Abstract
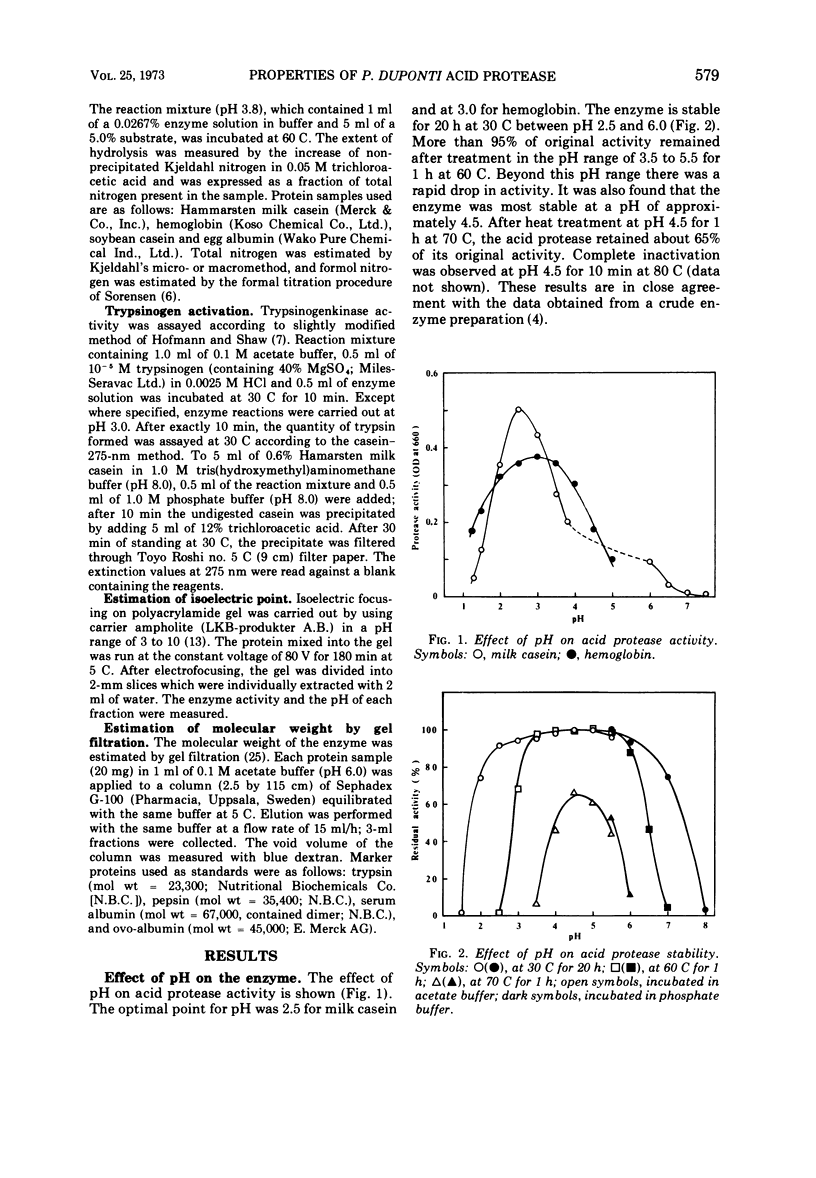
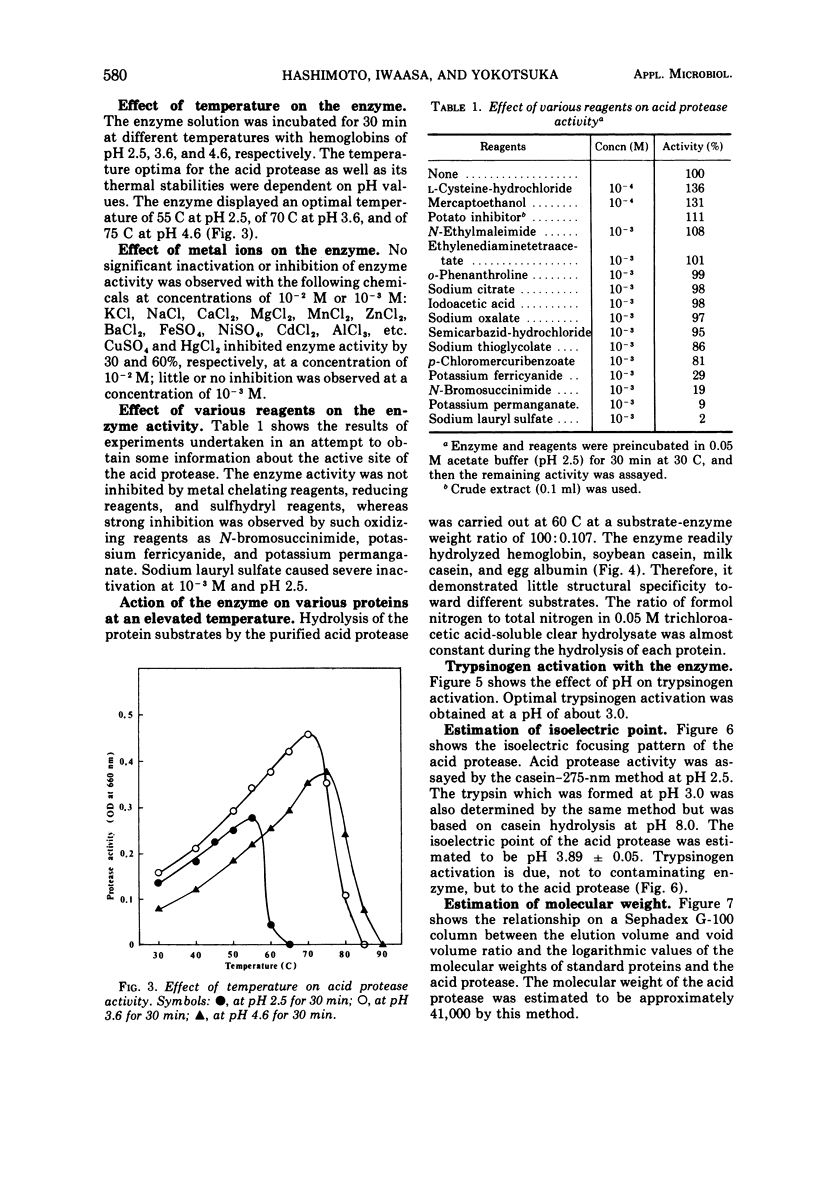
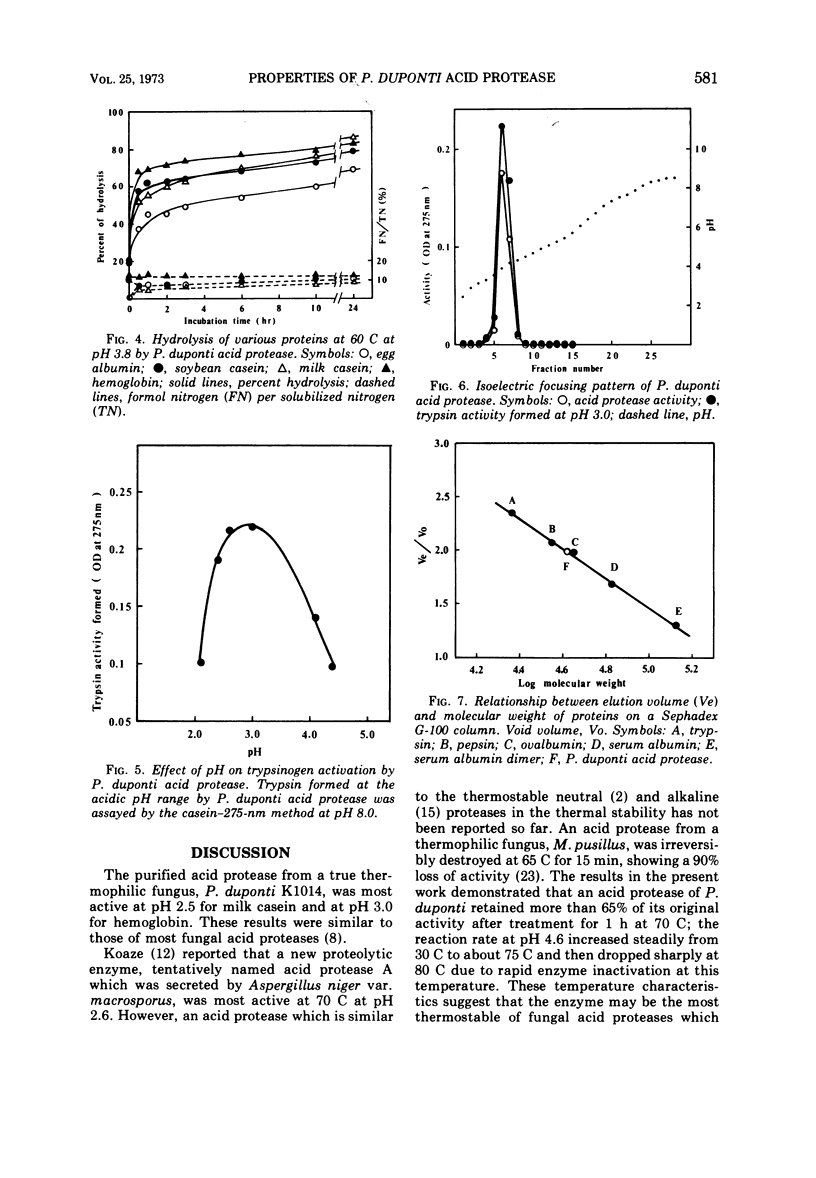
A purified acid protease from a true thermophilic fungus, Penicillium duponti K1014, was most active at pH 2.5 for milk casein and at pH 3.0 for hemoglobin. The enzyme was stable at a pH range of 2.5 to 6.0 at 30 C for 20 h. The acid protease retained full activity after 1 h at 60 C at a pH range between 3.5 and 5.5. At the most stable pH of 4.5, more than 65% of its activity remained after heat treatment for 1 h at 70 C. These thermal properties show the enzyme as a thermophilic protein. The enzyme activity was strongly inhibited by sodium lauryl sulfate and oxidizing reagents such as potassium permanganate and N-bromosuccinimide. No inhibition was caused by chelating reagents, potato inhibitor, and those reagents which convert sulfhydryl groups to mercaptides. Reducing reagents showed an activating effect. The enzyme showed the trypsinogen-activating property at an acidic pH range; optimal trypsinogen activation was obtained at a pH of approximately 3.0. The isoelectric point of the enzyme was estimated to be pH 3.89 by disk electrofocusing. By using gel filtration, an approximate value of 41,000 was estimated for the molecular weight.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima K., Yu J., Iwasaki S., Tamura G. Milk-clotting Enzyme from Microorganisms: V. Purification and Crystallization of Mucor Rennin from Mucor pusillus var. Lindt. Appl Microbiol. 1968 Nov;16(11):1727–1733. doi: 10.1128/am.16.11.1727-1733.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN T., SHAW R. PROTEOLYTIC ENZYMES OF PENICILLIUM JANTHINELLUM. I. PURIFICATION AND PROPERTIES OF A TRYPSINOGEN-ACTIVATING ENZYME (PEPTIDASE A). Biochim Biophys Acta. 1964 Dec 23;92:543–557. [PubMed] [Google Scholar]
- Hashimoto H., Iwaasa T., Yokotsuka T. Thermostable acid protease produced by Penicillium duponti K1014, a true thermophilic fungus newly isolated from compost. Appl Microbiol. 1972 Dec;24(6):986–992. doi: 10.1128/am.24.6.986-992.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Kaneko Y., Iwaasa T., Wokotsuka T. Production and purification of acid protease from the thermophilic fungus, Penicillium duponti K1014. Appl Microbiol. 1973 Apr;25(4):584–588. doi: 10.1128/am.25.4.584-588.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains G., Takahashi M., Sodek J., Hofmann T. The specificity of penicillopepsin. Can J Biochem. 1971 Oct;49(10):1134–1149. doi: 10.1139/o71-164. [DOI] [PubMed] [Google Scholar]
- Ottesen M., Rickert W. The isolation and partial characterization of an acid protease produced by Mucor miehei. C R Trav Lab Carlsberg. 1970;37(14):301–325. [PubMed] [Google Scholar]
- Rickert W. The degradation of the B-chain of oxidized insulin by Mucor miehei protease. C R Trav Lab Carlsberg. 1970;38(1):1–17. [PubMed] [Google Scholar]
- Sodek J., Hofmann T. A pepsin-like enzyme from Penicillium janthinellum. J Biol Chem. 1968 Jan 25;243(2):450–451. [PubMed] [Google Scholar]
- Sodek J., Hofmann T. Large-scale preparation and some properties of penicillopepsin, the acid proteinase of Penicillium janthinellum. Can J Biochem. 1970 Apr;48(4):425–431. doi: 10.1139/o70-069. [DOI] [PubMed] [Google Scholar]
- Somkuti G. A., Babel F. J. Purification and properties of Mucor pusillus acid protease. J Bacteriol. 1968 Apr;95(4):1407–1414. doi: 10.1128/jb.95.4.1407-1414.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


