Abstract
The bacterium Chlamydia trachomatis and the protozoan parasite Toxoplasma gondii are the causative agents of chlamydiosis and toxoplasmosis in humans, respectively. Both micro-organisms are obligate intracellular pathogens and notorious for extensively modifying the cytoskeletal architecture and the endomembrane system of their host cells to establish productive infections. This review highlights the similar tactics developed by these two pathogens to manipulate their host cell despite their genetic unrelatedness. By using an in vitro cell culture model whereby single fibroblasts are infected by C. trachomatis and T. gondii simultaneously, thus setting up an intracellular competition, we demonstrate that the solutions to the problem of intracellular survival deployed by the parasite and the bacterium may represent an example of convergent evolution, driven by the necessity to acquire nutrients in a hostile environment.
Introduction
Obligate intracellular pathogens represent a subset of consummate microbes typified by an overarching requirement for growth within an eukaryotic cell (reviewed in Casadevall, 2008; Kumar & Valdivia, 2009). Inherently to their intracellular residence and strategies to remain inside their host cells, these microbes induce damage to the host, either inadvertently or as a necessary condition for replication and transmission to a new host. As such, many obligate intracellular parasites, bacteria and viruses are indeed responsible for a significant negative impact on worldwide human health by causing debilitating or fatal disease. Among them, the apicomplexan parasite Toxoplasma gondii is one of the most successful parasites on earth; it is globally spread, affecting up to one-third of the human population. In healthy individuals, toxoplasmosis is usually asymptomatic and self-limiting, with the parasite remaining encysted during the whole lifetime of the host. However, Toxoplasma can cause severe and life-threatening disease (e.g., encephalitis, necrotizing retinochoroiditis, myocarditis) in immunocompromised individuals (Luft & Remington, 1992). In addition, Toxoplasma infection in pregnant women causes serious brain defects of the fetus (e.g., hydrocephalus, blindness). In the prokaryotic domain, Chlamydia trachomatis is widely distributed, affects ten percent of the human population and represents a major infectious cause of human genital (e.g., cervitis, urethritis) and eye disease (e.g., conjunctivitis, blindness) worldwide (Schachter, 1999). Table 1 summarizes commonalities and differences between T. gondii and C. trachomatis.
Table 1.
Comparative features of Toxoplasma gondii and Chlamydia trachomatis
| T. gondii | C. trachomatis | |
|---|---|---|
| Domain | Eukaryota | Bacteria |
| Potential hosts | All blood-warmed animals | Humans |
| Disease | Encephalitis, retinochoroiditis, blindness, abortion, hydrocephalus |
Trachoma, blindness, cervicitis, reactive arthritis |
| Infection | Ingestion of tissue cysts (bradyzoites) or oocysts (sporozoites) |
Sexual or eye contacts with reticulate bodies |
| Pathogen size | 2 × 7µm | 1 × 1µm |
| Parasitism | Obligate intracellular | Obligate intracellular |
| Mode of entry | Active invasion | Phagocytic-like event (elementary bodies) |
| Intracellular compartment | Parasitophorous vacuole | Inclusion |
| Residence in the body | Brain and muscle cells | Epithelial cells |
| Multiplication | Endodyogeny of tachyzoites (intestine) and bradyzoites (brain) |
Binary fission of reticulate bodies |
| Cycle | Lytic | Lytic |
| Stress response | Shift to tissue cyst | Persistence to elementary bodies |
| Lipids scavenged | Cholesterol, sphingolipids | Cholesterol, sphingolipids |
| Host structures recruited | Cytoskeleton, MTOC, ER, Golgi, mitochondria, endo-lysosomes, autophagosomes |
Cytoskeleton, MTOC, Golgi, endo-lysosomes, autophagosomes lipid bodies |
The luxury of an obligate intracellular life with its bountiful access to host resources and protection from immune confrontations comes, however, with a price. Survival within a host cell requires physiological adaptations of the microbe to the host. In fact, the strategies of intracellular pathogens are constantly co-evolving with their respective eukaryotic hosts over hundreds of millions of years. While adaptation and specialization increase the microbe’s fitness for interacting with the host, it can limit the ability of the microbe to thrive outside of a cell (Pallen & Wren, 2007). For instance, during evolution, the genomes of obligate intracellular pathogens have undergone dynamic remodeling, including the massive loss of genes that encode for entire metabolic pathways (Fraser-Liggett, 2005). As a part of their obligatory intracellular lifestyle, both T. gondii and C. trachomatis have undergone a massive condensation of their genome, compared to their non-parasitic relatives. The genome of T. gondii contains ~8,000 predicted genes while to the genome of Colpodellids, the free-living ancestors of Toxoplasma (Kuvardina et al., 2002), harbors at least 40,000 genes. Likewise, the genome of C. trachomatis totals only ~900 genes compared to ~2,000 present in the genome of environmental chlamydiae (Horn et al., 2004). For the two pathogens, loss of genes results in reduced central biosynthetic pathways. T. gondii and C. trachomatis possess only ~800 and ~200 genes coding for predicted metabolic enzymes respectively, compared to ~1,900 genes in humans (www.ToxoDB.org; Stephens et al., 1998; Dean et al., 2006). In return, genes promoting nutrient scavenging have expanded, and these genetic replacements have become indispensable for the pathogen to meet its basic metabolic requirements. Patho-adaptation to an obligatory lifestyle carries the danger that the loss of self, in the form of irreversible genome decay, may lead to extinction if the micro-organism narrows the cell types it invades and its subcellular replication niches; as it increases its reliance on host cell resources, the microbe ultimately links its own survival to the continued existence of its host (Andersson & Kurland, 1998). Thus, both T. gondii and C. trachomatis have become auxotrophic with maximal nutritional needs. As such, they have acquired redundant compensatory mechanisms to import nucleotides, sugars, amino acids, lipids, and several co-factors from the host cell. Proportionally to the number of genes, T. gondii contains three times more genes with similarity to known transporters or proteins with transporter-like properties than humans (820 transporter genes/8,000 total protein-coding genes in T. gondii vs. 1,000 transporter genes/30,000 total protein-coding genes in H. sapiens). Similarly, C. trachomatis harbors an extensive collection of transporters (summarized in Saka & Valdivia, 2010).
To access pools of host-derived nutrients, obligate intracellular pathogens must sculpt the intracellular environment of the host to their advantage. This situation is particularly challenging for pathogens that reside within a membrane-bound compartment in their host cell, which separates them from pools of soluble molecules present in the host cytosol and organelles. Despite this restriction, intravacuolar pathogens, such as T. gondii and C. trachomatis, covertly co-opt host resources to gain access to the needed nutrients by attracting host organelles to their vacuole or redirecting host transport vesicles (reviewed in Laliberté & Carruthers, 2008; Saka & Valdivia, 2010). Remarkably, T. gondii and C. trachomatis not only share a patho-adapted genome, but also operate in a similar way to manipulate the host cell and hijack organelles for their own benefit. Despite differences in their phylogenetic origin and their physico-chemical properties of their vacuolar compartments, both pathogens subvert host cytoskeleton elements, hijack the microtubule-organizing center (MTOC), attract endocytic and exocytic organelles, re-route Rab GTPases vesicle to their vacuole and engulf cytoplasmic organelles into their vacuoles (Table 1). In the first part of this review, we will summarize the extensive interactions that occur between host cell structures and the parasitophorous vacuole (PV) of Toxoplasma or the vacuole of C. trachomatis, termed the inclusion, and highlight the similar subversive tactics used by the two pathogens. In Figures 1 and 2, we illustrate the host cell interactions mediated by T. gondii and C. trachomatis, respectively. To evaluate the important contribution of host organelles to the intracellular development of T. gondii and C. trachomatis, and therefore the necessity for these pathogens to recruit host organelles to their vacuoles, we have established an in vitro cell culture model whereby single fibroblasts are infected by the two pathogens simultaneously (Romano et al., 2012; 2013a). In a co-infection system, the success and failure of an infection established by a pathogen depends on the skills of that pathogen to adhere to its normal developmental program. Since the parasite and the bacterium usurp the same host organelles, their presence in the same cell would lead to a severe competition for nutrients. In the second part of the review, we will collate our studies using the co-infection model and emphasize the unique opportunities provided by the dual infection system to evaluate the (in)compatibility of T. gondii and C. trachomatis and to gain fundamental knowledge on each of these pathogens. New aspects on the intracellular parasitism of C. trachomatis and T. gondii revealed by our co-infection assays will be discussed.
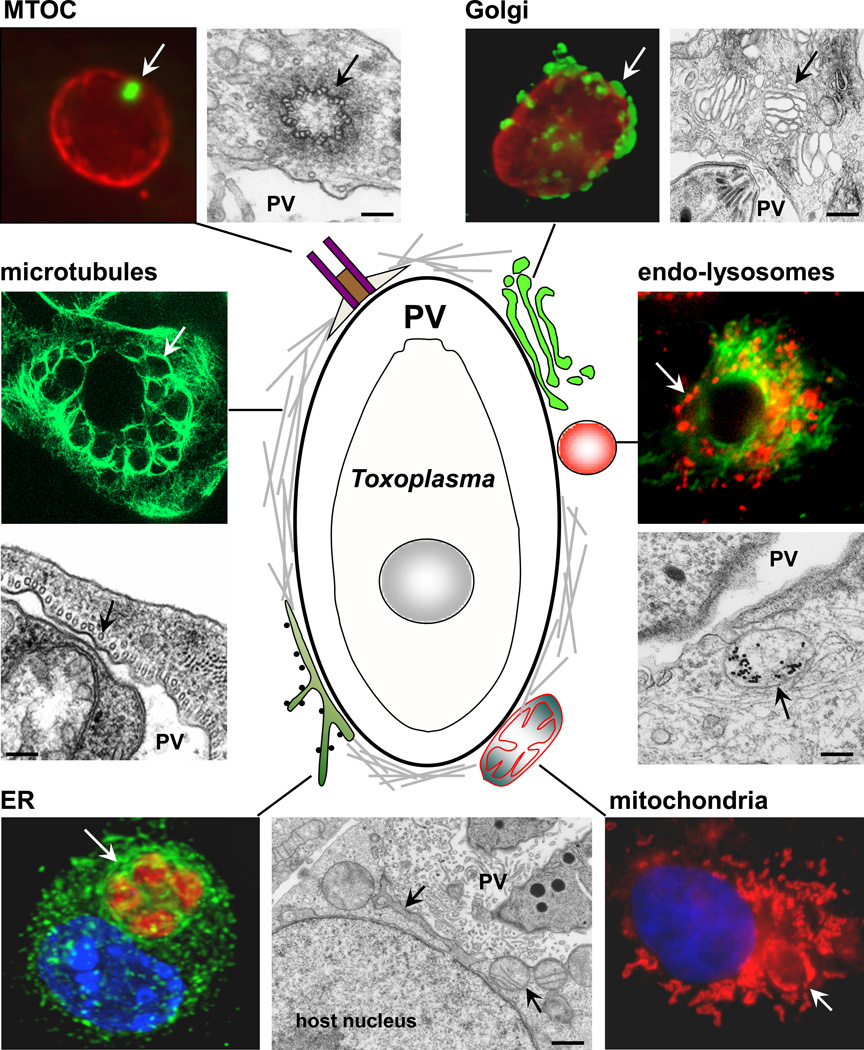
Figure 1. Model for the interaction of Toxoplasma gondii with host cells.
A schematic representation of the PV and the host cell structures that are recruited by the parasite is shown. These host cell-PV interactions are illustrated by immunofluorescence and electron microscopy images. Host structures are indicated by an arrow. The following antibodies and dyes used for staining are: anti-γ-tubulin for MTOC (green) and anti-GRA7 for the PV membrane (red); anti-giantin for Golgi (green) and anti-GRA7 (red); anti-α-tubulin for microtubules in a multi-infected cell; anti-calnexin for ER, anti-SAG1 for the parasite plasma membrane and DAPI for nuclei; mito-Tracker for mitochondria and DAPI; Texas-red EGF (red) for endo-lysosomes and anti-α-tubulin (green). EM picture shows an endocytic organelle containing LDL-gold particles. Scale bars are 150 nm.
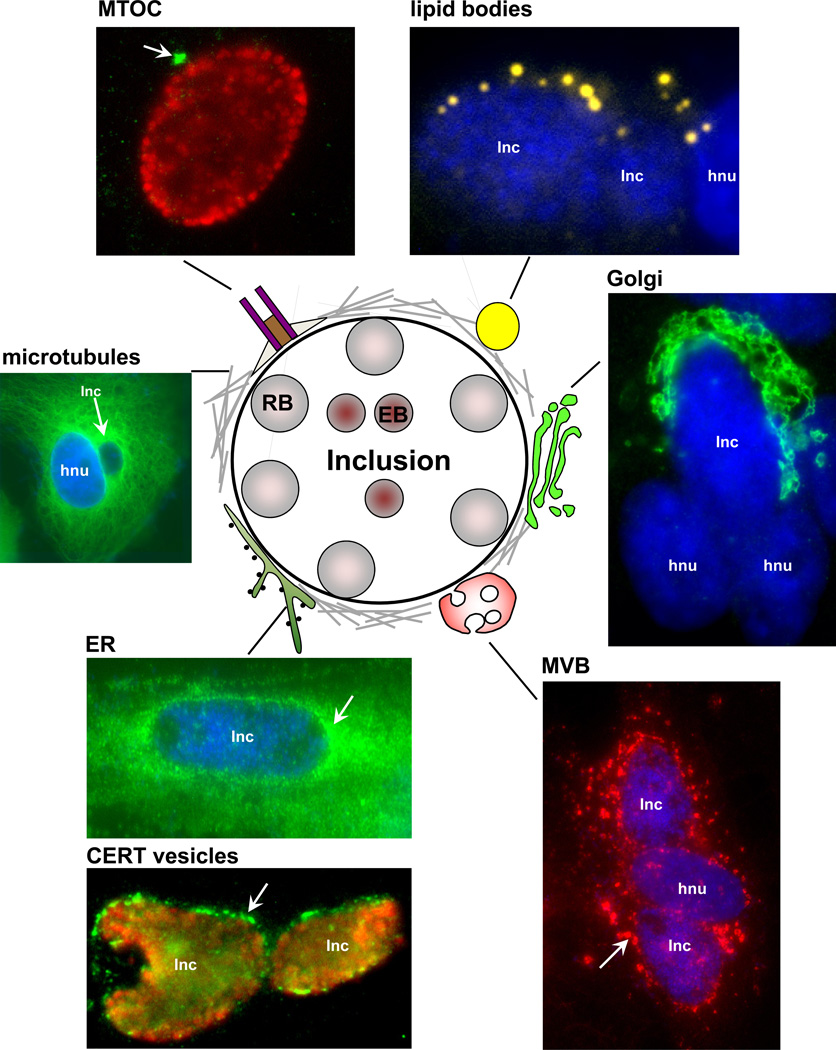
Figure 2. Model for the interaction of Chlamydia trachomatis with host cells.
A schematic representation of an inclusion and host cell structures that are recruited by the bacterium is shown. These host cell-inclusion interactions are illustrated by immunofluorescence images. Host structures are indicated by an arrow. The following antibodies and dyes used for staining are: anti-γ-tubulin for MTOC (green) and anti-EF-Tu for Chlamydia (red); Nile Red for lipid bodies (yellow) and DAPI; anti-giantin for Golgi (green) and DAPI; anti-α-tubulin for microtubules and DAPI; anti-calnexin for ER and DAPI; anti-CERT for ER-derived vesicles (green) and anti-EF-Tu (red); anti-CD-63 for multivesicular bodies (red) and DAPI; Nile Red for lipid bodies (yellow) and DAPI. Inc, inclusion; hnu, host nucleus.
Part I: Commonalities between Toxoplasma gondii and Chlamydia trachomatis in Host Cell Interactions and Nutrient Acquisition
Host cytoskeleton reorganization and MTOC capture
The initial phases of invasion by T. gondii and C. trachomatis differ completely from one another but both result in the formation of a specialized pathogen-containing vacuole whose the limiting membrane is derived from the host plasma membrane. Toxoplasma can invade any nucleated mammalian cell, irrespective of the cell type or host origin (Sibley, 2003). The parasite penetrates into mammalian cells in a rapid process called active invasion lasting about one minute (Carruthers & Boothroyd, 2007). Upon contact with the host cell surface, the parasite secretes proteins that induce the formation of a host cell-parasite junction (Aikawa et al., 1978; Besteiro et al., 2011) that mediates the contact between the parasite and the host cell plasma membranes. This structure is pulled toward the posterior end of the parasite as the latter invades the cell and forms the PV. As it forms its vacuole, the parasite excludes most host plasma membrane transmembrane proteins from the PV membrane and incorporates its own proteins into this membrane, creating a compartment unrecognizable by host endo-lysosomal organelles (Mordue, 1999; Charron and Sibley, 2004; Cesbron-Delauw et al 2008; Nam, 2009). In contrast, C. trachomatis invades predominantly epithelial cells and macrophages. The invasive form of C. trachomatis (or the elementary body, EB) is internalized into a phagosomal compartment in mammalian cells within ~10 min, in a process driven by both the host cell and the bacterium (Dautry-Varsat et al 2005). Comparatively, the bacterium disengages its inclusion from host degradative pathways by eliminating host proteins from the inclusion membrane and adding chlamydial proteins (reviewed in Fields and Hackstadt, 2002).
Regardless of the mechanism of microbial entry, the cortical host cell cytoskeleton, especially actin filaments, represents a physical and mechanical barrier for the entry of any intruder. At their site of entry, T. gondii and C. trachomatis actively induces a local re-organization of the cortical actin network to facilitate their penetration though using distinct mechanisms and specific effectors (Gonzalez et al., 2009; Carabeo et al., 2002). Upon invasion, Toxoplasma secretes a protein, toxofilin, from anterior organelles termed rhoptries, into the host cytoplasm at the entry site. Toxofilin binds and severs host actin filaments, thus aiding the parasite’s penetration into the host cell (Delorme-Walker et al., 2012). Afterwards, the parasite induces the formation of a stable ring-shaped F-actin structure at the parasite-cell junction, and recruits the Arp2/3 complex, an actin-nucleating factor essential for the formation of many F-actin-based structures, to the ring structure to provide a solid anchor for pulling the parasite inside the cell (Gonzalez et al., 2009). As opposed to the T. gondii situation, de novo polymerization of host actin is important for the entry of C. trachomatis. Within minutes upon attachment to the host cell, the bacterium transiently recruits actin to the site of invasion leading to the formation of an actin-rich pedestal underneath the attachment site. Chlamydial and host cell actin nucleators cooperate to increase the rate of actin filament formation for C. trachomatis internalization. Using a type III secretion system, C. trachomatis injects into the host cytoplasm a translocated actin recruiting phosphoprotein (Tarp) that, upon phosphorylation by the Abelson kinase (Abl kinase), associates with globular actin to nucleate the formation of linear actin filaments (Jewett et al., 2006; Elwell et al., 2008; Jiwani et al., 2012; 2013). Phosphorylated Tarp is implicated in the GTPase mediated activation of the host cell Arp2/3 complex (Lane et al., 2008). Binding of C. trachomatis to the host platelet Derived Growth Factor Receptor β (PDGFRβ) at the cell surface results in the activation of both PDGFRβ and Abl kinase signaling pathways, leading to the phosphorylation of VAv2, a Rac guanine nucleotide exchange factor, and the actin nucleators WAVE2 and cortactin, which are two activators of the Arp2/3 complex (Elwell et al., 2008).
The replicative cycle of C. trachomatis is initiated by the conversion of the EBs to reticulate bodies (RBs) that undergo successive binary fissions. During replication, the bacterium continues to remodel the host actin cytoskeleton and recruits intermediate filaments (Kumar and Valdivia, 2008a; 2008b). It encases its inclusion in a network of F-actin and intermediate filaments that act cooperatively to stabilize the pathogen-containing vacuole. Recruitment of F-actin at the inclusion is dependent on host RhoA, a regulator of the actin cytoskeleton that forms stress fibers. Disruption of RhoA leads to the loss of inclusion integrity, resulting in the leakage of the inclusion’s content into the host cytosol, and consequently the activation of cytoplasmic innate immune surveillance pathways (Buchholz and Stephens, 2008). Chlamydia also specifically modifies vimentin filaments around the inclusion by secreting the chlamydial proteasome like activity factor (CPAF). This protease cleaves the head domain of vimentin, which is essential for filament assembly, thus allowing for the expansion of the inclusion through the removal of the physical constraints imposed by these static cytoskeletal structures on the growing inclusion. Unlike Chlamydia, there is no evidence to date that Toxoplasma remodels the host actin cytoskeleton post-invasion but the parasite does rearrange vimentin filaments around its PV (Halonen and Weidner, 1994). As with the chlamydial inclusion, the intermediate filament network may serve to dock the PV to the host cell nuclear surface as its disruption results in displacement of the vacuole away from the host perinuclear region.
In addition to actin and intermediate filaments, host microtubules are largely exploited by Toxoplasma and C. trachomatis to penetrate into their host cell but the pathogens operate differently in accordance to their distinctive modes of invasion. Pharmacological disruption of the host microtubular network significantly increases the time before the parasite initiates invasion (Sweenet et al., 2010). Selectively concentrated on one side of the moving junction, host microtubules may help in stabilizing the site of parasite invasion, providing support to help withstand the compressive force provoked by Toxoplasma contacting the host cell. Once internalized, the parasite migrates to the host nucleus by an unknown mechanism. Concerning C. trachomatis, host microtubules are required for the phagocytosis of the bacterium into cells, the coalescence of vesicles containing chlamydiae EBs and the movement of the inclusion to the perinuclear region (Ward and Murray, 1984; Clausen et al., 1997; Grieshaber et al., 2003). The inclusion travels toward the host nucleus along microtubules by recruiting the microtubule-based motor dynein and kinesin II, and some components of the dynactin complex, which are required for the activity of dynein. Inhibitors of microtubules and dynein interfere with both chlamydial invasion and intracellular locomotion (Clausen et al., 1997). At the perinuclear region, the PV and the chlamydial inclusion are positioned at the center of the microtubular network and remain surrounded by host microtubules throughout infection (Coppens et al., 2006; Walker et al., 2008; Clausen et al., 1997). In addition, the parasite nucleates host microtubule growth via γ-tubulin-associated sites, which suggests a physical interaction between the PV membrane and host microtubules (Walker et al., 2008). In conclusion, due the unique mode of invasion of T. gondii and C. trachomatis, the host cytoskeletal components recruited by these pathogens and the microbial effectors used for the reorganization of cellular architecture are different for the parasite and the bacterium. However, what remains intriguing and unique to these two pathogens is the encasement of the PV and inclusion by both host microtubules and vimentin throughout replication.
Both pathogens actively hijack the host MTOC (or centrosome) by detaching it from the nuclear envelope and relocating the MTOC to their vacuole. Evidence for MTOC-PV association in Toxoplasma-infected cells is supported by the detection of centrosomal material at the PV surface, including pericentriolar matrix proteins and components of the γ-tubulin ring complex, which are critical for microtubule nucleation (Coppens et al., 2006; Walker et al., 2008; Wang et al., 2010; Romano et al., 2013b). Similarly, the host centrosomal foci associated with the chlamydial inclusion contain both centriolar and pericentriolar matrix proteins (Johnson et al., 2009). Host centrosome positioning at the Toxoplasma PV requires the function of the host mammalian target of rapamycin complex 2 (mTORC2), which activates the Akt signaling pathway (Wang et al., 2010). In mTORC2-deficient cells infected with the parasite, the host centrosome-PV association is abolished and the microtubules display an altered distribution. In addition, treatment of infected cells with an Akt inhibitor interferes with the interaction between the PV and host centrosomes. The Akt signaling pathway plays a pivotal role in growth factor regulation of microtubule stability, resulting in the phosphorylation of the glycogen synthase kinase 3β (GSK3β), which is a master regulator of the microtubule cytoskeleton (Kumar et al., 2008). Moreover, inhibition of GSK3β restores the host centrosome-PV association in infected cells treated with an Akt inhibitor and in mTORC2 deficient cells. Inhibition of GSK3β in untreated cells increases the association of the host centrosomes and the PV. The chlamydial inclusion also maintains a tight association with host centrosomes. While this association is dependent on host dynein (Grieshaber et al., 2006), chlamydial de novo transcription and translation is required to promote host MTOC-inclusion association, suggesting that chlamydial proteins at the inclusion membrane mediate this interaction. In silico predictions reveal that C. trachomatis may express up to ~50 inclusion membrane proteins (Incs). Furthermore, subdomains on the inclusion membrane that are enriched in cholesterol, active host Src-family kinases and four Inc proteins have been shown to associate with host centrosomes (Mital et al., 2010). One C. trachomatis Inc protein, Inc850, even co-localizes with host centrosomes when ectopically expressed in uninfected cells (Mital et al., 2010).
At the cellular level, controlling MTOC functions may allow intravacuolar pathogens to perturb the host cell cycle by creating centrosomal defects and/or disorganizing mitosis. By stalling host cell division prior to cytokinesis, these pathogens ensure for themselves a stable and spacious environment offered by a multinucleated cell (Walker et al., 2008;Brown et al 2012). To this point, infection of quiescent cells with T. gondii induces an increase in D and E cyclins, thus promoting progression through G1 and transition into S-phase, respectively (Molestina et al., 2008). C. trachomatis also modifies the host cell cycle but by targeting different cellular pathways, which include the dysregulation of the G2/M transition by cleavage of mitotic cyclin B1 and mitotic arrest via the destruction of microtubular networks by the chlamydial type III secreted effector CopN (Johnson et al., 2009; Knowlton et al., 2011; Archuleta et al., 2011; Brown et al., 2012). Therefore, in both Toxoplasma- and Chlamydia-infected cells, there is not only an induction of entry into S-phase triggered by the pathogens but also an arrest in cell cycle progression in the S/G2 transition, based on similar host cyclin expression levels and accumulation in the infected cells. More generally, this phenotype may be viewed as a vantage point of evolution for pathogens sequestered within a membrane-bound compartment as, contrarily, pathogens residing freely in the host cell cytoplasm, e.g., Theileria parasites are more prone to trigger the proliferation of the infected cell by interacting with the host mitotic machinery (Dobbelaere and Küenzi, 2004).
During Chlamydia infection, centrosomal abnormalities such as chromosomal segregation defects and supernumerary chromosomes that result from the dysregulation of centrosome duplication, are frequently observed (Grieshaber et al., 2006; Johnson et al., 2009). These dysfunctional centrosomes may be a factor in the epidemiological links between chlamydial infection and certain cancers (Koskela et al., 2000; Anttila et al., 2001; Wallin et al., 2002; Smith et al., 2004). Several studies report that Toxoplasma is also a possible oncogenic pathogen in humans, especially in the brain where the parasite resides (Wrensch et al., 1993; Ryan et al., 1993; Thomas et al., 2012). The underlying molecular mechanism of Toxoplasma- mediated brain carcinogenesis is not clearly understood but seems to be linked to a dysregulation in the host miRNA processing pathway by parasite effectors, which in turn promotes host cell survival (Thirugnanam et al., 2013). However, no information exists about a connection between host MTOC defects in Toxoplasma-infected brain cells and brain tumors. Finally, controlling MTOC functions may also permit intravacuolar pathogens to regulate the movement of host organelles and attract them to their vacuoles. Located at the intersection of the exocytic and endocytic pathway, the MTOC-Golgi region of the cell is rich in both endosomes and lysosomes. Therefore, the positioning of the PV and inclusion in the peri-Golgi/MTOC region of the cell could facilitate the interception of host vesicular trafficking, which may satisfy the pathogens’ requirements for essential nutrients.
Host exocytic pathway diversion
Two main organelles involved in the exocytic pathway comprised the ER and Golgi apparatus. The ER plays a central role in a number of cellular pathways: the synthesis of phospholipids, cholesterol and ceramides; the trafficking of proteins destined for secretion or transport to other organelles; the glycosylation of proteins via the addition of N-linked oligosaccharides or GPI anchors; and the processing and presentation of antigens via MHC class I. The Golgi is a major site of carbohydrate, glycolipid and sphingomyelin synthesis; the O-glycosylation and sorting of proteins; and the packaging of glycoproteins for delivery to other organelles. Both Toxoplasma and C. trachomatis have developed strategies to recruit ER and Golgi elements, and intercept the trafficking of host exocytic vesicles.
A- Endoplasmic reticulum
Rapidly after penetration into the cell, Toxoplasma induces a dramatic change in the distribution of the host rough ER. This organelle becomes concentrated around the PV, and by 4 h post-infection, about 50% of the PV membrane is covered by host ER structures (de Melo et al., 1992; Sinai et al., 1997). In fact, the PV is immobilized in the host perinuclear region of the cell by the anchorage of the PV membrane to the nuclear envelope, which is contiguous with the cytoplasmic ER network (Romano et al., 2008). These peri-vacuolar ER elements are closely apposed to the PV membrane (distance within ~20 nm) and ribosomes are restricted to the opposite face of the ER away from the PV. The retention of the ER at the PV has been proposed to be mediated by two parasite proteins that are anchored to the PV membrane: ROP2, which contains ER-targeting domains exposed to the host cytosol and GRA3, which interacts with the host ER type II transmembrane protein calcium modulating ligand (CAMLG) (Sinai and Joiner, 2001; Kim et al., 2008). By comparison, the interaction of the inclusion of C. trachomatis with the host ER is more intimate than for the Toxoplasma PV as the inclusion membrane forms direct Membrane Contact Sites (MCSs or zones of close apposition < 50 nm) with ER elements (Derre et al., 2011; Dumoux et al., 2012). These MCSs are numerous and persist for long distances along the inclusion membrane. The needle structures of the type III secretion system extend from the bacterial surface and connect to the inclusion membrane at these host ER-inclusion MCSs (Dumoux et al., 2012). Select host ER proteins are enriched, in patches, on the inclusion membrane. Located at ER-Golgi MCSs in mammalian cells, the ceramide transfer protein (CERT) is responsible for the transfer of ceramides from the ER to the Golgi (Lebiedzinska et al., 2009; Levine and Loewen, 2006). Interestingly, CERT is present at the host ER-inclusion MCSs (Derre et al., 2011). CERT bridges the host ER via association with the ER vesicle-associated membrane protein-associated proteins (VAP-A/VAP-B) and the inclusion via binding to IncD through its pleckstrin domain. Finally, these contact sites may represent portals for the selective influx of ER material into the inclusion. Indeed, host CERT-containing vesicles have been observed within the chlamydial inclusion, suggesting that C. trachomatis is able to engulf portions of the ER (Dumoux et al., 2012).
The host rough ER is essential for the biogenesis and integrity of the inclusion of C. trachomatis since disruption of ER architecture impedes chlamydial infectivity (Dumoux et al., 2012). In particular, host-derived sphingolipids play a crucial role in the replication of C. trachomatis as the bacterium does not grow in cells unable to produce sphingolipids (van Ooij et al., 2000). The close apposition of ER tubules to the inclusion membrane may represent a dynamic environment specialized in non-vesicular trafficking of lipids such as ceramides, leading to metabolism and signaling events that ensure proper bacterial development. Of interest, host CERT is required for bacterial replication and sphingolipid acquisition (Elwell et al., 2011). Similarly to Chlamydia infection, a possible function for associated ER to the PV would be to provide essential constituents for the parasite. To this point, Toxoplasma shows growth defect in cells impaired in ceramide biogenesis, suggesting the importance with host sphingolipid metabolism in the ER for the parasite (Romano et al., 2013b). Moreover, Toxoplasma makes N-glycans from a mixture of the 14-sugar precursor (Glc3Man9GlcNAc2) scavenged from the host ER and its own 10-sugar precursor (Glc3Man5GlcNAc2) (Bushkin et al., 2010) and incorporate them onto newly synthesized proteins (Garenaux et al., 2008).
The close association of Toxoplasma and C. trachomatis with the host ER may also be exploited by the pathogens to modify the host cell’s presentation of their antigens. In the case of Toxoplasma, the ER association may facilitate the presentation of parasite antigens at the host plasma membrane (Goldsmith et al., 2009), thereby eliciting a CD8+ T cell response and the secretion of INF-γ, which plays a role in the persistence of the parasite in the host. A molecular exchange between the host ER and the PV has been documented (Goldsmith et al., 2009), suggesting that host ER may be the export route of Toxoplasma-derived antigens into the cytosol via ERAD translocon subunit Sec61 before processing by the proteasome. Antigenic peptides produced by proteolysis in the cytoplasm are then transported back into the ER via TAP and further trimmed by the aminopeptidase ERAAP to the appropriate length for presentation by MHC class I molecules (Blanchard et al., 2008). The host ER is also a destination for chlamydial antigens, particularly the Major Outer Membrane Protein (MOMP), lipopolysaccharide (LPS) and IncA (Giles and Wyrick, 2008). These antigens are presented via MHC class I molecules and stimulate a CD8+ T cell response and IFN-γ secretion (Gervassi et al., 2003). Three possible scenarios for the release of chlamydial antigens from the inclusion have been proposed: i) IncA-laden fibers that are derived from the inclusion membrane and extend into the host cytosol (Brown et al., 2002); ii) the direct injection of LPS, MOMP and IncA into the host cytosol via the type III secretion machinery (Fields et al., 2005); and iii) vesicles derived from the inclusion membrane that contain LPS, MOMP and IncA that either protrude into the host cell or are detached from the inclusion (Giles et al., 2008). These latter vesicles may then fuse with the ER via the SNARE-like fusion properties of IncA, thereby delivering antigens directly into the ER for processing and presentation.
B- The Golgi apparatus
The morphology of the host Golgi apparatus is dramatically altered in cells infected either with Toxoplasma or C. trachomatis. Astonishingly, the Golgi alterations look very similar in both infections as the organelle is fragmented and sliced into functional mini-stacks that encircle the PV or the inclusion (Heuer et al., 2009; Romano et al., 2013b). In the case of a C. trachomatis infection, host matrix protein golgin84 is cleaved and this cleavage is responsible for the destabilization of the Golgi structure (Heuer et al., 2009). A secreted protease of bacterial origin has been proposed to be involved in the cleavage of host golgin84 but the nature of the protease is controversial (Christian et al., 2011; Chen et al., 2012). The process of Golgi fragmentation during a Toxoplasma infection, however, seems to involve a different process since no cleavage of host golgins is detected (Romano et al., 2013b).
As observed with the recruitment of host ER, the closeness of the pathogens to host Golgi elements may facilitate the scavenging of nutrients, e.g., sphingolipids from this organelle. As a case in point, C. trachomatis recruits two host Golgi enzymes implicated in sphingomyelin synthesis, the sphingomyelin synthase 1 and 2 (SMS1 and SMS2), to distinct compartments of the inclusion to produce its own sphingomyelin using host enzymes. Although Toxoplasma is capable of de novo sphingolipid synthesis (Azzouz et al., 2002; Sonda et al., 2005; Bisanz et al., 2006), its growth also relies on host sources of sphingolipids as exogenously added ceramides that are processed in the Golgi, enhance parasite replication (Romano et al., 2013b). Additionally, the destabilization and dispersion of the Golgi structure in Toxoplasma- and C. trachomatis- infected cells may be related to the interception of host Golgi-derived vesicles by these pathogens. Indeed, C. trachomatis has developed a vesicular-mediated access of ceramides to its inclusion (Hackstadt et al., 1995; 1996) involving the Golgi-specific brefeldin A resistance guanine nucleotide exchange factor 1 (GBF1), which is required for the assembly and maintenance of the Golgi stacks (Elwell et al., 2011). The bacterium selectively co-opts GBF1 within the cis-Golgi compartment for vesicle-mediated sphingolipid acquisition. Moreover, treatment of infected cells with brefeldin A that inhibits GBF1, depletion of host GBF1 or silencing of host golgin84 to prevent Golgi fragmentation lead to a decrease in sphingolipid scavenging and the formation of smaller inclusions (Hackstadt et al., 1995; Heuer et al., 2009; Elwell et al., 2011). Unlike chlamydial infection in epithelial cells where Golgi stacks are recruited by the pathogen, the efficiency of the inclusions to capture Golgi-derived vesicles is reduced significantly in macrophages, and parallels a reduction in the replication rate of the bacterium in macrophages, which underscores the importance of this process for the bacterium development (Sun et al., 2012). It has been proposed that the two trafficking paths for sphingolipid acquisition, molecular and vesicular, by C. trachomatis may operate simultaneously and contribute to distinct aspects of the developmental cycle of Chlamydia, the CERT pathway being important for bacterial replication while the GBF1 pathway may contribute to inclusion membrane integrity. Both Toxoplasma and C. trachomatis divert several host Golgi-derived vesicles to their vacuoles (Hackstadt et al., 1996; Capmany and Damiani, 2010; Pokrovskaya et al., 2012; Romano et al., 2013b). Rab14- associated Golgi vesicles, which deliver sphingomyelin to the plasma membrane, are intercepted by the two pathogens. In addition, Toxoplasma scavenges sphingolipids from Rab30-, or Rab43-associated Golgi vesicles that accumulate within the PV and the parasite incorporates these lipids into its plasma membrane and organelles.
Host endocytic network exploitation
Both the PV and chlamydial inclusion are impervious to the host endosomal/lysosomal degradative pathway. Yet, host endocytic organelles, by virtue of their catabolytic functions, represent a rich source of nutrients derived from the external medium, and both Toxoplasma and C. trachomatis have developed strategies to take advantage of the content of these organelles while avoiding destruction. Both pathogens localize near the MTOC in the perinuclear region of the cell where endo-lysosomes accumulate around their vacuoles. The host mTORC2-Akt signaling is exploited by Toxoplasma to maintain endo-lysosomes around the PV (Wang et al., 2010). The parasite manipulates the host microtubular network to create invaginations of the PV membrane, and through these microtubule-based invaginations, host endo-lysosomes are retained within the PV (Coppens et al., 2006). Mirroring the uptake of host Golgi-derived vesicles by the parasite, the sequestration of intact host endo-lysosomes into the PV lumen allows the parasite to acquire the needed nutrients by circumventing the fusion with host organelles. In case of C. trachomatis, host structures containing the transferrin receptor, a component of early endosomes, the cation-independent mannose-6-phosphate receptor (CI-M6PR) or LAMP-1 from late endosomes, and CD-63 and lysobisphosphatidic acid, two constituents of multivesicular bodies (MVB) gather around the inclusion. The bacterium can also inserts some of these proteins into the inclusion membrane and induce the fusion of host recycling endosomes with the inclusion (van Ooij et al., 1997; Carabeo et al., 2003; Ouellette et al., Beatty, 2006; 2008; Ouellette et al., 2011).
Interactions with the early and late endosomal compartments may provide a source of membrane or nutrients for the maintenance of a productive infection. Toxoplasma and C. trachomatis both acquire nutrients from the endo-lysosomal pathway. The parasite scavenges cholesterol from the host LDL receptor-mediated endocytic pathway, a process that is specifically increased in infected cells (Coppens et al., 2000). Interference with LDL endocytosis, lysosomal degradation of LDL or cholesterol translocation across lysosomal membranes results in blockade of cholesterol delivery to the PV and significantly reduces parasite replication. More generally, the sequestration of host endo-lysosomal organelles within the PV membrane invaginations allows the acquisition of a diverse range of molecules supplied by the endocytic circuit to the parasite. Plasma transferrin, LDL-derived cholesterol and albumin are detected within the inclusion, indicating that exogenously added ligands can be transported to the lumen of the vacuole (van Ooij et al., 1997; Carabeo et al., 2003; Ouellette et al., 2011). Blockade of lysosomal acidification and functions impairs the growth of C. trachomatis. The inclusion also interacts with host MVBs, which are temporary storage compartments enriched in sphingolipids and cholesterol. Inhibition of MVB biogenesis leads to disruption of sphingolipid and cholesterol trafficking to the chlamydial inclusion, and therefore delays inclusion maturation (Beatty, 2006; 2008). Of interest, Toxoplasma and Chlamydia are not only taking advantage of the heterophagic properties of its host cell to gain access to components present in the environment, but they also exploit the nutritive function of host autophagic compartments (Wang et al., 2008; Pachikara et al., 2009; Ouellette et al., 2011; Sun et al., 2012). The parasite induces autophagy in the host cell by a mechanism dependent on calcium but independent of mTOR, and attracts autophagosomes to its PV to enhance the flow of autophagic degradative products to the vacuole. Disabling of the host cell autophagy process by deleting autophagy-like proteins such asAtg5 abrogates the ability of the parasite to maintain optimal proliferation under nutrient limitation conditions. These observations illustrate the parasite’s ability to shift the balance from one source of nutrients (from heterophagy) to another one (from autophagy) in function of the anabolic resources of the host cell. C. trachomatis induces autophagy in the middle of its developmental cycle, in response to the declines in the nutrient pools of host cells. The bacterium attracts host autophagosomes to its inclusion but autophagosomes rarely fuse with the chlamydial inclusion. However, neither the augmentation nor blockade of host cell autophagic functions has a detectable effect on chlamydial infection, suggesting that unlike for T. gondii, host autophagic activities are dispensable for chlamydial growth.
Part II: Co-infection Toxoplasma and Chlamydia trachomatis
Relevance of co-infection models in vitro
For many decades, cells in culture have been infected with two different viruses, or with a viral and a nonviral pathogen, and these co-infection models in vitro have subserved fundamental discoveries such as the phenomena of recombination and phenotypic mixing, and the role of interferons in virus eradication. Cells dually infected with different prokaryotic and/or eukaryotic pathogens, however, have more rarely been reported (for examples, see Meirelles and De Souza, 1983; Black et al., 1990; Heinzen et al., 1996; Rabinovitch and Veras, 1996; de Chastellier et al., 1999; Sinai et al., 2000; Vanover et al., 2008; Borel et al., 2010; Real et al., 2010; Romano et al., 2012; 2013a). Although these instances represent artificially constructed in vitro model systems, dually infected cells with different nonviral pathogens may provide unique opportunities to evaluate the compatibility of two different pathogens during co-infection and to gain fundamental knowledge on each of the pathogens (Box 1). For example, a study on fibroblasts co-infected with Toxoplasma and the bacterium Coxiella burnetti, which flourishes within a mature phago-lysosomal compartment, highlights how refractory the parasite is to any interaction with the bacterium, despite the hyperactive fusion machinery associated with the C. burnetti vacuole (Sinai et al., 2000). A parallel study where cells are co-infected with C. trachomatis and C. burnetti demonstrates that both bacteria remain segregated from one another (Heinzen et al., 1996). In the C. burnetti-infected cells, Toxoplasma or Chlamydia replicate normally, suggesting that their intracellular survival depends on following their respective programs of infection, which are very different from that of Coxiella, to establish a permissive environment.
Box 1 - Some reasons to study cells co-infected with nonviral pathogens.
Capability of invasion, survival, multiplication, and/or differentiation
Compositional and functional features of the intracellular vacuole of the pathogens
Relative localization of one pathogen to the other one within the host cell
Direct interactions between intracellular pathogens
Indirect interactions between intracellular pathogens mediated by host cell factors
Competition for host organelles and cell-derived nutrients
Cooperation via exchange of substrates or growth factors
Antagonism via toxins or antibiotics secreted by one of the pathogens
Transfer or exchange of genetic elements between co-infection pathogens
Changes in differential gene expression of pathogens in co-infected versus singly infected cells
Induction or repression of host cell transduction cascades
Induction or repression of host protective/inhibitory cytokines
Induction or repression of host microbicidal/protective mechanisms
Modulation of pathogen antigen expression
Cohabitation of Toxoplasma and C. trachomatis - an evolving battle to catch host organelles
For a Toxoplasma and C. trachomatis co-infection, a completely different scenario would be expected since both pathogens have similar requirements for their host cells to support their intracellular growth. The shared ability of the parasite and the bacterium to usurp some host organelles would cause these pathogens to ferociously compete for the same pool of nutrients. To verify the hypothesis of rivalry between these two pathogens and address the importance of the manipulation of host cell pathways for them, we have established a novel co-infection model in which human cells are simultaneously infected with Toxoplasma and C. trachomatis. Using this system, we have examined each pathogen’s ability to exploit host cell resources and replicate (Romano et al., 2012; 2013a).
Figure 3 illustrates the morphology of an inclusion developing either alone or in the presence of a PV (panel A) and presents our model regarding the association of host structures with the inclusion and PV during 24-h of co-infection (panel B). A summary of the findings is as follows. A single mammalian cell can harbor both Chlamydia and Toxoplasma. The parasite and the bacterium never share the same vacuolar compartment in the host cell, which is consistent with their distinct modes of cell entry and their dissimilar vacuolar membrane composition that precludes mutual recognition and fusion. Following invasion, the two pathogen-containing vacuoles migrate independently to the host perinuclear region. Toxoplasma, which invades mammalian cells more rapidly than C. trachomatis, arrives the first at the host nucleus, hijacks the MTOC and distributes centrosomal microtubules around its vacuole. The parasite associates with host ER elements and the nuclear envelope. When the bacterium arrives in the perinuclear region, it is still able to recruit host microtubules, which enfold the inclusion, even though it is distant from the MTOC. This highlights that the control of the MTOC is not essential for the bacterium’s ability to recruit host microtubules. When C. trachomatis invades a cell before Toxoplasma, the host MTOC is systematically associated with the inclusion, suggesting that the targeting of the MTOC is a programmed post-invasion event for both pathogens. In co-infected cells, the host Golgi is severely fragmented into small mini-stacks by the two pathogens. The parasite and the bacterium attract and share the Golgi mini-stacks. C. trachomatis but not Toxoplasma, recruits CERT, and both pathogens efficiently diverts host sphingolipids. The redundancy in sphingolipid salvage pathways of both pathogens averts the need for severe competition for these lipids. Toxoplasma, though, is able to more effectively scavenge other nutrients from the host cell and outcompetes Chlamydia for cholesterol, essential amino acids, and to a lesser extent iron.
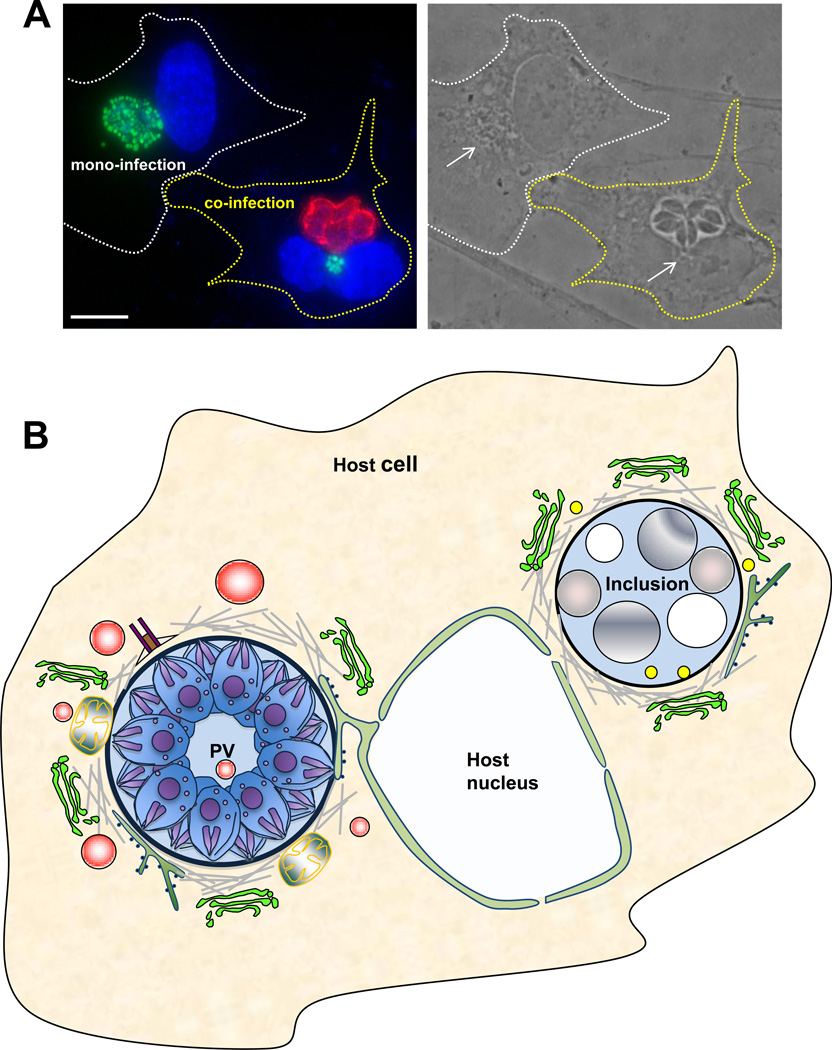
Figure 3. Co-infection of mammalian cells with Toxoplasma gondii and Chlamydia trachomatis, and host cell interactions with the PV and the inclusion during co-infection.
A. A phase and immunofluorescence image of a mono- and a co-infected epithelial cellsshowing the poor development of the inclusion (stained for EF-Tu in green; arrow) during a 24-h co-infection while the parasites (stained for GRA7 in red) develop normally. Both the inclusion and the PV are located in the perinuclear region of the host cell. Scale bar is 10 µm. B. A schematic representation of a PV and an inclusion occupying the same cell for 24-h summarizing their interaction with host cell structures. Similarly as in a mono-infection, the PV is anchored to the host nuclear envelope, associates with the host MTOC, microtubules, ER, mitochondria and attracts host Golgi fragments and endocytic organelles (in red) that are further delivered into the PV. In contrast, inclusions growing in a PV-containing cell shift to a stress-induced persistence state with large aberrant bodies despite its association with host microtubules (in the absence of the MTOC), Golgi fragments, ER and CERT vesicles, and the presence of lipid bodies in the vacuole.
Throughout co-infection, the parasite replicates normally, displaying a superior competitive fitness over the bacterium. Due to its poor ability to scavenge many host molecules in the presence of Toxoplasma, C. trachomatis shifts to a stress-induced persistent growth as a direct result from being barred from its normal nutrient supplies. Parasite killing engenders an ordered return to normal chlamydial development. Reintroduction of excess nutrients into the medium also results in a substantial recovery of chlamydial infectivity. Furthermore, co-infection of C. trachomatis and either slow-growing strains of Toxoplasma or a mutant impaired in nutrient acquisition, allows the bacterium to develop unhampered. Likewise, pre-infection of a cell with Chlamydia prior to Toxoplasma results in slower parasite growth than in a cell infected concurrently.
T. gondii and C. trachomatis: A case for convergent evolution?
Host-associated microbes, whether endosymbionts, commensals or pathogens, all require some adaptive capacity for intracellular life. There is no fossil record to estimate when some organisms have acquired the ability to survive inside other cells. Based on the endosymbiotic origin of some eukaryotic organelles (Margulis, 1971), the emergence of an intracellular lifestyle is likely ancient. Each attempt of microbes to adjust to an intracellular residence must have been subjected to many selection pressures that have contributed to various types of host-microbe relationships and to the development of diverse intracellular lifestyle strategies that are still constantly evolving to handle the stresses.
Similarities in intracellular pathogenic strategies between phylogenetically distant microbes suggest convergent evolution. Obligate intracellular pathogens, such as T. gondii and C. trachomatis, must evolve strategies to scavenge nutrients within a host cell and avoid host cytosolic assaults. Comparisons of these two pathogens uncover that both the parasite and the bacterium manifest similar traits in adapting to the interior of a mammalian cell, although their molecular and cellular mechanisms differ. Both microbes develop within vacuoles created from the host cell’s plasma membrane, which is subsequently modified by the pathogens. Despite different invasion strategies, which influences the development of their vacuolar membranes, both Toxoplasma and Chlamydia gather host vimentin and microtubules around their vacuoles and retain these associations throughout infection. The two microbes have evolved the ability to hijack the host MTOC, possibly to control host vesicular trafficking and organelle placement and/or to interfere with the host cell cycle. Host organelles, such as the ER, Golgi and endocytic vesicles, associate with the PV and inclusion presumably enabling the scavening of nutrients like lipids from these organelles, or in the case of the ER, controling antigen presentation. In addition, the host Golgi is fragmented into mini-stacks during an infection with either pathogen though the molecular mechanism used is different. In sum, both have evolved similar (thought not identical) strategies to exploit same host cell organelles and scavenge nutrients thereof (Laliberté and Carruthers, 2008; Saka and Valdivia, 2010; Cocchiaro and Valdivia, 2009; Romano et al., 2013b). Of note, these similarities are restricted to the subcellular level as Chlamydia and Toxoplasma infect different cell types in their mammalian hosts, which leads to different disease caused these pathogens.
Since the divergence of prokaryotes and protozoa is ancient (1–2 billion years ago) and antedated the emergence of mammals (400–500 million years ago), the selective pressure responsible for the similar adaptations to a mammalian cell of these phylogenetically unrelated microorganisms could not have been linked their pathogenicity in humans. As an alternative, it is tempting to hypothesize that a process of adaptive convergent evolution - possibly in response to interactions with hosts such as protozoan predators in the environment - may be at the origin of the similar survival strategies of nutrient scavenging developed by T. gondii and C. trachomatis.
Another intriguing case of convergent evolution between genetically unrelated pathogens is illustrated by the bacterium Yersinia pestis (human plague), the fungus Cryptococcus neoformans (cryptococcosis) and protozoan parasites from the Leishmania mexicana complex (cutaneous leishmaniasis) (Bliska and Casadevall, 2009; Antoine et al., 1998). These microorganisms are facultative intracellular pathogens. Inside their human hosts, Y. pestis, C. neoformans and L. mexicana parasites are taken up by macrophages wherein they can survive and replicate in a very large phagolysosome. To circumvent the cytocidal response of macrophages, these three different pathogens alter the intravacuolar environment of their phagolysosomes using analogous mechanisms: they interfere with phagosomal microbicidal activities by diluting lysosomal contents. The mechanism of spacious phagosome formation by Y. pestis has not been identified, but unidirectional fusion of endocytic compartments with the phagosome seems to occur (Grabenstein et al., 2006). C. neoformans secretes in the host cytoplasm capsular polysaccharide-containing vesicles that subsequently modify the membrane properties of phagosomes and promote their homotypic fusion, resulting into giant phagosomes sheltering the fungus (Alvarez and Casadevall, 2006). L. mexicana parasites expand their compartments by secreting proteophosphoglycans in the phagolysosomal lumen. Due to their polyanionic properties, these specific glycans induce the vacuolization of the phagosomes (Peters et al., 1997). Additionally, these three pathogens are protected by unique surface structures: Y. pestis has a modified coat of lipopolysaccharides, C. neoformans, a polysaccharide capsule, and L. mexicana parasites, high levels of glycosphingolipids (Bliska and Casadevall, 2009; McConville & Ralton, 1997). It could be interesting to analyze the outcome of the co-infection of a macrophage with the bacterium, the fungus and the protozoan. It is tempting to hypothesize that during a co-infection, the growth of Y. pestis, C. neoformans and L. mexicana parasites may be enhanced by some sort of cooperativity between themselves in coping with the defenses of their host cells. Altogether, these examples emphasize common adaptive strategies, such as the neutralization of lysosomal acidic contents by dilution, developed by intracellular pathogens that arise in distant evolutionary branches.
Concluding remarks
By comparing the program of infectivity of T. gondii and C. trachomatis, this review explores the strategies of these two intracellular pathogens for manipulating the host at a cellular level. First, the key role of cellular remodeling and host organelle interception for Toxoplasma and Chlamydia to gain access to host intracellular resources was highlighted. Second, a pathogen’s strict dependence on its ability to successfully adhere to a finely tuned developmental program to achieve productive infection was demonstrated. For instance, Toxoplasma and Chlamydia tend to conform to their respective intracellular developmental program regardless of the presence of the other organism within the cell. The normal growth of each pathogen (i.e., the production of infectious progeny) is highly dependent on the pathogen’s ability to maintain a threshold level of interaction between its vacuole and host cell organelles. Third, despite the uniqueness of each host-microbe interaction, there are relatively few solutions to the problem of intracellular entry, survival, and escape. For phylogenetically distant microbial species, similarities in intracellular pathogenic strategies are probably most easily explained by convergent evolution. For Toxoplasma and Chlamydia, the most fascinating example is certainly the hijacking of the host Golgi, which is further reorganized into mini-stacks that these pathogens similarly and conveniently align just outside their vacuole in the cell.
Acknowledgement
The authors are grateful to the members of the Coppens lab who provided comments during the course of these studies. We would like to thank Patrik P. Bavoil at the University of Maryland, our close collaborator who introduced us to the field of Chlamydia and intravacuolar bacteria. We thank Jose Carrasco at the University of Maryland for providing the seeds of Chlamydia. The work was supported by the grant from the NIH AI060767 to I.C.
References
- Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16:2161–2165. doi: 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- Andersson SG, Kurland CG. Reductive evolution of resident genomes. Trends Microbiol. 1998;6:263–268. doi: 10.1016/s0966-842x(98)01312-2. [DOI] [PubMed] [Google Scholar]
- Antoine JC, Prina E, Lang T, Courret N. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 1998;6:392–401. doi: 10.1016/s0966-842x(98)01324-9. [DOI] [PubMed] [Google Scholar]
- Anttila T, Saikku P, Koskela P, et al. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA. 2001;285:47–51. doi: 10.1001/jama.285.1.47. [DOI] [PubMed] [Google Scholar]
- Archuleta TL, Du Y, English CA, Lory S, Lesser C, Ohi MD, Ohi R, Spiller BW. The Chlamydia effector chlamydial outer protein N (CopN) sequesters tubulin and prevents microtubule assembly. J Biol Chem. 2011;286:33992–33998. doi: 10.1074/jbc.M111.258426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzouz N, Rauscher B, Gerold P, Cesbron-Delauw MF, Dubremetz JF, Schwarz RT. Evidence for de novo sphingolipid biosynthesis in Toxoplasma gondii . Int J Parasitol. 2002;32:677–684. doi: 10.1016/s0020-7519(02)00009-7. [DOI] [PubMed] [Google Scholar]
- Besteiro S, Dubremetz JF, Lebrun M. The moving junction of apicomplexan parasites: a key structure for invasion. Cell Microbiol. 2011;13:797–805. doi: 10.1111/j.1462-5822.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- Bisanz C, Bastien O, Grando D, Jouhet J, Maréchal E, Cesbron-Delauw MF. Toxoplasma gondii acyl-lipid metabolism: de novo synthesis from apicoplast-generated fatty acids versus scavenging of host cell precursors. Biochem J. 2006;394:197–205. doi: 10.1042/BJ20050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard N, Gonzalez F, Schaeffer M, Joncker NT, Cheng T, Shastri AJ, Robey EA, Shastri N. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat Immunol. 2008;9:937–944. doi: 10.1038/ni.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska JB, Casadevall A. Intracellular pathogenic bacteria and fungi--a case of convergent evolution? Nat Rev Microbiol. 2009;7:165–171. doi: 10.1038/nrmicro2049. [DOI] [PubMed] [Google Scholar]
- Brown HM, Knowlton AE, Grieshaber SS. Chlamydial infection induces host cytokinesis failure at abscission. Cell Microbiol. 2012;14:1554–1567. doi: 10.1111/j.1462-5822.2012.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz KR, Stephens RS. The cytosolic pattern recognition receptor NOD1 induces inflammatory interleukin-8 during Chlamydia trachomatis infection. Infect Immun. 2008;76:3150–3155. doi: 10.1128/IAI.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushkin GG, Ratner DM, Cui J, et al. Suggestive evidence for Darwinian Selection against asparagine-linked glycans of Plasmodium falciparum and Toxoplasma gondii . Eukaryot Cell. 2010;9:228–241. doi: 10.1128/EC.00197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capmany A, Damiani MT. Chlamydia trachomatis Intercepts Golgi-Derived Sphingolipids through a Rab14-Mediated Transport Required for Bacterial Development and Replication. PLoS ONE. 2010;5:e14084. doi: 10.1371/journal.pone.0014084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabeo RA, Mead DJ, Hackstadt T. Golgi-dependent transport of cholesterol to the Chlamydia trachomatis inclusion. Proc Natl Acad Sci USA. 2003;100:6771–6776. doi: 10.1073/pnas.1131289100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabeo RA, Grieshaber SS, Fischer E, Hackstadt T. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect Immun. 2002;70:3793–3803. doi: 10.1128/IAI.70.7.3793-3803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2007;10:83–89. doi: 10.1016/j.mib.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Chen AL, Johnson KA, Lee JK, Sütterlin C, Tan M. CPAF: A Chlamydial Protease in Search of an Authentic Substrate. PLoS Pathog. 2012;8:e1002842. doi: 10.1371/journal.ppat.1002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen JD, Christiansen G, Holst HU, Birkelund S. Chlamydia trachomatis utilizes the host cell microtubule network during early events of infection. Mol Microbiol. 1997;25:441–449. doi: 10.1046/j.1365-2958.1997.4591832.x. [DOI] [PubMed] [Google Scholar]
- Coppens I, Sinai AP, Joiner KA. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J Cell Biol. 2000;149:167–180. doi: 10.1083/jcb.149.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens I, Dunn JD, Romano JD, Pypaert M, Zhang H, Boothroyd JC, Joiner KA. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell. 2006;125:261–274. doi: 10.1016/j.cell.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Christian JG, Heymann J, Paschen SA, Vier J, Schauenburg L, Rupp J, Meyer TF, Häcker G, Heuer D. Targeting of a chlamydial protease impedes intracellular bacterial growth. PLoS Pathog. 2011;7:e1002283. doi: 10.1371/journal.ppat.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A, Subtil, Hackstadt T. Recent insights into the mechanisms of Chlamydia entry. Cell Microbiol. 2005;7:1714–1722. doi: 10.1111/j.1462-5822.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- Dean D, Myers GS, Read TD. Lessons and Challenges Arising from the “First Wave” of Chlamydia Genome Sequencing. In: Bavoil PM, Blakeney Wyrick P, editors. Chlamydia: Genomics and Pathogenesis. Norfolk, UK: Horizon Bioscience; 2006. pp. 1–24. [Google Scholar]
- Delorme-Walker V, Abrivard M, Lagal V, et al. Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion. J Cell Sci. 2012;125:4333–4342. doi: 10.1242/jcs.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo EJ, de Carvalho TU, de Souza W. Penetration of Toxoplasma gondii into host cells induces changes in the distribution of the mitochondria and the endoplasmic reticulum. Cell Struct Funct. 1992;17:311–317. doi: 10.1247/csf.17.311. [DOI] [PubMed] [Google Scholar]
- Derré I, Swiss R, Agaisse H. The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathog. 2011;7:e1002092. doi: 10.1371/journal.ppat.1002092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere DA, Küenzi P. The strategies of the Theileria parasite: a new twist in host-pathogen interactions. Curr Opin Immunol. 2004;16:524–530. doi: 10.1016/j.coi.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Dumoux M, Clare DK, Saibil HR, Hayward RD. Chlamydiae assemble a pathogen synapse to hijack the host endoplasmic reticulum. Traffic. 2012;13:1612–1627. doi: 10.1111/tra.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell CA, Ceesay A, Kim JH, Kalman D, Engel JN. RNA interference screen identifies Abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS Pathog. 2008;4:e1000021. doi: 10.1371/journal.ppat.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell CA, Jiang S, Kim JH, Lee A, Wittmann T, Hanada K, Melancon P, Engel JN. Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLoS Pathog. 2011;7:e1002198. doi: 10.1371/journal.ppat.1002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields KA, Hackstadt T. The chlamydial inclusion: escape from the endocytic pathway. Annu Rev Cell Dev Biol. 2002;18:221–245. doi: 10.1146/annurev.cellbio.18.012502.105845. [DOI] [PubMed] [Google Scholar]
- Fields KA, Fischer ER, Mead DJ, Hackstadt T. Analysis of putative Chlamydia trachomatis chaperones Scc2 and Scc3 and their use in the identification of type III secretion substrates. J Bacteriol. 2005;187:6466–6478. doi: 10.1128/JB.187.18.6466-6478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser-Liggett CM. Insights on biology and evolution from microbial genome sequencing. Genome Res. 2005;15:1603–1610. doi: 10.1101/gr.3724205. [DOI] [PubMed] [Google Scholar]
- Garénaux E, Shams-Eldin H, Chirat F, Bieker U, Schmidt J, Michalski JC, Cacan R, Guérardel Y, Schwarz RT. The dual origin of Toxoplasma gondii N-glycans. Biochemistry. 47:12270–12276. doi: 10.1021/bi801090a. [DOI] [PubMed] [Google Scholar]
- Gervassi AL, Probst P, Stamm WE, Marrazzo J, Grabstein KH, Alderson MR. Functional characterization of class Ia- and non-class Ia-restricted Chlamydia-reactive CD8+ T cell responses in humans. J Immunol. 2003;171:4278–4286. doi: 10.4049/jimmunol.171.8.4278. [DOI] [PubMed] [Google Scholar]
- Giles DK, Wyrick PB. Trafficking of chlamydial antigens to the endoplasmic reticulum of infected epithelial cells. Microbes Infect. 2008;10:1494–1503. doi: 10.1016/j.micinf.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles DK, Whittimore JD, LaRue RW, Raulston JE, Wyrick PB. Ultrastructural analysis of chlamydial antigen-containing vesicles everting from the Chlamydia trachomatis inclusion. Microbes Infect. 2006;8:1579–1591. doi: 10.1016/j.micinf.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Goldszmid RS, Coppens I, Lev A, Caspar P, Mellman I, Sher A. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med. 2009;206:399–410. doi: 10.1084/jem.20082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez V, Combe A, David V, Malmquist NA, Delorme V, Leroy C, Blazquez S, Ménard R, Tardieux I. Host cell entry by apicomplexa parasites requires actin polymerization in the host cell. Cell Host Microbe. 2009;5:259–272. doi: 10.1016/j.chom.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect Immun. 2006;74:3727–3741. doi: 10.1128/IAI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieshaber SS, Grieshaber NA, Hackstadt T. Chlamydia trachomatis uses host cell dynein to traffic to the microtubule-organizing center in a p50 dynamitin-independent process. J Cell Sci. 2003;116:3793–3802. doi: 10.1242/jcs.00695. [DOI] [PubMed] [Google Scholar]
- Grieshaber SS, Grieshaber NA, Miller N, Hackstadt T. Chlamydia trachomatis causes centrosomal defects resulting in chromosomal segregation abnormalities. Traffic. 2006;7:940–949. doi: 10.1111/j.1600-0854.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- Hackstadt T, Scidmore MA, Rockey DD. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci USA. 1995;92:4877–4881. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T, Rockey DD, Heinzen RA, Scidmore MA. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the Golgi apparatus to the plasma membrane. EMBO J. 1996;15:964–977. [PMC free article] [PubMed] [Google Scholar]
- Halonen SK, Weidner E. Overcoating of Toxoplasma parasitophorous vacuoles with host cell vimentin type intermediate filaments. J Eukaryot Microbiol. 1994;41:65–71. doi: 10.1111/j.1550-7408.1994.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Heinzen RA, Scidmore MA, Rockey DD, Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis . Infect Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer D, Rejman Lipinski A, Machuy N, Karlas A, Wehrens A, Siedler F, Brinkmann V, Meyer TF. Chlamydia causes fragmentation of the Golgi compartment to ensure reproduction. Nature. 2009;457:731–755. doi: 10.1038/nature07578. [DOI] [PubMed] [Google Scholar]
- Horn M, Collingro A, Schmitz-Esser S, et al. Illuminating the evolutionary history of chlamydiae . Science. 2004;304:728–730. doi: 10.1126/science.1096330. [DOI] [PubMed] [Google Scholar]
- Jewett TJ, Fischer ER, Mead DJ, Hackstadt T. Chlamydial TARP is a bacterial nucleator of actin. Proc Natl Acad Sci USA. 2006;103:15599–15604. doi: 10.1073/pnas.0603044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiwani S, Alvarado S, Ohr RJ, Romero A, Nguyen B, Jewett TJ. Chlamydia trachomatis Tarp harbors distinct G and F actin binding domains that bundle actin filaments. J Bacteriol. 2013;195:708–716. doi: 10.1128/JB.01768-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiwani S, Ohr RJ, Fischer ER, Hackstadt T, Alvarado S, Romero A, Jewett TJ. Chlamydia trachomatis Tarp cooperates with the Arp2/3 complex to increase the rate of actin polymerization. Biochem Biophys Res Commun. 2012;420:816–821. doi: 10.1016/j.bbrc.2012.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Tan M, Sütterlin C. Centrosome abnormalities during a Chlamydia trachomatis infection are caused by dysregulation of the normal duplication pathway. Cell Microbiol. 2009;11:1064–1073. doi: 10.1111/j.1462-5822.2009.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Ahn HJ, Ryu KJ, Nam HW. Interaction between parasitophorous vacuolar membrane-associated GRA3 and calcium modulating ligand of host cell endoplasmic reticulum in the parasitism of Toxoplasma gondii . Korean J Parasitol. 2008;46:209–216. doi: 10.3347/kjp.2008.46.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton AE, Brown HM, Richards TS, Andreolas LA, Patel RK, Grieshaber SS. Chlamydia trachomatis infection causes mitotic spindle pole defects independently from its effects on centrosome amplification. Traffic. 2011;12:854–866. doi: 10.1111/j.1600-0854.2011.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskela P, Anttila T, Bjørge T, et al. Chlamydia trachomatis infection as a risk factor for invasive cervical cancer. Int J Cancer. 2000;85:35–39. doi: 10.1002/(sici)1097-0215(20000101)85:1<35::aid-ijc6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Kumar Y, Valdivia RH. Reorganization of the host cytoskeleton by the intracellular pathogen Chlamydia trachomatis . Commun Integr Biol. 2008a;1:175–177. doi: 10.4161/cib.1.2.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Y, Valdivia RH. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell Host Microbe. 2008b;14:159–169. doi: 10.1016/j.chom.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Y, Valdivia Leading a sheltered life: intracellular pathogens and maintenance of vacuolar compartments. Cell Host Microbe. 2009;5:593–601. doi: 10.1016/j.chom.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Lyle KS, Gierke S, Matov A, Danuser G, Wittmann T. GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment. J Cell Biol. 2009;184:895–908. doi: 10.1083/jcb.200901042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuvardina ON, Leander BS, Aleshin VV, Myl’nikov AP, Keeling PJ, Simdyanov TG. The phylogeny of colpodellids (Alveolata) using small subunit rRNA gene sequences suggests they are the free-living sister group to apicomplexans. J Eukaryot Microbiol. 2002;49:498–504. doi: 10.1111/j.1550-7408.2002.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Laliberté J, Carruthers VB. Host cell manipulation by the human pathogen Toxoplasma gondii . Cell Mol Life Sci. 2008;65:1900–1915. doi: 10.1007/s00018-008-7556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarque MH, Papoin J, Finizio AL, Lentini G, Pfaff AW, Candolfi E, Dubremetz JF, Lebrun M. Identification of a new rhoptry neck complex RON9/RON10 in the Apicomplexa parasite Toxoplasma gondii . PLoS One. 2012;7:e32457. doi: 10.1371/journal.pone.0032457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane BJ, Mutchler C, Al Khodor S, Grieshaber SS, Carabeo RA. Chlamydial entry involves TARP binding of guanine nucleotide exchange factors. PLoS Pathog. 2008;4:e1000014. doi: 10.1371/journal.ppat.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebiedzinska M, Szabadkai G, Jones AW, Duszynski J, Wieckowski MR. Interactions between the endoplasmic reticulum, mitochondria, plasma membrane and other subcellular organelles. Int J Biochem Cell Biol. 2009;41:1805–1816. doi: 10.1016/j.biocel.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Levine T, Loewen C. Inter-organelle membrane contact sites: through a glass, darkly. Curr Opin Cell Biol. 2006;18:371–378. doi: 10.1016/j.ceb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- Manolea F, Claude A, Chun J, Rosas J, Melancon P. Distinct functions for Arf guanine nucleotide exchange factors at the Golgi complex: GBF1 and BIGs are required for assembly and maintenance of the Golgi stack and trans-Golgi network, respectively. Mol Biol Cell. 2008;19:523–535. doi: 10.1091/mbc.E07-04-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis L. Symbiosis and evolution. Sci. Am. 1971;225:48–57. doi: 10.1038/scientificamerican0871-48. [DOI] [PubMed] [Google Scholar]
- McConville MJ, Ralton JE. Developmentally regulated changes in the cell surface architecture of Leishmania parasites. Behring Inst Mitt. 1997;99:34–43. [PubMed] [Google Scholar]
- Mital J, Miller NJ, Fischer ER, Hackstadt T. Specific chlamydial inclusion membrane proteins associate with active Src family kinases in microdomains that interact with the host microtubule network. Cell Microbiol. 2010;12:1235–1249. doi: 10.1111/j.1462-5822.2010.01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molestina RE, El-Guendy N, Sinai AP. Infection with Toxoplasma gondii results in dysregulation of the host cell cycle. Cell Microbiol. 2008;10:1153–1165. doi: 10.1111/j.1462-5822.2008.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi K, Higuchi M, Asakura T, Masuyama N, Gotoh Y. The PI3K–Akt pathway promotes microtubule stabilization in migrating fibroblasts. Genes Cells. 2007;12:535–546. doi: 10.1111/j.1365-2443.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- Ouellette SP, Dorsey FC, Moshiach S, Cleveland JL, Carabeo RA. Chlamydia species-dependent differences in the growth requirement for lysosomes. PLoS One. 2011;6:e16783. doi: 10.1371/journal.pone.0016783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachikara N, Zhang H, Pan Z, Jin S, Fan H. Productive Chlamydia trachomatis lymphogranuloma venereum 434 infection in cells with augmented or inactivated autophagic activities. FEMS Microbiol Lett. 2009;292:240–249. doi: 10.1111/j.1574-6968.2009.01494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen MJ, Wren BW. Bacterial pathogenomics. Nature. 2007;449:835–842. doi: 10.1038/nature06248. [DOI] [PubMed] [Google Scholar]
- Peters C, Stierhof YD, Ilg T. Proteophosphoglycan secreted by Leishmania mexicana amastigotes causes vacuole formation in macrophages. Infect Immun. 1997;65:783–786. doi: 10.1128/iai.65.2.783-786.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrovskaya ID, Szwedo JW, Goodwin A, Lupashina TV, Nagarajan UM, Lupashin VV. Chlamydia trachomatis hijacks intra-Golgi COG complex-dependent vesicle trafficking pathway. Cell Microbiol. 2012;14:656–668. doi: 10.1111/j.1462-5822.2012.01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real F, Mortara RA, Rabinovitch M. Fusion between Leishmania amazonensis and Leishmania major parasitophorous vacuoles: live imaging of coinfected macrophages. PLoS Negl Trop Dis. 2010;4:e905. doi: 10.1371/journal.pntd.0000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano JD, Bano N, Coppens I. New host nuclear functions are not required for the modifications of the parasitophorous vacuole of Toxoplasma . Cell Microbiol. 2008;10:465–476. doi: 10.1111/j.1462-5822.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- Romano JD, de Beaumont C, Carrasco JA, Ehrenman K, Bavoil PM, Coppens I. A novel co-infection model with Toxoplasma and Chlamydia trachomatis highlights the importance of host cell manipulation for nutrient scavenging. Cell Microbiol. 2012 doi: 10.1111/cmi.12060. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano JD, de Beaumont C, Carrasco JA, Ehrenman K, Bavoil PM, Coppens I. Fierce competition between Toxoplasma and Chlamydia for host cell structures in dually infected cells. Eukaryot Cell. 2013a;12:265–277. doi: 10.1128/EC.00313-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano JD, Sonda S, Bergbower E, Smith ME, Coppens I. Toxoplasma gondii salvages sphingolipids from the host Golgi through the rerouting of selected Rab vesicles to the parasitophorous vacuole. Mol Biol Cell. 2013b doi: 10.1091/mbc.E12-11-0827. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P, Hurley SF, Johnson AM, Salzberg M, Lee MW, North JB, McNeil JJ, McMichael AJ. Tumours of the brain and presence of antibodies to Toxoplasma gondii . Int J Epidemiol. 1993;22:412–419. doi: 10.1093/ije/22.3.412. [DOI] [PubMed] [Google Scholar]
- Saka HA, Valdivia RH. Acquisition of nutrients by Chlamydiaeunique challenges of living in an intracellular compartment. Curr Opin Microbiol. 2010;13:4–10. doi: 10.1016/j.mib.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J. Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia Intracellular Biology, Pathogenesis, and Immunity. Washington, DC: ASM Press; 1999. pp. 139–169. [Google Scholar]
- Schwab JC, Beckers CJ, Joiner KA. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci USA. 1994;91:509–513. doi: 10.1073/pnas.91.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scidmore MA, Fischer ER, Hackstadt T. Restricted fusion of Chlamydia trachomatis vesicles with endocytic compartments during the initial stages of infection. Infect Immun. 2003;71:973–984. doi: 10.1128/IAI.71.2.973-984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley LD. Toxoplasma gondii: perfecting an intracellular life style. Traffic. 2003;4:581–586. doi: 10.1034/j.1600-0854.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Niesman IR, Asai T, Takeuchi T. Toxoplasma gondiisecretion of a potent nucleoside triphosphate hydrolase into the parasitophorous vacuole. Exp Parasitol. 1994;79:301–311. doi: 10.1006/expr.1994.1093. [DOI] [PubMed] [Google Scholar]
- Sinai AP, Joiner KA. The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane. J Cell Biol. 2001;154:95–108. doi: 10.1083/jcb.200101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai AP, Webster P, Joiner KA. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J Cell Sci. 1997;110:2117–2128. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- Sinai AP, Paul S, Rabinovitch M, Kaplan G, Joiner KA. Coinfection of fibroblasts with Coxiella burnetti and Toxoplasma gondii to each their own. Microbes Infect. 2000;2:727–736. doi: 10.1016/s1286-4579(00)90362-9. [DOI] [PubMed] [Google Scholar]
- Smith JS, Bosetti C, Muñoz N, Herrero R, Bosch FX, Eluf-Neto J, Meijer CJ, Van Den Brule AJ, Franceschi S, Peeling RW. Chlamydia trachomatis and invasive cervical cancer: a pooled analysis of the IARC multicentric case-control study. Int J Cancer. 2004;111:431–439. doi: 10.1002/ijc.20257. [DOI] [PubMed] [Google Scholar]
- Sonda S, Sala G, Ghidoni R, Hemphill A, Pieters J. Inhibitory effect of aureobasidin A on Toxoplasma gondii . Antimicrob Agents Chemother. 2005;49:1794–1801. doi: 10.1128/AAC.49.5.1794-1801.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Kalman S, Lammel C, Fanc J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov RL, Zhao Q, Koonin EV, Davis RW. Genome Sequence of an Obligate Intracellular Pathogen of Humans: Chlamydia trachomatis . Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- Sun HS, Eng EW, Jeganathan S, Sin AT, Patel PC, Gracey E, Inman RD, Terebiznik MR, Harrison RE. Chlamydia trachomatis vacuole maturation in infected macrophages. J Leukoc Biol. 2012;92:815–827. doi: 10.1189/jlb.0711336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchland RJ, Rockey DD, Weeks SK, Alzhanov DT, Stamm WE. Development of secondary inclusions in cells infected by Chlamydia trachomatis . Infect Immun. 2005;73:3954–3962. doi: 10.1128/IAI.73.7.3954-3962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney KR, Morrissette NS, LaChapelle S, Blader IJ. Host cell invasion by Toxoplasma gondii is temporally regulated by the host microtubule cytoskeleton. Eukaryot Cell. 2010;9:1680–1689. doi: 10.1128/EC.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirugnanam S, Rout N, Gnanasekar M. Possible role of Toxoplasma gondii in brain cancer through modulation of host microRNAs. Infect Agent Cancer. 2013;8:8. doi: 10.1186/1750-9378-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F, Lafferty KD, Brodeur J, Elguero E, Gauthier-Clerc M, Missé D. Incidence of adult brain cancers is higher in countries where the protozoan parasite Toxoplasma gondii is common. Biol Lett. 2012;8:101–103. doi: 10.1098/rsbl.2011.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooij C, Apodaca G, Engel J. Characterization of the Chlamydia trachomatis vacuole and its interaction with the host endocytic pathway in HeLa cells. Infect Immun. 1997;65:758–766. doi: 10.1128/iai.65.2.758-766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooij C, Kalman L, van Ijzendoorn, Nishijima M, Hanada K, Mostov K, Engel JN. Host cell-derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis . Cell Microbiol. 2000;2:627–637. doi: 10.1046/j.1462-5822.2000.00077.x. [DOI] [PubMed] [Google Scholar]
- Walker ME, Hjort EE, Smith SS, Tripathi A, Hornick JE, Hinchcliffe EH, Archer W, Hager KM. Toxoplasma gondii actively remodels the microtubule network in host cells. Microbes Infect. 2008;10:1440–1449. doi: 10.1016/j.micinf.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin KL, Wiklund F, Luostarinen T, et al. A population-based prospective study of Chlamydia trachomatis infection and cervical carcinoma. Int J Cancer. 2002;101:371–374. doi: 10.1002/ijc.10639. [DOI] [PubMed] [Google Scholar]
- Wang Y, Weiss LM, Orlofsky A. Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth. J Biol Chem. 2009;284:1694–701. doi: 10.1074/jbc.M807890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Weiss LM, Orlofsky A. Coordinate control of host centrosome position, organelle distribution, and migratory response by Toxoplasma gondii via host mTORC2. J Biol Chem. 2010;285:15611–15618. doi: 10.1074/jbc.M109.095778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward ME, Murray A. Control mechanisms governing the infectivity of Chlamydia trachomatis for HeLa Cells: Mechanisms of Endocytosis. J Gen Microbiol. 1984;130:1765–1780. doi: 10.1099/00221287-130-7-1765. [DOI] [PubMed] [Google Scholar]
- Wrensch M, Bondy ML, Wiencke J, Yost M. Environmental risk factors for primary malignant brain tumors: a review. J Neurooncol. 1993;17:47–64. doi: 10.1007/BF01054274. [DOI] [PubMed] [Google Scholar]