Abstract
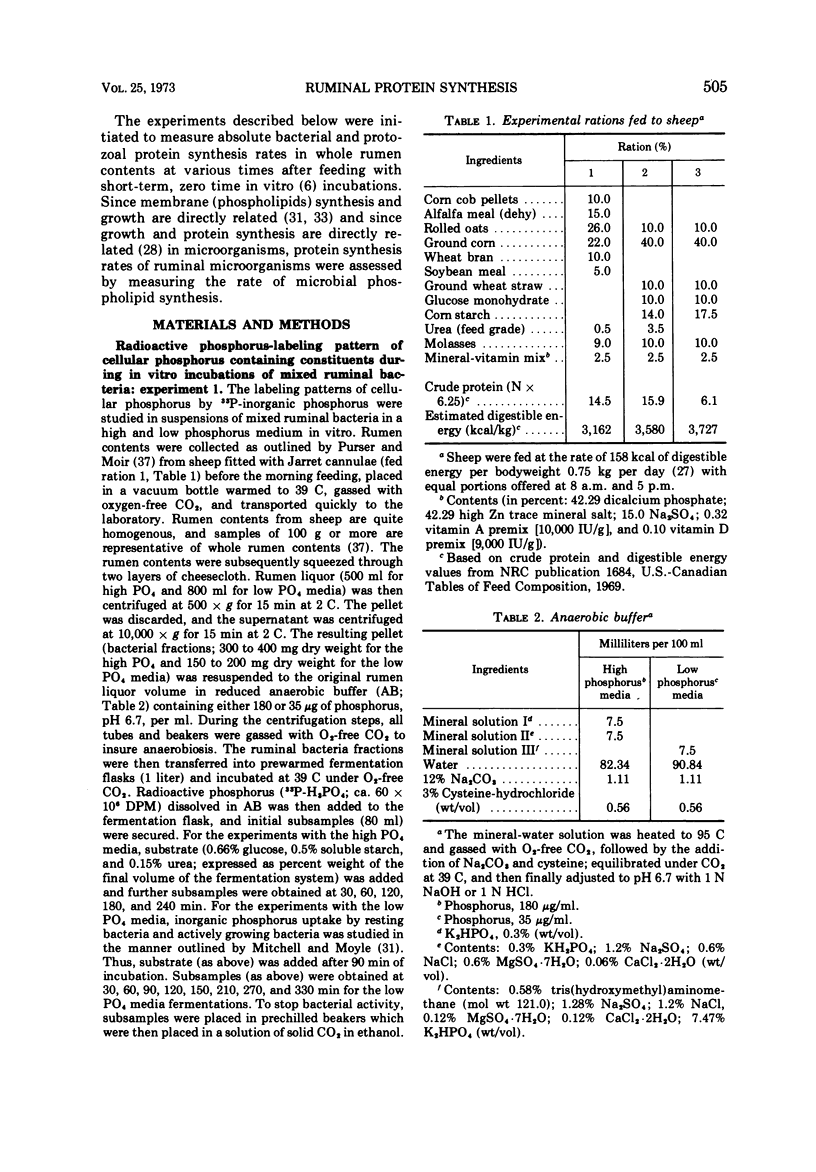
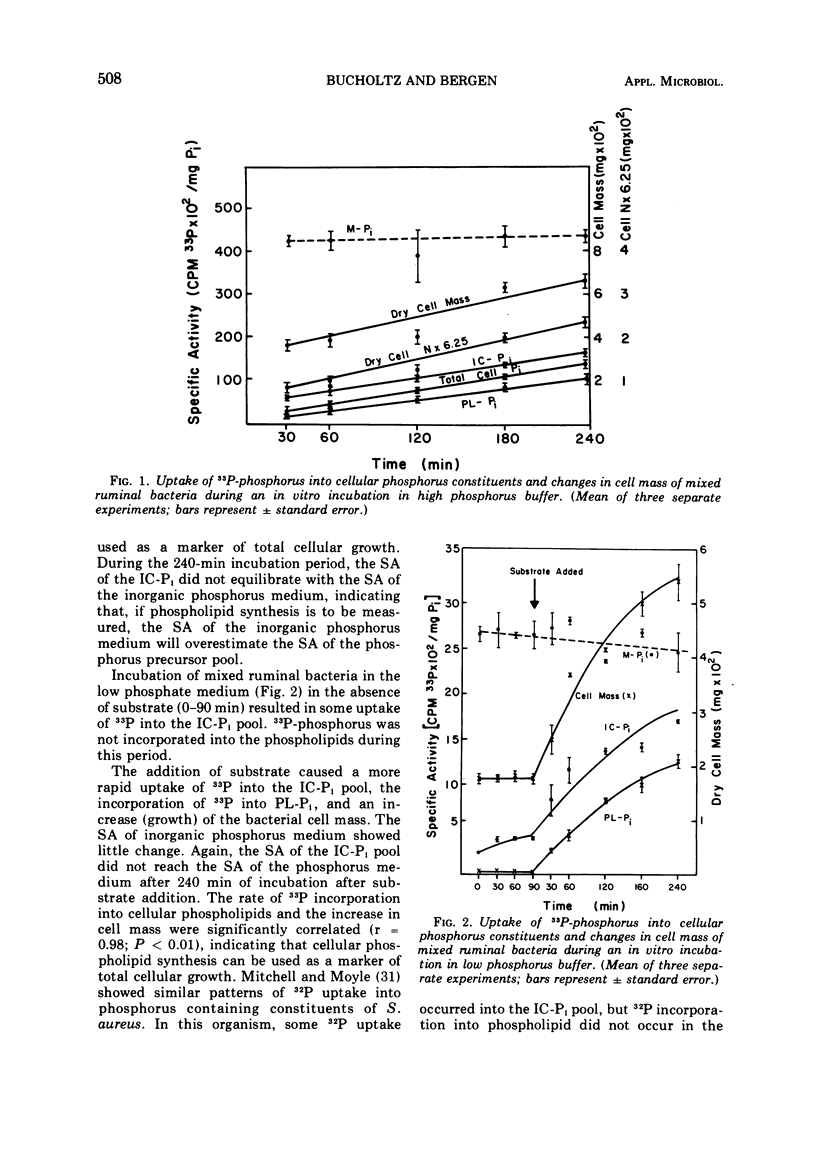
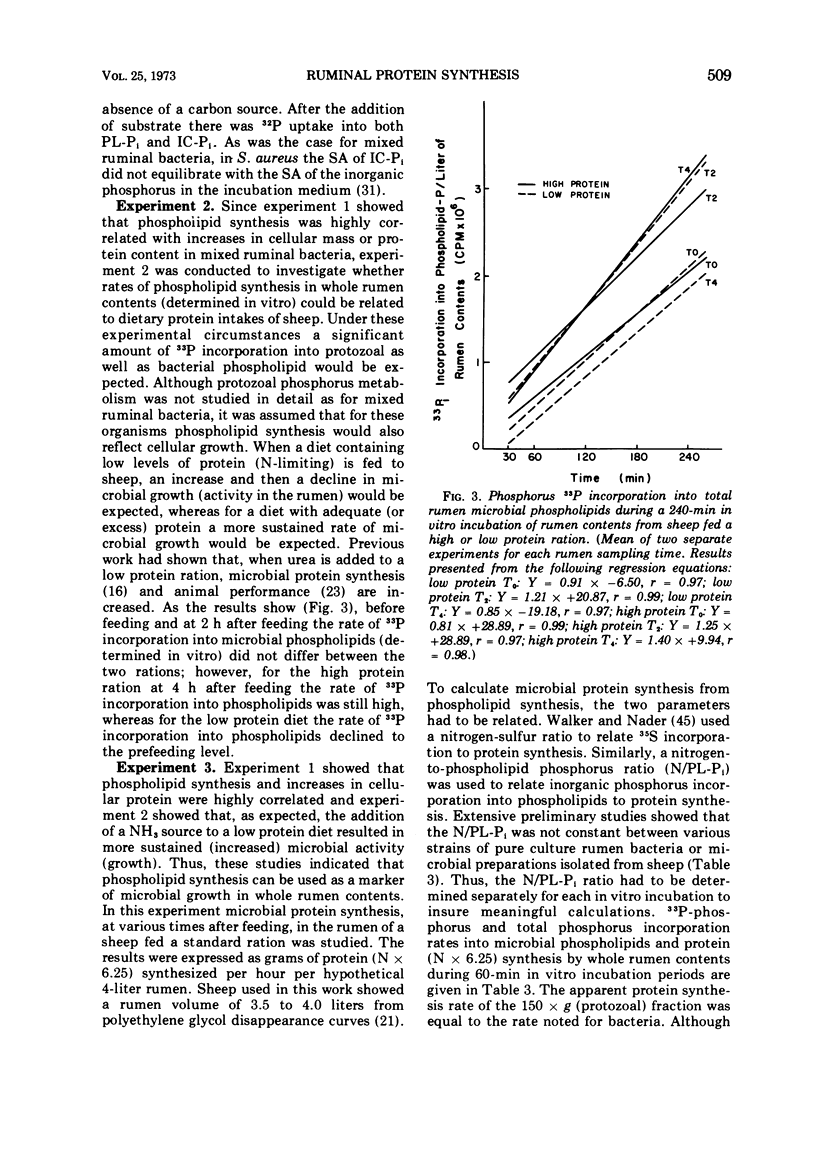
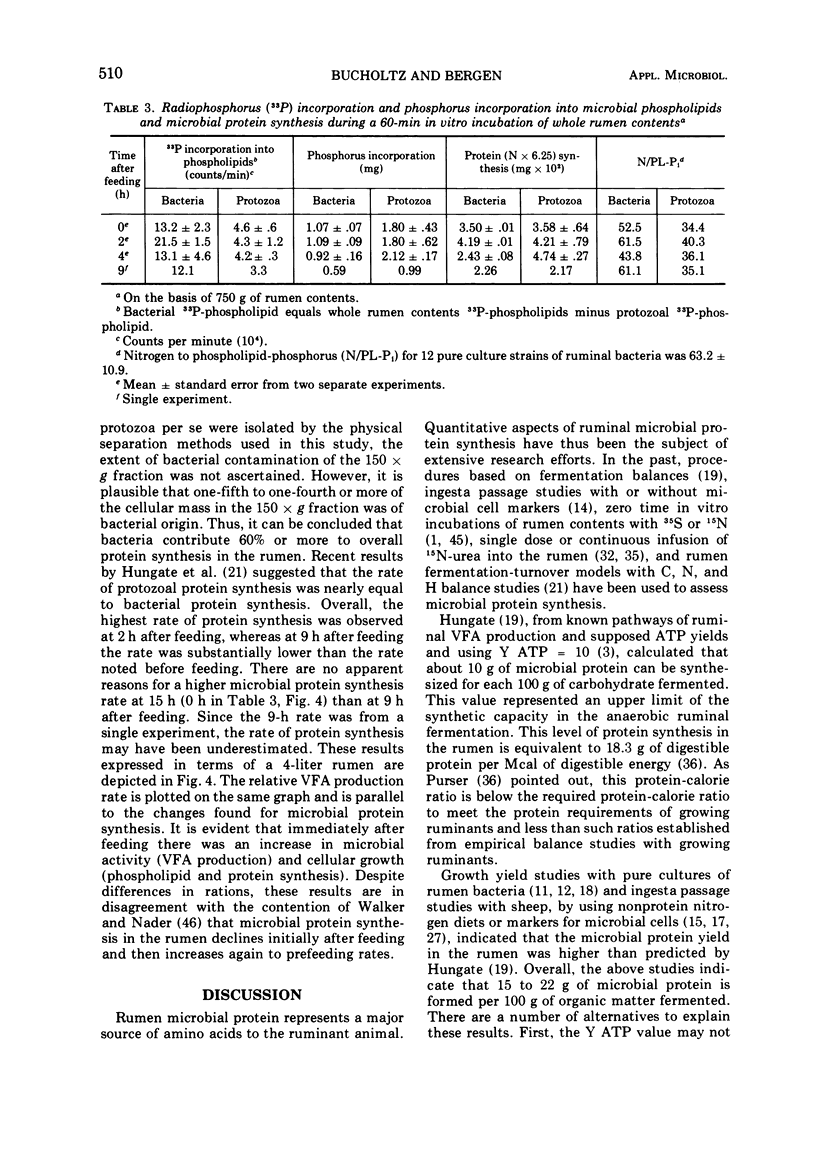
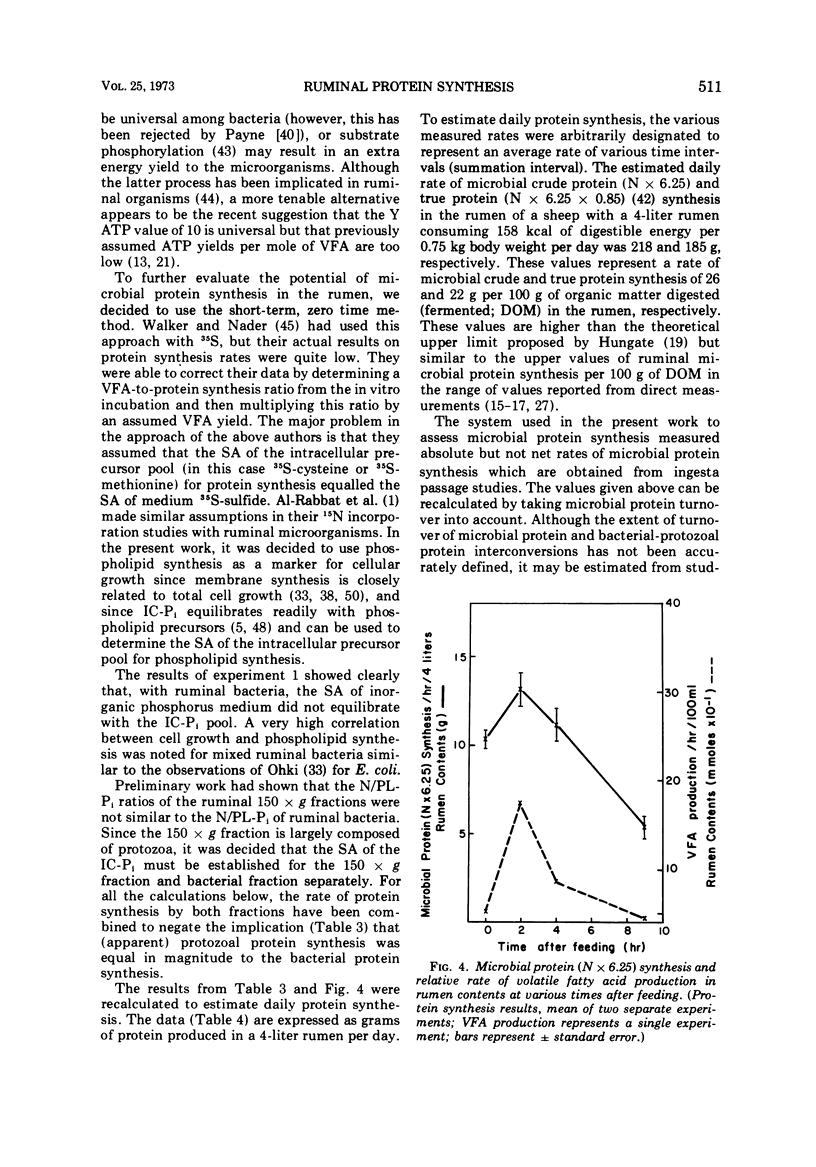
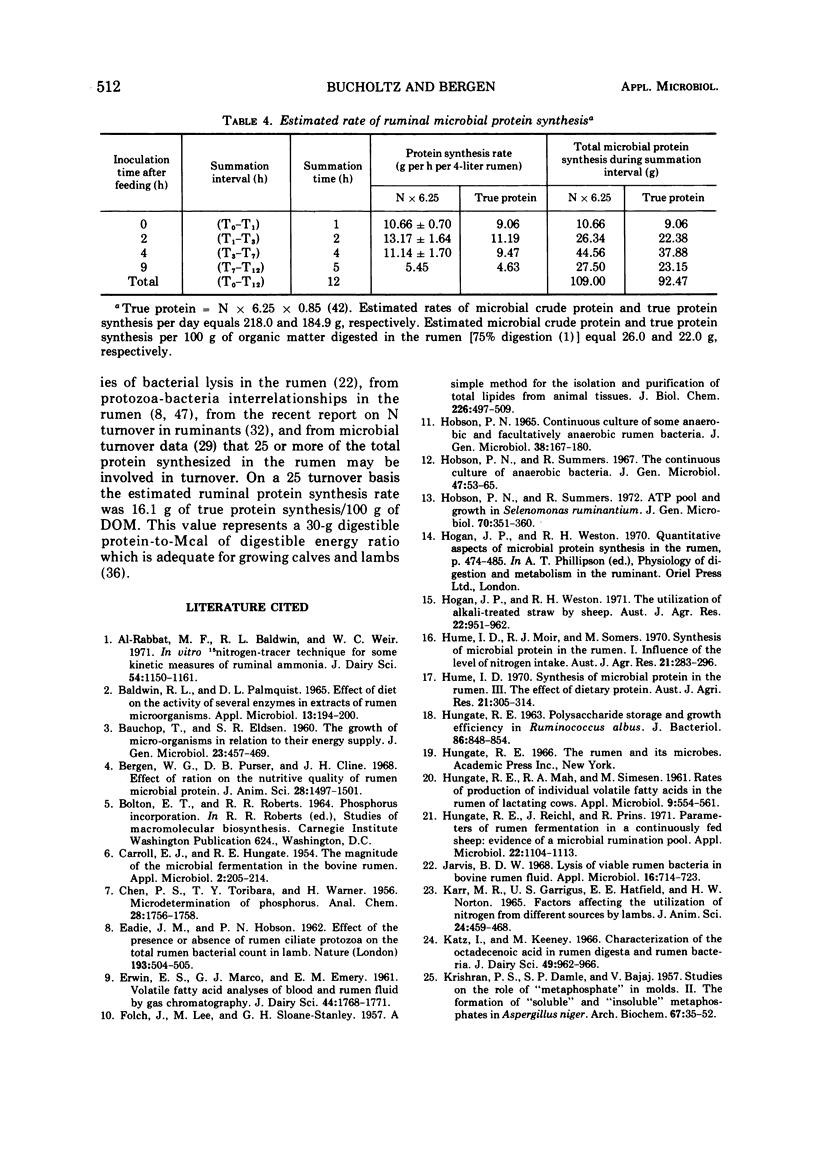
Phosphate uptake into intracellular inorganic phosphorus and cellular phospholipids and the relationship between cell growth and phospholipid synthesis were studied with suspensions of washed ruminal bacteria in vitro with 33P-phosphorus. It was shown that ruminal bacteria accumulated inorganic phosphate at a low rate when incubated without substrate. Upon the addition of substrate, the rate of inorganic phosphorus uptake into the cells increased markedly, and phospholipid synthesis and cell growth commenced. There was a highly significant relationship (r = 0.98; P < 0.01) between phospholipid synthesis and cell growth. The specific activity of the intracellular inorganic phosphorus did not equilibrate with phosphorus medium. When ruminal contents from sheep fed a high or low protein diet were incubated in vitro, the rate of 33P incorporation into microbial phospholipids was higher for the high protein diet. Since there was a high relationship between phospholipid synthesis and growth, rumen contents were collected before and various times after feeding and incubated with 33P-phosphorus in vitro. The short-term, zero time approach was used to measure the rate of microbial phospholipid synthesis in whole rumen contents. In these studies the average specific activity of the intracellular inorganic phosphorus was used to represent the precursor pool specific activity. Microbial phospholipid synthesis was then related to protein (N × 6.25) synthesis with appropriate nitrogen-to-phospholipid phosphorus ratios. Daily true protein synthesis in a 4-liter rumen was 185 g. This represents a rate of 22 g of protein synthesized per 100 g of organic matter digested. These data were also corrected for ruminal turnover. On this basis the rate of true protein synthesis in a 4-liter rumen was 16.1 g of protein per 100 g of organic matter digested. This value represents a 30-g digestible protein-to-Mcal digestible energy ratio which is adequate for growing calves and lambs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALDWIN R. L., PALMQUIST D. L. EFFECT OF DIET ON THE ACTIVITY OF SEVERAL ENZYMES IN EXTRACTS OF RUMEN MICROORGANISMS. Appl Microbiol. 1965 Mar;13:194–200. doi: 10.1128/am.13.2.194-200.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- CARROLL E. J., HUNGATE R. E. The magnitude of the microbial fermentation in the bovine rumen. Appl Microbiol. 1954 Jul;2(4):205–214. doi: 10.1128/am.2.4.205-214.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EADIE J. M., HOBSON P. N. Effect of the presence or absence of rumen ciliate protozoa on the total rumen bacterial count in lambs. Nature. 1962 Feb 3;193:503–505. doi: 10.1038/193503a0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- HOBSON P. N. CONTINUOUS CULTURE OF SOME ANEROBIC AND FACULTATIVELY ANAEROBIC RUMEN BACTERIA. J Gen Microbiol. 1965 Feb;38:167–180. doi: 10.1099/00221287-38-2-167. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E., MAH R. A., SIMESEN M. Rates of production of individual volatile fatty acids in the rumen of lactating cows. Appl Microbiol. 1961 Nov;9:554–561. doi: 10.1128/am.9.6.554-561.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. POLYSACCHARIDE STORAGE AND GROWTH EFFICIENCY IN RUMINOCOCCUS ALBUS. J Bacteriol. 1963 Oct;86:848–854. doi: 10.1128/jb.86.4.848-854.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson P. N., Summers R. The continuous culture of anaerobic bacteria. J Gen Microbiol. 1967 Apr;47(1):53–65. doi: 10.1099/00221287-47-1-53. [DOI] [PubMed] [Google Scholar]
- Hungate R. E., Reichl J., Prins R. Parameters of rumen fermentation in a continuously fed sheep: evidence of a microbial rumination pool. Appl Microbiol. 1971 Dec;22(6):1104–1113. doi: 10.1128/am.22.6.1104-1113.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis B. D. Lysis of viable rumen bacteria in bovine rumen fluid. Appl Microbiol. 1968 May;16(5):714–723. doi: 10.1128/am.16.5.714-723.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARR M. R., GARRIGUS U. S., HATFIELD E. E., NORTON H. W. FACTORS AFFECTING THE UTILIZATION OF NITROGEN FROM DIFFERENT SOURCES BY LAMBS. J Anim Sci. 1965 May;24:459–468. doi: 10.2527/jas1965.242459x. [DOI] [PubMed] [Google Scholar]
- KRISHNAN P. S., DAMLE S. P., BAJAJ V. Studies on the role of metaphosphate in molds. II. The formation of soluble and insoluble metaphosphates in Aspergillus niger. Arch Biochem Biophys. 1957 Mar;67(1):35–52. doi: 10.1016/0003-9861(57)90243-6. [DOI] [PubMed] [Google Scholar]
- Katz I., Keeney M. Characterization of the octadecenoic acids in rumen digesta and rumen bacteria. J Dairy Sci. 1966 Aug;49(8):962–966. doi: 10.3168/jds.S0022-0302(66)87990-0. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. M. Paths of phosphate transfer in Micrococcus pyogenes: phosphate turnover in nucleic acids and other fractions. J Gen Microbiol. 1953 Oct;9(2):257–272. doi: 10.1099/00221287-9-2-257. [DOI] [PubMed] [Google Scholar]
- Nolan J. V., Leng R. A. Dynamic aspects of ammonia and urea metabolism in sheep. Br J Nutr. 1972 Jan;27(1):177–194. doi: 10.1079/bjn19720081. [DOI] [PubMed] [Google Scholar]
- Oki M. Correlation between metabolism of phosphatidylglycerol and membrane synthesis in Escherichia coli. J Mol Biol. 1972 Jul 21;68(2):249–264. doi: 10.1016/0022-2836(72)90212-4. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- Pilgrim A. F., Weller R. A., Gray F. V., Belling C. B. Synthesis of microbial protein from ammonia in the sheep's rumen and the proportion of dietary nitrogen converted into microbial nitrogen. Br J Nutr. 1970 Jun;24(2):589–598. doi: 10.1079/bjn19700057. [DOI] [PubMed] [Google Scholar]
- Randle C. L., Albro P. W., Dittmer J. C. The phosphoglyceride composition of Gram-negative bacteria and the changes in composition during growth. Biochim Biophys Acta. 1969;187(2):214–220. doi: 10.1016/0005-2760(69)90030-7. [DOI] [PubMed] [Google Scholar]
- Rhee K. S., Dugan L. R., Jr The implication of acid concentration in lipid phosphorus determinations. Anal Biochem. 1967 Apr;19(1):157–165. doi: 10.1016/0003-2697(67)90145-5. [DOI] [PubMed] [Google Scholar]
- Robinson J. R. 33P: a superior radiotracer for phosphorus? Int J Appl Radiat Isot. 1969 Jul;20(7):531–540. doi: 10.1016/0020-708x(69)90007-6. [DOI] [PubMed] [Google Scholar]
- SCHERBAUM O. H. ACID-SOLUBLE PHOSPHATES IN NUCLEATE AND ENUCLEATE ACETABULARIA. I. PAPER-CHROMATOGRAPHIC PATTERNS. Biochim Biophys Acta. 1963 Aug 20;72:509–515. [PubMed] [Google Scholar]
- WELLER R. A., GRAY F. V., PILGRIM A. F. The conversion of plant nitrogen to microbial nitrogen in the rumen of the sheep. Br J Nutr. 1958;12(4):421–429. doi: 10.1079/bjn19580056. [DOI] [PubMed] [Google Scholar]
- Walker D. J. Energy utilization for polysaccharide synthesis by mixed rumen organisms fermenting soluble carbohydrates. Appl Microbiol. 1968 Nov;16(11):1672–1677. doi: 10.1128/am.16.11.1672-1677.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. J., Nader C. J. Method for measuring microbial growth in rumen content. Appl Microbiol. 1968 Aug;16(8):1124–1131. doi: 10.1128/am.16.8.1124-1131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis O. C., Coleman G. S. Incorporation of 14C-labelled components of Escherichia coli and of amino acids by Isotricha intestinalis and Isotricha prostoma from the sheep rumen. J Gen Microbiol. 1967 Nov;49(2):315–323. doi: 10.1099/00221287-49-2-315. [DOI] [PubMed] [Google Scholar]
- Weissbach H., Thomas E., Kaback H. R. Studies on the metabolism of ATP by isolated bacterial membranes: formation and metabolism of membrane-bound phosphatidic acid. Arch Biochem Biophys. 1971 Nov;147(1):249–254. doi: 10.1016/0003-9861(71)90332-8. [DOI] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during bacterial growth. J Lipid Res. 1969 Mar;10(2):220–233. [PubMed] [Google Scholar]


