Abstract
The fruit of the strawberry Fragaria×ananassa has traditionally been classified as non-climacteric because its ripening process is not governed by ethylene. However, previous studies have reported the timely endogenous production of minor amounts of ethylene by the fruit as well as the differential expression of genes of the ethylene synthesis, reception, and signalling pathways during fruit development. Mining of the Fragaria vesca genome allowed for the identification of the two main ethylene biosynthetic genes, 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase. Their expression pattern during fruit ripening was found to be stage and organ (achene or receptacle) specific. Strawberry plants with altered sensitivity to ethylene could be employed to unravel the role of ethylene in the ripening process of the strawberry fruit. To this end, independent lines of transgenic strawberry plants were generated that overexpress the Arabidopsis etr1-1 mutant ethylene receptor, which is a dominant negative allele, causing diminished sensitivity to ethylene. Genes involved in ethylene perception as well as in its related downstream processes, such as flavonoid biosynthesis, pectin metabolism, and volatile biosynthesis, were differently expressed in two transgenic tissues, the achene and the receptacle. The different transcriptional responsiveness of the achene and the receptacle to ethylene was also revealed by the metabolic profiling of the primary metabolites in these two organs. The free amino acid content was higher in the transgenic lines compared with the control in the mature achene, while glucose and fructose, and citric and malic acids were at lower levels. In the receptacle, the most conspicuous change in the transgenic lines was the depletion of the tricarboxylic acid cycle intermediates at the white stage of development, most probably as a consequence of diminished respiration. The results are discussed in the context of the importance of ethylene during strawberry fruit ripening.
Key words: Ethylene, fruit, metabolic profiling, non-climacteric, ripening, strawberry.
Introduction
The strawberry (Fragaria×ananassa, Duch.) is an important crop worldwide, and the potential benefits of its consumption in the prevention of cardiovascular and neurodegenerative diseases, obesity, cancer, and ageing are currently under investigation (Seeram, 2008; Giampieri et al., 2012). Despite the increasing amount of information regarding the processes involved in strawberry fruit ripening (Medina-Escobar et al., 1997; Aharoni and O’Connell, 2002; Bombarely et al., 2010; Csukasi et al., 2011, 2012), little is known about the regulation of these processes, including the role played by the various phytohormones. The main reason for this relatively poor understanding in comparison with other fruits is that what is commonly referred to as the strawberry fruit is in fact a false fruit that is composed of achenes (true fruits that evolve from ovaries), and the engrossed flower receptacle (fleshy part), and both organs are connected through vascular bundles (Perkins-Veazie, 1995). These two organs, the achene and the receptacle, although highly interconnected throughout their developmental programmes, particularly at early developmental stages, are very different in terms of cell ontogeny and function, as has been revealed by both gene expression studies (Aharoni and O’Connell, 2002; Csukasi et al., 2012) and metabolic profiling (Fait et al., 2008). Therefore, the underlying regulatory processes that occur during development and ripening are expected to differ for each organ. Importantly, studies on the whole fruit, without dissection into its two composite parts, mask tissue-specific differences, the knowledge of which is required to draw reliable conclusions.
Ethylene, the simplest olefin, plays a key role in many aspects of the plant life cycle, regulating various biological processes, including seed germination, cell elongation, root initiation, flower development, sex determination, fruit ripening, senescence, and responses to biotic and abiotic stresses (Bleecker and Kende, 2000). Depending on the role of ethylene in ripening, fruits are classified as either climacteric, such as the tomato, apple, banana, and avocado, all of which present increased respiration and a burst of ethylene biosynthesis at the onset of ripening, or non-climacteric, such as the grape and pepper, which show no relevant increase in respiration and ethylene production during ripening (Giovannoni, 2004). In climacteric fruits, ethylene is the key hormone that controls fruit ripening (Giovannoni, 2007; Klee and Giovannoni, 2011; Osorio et al., 2011). In plants, ethylene is synthesized from methionine, and the last two steps in ethylene biosynthesis are the main regulatory control points. These steps consist of the conversion of S-adenosyl-l-methionine into 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS) and the oxidation of ACC to ethylene by ACC oxidase (ACO) (Yang and Hoffman, 1984). Both enzymes are encoded by multigene families in many plant species, with differences in the expression and regulation of the various members (Lin et al., 2009). This pathway has been studied in detail in climacteric tomato fruits, and different family members have been associated with specific developmental stages (Cara and Giovannoni, 2008; Itkin et al., 2009; Yokotani et al., 2009; Kamiyoshihara et al., 2010; Klee and Giovannoni, 2011).
The strawberry fruit has traditionally been classified as non-climacteric, based on its low endogenous production of ethylene compared with standard climacteric fruits and because of the inability to accelerate strawberry fruit ripening by the external application of ethylene or ethylene-releasing compounds (Perkins-Veazie, 1995). However, despite the low levels of this hormone in the strawberry fruit (Leshem and Pinchasov, 2000), recent studies have shown that the situation is not that simple and have generated some controversy in the field (Trainotti et al., 2005; Iannetta et al., 2006). It has been reported that, despite its low concentration, ethylene presents a characteristic pattern of production during different developmental stages; it is relatively high in green fruits, decreases in white fruits, and finally increases at the red stage of ripening (Perkins-Veazie et al., 1996; Iannetta et al., 2006). Interestingly, this last increase is accompanied by an enhanced respiration rate that resembles that which occurs in climacteric fruits at the onset of ripening (Iannetta et al., 2006). In the strawberry, two ACO genes (FaACO1 and FaACO2) and three ethylene receptor genes, two type I (FaEtr1 and FaErs1) and one type II (FaEtr2), have been identified. Moreover, their expression during ripening as well as their response to different hormone treatments have been studied (Trainotti et al., 2005). In general, a good correlation exists between the expression of all of these genes and ethylene production (Iannetta et al., 2006). However, the expression and the ethylene production data corresponded to the whole berry; that is, the achenes and receptacle (Trainotti et al., 2005).
The external application of ethylene or its precursors, and the application of inhibitors of ethylene synthesis or perception, have been utilized to discern the function of this hormone in the growth and ripening of the strawberry. Although the application of ethylene to strawberry fruits does not have an obvious effect on ripening, it does have an effect on the expression of a subset of ripening-related genes (Trainotti et al., 2001; Castillejo et al., 2004; Bustamante et al., 2009).
Ethylene causes the down-regulation of several cell wall-related genes that are involved in fruit softening, such as β-galactosidase, pectin methylesterase, and β-xylosidase (Trainotti et al., 2001; Castillejo et al., 2004; Bustamante et al., 2009), while the expression of other cell wall-related genes, such as expansin FaEXP2 (Civello et al., 1999), is ethylene insensitive. Blocking ethylene action with 1-methylcyclopropene (1-MCP) results in the down-regulation of the polygalacturonase gene (Villarreal et al., 2009). In addition to its effects on gene expression, ethylene treatment also decreases the enzymatic activities of endo-1,4-β-glucanase and β-xylosidase (Villarreal et al., 2010). These results indicate that although it is not as relevant as in climacteric fruits, ethylene may play a role in strawberry fruit ripening.
To gain further insight into ethylene production during strawberry ripening, the expression of the key genes involved in ethylene biosynthesis, ACS and ACO, was analysed in both the achenes and receptacles at different developmental stages. Next, the role of ethylene during strawberry ripening was investigated by generating transgenic strawberry plants with reduced ethylene sensitivity. These plants were generated through ectopic expression of the dominant negative mutant allele of the Arabidopsis ethylene receptor gene etr1-1, which has been shown to diminish ethylene insensitivity in different plant species, such as Arabidopsis, tomato, petunia, tobacco, and birch (Chang et al., 1993; Wilkinson et al., 1997; Vahala et al., 2003; Knoester et al., 2005). Transcriptional and metabolic analyses of the transgenic fruits with respect to organ and developmental specificity revealed different roles for ethylene in the growth and ripening of the achene and receptacle. The results are discussed in terms of the specific development patterns for these two organs, which together form the edible fruit.
Materials and methods
Plant material, transformation, and sampling
Strawberry plants (Fragaria×ananassa Duch.) were grown in a greenhouse under natural light conditions in southern Spain (Málaga). Transformation of F.×ananassa cv. Chandler plants was performed according to the protocol described by El-Mansouri et al. (1996). Strawberry leaf discs were transformed with Agrobacterium tumefaciens LBA4404 carrying a pCD-2 plasmid that contained the kanamycin resistance gene nptII and the cDNA for the mutant allele of etr1-1 (At1g66340) in the sense orientation under the control of a single constitutive [Cauliflower mosiac virus (CaMV) 35S] promoter (Wilkinson et al., 1997). All the F.×ananassa plants were vegetatively propagated each season using stolons. The plants used in this work corresponded to the first, second, and third vegetative generations, and the transgenic lines were evaluated in consecutive years.
Fruits from wild-type and transgenic plants were harvested at three different developmental stages: green fruit (G; green achenes and receptacle), white fruit (W; green achenes and white receptacle), and red fruit (R; red achenes and receptacle), corresponding to 12, 21, and 35 d post-anthesis, respectively, as previously described by de la Fuente et al. (2006). Prior to removing the achenes from the receptacle, whole fruits were collected and immediately frozen in liquid nitrogen to avoid changes in gene transcription due to wounding. Then, for the achene and receptacle analysis, the achenes of G, W, and R fruits were carefully removed from the corresponding receptacles with a scalpel tip. Analyses of the fruits were performed on a minimum of four or five separate pools of 30 fruits each for each ripening stage. Each pool was from one individual plant.
Fruit firmness was measured using a penetrometer with 3mm2 surface needles. Three punctures were made in opposite sites per fruit. Between 20 and 25 fruits per line were used.
Metabolome analysis
Metabolite extraction, derivatization, standard addition, and sample injection for gas chromatography–mass spectrometry (GC-MS) were performed according to Osorio et al. (2012). The mass spectra were cross-referenced with those in the Golm Metabolome database (Kopka et al., 2005). The content of each metabolite was determined by normalizing the integration area of a characteristic fragment ion trace to the integration area of the internal standard, ribitol (m/z 319), and the fresh weight of the plant material was extracted.
Total phenolics
Total phenolics were determined according to the Folin–Ciocalteu procedure (Waterhouse, 2001). Total phenolic content was expressed as gallic acid equivalents (GAE) in milligrams per 100g of fresh weight of achenes.
RNA extraction and quantification for qRT–PCR
The RNA extraction and quantitative reverse transcription–PCR (qRT–PCR) were performed as described by Osorio et al. (2008). The expression of the genes encoding various ethylene receptors (etr1-1, FaETR1, FaETR2, and FaERS1), phenylalanine ammonia lyase (FaPAL), chalcone synthase (FaCHS), 1-aminocyclopropane-1-carboxylate oxidase 1 (FaACO1), 1-aminocyclopropane-1-carboxylate oxidase 2 (FaACO2), 1-aminocyclopropane-1-carboxylate oxidase 3(FaACO3), 1-aminocyclopropane-1-carboxylate synthase 1 (FaACS1), 1-aminocyclopropane-1-carboxylate synthase 2 (FaACS2), 1-aminocyclopropane-1-carboxylate synthase 3 (FaACS3), 1-aminocyclopropane-1-carboxylate synthase 4 (FaACS4), pectin methyl esterase (FaPE1), pectate lyase A (FaPLA), polygalacturonase 1 and 2 (FaPG1 and FaPG2), O-methyltransferase (FaOMT), quinone reductase (FaQR), MYB transcription factors (FaMYB1 and FaMYB10), and d-galacturnonate reductase (FaGalUR), was analysed by real-time qRT–PCR using the fluorescent intercalating dye EvaGreen in an iCycler detection system (Bio-Rad). Relative quantification of the target expression level was performed using the comparative Ct method (Pfaffl, 2001). To normalize gene expression for differences in the efficiency of cDNA synthesis, the transcript levels of the constitutively expressed glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) were measured (Opazo et al., 2010; Salvatierra et al., 2010). The primers used are shown in Supplementary Table S1 available at JXB online.
Phylogenetic analysis
Sequence analysis was performed with ClustalW, and the Neighbor–Joining method was used to generate the tree. The percentages of replicate trees in which the associated proteins clustered together in the bootstrap test (1000 trials) are shown next to the branches. The trees are drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the p-distance method, and the scale is the number of amino acid differences per site. The evolutionary analyses were conducted in MEGA5 (Tamura et al., 2011).
Accession numbers
Sequence data from this article can be found at http://www.strawberrygenome.org under the following accession numbers: gene 31839 (FvACS1), gene 19023 (FvACS2), gene 30682 (FvACS3), gene 11392 (FvACS4), gene 01202 (FvACO1), gene 19733 (FvACO2), and gene 11261 (FvACO3). Other sequence data can be found in GenBank under the following accession numbers: AAT77723 (FaACS1), AAF97614 (SlACS1A), AAF97615 (SlACS1B), AAP96918 (SlACS2), AAA78789 (SlACS3), P29535 (SlACS4), AAK72430 (SlACS5), AAK72433 (SlACS6), AAC32317 (SlACS7), AAK72431 (SlACS8), CAH65482 (FaACO1), CAH65483 (FaACO2), ADO32577 (SlACO1), Y00478 (SlACO2), Z54199 (SlACO3), BAA34924 (SlACO4), AJ715790 (SlACO5), AB201756 (FaPAL), AB360390 (FaCHS), AY324809 (FaPE1), AF339025 (FaPLA), AF280662 (FaPG1) and AF380299 (FaPG2), AJ297511 (FaETR1), AJ297513 (FaETR2), AJ297512 (FaERS1), EU155162 (FaMYB10), AF401220 (FaMYB1), AF220491 (FaOMT), AY158836 (FaQR), and AF039182 (FaGalUR).
Statistical analysis
Statistical analysis was performed with SPSS Statistics v20 (IBM Corp.) and the R environment (http://www.r-project.org) using analysis of variance (ANOVA). The means were compared by Student’s t-test and Tukey HSD for metabolite and expression analysis, respectively. Correlation analysis based on Pearson correlation was performed using R software (Ihaka and Gentleman, 1996).
Results
Expression of genes encoding ethylene biosynthesis enzymes in strawberry fruits display a tissue-/stage-specific pattern
Previous studies of ethylene production in strawberry fruits have been performed in the so-called fruits, which are a combination of both the achenes and the receptacle. However, this type of analysis provides ambiguous information due to the heterogeneity of the sample. To be consistent with the previous literature, the term ‘fruit’ has been used to describe the complete berry, but a distinction is made between the receptacles and achenes when they are studied separately.
As previously mentioned, ethylene production has been measured in strawberry fruits during ripening. However, the focus of the present investigation was the independent ethylene production of the achenes and receptacles. It is important to note that this is technically difficult because ethylene should be produced after the physical damage resulting from the separation of the achenes. A reasonable correlation between the transcription of the genes encoding ACS and ethylene production has, however, been reported (Lin et al., 2009). Therefore, it was decided to analyse the expression of ACS and ACO in the achenes and receptacles separately. In the strawberry, one ACS (FaACS1, GenBank accession no. AY661301.1) and two ACO genes have been reported (FaACO1 and FaACO2; GenBank accession nos CAH65482 and CAH65483; Trainotti et al., 2005). Previous studies have shown that the diploid F. vesca genome contributes to the octoploid genome of the strawberry F.×ananassa (Rousseau-Gueutin et al., 2009) with an extremely high sequence identity (Bombarely et al., 2010). Using the available sequence of the wild strawberry Fragaria vesca genome (Shulaev et al., 2011), three new ACS genes (FvACS2, FvACS3, FvACS4) and one new ACO gene (FvACO3) were identified, in addition to those that have been previously reported in F.×ananassa. Therefore, the F. vesca sequences were used to design gene-specific primers for the members of the FaACS and FaACO gene families that were not previously reported in F.×ananassa.
Expression analyses using qRT–PCR were performed separately in the achenes and receptacles at three developmental stages, namely green, white, and red, based on the colour of the receptacle (Supplementary Fig. S1 at JXB online). At the green stage, the seed in the achene has reached the cotyledon stage, and the subsequent main events include preparation for the dormancy of the seed and changes associated with the ripening of the dry pericarp (Hollender et al., 2012). In the receptacle, the main change between the green and white stages is cell enlargement, and those between the white and red stages are cell wall disassembly and metabolism associated with ripening (Rose and Bennett, 1999; Brummell, 2006; Fait et al., 2008; Zhang et al., 2011).
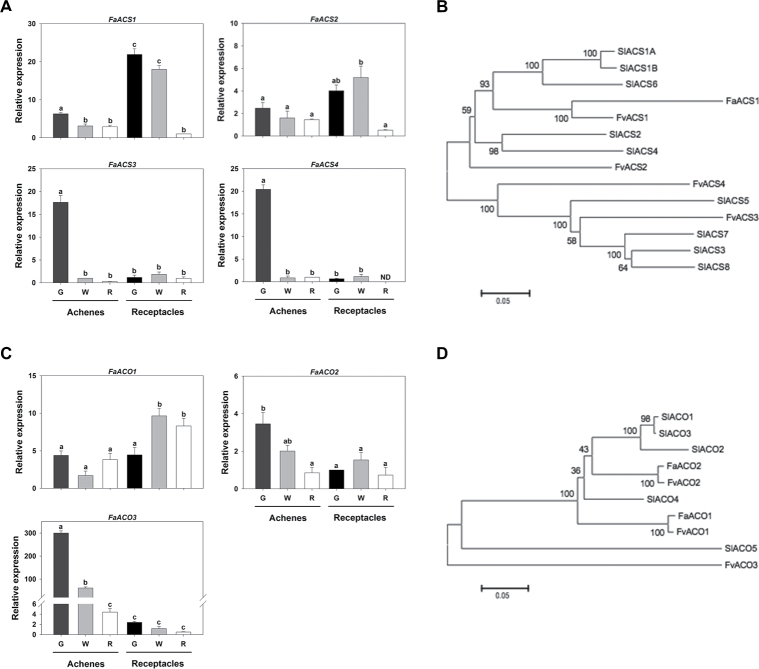
The expression of FaACS1 and FaACS2 was higher in the green and white receptacles than in any other tissue/stage, whereas the expression of FaACS3 and FaACS4 was mostly limited to the green achenes (Fig. 1A). A phylogenetic tree of the four F. vesca and F. ananassa ACS enzymes with the tomato ACS enzymes revealed that FvACS1 and FaACS1 were grouped with the tomato ACS enzymes involved in fruit ripening (SlACS1, SlACS2, SlACS4, and SlACS6) (Cara and Giovannoni, 2008). FvACS2 was also grouped, at a greater distance, with the same tomato ACS. However, FvACS3 and FvACS4 were grouped apart from the other tomato ACS proteins (Fig. 1B). Regarding the FaACO genes, the highest expression of FaACO1 occurred in the white and red receptacles, while FaACO2 and FaACO3 showed the highest expression in the green achenes (Fig. 1C). Their comparison with the corresponding tomato proteins and the generation of a phylogenetic tree showed that FaACO2 and FvACO2 grouped closely with the fruit-specific SlACO1, SlACO3, and SlACO4 (Cara and Giovannoni, 2008), while FaACO1 and FvACO1 grouped with the same tomato enzymes at a greater distance. FvACO3 was found to be less closely related to the tomato ACO proteins (Fig. 1D). Overall, the expression patterns of FaACS and FaACO are indicative of two organ-specific stages, with the highest expression values in the green achenes (FaACS3, FaACS4, and FaACO3) and the green/white receptacles (FaACS1).
Fig. 1.
Analysis of expression of the genes encoding ACC synthase (ACS) and ACC oxidase (ACO) in the strawberry Fragaria×ananassa. (A and C) Expression of ACS and ACO, as determined by qRT–PCR, in the achenes and receptacles of strawberry fruits at three developmental stages (G, green; W, white; R, red). (B and D) Unrooted phylogenetic tree of the ACS and ACO proteins of F.×ananassa (Fa), F. vesca (Fv), and Solanum lycopersicum (Sl). Different letters indicate significant differences, in achenes and receptacles, for each gene using ANOVA and the Tukey HSD test adjusted to a 95% significance level.
Strawberry plants overexpressing etr1-1 from Arabidopsis thaliana have a diminished sensitivity to ethylene
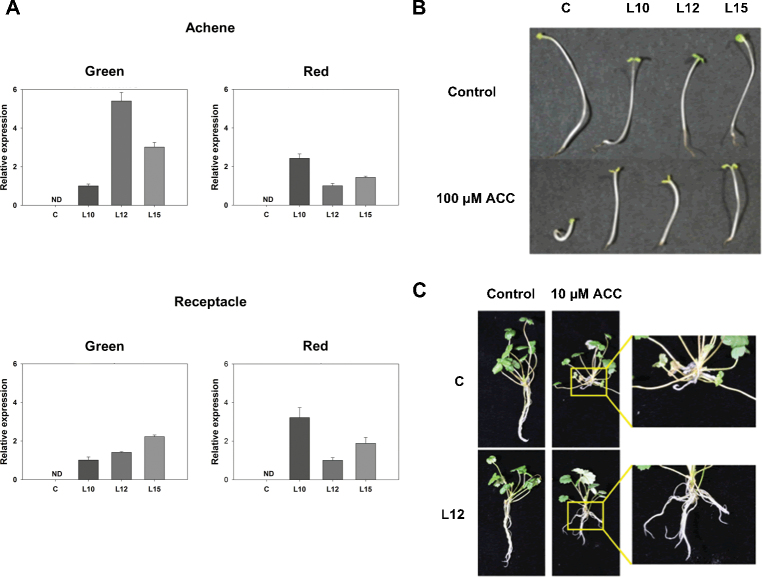
The generation of transgenic strawberry plants displaying reduced ethylene sensitivity was instrumental in this work to investigate the role of ethylene during fruit ripening. Strawberry leaf discs were transformed with a construct containing the etr1-1 gene under the control of the CaMV 35S promoter. This construct has been shown to confer ethylene insensitivity in heterologous plant species (Wilkinson et al., 1997). The leaves of 32 independent lines that were resistant to antibiotic selection were analysed for the expression of the transgene. Most of the selected lines expressed the etr1-1 gene (Supplementary Fig. S2 at JXB online). The primers were specific for the transgene. Therefore, no expression was detected in the non-transformed control plants. Three lines that displayed high expression of the transgene in the leaves were selected for further analysis (lines 10, 12, and 15). Next, it was confirmed that the transgene was expressed in the achenes and receptacles of the green and red stages using qRT–PCR. As shown in Fig. 2A, the expression of the transgene was dependent on the tissue and developmental stage, with a relatively good correlation in the expression levels between the achenes and receptacles in the three lines (Fig. 2A). It was previously reported that the 35S promoter was effective in driving gene expression in strawberry fruits (Agius et al., 2005). Here, it can be added that this promoter is functional in both the achenes and the receptacle.
Fig. 2.
Expression of the Arabidopsis etr1-1 gene in the fruits of the transgenic lines and analysis of the lower sensitivity of these lines to ethylene. (A) Relative expression of etr1-1, as determined by qRT–PCR, in the achenes and receptacles of the green and ripe stages of the transgenic lines (L10, L12, and L15) and control (C). (B) Photographs of control and etr1-1 plantlets obtained after germination of achenes in normal medium (MS) and medium supplemented with 100 μM ACC. (C) Photographs of 4-week-old in vitro control and L12 plants grown in standard (N30K) medium and medium supplemented with 10 μM ACC.
During the generation of the primary transformants, there was evidence of low sensitivity to ethylene as the transgenic lines showed improved growth in vitro compared with the control lines. This is most probably due to ethylene accumulation in the culture flask (data not shown). The effect of increasing the ethylene production in plants grown in vitro by the addition of ACC, which is readily converted to ethylene by the action of ACO, was therefore investigated. As shown in Fig. 2C, while 10 μM ACC inhibited the root growth of the in vitro control plants, the roots of the transgenic lines exhibited noticeable growth. Next, the decreased sensitivity of the transgenic lines to ethylene was investigated by observing the ethylene-mediated effects on the hypocotyl and root phenotype. As shown in Fig. 2B, the control and transgenic seedlings showed similar growth under the control conditions. However, in the presence of 100 μM ACC, while the control line showed the characteristic triple response [i.e. the inhibition of stem elongation, radial swelling of the stem, and absence of a normal geotropic response (Guzmán and Ecker, 1990)], the transgenic seedlings displayed reduced sensitivity. It is important to note that the concentration required to induce the triple response was unusually high compared with that used in other species (Smalle et al., 1997; Lohar et al., 2009).
Reduced ethylene sensitivity alters strawberry fruit ripening
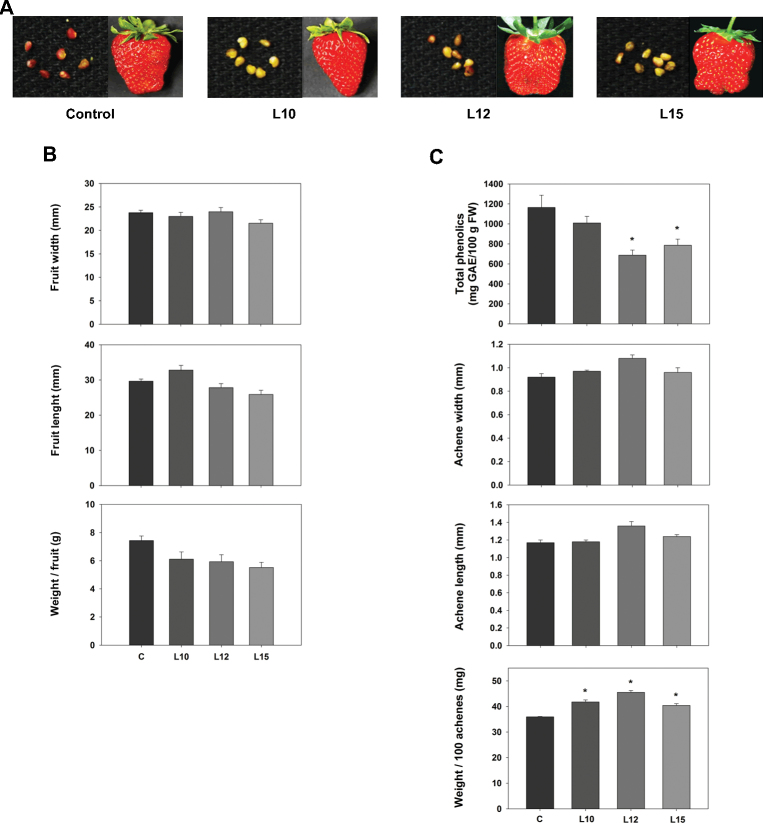
The expression of etr1-1 in the tomato and petunia causes extreme phenotypes, with tomato fruits remaining unripened for >3 months and petunias showing an extended flower life (Wilkinson et al., 1997). In contrast, the flowers and fruits produced by the three transgenic lines used here did not show any apparent visible differences in colour or size compared with the control at the developmental stages analysed (Fig. 3A). This result was corroborated by statistical analyses of the fruits in terms of width, length, and weight (Fig. 3B). However, differences in the colour of the achenes between the control and transgenic lines were found in ripe fruits. As shown in Fig. 3A, while the achenes showed a dark red colour in the control fruits, they displayed a green or light red colour in the transgenic lines. The content of phenolics was lower in these transgenic lines compared with the control, being significant in two of the lines (Fig. 3C). The achene size and weight were also determined at the fully ripe stage. The etr1-1 transformants exhibited a significantly higher weight, which was consistent across all three transgenic lines (Fig. 3C). In contrast, the achene size was unaltered (Fig. 3C).
Fig. 3.
Phenotypic analysis of the ripe fruits of the control and etr1-1 transgenic plants (L10, L12, and L15). (A) Photographs of the ripe fruits and achenes of the control and etr1-1 transgenic lines. (B) Width, length, and weight of the ripe fruits. (C) Total phenolics. Width, length, and weight of the ripe achenes. Asterisks indicate differences at a 95% significance level using ANOVA and Tukey HSD as a post-hoc test.
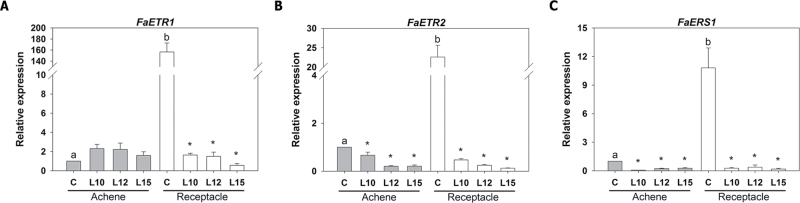
In strawberry fruits, the expression of the three ethylene receptors as well as their response to external ethylene have been studied during fruit ripening (Trainotti et al., 2005). The expression of these genes during the ripening of the achenes and receptacle was therefore analysed. The results in ripe fruits indicate that the expression of the three genes is much higher in the receptacle than in the achene, and, interestingly, they are down-regulated in the etr1-1 transgenic lines (Fig. 4A–C). This effect was dramatic in the receptacle.
Fig. 4.
Relative expression of ethylene receptor genes (FaETR1, FaETR2, and FaERS1), as determined by qRT–PCR, in the achenes and receptacles of ripe strawberry fruits. Asterisks indicate significant differences between the transgenic lines and the control for each sample using ANOVA and the Tukey HSD test adjusted to a 95% significance level. Different letters indicate significant differences within the control lines (achene and receptacle) for each gene using ANOVA and the Tukey HSD test adjusted to a 95% significance level.
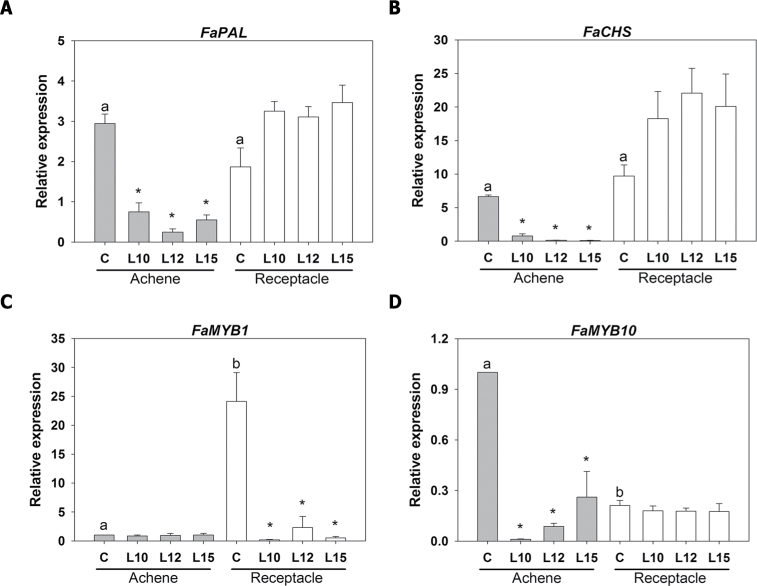
The reduced redness in colour in the transgenic achenes is probably due to the diminished synthesis of flavonoids, which constitute the group of phenylpropanoids that accumulate in this organ during ripening (Fait et al., 2008). In the biosynthesis of flavonoids, the expression of the genes encoding the key enzymes phenylalanine ammonia lyase (PAL) and chalcone synthase (CHS) plays a major role due to tight transcriptional regulation in this metabolic pathway (Salvatierra et al., 2010; Gou et al., 2011; Hichri et al., 2011; Muñoz et al., 2011). Therefore, the expression level of these genes was investigated in the control and transgenic achenes and receptacles during the red stage. As shown in Fig. 5A and B, no differences in the expression of FaPAL and FaCHS were found between the control and transgenic lines in the receptacles, which is consistent with the lack of visual differences between the control and transgenic receptacles. In fact, although no significant differences were found, a tendency for higher expression in the transgenic receptacle was observed (Fig. 5A, B). However, the expression of FaPAL and FaCHS was strongly reduced in the achenes of the transgenic fruits (Fig. 5A, B), which is consistent with their decreased pigmentation (Fig. 3A). Some genes of the MYB family have been reported to play a regulatory role in the flavonoid pathway. In the strawberry, the FaMYB1 gene is suggested to be a transcriptional repressor in the regulation of anthocyanin biosynthesis (Aharoni et al., 2001; Salvatierra et al., 2013). The study of the relative expression of this gene in the achenes and receptacle of control and etr1-1 transgenic ripe fruits showed a higher expression in the receptacle in comparison with the achene, and, as expected from its putative role in anthocyanin biosynthesis, it was significantly down-regulated in the transgenic receptacle (Fig. 5C). More recently, FaMYB10 has been positively associated with colour development in strawberry fruits (Lin-Wang et al., 2010). The analysis of the relative expression of this gene in the ripe fruits of the control and transgenic etr1-1 lines showed that its expression was down-regulated only in the transgenic achenes (Fig. 5D), whereas the expression of this gene was >3-fold higher in the receptacle. The effect of ethylene on the expression of the two genes encoding the MYB transcription factors (FaMYB1 and FaMYB10), as deduced from analysis of the etr1-1 fruits, is in agreement with the changes in the expression of the FaPAL and FaCHS genes (Fig. 5A, B) and the less coloured phenotype of the transgenic achenes (Fig. 3A).
Fig. 5.
Relative expression of strawberry genes (FaPAL, FaCHS, FaMYB1, and FaMYB1), as determined by qRT–PCR, in the achenes and receptacles of ripe strawberry fruits. Asterisks indicate significant differences between the transgenic lines and the control for each sample using ANOVA and the Tukey HSD test adjusted to a 95% significance level. Different letters indicate significant differences within the control lines (achene and receptacle) for each gene using ANOVA and the Tukey HSD test adjusted to a 95% significance level.
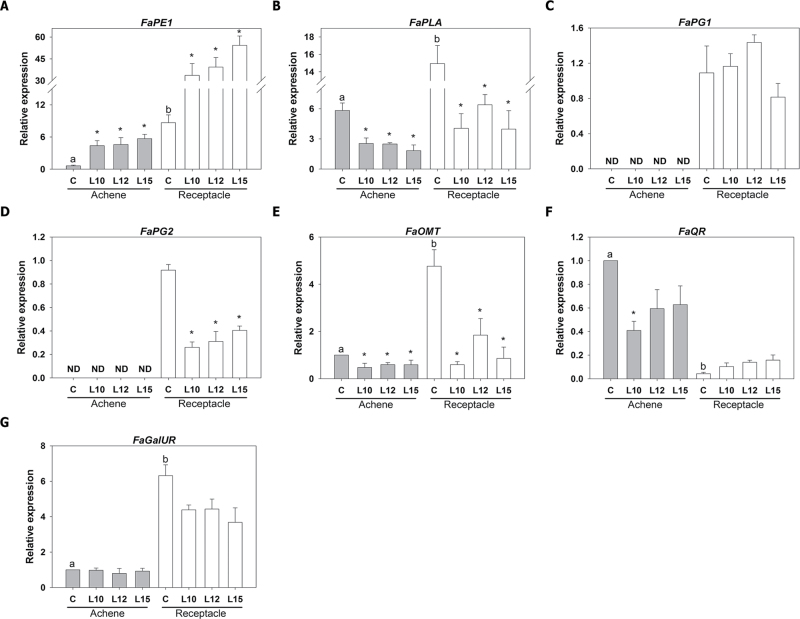
A number of genes involved in cell wall restructuring and disassembly are canonically associated with fruit ripening (Rose and Bennett, 1999; Brummell, 2006). Therefore, to investigate the effect of altering the ethylene sensitivity on cell wall metabolism, the expression level of three cell wall-related genes was analysed in the achenes and receptacles of ripe fruits. The genes pectin methyl esterase 1 (FaPE1) (Castillejo et al., 2004), pectate lyase A (FaPLA) (Benitez-Burraco et al., 2003), and polygalacturonase 1 and 2 (FaPG1 and FaPG2) (Quesada et al., 2009) were selected. These genes have been shown to play important roles in the ripening of strawberry fruit through functional analyses (Osorio et al., 2008; Santiago-Domenech et al, 2008; Quesada et al., 2009). All of these pectin-depolymerizing genes showed higher expression in the receptacle than in the achenes in the control fruits, but ethylene was found to affect their expression in the two organs (Fig. 6A–D). FaPE1 expression was enhanced in both the achenes and receptacle in the three transgenic lines (Fig. 6A), which is in agreement with its previously reported down-regulation by ethylene and induction by 1-MCP, an inhibitor of ethylene action (Castillejo et al., 2004). In contrast, the diminished ethylene sensitivity of the transgenic lines caused a decrease in the expression of FaPLA in both the achenes and receptacles, indicating that this gene is positively regulated by ethylene. Interestingly, the FaPG2 transcript was also down-regulated in the receptacle in the three transgenic lines (Fig. 6D), but no changes were observed in FaPG1 expression (Fig. 6C). It has been described that these two PG genes are up-regulated during fruit ripening, but their expression patterns and cell wall targets are different (Quesada et al., 2009). Given the different expression levels of the cell wall-related genes observed in the transgenic fruits, the firmness was next determined in ripe control and etr1-1 fruits. Non-significant differences in firmness were observed (38.2±0.9, 37.1±0.8, 36.8±0.9, and 38.1±0.7g mm–2 in the control, L10, L12, and L15, respectively; values are the means ±SE).
Fig. 6.
Relative expression of strawberry genes (FaPE1, FaPLA, FaPG1, FaPG2, FaOMT, FaQR, and FaGalUR), as determined by qRT–PCR, in the achenes and receptacles of ripe strawberry fruits. Asterisks indicate significant differences between the transgenic lines and the control for each sample using ANOVA and the Tukey HSD test adjusted to a 95% significance level. Different letters indicate significant differences within the control lines (achene and receptacle) for each gene using ANOVA and the Tukey HSD test adjusted to a 95% significance level.
The ripening of strawberry fruit is accompanied by the production of volatile compounds that account for the aroma of the fruit. Among these, furaneol is known to be important due to its high production by ripe fruits and its low odour threshold, quinone oxidoreductase (FaQR) being a key enzyme in its production (Raab et al., 2006). Analysis of the expression of the FaQR gene in the control and transgenic etr1-1 lines showed a non-significant decrease in the expression of FaQR in the achenes as a result of ethylene insensitivity (Fig. 6F), and the expression of this gene was significantly lower in the receptacle in all cases. Mesifurane is another important contributor to strawberry fruit aroma, which is controlled by O-methyl transferase (FaOMT) (Lunkenbein et al., 2006; Zorrilla-Fontanesi et al., 2012). Transcript analysis showed that the expression of FaOMT was down-regulated in the transgenic etr1-1 lines compared with the control in both the achenes and receptacle (Fig. 6E). Additionally, the expression levels of the gene encoding d-galacturonate reductase (FaGalUR), which is involved in ascorbic acid biosynthesis, were not significantly different at the red stage in the transgenic lines in comparison with the control (Fig. 6G).
Specific metabolites are differentially regulated in the achenes and receptacle of the etr1-1 strawberry fruits
To investigate further the role of ethylene in fruit ripening, metabolic profiling of the primary metabolites was performed using GC-MS (Schauer et al., 2006). The achenes and receptacles of the control and the three transgenic lines were analysed at the green, white, and red ripening stages (Supplementary Fig. S1 at JXB online).
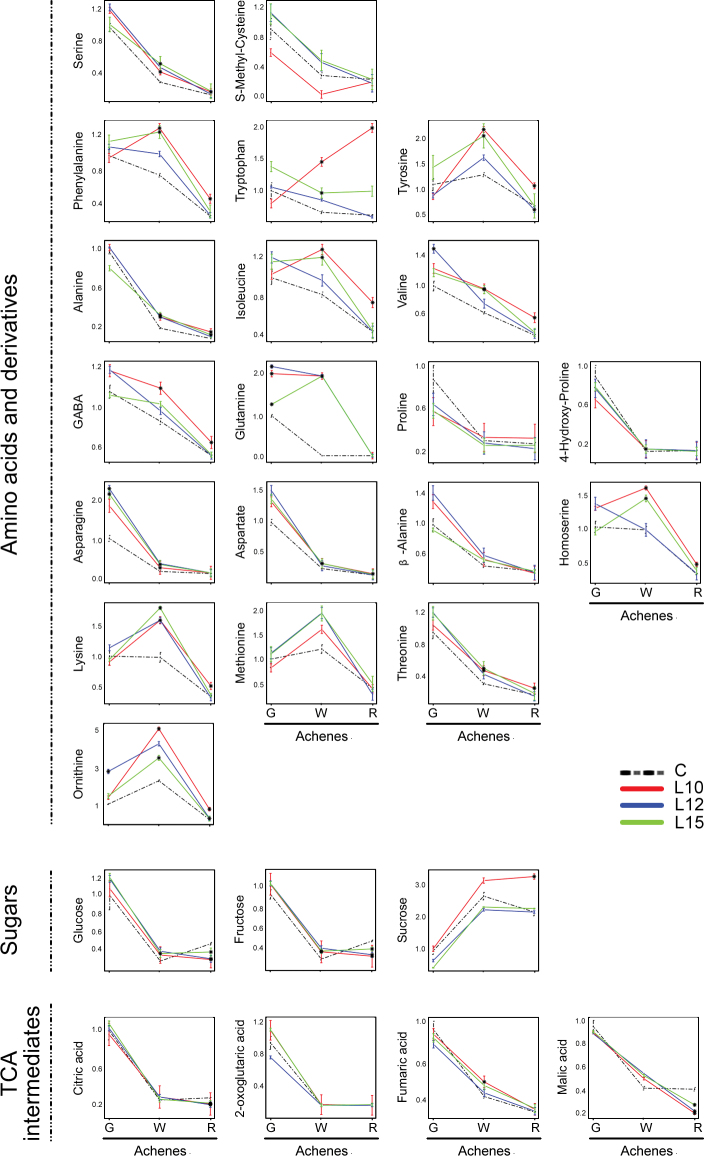
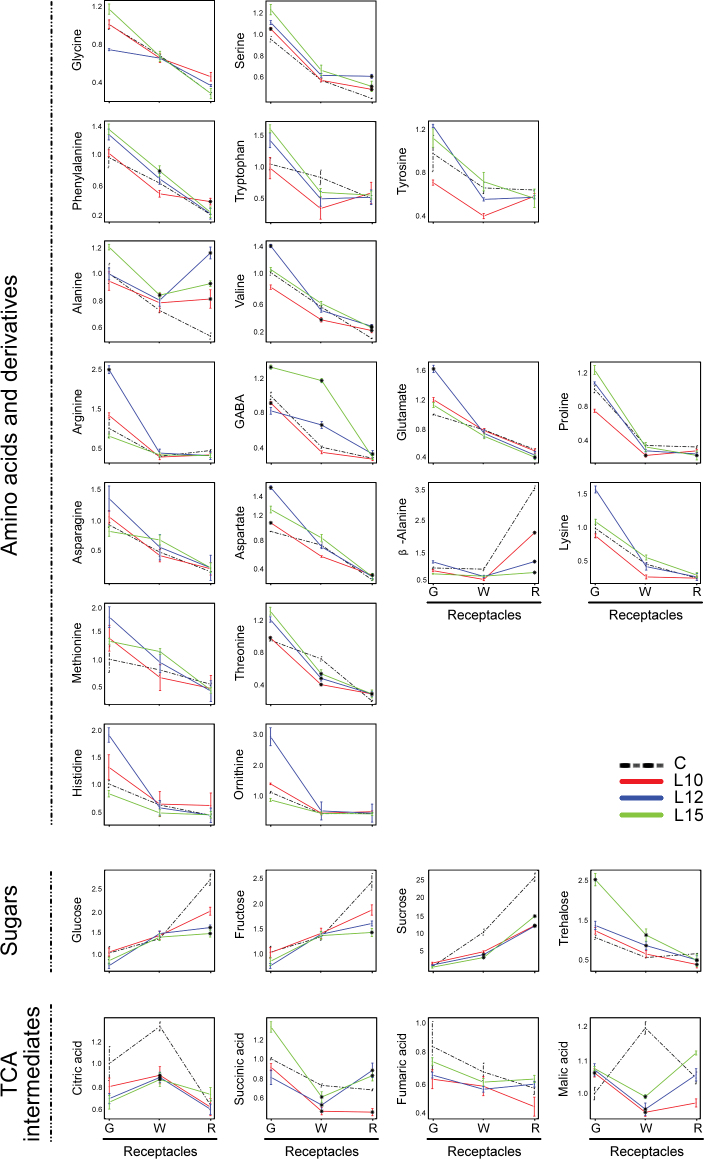
In the control achenes, the amino acid levels generally declined during ripening, as has been previously described (Fait et al., 2008). While this pattern was conserved in the transgenic lines, it is important to note that most of the free amino acids were at higher levels in the achenes of the three transgenic lines than in the control (Fig. 7). Such differences were particularly prominent at the green stage for the N-rich amino acids asparagine and glutamine (three lines) and extended to most of the other amino acids in the white stage and, in some cases, also in the red stage. There are some amino acids that showed even higher accumulation from the green stage to the white stage in the transgenic achenes compared with the control. These include the two aromatic amino acids phenylalanine and tyrosine (L10 and L15), and the basic N-rich amino acids lysine and ornithine (L10 and L15).
Fig. 7.
Primary metabolite levels in the achenes of the control (C) and etr1-1 transgenic lines (L10, L12, and L15) at the three developmental stages (G, green; W, white; R, red). Data, calculated as described in the Materials and Methods, are relative to the value obtained for the control at the G stage. Values are the means ±SE of three replicates. Asterisks indicate significant differences (t-test, P < 0.01) between the transgenic lines and the control at the same developmental stage.
The sugar and organic acid contents in the achenes were not altered in the transgenic lines compared with the control at the green stage. However, a decrease was observed in the levels of citric acid and malic acid at the red stage (all lines; Fig. 7). Similarly, all lines showed a decrease in the levels of the major sugars glucose and fructose at the red stage. Additionally, the etr1-1 lines displayed an increase in the levels of maltose and trehalose at the red stage (lines 10 and 15; Fig. 7)
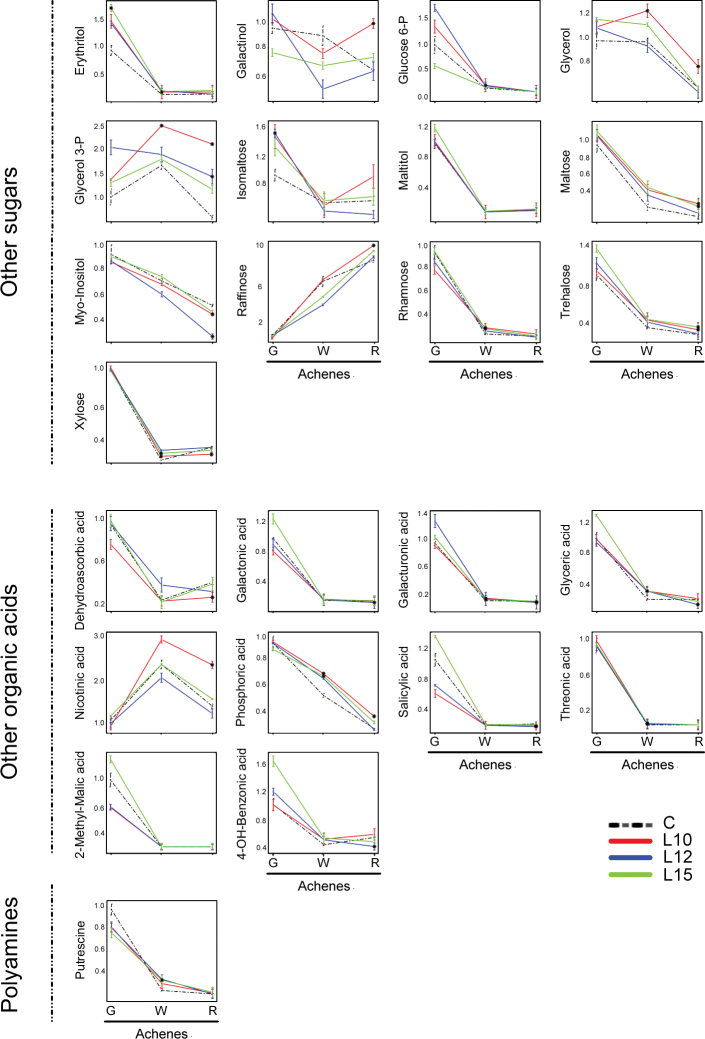
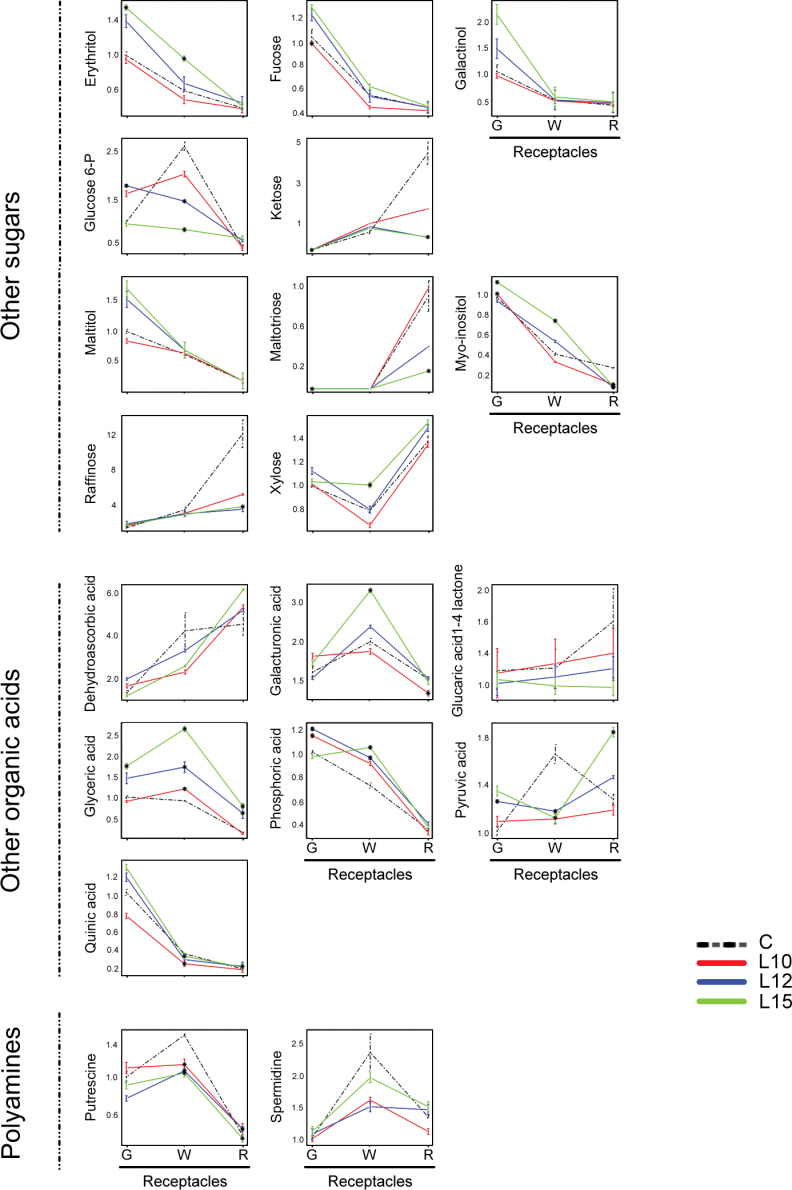
In the receptacle, the general analysis of the amino acid contents revealed that significant differences between the control and the three transgenic lines were rather limited, with only certain amino acids affected and only at specific stages (Fig. 8). A decrease in the threonine content was observed at the white stage (all three lines) and an increase at the red stage (all three lines). Similarly, an increase in the levels of alanine, valine, and serine (all three lines) was observed (Fig. 8).
Fig. 8.
Primary metabolite levels in the receptacles of the control (C) and etr1-1 transgenic lines (L10, L12, and L15) at the three developmental stages (G, green; W, white; R, red). Data, calculated as described in the Materials and Methods, are relative to the value obtained for the control at the G stage Values are the means ±SE of three replicates. Asterisks indicate significant differences (t-test, P < 0.01) between the transgenic lines and the control at the same developmental stage.
Differences in the sugar and organic acid contents between the transgenic and control receptacles were also found at the white and red stages (Fig. 8). At the white stage, a significant reduction in the tricarboxylic acid (TCA) cylce intermediates citric acid, succinic acid, and malic acid (all lines) was observed compared with the control. This reduction was accompanied by a significant decrease in the most important sugars (glucose, fructose, sucrose, and trehalose) at the red stage (Fig. 8). The metabolic analysis also showed that the transgenic receptacles were characterized by decreases in glucose-6P (L12 and L15), pyruvic acid (all lines), and putrescine (all lines) at the white stage, as well as decreased levels of β-alanine at the red stage (all lines) (Fig. 8).
Correlation analysis of metabolites in the achenes and receptacles
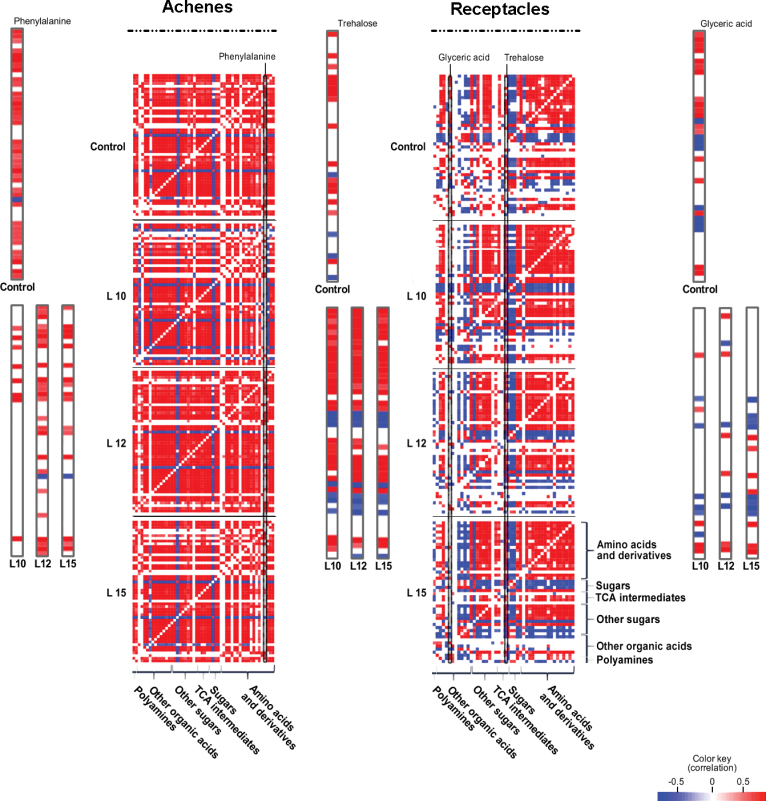
Next the coordinated metabolic changes were identified by performing a pair-wise correlation analysis using Pearson’s correlation at a strict stringency threshold (P < 0.05; values in Supplementary Tables S2–S9 at JXB online). This analysis reflects the degree of coordination of the metabolic changes in the corresponding organ. Here, this analysis was performed in the achenes and receptacles of each transgenic line and the control to identify any interference in the correlations caused by the diminished sensitivity to ethylene of the transgenic lines. However, it is important to note that any conclusions drawn from these data must be made cautiously, given the low number of time points used in this study. In general, it was observed that the total number of correlations was higher in the achenes than in the receptacles (Fig. 9). The number of correlations in the achenes was 963 in the control, and 933, 1030, and 897 correlations were observed in L10, L12, and L15, respectively. In the receptacle, 559 correlations were observed in the control, and 638, 558, and 650 were observed in L10, L12, and L15, respectively. The results for the control plants were anticipated because they agree with previous reports (Fait et al., 2008). However, it is interesting that the transgenic lines displayed very similar coordination. Focusing on specific changes associated with the transformation event revealed some interesting changes. For example, in the achenes, the most conspicuous change was the loss of the positive correlation of phenylalanine with most of the metabolites in the transgenic lines compared with the control (Fig. 9). This is most probably due to the down-regulation of FaPAL in the transgenic lines (Fig. 5A). Regarding the receptacles, there were two dramatic changes in the transgenic lines (Fig. 9). The first is the loss of the strong positive correlation of glyceric acid with the majority of the amino acids in the transgenic lines. This is probably due to the dramatically enhanced content of 3C-derived amino acids (serine, alanine, and valine) in the transgenic lines. Secondly, trehalose was negatively correlated with glucose, fructose, sucrose, fumaric acid, and glucose-6P, and was positively correlated with amino acids in the transgenic lines. This observation is in keeping with the main role hypothesized for this disaccharide as a result of changes in carbon allocation between the sink and source tissues (Eveland and Jackson, 2012). However, given that trehalose-6-phosphate has been proposed as a signalling molecule whose metabolism is regulated by sucrose (Ma et al., 2011), it cannot be disregarded that it mediates the changes observed in the sucrose content in the transgenic receptacles.
Fig. 9.
Visualization of metabolite–metabolite correlations. Heat maps of the metabolite–metabolite correlations across ripening stages (G, W, and R) for the achenes (A) and receptacles (B) of the control and transgenic lines are shown. The metabolites are grouped by compound class, and each square represents the correlation between the metabolite heading the column and the metabolite heading the row. Correlation coefficients and significances were calculated by applying the Pearson algorithm using R-environment. Here, only the significant correlations are presented (P < 0.05). Positive and negative correlations are presented in red and blue, respectively.
Discussion
The strawberry fruit has traditionally been considered to be non-climacteric because its ripening process does not follow the characteristic ethylene production and respiration of the so-called climacteric fruits (Given et al., 1988). However, changes in the endogenous production of ethylene, albeit very small, have been reported in the intact fruit (Iannetta et al., 2006). In addition, there are several reports showing that some molecular responses associated with fruit development are altered by treatment with ethylene, ethylene precursors, or ethylene inhibitors (Trainotti et al., 2001; Castillejo et al., 2004; Bustamante et al., 2009: Villareal et al., 2009, 2010). These data suggest a role for ethylene in fruit development and/or ripening. However, there are several aspects of the strawberry fruit that must be taken into consideration. First, the strawberry fruit is composed of two organs that are very different in terms of origin, physiological role, and prevailing metabolic networks; these organs are the achenes, which are the true fruits, and the receptacles, which are derived from the flower receptacles (Fait et al., 2008; Csukasi et al., 2011). Analysis of the complete berry, which includes both parts, makes it difficult to interpret the results obtained due to the heterogeneity of the sample. Secondly, the relative contribution of each organ to the whole berry is highly dependent on the developmental stage of the fruit. While the relative contribution of the achenes to the biomass of the whole fruit is high in green fruits, this contribution is considerably smaller in red fruits (see Supplementary Fig. S1 at JXB online). A third consideration is the inherent difficulty in measuring the independent endogenous production of ethylene in the achenes and receptacle, which is exacerbated by the low level of internal production of this hormone by the strawberry fruit (Iannetta et al., 2006). Therefore, the strategy used here for overcoming these issues was to generate transgenic lines with reduced sensitivity to endogenous ethylene and to perform separate analyses of the achenes and receptacles. These aims were achieved by overexpressing the mutant Arabidopsis allele etr1-1, as exhaustive studies on the function and interaction of the various ethylene receptors in Arabidopsis have demonstrated that the dominant mutant receptor gene etr1-1 prevents the ethylene response independently of other wild-type receptors (Liu and Wen, 2012). This means that the transgenic strawberry etr1-1 lines are partially insensitive to ethylene, which was apparent from its phenotype (Fig. 2), despite the occurrence of other active endogenous ethylene receptors. Interestingly, the endogenous ethylene receptors were down-regulated in the transgenic lines, which agrees with the positive response to ethylene treatment reported for these three genes in ripe fruits (Trainotti et al., 2005).
Full ripening of the achene requires ethylene action
The expression of some of the key genes involved in ethylene biosynthesis (i.e. FaACS3, FaACS4, and FaACO3) was highest in the green achenes. A previous proteomic study in achenes identified S-adenosylmethionine synthetase as one of the most abundant proteins in green achenes (Aragüez et al., 2013). This enzyme is involved in the biosynthetic ethylene pathway (Yang and Hoffman, 1984). All of this information suggests that ethylene production is important in the achene at this stage, a hypothesis that is supported by the changes in gene expression and metabolites that were observed when the ethylene action was reduced in the etr1-1 transgenic plants. This stage corresponds to fruits at 12 d post-anthesis, at which time the embryo is in the cotyledon stage and the dry pericarp of the achene is developing. This is consistent with the role proposed for ethylene during early seed development (Lombardo et al., 2011). Later developmental changes are dominated by the function of the mature achenes as dispersal units, namely lignification, the synthesis of secondary defence metabolites, and the accumulation of storage compounds (Aharoni and O’Connell, 2002; Fait et al., 2008). The key genes involved in the biosynthesis of phenolics and flavonoids, FaPAL and FaCHS, showed reduced expression in the etr1-1 achenes, indicating a regulatory role for ethylene in the synthesis of these compounds. Consistent with this, a visible outcome was the diminution of coloured flavonoids in the mature achene, which also showed a lower content of phenolics. The fact that regulatory FaMYB10 is significantly down-regulated in transgenic achenes indicates that this gene might be involved in the ethylene signalling pathway in this organ.
There were significant changes in the levels of the primary metabolites. The sharp decline in the free amino acid content, which characterizes the ripening of the achenes (Fait et al., 2008), was somewhat altered in the three transgenic lines; the free amino acid level was higher than in the control. This difference might result from the impaired synthesis of storage proteins in the transgenic achenes. The higher accumulation of phenylalanine and tyrosine in the white achenes of the transgenic lines was most probably due to the diminished synthesis of phenylpropanoids, which was caused by the down-regulation of FaPAL and FaCHS. There is also a significant increase of lysine in transgenic achenes at the white stage. Since this amino acid is in the pathway leading to the synthesis of ethylene, this might be indicative of an altered ethylene biosynthetic pathway in transgenic achenes. When analysing the correlations between metabolites, the positive correlation between phenylalanine and most of the analysed metabolites was absent in the transgenic lines. Ethylene was also important for normal energy metabolism in the achenes. Thus, the effects of diminished sensitivity to this hormone were also evident in the red stage, which exhibited lower levels of sugars, such as glucose, frructose, and sucrose, as well as TCA cycle intermediates, such as citric acid and malic acid. The late stages of achene maturation are characterized by a depletion in the metabolites of the major energy pathways (Fait et al., 2008), an event that was enhanced in the etr1-1 achenes.
Taken together, the present results demonstrate that ethylene is involved, either directly or indirectly, in the developmental changes that occur in both the early and late developmental stages of the achenes. Its involvement in the ripening of the achenes appears critical for phenylpropanoid metabolism and strawberry aroma production. However, the presence of a dry pericarp reveals that the major changes that take place during the ripening of this organ, such as the accumulation of storage and protective compounds, are not comparable with those of fruits with a fleshy pericarp. Therefore, despite the fact that the achene is a true fruit, given the low endogenous production of ethylene by the whole fruit (Iannetta et al., 2006) and its lack of involvement in some changes that are commonly associated with the ripening of fleshy fruits (Seymour, 1993), it cannot be considered a canonical climacteric fruit.
Ripening of the receptacle partially resembles the climacteric ripening of fleshy fruits
Although the fleshy part of the strawberry is not a true fruit because it does not develop from the ovary and is the result of the enlargement of the flower receptacle, it is important for seed dispersal and human consumption. This functional evolutionary convergence with true fleshy fruits reflects that, despite some differences, changes in the receptacle during ripening, such as those related to cell wall disassembly as well as primary and secondary metabolism, are similar to those of true fruits (Castillejo et al., 2004; Rosli et al., 2004; Fait et al., 2008; Quesada et al., 2009; Osorio et al., 2011).
The highest expression of FaACS1 occurred at the green/white stage, when cell expansion is very active and just before the changes associated with the ripening of this tissue are triggered. In the tomato, the sequential expression of the members of the ACS and ACO gene families during fruit development has been reported (Cara and Giovannoni, 2008; Yokotani et al., 2009). Some of these genes are associated with the early production of ethylene (SlACS1a and SlACS6), while others are associated with the climacteric production of this hormone (SlACS2 and SlACS4). Interestingly, the protein sequence analysis showed the grouping of FaACS1 with SlACS2 and SlACS4, indicating a parallelism between ripening in the strawberry receptacle and the tomato fruit.
Changing the ethylene sensitivity resulted in considerable changes in the levels of the ethylene receptor genes FaETR1, FaETR2, and FaERS1, whose expression in the control fruits was higher in the receptacle than in the achenes. Previous studies in the tomato showed a significant down-regulation of transcripts related to ethylene perception in the nor (non-ripening) mutant, in which ripening is almost completely inhibited (Osorio et al., 2011). Additionally, in the receptacle, changes in the expression of some pectin-related genes were observed. The involvement of these genes in cell wall disassembly at the ripe fruit stage has been previously reported (Jiménez-Bermúdez et al., 2002; Castillejo et al., 2004; Quesada et al., 2009). It was found that FaPE1 was up-regulated while FaPLA was down-regulated in the transgenic receptacles. Interestingly, the peaks of highest expression for these two genes during strawberry development and ripening are not coincident (Benitez-Burraco et al., 2003; Castillejo et al., 2004). This might explain the opposite effects for these two genes in the transgenic receptacle in this study, which resulted from nullifying/mitigating the action of endogenous ethylene, which was produced at specific developmental stages. In addition, the expression of FaPG2 but not FaPG1 was altered in the transgenic receptacles, indicating that ethylene does not have a general effect on all ripening-associated genes. This explains why no effect was found on fruit firmness, which is controlled by the coordinated action of all cell wall-degrading enzymes.
The metabolic programme associated with receptacle ripening is also regulated by ethylene. Decreasing ethylene perception caused a lower content of TCA cycle intermediates, including citric acid, succinic acid, and malic acid, at the white stage. It is possible that this is a consequence of the down-regulation of the alternative respiratory pathway because this pathway has demonstrated sensitivity to changes in ethylene in other plant systems (Wang et al., 2010). The lower content of TCA cycle intermediates at the white stage is accompanied by a higher content of some amino acids, such as serine, phenylalanine, and alanine, and a diminished content of the major sugars fructose, glucose, and sucrose in the red transgenic receptacle compared with the control. Interestingly, similar changes in sugars and TCA cycle intermediates have also been observed during the ripening of two ripening mutants of the tomato, nor and Nr, in comparison with the wild-type fruit (Osorio et al., 2011). The Nr gene mutation results in the expression of an ethylene receptor with an impaired ability to bind ethylene (Lanahan et al., 2009), while the nor mutation has been suggested to be involved in ethylene biosynthesis (Tigchelaar et al., 1973). These orchestrated metabolic changes found in the strawberry receptacle are expected due to the connectivity reported between the different metabolic pathways in the ripening receptacle (Fait et al., 2008). Analysis of correlations among metabolites in the receptacles identified specific changes that were common to all transgenic lines in comparison with the control. Independently of their metabolic significance, which deserves further study, these changes reveal a role for ethylene in the metabolism of the ripening receptacle. This role most probably extends to the production of strawberry aroma compounds. Although the production of volatiles was not evaluated in the transgenic receptacles, the decreased expression of FaOMT supports a change in mesifurane production. It has been reported that natural variation of mesifurane is associated with the expression of the FaOMT gene (Zorrilla-Fontanesi et al., 2012).
The analysis of mutants that are affected by ethylene signalling has been a useful tool to investigate ethylene-dependent or ethylene-independent processes during tomato ripening at the systems biology level (Osorio et al., 2011). In the strawberry, there are no previous reports on the occurrence of mutants in ethylene synthesis and/or signalling. However, the transformation of the strawberry with the ethylene receptor allele etr1-1 and the subsequent dominant-negative effect in the ethylene perception have allowed for the examination of the role of ethylene in strawberry fruit ripening. The data presented here indicate that ethylene is required for the normal development of the strawberry fruit, in which it acts differently in the achenes and receptacle. In achenes, it acts at the green and red stages, while in the receptacle it acts at the green/white stages. In both organs, ethylene selectively affects the expression of genes involved in ethylene reception, phenylpropanoid metabolism, cell wall degradation, and strawberry aroma production. Further dissection of its differential regulatory role in the two organs would require a more exhaustive time and cell type analysis. However, the strawberry fruits cannot be considered as canonical climacteric fruits. Finally, this study supports the need to study the growth and ripening process of these two strawberry fruit components independently to answer key questions, such as the role of hormones. The two organs follow parallel yet distinctive developmental programmes (Fait et al., 2008; Csukasi, 2012), and their connectivity at early developmental stages has been known for a long time (Nitsch, 1950). However, this relationship remains to be further explored during ripening. Such an exploration must be extended to the role played by other hormones, such as abscisic acid (ABA), whose involvement in strawberry fruit ripening has recently been reported (Chai et al., 2011; Jia et al., 2011). This result is particularly interesting due to the connections that were previously established between ethylene and ABA signalling. For example, in grape berry ripening, a sequential action has been proposed for ethylene and ABA in the regulation of the ripening process (Ziliotto et al., 2012). More interestingly, in the promoter of the FaOMT gene, which was found here to be down-regulated upon diminution of ethylene sensitivity, an ABA response element was identified (Zorrilla-Fontanesi et al., 2012).
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Photographs of fruits at the three studied ripening stages (G, green; W, white; R, red). Cross-sections of the receptacles at these three stages and of the achenes at the green stage are shown.
Figure S2. Screening of transgenic lines expressing the etr1-1 gene under the control of the CaMV 35S promoter.
Table S1. List of the primers used in the qRT–PCRs.
Table S2. Pearson correlation coefficients between the metabolite contents in the three ripening stages of control strawberry achenes.
Table S3. Pearson correlation coefficients between the metabolite contents in the three ripening stages of L10 strawberry achenes.
Table S4. Pearson correlation coefficients between the metabolite contents in the three ripening stages of L12 strawberry achenes.
Table S5. Pearson correlation coefficients between the metabolite contents in the three ripening stages of L15 strawberry achenes.
Table S6. Pearson correlation coefficients between the metabolite contents in the three ripening stages of control strawberry receptacles.
Table S7. Pearson correlation coefficients between the metabolite contents in the three ripening stages of L10 strawberry receptacles.
Table S8. Pearson correlation coefficients between the metabolite contents in the three ripening stages of L12 strawberry receptacles.
Table S9. Pearson correlation coefficients between the metabolite contents in the three ripening stages of L15 strawberry receptacles.
Acknowledgements
We thank Dr Harry Klee for providing the pCD-2 plasmid with the construct 35S-etr1-1. We are grateful to the Churriana-IFAPA Center (Málaga, Spain) for granting access to the greenhouse facilities. This work was supported by the Ministerio de Ciencia e Innovación (MICINN, Spain) [grant nos BIO2010-15630 and AR2009-0004]. CM was supported by a FPI fellowship from Junta de Andalucía (Spain), and IA by a FPU fellowship (MICINN, Spain). SO and NME acknowledge the support by Ministerio de Ciencia e Innovación, Spain (Ramón and Cajal contract).
References
- Agius F, Amaya I, Botella MA, Valpuesta V. 2005. Functional analysis of homologous and heterologous promoters in strawberry fruits using transient expression. Journal of Experimental Botany 56, 37–46 [DOI] [PubMed] [Google Scholar]
- Aharoni A, O’Connell AP. 2002. Gene expression analysis of strawberry achene and receptacle maturation using DNA microarrays. Journal of Experimental Botany 53, 2073–2087 [DOI] [PubMed] [Google Scholar]
- Aharoni A, De Vos CHR, Wein M, Sun Z, Greco R, Kroon A, Mol JNM, O’Connell AP. 2001. The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. The Plant Journal 28, 319–332 [DOI] [PubMed] [Google Scholar]
- Aragüez I, Cruz-Rus E, Botella MA, Medina-Escobar N, Valpuesta V. 2013. Proteomic analysis of strawberry achenes reveals active synthesis and recycling ofl-ascorbic acid. Journal of Proteomics 83, 160–179 [DOI] [PubMed] [Google Scholar]
- Benítez-Burraco A, Blanco-Portales R, Redondo-Nevado J, Bellido ML, Moyano E, Caballero JL, Munoz-Blanco J. 2003. Cloning and characterization of two ripening-related strawberry (Fragaria×ananassa cv. Chandler) pectate lyase genes. Journal of Experimental Botany 54, 633–645 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H. 2000. Ethylene: a gaseous signal molecule in plants. Annual Review of Cell and Developmental Biololgy 16, 1–18 [DOI] [PubMed] [Google Scholar]
- Bombarely A, Merchante C, Csukasi F, et al. 2010. Generation and analysis of ESTs from strawberry (Fragaria×ananassa) fruits and evaluation of their utility in genetic and molecular studies. BMC Genomics 11, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA. 2006. Cell wall disassembly in ripening fruit. Functional Plant Biology 33, 103–119 [DOI] [PubMed] [Google Scholar]
- Bustamante CA, Civello PM, Martinez GA. 2009. Cloning of the promoter region of beta-xylosidase (FaXyl1) gene and effect of plant growth regulators on the expression of FaXyl1 in strawberry fruit. Plant Science 177, 49–56 [Google Scholar]
- Cara B, Giovannoni JJ. 2008. Molecular biology of ethylene during tomato fruit development and maturation. Plant Science 175, 106–111 [Google Scholar]
- Castillejo C, de la Fuente JI, Iannetta P, Botella MA, Valpuesta V. 2004. Pectin esterase gene family in strawberry fruit: study of FaPE1, a ripening-specific isoform. Journal of Experimental Botany 55, 909–918 [DOI] [PubMed] [Google Scholar]
- Chai Y-M, Jia H-F, Li C-L, Dong Q-H, Shen Y-Y. 2011. FaPYR1 is involved in strawberry fruit ripening. Journal of Experimental Botany 62, 5079–5089 [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. 1993. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262, 539–544 [DOI] [PubMed] [Google Scholar]
- Civello PM, Powell A, Sabehat A, Bennett AB. 1999. An expansin gene expressed in ripening strawberry fruit. Plant Physiology 121, 1273–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csukasi F, Donaire L, Casañal A, Martínez-Priego L, Botella MA, Medina-Escobar N, Llave C, Valpuesta V. 2012. Two strawberry miR159 family members display developmental-specific expression patterns in the fruit receptacle and cooperatively regulate Fa-GAMYB. New Phytologist 195, 47–57 [DOI] [PubMed] [Google Scholar]
- Csukasi F, Osorio S, Gutierrez JR, et al. 2011. Gibberellin biosynthesis and signalling during development of the strawberry receptacle. New Phytology 191, 376–390 [DOI] [PubMed] [Google Scholar]
- de la Fuente JI, Amaya I, Castillejo C, Sanchez-Sevilla JF, Quesada MA, Botella MA, Valpuesta V. 2006. The strawberry gene FaGAST affects plant growth through inhibition of cell elongation. Journal of Experimental Botany 57, 2401–2411 [DOI] [PubMed] [Google Scholar]
- El-Mansouri I, Mercado JA, Valpuesta V, López-Aranda JM, Pliego-Alfaro F, Quesada MA. 1996. Shoot regeneration and Agrobacterium-mediated transformation of Fragaria vesca L. Plant Cell Reports 15, 642–646 [DOI] [PubMed] [Google Scholar]
- Eveland AL, Jackson DP. 2012. Sugars, signalling, and plant development. Journal of Experimental Botany 63, 3367–3377 [DOI] [PubMed] [Google Scholar]
- Fait A, Hanhineva K, Beleggia R, Dai N, Rogachev I, Nikiforova VJ, Fernie AR, Aharoni A. 2008. Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant Physiology 148, 730–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampieri F, Tulipani S, Alvarez-Suarez JM, Quiles JL, Mezzetti B, Battino M. 2012. The strawberry: composition, nutritional quality, and impact on human health. Nutrition 28, 9–19 [DOI] [PubMed] [Google Scholar]
- Giovannoni JJ. 2004. Genetic regulation of fruit development and ripening. The Plant Cell 16 (Suppl), S170–S180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni JJ. 2007. Fruit ripening mutants yield insights into ripening control. Current Opinion in Plant Biology 10, 283–289 [DOI] [PubMed] [Google Scholar]
- Given NK, Venis MA, Grierson D. 1988. Hormonal-regulation of ripening in the strawberry, a non-climateric fruit. Planta 174, 402–406 [DOI] [PubMed] [Google Scholar]
- Gou JY, Felippes FF, Liu CJ, Weigel D, Wangb JW. 2011. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. The Plant Cell 23, 1512–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. 1990. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell 2, 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hichri I, Barrieu F, Bogs J, Kappel C, Delrot S, Lauvergeat V. 2011. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. Journal of Experimental Botany 62, 2465–2483 [DOI] [PubMed] [Google Scholar]
- Hollender C, Geretz A, Slovin J, Liu Z. 2012. Flower and early fruit development in a diploid strawberry, Fragaria vesca. Planta 235, 1123–1139 [DOI] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. 1996. R: a language for data analysis and graphics. Journal of Computational and Graphical Statistics 5, 299–314 [Google Scholar]
- Iannetta PPM, Laarhovenb LJ, Medina-Escobar N, James EK, McManuse MT, Davies HV, Harren FJM. 2006. Ethylene and carbon dioxide production by developing strawberries show a correlative pattern that is indicative of ripening climacteric fruit. Physiologia Plantarum 127, 247–259 [Google Scholar]
- Itkin M, Seybold H, Breitel D, Rogachev I, Meir S, Aharoni A. 2009. Tomato AGAMOUS-Like 1 is a component of the fruit ripening regulatory network. The Plant Journal 60, 1081–1095 [DOI] [PubMed] [Google Scholar]
- Jia H-F, Chai Y-M, Li C-L, Lu D, Luo J-J, Qin L, Shen Y-Y. 2011. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiology 157, 188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Bermúdez S, Redondo-Nevado J, Muñoz-Blanco J, Caballero JL, López-Aranda JM, Valpuesta V, Pliego-Alfaro F, Quesada MA, Mercado JA. 2002. Manipulation of strawberry fruit softening by antisense expression of a pectate lyase gene. Plant Physiology 128, 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyoshihara Y, Iwata M, Fukaya T, Tatsuki M, Mori H. 2010. Turnover of LeACS2, a wound-inducible 1-aminocyclopropane-1-carboxylic acid synthase in tomato, is regulated by phosphorylation/dephosphorylation. The Plant Journal 64, 140–150 [DOI] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ. 2011. Genetics and control of tomato fruit ripening and quality attributes. Annual Review of Genetics 45, 41–59 [DOI] [PubMed] [Google Scholar]
- Knoester M, van Loon LC, van den Heuvel J, Hennig J, Bol JF, Linthorst HJM. 2005. Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proceedings of the National Academy of Sciences, USA 95, 1933–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopka J, Schauer N, Krueger S, et al. 2005. GMD@CSB. DB: the Golm Metabolome Database. Bioinformatics 21, 1635–1638 [DOI] [PubMed] [Google Scholar]
- Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. 1994. The never ripe mutation blocks ethylene perception in tomato. The Plant Cell 6, 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem YY, Pinchasov Y. 2000. Non-invasive photoacoustic spectroscopic determination of relative endogenous nitric oxide and ethylene content stoichiometry during the ripening of strawberries Fragaria anannasa (Duch.) and avocados Persea americana (Mill.). Journal of Experimental Botany 51, 1471–1473 [PubMed] [Google Scholar]
- Lin Z, Zhong S, Grierson D. 2009. Recent advances in ethylene research. Journal of Experimental Botany 60, 3311–3336 [DOI] [PubMed] [Google Scholar]
- Lin-Wang K, Bolitho K, Grafton K, Kortstee A, Karunairetnam S, McGhie TK, Espley RV, Hellens RP, Allan AC. 2010. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biology 10, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wen CK. 2012. Arabidopsis ETR1 and ERS1 differentially repress the ethylene response in combination with other ethylene receptor genes. Plant Physiology 158, 1193–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohar D, Stiller J, Kam J, Stacey G, Gresshoff PM. 2009. Ethylene insensitivity conferred by a mutated Arabidopsis ethylene receptor gene alters nodulation in transgenic Lotus japonicus . Annals of Botany 104, 277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo VA, Osorio S, Borsani J, Lauxmann MA, Bustamante CA, Budde CO, Andreo CS, Lara MV, Fernie AR, Drincovich MF. 2011. Metabolic profiling during peach fruit development and ripening reveals the metabolic networks that underpin each developmental stage. Plant Physiology 157, 1696–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunkenbein S, Salentijn EMJ, Coiner HA, Boone MJ, Krens FA, Schwab W. 2006. Up- and down-regulation of Fragaria×ananassa O-methyltransferase: impacts on furanone and phenylpropanoid metabolism. Journal Experimental Botany 57, 2445–2453 [DOI] [PubMed] [Google Scholar]
- Ma J, Hanssen M, Lundgren K, et al. 2011. The sucrose-regulated Arabidopsis transcription factor bZIP11 reprograms metabolism and regulates trehalose metabolism. New Phytologist 191, 733–745 [DOI] [PubMed] [Google Scholar]
- Medina-Escobar N, Cárdena J, Valpuesta V, Muñoz-Blanco J, Caballero JL. 1997. Cloning and characterization of cDNAs from genes differentially expressed during the strawberry fruit ripening process by a MAST-PCR-SBDS method. Analytical Biochemistry 248, 288–296 [DOI] [PubMed] [Google Scholar]
- Muñoz C, Sanchez-Sevilla JF, Botella MA, Hoffmann T, Schwab W, Valpuesta V. 2011. Polyphenol composition in the ripe fruits of Fragaria species and transcriptional analyses of key genes in the pathway. Journal of Agricultural and Food Chemistry 59, 12598–12604 [DOI] [PubMed] [Google Scholar]
- Nitsch J. 1950. Growth and morphogenesis of the strawberry as related to auxin. American Journal of Botany 37, 211–215 [Google Scholar]
- Opazo MC, Figueroa CR, Henriquez J, Herrera R, Bruno C, Valenzuela PD, Moya-Leon MA. 2010. Characterization of two divergent cDNAs enconding xyloglucan endotransglycosylase/hydrolase (XTH) expressed in Fragaria chiloensis fruit. Plant Science 179, 479–488 [DOI] [PubMed] [Google Scholar]
- Osorio S, Alba R, Damasceno CM, et al. 2011. Systems biology of tomato fruit development: combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiology 157, 405–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio S, Alba R, Nikoloski Z, Kochevenko A, Fernie AR, Giovannoni JJ. 2012. Integrative comparative analyses of transcript and metabolite profiles from pepper and tomato ripening and development stages uncovers species-specific patterns of network regulatory behavior. Plant Physiology 159, 1713–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio S, Castillejo C, Quesada MA, Medina-Escobar N, Brownsey G, Suau R, Heredia A, Botella MA, Valpuesta V. 2008. Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defense responses in wild strawberry (Fragaria vesca). The Plant Journal 54, 43–55 [DOI] [PubMed] [Google Scholar]
- Perkins-Veazie P. 1995. Growth and ripening of strawberry fruit. Horticultural Reviews 17, 267–297 [Google Scholar]
- Perkins-Veazie PM, Huber DJ, Brecht JK. 1996. In vitro growth and ripening of strawberry fruit in presence of ACC, STS or propylene. Annals of Applied Biology 128, 105–116 [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada MA, Blanco-Portales R, Posé S, García-Gago JA, Jiménez-Bermúdez S, Muñoz-Serrano A, Caballero JL, Pliego-Alfaro F, Mercado JA, Muñoz-Blanco J. 2009. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiology 150, 1022–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab T, López-Ráez JA, Klein D, Caballero JL, Moyano E, Schwab W, Muñoz-Blanco J. 2006. FaQR, required for the biosynthesis of the strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone, encodes an enone oxidoreductase. The Plant Cell 18, 1023–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Bennett AB. 1999. Cooperative disassembly of the cellulose-xyloglucan network of plant cell walls: parallels between cell expansion and fruit ripening. Trends in Plant Science 2, 176–183 [DOI] [PubMed] [Google Scholar]
- Rosli HG, Civello PM, Martínez GA. 2004. Changes in cell wall composition of three Fragaria×ananassa cultivars with different softening rate during ripening. Plant Physiology and Biochemistry 42, 823–831 [DOI] [PubMed] [Google Scholar]
- Rousseau-Gueutin M, Gaston A, Aïnouche A, Aïnouche ML, Olbricht K, Staudt G, Richard L, Denoyes-Rothan B. 2009. Tracking the evolutionary history of polyploidy in Fragaria L. (strawberry): new insights from phylogenetic analyses of low-copy nuclear genes. Molecular Phylogenetics and Evolution 51, 515–530 [DOI] [PubMed] [Google Scholar]
- Salvatierra A, Pimentel P, Moya-Leon MA, Caligari PDS, Herrera R. 2010. Comparison of transcriptional profiles of flavonoid genes and anthocyanin contents during fruit development of two botanical forms of Fragaria chiloensis ssp. chiloensis. Phytochemistry 71, 1839–1847 [DOI] [PubMed] [Google Scholar]
- Salvatierra A, Pimentel P, Moya-Leon MA, Herrera R. 2013. Increased accumulation of anthocyanins in Fragaria chiloensis fruits by transient suppression of FcMYB1 gene. Phytochemistry 90, 25–36 [DOI] [PubMed] [Google Scholar]
- Santiago-Doménech N, Jiménez-Bermúdez S, Matas AJ, Rose JKC, Muñoz-Blanco J, Mercado JA, Quesada MA. 2008. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. Journal of Experimental Botany 59, 2769–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer N, Semel Y, Roessner U, et al. 2006. Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nature Biotechnology 24, 447–454 [DOI] [PubMed] [Google Scholar]
- Seeram NP. 2008. Berry fruits for cancer prevention: current status and future prospects. Journal of Agricultural and Food Chemistry 56, 630–635 [DOI] [PubMed] [Google Scholar]
- Seymour GB. 1993. Biochemistry of fruit ripening. London: Chapman & Hall, 1–45 [Google Scholar]
- Shulaev V, Sargent DJ, Crowhurst RN, et al. 2011. The genome of woodland strawberry (Fragaria vesca). Nature Genetics 43, 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, van Montagu N, van der Straeten D. 1997. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proceedings of the National Academy of Sciences, USA 94, 2756–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28, 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigchelaar EC, Tome M, Kerr EA, Barman RJ. 1973. A new fruit ripening mutant, non-ripening (nor). Report of the Tomato Genetics Cooperative 23, 33–34 [Google Scholar]
- Trainotti L, Pavanello A, Casadoro G. 2005. Different ethylene receptors show an increased expression during the ripening of strawberries: does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? Journal of Experimental Botany 56, 2037–2046 [DOI] [PubMed] [Google Scholar]
- Trainotti L, Spinello R, Piovan A, Spolaore S, Casadoro G. 2001. β-Galactosidases with a lectin-like domain are expressed in strawberry. Journal of Experimental Botany 52, 1635–1645 [PubMed] [Google Scholar]
- Vahala J, Ruonala R, Keinanen M, Tuominen H, Kangasjarvi J. 2003. Ethylene insensitivity modulates ozone-induced cell death in birch. Plant Physiology 132, 185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal NM, Bustamante CA, Civello PM, Martínez GA. 2010. Effect of ethylene and 1-MCP treatments on strawberry fruit ripening. Journal of the Science of Food and Agriculture 90, 683–689 [DOI] [PubMed] [Google Scholar]
- Villarreal NM, Martínez GA, Civello PM. 2009. Influence of plant growth regulators on polygalacturonase expression in strawberry fruit. Plant Science 176, 749–757 [Google Scholar]
- Wang H, Liang X, Huang J, Zhang D, Lu H, Liu Z, Bi Y. 2010. Involvement of ethylene and hydrogen peroxide in induction of alternative respiratory pathway in salt-treated Arabidopsis calluses. Plant and Cell Physiology 51, 1754–1765 [DOI] [PubMed] [Google Scholar]
- Waterhouse AL. 2002. Determination of total phenolics. In: Wrolstad RE, ed. Current protocols in food analytical chemistry. New York: John Wiley & Sons, 1.1.1–11.1.8 [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ. 1997. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nature Biotechnology 15, 444–447 [DOI] [PubMed] [Google Scholar]
- Yang S, Hoffman N. 1984. Ethylene biosynthesis and its regulation in higher plants. Annual Review of Plant Physiology 35, 155–189 [Google Scholar]
- Yokotani N, Nakano R, Imanishi S, Nagata M, Inaba A, Kubo Y. 2009. Ripening-associated ethylene biosynthesis in tomato fruit is autocatalytically and developmentally regulated. Journal of Experimental Botany 60, 3433–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang X, Yu O, Tang J, Gu X, Wan X, Fang C. 2011. Metabolic profiling of strawberry (Fragaria×ananassa Duch.) during fruit development and maturation. Journal of Experimental Botany 62, 1103–1118 [DOI] [PubMed] [Google Scholar]
- Ziliotto F, Corso M, Rizzini FM, Rasori A, Botton A, Bonghi C. 2012. Grape berry ripening delay induced by a pre-véraison NAA treatment is paralleled by a shift in the expression pattern of auxin- and ethylene-related genes. BMC Plant Biology 12, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla-Fontanesi Y, Rambla JL, Cabezaa A, Medina JJ, Sánchez-Sevilla JF, Valpuesta V, Botella MA, Granell A, Amaya I. 2012. Genetic analysis of strawberry fruit aroma and identification of O-methyltransferase FaOMT as the locus controlling natural variation in mesifurane content. Plant Physiology 159, 851–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.