleagues (2005) propose that the behavioral characteristics associated with ADHD, including delay aversion, impulsiveness, development of hyperactivity in novel situations, increased behavioral variability, deficient sustained attention, and disinhibition, are associated with dysfunction in dopamine transmission in the fronto-limbic brain circuitry. In addition, Tripp & Wickens (2008) propose that altered activity of the phasic dopaminergic response result in altered reinforcement sensitivity in ADHD. Furthermore, the use of contingency management (i.e., reward and response cost) to modify behavior is an important component of evidence-based treatments for ADHD (American Academy of Pediatrics, 2001; Pelham & Fabiano, 2008). Reward also improves the performance of children with ADHD on a variety of cognitive tasks (see reviews by Luman, Oosterlaan, & Sergeant, 2005; Luman et al., 2010; e.g., Shiels et al., 2008; Strand et al., 2012). Improving our understanding of the impact of reward on cognitive deficits in children with ADHD has important theoretical and clinical implications, particularly for those individuals with clinically significant hyperactive/impulsive symptoms, as is the focus of the current study.
A comprehensive understanding the mechanisms by which reward improves the performance of children with ADHD is gained through the analysis of behavior (e.g., accuracy and reaction time) as well as the neural correlates of information processing reflected in event-related potentials (ERPs). This includes examination of ERPs associated with processing of the primary task stimuli as well as responses to errors and feedback thought to reflect performance monitoring. Relevant stimulus processing ERP components include a frontal N2 component occurring approximately 200-300 ms post-stimulus onset, associated with conflict monitoring (Donkers & van Boxtel, 2004), and a parietal P3 component occurring approximately 300-600 ms post-stimulus, associated with stimulus evaluation/categorization (Polich, 2007; Ridderinkhof & Vandermolen, 1995). ERPs associated with error-processing include the error-related negativity (ERN or Ne; Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993), a response-locked ERP that occurs approximately 0-100 ms post-response onset associated with early error detection (Holroyd & Coles, 2002), and the error positivity (Pe; Falkenstein, Hoormann, Christ, & Hohnsbein, 2000), a positive deflection that frequently follows the ERN (250-500 ms post-response) associated with conscious error recognition/evaluation (see review by Overbeek, Nieuwenhuis, & Ridderinkhof, 2005). In addition, the feedback-related negativity (FRN), a negative deflection in the ERP that occurs approximately 250-500 ms post-feedback onset (Miltner, Braun, & Coles, 1997), is also related to performance monitoring. Understanding where reward is having an influence on performance involves simultaneous examination of ERPs associated with processing of the primary task stimuli and performance monitoring within a single task.
Establishing that children with ADHD display deficient processing of the primary task stimuli, errors and feedback is an important first step when considering the impact of reward on performance. Children with ADHD typically display greater difficulty processing stimuli when conflicting distractors are present (e.g., incongruent compared to congruent stimuli), as reflected in their behavioral (e.g., Crone, Jennings, & van der Molen, 2003) and neurophysiological responses (e.g., Johnstone, Barry, & Clarke, 2012; Johnstone, Barry, Markovska, Dimoska, & Clarke, 2009; Jonkman et al., 1999). In contrast, the rapidly emerging body of research examining neurophysiological indices of error-processing (ERN and Pe) among children with ADHD has produced equivocal results as demonstrated in a recent review (Shiels & Hawk, 2010) and meta-analysis (Geburek, Rist, Gediga, Stroux, & Pedersen, 2012). Studies examining ERN amplitude in children with ADHD report diminished (e.g., Senderecka, Grabowska, Szewczyk, Gerc, & Chmylak, 2012), equivalent (e.g., Groom, Cahill, et al., 2010; Shen, Tsai, & Duann, 2011), and increased ERN amplitude in ADHD (Burgio-Murphy et al., 2007). As discussed in our review (Shiels & Hawk, 2010), the inconsistent results regarding ERN amplitude in ADHD may be due to a number of methodological issues, including: small sample sizes with modest statistical power, variation in task difficulty and duration, wide age ranges contributing to within-group variability, and failure to consider or exclude comorbid internalizing psychopathology, which is generally associated with increased ERN (Meyer, Weinberg, Klein, & Hajcak, 2012; Olvet & Hajcak, 2008). Indeed, ERN amplitude was reduced among children with ADHD in studies that had a large sample size (n=68; Albrecht et al., 2008), excluded participants with internalizing psychopathology (Liotti, Pliszka, Perez, Kothmann, & Woldorff, 2005; Senderecka et al., 2012), or used a relatively long task duration (Groen et al., 2008; van Meel, Heslenfeld, Oosterlaan, & Sergeant, 2007). The results appear more consistent for Pe, with the majority of studies reporting diminished Pe among children with ADHD (e.g., Senderecka et al., 2012; Shen et al., 2011), and only two studies reporting equivalent Pe amplitude in ADHD and control children (Albrecht et al., 2008; Burgio-Murphy et al., 2007).
Studies that have examined the FRN as an index of feedback-processing in children with ADHD have also produced inconsistent results. However, these studies have employed a very different set of tasks compared with standard measures of attention or inhibition, such as a traditional flanker or Go/No-Go task. Instead, probabilistic or maze-learning tasks have been used, in which feedback was provided in response to a guess rather than a task in which the accuracy of the child's response to the primary task stimuli is evaluated and feedback depends on the accuracy of their response. This is a crucial distinction as responses to feedback may differ depending on whether the task allows the participant to form an expectation regarding the type of feedback that will be presented (Bismark, Hajcak, Whitworth, & Allen, 2013). For example, the explicit stimulus-response mappings provided during a flanker task (i.e., press right if central arrow points right) allows participants to perfectly predict the feedback they will receive if they are monitoring the accuracy of their response. However, during a probabilistic or maze-learning task, participants are unable to determine the accuracy of their response until they learn the associations and are therefore more dependent on feedback for performance monitoring. For the three studies that have examined FRN amplitude during a probabilistic or maze-learning task in children with ADHD, one study did not find evidence of a typical FRN in children with or without ADHD (Groen et al., 2008), another reported a larger FRN in children with ADHD (van Meel, Oosterlaan, Heslenfeld, & Sergeant, 2005), and the third study reported that the response to negative feedback in children with ADHD was influenced by reward salience (Holroyd, Baker, Kerns, & Muller, 2008). In addition, van Meel and colleagues (2011) found that the FRN was absent in children with ADHD during a time estimation task, adding to the inconsistent FRN results. The current study is the first to examine ERP responses to feedback during a traditional flanker task, allowing for consideration of processing of the primary task stimuli measuring attentional processes, as well as errors and feedback, associated with performance monitoring, in a single paradigm.
The available literature examining impact of reward on the ERPs associated with the various information processing stages, particularly stimulus- and error-processing, as well as studies examining neural activation during reward tasks using fMRI, also informed the predictions for this study. Reward has been shown to improve stimulus processing and increase attention for task relevant stimuli, resulting in increased N2 and P3 amplitude in healthy adults (Goldstein et al., 2006) and in children with ADHD (Groom, Scerif, et al., 2010). Studies have also shown that neurophysiological correlates of error processing are influenced by factors thought to alter one's motivational state (Boksem, Meijman, & Lorist, 2006; Dikman & Allen, 2000; Gehring & Willoughby, 2002; Hajcak, Moser, Yeung, & Simons, 2005; Potts, 2011). These studies suggest that the ERN is larger when motivation is increased, such as when monetary incentives are offered for accurate performance (Boksem et al., 2006; Hajcak et al., 2005), consistent with the claim that the ACC monitors the motivational significance of stimuli and events (Holroyd & Yeung, 2011) and with reinforcement learning theories of the ERN (Holroyd & Coles, 2002). The impact of motivational factors on ERN or Pe amplitude has only been examined in one study involving children with ADHD (Groom et al., 2013), despite the evidence of altered reinforcement mechanisms in ADHD (Luman et al., 2010; Tripp & Wickens, 2008) and that the ERN is influenced by reward (Sturmer, Nigbur, Schacht, & Sommer, 2011) and associated with reinforcement learning (Holroyd & Coles, 2002). The findings from the recent Groom et al. (2013) study suggest that ERN amplitude was greatest in children with ADHD as response cost magnitude increased whereas Pe amplitude was greatest in children with ADHD as reward magnitude increased. However, this study did not include a condition without motivational incentives and immediate feedback regarding accuracy on each trial was not provided, preventing an examination of group differences in performance without incentives and responses to feedback.
Consideration of the neuroimaging literature examining activation of brain regions associated with reward processing during tasks involving reward anticipation or delivery in individuals with ADHD is also informative, with accumulating evidence of general disruption in fronto-striatal circuits in ADHD (Cubillo, Halari, Smith, Taylor, & Rubia, 2012; Durston, van Belle, & de Zeeuw, 2011). Studies involving a reward task have reported reduced neural activation in children with ADHD in the ventral striatum during reward anticipation (Plichta et al., 2009; Scheres, Milham, Knutson, & Castellanos, 2007), reduced reactivity of the anterior cingulate cortex to non-reward (Gatzke-Kopp et al., 2009), increased ventral striatal response to successful versus unsuccessful outcomes (Paloyelis, Mehta, Faraone, Asherson, & Kuntsi, 2012), and reduced activation of the orbitofrontal cortex to reward feedback (Edel et al., 2013). These studies have primarily focused on neural activation during reward anticipation or outcome, rather than the impact of reward on neural activation during stimulus-, error-, and feedback-processing, thereby limiting the comparisons that can be made, although an atypical response to feedback (i.e., reward outcomes) is expected based on this literature.
The current study examined neurophysiological measures of stimulus-, error- and feedback-processing in children diagnosed with ADHD and typically developing children in the context of a cognitive task with performance-based rewards. To address the concerns from previous research, the present study includes participants within a narrow age range and diagnosed with ADHD Combined Type without comorbid internalizing problems to create a relatively homogeneous sample (Shiels & Hawk, 2010). We did not include children with ADHD Hyperactive/Impulsive Type, due to the low prevalence of this subtype which prevents a comparison of the two groups, or children with ADHD Inattentive Type, due to the lack of evidence for reward processing deficits in these children. Another important aspect of our study design is the use of an individualized response deadline to adjust the difficulty of the task to each participant's ability level (Groom et al., 2013; Spronk, Dumont, Verkes, & de Bruijn, 2011; van Meel et al., 2007) and increase the error rate (Potts, 2011), thereby reducing the potential influence of group differences in task performance on ERP measures and producing enough error trials to reliably examine the ERN and FRN. This manipulation provides a cleaner interpretation of ERN amplitude, which is sensitive to error rate (Holroyd & Coles, 2002; Pailing & Segalowitz, 2004; Santesso, Segalowitz, & Schmidt, 2005). The primary hypotheses included predictions that children with ADHD, compared to typically developing children, will exhibit (1) reduced N2, P3, ERN and Pe amplitude, (2) greater improvement in performance with reward, (3) greater increase in N2, P3, ERN and Pe amplitude with reward, and (4) an atypical response to performance feedback (FRN amplitude).
Method
Participants
The study included 10-12 year-old children with a clinical diagnosis of ADHD-Combined type (n=30, 6 female) and typically developing controls (CTRL; n=25, 6 female) (see Table 1). Participants were recruited from a larger study on the effects of medication and motivation on cognitive performance. Recruitment methods included letters to families that have visited the University at Buffalo Center for Children and Families for research or clinical services, advertisements in local newspapers, and flyers posted in pediatrician offices and at schools. The study procedures were approved by the University at Buffalo Institutional Review Board.
Table 1.
Sample Characteristics
| ADHD (n=30) | CTRL (n=25) | p-value | |||
|---|---|---|---|---|---|
| Mean(SD) | Range | Mean(SD) | Range | ||
| Age (years) | 11.2 (0.8) | 10.1-12.7 | 11.5 (1.0) | 10.0-12.9 | .20 |
| Sex (% male) | 80% | 76% | .73 | ||
| Estimated IQ | 106.1 (11.9) | 83-123 | 114.4 (12) | 94-135 | .02 |
| Race (% white) | 87% | 92% | .90 | ||
| Parent's Education (median) | College Graduate | College Graduate | .94 | ||
| Income (median) | 50-59K | 10-100K+ | 70-79K | 40-100K+ | .01 |
| DBD Inattention Symptomsa | 8.2 (1.5) | 3-9 | 0.4 (0.2) | 0-1 | <.001 |
| DBD Hyperactivity/Impulsivity Symptomsa | 7.7 (1.5) | 4-9 | 0.1 (0.3) | 0-1 | <.001 |
| DBD ODD Symptomsa | 3.8 (2.2) | 0-7 | 0.0 (0.0) | 0-0 | <.001 |
| DBD CD Symptomsa | 0.9 (1.1) | 0-4 | 0.0 (0.0) | 0-0 | <.001 |
| CDI T-score | 46.5 (6.9) | 40-62 | 44.4 (5.1) | 40-59 | .20 |
| RCMAS T-score | 49.8 (9.2) | 24-64 | 45.0 (10.3) | 24-65 | .07 |
Notes. IQ = Estimated Full Scale IQ based on prorated scores from the vocabulary and block design subtests of the WISC-IV; DBD = Disruptive Behavior Disorder Rating Scale; ODD = Oppositional Defiant Disorder; CD = Conduct Disorder; CDI = Children's Depression Inventory; RCMAS = Revised Children's Manifest Anxiety Scale.
Combined parent and teacher ratings on the DBD Rating Scale, mean (SD) and range of total number of items rated as “pretty much” or “very much” by either the parent or the teacher, indicating a symptom was present.
Recruitment for the larger study consisted of a phone screen followed by completion and review of parent and teacher rating forms and an intake visit with the parent and child. Inclusion criteria for children in the ADHD group included the presence of at least 6 symptoms of inattention or 6 symptoms of hyperactivity/impulsivity based on the combined parent and teacher ratings on the Disruptive-Behavior Disorder Rating Scale (DBD-RS; Pelham, Gnagy, Greenslade, & Milich, 1992) and functional impairment (i.e., score of 3 or greater in at least one domain) according to the Impairment Rating Scale (IRS; Fabiano et al., 2006). A diagnosis of ADHD-combined subtype was also required on the Diagnostic Interview Schedule for Children, 4th edition (DISC-IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000), which was administered by research assistants who had obtained either a bachelors or masters degree in psychology after receiving training from a licensed clinical psychologist. Symptoms of oppositional defiant disorder (ODD) and conduct disorder (CD) were permissible for children in the ADHD group given the high comorbidity rates of these disorders.
In contrast, inclusion criteria for children in the control group were (1) subclinical levels of inattention (<4 symptoms), hyperactivity/impulsivity (<4 symptoms), ODD (<3 symptoms), and CD (<2 symptoms) according to the combined parent and teacher DBD ratings, (2) the absence of DSM-IV disruptive behavior disorders on the DISC-IV, and (3) no first-degree relative with ADHD (per parent report). Finally, they could not score in the clinical range (T-score > 65) on the externalizing subscale of the Child-Behavior Checklist and Teacher Report Form (CBCL or TRF; Achenbach & Rescorla, 2001), or exhibit functional impairment according to the IRS.
Exclusion criteria for both groups included: (1) estimated IQ less than 80 based on prorated scores from the vocabulary and block design subtests from the WISC-IV (Kaplan, Fein, Kramer, Delis, & Morris, 2004; Sattler, 1992),1 (2) parent-reported developmental disorder (Tourette's syndrome, epilepsy, Autism or Asperger's syndrome, pervasive developmental disorder), (3) clinically elevated internalizing symptoms as indicated by a T-score > 65 on self-reported anxiety (Revised Children's Manifest Anxiety Scale, RCMAS; Gerard & Reynolds, 1999), depression (Children's Depression Inventory, CDI; Sitarenios & Kovacs, 1999), or parent and teacher report on the internalizing subscale for both the CBCL and TRF since internalizing psychopathology may enhance the ERN (Olvet & Hajcak, 2008; Vaidyanathan, Nelson, & Patrick, 2012; Weinberg, Luhmann, Bress, & Hajcak, 2012), (4) current psychotropic medications other than stimulants, (5) history of traumatic brain injury and/or concussion, and (6) uncorrected auditory or visual problems. Participation in the current study occurred within 6 months from when the diagnostic information was obtained for the larger study. In addition, updated parent ratings of ADHD symptoms and impairment and child self-report ratings of anxiety and depression were obtained during the study visit to confirm diagnostic status. Parents were also asked to complete a brief measure indicating socioeconomic status.
Procedures
Eligible participants visited the lab for a single session lasting up to 3 hours consisting of the following: (1) consent/assent procedures, (2) explanation of the EEG application procedures and the behavior system, which involved earning points for following four basic behavioral rules and was established to encourage cooperation from the child throughout the visit, (3) application of the electrode cap, (4) completion of the cognitive task (including a break halfway through the task), (5) recording of resting EEG for 10 minutes, (6) completion of questionnaires and (7) spending points on preferred prizes and gift cards in a small lab “store”.
Eighteen participants within the ADHD group (60%) were regularly taking a stimulant medication. Participants were asked to discontinue their medication at least 24 hours prior to the visit, as in prior work on long-acting stimulants (e.g., Ashare et al., 2010; Shiels et al., 2009; Strand et al., 2012) Most medicated children were prescribed a formulation of methylphenidate (Concerta [n=9], Ritalin [n=2], Daytrana [n=1], Focalin [n=1]), which has a half-life of approximately 3.5 hours. The average reported time since last dose for these participants was 31.5 hours (SD=8.8 hours; range = 25-52 hours) such that these children were essentially medication free at the time of testing. Amphetamine-based medications were taken by about one quarter of the medicated children (Adderall [n=3], Vyvanse [n=2]), with the average reported time since last dose as 31.5 hours (SD=0.6 hours; range = 31-32 hours). Given the elimination half-life of approximately 7-10 hours for d- and l-amphetamine in children with ADHD (e.g., Greenhill et al., 2003) the vast majority of medication was also eliminated from these participants at the time of testing.
Experimental task
During the cognitive task, participants were comfortably seated in an IAC (Bronx, NY) 2.5- × 2.3-m acoustically isolated chamber. Stimuli were presented on a 43 cm CRT monitor placed ~70 cm away from the participant's face. The participant held a response box (Psychology Software Tools) with both hands with their thumbs positioned on the outermost response buttons. The experimenter sat outside of the room and constantly monitored the child via video cameras and two-way intercom.
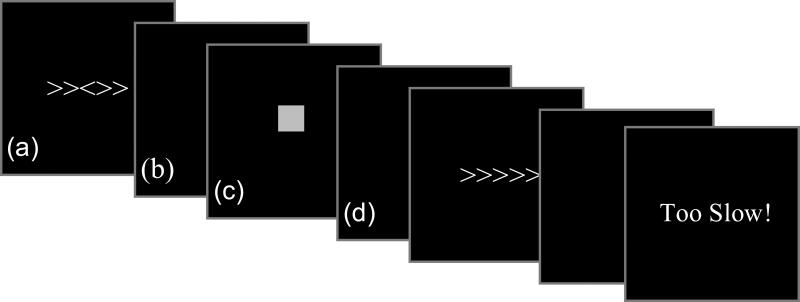
The Eriksen Flanker Task (Eriksen & Eriksen, 1974) was programmed and presented with E-Prime 1.1. Stimulus array was five “arrows” with central target flanked by incongruent arrows pointing in the opposite direction from the central target arrow (>><>>, <<><<) or congruent trials with identical flankers (<<<<<, >>>>>). Congruent and incongruent trials were presented with equal probability in a unique pseudorandom order for each participant. Participants were instructed to respond by pressing a button indicating the direction of the central arrow. The trial structure consisted of the flanker stimulus array (150 ms), a variable interval response window (1200-1600 ms), feedback stimulus (300 ms) and a variable intertrial interval (1000-1400 ms) (see Figure 1).
Figure 1. Task Structure.
The task included reward and no-reward conditions, each of which consisted of 2 blocks with 200 trials per block and a short break in between. During the reward condition, a red box appeared when an error occurred indicating no points were earned on the trial and a green box appeared for a correct response indicating 2 points were earned on the trial. During the no reward condition, a yellow box appeared regardless of the accuracy of the response and no points could be earned during the no reward condition. During both conditions, the words ‘Too Slow!’ appeared on the screen if the participant's response exceeded their individualized response deadline indicating that no points were earned. The timing of each component of the trial was identical across conditions: (a) stimulus (150 ms), (b) variable blank response screen (1200-1600 ms), (c) feedback (300 ms), and (d) variable ITI (1000-1400 ms).
Participants completed several practice blocks to ensure they understood the task, including a practice block (48 trials) used to determine an individualized response deadline (Spronk et al., 2011; van Meel et al., 2007), and a reward practice (24 trials) to explain the accuracy feedback and earning points. The response deadline was determined for each individual as their mean reaction time (MRT) plus ½ of the standard deviation of their reaction time (SDRT) with a minimum deadline of 400 ms and a maximum of 800 (Potts, 2011; Spronk et al., 2011; van Meel et al., 2007). After completing practice, the task consisted of four experimental blocks (200 trials, 10 minutes per block) that alternated between reward and no-reward conditions with the starting condition counterbalanced across participants. There was a short break between blocks, with a longer break (5-10 minutes) halfway through the task.
During the reward condition, feedback regarding response accuracy was provided for responses that occurred prior to the individualized response deadline (Figure 1). Correct fast responses resulted in the presentation of a green square indicating a gain of two points. For incorrect responses which occurred prior to the response deadline and omission errors, a red square indicated no points were earned. Participants could earn a maximum of 1000 points (equivalent to US$10). During the no-reward condition, participants did not receive feedback regarding accuracy. Instead, a yellow square was presented for correct and incorrect responses that occurred prior to the response deadline. During both the reward and no-reward conditions, participants received ‘Too Slow!’ feedback for responses exceeding their individualized deadline.
Computation of Performance Measures
Responses within 150-ms post-stimulus onset were considered invalid and were excluded from all analyses. To investigate the effect of reward on task performance, percent correct and MRT for correct responses were computed according to congruency (congruent, incongruent) and reward condition (no-reward, reward).
Electroencephalographic (EEG) Recording
The EEG was recorded using a lycra stretch cap (ECI, Eaton, OH) with electrodes placed at Fz, FCz, Cz, Pz, F3, F4, C3, C4, P3, and P4 according to the 10-20 system (Jasper, 1958) and the ground electrode positioned on the center of the forehead. Vertical electrooculographic (EOG) activity was recorded with electrodes above and below the left eye. For all channels, Ag-AgCl electrodes were used and impedance levels were kept below 10 kΩ. Continuous EEG data was on-line referenced to left mastoid. Neuroscan SynAmps2 bioamplifiers with a gain of 10 in DC mode were used to continuously digitize (1000 Hz sampling rate) and amplify the raw EEG signal with a low-pass cut-off of 200 Hz. Using Matlab (version 7.9) EEGlab software (version7.2.9.20b), the signals were then resampled at 250 Hz, filtered at 0.5 to 30 Hz, and re-referenced to the average of both mastoids. Eye blink VEOG activity was removed from all scalp sites via a modified regression procedure based on Semlitsch, Anderer, Schuster, & Presslich (1986).
Computation of ERP Measures
Only midline electrode sites (Fz, FCz, Cz, and Pz) were examined because the effects of interest were expected to be maximal at the midline sites (Holroyd & Coles, 2002; Johnstone & Galletta, 2012). Epochs were created from 200 ms before to 1000 ms after the onset of the flanker and feedback stimuli and from 300 ms before to 1000 ms after the response. Average amplitude from −200 to 0 ms pre-stimulus onset and −300 to −100 ms pre-response onset served as the baseline. Epochs with deflections of greater than 100 μV at any scalp site were rejected as artifact.
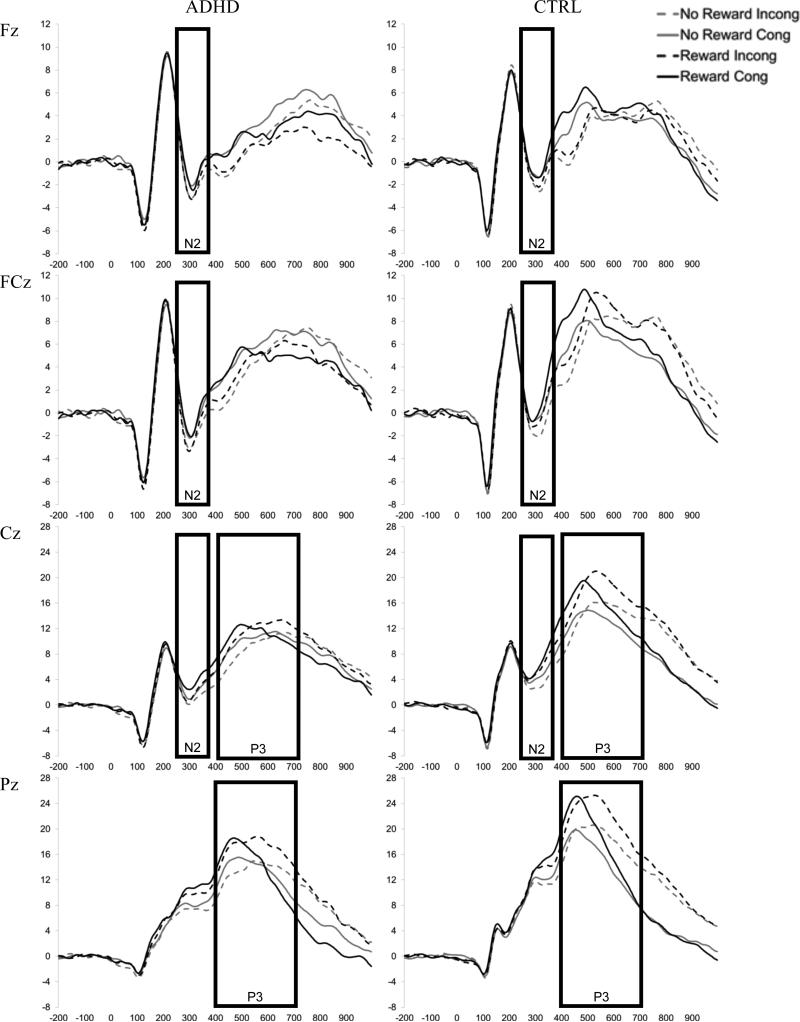
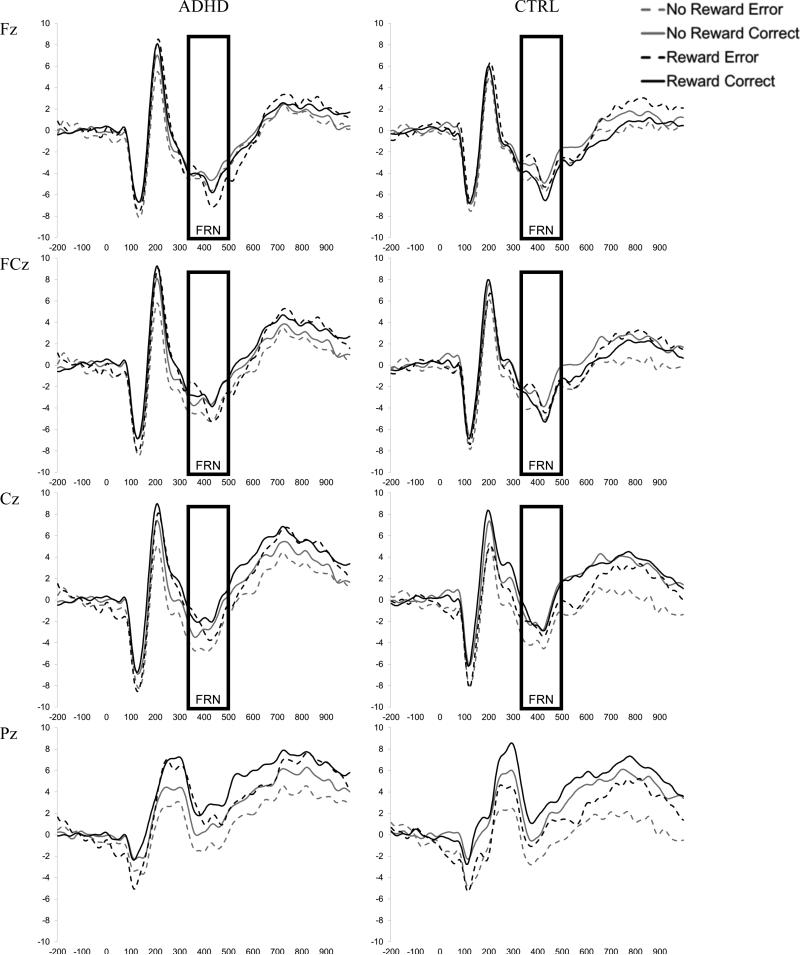
Processing of the primary task stimuli was examined with two flanker-locked ERPs: the frontal N2 ERP component thought to reflect conflict monitoring (Donkers & van Boxtel, 2004), and parietal P3 component typically associated with stimulus evaluation/categorization (Ridderinkhof & Vandermolen, 1995). Within-subject average waveforms were created for each Reward X Congruency condition at each site. All subjects exceeded our criterion of at least 30 trials per condition and groups did not differ in the number of trials included in the average waveforms for any of the Reward X Congruency conditions (see Table 2). Visual inspection of the grand average waveforms collapsed across group, reward, and congruency suggested maximal N2 from 250 to 350 ms and P3 from 400-700 ms. The mean amplitude within this window was used to quantify each component. Figure 2 presents average flanker-locked waveforms for all Group X Site X Reward X Congruency conditions.
Table 2.
Average number of trials included in ERP averages.
| ADHD | CTRL | p-value | |||
|---|---|---|---|---|---|
| Mean(SD) | Range | Mean(SD) | Range | ||
| Flanker | |||||
| No Reward Incongruent | 133 (35) | 70-184 | 147 (33) | 75-194 | .12 |
| No Reward Congruent | 132 (37) | 71-192 | 147 (34) | 71-193 | .14 |
| Reward Incongruent | 135 (33) | 73-182 | 149 (34) | 78-195 | .12 |
| Reward Congruent | 136 (36) | 75-185 | 149 (35) | 75-195 | .18 |
| Response | |||||
| No Reward Error | 67 (30) | 23-148 | 74 (33) | 12-152 | .44 |
| No Reward Correct | 155 (64) | 55-299 | 201 (63) | 77-335 | .01 |
| Reward Error | 69 (36) | 19-164 | 79 (42) | 8-161 | .36 |
| Reward Correct | 171 (62) | 77-301 | 201 (59) | 78-317 | .08 |
| Feedback | |||||
| No Reward Error | 90 (48) | 26-244 | 85 (33) | 48-154 | .70 |
| No Reward Correct | 167 (63) | 64-268 | 199 (52) | 80-298 | .06 |
| Reward Error | 82 (43) | 26-177 | 90 (44) | 39-180 | .54 |
| Reward Correct | 194 (61) | 103-306 | 211 (55) | 70-311 | .32 |
Figure 2. Grand average waveforms for flanker-locked ERPs: N2 and P3.
Grand average waveforms for each Reward X Congruency condition time-locked to the onset of the flanker stimulus for the ADHD (left) and Control (right) groups at Fz, FCz, Cz and Pz. The boxes reflect the window selected to calculate mean amplitude for each component (N2 and P3).
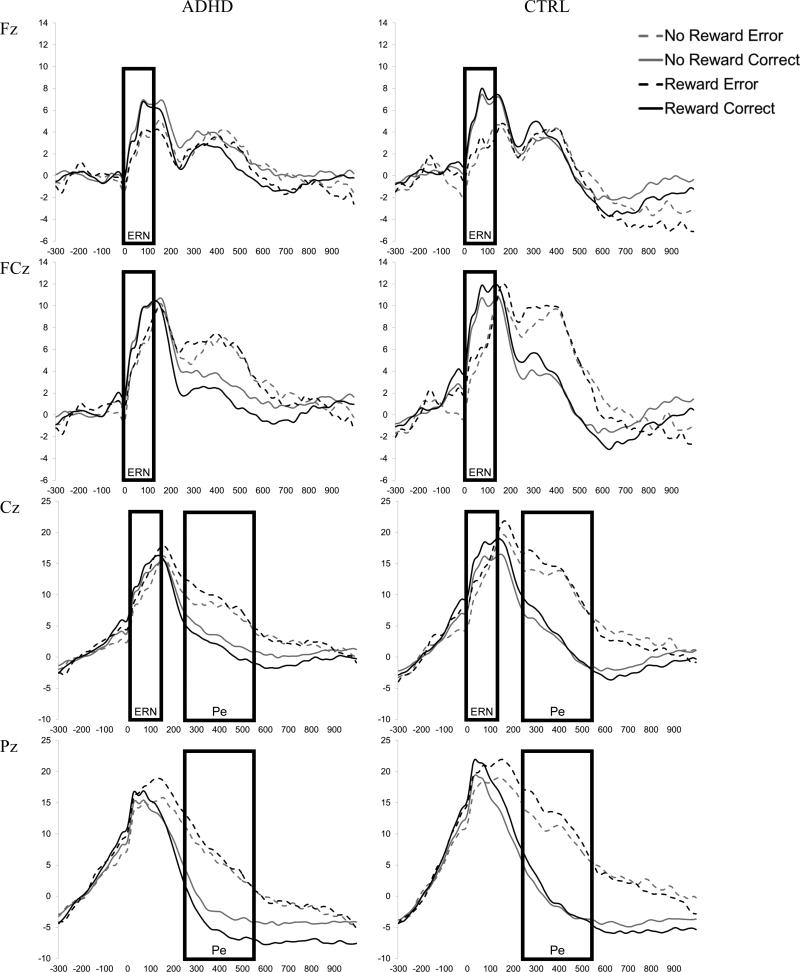
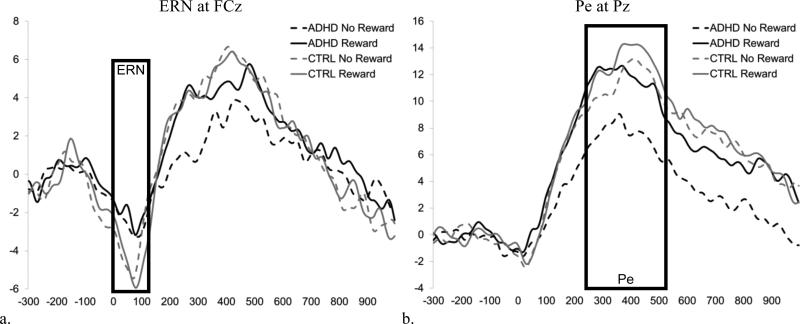
For the response-locked ERPs (ERN and Pe), within-subject average waveforms were created for each Reward X Accuracy condition. Congruency was not included as a factor due to the low number of errors on congruent trials. Only correct responses that preceded the participant's response deadline were included in the correct trials average to reduce the potential contributions of error-related activity as a result of the late response (Heldmann, Ruesseler, & Muente, 2008; Luu, Flaisch, & Tucker, 2000). All subjects were required to have at least 6 trials included in each average (Olvet & Hajcak, 2009) resulting in the exclusion of one control participant due to too few errors. Control children had a greater number of correct trials included in their average for the no reward condition, but groups did not differ in the number of error trials in the average waveform (see Table 2). To determine the window to be used for analysis of the ERN and Pe, difference waves were created by subtracting the correct waveform from the error waveform collapsed across reward and group to provide a relatively pure measure of the brain's differential response as a function of response accuracy (Luck, 2005). Examination of the grand average difference waves suggested ERN was maximal at frontocentral sites (Fz, FCz, Cz) from 0 to 100-ms post-response and Pe was maximal at centroparietal sites (Cz, Pz) from 250- to 550-ms post-response. Grand averages for Group X Site X Reward X Accuracy conditions for the response-locked ERPs are presented in Figure 3. The mean amplitude in the respective window for the average waveform created for each reward condition was used for analysis of ERN and Pe. The Group X Reward error-minus-correct difference waves for ERN at site FCz and Pe at site Pz are presented in Figures 4a and 4b, respectively.
Figure 3. Grand average waveforms for response-locked ERPs: ERN and Pe.
Grand average waveforms for each Reward X Accuracy condition time-locked to the onset of the response for the ADHD (left) and Control (right) groups at Fz, FCz, Cz and Pz. The boxes reflect the window selected to calculate mean amplitude for each component (ERN and Pe).
Figure 4. Error-correct difference waves for the response-locked ERPs: ERN and Pe.
Grand average waveforms for the error-correct difference wave for each Group X Reward Condition for (a) ERN at FCz and (b) Pe at Pz. The boxes reflect the window selected to calculate mean amplitude for each component (ERN and Pe).
For the feedback-locked ERP (FRN), within-subject average waveforms were created for each Reward × Accuracy condition. Only trials with correct (green square) or error (red square) feedback during the reward condition and with neutral feedback (yellow square) during the no-reward condition were examined. Trials on which “Too Slow!” feedback was presented were excluded due to the low number of trials in this condition. Responses to neutral feedback during the no-reward condition were categorized depending on response accuracy (e.g., neutral-correct, neutral-error), although the feedback was identical regardless of response accuracy (yellow square). This allowed for a direct comparison of ERPs in response to positive and negative feedback in the reward compared to the no-reward condition while accounting for activity related to response accuracy or other factors that may vary as a function of response accuracy. All subjects were required to have at least 20 trials included in each average, resulting in the exclusion of 5 participants (3 CTRL, 2 ADHD) for the analysis of responses to feedback. Groups did not significantly differ in the number of trials included in the average waveform for each condition (Table 2). Visual inspection of the grand average waveforms for each feedback type suggested the FRN was present at frontocentral sites (Fz, FCz, Cz) from 350 to 500 ms post-feedback onset and was measured as the mean amplitude. Figure 5 presents the average waveform for Group X Site X Reward X Accuracy conditions for the FRN.
Figure 5. Grand average waveforms for the feedback-locked ERP: FRN.
Grand average waveforms for each Reward X Accuracy condition time-locked to the onset of the feedback stimuli (i.e., green square for reward/correct, red square for reward/error, yellow square for no reward/correct and error) for the ADHD (left) CTRL (right) groups at Fz, FCz, Cz and Pz. The boxes reflect the window selected to calculate mean amplitude for the FRN.
Statistical Analysis
Repeated Measures ANOVAs were employed with Group (ADHD v. CTRL) and reward order (Order 1: reward/no-reward/reward/no-reward; Order 2: no-reward/reward/no-reward/reward) as between-subjects factors. Due to the small number of participants in each Group X Reward Order condition, we did not have sufficient power to reliably test for a 2×2 between-subjects interaction and this interaction term was omitted from the analytical models. Within-subjects factors were site (Fz, FCz, Cz for N2, ERN, and FRN; Cz, Pz for P3 and Pe) and reward condition (no-reward, reward) for all measures. Congruency (incongruent, congruent) was also a within-subjects factor for the following measures: percent correct, MRT and flanker-locked ERPs (N2 and P3). Examination of electrode site effects was used to identify the location at which the expected effects of congruency (for N2 and P3) and accuracy (for ERN, Pe, and FRN) were maximal. Significant interactions with site beyond the potential two-way interactions with congruency and accuracy were not reported. Simple effects were used to follow-up significant interactions, and effect sizes for significant effects are reported as Cohen's d (Cohen, 1988).
Results
Sample Characteristics
On average, children with ADHD did not differ from typically developing children on several important demographic measures, including age, sex, and race (see Table 1). However, children with ADHD had lower average estimated IQ, F (1, 54) = 6.3, p = .02, and family income, F (1, 54) = 7.1, p = .01, compared to the control group. The decrement in IQ in the ADHD group is commonly reported (Frazier, Demaree, & Youngstrom, 2004) and is considered to be an inherent group characteristic, suggesting that the inclusion of IQ as a covariate is inappropriate due to violations of the assumptions of ANCOVA (Miller & Chapman, 2001) and because this approach often produces overcorrected and counterintuitive findings about neurocognitive function (Dennis et al., 2009). However, we did run all of the analyses excluding 5 participants from the ADHD group with the lowest IQ, which eliminated the group difference in IQ (p = .15), and the pattern of results did not change although some results were slightly weaker likely due to reduced power. Consistent with group selection, the ADHD group exhibited markedly higher levels of symptoms of ADHD, ODD and CD according to parent and teacher report. However, again due to our inclusion criteria, the groups did not reliably differ in self-reported depression (CDI) or anxiety (RCMAS).
Behavioral Results
Not surprisingly, the response deadline was shorter for control participants, group F(1, 52) = 5.3, p = .03, d = 0.61. All participants had fewer “Too Slow” responses during the reward condition, reward: F(1, 52) = 19.1, p < .001, d = 0.60, but this effect did not vary by group, nor did children with ADHD have a greater percentage of responses that exceeded their individualized response deadline, group: F(1, 52) = 1.3, p = .26, and Group X Reward: F(1, 52) = 0.28, p = .60 (see Table 3).
Table 3.
Group differences on behavioral measures of performance.
| ADHD Mean(SD) | CTRL Mean(SD) | p-value | Effect Size (Cohen's d) | |
|---|---|---|---|---|
| Accuracy (%) | ||||
| No Reward | 66.3 (14.3) | 72.0 (11.9) | .12 | 0.43 |
| Reward | 70.8 (13.5) | 72.2 (14.3) | .73 | 0.10 |
| No Reward Congruent | 80.5 (17.6) | 88.9 (9.6) | .04 | 0.56 |
| No Reward Incongruent | 52.1 (13.9) | 55.2 (17.1) | .46 | 0.20 |
| Reward Congruent | 86.4 (14.2) | 89.2 (11.7) | .63 | 0.22 |
| Reward Incongruent | 55.3 (15.3) | 55.3 (19.8) | .99 | 0.01 |
| Too Slow Response Deadline | 666.0 (103.2) | 602.0 (99.2) | .02 | 0.61 |
| No Reward (% TS) | 12.8 (9.3) | 10.3 (7.0) | .27 | 0.30 |
| Reward (% TS) | 9.6 (7.6) | 7.8 (5.7) | .33 | 0.26 |
| Reaction Time (ms) | ||||
| No Reward Congruent | 576.6 (109.6) | 504.2 (104.9) | .02 | 0.64 |
| No Reward Incongruent | 625.7 (112.1) | 556.6 (101.7) | .02 | 0.62 |
| Reward Congruent | 539.4 (115.9) | 471.5 (105.0) | .03 | 0.59 |
| Reward Incongruent | 595.5 (110.0) | 533.3 (114.3) | <.05 | 0.54 |
Children were generally less accurate and slower on incongruent compared to congruent trials, percent correct: congruency F(1, 52) = 314.7, p < .001, d = 2.39 and MRT: congruency F(1, 52) = 31.7, p < .001, d = 0.73. The effect of congruency on accuracy and response speed did not vary by group, percent correct: Group X Congruency F(1, 52) = 1.3, p = .26, and MRT: Group X Congruency F(1, 52) = 0.26, p = .61. Accuracy and response speed improved modestly during the reward condition, percent correct: reward F(1, 52) = 6.6, p = .01, d = 0.35, and MRT: reward F(1, 52) = 4.3, p = .04, d = 0.26. Children with ADHD were more accurate during the reward compared to the no reward condition, p < .001, whereas the control group performed similarly during the reward and no reward conditions, p = .95, Group X Reward: F(1, 52) = 6.2, p = .02, d = 0.60. Importantly, accuracy did not significantly differ between groups in either reward condition, no-reward p = .12 and reward p = .73, suggesting the response deadline effectively reduced group differences in performance (see Table 3).
ERP Results
Flanker-Locked ERPs: N2 and P3
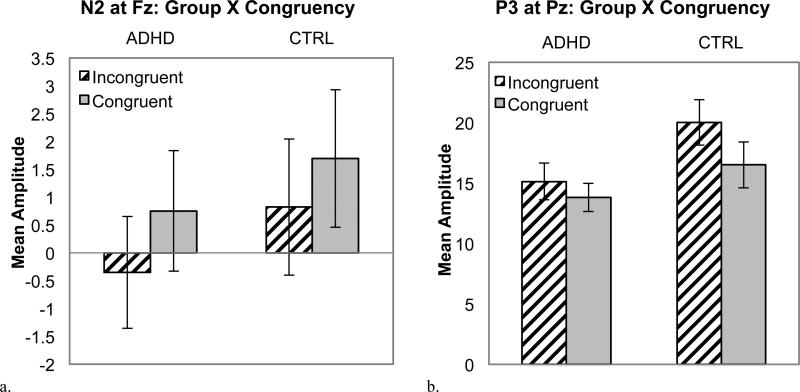
As expected, N2 amplitude (250-350 ms) was greatest at Fz, site linear: F(1, 52) = 36.9, p <.001, d = 0.80, and for incongruent than congruent trials, congruency: F (1, 52) = 11.3, p = .001, d = 0.45. The effect of congruency was similar across sites, Site linear X Congruency, F(1, 52) = 0.57, p = .46. Groups did not differ in N2 amplitude, group: F(1, 52) = 0.45, p = .50 and Group X Congruency: F(1, 52) = 0.13, p = .72 (see Figure 6a). Rewards did not significantly impact N2 amplitude, reward: F(1, 52) = 2.0, p =.16, Reward X Congruency: F(1, 52) = 0.03, p =.87, and Group X Reward: F(1, 52) = 0.41, p =.52.
Figure 6.
Mean amplitude for each Group X Congruency condition for the flanker-locked ERPs: N2 (a) and P3 (b).
P3 amplitude (400-700 ms) was greatest at Pz, site: F (1, 52) = 25.0, p < .001, d = 0.70. As expected, overall P3 amplitude was reduced in children with ADHD, group: F(1, 52) = 4.7, p = .04, d = 0.56. In addition, P3 was larger for incongruent compared to congruent stimuli, congruency: F (1, 52) = 9.8, p = .003, d = 0.39, but only for the control group, Group X Congruency: F(1, 52) = 4.4, p = .04, d = 0.56 (see Figure 6b). Moreover, P3 amplitude was enhanced during the reward condition, reward: F(1, 52) = 23.5, p < .001, d = 0.64. The effect of reward was greatest for incongruent stimuli, Reward X Congruency: F (1, 52) = 7.5, p =.008. A differential effect of reward on P3 amplitude for children with ADHD was not observed, Group X Reward: F (1, 52) = 1.4, p =.24 and Group X Reward X Congruency: F (1, 52) = 1.8, p = .19.
Response-Locked ERPs: ERN and Pe
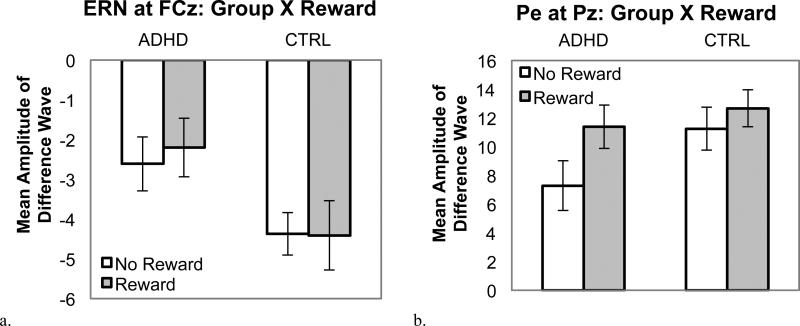
Error responses resulted a greater response-related negativity than did correct responses for the ERN (0-100 ms), accuracy: F (1, 51) = 62.2, p < .001, d = 1.0. The ERN (i.e., difference between error and correct amplitude) was greatest at FCz, Site quadratic X Accuracy: F(1, 51) = 20.6, p < .001, d = 0.19. As expected, ERN was reduced in the ADHD group compared to controls, Group X Accuracy: F(1, 51) = 4.6 p = .04, d = 0.60 (see Figure 7a). Contrary to our predictions, the ERN was not influenced by reward, Reward X Accuracy: F(1, 51) = 0.17 p = .68 and Group X Reward X Accuracy: F(1, 51) = 0.10 p = .75.
Figure 7.
Mean amplitude of the error-correct difference wave for each Group X Reward condition for the response-locked ERPs: ERN (a) and Pe (b).
Error responses also resulted in a greater response-related negativity than did correct responses for the Pe (250-550ms), accuracy: F (1, 52) = 108.1, p < .001, d = 1.4. The Pe was greatest at Pz, Site X Accuracy: F (1, 51) = 29.8, p < .001, d = 0.47. Consistent with predictions, Pe tended to be reduced in the ADHD group compared to controls, Group X Accuracy: F (1, 51) = 3.2, p =.08, d = 0.48. In addition, Pe was significantly enhanced by reward, Reward X Accuracy: F (1, 51) = 7.3, p = .009, d = 0.36. However, this effect tended to vary by group such that only children with ADHD showed a significant increase in Pe amplitude with reward (p = .002), whereas controls did not (p =.50), Group X Reward X Accuracy: F(1, 51) = 2.8, p=.10, d = 0.45 (see Figure 7b). Analysis of the mean amplitude of the error-correct difference wave indicated that Pe amplitude was reduced in children with ADHD compared to controls during the no-reward condition (ADHD v. CTRL difference = 4.5 μV, p = .03, d = 0.58), but not in the presence of reward (ADHD v. CTRL difference = 1.8 μV, p = .34, d = 0.27).
Feedback-locked ERP: FRN
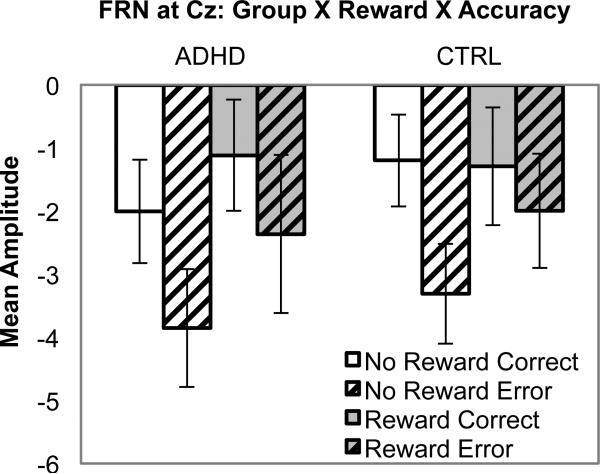
Responses to feedback as reflected in the FRN (350-500 ms) was greater on error trials, accuracy: F (1, 47) = 4.0, p = .05, d = 0.28. Differences in response to feedback on error versus correct trials varied by electrode site, with the largest difference observed at Cz , Site linear X Accuracy: F(1, 47) = 11.2, p = .002, d = 0.46. Surprisingly, the FRN to positive versus negative feedback during the reward condition did not differ from the ‘FRN’ to neutral feedback (i.e., identical yellow squares) provided for correct versus error responses, Reward X Accuracy: F(1, 47) = 2.1, p = .16, and Group X Reward X Accuracy: F(1, 47) = 1.1, p = .30 (see Figure 8).
Figure 8.
Mean amplitude for each Group X Reward X Accuracy condition for the feedback-locked ERP: FRN.
Discussion
To improve our understanding of how reward impacts the performance of children with ADHD, we examined the impact of reward on behavioral and neurophysiological correlates associated with processing of the primary task (flanker) stimuli, errors, and feedback within the context of a similar level of task performance between groups. We expected children with ADHD, compared to typically developing children, to exhibit (1) reduced ERP responses to the flanker stimuli (N2 and P3) and errors (ERN and Pe), (2) greater improvement in performance with reward, (3) greater increase in ERP responses to the flanker stimuli (N2 and P3) and errors (ERN and Pe) with reward, and (4) an atypical ERP response to performance feedback (FRN). The results suggest that children with ADHD exhibited reduced processing of the flanker stimuli as reflected in P3 amplitude (but not N2), and reduced error-processing as reflected in both ERN and Pe amplitude. Responses to feedback did not differ between groups in either the reward or the no-reward condition. Performance-based rewards enhanced P3 amplitude to the flanker stimuli and Pe amplitude after making an error. However, the increase in Pe amplitude with reward was only observed in children with ADHD, such that performance-based rewards eliminated group differences in Pe amplitude that were present during the no-reward condition. Surprisingly, the ERP response to positive versus negative feedback during the reward condition did not differ from the ERP response to the neutral feedback (i.e., identical yellow squares) provided for correct versus error responses during the no-reward condition. Although our results suggest the FRN was larger to both negative and neutral feedback provided after making an error, our prediction that the FRN would be heightened for negative feedback during the reward condition (indicating that an error was made and points were not earned) compared to neutral, uninformative feedback was not supported. Further consideration of each component of information processing examined during this task is provided below.
Stimulus Processing
Information processing during the flanker task began with processing of the primary task stimuli (i.e., the flanker stimulus). Examination of behavioral (accuracy and RT) and physiological (N2 and P3) measures of stimulus processing suggested that there was a strong effect of stimulus congruency. Participants were less accurate and slower on incongruent compared to congruent trials. Similarly, the flanker-locked N2, thought to reflect conflict monitoring (Donkers & van Boxtel, 2004), and flanker-locked P3, which is typically associated with stimulus evaluation/categorization (Ridderinkhof & Vandermolen, 1995) were enhanced for incongruent compared to congruent trials. These findings are consistent with the interpretation that incongruent flanker stimuli caused significant conflict, resulting in the need for greater interference control to execute a correct response (e.g., Crone et al., 2003).
Consistent with previous research, children with ADHD may have experienced greater difficulty processing incongruent compared to congruent stimuli as reflected in reduced P3 amplitude (e.g., Johnstone et al., 2009; Jonkman et al., 1999), such that a differential P3 response for incongruent compared to congruent stimuli was not observed in children with ADHD whereas controls demonstrated this effect. However, group differences in N2 amplitude were not observed, suggesting that early conflict detection for children with ADHD was comparable to that of control children. One plausible explanation for this is the inclusion of an individualized response deadline, which adjusted the difficulty of the task to each child's ability level. This task manipulation likely attenuated group differences in the effect of congruency for group differences in behavioral measures (i.e., accuracy and response speed) and early conflict detection, whereas processing of the primary task stimuli (i.e., P3) remained sensitive to ADHD.
We expected that reward would improve stimulus processing and increase attention for task relevant stimuli resulting in faster and more accurate responses (Crone et al., 2003) and increased N2 and P3 amplitude (e.g., Groom, Scerif, et al., 2010). In addition, reward was hypothesized to exert greater effects for children with ADHD given the motivational deficits associated with this disorder. Consistent with this prediction, reward improved accuracy for children with ADHD but not for typically developing children, although the performance did not significantly differ between groups in either reward condition. Reward did not significantly impact N2 amplitude, whereas P3 amplitude increased with reward consistent with prior research (Goldstein et al., 2006). However, the increase in P3 amplitude with reward was similar for ADHD and control participants, suggesting it did not have a differential impact on stimulus processing for children with ADHD. In addition, the impact of rewards on P3 amplitude was greatest for incongruent stimuli which required more effortful processing than congruent stimuli, suggesting that reward may selectively impact processes which require effortful control of attention (see Ashare et al., 2010, for similar results with stimulant medication; Sarter, Gehring, & Kozak, 2006). In sum, performance-based rewards did not differentially impact processing of the primary task stimuli in children with ADHD, although improved processing of the flanker stimuli was observed across groups.
Error Processing
Our hypotheses that children with ADHD would exhibit reduced ERN and Pe amplitude, thought to reflect deficient early error detection and conscious error evaluation, were both supported in the current study. These findings are particularly important given the inconsistencies in the ERN literature for children with ADHD (Shiels & Hawk, 2010), suggesting that contextual factors and task parameters should be considered when interpreting the results of the current study in relation to the existing error-processing in ADHD literature which has produced inconsistent results, particularly for ERN. The sample included in this study was relatively large and selective, limiting children in the ADHD group to those with the combined subtype, excluding children for comorbid internalizing psychopathology, carefully selecting control participants, and restricting participants to a small age range. Although these criteria limit the generalizability of the results, they enhance the internal validity of the study. In addition, the task was designed in consideration of factors that may influence motivation and self-regulation in ADHD (e.g., time on task, task difficulty, reward manipulation, event rate). Therefore, these results may suggest that ERN and Pe amplitude is diminished among ADHD participants when task and sample characteristics are carefully considered, and they are more broadly consistent with the hypothesis that the ERN is an endophenotype for externalizing proneness (Nelson, Patrick, & Bernat, 2010).
In addition to the predicted group differences in ERN and Pe amplitude, we also hypothesized that performance-based rewards would improve error processing, thereby enhancing ERN and Pe amplitude, and that the impact of reward would be strongest for children with ADHD. This hypothesis is based in part on the error-processing literature, which has fairly consistently shown that motivational factors influence ERN amplitude (Boksem et al., 2006; Groom et al., 2013; Hajcak et al., 2005; Pailing & Segalowitz, 2004) and the ADHD literature that has shown improved behavior and cognitive task performance with reward (see reviews by Luman et al., 2005; Luman et al., 2010; e.g., Shiels et al., 2008; Strand et al., 2012). Therefore, it was surprising to see that rewards did not significantly increase ERN amplitude for either group of children, and this does not appear to be an issue of insufficient power because the means were in the opposite direction for the ADHD group. This finding is in contrast to the results of a recent study reporting increased ERN amplitude in children with ADHD as the magnitude of the motivational incentive increased (Groom et al., 2013), In that study, the ERN was largest in children with ADHD when errors resulted in response cost (i.e., lose 5 points for failed inhibitions), suggesting that modulation of the ERN in children with ADHD may depend on the incentive structure. In addition, immediate feedback was not provided in the Groom et al. study and the type of error differed (inhibition versus discrimination errors), which may have impacted ERN amplitude. The failure to find an effect of reward, specifically failure to obtain a reward when an error was made, on ERN amplitude is consistent with evidence of reduced activation of the anterior cingulate cortex (ACC), the neural generator of the ERN, during omission of expected reward (Gatzke-Kopp et al., 2009) suggesting dysfunction of the ACC within a reward context. However, the finding that reward did not impact ERN amplitude in either group of children may suggest that earlier error detection is less influenced by motivational factors in children as this ability is still developing, whereas the Pe is relatively stable by this age (Davies, Segalowitz, & Gavin, 2004). Interestingly, rewards did increase Pe amplitude in children with ADHD, suggesting a differential effect of reward on conscious error evaluation and consistent with previous research (Groom et al., 2013). Integration of the results across our study and Groom et al. (2013) suggests that the ERN and Pe may be differentially sensitive to reward and response cost in children with ADHD. This may be related to evidence that neural generator of these error processing components differs in terms of the specific region of the ACC, with the ERN localized to the caudal ACC and Pe to the rostral ACC (van Veen & Carter, 2002). Conceptually, it may be that children with ADHD allocated greater attentional resources to error processing during the reward condition because the errors were more significant and meaningful to them, as they were associated with not earning points. In sum, during a difficult task rewards may improve performance through increased error awareness, whereas rewards do not impact early error detection. The impact of rewards on conscious error evaluation may in turn improve processing of the primary task stimuli on the subsequent trial or lead to adaptive adjustments in responding.
Feedback Processing
This is the first study to examine ERPs associated with feedback processing in children during a standard cognitive task, as opposed to a guessing or gambling task, which enables participants to monitor the accuracy of their response while they are pressing the button (ERN and Pe) and when feedback is provided (FRN). During a guessing or gambling task, the participant is unable to independently determine whether their response is correct until feedback is provided, preventing simultaneous examination of the ERN/Pe and the FRN. The system that produces the FRN is thought to be differentially sensitive to positive and negative feedback reflecting classification of outcomes into those that indicate a goal has been satisfied and those that do not (Holroyd, Hajcak, & Larsen, 2006) and is larger for immediate feedback (Weinberg et al., 2012). Thus, if an error was not detected before feedback was provided, the response to negative feedback would be heightened because the feedback received was worse than expected (Bismark et al., 2013). In the current study, the FRN was examined in response to error and correct feedback during a condition with performance-based rewards in comparison to a condition in which rewards could not be earned and an identical neutral stimulus was presented for error and correct trials. This allowed for a direct comparison of responses to feedback while accounting for differences that may emerge as a result of response accuracy (i.e., whether FRN differs in response to an identical stimulus that was presented following error versus correct responses). Contrary to our prediction and to the existing neuroimaging literature suggesting abnormal neural activation in response to reward outcomes (e.g., Gatzke-Kopp et al., 2009; Paloyelis et al., 2012), children with ADHD displayed a similar neural response to negative and positive feedback during the reward condition as typically developing children. In addition, we expected the FRN in response to identical neutral feedback during the no-reward condition would not differ for error versus correct trials nor would group differences emerge. However, the response to neutral feedback during the no-reward condition was different for error and correct responses, despite the use of identical feedback for error and correct trials (yellow square).
The findings for feedback processing raise several intriguing questions, particularly because comparisons to the available literature are limited due to the novel task design. Specifically, the explicit stimulus-response mappings provided during a flanker task (i.e., press right if central arrow points right) allows participants to perfectly predict the feedback they will receive if they are monitoring the accuracy of their response. It is important to make this distinction from the probabilistic or maze-learning tasks that have previously been used to examine the FRN in children with ADHD, during which participants are unable to determine the accuracy of their response until they learn the associations and are therefore more dependent on feedback for performance monitoring. The most surprising result is that there was a differential response to identical neutral feedback depending on whether the participant's response was correct. The pattern of results suggests that neural activity related to making an error may have impacted processing of the feedback stimuli. The FRN is often examined within the context of a probabilistic learning or gambling task, in which the proportion of positive and negative feedback can be controlled and is often equivalent (Holroyd et al., 2008). In this study, we were interested in how children responded to feedback during a task which allowed them to accurately predict the feedback depending on their response. Thus, there were fewer error trials than correct trials, which may have impacted FRN amplitude. The failure to find a differential response to feedback among children with ADHD during the reward condition may be due to the impact of reward on error awareness (Pe), such that children were aware that they made an error prior to the feedback being provided because they engaged in greater self-monitoring of their response. Therefore the feedback was redundant and consistent with their expectations. The inclusion of a condition in which children receive immediate feedback but are not rewarded may disentangle the effect of the information provided by feedback and the associated reward, providing clarification of these results. Furthermore, the results of this study suggest there is a need for additional research on the FRN in tasks such as this in order to understand how individuals respond to feedback when the task permits independent identification of errors and correct responses through intact self-monitoring.
Conclusions
The current study examined the influence of reward on neurophysiological correlates of stimulus, error and feedback processing in children with and without ADHD. Consistent with motivational models of ADHD, immediate reward resulted in greater improvement in performance accuracy in children with ADHD compared to typically developing controls. Importantly, the individualized response deadline also minimized group differences in performance allowing for a cleaner interpretation of the ERPs associated with information processing during this task. The results suggest that children with ADHD exhibited reduced processing of the stimulus, as well as deficient early error detection, and conscious error evaluation, whereas their response to feedback was similar to that of controls. Furthermore, rewards enhanced ERPs associated with processing of the primary task stimuli (P3) and conscious error evaluation (Pe), with the latter effect being greatest for children with ADHD. Thus, rewards may enhance conscious error evaluation or the subjective salience of making errors, among children with ADHD. This heightened error awareness may in turn indicate the need for an adjustment of response strategies to improve performance on a subsequent trial, including enhanced attention to the primary task stimuli. In addition, the current paradigm and associated results may inform future research aimed at characterizing the altered reward mechanisms observed in children with ADHD (Sagvolden et al., 2005; Tripp & Wickens, 2008). Specifically, examining the impact of variations in the consistency of reward delivery and magnitude on ERN and Pe amplitude in particular may inform self-monitoring theories. The integration of paradigms such as this with neuroimaging methodology will also inform our understanding of ‘where’ in addition to ‘when’ in the information processing chain reward influences performance. Finally, extension of these results into the clinical realm may elucidate the mechanisms by which reward improves the behavior of children with ADHD, as evident in the effectiveness of behavior modification treatment (Pelham & Fabiano, 2008).
Acknowledgments
Keri Shiels Rosch is now at the Kennedy Krieger Institute, Johns Hopkins School of Medicine, Baltimore, MD. This research was supported in part by grants from the Mark Diamond Research Foundation awarded to Keri Shiels Rosch and the National Institute of Mental Health (R01MH069434-05) awarded to Larry W. Hawk. We thank Nicholas Albino for assistance with data collection, Mark Kutgowski for computer programming support, and John Curtin, Sidney Segalowitz, Craig Colder, Jerry Richards, William Pelham, Rebecca Houston, and Peter Pfordresher for their assistance with the study design and feedback regarding data reduction, analysis and interpretation.
Footnotes
The reliability and validity of this short form is excellent (Sattler, 1992) with the sum of the vocabulary and block design subtests correlated .88 with full scale IQ (Mercer & Smith, 1972). In addition, the practice of estimating IQ for screening purposes based on performance on select subtests from the WISC is not uncommon in studies involving children with ADHD (Banaschewski et al., 2012; Brackenridge, McKenzie, Murray, & Quigley, 2011; Yordanova et al., 2011).
Contributor Information
Keri Shiels Rosch, Department of Psychology, University at Buffalo, Buffalo, New York.
Larry W. Hawk, Jr., Department of Psychology and the Center for Children and Families, University at Buffalo, Buffalo, New York
References
- Achenbach TM, Rescorla L. Manual for ASEBA School-Age Forms and Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- Albrecht B, Brandeis D, Uebel H, Heinrich H, Mueller UC, Hasselhorn M, Banaschewski T. Action Monitoring in Boys With Attention-Deficit/Hyperactivity Disorder, Their Nonaffected Siblings, and Normal Control Subjects: Evidence for an Endophenotype. Biological Psychiatry. 2008 doi: 10.1016/j.biopsych.2007.12.016. doi: 10.1016/j.biopsych.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Clinical practice guideline: treatment of the school-aged child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108(4):1033–1044. doi: 10.1542/peds.108.4.1033. doi: 10.1542/peds.108.4.1033. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Ashare RL, Hawk LW, Jr., Shiels K, Rhodes JD, Pelham WE, Jr., Waxmonsky JG. Methylphenidate enhances prepulse inhibition during processing of task-relevant stimuli in attention-deficit/hyperactivity disorder. Psychophysiology. 2010 doi: 10.1111/j.1469-8986.2010.01001.x. doi: 10.1111/j.1469-8986.2010.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T, Jennen-Steinmetz C, Brandeis D, Buitelaar JK, Kuntsi J, Poustka L, Asherson P. Neuropsychological correlates of emotional lability in children with ADHD. Journal of Child Psychology and Psychiatry. 2012;53(11):1139–1148. doi: 10.1111/j.1469-7610.2012.02596.x. [Article]. doi: 10.1111/j.1469-7610.2012.02596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismark AW, Hajcak G, Whitworth NM, Allen JJ. The role of outcome expectations in the generation of the feedback-related negativity. Psychophysiology. 2013;50(2):125–133. doi: 10.1111/j.1469-8986.2012.01490.x. [Research Support, N.I.H., Extramural]. doi: 10.1111/j.1469-8986.2012.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksem MA, Meijman TF, Lorist MM. Mental fatigue, motivation and action monitoring. Biological Psychology. 2006;72(2):123–132. doi: 10.1016/j.biopsycho.2005.08.007. doi: 10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Brackenridge R, McKenzie K, Murray GC, Quigley A. An examination of the effects of stimulant medication on response inhibition: a comparison between children with and without attention deficit hyperactivity disorder. Research in Developmental Disabilities. 2011;32(6):2797–2804. doi: 10.1016/j.ridd.2011.05.027. doi: 10.1016/j.ridd.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Burgio-Murphy A, Klorman R, Shaywitz SE, Fletcher JM, Marchione KE, Holahan J, Shaywitz BA. Error-related event-related potentials in children with attention-deficit hyperactivity disorder, oppositional defiant disorder, reading disorder, and math disorder. Biological Psychology. 2007;75(1):75–86. doi: 10.1016/j.biopsycho.2006.12.003. doi: 10.1016/j.biopsycho.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. Statistical power analyses for the behavioral sciences. 2nd ed. Lawrence Earlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Crone EA, Jennings JR, van der Molen MW. Sensitivity to interference and response contingencies in attention-deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2003;44(2):214–226. doi: 10.1111/1469-7610.00115. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48(2):194–215. doi: 10.1016/j.cortex.2011.04.007. [Research Support, Non-U.S. Gov't]. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7-to 25-year-olds. Developmental Neuropsychology. 2004;25(3):355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikman ZV, Allen JJ. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37(1):43–54. [PubMed] [Google Scholar]
- Donkers, Franc CL, van Boxtel, Geert JM. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain and Cognition. 2004;56(2):165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Durston S, van Belle J, de Zeeuw P. Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2011;69(12):1178–1184. doi: 10.1016/j.biopsych.2010.07.037. doi: 10.1016/j.biopsych.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Edel MA, Enzi B, Witthaus H, Tegenthoff M, Peters S, Juckel G, Lissek S. Differential reward processing in subtypes of adult attention deficit hyperactivity disorder. Journal of Psychiatric Research. 2013;47(3):350–356. doi: 10.1016/j.jpsychires.2012.09.026. doi: 10.1016/j.jpsychires.2012.09.026. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a nonsearch task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Fabiano, Gregory A, Pelham WE, Waschbusch, Daniel A, Gnagy, Elizabeth M, Lahey, Benjamin B, Chronis, Andrea M, Burrows-MacLean, Lisa A Practical Measure of Impairment: Psychometric Properties of the Impairment Rating Scale in Samples of Children With Attention Deficit Hyperactivity Disorder and Two School-Based Samples. Journal of Clinical Child & Adolescent Psychology. 2006;35(3):369–385. doi: 10.1207/s15374424jccp3503_3. doi: doi:10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51(2-3):87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–555. doi: 10.1037/0894-4105.18.3.543. [Meta-Analysis]. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM, Beauchaine TP, Shannon KE, Chipman J, Fleming AP, Crowell SE, Aylward E. Neurological correlates of reward responding in adolescents with and without externalizing behavior disorders. Journal of Abnormal Psychology. 2009;118(1):203–213. doi: 10.1037/a0014378. doi: 10.1037/a0014378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geburek AJ, Rist F, Gediga G, Stroux D, Pedersen A. Electrophysiological indices of error monitoring in juvenile and adult attention deficit hyperactivity disorder (ADHD)-A meta-analytic appraisal. International Journal of Psychophysiology. 2012 doi: 10.1016/j.ijpsycho.2012.08.006. doi: 10.1016/j.ijpsycho.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4(6):385–390. [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gerard AB, Reynolds CR. Characteristics and Applications of the Revised Children's Manifest Anxiety Scale (RCMAS). In: Maruish M, editor. The use of psychological testing for treatment planning and outcome assessment. Lawrence Erlbaum Associates; Mahwah, NJ: 1999. pp. 323–340. [Google Scholar]
- Goldstein RZ, Cottone LA, Jia Z, Maloney T, Volkow ND, Squires NK. The effect of graded monetary reward on cognitive event-related potentials and behavior in young healthy adults. Int J Psychophysiol. 2006;62(2):272–279. doi: 10.1016/j.ijpsycho.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill LL, Swanson JM, Steinhoff K, Fried J, Posner K, Lerner M, Tulloch S. A pharmacokinetic/pharmacodynamic study comparing a single morning dose of adderall to twice-daily dosing in children with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(10):1234–1241. doi: 10.1097/00004583-200310000-00015. doi: 10.1097/00004583-200310000-00015. [DOI] [PubMed] [Google Scholar]
- Groen Y, Wijers AA, Mulder LJ, Waggeveld B, Minderaa RB, Althaus M. Error and feedback processing in children with ADHD and children with Autistic Spectrum Disorder: An EEG event-related potential study. Clinical Neurophysiology. 2008;119(11):2476–2493. doi: 10.1016/j.clinph.2008.08.004. doi: 10.1016/j.clinph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Groom MJ, Cahill JD, Bates AT, Jackson GM, Calton TG, Liddle PF, Hollis C. Electrophysiological indices of abnormal error-processing in adolescents with attention deficit hyperactivity disorder (ADHD). Journal of Child Psychology and Psychiatry. 2010;51(1):66–76. doi: 10.1111/j.1469-7610.2009.02128.x. doi: 10.1111/j.1469-7610.2009.02128.x. [DOI] [PubMed] [Google Scholar]
- Groom MJ, Liddle EB, Scerif G, Liddle PF, Batty MJ, Liotti M, Hollis CP. Motivational incentives and methylphenidate enhance electrophysiological correlates of error monitoring in children with attention deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2013 doi: 10.1111/jcpp.12069. doi: 10.1111/jcpp.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom MJ, Scerif G, iddle PF, Batty MJ, Liddle EB, Roberts KL, Hollis C. Effects of motivation and medication on electrophysiological markers of response inhibition in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010;67(7):624–631. doi: 10.1016/j.biopsych.2009.09.029. [Research Support, Non-U.S. Gov't]. doi: 10.1016/j.biopsych.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenlein M, Caul WF. Attention deficit disorder with hyperactivity: a specific hypothesis of reward dysfunction. Journal of the American Academy of Child and Adolescent Psychiatry. 1987;26(3):356–362. doi: 10.1097/00004583-198705000-00014. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42(2):151–160. doi: 10.1111/j.1469-8986.2005.00270.x. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Heldmann M, Ruesseler J, Muente TF. Internal and external information in error processing. BMC Neurosci. 2008;9(1):33. doi: 10.1186/1471-2202-9-33. doi: 10.1186/1471-2202-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Baker TE, Kerns KA, Muller U. Electrophysiological evidence of atypical motivation and reward processing in children with attention-deficit hyperactivity disorder. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2008.02.011. doi: 10.1016/j.neuropsychologia.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. doi: 10.1037//0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Hajcak G, Larsen JT. The good, the bad and the neutral: electrophysiological responses to feedback stimuli. Brain Research Bulletin. 2006;1105(1):93–101. doi: 10.1016/j.brainres.2005.12.015. doi: 10.1016/ j.brainres.2005.12.01 5. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. An Integrative Theory of Anterior Cingulate Cortex Function: Option Selection in Hierarchical Reinforcement Learning. In: Mars RB, Sallet J, Rushworth MFS, Yeung N, editors. Neural Basis of Motivational and Cognitive Control. The MIT Press; Cambridge, Massachusetts: 2011. pp. 333–349. [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, Clarke AR. Ten years on: A follow-up review of ERP research in attention-deficit/hyperactivity disorder. Clinical Neurophysiology. 2012 doi: 10.1016/j.clinph.2012.09.006. doi: 10.1016/j.clinph.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, Markovska V, Dimoska A, Clarke AR. Response inhibition and interference control in children with AD/HD: a visual ERP investigation. International Journal of Psychophysiology. 2009;72(2):145–153. doi: 10.1016/j.ijpsycho.2008.11.007. doi: 10.1016/j.ijpsycho.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Galletta D. Event-rate effects in the flanker task: ERPs and task performance in children with and without AD/HD. International Journal of Psychophysiology. 2012 doi: 10.1016/j.ijpsycho.2012.07.170. doi: 10.1016/j.ijpsycho.2012.07.170. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Kemner C, Verbaten MN, Van Engeland H, Kenemans JL, Camfferman G, Koelega HS. Perceptual and response interference in children with attention-deficit hyperactivity disorder, and the effects of methylphenidate. Psychophysiology. 1999;36(4):419–429. [PubMed] [Google Scholar]
- Kaplan E, Fein D, Kramer J, Delis D, Morris R. WISC-IV Integrated. The Psychological Corporation; San Antonio, TX: 2004. [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV Subtypes of ADHD from preschool through elementary school. Archives of General Psychiatry. 2005;62(8):896–902. doi: 10.1001/archpsyc.62.8.896. [Comparative Study]. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41(3):377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Luck, Steven J. An Introduction to the Event-Related Potential Technique. The MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clinical Psychology Review. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neuroscience and Biobehavioral Reviews. 2010;34(5):744–754. doi: 10.1016/j.neubiorev.2009.11.021. [Review] doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. Journal of Neuroscience. 2000;20(1):464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JR, Smith JM. Subtest estimates of the WISC full scale IQ's for children. Vital and Health Statistics. Series 2: Data Evaluation and Methods Research. 1972;(47):1–42. [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Dev Cogn Neurosci. 2012;2(1):152–161. doi: 10.1016/j.dcn.2011.09.005. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, Gregory A, Chapman, Jean P. Misunderstanding Analysis of Covariance. Journal of Abnormal Psychology. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9(6):788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Bernat EM. Operationalizing proneness to externalizing psychopathology as a multivariate psychophysiological phenotype. Psychophysiology. 2010 doi: 10.1111/j.1469-8986.2010.01047.x. doi: PSYP1047 [pii] 10.1111/j.1469-8986.2010.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: Toward an endophenotype. Clinical Psychology Review. 2008 doi: 10.1016/j.cpr.2008.07.003. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009 doi: 10.1111/j.1469-8986.2009.00848.x. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Overbeek, Therese JM, Nieuwenhuis, Sander, Ridderinkhof K, Richard Dissociable components of error processing: on the functional significance of the Pe vis-a-vis the ERN/Ne. Federation of European Psychophysiology Societies. 2005;19(4):319–329. doi: 10.1027/0269-8803.19.4.319. [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41(1):84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Faraone SV, Asherson P, Kuntsi J. Striatal sensitivity during reward processing in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(7):722–732. e729. doi: 10.1016/j.jaac.2012.05.006. doi: 10.1016/j.jaac.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. Journal of Clinical Child & Adolescent Psychology. 2008;37(1):184–214. doi: 10.1080/15374410701818681. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Vasic N, Wolf C, Lesch KP, Brummer D, Jacob C, Gron G. Neural Hyporesponsiveness and Hyperresponsiveness During Immediate and Delayed Reward Processing in Adult Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2009;65(1):5–6. doi: 10.1016/j.biopsych.2008.07.008. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. American Journal of Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. doi: 10.1176/appi.ajp.164.6.942. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts GF. Impact of reward and punishment motivation on behavior monitoring as indexed by the error-related negativity. International Journal of Psychophysiology. 2011;81(3):324–331. doi: 10.1016/j.ijpsycho.2011.07.020. doi: 10.1016/j.ijpsycho.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Vandermolen MW. A Psychophysiological Analysis of Developmental Differences in the Ability to Resist Interference. Child Development. 1995;66(4):1040–1056. [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28(3):397–419. doi: 10.1017/S0140525X05000075. discussion 419-368. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. ERP correlates of error monitoring in 10-year olds are related to socialization. Biological Psychology. 2005;70(2):79–87. doi: 10.1016/j.biopsycho.2004.12.004. doi: 10.1016/j.biopsycho.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Brain Res Rev. 2006;51(2):145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: WISC-III and WPPSI-R supplement. Jerome M. Sattler; San Diego, CA: 1992. [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral Striatal Hyporesponsiveness During Reward Anticipation in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2007;(61):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Senderecka M, Grabowska A, Szewczyk J, Gerc K, Chmylak R. Response inhibition of children with ADHD in the stop-signal task: an event-related potential study. International Journal of Psychophysiology. 2012;85(1):93–105. doi: 10.1016/j.ijpsycho.2011.05.007. doi: 10.1016/j.ijpsycho.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shen IH, Tsai SY, Duann JR. Inhibition control and error processing in children with attention deficit/hyperactivity disorder: an event-related potentials study. International Journal of Psychophysiology. 2011;81(1):1–11. doi: 10.1016/j.ijpsycho.2011.03.015. doi: 10.1016/j.ijpsycho.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW., Jr. Self-regulation in ADHD: the role of error processing. Clinical Psychology Review. 2010;30(8):951–961. doi: 10.1016/j.cpr.2010.06.010. doi: S0272-7358(10)00104-2 [pii] 10.1016/j.cpr.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Jr., Reynolds B, Mazzullo RJ, Rhodes JD, Pelham WE, Jr., Gangloff BP. Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol. 2009;17(5):291–301. doi: 10.1037/a0017259. doi: 10.1037/a0017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Lysczek CL, Tannock R, Pelham WE, Spencer SV, Waschbusch DA. The Effects of Incentives on Visual-Spatial Working Memory in Children with Attention-deficit/Hyperactivity Disorder. J Abnorm Child Psychol. 2008;36(6):903–913. doi: 10.1007/s10802-008-9221-0. doi: 10.1007/s10802-008-9221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitarenios G, Kovacs M. Use of the Children's Depression Inventory. In: Maruish M, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Lawrence Erlbaum Associates; Mahwah, New Jersey: 1999. pp. 267–298. [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neuroscience and Biobehavioral Reviews. 2003;27(7):593–604. doi: 10.1016/j.neubiorev.2003.08.005. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Spronk D, Dumont GJ, Verkes RJ, de Bruijn ER. Acute effects of delta-9-tetrahydrocannabinol on performance monitoring in healthy volunteers. Front Behav Neurosci. 2011;5:59. doi: 10.3389/fnbeh.2011.00059. doi: 10.3389/fnbeh.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand MT, Hawk LW, Jr., Bubnik M, Shiels K, Pelham WE, Jr., Waxmonsky JG. Improving working memory in children with attention-deficit/hyperactivity disorder: the separate and combined effects of incentives and stimulant medication. Journal of Abnormal Child Psychology. 2012;40(7):1193–1207. doi: 10.1007/s10802-012-9627-6. doi: 10.1007/s10802-012-9627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmer B, Nigbur R, Schacht A, Sommer W. Reward and punishment effects on error processing and conflict control. Front Psychol. 2011;2:335. doi: 10.3389/fpsyg.2011.00335. doi: 10.3389/fpsyg.2011.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2008;49(7):691–704. doi: 10.1111/j.1469-7610.2007.01851.x. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan U, Nelson LD, Patrick CJ. Clarifying domains of internalizing psychopathology using neurophysiology. Psychological Medicine. 2012;42(3):447–459. doi: 10.1017/S0033291711001528. doi: 10.1017/S0033291711001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meel C, Heslenfeld DJ, Oosterlaan J, Luman M, Sergeant JA. ERPs associated with monitoring and evaluation of monetary reward and punishment in children with ADHD. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2011 doi: 10.1111/j.1469-7610.2010.02352.x. doi: 10.1111/j.1469-7610.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- van Meel C, Heslenfeld D, Oosterlaan J, Sergeant J. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): The role of error processing. Psychiatry Research. 2007;151(3):211–220. doi: 10.1016/j.psychres.2006.05.011. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- van Meel C, Oosterlaan J, Heslenfeld D, Sergeant J. Telling good from bad news: ADHD differentially affects processing of positive and negative feedback during guessing. Neuropsychologia. 2005;43(13):1946–1954. doi: 10.1016/j.neuropsychologia.2005.03.018. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002;14(4):593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Luhmann CC, Bress JN, Hajcak G. Better late than never? The effect of feedback delay on ERP indices of reward processing. Cogn Affect Behav Neurosc.i. 2012 doi: 10.3758/s13415-012-0104-z. doi: 10.3758/s13415-012-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Albrecht B, Uebel H, Banaschewski T, Rothenberger A. May posterror performance be a critical factor for behavioral deficits in attention-deficit/hyperactivity disorder? Biological Psychiatry. 2011;70(3):246–254. doi: 10.1016/j.biopsych.2011.02.026. doi: S0006-3223(11)00201-0 [pii]10.1016/j.biopsych.2011.02.026. [DOI] [PubMed] [Google Scholar]