Abstract
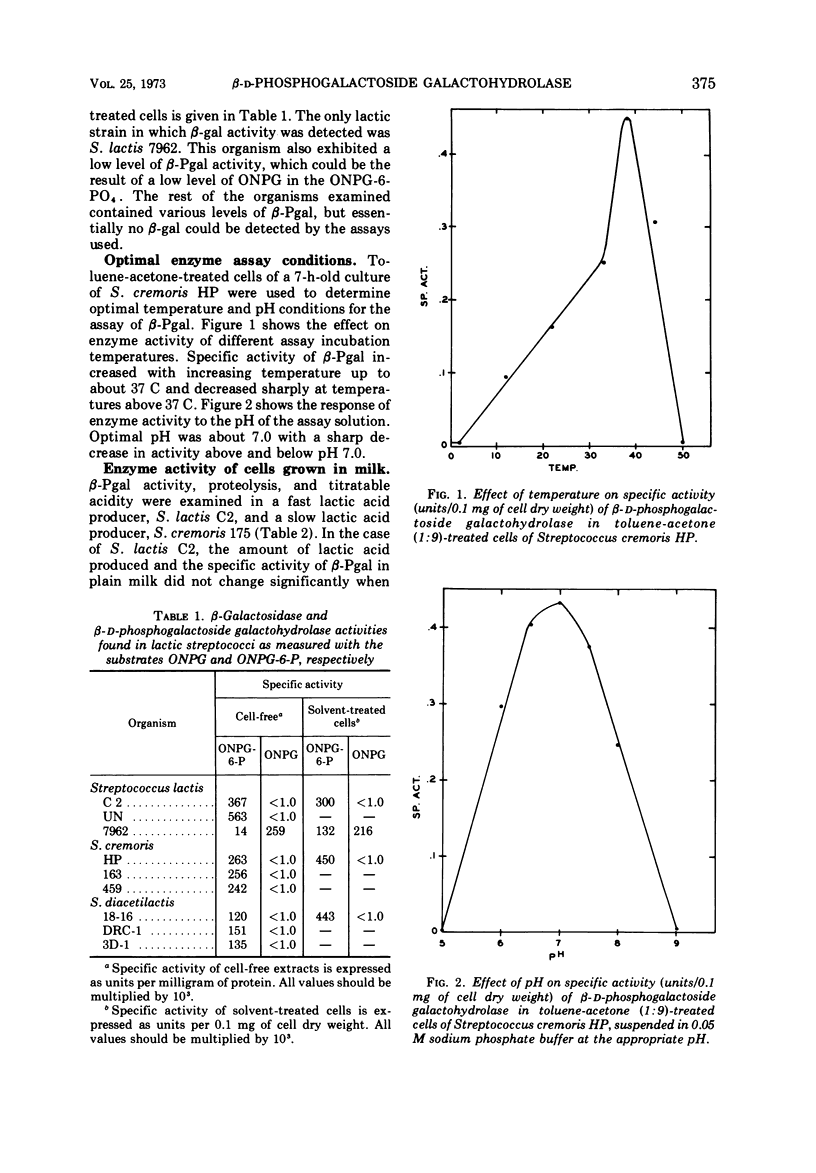
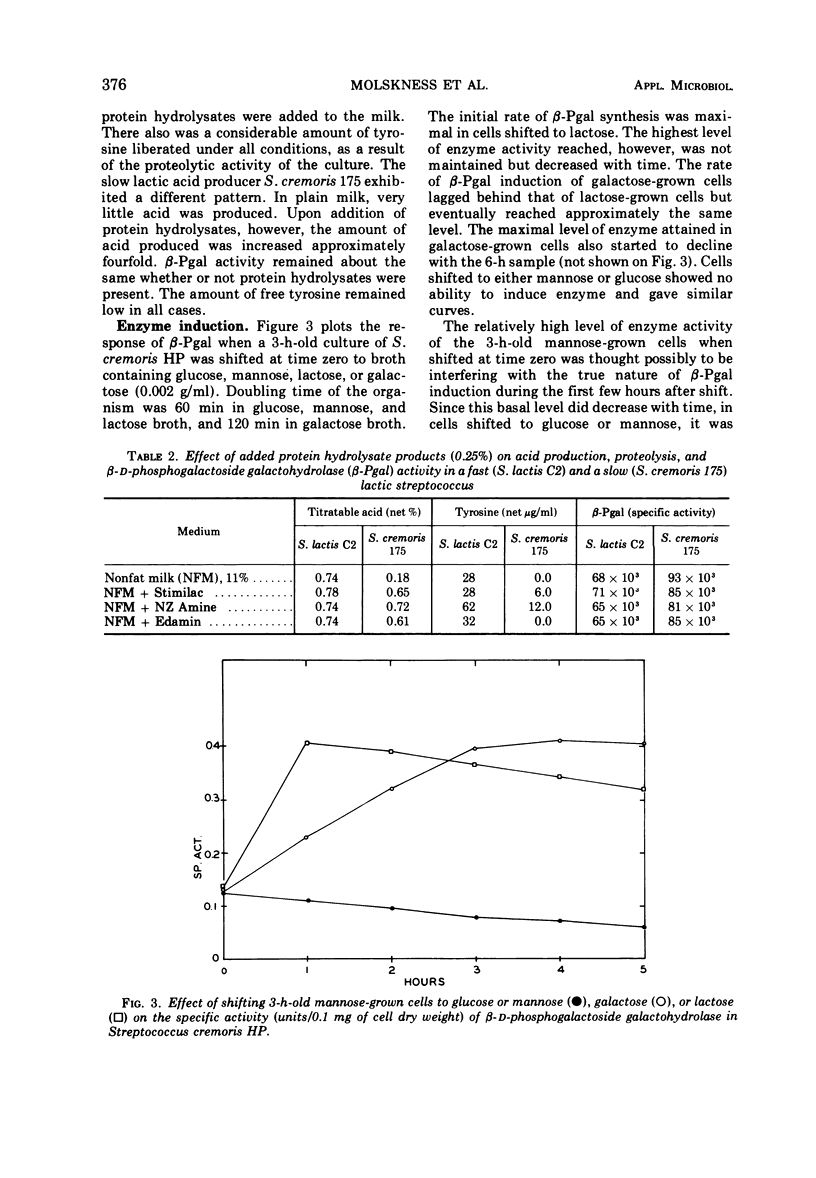
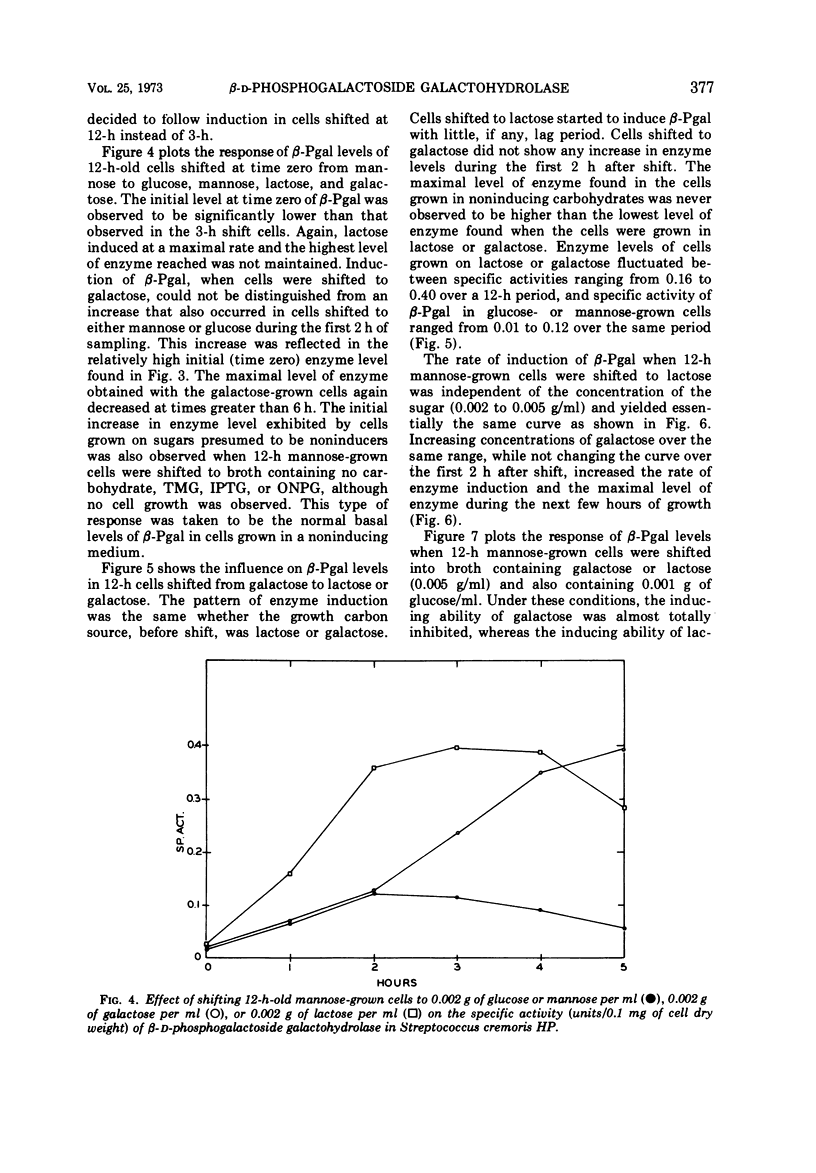
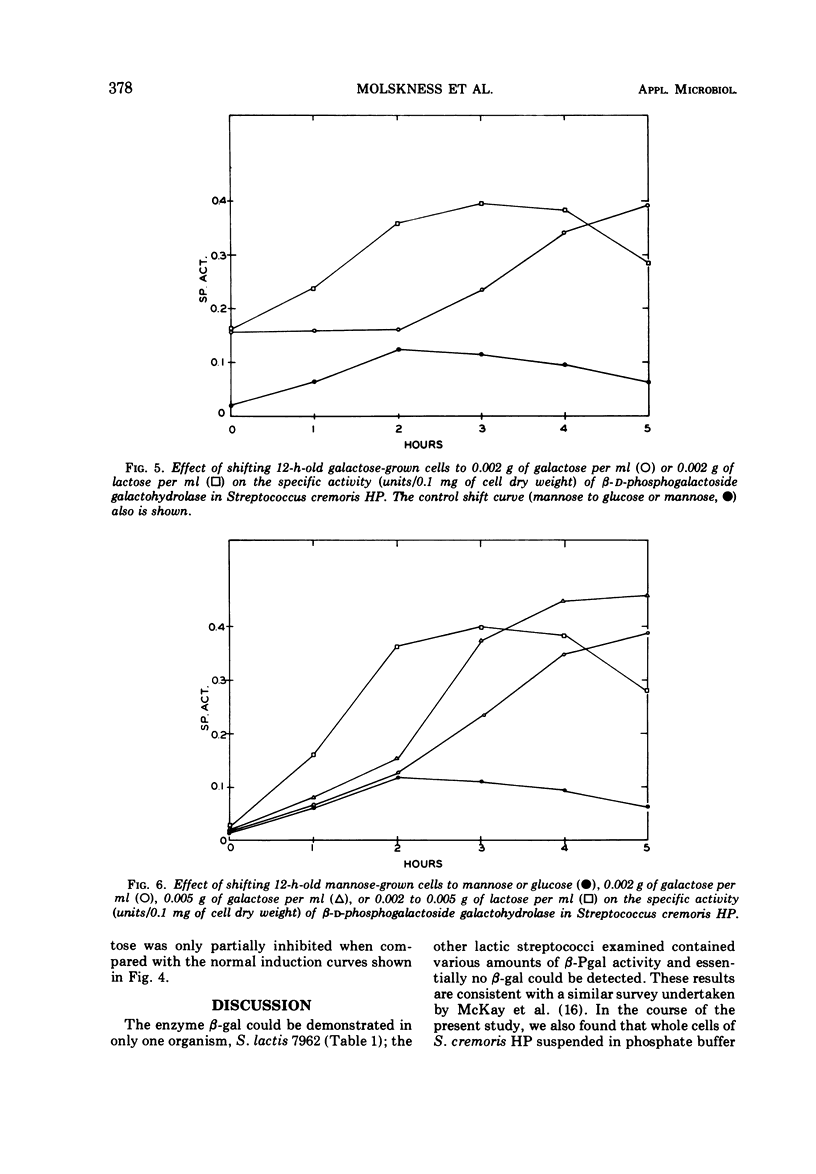
β-D-Phosphogalactoside galactohydrolase (β-Pgal) was examined in a number of lactic streptococci by use of the chromogenic substrate o-nitrophenyl-β-D-galactopyranoside-6-phosphate. Specific activity of β-Pgal ranged from 0.563 units/mg of protein in Streptococcus lactis UN, to 0.120 in S. diacetilactics 18-16. Essentially no β-D-galactoside galactohydrolase (β-gal) was found in these organisms when o-nitrophenyl-β-D-galactopyranoside served as the chromogenic substrate. S. lactis 7962 was the one exception found. This organism contained rather high levels of β-gal, and very little β-Pgal could be detected. β-Pgal activity was examined in streptococci that differed widely in both their proteolytic ability and rates of lactic acid production during growth in milk. Differences in proteolytic ability did not influence β-Pgal synthesis; also, the rate of lactic acid production was independent of the level of β-Pgal present in the cell, since the rate of lactic acid production could be increased approximately fourfold without changing the amount of β-Pgal present in the cell. Various carbohydrates were tested as potential inducers of the enzyme. Although galactose, either as the free sugar or combined with glucose in lactose, was the only inducer, noninducing sugars such as mannose or glucose showed some ability to cause fluctuations in the basal level of β-Pgal. Cells growing in mannose or glucose exhibited about 30% of the maximal enzyme levels found in cells growing in lactose or galactose. No gratuitous inducers were found.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CITTI J. E., SANDINE W. E., ELLIKER P. R. BETA-GALACTOSIDASE OF STREPTOCOCCUS LACTIS. J Bacteriol. 1965 Apr;89:937–942. doi: 10.1128/jb.89.4.937-942.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. V. The accumulation of phosphorylated carbohydrate derivatives, and evidence for a new enzyme-splitting lactose phosphate. Proc Natl Acad Sci U S A. 1967 Jul;58(1):274–279. doi: 10.1073/pnas.58.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. VI. The nature of the derivatives accumulated. J Biol Chem. 1968 Apr 25;243(8):1881–1885. [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Hill K. L., Morse M. L. Metabolism of lactose by Staphylococcus aureus. J Bacteriol. 1968 Dec;96(6):2187–2188. doi: 10.1128/jb.96.6.2187-2188.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Hill K. L., Morse M. L. Phosphotransferase system of Staphylococcus aureus: its requirement for the accumulation and metabolism of galactosides. J Bacteriol. 1969 Aug;99(2):383–388. doi: 10.1128/jb.99.2.383-388.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBURGER J. A., SPECK M. L., AURAND L. W. IDENTIFICATION OF GROWTH STIMULANTS FOR STREPTOCOCCUS LACTIS. J Bacteriol. 1963 May;85:1051–1055. doi: 10.1128/jb.85.5.1051-1055.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Scarborough G. A. Mechanism of hydrolysis of O-nitrophenyl-beta-galactoside in Staphylococcus aureus and its significance for theories of sugar transport. Proc Natl Acad Sci U S A. 1967 Jul;58(1):225–228. doi: 10.1073/pnas.58.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. L., Hill K. L., Egan J. B., Hengstenberg W. Metabolism of lactose by Staphylococcus aureus and its genetic basis. J Bacteriol. 1968 Jun;95(6):2270–2274. doi: 10.1128/jb.95.6.2270-2274.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


