Abstract
Asthma has been considered a T helper 2 (TH2) cell-associated inflammatory disease, and TH2-type cytokines, such as interleukin-4 (IL-4), IL-5 and IL-13, are thought to drive the disease pathology in patients. Although atopic asthma has a substantial TH2 cell component, the disease is notoriously heterogeneous, and recent evidence has suggested that other T cells also contribute to the development of asthma. Here, we discuss the roles of different T cell subsets in the allergic lung, consider how each subset can contribute to the development of allergic pathology and evaluate how we might manipulate these cells for new asthma therapies.
Asthma is classically thought to be a T helper 2 (TH2)-cell driven inflammatory disease, characterized by eosinophilic inflammation, TH2-cell associated cytokine production and airway hyperresponsiveness (AHR). Although many animal models have suggested that TH2 cells promote allergic airway inflammation, these studies have not led to new therapies for the increasing number of patients with asthma. Recent advances indicate that asthma can no longer be considered solely as a disease of the adaptive immune system, but that crosstalk between the innate and adaptive immune system is crucial for the initiation and propagation of the allergic immune response. Indeed, the typical clinical heterogeneity of asthma (BOX 1) might reflect the varied inter actions that occur among distinct populations of stromal cells, epithelial cells and leukocytes in the allergic lung, as well as the contribution of T cell subsets.
Box 1. Asthma heterogeneity.
Asthma is recognized to be a heterogeneous disease with a number of distinct clinical phenotypes. The most common form of asthma is allergic asthma, which results from an inappropriate immune response to common inhaled proteins (or allergens) in genetically susceptible individuals. These individuals are termed atopic asthmatics and exhibit IgE reactivity to specific antigens. By contrast, non-atopic asthmatic patients have disease that is not driven by a specific allergen. Although most individuals present with eosinophilic diseases, a number of patients with particularly severe asthma show a substantial neutrophil component to airway infiltrates. These patients often have resistance to treatment with steroids. More recently, detailed statistical cluster analysis has revealed defined phenotypes of patients that differ on the basis of lung function, age of asthma onset, disease duration, atopy, gender, symptoms, medication use and health care usage. A better understanding of these clinical phenotypes will provide the opportunity to develop tailored therapies aimed specifically at each phenotype.
In this Review, we discuss the roles of different T cell subsets in allergic asthma that have led to the view that asthma is more than just a TH2-type disease. Although the classical TH1–TH2 paradigm remains a useful frame-work for understanding T cell heterogeneity, it has been extended by the discovery of several of other T cell subsets, such as TH17 cells, TH9 cells and regulatory T (TReg) cells (FIG. 1). New data suggest that T cells do not become fully committed to a specific subset following encounter with antigen but may retain plasticity and are influenced by the surrounding microenvironment. These new concepts will be discussed in the context of the unique immunological milieu of the allergic lung (BOX 2).
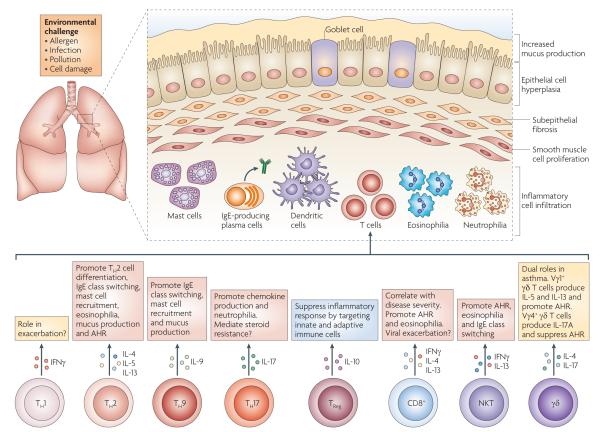
Figure 1. T cells involved in the induction of the allergic phenotype.
Asthma is a heterogeneous disease that is characterized by airway hyperresponsiveness (AHR), recruitment of inflammatory leukocytes to the lung and tissue remodelling, including mucus production and airway smooth muscle changes. A number of different T cell subsets are thought to influence the nature and magnitude of the allergic immune response by the cytokines that they secrete. T helper 2 (TH2) cells are thought to promote eosinophil recruitment, in conjunction with nature killer T (NKT) cells and CD8+ T cells. By contrast, TH1 cells and TH17 cells are thought to be associated with severe, steroid-resistant asthma, which is often marked by neutrophilic infiltrates. Regulatory T (TReg) cells and subtypes of γδ T cells are able to downregulate pulmonary immune responses and are thought to be important for maintenance of immune homeostasis in the lungs. The nature and magnitude of allergic inflammation in the lung is influenced by external environmental stimuli, such as exposure to allergens and pollution as well as infection with pathogens. IFNγ, interferon-γ; IL, interleukin.
Box 2. The lung represents a unique mucosal environment.
The primary function of the lung is gaseous exchange. There are a number of mechanisms in place to ensure that this function is not compromised. The large surface area of the lung is constantly exposed to the external environment, which contains airborne particles that, although immunogenic, do not generally represent a threat to the host — that is, allergens. A number of specialized control mechanisms ensure that de novo immune responses are not continually instigated. This is of vital importance as repeated stimulation of the airway immune system would generate effector memory cells that promote chronic inflammation and damage to the epithelial barrier, thus compromising gaseous exchange. An absolute requirement of these control mechanisms is the ability to discriminate between harmless airborne particles and infectious agents.
Effector CD4+ T cells in allergic inflammation
TH1 and TH2 cells
The division of CD4+ T cells into two distinct functional subsets, namely the TH1 and TH2 cell subsets, prompted much research into the roles of both subsets in disease1, and the increased presence of TH2 cells in the airways of patients with asthma categorized asthma as a TH2 cell-driven disease2,3. Subsequently, a large body of data from both humans and animal models (BOX 3) provided evidence that TH2 cells, through the production of cytokines such as interleukin-4 (IL-4), IL-5 and IL-13, can initiate and maintain key pathophysiological features of the disease4,5. Whereas IL-4 is important for allergic sensitization and IgE production, and IL-5 is crucial for eosinophil survival, IL-13 has pleiotropic effects in the lungs, including a central role in the development of AHR and tissue remodelling6.
Box 3. Animal models of asthma.
The majority of animal models of asthma are initiated by one or more intraperitoneal injections of a protein allergen (most commonly ovalbumin) in conjunction with the T helper 2 (TH2)-type skewing adjuvant aluminium hydroxide (alum). This sensitization phase is followed 7–14 days later by direct administration of allergen to the lung either in a soluble form via the intranasal route, or by aerosolization. This protocol results in airway hyperresponsiveness (AHR), eosinophilic inflammation in the lung, serum IgE production and pulmonary production of TH2-type cytokines, including interleukin-4 (IL-4), IL-5 and IL-13. These models have focused almost exclusively on the role of TH2-type inflammation and do not adequately characterize asthma that is not driven by TH2 cell responses. However, more recently, models have been developed whereby environmental allergen, such as house dust mite extract, is administered directly to the airways in the absence of systemic sensitization with allergen. This model also results in AHR and eosinophilia, but the pathology is less TH2-skewed, and neutrophils are also present in the lung. Disease mechanisms are likely to be different in this inhaled model as the first contact of allergen with the immune system is through pulmonary, rather than peritoneal, dendritic cells, and also depends on contribution from the respiratory epithelium. Other models have used neonatal mice to take age into account, and others have combined allergen exposure with viral challenge or particulate matter.
In an attempt to inhibit the unwanted effects of a TH2-type response, much effort has been invested in elucidating the factors that are involved in TH2 cell development. Although IL-4 has long been recognized as an important cytokine in the development of TH2 cells, recent progress demonstrates that IL-25, IL-33 and thymic stromal lymphopoeitin (TSLP) can induce many of the key features of an allergen or helminth-induced TH2-type response, including eosinophilia and the production of IL-4, IL-5, IL-13 and IgE antibodies. Recent reports show that the cellular sources of these TH2-type cytokines include innate lymphoid cells that do not express surface markers for any known leukocyte lineage and have been termed ‘innate helper’ cells7–9. In mice, these cells are found in many different tissues in the absence of immune challenge, and they are particularly prevalent in the mesenteric lymph nodes, spleen, gut and liver, as well as in the lungs10. A functional role for these innate helper cells in pulmonary immune responses is inferred from the observation that TH2-type disease still develops in mice in the absence of T and B cells11 (FIG. 2). The fact that a TH2-type response can develop in the absence of TH2 cells raises the question of what the potential role of these innate helper cells may be during allergic immune responses.
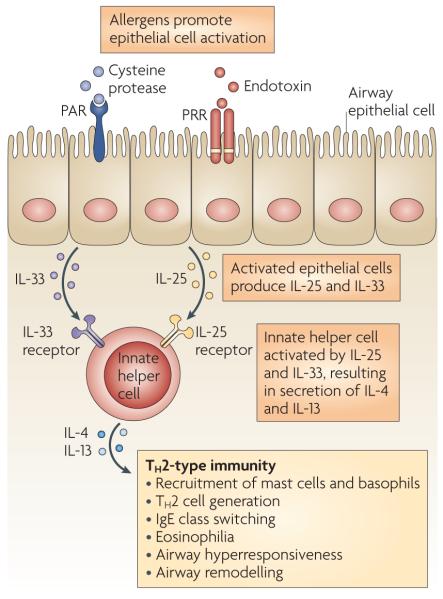
Figure 2. Alternative pathway to a TH2-type response in the airways.
Cysteine protease activity and endotoxin within allergens can activate lung epithelial cells through protease-activated receptors (PARs) and pattern-recognition receptors (PRRs), such as Toll-like receptors. Recent experimental data indicate that a population of ‘innate helper’ cells can secrete interleukin-4 (IL-4) and IL-13 in response to epithelial cell-derived cytokines, such as IL-33 and IL-25, and promote T helper 2 (TH2)-type immune responses. Thymic stromal lymphopoietin (TSLP) can promote TH2-type responses in the lung, but a direct association with this cytokine and innate helper cells in the lung has not yet been found. Although these innate helper cells have been identified in a number of different tissues, including in the resting lungs, evidence for their involvement in allergic airway inflammation remains indirect. Therefore the model described here is theoretical and remains to be tested in vivo.
Allergen challenge in patients with asthma provokes the influx of activated TH2 cells into the airways and this is accompanied by an increase in the levels of TH2-type cytokines and recruitment of eosinophils (reviewed in REF. 12). Notably, the number of TH2 cells present in the airways positively correlates with disease severity (reviewed in REF. 12). During asthma exacerbation, both TH2 and TH1 cells increase in number in blood and induced sputum13. TH1 cells could perhaps be expected to have an inhibitory role in asthma as they can directly inhibit the development of TH2 cells11. In support of this, deletion of the TH1 cell master transcription factor T-bet in mice results in the development of spontaneous AHR and IL-13-dependent eosinophilia14. However, when the signature TH1-type cytokine, interferon-γ (IFNγ), is administered to the airways of patients with asthma, there is no improvement of disease symptoms15. The correlation between TH2 cell numbers and disease severity suggests an essential role for TH2 cells in human asthma. However, recent cluster analysis of asthma clinical phenotypes shows that eosinophilic and noneosinophilic subtypes exist, suggesting that mechanisms other than TH2 cells may be important for pathology in some patients16.
TH17 cells
TH17 cells are a distinct lineage of CD4+ effector T cells that express IL-17 and the key transcription factors retinoic acid receptor-related orphan receptor-γt (RoRγt) and RoRα17,18. Although transforming growth factor-β (TGFβ) and the inflammatory cytokines IL-6, IL-21 and IL-23 are thought to be crucial for TH17 cell development in mice, it is less clear which cytokines are required for TH17 development in humans19–21. IL-17A is upregulated in the lungs of patients with asthma, and levels of IL-17 correlate with disease severity, particularly in patients with neutrophilic, steroid-resistant asthma22,23. Furthermore, tissue-infiltrating CD4+IL-17+ T cells have been documented in the lungs of patients with asthma24.
Careful studies of CD4+IL-17+ T cells have revealed an important role for TH17 cells in allergic inflammation. Epicutaneous or airway sensitization with allergen primes modest TH2 cell responses, but it also primes strong TH17 cell responses that promote airway neutrophilia and acute AHR25,26. TH17 cells also exacerbate TH2 cell-mediated eosinophilic airway inflammation27. Interestingly, transfer of antigen-specific TH17 cells promotes steroidresistant airway inflammation and AHR in mice28, further strengthening the association between TH17 cells and severe asthma.
However, blocking studies in vivo have revealed a complex role for IL-17 in the allergic lung. Although abrogation of IL-17 function during allergic sensitization is protective in mouse models of asthma, delivery of exogenous IL-17A during allergen rechallenge decreases airway inflammation, suggesting that there is also a regulatory role for IL-17 in the allergic lung29–31.
TH9 cells
The complexity surrounding T cell subsets increased further with the recent description of a discrete population of IL-9-secreting CD4+ T cells that depend on TGFβ for their development32. Generation of these TH9 cells requires expression of the transcription factor Pu.1, and secretion of IL-9 from TH9 cells is upregulated by IL-25 (REFS 33,34). There is a wealth of evidence to suggest that IL-9 is involved in the development of allergic inflammation in the lungs. IL-9 is detected in biopsies from patients with asthma and localizes to CD4+ T cells, although there are several other cellular sources of IL-9, including mast cells and eosinophils35–37. Although IL-9-transgenic mice exhibit baseline pulmonary eosinophilia and AHR in the absence of allergen exposure, IL-9-deficient mice have produced mixed results, indicating a degree of redundancy in the functions of IL-9 (REFS 38,39).
Manipulation of TH9 cell functions in vivo changes the course of allergic inflammation. For example, compared with wild-type mice, mice with Pu.1-deficient T cells developed normal TH2 cell responses after allergen exposure in vivo, but they showed reduced allergic pulmonary inflammation, which was associated with decreased IL-9 expression33. The discovery of a potentially distinct lineage of IL-9-producing CD4+ T cells may well clarify this situation; however, direct in vivo evidence for the existence of TH9 cells in the allergic lung of mice or humans is still lacking. It is also not completely clear whether TH9 cells are anti- or pro-inflammatory in vivo. Further analysis of the function of these cells in the allergic lung is warranted.
CD8+ T cells in asthma
The presence of CD8+ T cells in asthmatic airways is well documented; bronchial biopsies from patients with atopic and non-atopic asthma contain CD8+ T cells that produce IL-4 and IL-5 (REF. 40), and spontaneous IL-4, IL-5 and IFNγ production by sputum-derived CD8+ T cells is higher than in healthy subjects41. It is now clear that, particularly in patients with asthma, the behaviour of peripheral blood-derived CD8+ T cells does not resemble that of airway CD8+ T cells41. This may reflect preferential homing or an increased or altered activation state induced by the local airway environment. CD8+ αβ T cells are particularly well represented among intraepithelial T cells and are present in increased proportions in patients with asthma42.
In the past decade, many animal models have shown that the actions of CD8+ T cells in asthmatic airways can be beneficial or deleterious (reviewed in REF. 43). Although this body of data has provided a stepwise improvement in our understanding of the potential roles of CD8+ T cells in asthma, our understanding of the role (or roles) these cells actually have in the airways of patients with asthma remains limited. Depletion of CD8α+ cells (using oX-8 antibody) in previously sensitized rats leads to enhancement of airway inflammation44, an increase in the late-phase response45 (BOX 4) and enhancement of various airway remodelling parameters, including airway smooth muscle mass, epithelial cell proliferation and mucus production46, which all suggest a protective role for CD8+ T cells. However, in addition to T cells, several other cell types can express CD8α, including macrophages, dendritic cell (DC) subsets, natural killer (NK) cells, and invariant NKT (iNKT) cells, leaving these depletion studies open for several potential interpretations.
Box 4. Phases of asthma.
The specific timing and mechanisms of sensitization of patients with asthma to allergens remains unclear; however, various factors influencing the process of sensitization have been discovered, including genetic predisposition, diet, infection history, gender and age. After sensitization, exposure to allergen leads to early-phase reactions in most patients; these reactions involve IgE-mediated degranulation of mast cells and subsequent constriction of the airway smooth muscle. This is followed 4–18 hours later by the late-phase reaction, which is characterized by recruitment of eosinophils and T cells. In a number of patients, this leads to chronic inflammation and airway remodelling that remains difficult to treat.
By contrast, a variety of cell transfer studies using CD8+ αβ T cells show that these cells worsen asthma disease symptoms, either directly47 or, as the majority of studies indicate, in concert with sensitized CD4+ T cells48–51. CD8-deficient mice are less susceptible to allergic airway inflammation, but the transfer of in vitro-generated antigen-primed effector memory CD8+ αβ T cells into sensitized CD8-deficient mice increased AHR, eosinophilic inflammation and the levels of IL-13 in bronchoalveolar lavage (BAL) fluid. Interestingly, this does not occur when the transferred CD8+ T cells are derived from IL-13-deficient mice or in the absence of subsequent allergen challenge49,50, indicating that these deleterious CD8+ αβ T cells are activated within the airways and produce IL-13 locally.
In the presence of TH2 cells, virus-specific CD8+ T cells become potent IL-5 producers; they switch off their IFNγ production and contribute to increased airway eosinophilia in mice52. This observation may in part explain how viral infections contribute to asthma exacerbation. In turn, impaired secretion of IFNγ and a non-cytolytic CD8+ T cell phenotype may prolong the resolution of viral infection, an event that has been documented in rhinovirus-infected patients with asthma. Compared with healthy counterparts, patients with asthma have an enhanced frequency, severity and duration of their rhinovirus infections53. Intriguingly, the annual decline in forced expiratory volume in 1 second (FEV1) in patients with asthma can be predicted by the numbers of CD8+ T cells in the bronchial infiltrate54, and cytokine production by sputum CD8+ T cells correlates with asthma severity41. Also, patients who die from asthma have a larger proportion of activated bronchial CD8+ T cells than patients with asthma who die from other causes55; this suggests a detrimental role for CD8+ T cells in the pathogenesis of human asthma. However, in spite of these existing correlations, the question remains whether CD8+ cells are non-participating bystanders or acquire a more active role in driving more chronic and severe disease.
TReg cells in asthma
Reduced or altered function of TReg cell populations provides a possible explanation for the inappropriate immune response to allergens observed in patients with asthma. Several TReg cell subsets have been described, including naturally occurring forkhead box P3 (FoXP3)+CD4+CD25+ cells and inducible TReg cells, which develop in vitro or in vivo following antigen stimulation (reviewed in REF. 56). Transfer of CD4+CD25+ TReg cells ameliorates the development of airway inflammation and AHR and prevents the allergen-induced activation of DCs in the airways57–61. TReg cells exert their inhibitory action through direct and indirect mechanisms: they produce antiinflammatory cytokines, such as IL-10 and TGFβ, express inhibitory molecules, such as cytotoxic T lymphocyte antigen 4 (CTLA4)57,58 and induce the downregulation of mHC class II and the co-stimulatory molecules CD80 and CD86 by antigen-presenting cells62. Interestingly, TReg cells do not necessarily produce IL-10 themselves but, instead, can induce IL-10 production from bystander CD4+ T cells, which then mediate the suppressive effect58. TReg cell-mediated suppression of DCs is dependent on direct cell contact: TReg cells form lymphocyte functionassociated antigen 1 (LFA1)-dependent aggregates with DCs, which leads to downregulation of DC co-stimulatory molecules in response to TReg cell-expressed CTLA4 (REF. 63). Consistent with this, higher frequencies of airway DCs with increased levels of co-stimulatory molecules and an improved stimulatory capacity are detected in TReg cell-depleted mice64.
Both mouse and human data show that TReg cells need to be maintained by chronic local antigen exposure. If not, the suppressive effect diminishes and airway responses to allergen return61,65. Waxing and waning of a TReg cell response owing to discontinuous allergen exposure may contribute to the intermittent pattern of exacerbations observed in patients with asthma. The presence and functional status of TReg cells has been investigated in human blood and airway compartments. Depletion of CD4+CD25+ T cells from peripheral blood cultures of non-atopic, non-allergic control mice shows that the remaining CD4+CD25− T cells are fully capable of proliferating in response to allergen66. CD4+CD25+ T cells suppress this response: the highest levels of suppression occur in non-atopic controls, but suppression is reduced in atopic patients. Suppression is lowest when CD4+CD25+ T cells come from atopic individuals during the active pollen season66–68. Similar observations have been made in both adult and paediatric patients66–68. In the airways of children with asthma, the percentage of CD4+CD25+ T cells is lower than in healthy children, which might be explained by the reduced response to chemokines of peripheral blood CD4+CD25+ T cells noted in patients with asthma71. The presence of CD4+CD25+ T cells in the airways is positively correlated with FEV1 and, interestingly, corticosteroid treatment increases the number of CD4+CD25+ T cells in the airways of patients with asthma70.
Several immunotherapy regimens have been shown to increase TReg cell responses (reviewed in REF.56). The combination of corticosteroid and vitamin D induces IL-10-producing TReg cells in vitro (reviewed in REF. 72), and vitamin D by itself restores the defective in vitro production of IL-10 that is seen in T cells from patients with corticosteroid-resistant asthma73. Interestingly, in a small clinical study, administration of calcitriol (the active form of vitamin D) to corticosteroid-resistant asthmatics for one week restored the ex vivo IL-10 response of T cells from these patients74. Clinical research designed to evaluate TReg cell-inducing therapies will greatly benefit from specific surface markers that allow definitive identification of TReg cells. A recent genome-wide screen of human FoXP3 target genes showed that 739 target genes are differentially expressed in FoXP3+ TReg cells and that peptidase inhibitor 16, in conjunction with CD25, may serve as a useful marker for TReg cells (these molecules are expressed by >80% of TReg cells)75. An intriguing observation is that TReg cells often have several transcriptional control elements in common with the cells they regulate, which may ensure expression of the same homing receptors and facilitate homing of both the ‘regulator’ and the ‘regulatee’ to the same organ76.
Innate-like T cells in asthma
NKT cells
NKT cells express an invariant T cell receptor (TCR): Vα14 in mice and Vα24 in humans. This TCR recognizes glycolipids, instead of conventional peptide antigens, in the context of the mHC class I-like molecule CD1d, and it is highly conserved between species, suggesting that it behaves like a pattern-recognition receptor and that NKT cells contribute to innate immune responses. Activation of the invariant TCR of NKT cells leads to the rapid production of a range of inflammatory cytokines, including IL-4 and IFNγ, providing early support to drive adaptive immune responses. Another population of NKT cells, referred to as non-classical or type II NKT cells, also recognize CD1d-associated antigens, but do not express the invariant TCR.
Evidence from animal models strongly implicates NKT cells in the development of allergic airway disease. Mice that are deficient in NKT cells (Jα18−/− or CD1d−/− mice) retain some degree of eosinophilic inflammation, but fail to develop AHR77. Conversely, pulmonary instillation of the NKT cell TCR ligand glycolipid α-galactosylceramide (α-GalCer) promotes NKT cell production of IL-13 and TSLP and promotes AHR78. Moreover, exposure of mice to α-GalCer in the presence of antigen promotes NKT cell-dependent allergen sensitization following subsequent allergen exposure79. Similar results were obtained following α-GalCer challenge of non-human primates80. However, the role of NKT cells in the development of human asthma has been controversial as the reported incidence of NKT cells in allergic airways varies from 2% to 60% of BAL cells81,82. Although these differences probably reflect variances in experimental techniques and patient populations, the fact remains that NKT cells are increased in asthmatic patients following allergen challenge83,84. NKT cells may amplify allergic responses in the presence of TH2 cells, but may also drive AHR in the absence of adaptive immune responses, particularly during asthma associated with viral infections, pollution or neutrophils85.
γδ T cells
A local population of γδ T cells resides in close association with the lung epithelium, where they have an immunosurveillance function, responding to endogenous stress signals and localizing to sites of tissue damage86,87. The proportion of γδ T cells in BAL is higher in patients with asthma than in healthy controls. Moreover, γδ T cells from patients with asthma, but not from controls, secrete IL-4 and proliferate to specific, but not unrelated allergen88. Furthermore, a high proportion of γδ T cells in the nasal mucosa of patients with allergic rhinitis express IL-4 but little IFNγ89. This correlation was not observed in peripheral blood cells, underscoring the unique milieu of the lungs and the need to assess tissue-resident cells. However, γδ T cell function has not yet been studied in the lungs of allergic patients.
Studies in mouse models suggest that γδ T cells have dual contrasting roles in the development of allergic inflammation. Analysis of subsets of pulmonary γδ T cells shows that there is a pro-inflammatory γδ T cell subset that expresses Vγ1+ TCR and a suppressive subset that expresses Vγ4+ TCR90,91. Similarly to TH2 cells, Vγ1+ γδ T cells produce IL-5 and IL-13 in the airways and synergize with NKT cells in mediating AHR. The capacity of Vγ4+ γδ T cells to suppress established allergic inflammation and AHR depends on their ability to secrete IL-17A31. These data suggest that depending on its cellular source (either TH17 or γδ T cells), IL-17A can have distinct functions in the airways, thereby further emphasizing the site- and context-specific nature of cytokine production within the lung. Interestingly, AHR occurs in non-allergic mice that lack γδ T cells, suggesting a role for these cells in the maintenance of normal airway function92.
Collectively, these experiments suggest that the tissue localization of γδ T cells and their ability to recognize stress or damage-associated antigens allows them to initiate protective immune responses, but signals from the local inflammatory environment may promote regulatory activity during established responses in order to control inflammation and stimulate resolution.
T cell trafficking in asthma
Similar to their cytokine expression, T cell subsets have distinct chemokine receptor expression patterns (FIG. 3). For example, TH2 cells have been delineated by expression of CC-chemokine receptor 4 (CCR4) and CCR8, and TH1 cells by expression of CCR5 and CXC-chemokine receptor 3 (CXCR3). However, given the opposing functions of TH2 cells and TReg cells, it is perhaps surprising that they share expression of CCR4 and CCR8. Recruitment of TReg cells is abrogated in CCR4-deficient mice93. Mice with a specific loss of CCR4 in their TReg cell compartment develop lymphocytic infiltration and severe pulmonary inflammatory disease. Analogous expression patterns have been seen in human TReg cells56. A similar shared pattern of chemokine receptor expression is observed between TH17 and γδ T cells: both populations express CCR6 and migrate towards CC-chemokine ligand 20 (CCL20) in vitro and in vivo94–97. Whether this shared receptor expression is another facet of flexibility is not clear but, again, it is likely to be determined by the local cytokine milieu. The chemokine receptor repertoire of TH9 cells has yet to be determined.
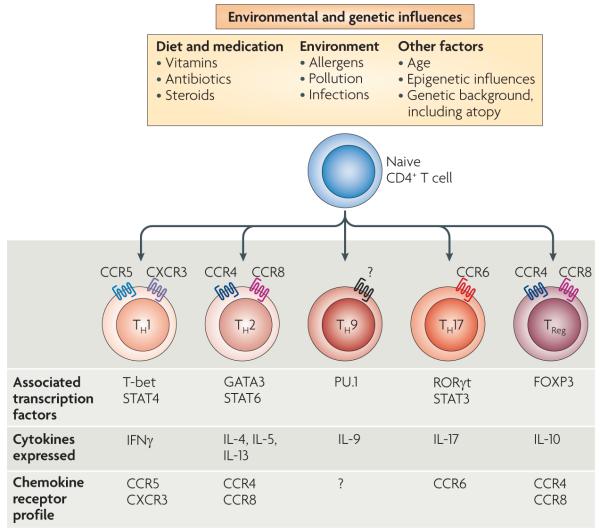
Figure 3. T cell subset signatures are affected by a variety of genetic and environmental influences.
T cell subsets can be distinguished by their cytokine secretion patterns, as well as by their chemokine receptor expression. Distinct T cell lineages are determined by the expression of ‘master’ transcription factors. Although once thought to be committed stable lineages, it is becoming apparent that a range of environmental and genetic influences are able to promote flexibility in T cell programmes. Thus, effector subsets can rapidly react to changing environmental circumstances to promote effective immune responses. CCR, CC-chemokine receptor; CXCR, CXC-chemokine receptor; FOXP3, forkhead box P3; GATA3, GATA-binding protein 3; IFNγ, interferon-γ; IL, interleukin; RORγt, retinoic acid receptor-related orphan receptor-γt; STAT, signal transducer and activator of transcription; TH, T helper; TReg, regulatory T.
It is generally accepted that T cell responses are induced following the uptake of antigen by local DCs and the transport of this antigen to the draining lymph node for presentation to T cells. However, there is evidence that effector T cells constantly recirculate through the lungs and have an immunosurveillance role. Moreover, resident pulmonary antigen-presenting cells can present allergen long after cessation of allergen exposure and have been shown to promote TH2 cell differentiation in situ, indicating that migration to secondary lymphoid organs is not crucial for T cell priming within the respiratory tract98,99. With the growing acceptance of T cell plasticity and flexibility, it is possible that T cells can de-differentiate or be reprogrammed locally. The subsequent change in cytokine secretion pattern probably evokes a change in chemokine receptor expression to allow correct migration within the surrounding airways.
Flexibility of T cell subsets in the lung
The discovery of several potential new T cell lineages suggests that the fate of CD4+ T cell subsets may be more flexible than previously thought. Immunological dogma dictates that, following antigen stimulation and several rounds of division, TH1 and TH2 cells become irreversibly committed to these lineages. However, the finding that TGFβ can subvert TH2 cells to the TH9 cell lineage has led to the understanding that effector CD4+ T cell populations might be more plastic than originally thought32,100. Recent studies have also shown that TReg and TH17 cells are not stable populations and have the capacity for re-differentiation101. Given that most of the original evidence for the commitment of these cells stems from in vitro analysis, it is perhaps not surprising that the situation is different in vivo.
Flexibility of cytokine secretion potentially enables T cells to respond efficiently and effectively to the many immunological insults that they encounter. Thus the influence of molecular mediators produced by the local microenvironment is probably the crucial cue for change; this fact is particularly pertinent to the lungs, as the respiratory environment is a uniquely specialized site that is constantly exposed to the external atmosphere. The lung needs to limit immunological responses to airborne antigens, most of which do not represent a threat to the host. The mechanisms that discriminate these allergens from pathogens involve both innate and adaptive immune responses, including activation and differentiation of T cell subsets. It is clear that flexibility of T cell subsets within the lung would permit rapid reaction to changing circumstances. In addition, although cooperation among all of the different T cell subsets present in the asthmatic lung is easily envisaged, evidence for these interactions is not clearly documented in the literature. In the future, it will be vital to study these local T cell networks and their interactions.
Environmental influences on airway T cells
Several factors are likely to influence the development of T cell responses to allergen, including age, gender, obesity, infection history, atopic status, allergen exposure levels, antibiotic use, environmental factors (such as exposure to diesel) and nutrition (for example, vitamin levels and hormone exposure) (FIG. 3), and these factors probably contribute to the heterogeneity of clinical disease (BOX 1). Although asthma was once thought to be primarily a disease of aberrant TH2-cell directed immune responses, clinical studies now indicate that individuals with asthma can be phenotyped according to their symptoms and that these clinical phenotypes are closely associated with environmental factors102,103. Moreover, interaction between the epithelium and the immune system is crucial in promoting disease, and the genetic signature of airway epithelial cells may influence clinical phenotype.
Epithelial cells
Evidence that epithelial cell function affects the degree of TH2-type inflammation has come from studies of the epithelium from patients with asthma. The TH2-type cytokine induced genes POSTN (encoding periostin), SERPINB2 and CLCA1 have previously been identified as epithelial cell-expressed genes that are specifically induced in patients with asthma; these genes are proposed to represent an asthma-specific mRNA signature for epithelial cells104. At least two distinct molecular phenotypes of asthma are defined by the degree of TH2-type inflammation105. Direct experimental evidence linking epithelial gene expression to the development of TH2-type immunity comes from mice with a specific deletion of Foxa2. The loss of this transcription factor from the pulmonary epithelium leads to spontaneous eosinophilic inflammation and mucous metaplasia106. Interestingly, Foxa2 mice show reduced levels of both IL-33 and CCL20, both of which are important for immune responses against inhaled allergens. These findings may explain, to some extent, the clinical heterogeneity of asthma, as the epithelial gene signature of an individual may influence the strength of TH2-type inflammatory responses to allergen.
Age and diet
Age is also a confounding factor in development of asthma with early onset and late onset disease showing apparently different phenotypes102. The majority of patients with asthma are diagnosed by the age of 6, and atopic disease and viral wheezing illness in infancy are increased risk factors for, and predictive of, childhood asthma107,108. These factors suggest that early life events influence the development of protective regulatory mechanisms that are required for the suppression of inappropriate immune responses to innocuous particles such as allergens. In support of this, TReg cell function is already impaired in cord blood in neonates that have a hereditary risk for allergy109. Interestingly, cluster analysis defines a group of older, mostly female, obese asthmatics with adult-onset asthma and less atopy, comprising about 10% of patients with severe asthma102. These studies strengthen the growing belief that diet and metabolism may affect the degree of severity of asthma.
Nutrients such as vitamin D have notable effects on the immune system, with a vitamin D-deficient diet being associated with decreased regulatory responses to allergen110,111. Importantly, these effects occur even prenatally112. The apparent divergent phenotypic characteristics observed in all of these studies suggest different pathophysiological processes that may determine responses to therapy responses and thus affect asthma control. In the future it will be important to determine whether these clusters of patients are representative of different T cell responses in their lungs.
Infection
Infection history influences the shaping of pulmonary immune responses from the very early stages of life. Recurrent wheeze during the first 3 years of life is considered a major risk factor for asthma. As the majority of lower respiratory tract wheeze in childhood is caused by viruses, it has been postulated that early viral exposure has long-term effects on airway immunity113. By contrast, the ‘hygiene hypothesis’ proposes that early childhood infections inhibit development of allergic disease114, and early infection is thus protective. However, most of the evidence relates to protection against atopy and atopic diseases rather than asthma itself. It has been shown that prenatal exposure to infections influences the development of the innate immune system. Maternal exposure to an environment rich in microbial compounds during pregnancy is associated with higher levels of Toll-like receptor 2 (TLR2), TLR4 and CD14 expression in children115. This suggests that early exposure might prevent allergic sensitization of children owing to the development of a transient local TH1 response. More directly, farm exposure during pregnancy is associated with increased number and function of TReg cells within cord blood, as well as reduced production of TH2-type cytokines and decreased lymphocyte proliferation following innate restimulation116. Studies in mice have shown that exposure of mothers to endotoxin from the cowshed-derived bacterium Acinetobacter lwoffii F78 prevents subsequent allergen-induced sensitization and airway inflammation in the pups117. Whether infection is beneficial or harmful, it is clear that both the timing and nature of the infection have crucial implications for the development of T cell responses in the lung.
Potential of new therapies
Limitations of existing therapies
The discovery of new T cell subsets and of alternative ways to induce a TH2-type response, together with a deeper understanding of the lung microenvironment, has provided us with new insights into the pathology of asthma, and thus with opportunities for innovation in therapy. one important area of unmet need in asthma is curative therapies for patients with mild to moderate asthma (discussed in REF. 118), which would be a welcome replacement for the symptomatic treatment that most patients need to take for many consecutive years. The second area of unmet need in asthma is symptom control in patients with severe asthma (discussed in REF. 118). Current management of asthma is by inhaled corticosteroids with or without long-acting β2-agonists. Although steroids are relatively safe, inexpensive to manufacture and highly effective in clinical trial settings, their use in the asthma population at large leaves over half of patients with poorly controlled disease119. Poor adherence to therapy seems to be an important factor, particularly in patients with difficult-to-treat asthma120.
Targeting cytokines
The effectiveness of steroids in a clinical trial setting sets a high bar for any new therapeutic or treatment regimens that seek to improve or replace them. In spite of recent progress, the increase in our understanding of TH2-type responses and new T cell subsets may not easily lead to a new ‘blockbuster’ therapy; however, there is a growing realization in the field that improvement in therapy may be achievable when approaches become more tailored to distinct clinical phenotypes that are only just being recognized16,102. Moreover, treatments targeting a single mediator are less likely to be effective in the general asthma population, and may be of greater value in specific subgroups of patients or as an add-on to current therapy. This may be particularly true for anti-cytokine treatment approaches (Supplementary information S1 (table)).
A relevant example of this is the experience with the IL-5-specific antibodies mepoluzimab (Bosatria; GlaxoSmithKline) and reslizumab (Ception Therapeutics). Initial clinical trials in patients with both mild and severe asthma showed that these antibodies effectively reduce the number of eosinophils in blood and sputum, but failed to improve other disease symptoms121–123. However, in these initial trials with IL-5-specific antibodies, a trend was noted for improved efficacy of this therapy in patients with asthma exacerbations. Subsequent trials focused on a more specific clinical phenotype and patients were pre-selected for high sputum eosinophilia. Mepoluzimab significantly reduced the number of blood and sputum eosinophils and allowed the use of lower doses of the steroid prednisone in these patients124,125.
Another new approach to asthma therapy is the development of antibodies targeting IL-9. Expression of IL-9 is detected in biopsies from patients with asthma and localizes to CD4+ T cells, mast cells and eosinophils, and several observations point to a role for IL-9 in the development of allergic inflammation35. Efficacy trials with IL-9-specific antibodies are currently ongoing, and these studies should identify the precise role of IL-9 in asthma patients and reveal whether targeting IL-9 may perhaps be especially beneficial to asthma patients with certain clinical phenotypes (ClinicalTrials.gov Identifier: NCT00968669).
As IL-17A and IL-17F production is upregulated in patients with neutrophilic, steroid-resistant asthma and correlates with disease severity, IL-17 family members may be an interesting therapeutic target for severe asthma22,23. Data from animal models, however, show that IL-17 can have both beneficial and detrimental effects in the allergic airway, and it may be necessary to specifically inhibit IL-17 production by CD4+ T cells to obtain any advantage. Antibody approaches targeting IL-17 are currently in the preclinical development stages (discussed in REF. 118).
Promoting TReg cell responses
When contemplating new therapeutic directions for patients with asthma, great emphasis ought to be placed on new therapies that could potentially result in disease modification. In search of this ‘Holy Grail’ of disease modifiers for asthma, it may be desirable to stimulate regulatory pathways or mechanisms that are already present in the airways, in particular those that have the potential to induce long-term suppression or lasting changes in T cell populations. Examples may include induction of TReg cells and stimulation of various TLR pathways. It is suggested that allergen-specific immunotherapy regimens increase the numbers of both CD25+ TReg cells and IL-10-producing TReg cells126,127. Although long-term inhibition is induced by immunotherapy, ultimately symptoms do return128. As TReg cells are maintained by continued antigen exposure, frequent repetition of immunotherapy regimens may be required61,65. Further understanding of the mechanisms responsible for long-term inhibition will be an important aspect of improving immunotherapy for asthma.
Interestingly, corticosteroid treatment in vivo enhances CD4+CD25+ TReg cell function70 and can, in combination with vitamin D, also induce IL-10 producing TReg cells in vitro. of note is the finding that β2-agonists enhance the production of IL-10 by these cells129. of course, it is well known that the beneficial effects of corticosteroids quickly dissipate when treatment stops. Vitamin D treatment itself may be an approach that is particularly promising for the treatment of patients with severe corticosteroid-resistant asthma. Treatment with calcitriol (the active form of vitamin D) restores the ex vivo steroid responsiveness in T cells from patients with steroidresistant asthma, and a trial investigating the efficacy of calcitriol in patients with severe asthma is underway (ClinicalTrials.gov Identifier: NCT00712205).
Indirect targeting of T cell response
An immediate and effective way to silence allergen-induced activation of TH2 cells and the downstream TH2-type response in the airways is through stimulation of TLR7 or TLR9 pathways130–132. A large number of experiments in animal models have shown successful inhibition of both acute and existing asthma responses following TLR targeting, along with long-lasting duration of effects131–132. Stimulation of the TLR9 pathway leads to downregulation of co-stimulatory molecules from airway antigen-presenting cells and subsequent silencing of TH2 cell function131. An initial 4-week clinical trial with weekly dosing of the TLR9 agonist 1018 ISS failed to show clinical benefit133; however, exploration of this pathway in humans is being continued with second-generation compounds and longer treatment regimens. Encouraging data were observed with the TLR9 agonist 1018 ISS conjugated to the ragweed protein Amb a1; patients with ragweed allergic rhinitis treated before the ragweed season showed substantial benefit during the next ragweed season 1 year later134. Interestingly, treatment induces a significant increase in the number of CD4+CD25+ T cells in the nasal mucosa of these patients when compared with placebo135. Further studies are needed to determine whether similar beneficial effects would be observed in patients with asthma.
The discovery of alternative ways to induce a TH2-type response (FIG. 2) is a large step forward in the field, and it may provide an explanation for why therapies solely aimed at T cell depletion have shown limited success in patients with asthma. Initially, these new pathways were observed mostly in the gut mucosa; however, experiments in recombination-activating gene 2 (RAG2)-knockout mice indirectly indicate that the same phenomena also occur in the lungs10,136,137. It will be exciting to test the contribution of the newly described innate helper cells in the airways of patients with asthma, and this effort has already started with the development of antibody approaches targeting IL-25 and IL-33, which are currently in preclinical stages (discussed in REF 118). In addition, a clinical study aims to characterize IL-33 expression in different clinical phenotypes by investigating emergency room patients with breathing difficulties (ClinicalTrials.gov Identifier: NCT00707811).
The fact that asthma is a chronic disease makes the therapeutic application of microRNA of particular interest. MicroRNAs are involved in the fine-tuning of expression levels of networks involving hundreds of proteins, and inhibiting a particular microRNA is therefore the antithesis of a ‘single mediator’ approach138. Expression profiling of microRNAs in both mouse and human airways has identified several microRNAs that may be of interest in asthma. Recent studies in which miRNA-126 and Let-7a were inhibited reported beneficial effects: that is, TH2 cell numbers and TH2-type responses were decreased in animal models of asthma139,140. However, the extent to which these observations translate to humans or whether this approach will have long-term benefits remains to be determined.
Concluding remarks
In summary, T cell subsets and the cytokines that they secrete are undoubtedly central to the pathogenesis and regulation of immune responses to allergen, and the new T cell subsets that have emerged extend the existing paradigm of asthma as a TH2-type disease. The need for tight control of immune responses in the airways makes the lung a specialized environment where recognition of pathogens elicits appropriate inflammatory responses to limit infection while discriminating from harmless inhaled particles, a process that becomes dysregulated in patients with asthma. Factors that damage the pulmonary epithelium — including pathogens, air pollution and allergen protease activity — result in the production of cytokines such as IL-33, IL-25 and TSLP, which are able to promote the development of TH2-type responses in the airways through activation of specific effector cells that can produce large amounts of IL-4, IL-5 and IL-13. The presence of these alternative TH2-type response pathways may contribute to asthma being a heterogeneous collection of clinical phenotypes. The influence of environmental factors and the genetic makeup of an individual determine the specific clinical phenotype in each patient. More research is needed to understand the molecular mechanisms underlying phenotypic clusters, along with the development of validated biomarkers and clinical read-outs to efficiently identify the various phenotypes in the clinic. In the future, asthma therapy may need a more sophisticated approach with new treatments that are more tailored to specific patient groups in order to achieve improvement over standard of care.
Acknowledgements
The authors acknowledge R. Coffman for his critical reading and input, A. Calver for his contribution to the supplementary information and K. Alexander for her help with the manuscript.
Glossary
- Airway hyperresponsiveness (AHR)
A hyperreactivity of the airways, initiated by exposure to a defined stimulus that is usually tolerated by normal individuals; it causes bronchoconstriction and inflammatory-cell infiltration in allergic individuals. This is a defining physiological characteristic of asthma
- Forced expiratory volume in 1 second (FeV1)
A primary indicator of lung function
- Hygiene hypothesis
This hypothesis originally proposed that the increased incidence of atopic diseases in westernized countries was a consequence of living in an overly clean environment, resulting in an under-stimulated immune system that responded inappropriately to harmless antigens. More recently it has been proposed that an absence of exposure to pathogens, in particular helminths, may predispose to both increased allergy and autoimmune disease.
- β2-agonist
A class of medication aimed at dilating the airways by the agonism of the β-adrenoreceptor pathway.
- Allergen-specific immunotherapy
Allergen immunotherapy was introduced in the early 1900s. In general, it involves subcutaneous injection of increasing doses of specific allergen into the patient. This is carried out under carefully controlled clinical conditions because of the potential for life-threatening adverse reactions. On average, it results in ~50% reduction of clinical symptoms and medication usage, and it also results in beneficial modifications of the patient’s immune response to allergen. Following the initial course of injections (either conventional or rushed), patients receive maintenance injections (less frequently) of allergen for optimal clinical benefit.
- MicroRNA
Small, non-coding RNA molecules that regulate the expression of several genes by binding to the 3′ Guntranslated regions of specific mRNAs.
Footnotes
Competing interests statement E.M.H. declares competing financial interests: see Web version for details.
DATABASE ClinicalTrials.gov: http://clinicaltrials.gov NCT00707811 ∣ NCT00712205 ∣ NCT00968669
FURTHER INFORMATION Clare M. Lloyd’s homepage: http://www1.imperial.ac.uk/medicine/about/divisions/nhli/respiration/leukocyte
SUPPLEMENTARY INFORMATION See online article: S1 (table) ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv. Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 2.Robinson DS, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 3.Bentley AM, et al. Identification of T lymphocytes, macrophages, and activated eosinophils in the bronchial mucosa in intrinsic asthma. Relationship to symptoms and bronchial responsiveness. Am. Rev. Respir. Dis. 1992;146:500–506. doi: 10.1164/ajrccm/146.2.500. [DOI] [PubMed] [Google Scholar]
- 4.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu. Rev. Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 5.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 6.Finkelman FD, Hogan SP, Hershey GKK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J. Immunol. 2010;184:1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saenz SA, et al. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moro K, et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 9.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. References 7–10 are a series of landmark papers describing the populations of innate immune cells that are able to produce TH2-type cytokines.
- 11.Paul WE, Zhu J. How are TH2-type immune responses initiated and amplified? Nature Rev. Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 2003;111:450–463. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 13.Mamessier, et al. T-cell activation during exacerbations: a longitudinal study in refractory asthma. Allergy. 2008;63:1202–1210. doi: 10.1111/j.1398-9995.2008.01687.x. [DOI] [PubMed] [Google Scholar]
- 14.Finotto S, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–338. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 15.Boguniewicz M, et al. The effects of nebulized recombinant interferon-γ in asthmatic airways. J. Allergy Clin. Immunol. 1995;95:133–135. doi: 10.1016/s0091-6749(95)70162-1. [DOI] [PubMed] [Google Scholar]
- 16.Haldar P, Pavord ID. Noneosinophilic asthma: a distinct clinical and pathologic phenotype. J. Allergy Clin. Immunol. 2007;119:1043–1052. doi: 10.1016/j.jaci.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 17.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 18.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangan PR, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 20.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Alcorn JF, Crowe CR, Kolls JK. TH17 Cells in Asthma and COPD. Annu. Rev. Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 22.Molet S, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 23.Wisam A, et al. TH17-associated cytokines (IL-17A and IL-17F) in severe asthma. J. Allergy Clin. Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Pene J, et al. Chronically inflamed human tissues are infiltrated by highly differentiated TH17 lymphocytes. J. Immunol. 2008;180:7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 25.He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a TH17 response that drives airway inflammation after inhalation challenge. Proc. Natl Acad. Sci. USA. 2007;104:15817–15822. doi: 10.1073/pnas.0706942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson RH, et al. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakashin H, et al. IL-23 and TH17 cells enhance TH2 cell-mediated eosinophilic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 2008;178:1023–1032. doi: 10.1164/rccm.200801-086OC. [DOI] [PubMed] [Google Scholar]
- 28.McKinley L, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J. Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 30.Schnyder-Candrian S, et al. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murdoch JR, Lloyd CM. Resolution of allergic airway inflammation and airway hyperreactivity is mediated by IL-17 producing γδ T cells. Am. J. Respir. Crit. Care Med. 2010;182:464–476. doi: 10.1164/rccm.200911-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veldhoen M, et al. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. This paper provides evidence that T cells are not necessarily committed lineages and that there is plasticity among T cell subsets.
- 33.Chang HC, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nature Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nature Immunol. 2010;11:250–256. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pejman S, Taylor AD. TH9 and allergic disease. Immunology. 2009;127:450–458. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erpenbeck VJ, et al. Segmental allergen challenge in patients with atopic asthma leads to increased IL-9 expression in bronchoalveolar lavage fluid lymphocytes. J. Allergy Clin. Immunol. 2003;111:1319–1327. doi: 10.1067/mai.2003.1485. [DOI] [PubMed] [Google Scholar]
- 37.Shimbara A, et al. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J. Allergy Clin. Immunol. 2000;105:108–115. doi: 10.1016/s0091-6749(00)90185-4. [DOI] [PubMed] [Google Scholar]
- 38.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J. Exp. Med. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMillan SJ, Bishop B, Townsend MJ, McKenzie AN, Lloyd CM. The absence of interleukin 9 does not affect the development of allergen-induced pulmonary inflammation nor airway hyperreactivity. J. Exp. Med. 2002;195:51–57. doi: 10.1084/jem.20011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ying S, et al. Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils, and mast cells in bronchial biopsies obtained from atopic and nonatopic (intrinsic) asthmatics. J. Immunol. 1997;158:3539–3544. [PubMed] [Google Scholar]
- 41.Cho SH, Stanciu LA, Holgate ST, Johnston SL. Increased interleukin-4, interleukin-5, and interferon-γ in airway CD4+ and CD8+ T cells in atopic asthma. Am. J. Respir. Crit. Care Med. 2005;171:224–230. doi: 10.1164/rccm.200310-1416OC. [DOI] [PubMed] [Google Scholar]
- 42.Hirosako S, et al. CD8 and CD103 are highly expressed in asthmatic bronchial intraepithelial lymphocytes. Int. Arch. Allergy Immunol. 2010;153:157–165. doi: 10.1159/000312633. [DOI] [PubMed] [Google Scholar]
- 43.Betts RJ, Kemeny DM. CD8+ T cells in asthma: friend or foe? Pharmacol. Ther. 2009;121:123–131. doi: 10.1016/j.pharmthera.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Laberge S, et al. Depletion of CD8+ T cells enhances pulmonary inflammation but not airway responsiveness after antigen challenge in rats. J. Allergy Clin. Immunol. 1996;98:617–627. doi: 10.1016/s0091-6749(96)70096-9. [DOI] [PubMed] [Google Scholar]
- 45.Isogai S, Jedrzkiewicz S, Taha R, Hamid Q, Martin JG. Resident CD8+ T cells suppress CD4+ T cell-dependent late allergic airway responses. J. Allergy Clin. Immunol. 2005;115:521–526. doi: 10.1016/j.jaci.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 46.Tsuchiya K, et al. Depletion of CD8+ T cells enhances airway remodelling in a rodent model of asthma. Immunology. 2009;126:45–54. doi: 10.1111/j.1365-2567.2008.02876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sawicka E, Noble A, Walker C, Kemeny DM. Tc2 cells respond to soluble antigen in the respiratory tract and induce lung eosinophilia and bronchial hyperresponsiveness. Eur. J. Immunol. 2004;34:2599–2608. doi: 10.1002/eji.200425018. [DOI] [PubMed] [Google Scholar]
- 48.Isogai S, et al. CD8+ αβ T cells can mediate late airway responses and airway eosinophilia in rats. J. Allergy Clin. Immunol. 2004;114:1345–1352. doi: 10.1016/j.jaci.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 49.Miyahara N, et al. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nature Med. 2004;10:865–869. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 50.Miyahara N, et al. Contribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-13. J. Immunol. 2004;172:2549–2558. doi: 10.4049/jimmunol.172.4.2549. [DOI] [PubMed] [Google Scholar]
- 51.Koya T, et al. CD8+ T cell-mediated airway hyperresponsiveness and inflammation is dependent on CD4+IL-4+ T cells. J. Immunol. 2007;179:2787–2796. doi: 10.4049/jimmunol.179.5.2787. [DOI] [PubMed] [Google Scholar]
- 52.Coyle AJ, et al. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J. Exp. Med. 1995;181:1229–1233. doi: 10.1084/jem.181.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corne JM, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 54.van Rensen EL, et al. Bronchial CD8 cell infiltrate and lung function decline in asthma. Am. J. Respir. Crit. Care Med. 2005;172:837–841. doi: 10.1164/rccm.200504-619OC. [DOI] [PubMed] [Google Scholar]
- 55.O’Sullivan S, et al. Activated, cytotoxic CD8+ T lymphocytes contribute to the pathology of asthma death. Am. J. Respir. Crit. Care Med. 2001;164:560–564. doi: 10.1164/ajrccm.164.4.2102018. [DOI] [PubMed] [Google Scholar]
- 56.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31:438–449. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ostroukhova M, et al. Tolerance induced by inhaled antigen involves CD4+ T cells expressing membranebound TGF-β and FOXP3. J. Clin. Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leech MD, et al. Resolution of Der p1-Induced allergic airway inflammation is dependent on CD4+CD25+Foxp3+ regulatory cells. J. Immunol. 2007;179:7050–7058. doi: 10.4049/jimmunol.179.10.7050. [DOI] [PubMed] [Google Scholar]
- 60.Joetham A, et al. Naturally occurring lung CD4+CD25+ T cell regulation of airway allergic responses depends on IL-10 induction of TGF-β. J. Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 61.Strickland DH, et al. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J. Exp. Med. 2006;203:2649–2660. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur. J. Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 63.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl Acad. Sci. USA. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewkowich IP, et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meiler F, et al. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J. Exp. Med. 2008;205:2887–2898. doi: 10.1084/jem.20080193. This paper shows that T cells can be successfully reprogrammed as a result of allergen immunotherapy.
- 66.Ling EM, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 67.Bellinghausen I, Klostermann B, Knop J, Saloga J. Human CD4+CD25+ T cells derived from the majority of atopic donors are able to suppress TH1 and TH2 cytokine production. J. Allergy Clin. Immunol. 2003;111:862–868. doi: 10.1067/mai.2003.1412. [DOI] [PubMed] [Google Scholar]
- 68.Grindebacke H, et al. Defective suppression of TH2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season. Clin. Exp. Allergy. 2004;34:1364–1372. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 69.Lin Y-L, Shieh C-C, Wang JY. The functional insufficiency of human CD4+CD25+ T-regulatory cells in allergic asthma is subjected to TNF-α modulation. Allergy. 2008;63:67–74. doi: 10.1111/j.1398-9995.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 70.Hartl D, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J. Allergy Clin. Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 71.Khoa DN, Christopher V, Alison F, Kari CN. Selective deregulation in chemokine signaling pathways of CD4+CD25hiCD127lo regulatory T cells in human allergic asthma. J. Allergy Clin. Immunol. 2009;123:933–939. doi: 10.1016/j.jaci.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryanna K, Stratigou V, Safinia N, Hawrylowicz C. Regulatory T cells in bronchial asthma. Allergy. 2009;64:335–347. doi: 10.1111/j.1398-9995.2009.01972.x. [DOI] [PubMed] [Google Scholar]
- 73.Hawrylowicz C, et al. A defect in corticosteroidinduced IL-10 production in T lymphocytes from corticosteroid-resistant asthmatic patients. J. Allergy Clin. Immunol. 2002;109:369–370. doi: 10.1067/mai.2002.121455. [DOI] [PubMed] [Google Scholar]
- 74.Xystrakis E, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoidresistant asthma patients. J. Clin. Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sadlon TJ, et al. Genome-wide identification of human FOXP3 target genes in natural regulatory T cells. J. Immunol. 2010;185:1071–1081. doi: 10.4049/jimmunol.1000082. [DOI] [PubMed] [Google Scholar]
- 76.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nature Immunol. 2009;10:689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 77.Akbari O, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 78.Nagata Y, Kamijuku H, Taniguchi M, Ziegler S, Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int. Arch. Allergy Immunol. 2007;144:305–314. doi: 10.1159/000106319. [DOI] [PubMed] [Google Scholar]
- 79.Jae-Ouk K, et al. Asthma is induced by intranasal coadministration of allergen and natural killer T-cell ligand in a mouse model. J. Allergy Clin. Immunol. 2004;114:1332–1338. doi: 10.1016/j.jaci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Ponpan M, et al. Direct activation of natural killer T cells induces airway hyperreactivity in nonhuman primates. J. Allergy Clin. Immunol. 2008;121:1287–1289. doi: 10.1016/j.jaci.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vijayanand P, et al. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N. Engl. J. Med. 2007;356:1410–1422. doi: 10.1056/NEJMoa064691. [DOI] [PubMed] [Google Scholar]
- 82.Akbari O, et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N. Engl. J. Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 83.Reynolds C, et al. Natural killer T cells in bronchial biopsies from human allergen challenge model of allergic asthma. J. Allergy Clin. Immunol. 2009;124:860–862. doi: 10.1016/j.jaci.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 84.Matangkasombut P, et al. Natural killer T cells in the lungs of patients with asthma. J. Allergy Clin. Immunol. 2009;123:1181–1185. doi: 10.1016/j.jaci.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nature Immunol. 2010;11:577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wands JM, et al. Distribution and leukocyte contacts of γδ T cells in the lung. J. Leukoc. Biol. 2005;78:1086–1096. doi: 10.1189/jlb.0505244. [DOI] [PubMed] [Google Scholar]
- 87.Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nature Rev. Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 88.Spinozzi F, et al. Increased allergen-specific, steroidsensitive γδ T cells in bronchoalveolar lavage fluid from patients with asthma. Ann. Intern. Med. 1996;124:223–227. doi: 10.7326/0003-4819-124-2-199601150-00005. [DOI] [PubMed] [Google Scholar]
- 89.Pawankar RU, et al. Phenotypic and molecular characteristics of nasal mucosal γδ T cells in allergic and infectious rhinitis. Am. J. Respir. Crit. Care Med. 1996;153:1655–1665. doi: 10.1164/ajrccm.153.5.8630617. [DOI] [PubMed] [Google Scholar]
- 90.Lahn M, et al. MHC class I-dependent Vγ4+ pulmonary T cells regulate αβ T cell-independent airway responsiveness. Proc. Natl Acad. Sci. USA. 2002;99:8850–8855. doi: 10.1073/pnas.132519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hahn YS, et al. Vγ4+ γδ T cells regulate airway hyperreactivity to methacholine in ovalbuminsensitized and challenged mice. J. Immunol. 2003;171:3170–3178. doi: 10.4049/jimmunol.171.6.3170. [DOI] [PubMed] [Google Scholar]
- 92.Born W, et al. Immunoregulatory functions of γδ T cells. Adv. Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 93.Sather BD, et al. Altering the distribution of Foxp3+ regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Annunziato F, et al. Phenotypic and functional features of human TH17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 96.Lim HW, Lee J, Hillsamer P, Kim CH. Human TH17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J. Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 97.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 98.Julia V, et al. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–283. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 99.Constant SL, et al. Resident lung antigen-presenting cells have the capacity to promote TH2 T cell differentiation in situ. J. Clin. Invest. 2002;110:1441–1448. doi: 10.1172/JCI16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dardalhon V, et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3– effector T cells. Nature Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moore WC, et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am. J. Respir. Crit. Care Med. 2009;181:315–323. doi: 10.1164/rccm.200906-0896OC. This paper describes the results of hierarchical cluster analysis, which reveals distinct but overlapping clinical phenotypes in asthma; this should facilitate the development of new therapeutic approaches tailored towards specific patient groups.
- 103.Lloyd CM, Saglani S. Asthma and allergy: the emerging epithelium. Nature Med. 2010;16:273–274. doi: 10.1038/nm0310-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Woodruff PG, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc. Natl Acad. Sci. USA. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. This paper highlights the importance of epithelial gene expression as a driving force in asthma and describes potential epithelial gene signatures that predispose towards particular clinical phenotypes.
- 105.Woodruff PG, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen G, et al. Foxa2 programs TH2 cell-mediated innate immunity in the developing lung. J. Immunol. 2010;184:6133–6141. doi: 10.4049/jimmunol.1000223. [DOI] [PubMed] [Google Scholar]
- 107.Martinez FD, et al. Asthma and wheezing in the first six years of life. N. Engl. J. Med. 2009;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 108.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nature Rev. Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 109.Haddeland U, et al. Putative regulatory T cells are impaired in cord blood from neonates with hereditary allergy risk. Pediatr. Allergy Immunol. 2005;16:104–112. doi: 10.1111/j.1399-3038.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 110.Willers SM, et al. Maternal food consumption during pregnancy and the longitudinal development of childhood asthma. Am. J. Respir. Crit. Care Med. 2008;178:124–131. doi: 10.1164/rccm.200710-1544OC. [DOI] [PubMed] [Google Scholar]
- 111.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J. Allergy Clin. Immunol. 2007;120:1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 112.Miller RL. Prenatal maternal diet affects asthma risk in offspring. J. Clin. Invest. 2008;118:3265–3268. doi: 10.1172/JCI37171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holt PG, Upham JW, Sly PD. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies. J. Allergy Clin. Immunol. 2005;116:16–24. doi: 10.1016/j.jaci.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 114.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ege MJ, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J. Allergy Clin. Immunol. 2006;117:817–823. doi: 10.1016/j.jaci.2005.12.1307. [DOI] [PubMed] [Google Scholar]
- 116.Bianca S, et al. Impairment of T-regulatory cells in cord blood of atopic mothers. J. Allergy Clin. Immunol. 2008;121:1491–1499. doi: 10.1016/j.jaci.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 117.Conrad ML, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J. Exp. Med. 2009;206:2869–2877. doi: 10.1084/jem.20090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barnes PJ. New therapies for asthma: is there any progress? Trends Pharmacol. Sci. 2010;31:335–343. doi: 10.1016/j.tips.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 119.Partridge M, van der Molen T, Myrseth SE, Busse W. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm. Med. 2006;6:13. doi: 10.1186/1471-2466-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gamble J, Stevenson M, McClean E, Heaney LG. The prevalence of nonadherence in difficult asthma. Am. J. Respir. Crit. Care Med. 2009;180:817–822. doi: 10.1164/rccm.200902-0166OC. [DOI] [PubMed] [Google Scholar]
- 121.Leckie MJ, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyperresponsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 122.Kips JC, et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am. J. Respir. Crit. Care Med. 2003;167:1655–1659. doi: 10.1164/rccm.200206-525OC. [DOI] [PubMed] [Google Scholar]
- 123.O’Byrne PM. The demise of anti IL-5 for asthma, or not. Am. J. Respir. Crit. Care Med. 2007;176:1059–1060. doi: 10.1164/rccm.200708-1264ED. [DOI] [PubMed] [Google Scholar]
- 124.Nair P, et al. Mepolizumab for prednisone-dependent asthma with Sputum Eosinophilia. N. Engl. J. Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 125.Haldar P, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. References 124 and 125 describe the results from clinical trials in which patients were preselected on the basis of a specific asthma phenotype: the authors show that this improves the efficacy of treatment.
- 126.Suzana R, Mikila RJ, Stephen RD, Kayhan TNA. Grass pollen immunotherapy induces Foxp3-expressing CD4+CD25+ cells in the nasal mucosa. J. Allergy Clin. Immunol. 2008;121:1467–1472. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 127.Akdis M, Akdis CA. Therapeutic manipulation of immune tolerance in allergic disease. Nature Rev. Drug Discov. 2009;8:645–660. doi: 10.1038/nrd2653. [DOI] [PubMed] [Google Scholar]
- 128.Stephen RD, et al. Long-term clinical efficacy in grass pollen-induced rhinoconjunctivitis after treatment with SQ-standardized grass allergy immunotherapy tablet. J. Allergy Clin. Immunol. 2010;125:131–138. doi: 10.1016/j.jaci.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 129.Peek EJ, et al. Interleukin-10-secreting “regulatory” T cells induced by glucocorticoids and β2-agonists. Am. J. Respir. Cell. Mol. Biol. 2005;33:105–111. doi: 10.1165/rcmb.2005-0100OC. [DOI] [PubMed] [Google Scholar]
- 130.Xirakia C, et al. Toll-like receptor 7-triggered immune response in the lung mediates acute and long-lasting suppression of experimental asthma. Am. J. Respir. Crit. Care Med. 2010;181:1207–1216. doi: 10.1164/rccm.200908-1255OC. [DOI] [PubMed] [Google Scholar]
- 131.Hessel EM, et al. Immunostimulatory oligonucleotides block allergic airway inflammation by inhibiting TH2 cell activation and IgE-mediated cytokine induction. J. Exp. Med. 2005;202:1563–1573. doi: 10.1084/jem.20050631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sur S, et al. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J. Immunol. 1999;162:6284–6293. [PubMed] [Google Scholar]
- 133.Gauvreau GM, Hessel EM, Boulet LP, Coffman RL, O’Byrne PM. immunostimulatory sequences regulate interferon-inducible genes but not allergic airway responses. Am. J. Respir. Crit. Care Med. 2006;174:15–20. doi: 10.1164/rccm.200601-057OC. [DOI] [PubMed] [Google Scholar]
- 134.Meri KT, et al. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J. Allergy Clin. Immunol. 2004;113:235–241. doi: 10.1016/j.jaci.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 135.Asai K, et al. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy increases CD4+CD25+ T cells in the nasal mucosa of subjects with allergic rhinitis. Allergol. Int. 2008;57:377–381. doi: 10.2332/allergolint.O-07-528. [DOI] [PubMed] [Google Scholar]
- 136.Fort MM, et al. IL-25 Induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 137.Kondo Y, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int. Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 138.Mattes J, Yang M, Foster PS. Regulation of microRNA by antagomirs: a new class of pharmacological antagonists for the specific regulation of gene function? Am. J. Respir. Cell. Mol. Biol. 2007;36:8–12. doi: 10.1165/rcmb.2006-0227TR. [DOI] [PubMed] [Google Scholar]
- 139.Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc. Natl Acad. Sci. USA. 2009;106:18704–18709. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Polikepahad S, et al. Pro-inflammatory role for let-7 microRNAs in experimental asthma. J. Biol. Chem. 2010;285:30139–30149. doi: 10.1074/jbc.M110.145698. [DOI] [PMC free article] [PubMed] [Google Scholar]