Abstract
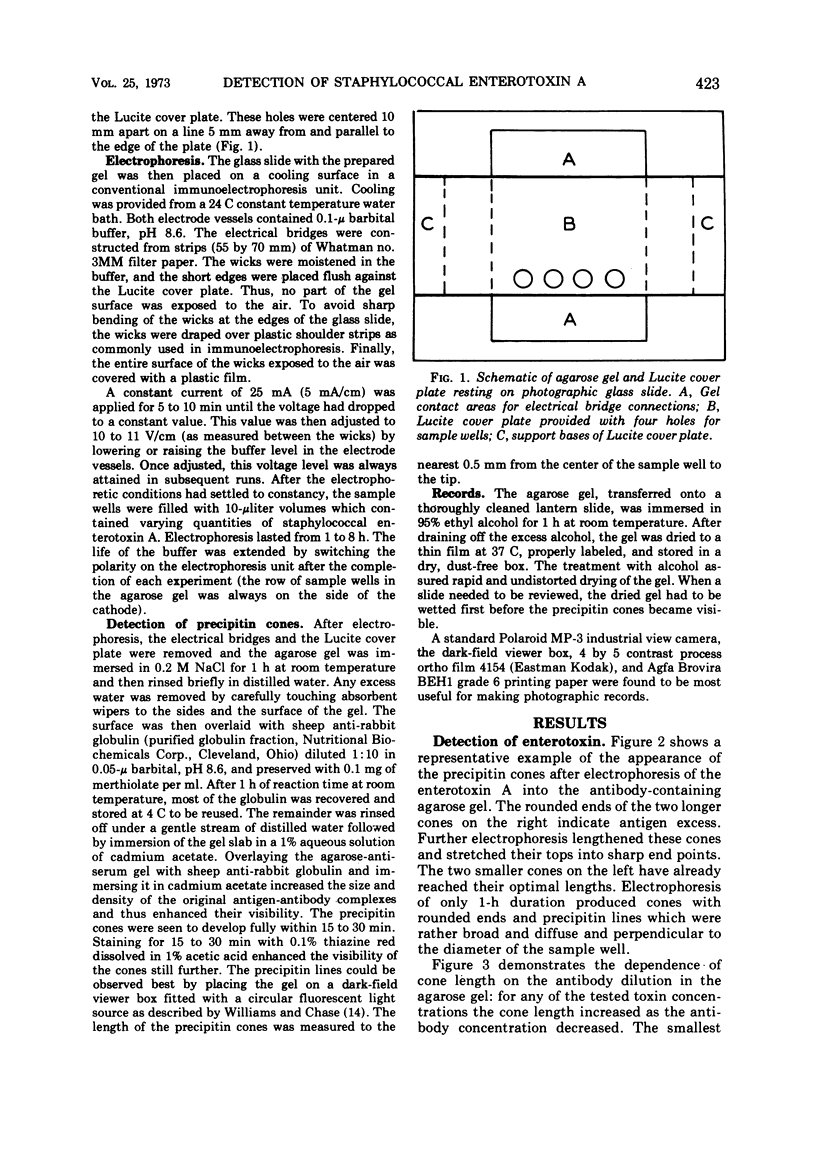
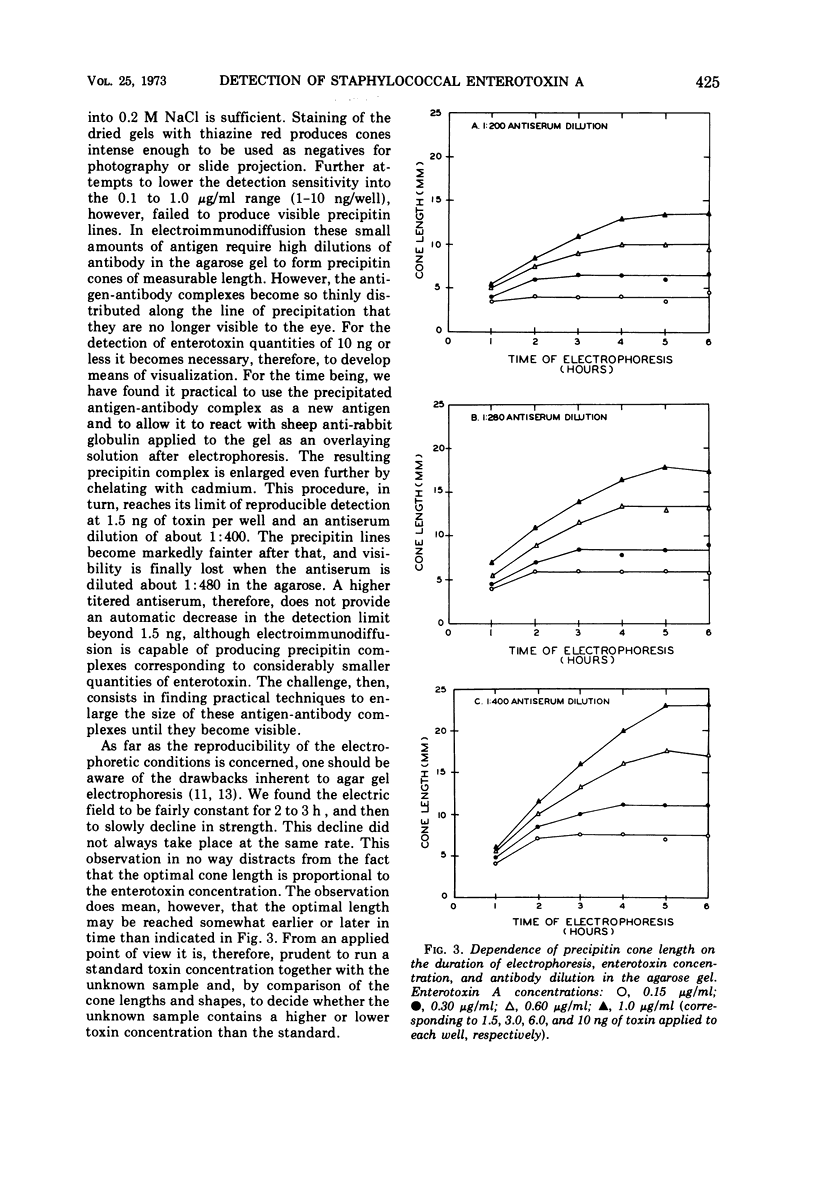
The detection of staphylococcal enterotoxin A by the quantitative technique of electroimmunodiffusion is described. High dilutions of type-specific rabbit antiserum were used in 1% agarose gels, 1 mm thick, and prepared in 0.05-μg barbital buffer, pH 8.6. Volumes of 10 μliters containing 1.5 to 10 ng of toxin were electrophoresed out of 4-mm diameter wells at 5 mA/cm width of gel. The precipitin cones formed were made visible by first immersing the agarose gels in 0.2 M NaCl and then overlaying the surface with the purified globulin fraction of sheep serum against rabbit globulin, followed by soaking of the gels in 1% aqueous cadmium acetate and staining with 0.1% thiazine red in 1% glacial acetic acid. Fully extended cones, 4 to 23 mm in length depending on toxin concentration and antiserum dilution, were developed in 2 to 5 h of electrophoresis, and visualization was achieved within 2 to 3 h. Because the method is qualitative, quantitative, simple, rapid, and sensitive, it offers a practical tool for the detection of small amounts of bacterial toxins in contaminated foods. The method should also qualify as a sensitive detection device in biochemical procedures which attempt to trace, detect, and identify biological substances in nanogram quantities, provided these substances are antigenic and capable of forming a precipitate with their specific antibodies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASMAN E. P., BENNETT R. W. DETECTION OF STAPHYLOCOCCAL ENTEROTOXIN IN FOOD. Appl Microbiol. 1965 Mar;13:181–189. doi: 10.1128/am.13.2.181-189.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung D. Y., Wagner J. Capillary tube assay for staphylococcal enterotoxins A, B, and C. Appl Microbiol. 1971 Mar;21(3):559–561. doi: 10.1128/am.21.3.559-561.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi N. R., Richardson G. H. Capillary tube immunological assay for staphylococcal enterotoxins. Appl Microbiol. 1971 Apr;21(4):626–627. doi: 10.1128/am.21.4.626-627.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Bukovic J. A., Kauffman P. E. Antigenic cross-reactivity of staphylococcal enterotoxins. Infect Immun. 1972 May;5(5):645–647. doi: 10.1128/iai.5.5.645-647.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Lopez M., Tsu T., Hyslop N. E., Jr Studies of electroimmunodiffusion: immunochemical quantitation of proteins in dilute solutions. Immunochemistry. 1969 Jul;6(4):513–526. doi: 10.1016/0019-2791(69)90191-8. [DOI] [PubMed] [Google Scholar]
- Miller C. A., Anderson A. W. Rapid detection and quantitative estimation of type A botulinum toxin by electroimmunodiffusion. Infect Immun. 1971 Aug;4(2):126–129. doi: 10.1128/iai.4.2.126-129.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D. Neglected Aspects of Electroosmosis in Porous Bodies. Science. 1955 Aug 26;122(3165):373–374. doi: 10.1126/science.122.3165.373. [DOI] [PubMed] [Google Scholar]
- Roberts D. B., Wright G. L., Jr, Affronti L. F., Reich M. Characterization and comparison of mycobacterial antigens by two-dimensional immunoelectrophoresis. Infect Immun. 1972 Oct;6(4):564–573. doi: 10.1128/iai.6.4.564-573.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



