Abstract
Neuropathic pain is a debilitating condition resulting from damage to sensory transmission pathways in the peripheral and central nervous system. A potential new way of treating chronic neuropathic pain is to target specific pain processing neurons based on their expression of particular receptor molecules. We hypothesized that a toxin-neuropeptide conjugate would alter pain by first being taken up by specific receptors for the neuropeptide expressed on the neuronal cells. Then, once inside the cell the toxin would inhibit the neurons’ activity without killing the neurons, thereby providing pain relief without lesioning the nervous system. In an effort to inactivate the nociceptive neurons in the trigeminal nucleus caudalis in mice we targeted the NK1 receptor (NK1R) using substance P (SP). The catalytically active light chain of botulinum neurotoxin type A (LC/A) was conjugated with SP. Our results indicate that the conjugate BoNT/A-LC:SP is internalized in cultured NK1R expressing neurons and also cleaves the target of botulinum toxin, a component docking motif necessary for release of neurotransmitters called SNAP-25. The conjugate was next tested in a mouse model of Taxol induced neuropathic pain. An intracisternal injection of BoNT/A-LC:SP decreased thermal hyperalgesia as measured by the operant orofacial nociception assay. These findings indicate that conjugates of the light chain of botulinum toxin are extremely promising agents for use in suppressing neuronal activity for extended time periods and that BoNT/A-LC:SP may be a useful agent for treating chronic pain.
Keywords: Neuropathic pain, Neurokinin receptor, Botulinum neurotoxin
Introduction
Neuropathic pain syndromes such as diabetic neuropathy, post-herpetic neuralgia, trigeminal neuralgia and chemotherapy induced neuropathy lack consistently effective therapies (1). The exact mechanism of neuropathic pain is unknown. Neuropathic pain signals, whether arising from an axonal site on primary sensory neurons or extra-junctional sites, are transmitted to the spinal dorsal horn or trigeminal nucleus caudalis (2), frequently leading to central sensitization, which is symptomatically expressed as allodynia, and hyperalgesia (3). Among the various neurotransmitter receptors the neurokinin 1 receptor (NK1R) plays an important role in the transmission of neuropathic pain. The role of NKR1 in neuropathic pain is supported by the ability of NKR1 antagonists to suppress pain in a chronic constriction injury model (4). NK1Rs are widespread in nociceptive pathways in the central nervous system and the ablation of spinal NK1R-expressing neurons is an effective strategy to reduce most types of pain. Previous research has demonstrated that delivering Saporin, a ribosome-inactivating neurotoxin, conjugated with SP (SP-SAP) can selectively kill NK1R-expressing cells. This lesion results in attenuation of hyperalgesia and allodynia by interrupting the nociceptive pathway (5–8). On the basis of this principle a variety of lethal toxins like diphtheria and pseudomonas exotoxin were conjugated with SP by various groups as potential therapeutics (9;10). Also, a non-lethal cholera toxin and SP conjugate has also been reported to selectively activate NK1R expressing neurons producing allodynia and hyperalgesia (11–13). Thus delivering targeted toxins to NK1R expressing cells is a useful means of manipulating pain.
Botulinum neurotoxin (BoNT) is the most potent among the clostridial neurotoxins and is expressed as seven serologically distinct types (BoNT/A-F) (14). Among these BoNT/A (serotype A) is considered to have more sustained action due to its ability to cleave SNAP-25 for a longer period of time (15). SNAP-25 is required to form soluble n-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complexes which are necessary for normal neurotransmitter release (16). BoNT itself is not lethal to neurons but halts Ca+2 dependent neurotransmitter release for approximately three months. The toxin is composed of a heavy chain domain and a light chain (LC) domain. The endoprotease activity of BoNT comes from the LC domain while the heavy chain acts as the cell binding and translocation domain (14–16). The LC without the heavy chain is unable to be internalized by cells (17). Here we demonstrate that a conjugate of the BoNT/A-LC and SP selectively targets NK1R expressing neurons and delivers the LC inside the NK1R neurons. Our results indicate that internalized LC cleaves the SNAP-25 of NK1R expressing neurons in vitro and we also demonstrate that this conjugate can alleviate nociceptive behavior in a chemotherapy induced neuropathic pain model as measured by a thermal operant system.
Methods
Animals
Male hairless SKH1-Hrhr mice (25–40 g) (The Jackson Laboratories, Bar Harbor, ME) and Sprague-Dawley dams (Harlan, Tampa, FL) were used for these studies. Animals were maintained in AAALAC-approved facilities with 12-hour light/dark cycles, with ad libitum access to chow and water. All experiments were reviewed and approved by the University of Florida Institutional Animal Care and Use Committee and conformed to the standards of the International Association for the Study of Pain guidelines for animal research.
Synthesis of BoNT/A-LC:SP
The gene segment encoding the light chain of botulinum neurotoxin type A was cloned into 6x His vector pET30 a+ (Novagen; Gibstown, NJ) to construct the expression plasmid for LC/A (18). The Escherichia coli [BL21-codon plus (DE3)-RIL (Stratagene: La Jolla, CA) was used as host strain for expression of recombinant polypeptide. After transformation of the bacteria by the BoNT/A-LC expression plasmid, the bacteria were grown in Terrific Broth (Sigma-Aldrich, St. Louis, MO). A final concentration of 0.5 mM of Isopropyl β-D-thiogalactopyranoside (Sigma-Aldrich, St. Louis, MO) was added in the bacterial culture when a density, measured at A600, of 0.6–0.8 was reached to induce the expression of recombinant protein. After cultivation the bacterial protein was extracted by protein extract reagents, B-PER, 0.2 mg/ml, Lysozyme (Sigma, St. Louis, MO), 0.01 mg/ml DNAse (Sigma, St. Louis, MO) and protease inhibitor cocktail kit (Thermo Scientific, Rockford, IL). The successive isolation and the purification of BoNT/A-LC by two columns [Ni-NTA agarose (Invitrogen, Grand Island, NY) and Q sepharose fast flow (GE Healthcare, USA) respectively] were done according to the methods described by Takahashi et al., (18). The purified fractions were pooled and dialyzed against phosphate buffered saline (PBS, pH 7.4). For the conjugation of the SP and BoNT/A-LC the same procedure was followed as was described by Caudle et al., (11). Briefly, the N terminal amino groups of SP located on the arginine and lysine residues (RPKPQQFFGLM-Amide) (Ana Spec Inc, CA, USA) were linked to maleimide by combining with a 5 fold excess of sulfosuccinimidyl-4-N-maleimidomethyl cyclohexane-1-carboxylte (Sulfo-SMCC) (Pierce Biotechnology Inc, USA) in PBS, pH 7.4. The mixture was incubated at room temperature for 1 hour. Unreacted Sulfo-SMCC was separated from the conjugated SP and maleimide by a sephadex G-10 (30 × 1.5 cm) column eluted with PBS. A 10 fold excess of purified conjugate was then incubated with purified BoNT/A-LC in PBS containing 5 mM β-mercaptoethanol overnight at room temperature. The BoNT/A-LC:SP conjugate was separated from unreacted SP maleimide conjugate by washing with PBS three times and concentrating by Amicon ultra (Millipore Inc, USA) with a cutoff of 10 kDa. All steps of the purified protein and conjugate were confirmed by SDS-Polyacrylamide gel electrophoresis (SDS-PAGE) and western blot using antibodies against BoNT/A-LC and SP.
Cell Culture
Primary neuronal cell cultures were prepared with protocols based on Hilgenberg and Smith (19). Cell culture vials [12 well plates (Costar) with added 12 mm round glass coverslips (Warner Instrument Corp, Hamden, CT), or 60×12mm plates (Falcon)] were coated with 0.001% Poly-L-ornithine (Sigma) and two hours later were rinsed twice with ddH20 then coated with 5μg/mL Laminin (Invitrogen). The plates and coverslips were kept in a 37°C incubator in 5% CO2 overnight. The next day an E17 rat was euthanized with CO2 followed by decapitation. Embryos were removed and placed into ice cold sterile dissecting solution (6.85mM NaCl, 0.27mM KCl, 8.5μM Na2HPO4, 11μM KH2PO4, 0.27mM Hepes, 33.3mM D (+)-Glucose, 43.8mM Sucrose, pH 7.4). Frontal areas of the fetal brains containing the diencephalon were removed and cut into pieces with razor blades and placed in 37°C dissociation solution (5mL TrypLE, and 500μL 1M Hepes, Gibco) for 10mins. Cells were dissociated with a fire polished glass pipette, incubated for 5mins, then re-dissociated and re-incubated for 5mins. After a final dissociation, the solution was centrifuged at 150g for 5mins at 4°C. The supernatant was discarded and cells were re-suspended in 5mL of 37°C media [Neurobasal, 1mM Na+ Pyruvate, 2mM L-Glutamine (Cellgro), Pen-Strep, B27 (Gibco), with 5% Fetal Bovine Serum (FBS) (Hyclone)]. Cells were counted with a hemocytometer (Hausser Scientific, Horsham, PA) and plated on either 12 well plates or 60×12 mm plates with a density of 1×106 cells/ml. The next day the media was replaced with media without FBS for the duration of the experiment.
SNAP-25 cleavage assay
Culture plates (60 mm × 12 mm) were washed with phosphate-buffered saline (PBS; 137 mM NaCl, 10 mM NaH2PO4, 2.7 mM KCl, pH 7.4) and then 200 μl of ice cold RIPA buffer (10% 10X RIPA buffer (Cell Signaling, Danvers, MA), 1% protease inhibitor cocktail (Thermo), 1% phosphatase inhibitor cocktail (Sigma), 1μM PMSF (dissolved in EtOH) (Sigma) in ddH20) was added and cells were loosened with a sterile scraper and pipetted into a 1.5mL tube. To analyze SNAP-25 cleavage, BoNT/A-LC:SP was added directly to the cultured cells at a concentration of 100 ng/ml. As a control the same amount of BoNT-LC/A was added directly in a 1.5 ml tube containing the cultured cells and incubated for 48 hrs at 25 °C in mild rotating condition. Cells were then sonicated with a Sonics Vibra-Cell Sonicator (Danbury, CT, USA) at 20 Amps for 10 seconds. Samples were centrifuged at 14,000RPM then resonicated, recentrifuged and the supernatant was retained. Protein quantification and western blot analysis were then performed as described below.
Immunocytochemistry
Primary neuronal cell cultures on coverslips were transferred to a fresh 12 well Corning plate with FBS. A concentration of 50 ng/ml of either BoNT/A-LC:SP or BoNT/A-LC (control) was added directly in the wells and incubated for 48 hrs at 25 °C. To remove unbound LC or LC:SP cells were washed 3 times with PBS and fixed in 4°C 10% Buffered Formalin Phosphate (Fisher) for 10mins at RT (room temperature), rinsed 3X for 10mins in rinse buffer (PBS with 0.1% TritonX (Fisher)), and blocked for 60mins with blocking buffer (5% Bovine Serum Albumin (Sigma) in PBS with 0.1% TritonX). Primary antibodies to the LC (1:100, anti-rabbit, generously provided by Dr. MD. Elias) and NK1R (1:100, goat, Abcam) were added to new blocking buffer and left overnight at 4°C. Cells were washed with rinse buffer 3X for 10mins, then fluorescent secondary antibodies (1:1000, Alexa Fluor 594 donkey anti-rabbit and Alexa Fluor 488 chicken anti-goat (Invitrogen)) in new blocking buffer were added. After an hour of dark incubation at RT and 3X 10mins washes in rinse buffer, cover-slips were flipped onto a slide with Mowiol mounting media (0.1% Mowiol 4–88 (Millipore), 25% glycerol, 0.1M tris, in ddH20, pH 8.5). Pictures were taken with a Photometrics cascade-cooled EMCCD camera using the Open Source software package MicroManager connected to a spinning disk confocal system with a Leica DMIRB microscope with a 63X oil immersion objective. The images were processed with ImageJ software.
Chemotherapy induced neuropathic pain model
We were initially interested in reproducing the heat hyperalgesia due to chemotherapy induced peripheral neuropathy (CIPN) (20; 21) in our operant assay. We administered the chemotherapeutic agent Taxol® (paclitaxol) (Santa Cruz Biotechnology, USA) via intraperitoneal injections (i.p.) every other day for five days with a cumulative dose of 2 mg/kg. The control group was injected with vehicle (PBS; pH 7.4 containing 1% Tween-40).
Thermal nociception assay
A reward-conflict operant testing paradigm (see Neubert et al. (22) for full methodology) was used for orofacial pain testing. In this paradigm the mice may choose to experience a nociceptive thermal stimulation in order to obtain a sweetened milk reward. They may also choose not to experience nociception by abstaining from the reward. Briefly, mice were trained to drink diluted sweetened condensed milk (1:2, milk: water) while making facial contact with two thermodes. During the training period mice were fasted for 13–15 h prior to testing. Training occurred three times per week for 4 weeks to obtain baseline behavioral data. Baseline data was obtained during two separate sessions for each stimulus temperature (37°C, and 46°C). The number of licks on the reward bottle and the number of stimulus contacts were recorded. Additionally we calculated the pain index for each mouse as described previously (22), which is the number of licks divided by the number of stimulus contacts. After baseline data acquisition we gave each animal a dose of 1μg of BONT/A-LC:SP or 1μl of PBS as control via an injection into the cisterna magna (i.c.m.). After forty eight hours of recovery the behavioral testing at each temperature was repeated.
Statistics
Data were analyzed using GraphPad Prism 4 software (La Jolla, CA). The methods of analysis were One Way ANOVA followed by Dunnett’s post-hoc test and students t-test. Significance was assigned for p≤ 0.05.
Results
Synthesis of BoNT/A-LC:SP
The neuropeptide substance P was coupled to the catalytic domain (LC) of BoNT/A by using the bifunctional linking agent Sulfo-SMCC as indicated in Figure 1A. First the Sulfo-SMCC reacted with the N-terminal amino groups on the arginine and lysine residues of substance P to form a linkage to the maleimide group. The substance P-maleimide was then conjugated to the LC of BoNT/A through the free cysteine residues on surface of the LC. The final product was washed to remove unconjugated residues and concentrated by membrane filters with a cutoff of 10 kDa. The conjugation was confirmed by western blot using an antibody against substance P and one against the LC of BoNT/A. As demonstrated in Figure 1B, bands appear on the blot with a molecular weight of approximately 50 kDa with both antibodies.
Figure 1.
Synthesis of BoNT/A-LC:SP. A Schematic representation of the procedure used to synthesize BoNT/A-LC:SP. B. Western blots of the final BoNT/A-LC:SP product. The concentrated conjugate was run on western blot and probed with both anti-BoNT/A-LC and anti SP.
In vitro analysis of BoNT/A-LC:SP
BoNT/A-LC:SP was tested on neuronal cells cultured from the embryonic diencephalon area of the brain. This area expresses NK1R (23). To verify selective uptake of SP-LC/A, the cells were incubated with either BoNT/A-LC:SP (50 ng/ml) or BoNT/A-LC (50 ng/ml). The neuronal cells were then fixed and prepared for immunocytochemistry with antibodies to LC/A and NK1R. Fig 2A (upper panel) demonstrates the internalization of BoNT/A-LC:SP in the neuronal cells where the conjugate became localized in the periplasmic area. The BoNT/A-LC alone was not internalized by the neuronal cells as illustrated by Fig 2A (lower panel). This result implies that LC/A can be selectively targeted to NK1R expressing cells by conjugating it to SP. Figure 2B also indicates the conjugate was able to cleave SNAP-25 as there was a significant decrease in SNAP-25 in BoNT/A-LC:SP treated sample.
Figure 2.
In situ evaluation of BoNT/A-LC:SP. A. Cultured neuronal cells from the diencephalon of rat embryos were treated with either BoNT/A-LC:SP (50 ng/ml) or BoNT/A-LC (50 ng/ml) only. The cells were incubated at 25 °C overnight. The immunocytochemistry of the cells was performed using antibodies against LC/A and NK1R. The arrow head indicates the area where LC/A localized after internalization. B. Cleavage of SNAP-25 by BoNT/A-LC:SP. Cultured neuronal cells were incubated with either BoNT/A-LC (100 ng/ml) or BoNT/A-LC:SP (100 ng/ml) at 25 °C for 48 hours. A control was run treating the cells with PBS only. The unbound toxin or toxin conjugate was washed away with PBS. The cells were sonicated and the SNAP-25 cleavage was analyzed by western blot using anti-SNAP-25 antibodies.
Assessment of CIPN in thermal operant system
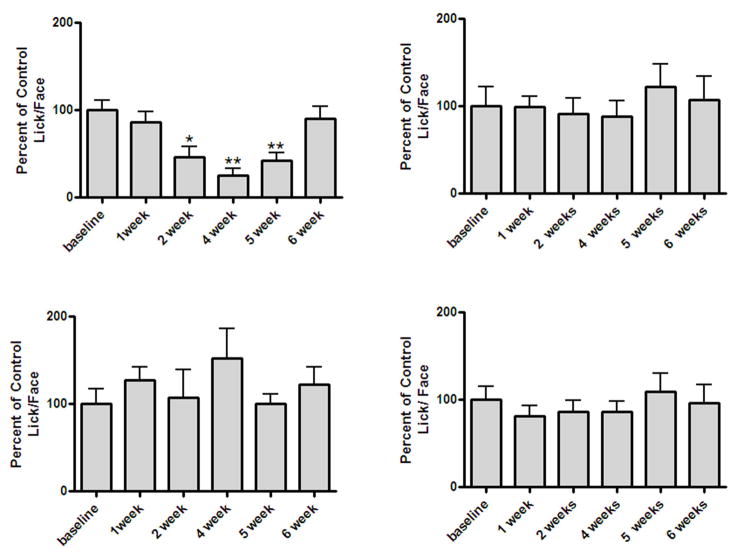
The thermal operant assay showed the development of CIPN induced hyperalgesia when the baseline behavior was compared to the post Taxol® treatment behavior. Figure 3A and figure 3B demonstrate that Taxol® injections induced the development of thermal hyperalgesia at 46 °C in mice by week 2 and that the hyperalgesia lasts until week 5 (Fig. 3A ), one way ANOVA (F(5, 85) =10.43, p < 0.05). No significant change was observed for the group treated with vehicle (Fig. 3B), one way ANOVA (F(5, 35) = 1.037, p > 0.05). At the non-aversive temperature (37 °C) there was no significant change in their L/F behavior observed for both Taxol® and vehicle treated mice (Fig. C and Fig. D); one way ANOVA (F(5, 85) =0.4318, p > 0.05) and one way ANOVA (F(5, 35) =2.081, p > 0.05).
Figure 3.
Hyperalgesic effect of Taxol in mice at 46 °C. A. Mice (N=16) received a cumulative 2 mg/kg dose of Taxol and were tested in a thermal operant system as described in the methods section. A * indicates p<0.05 and a ** indicates p<0.01 for week 3 and week 4 respectively in a Repeated Measures One-Way ANOVA followed by Dunnett’s test. B. Mice (N=8) received vehicle for Taxol and tested in the same device. There was no significant change in behavior at 46 °C for the vehicle group as evidenced by P>0.05 in a One-Way Repeated Measures ANOVA followed by Dunnett’s test. C and D. Thermal operant response at 37 °C for the above Taxol receiving group (N=16) and vehicle group (N=8) with a P>0.05 for both groups indicating no significant change in behavior at non-nociceptive temperature.
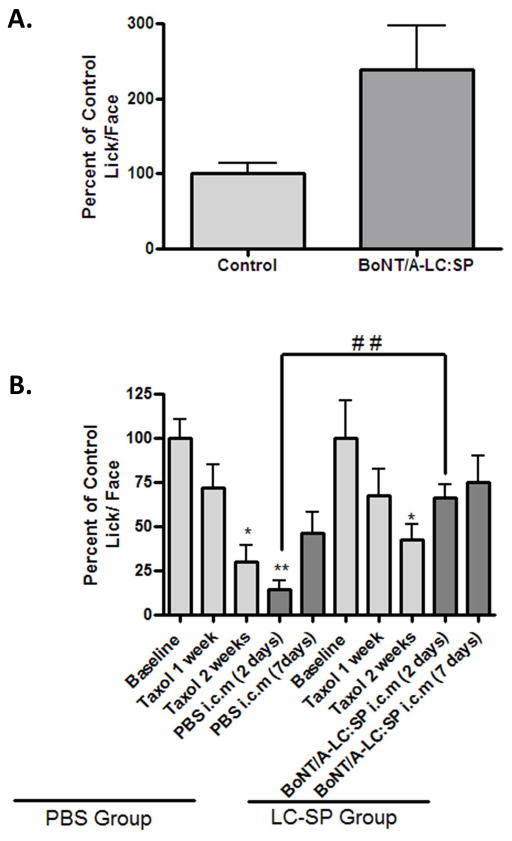
Anti-nociceptive effect of BoNT/A-LC:SP
The conjugate BoNT/A-LC:SP was found to be anti-nociceptive when comparing the outcome measures of a group of mice that received a 200 ng dose of conjugate i.c.m (n=9) to that of another group (n=3) given PBS i.c.m. as control at 46°C (Fig. 4A, t=2.336; df=8, p<0.05). To evaluate if BoNT/A-LC:SP can reduce neuropathic pain we used a new cohort of mice separated into two groups, PBS group and LC-SP group, all of which received 2 mg/Kg of Taxol® as described above. After two weeks the animals in both of the groups developed significant neuropathic pain which was evident from their increased sensitivity to an aversive temperature (46 °C) (Fig. 4B). For the PBS treatment group ANOVA F(4, 28) =1.045 and for LC-SP group F(4, 28)=2.269. Following testing at the two week time point the LC-SP group of animals were injected with 2μl PBS containing 1 μg BoNT/A-LC:SP (i.c.m) and the PBS group was injected with 2 μl PBS. Forty eight hours following the injections the SP-LC/A group demonstrated significant improvement in their L/F ratio compared to the PBS injected group. An unpaired t test between 2 days after the PBS i.c.m (14.26 ± 4.981) and BoNT/A-LC:SP i.c.m (66.10 ±7.705) showed significant recovery from acute Taxol® induced hypersensitivity [ t =5.650, df=14, P<0.0001 ].
Figure 4.
Antinociceptive effect of SP-LC/A. A. Mice received BoNT/A-LC:SP (N=9) or PBS (N=3) by i.c.m were analyzed for their behavioral response at noxious temperature (46 °C) in the thermal operant system after 2 weeks of training at 37 °C and 46 °C alternatively. A* indicates p<0.05 in an unpaired t-test with Welch’s correction. B. Both the BoNT/A-LC:SP (N=8) and PBS group (N=8) were treated with Taxol and their behavior to a noxious temperature (46 °C) was measured with the thermal operant system for two weeks. After 2 weeks each mouse of the LC-SP group received 1μg of BoNT/A-LC:SP in 2μl i.c.m and each mouse in the PBS group received 2 μl of PBS i.c.m. After 48 hours both groups were tested for pain response in thermal operant system for up to two consecutive weeks. A* indicate P<0.05 and a ** indicates P<0.001 in a Repeated Measures One-Way ANOVA followed by Dunnett’s test. A ## indicates significant comparison in an unpaired t-test (P< 0.0001) between the BoNT/A-LC:SP and PBS treated group 2 days after the treatment.
Discussion
The targeted delivery of toxins to specific neuronal cells is a relatively new approach in pain management. The specificity of receptor and ligand enable this targeted delivery where the ligand acts as the carrier for the toxin. Among these receptors g-protein coupled receptors (GPCRs) are of particular interest because they have the ability to bind a large variety of ligands. The range of these ligands comprises lipids (e.g., prostanoids), sugars recognized by taste receptors, volatile organic acid recognized by odorant receptors, amino acids (e.g., glutamate), inorganic ions (e.g., Ca2+), nucleosides and nucleotides (e.g., adenosine, ATP), biogenic amines (e.g., dopamine, norepinephrine), peptides (e.g., secretin, gastrin, vasopressin, oxytocin), large proteins (e.g., TSH/thyrotropin, the gonadotropins) (24;25). Following binding the complex is internalized by receptor mediated endocytosis (9;11;12). This particular ability of GPCRs has been exploited to deliver bacterial toxins to inhibit the neuronal action of nociceptors. The first use of this approach was a SAP-SP conjugate and it was reported as a novel therapeutic approach to reduce pain (9). Since then a number of lethal and non-lethal toxins (i.e. diptheria toxin and cholera toxin ) were used to fortify this concept of targeting cells via their g-protein coupled receptors (GPCRs) (9–12). Similarly clostridial neurotoxins like BoNTs and Tetanus neurotoxins have the potential to be used in this field. BoNT/A is considered the most potent neurotoxin and has been used to a limited extent in pain management; however, it can cause the dysfunction of both motor neurons and sensory neurons (26). Previous studies have found that the targeting of botulinum toxin to both types of neurons is due to its ganglioside receptors binding domain, also known as the heavy chain domain. The intracellular toxicity is caused by the light chain domain’s endoproteolytic activity (14). Both of these domains have been cloned and expressed separately. Taking advantage of the ability to express light chain alone we developed a conjugate of SP and the LC of Botulinum toxin serotype A in order to target NK1R expressing cells. The LC/A region of the conjugate retained the endoproteolytic activity, which was evidenced by its ability to degrade SNAP-25 once it was internalized in our primary neuronal cell cultures. The rationale behind constructing this conjugate is that while saporin and some other toxins kill the targeted cells, BoNT/A-LC inactivates the cells and halts neurotransmitter release for long periods of time without causing cell death. BoNT/A can suppress neurotransmitter release for several months (14; 27). Therefore, BoNT/A-LC: SP is an alternative to using lethal toxins that may prove more advantageous in producing long lasting analgesia by avoiding the unintended consequences of neuronal cell death.
The recombinant BoNT/A-LC was expressed in Escherichia coli and purified by affinity and gel chromatography to achieve a homogenous product. The treatment did not alter the endoproteolytic activity of the BoNT/A-LC as evidenced by the in vitro SNAP-25 cleavage assay (Fig 2B). Although it has not been determined where SP attaches to BoNT/A-LC, this conjugation method binds SP to exposed cysteine residues (28). In cultured neurons the NK1R expressing cells internalized the conjugate and immunocytochemistry demonstrated that the conjugate was localized in the plasma membrane (Fig 2A). The subcellular localization of BoNT/A-LC in neuronal cells was previously demonstrated in the plasma membrane by Ester et al (29). Similar results were observed with retargeted clostridial neuropeptidase as these have been reported to be anti-nociceptive in in-vivo models of pain (30). In this study a dimodular catalytically active BoNT/A consisting of the N-terminal domain of the heavy chain and light chain of BoNT/A (100 kDa) was conjugated with Erythrina cristagalli lectin (ECL) to make LHN/A-ECL. This conjugate was shown to be an effective long-term analgesic when administered intrathecally in mice (26). However, it has been suggested that part of the heavy chain of BoNT/A is enough to translocate the toxin into the cytosol (31). The presence of part of the heavy chain of BoNT/A in a conjugate may target it to both motor and sensory neurons. Therefore, the use of the light chain (LC) domain alone would be a better choice since it is incapable of translocation on its own. Conjugation of SP to the LC of BoNT/A makes it specific to the NK1R expressing cells. The success of such conjugates or toxin-SP fusion proteins has already been reported (5; 6; 9). We next demonstrated efficacy of this conjugate to treat an animal model of Taxol®-induced chronic neuropathic pain. Taxol has been reported to cause neuropathic pain in both rodents and humans (32–34). The thermal operant system has been proven successful in assessing orofacial pain in a variety of pain models(22; 35–40). Previously Taxol was reported to induce mechanical allodynia and heat hyperalgesia. However, those data came from reflex assessments of nociception like the Hargreaves apparatus, Plantar test, and the tail immersion test (20;21). No study so far has been performed using a similar operant system in the assessment of CIPN in an animal model. Our data indicate Taxol causes a significant level of thermal hyperalgesia in mice at the 3rd, 4th and 5th weeks at 46 °C (Fig 2A), While the control group had no significant alteration in their L/F behavior (Fig 2B). It should be noted that the average paclitaxel neuropathy in humans start in the 5th week of their treatment (32). Our results are comparable with a 4 mg/kg i.p. dose of paclitaxel which caused neuropathic pain in mice after 2 weeks (41) and also 4.5 mg/kg i.p. dose after 2 weeks (46). Our operant based system is able to detect CIPN after just 2 weeks with a lower dose of Taxol® (2 mg/kg i.p.). Hence, this method is more sensitive than other indirect assay methods in assessing CIPN. In a new set of Taxol® treated neuropathic mice we treated half with the conjugate and half with vehicle after two weeks. The BoNT/A-LC:SP group recovered from neuropathic pain faster than the PBS treated group (Fig 4B). The greater sensitivity of our operant assay allowed us to use a significantly lower cumulative dose to induce signs of CINP in this rodent model. This is likely the reason that our animal model recovered back to the baseline response by 5 weeks. The rapid recovery thus prevented the assessment of longer duration of activity of BoNT/A-LC. However, our study suggests that a conjugate of BoNT/A-LC:SP has the potential to reduce CIPN long term when injected intracisternally. Work by Chaddock et al. suggests that the duration of anti-nociceptive activity should be in excess of 40 days (30).
Although some drugs have been reported to prevent CIPN in rodents and other toxin-based drugs exist, they all have disadvantages which our conjugate does not. Gabapentin, thalidomide and minocycline have been reported to prevent Taxol induced neuropathic pain in rodents(21;42). Gabapentin is an anticonvulsant drug that inhibits high threshold Ca+2 channels and modulates synaptic neurotransmission. Thalidomide and minocycline are both immunomodulatory drugs. A therapeutic concern in the use of these drugs is that they would be inappropriate to use in neurologically impaired and/or immunosuppressed patients. In contrast, any therapeutic approach that can block the ascending pain signals by blocking the neurotransmission from specific pain sensing fibers would be a better approach. Our conjugate also has advantages over other analgesia-producing toxin-based drugs. Unlike saporin and diphtheria toxin which kill neurons by inhibiting ribosomes, the endoprotease activity of the LC only blocks neurotransmitter release by the inhibition of SNARE complex formation leaving the neurons intact and alive (9–15).
In conclusion, here we report a new therapeutic candidate BoNT/A-LC:SP to treat neuropathic pain by inactivating NK1R type neuronal cells. Our rodent model demonstrates that this conjugate has significant therapeutic potential for the treatment of neuropathic pain, including those induced by chemotherapeutic agents, due to its low potential for adverse effects as previously discussed. This promising area warrants further investigation based on these preliminary data.
Acknowledgments
This work was supported by National Institutes of Health DA030044. The authors would like to thank Dr. MD Elias for kindly providing the plasmid and rabbit anti serum for BoNT/A-LC and Mrs. Jenny Holt for her assistance in behavioral procedure.
Footnotes
Conflict of interest statement
All authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Dray A. Neuropathic pain: emerging treatments. British Journal of Anaesthesia. 2008;101(1):48–58. doi: 10.1093/bja/aen107. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429(1–3):23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 3.Caudle R, Perez F, Valle-Pinero A, Iadarola M. Spinal cord NR1 serine phosphorylation and NR2B subunit suppression following peripheral inflammation. Molecular Pain. 2005;1(1):25. doi: 10.1186/1744-8069-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill CM, Coderre TJ. Attenuation of hyperalgesia in a rat model of neuropathic pain after intrathecal pre-or post –treatment with a neurokinin-1 antagonist. Pain. 2002;95 (3):277–285. doi: 10.1016/S0304-3959(01)00410-9. [DOI] [PubMed] [Google Scholar]
- 5.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, et al. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 6.Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. Journal of Neuroscience. 2002;22:9086–9098. doi: 10.1523/JNEUROSCI.22-20-09086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286(5444):1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- 8.Vierck CJ, Kline RH, Wiley RG. Intrathecal substance p-saporin attenuates operant escape from nociceptive thermal stimuli. Neuroscience. 2003;119(1):223–232. doi: 10.1016/s0306-4522(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 9.Benoliel R, Eliav E, Mannes AJ, Caudle RM, Leeman S, Iadarola MJ. Actions of intrathecal diphtheria toxin-substance P fusion protein on models of persistent pain. Pain. 1999;79 (2–3):243–253. doi: 10.1016/s0304-3959(98)00170-5. [DOI] [PubMed] [Google Scholar]
- 10.Saka E, Iadarola M, FitzGerald DJ, Graybiel AM. Local circuit neurons in the striatum regulate neural and behavioral responses to dopaminergic stimulation. Proc Natl Acad Sci USA. 2002;99:9004–9009. doi: 10.1073/pnas.132212499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caudle R, Mannes A, Keller J, Perez F, Suckow S, Neubert J. Sensitization of spinal cord nociceptive neurons with a conjugate of substance P and cholera toxin. BMC Neuroscience. 2007;8(1):30. doi: 10.1186/1471-2202-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caudle RM, Mannes AJ, Benoliel R, Eliav E, Iadarola MJ. Intrathecally administered cholera toxin blocks allodynia and hyperalgesia in persistent pain models. Journal of Pain. 2001;2:118–127. doi: 10.1054/jpai.2000.19948. [DOI] [PubMed] [Google Scholar]
- 13.Caudle RM, King C, Nolan TA, Suckow SK, Vierck J, Neubert JK. Central sensitization in the trigeminal nucleus caudalis produced by a conjugate of substance P and the A Subunit of Cholera Toxin. The Journal of Pain. 2010;11(9):838–846. doi: 10.1016/j.jpain.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson LL. Identification of the characteristics that underlie botulinum toxin potency: Implications for designing novel drugs. Biochimie. 2000;82(9–10):943–953. doi: 10.1016/s0300-9084(00)01169-x. [DOI] [PubMed] [Google Scholar]
- 15.Keller JE, Neale EA. The Role of the Synaptic Protein SNAP-25 in the Potency of Botulinum Neurotoxin Type A. Journal of Biological Chemistry. 2001;276(16):13476–13482. doi: 10.1074/jbc.M010992200. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Zurawski TH, Meng J, Lawrence G, Olango WM, Finn DP, et al. A dileucine in the protease of Botulinum toxin A underlies its long-lived neuroparalysis: transfer of longevity to a novel potential therapeutic. Journal of Biological Chemistry. 2011;286(8):6375–6385. doi: 10.1074/jbc.M110.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunger AT, Breidenbach MA, Jin R, Fischer A, Santos JS, Montal M. Botulinum neurotoxin heavy chain belt as an intramolecular chaperone for the light chain. Plos Pathogens. 2007;3(9):1191–1194. doi: 10.1371/journal.ppat.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi T, Joshi SG, Al Saleem F, Ancharski D, Singh A, Nasser Z, et al. Localization of the sites and characterization of the mechanisms by which anti-light chain antibodies neutralize the actions of the botulinum holotoxin. Vaccine. 2009;27(19):2616–2624. doi: 10.1016/j.vaccine.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilgenberg LG, Smith MA. Preparation of dissociated mouse cortical neuron cultures. J Vis Exp. 2007;(10):562. doi: 10.3791/562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. Description of a short-term Taxol-induced nociceptive neuropathy in rats. Brain Research. 2000;887(2):239–249. doi: 10.1016/s0006-8993(00)02910-3. [DOI] [PubMed] [Google Scholar]
- 21.Cata JP, Weng HR, Dougherty PM. The effects of thalidomide and minocycline on taxol-induced hyperalgesia in rats. Brain Research. 2008;1229:100–110. doi: 10.1016/j.brainres.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116(3):386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Delgado-Morales R, del Rio E, Gomez-Roman A, Bisagno V, Nadal R, De Felipe C. Adrenocortical and behavioural response to chronic restraint stress in neurokinin-1 receptor knockout mice. Physiol Behav. 2012;105(3):669–675. doi: 10.1016/j.physbeh.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov. 2012;11(8):603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- 25.De Amici M, Dallanoce C, Holzgrabe U, Tränkle C, Mohr K. Allosteric ligands for G protein-coupled receptors: A novel strategy with attractive therapeutic opportunities. Med Res Rev. 2010;30(3):463–549. doi: 10.1002/med.20166. [DOI] [PubMed] [Google Scholar]
- 26.Stancombe PR, Masuyer G, Birch-Machin I, Beard M, Foster KA, Chaddock JA. Engineering botulinum neurotoxin domains for activation by toxin light chain. Febs Journal. 2012;279(3):515–523. doi: 10.1111/j.1742-4658.2011.08444.x. [DOI] [PubMed] [Google Scholar]
- 27.Sloop RR, Cole BA, Escutin RO. Human response to botulinum toxin injection: Type B compared with type A. Neurology. 1997;49(1):189–194. doi: 10.1212/wnl.49.1.189. [DOI] [PubMed] [Google Scholar]
- 28.Antharavally B, Tepp W, DasGupta BR. Status of Cys Residues in the Covalent Structure of Botulinum Neurotoxin Types A, B, and E. Journal of Protein Chemistry. 1998;17(3):187–196. doi: 10.1023/a:1022572332150. [DOI] [PubMed] [Google Scholar]
- 29.Fernández-Salas E, Ho H, Garay P, Steward LE, Aoki KR. Is the light chain subcellular localization an important factor in botulinum toxin duration of action? Mov Disord. 2004;19(S8):S23–S34. doi: 10.1002/mds.20006. [DOI] [PubMed] [Google Scholar]
- 30.Chaddock JA, Purkiss JR, Alexander FCG, Doward S, Fooks SJ, Friis LM. Retargeted clostridial endopeptidases: Inhibition of nociceptive neurotransmitter release in vitro, and antinociceptive activity in in vivo models of pain. Mov Disord. 2004;19(S8):S42–S47. doi: 10.1002/mds.20008. [DOI] [PubMed] [Google Scholar]
- 31.Fischer A, Mushrush DJ, Lacy DB, Montal M. Botulinum Neurotoxin devoid of receptor binding domain translocates active protease. PLoS Pathog. 2008;4(12):e1000245. doi: 10.1371/journal.ppat.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudhry V, Cornblath DR, Polydefkis M, Ferguson A, Borrello I. Characteristics of bortezomib- and thalidomide- induced peripheral neuropathy. J Periph Nerv Sys. 2008;13:275–282. doi: 10.1111/j.1529-8027.2008.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsyth PA, Balmaceda C, Peterson K, Seidman AD, Brasher P, DeAngelis LM. Prospective study of paclitaxel-induced peripheral neuropathy with quantitative sensory testing. J Neurooncol. 1997;35(1):47–53. doi: 10.1023/a:1005805907311. [DOI] [PubMed] [Google Scholar]
- 34.Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sciences. 2004;74(21):2593–2604. doi: 10.1016/j.lfs.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Rossi HL, Jenkins AC, Kaufman J, Bhattacharyya I, Caudle RM, Neubert JK. Characterization of bilateral trigeminal constriction injury using an operant facial pain assay. Neuroscience. 2012;224:294–306. doi: 10.1016/j.neuroscience.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nolan TA, Price DD, Caudle RM, Murphy NP, Neubert JK. Placebo-induced analgesia in an operant pain model in rats. Pain. 2012;153(10):2009–2016. doi: 10.1016/j.pain.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolan TA, Hester J, Bokrand-Donatelli Y, Caudle RM, Neubert JK. Adaptation of a novel operant orofacial testing system to characterize both mechanical and thermal pain. Behavioural Brain Research. 2011;217(2):477–480. doi: 10.1016/j.bbr.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neubert J, King C, Malphurs W, Wong F, Weaver J, Jenkins A, et al. Characterization of mouse orofacial pain and the effects of lesioning TRPV1-expressing neurons on operant behavior. Molecular Pain. 2008;4(1):43. doi: 10.1186/1744-8069-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neubert JK, Rossi HL, Malphurs W, Vierck J, Caudle RM. Differentiation between capsaicin-induced allodynia and hyperalgesia using a thermal operant assay. Behavioural Brain Research. 2006;170(2):308–315. doi: 10.1016/j.bbr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Rossi HL, Neubert JK. Effects of environmental enrichment on thermal sensitivity in an operant orofacial pain assay. Behavioural Brain Research. 2008;187(2):478–482. doi: 10.1016/j.bbr.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumoto M, Inoue M, Hald A, Xie W, Ueda H. Inhibition of Paclitaxel-Induced A-Fiber Hypersensitization by Gabapentin. Journal of Pharmacology and Experimental Therapeutics. 2006;318(2):735–740. doi: 10.1124/jpet.106.103614. [DOI] [PubMed] [Google Scholar]
- 42.Mo M, Erdelyi I, Szigeti-Buck K, Benbow JH, Ehrlich BE. Prevention of paclitaxel-induced peripheral neuropathy by lithium pretreatment. FASEB J. 2012;26(11):4696–4709. doi: 10.1096/fj.12-214643. [DOI] [PMC free article] [PubMed] [Google Scholar]