Significance
Myosin V, a two-headed motor protein, ferries cellular cargo by walking hand-over-hand on actin filaments. Interplay between ATP-driven conformational changes in the heads and stress due to load produces a variety of stepping dynamics: The motor can step forward or backward, or “stomp,” where one head detaches and rebinds to the same site. We created an analytically solvable theory capturing all these behaviors, quantitatively matching a wide array of single-molecule experiments. We describe the structural and chemical design principles underlying the motor’s robust function, providing a guide for how bioengineering might alter its dynamics.
Keywords: functional robustness, polymer physics, architectural basis, stall force expression
Abstract
The molecular motor myosin V (MyoV) exhibits a wide repertoire of pathways during the stepping process, which is intimately connected to its biological function. The best understood of these is the hand-over-hand stepping by a swinging lever arm movement toward the plus end of actin filaments. Single-molecule experiments have also shown that the motor “foot stomps,” with one hand detaching and rebinding to the same site, and back-steps under sufficient load. The complete taxonomy of MyoV’s load-dependent stepping pathways, and the extent to which these are constrained by motor structure and mechanochemistry, are not understood. Using a polymer model, we develop an analytical theory to describe the minimal physical properties that govern motor dynamics. We solve the first-passage problem of the head reaching the target-binding site, investigating the competing effects of backward load, strain in the leading head biasing the diffusion in the direction of the target, and the possibility of preferential binding to the forward site due to the recovery stroke. The theory reproduces a variety of experimental data, including the power stroke and slow diffusive search regimes in the mean trajectory of the detached head, and the force dependence of the forward-to-backward step ratio, run length, and velocity. We derive a stall force formula, determined by lever arm compliance and chemical cycle rates. By exploring the MyoV design space, we predict that it is a robust motor whose dynamical behavior is not compromised by reasonable perturbations to the reaction cycle and changes in the architecture of the lever arm.
Myosin V (MyoV), a cytoskeletal motor protein belonging to the myosin superfamily (1), converts energy from ATP hydrolysis into the transport of intracellular cargo, such as mRNA and organelles along actin filaments (2). In its dimeric form, the motor has two actin-binding, ATPase heads connected to α-helical lever arm domains stiffened by attached calmodulins or essential light chains (Fig. 1). The nucleotide-driven mechanochemical cycle of the heads produces two changes in the lever arm orientation: a power stroke, where an actin-bound head swings the lever arm forward toward the plus (barbed) end of the filament, and a recovery stroke, which returns the arm to its original configuration when the head is detached from actin (3). The motor translates these changes into processive plus end-directed movement (4–6). By alternating head detachment, MyoV walks hand-over-hand (7, 8), taking one step of  for each ATP consumed (9). At small loads, the motor can complete
for each ATP consumed (9). At small loads, the motor can complete  forward steps before dissociating from actin (6, 10, 11). Such a high unidirectional processivity requires coordination in the detachment of the two heads, a “gating” mechanism, which is believed to arise from the strain within the molecule when both heads are bound to actin (12–15). Sufficiently large opposing loads can counteract the plus end-directed bias, resulting in an increase in the probability of back-stepping (16) until the motor velocity goes to zero at a stall force of
forward steps before dissociating from actin (6, 10, 11). Such a high unidirectional processivity requires coordination in the detachment of the two heads, a “gating” mechanism, which is believed to arise from the strain within the molecule when both heads are bound to actin (12–15). Sufficiently large opposing loads can counteract the plus end-directed bias, resulting in an increase in the probability of back-stepping (16) until the motor velocity goes to zero at a stall force of  pN (4, 12, 16–19). Although MyoV is among the most extensively studied motor proteins, improvements in experimental resolution continue to provide new and surprising insights into the details of its dynamics. A beautiful recent example is the high-speed atomic force microscopy (AFM) of Kodera et al. (20), which was used to visualize not only the expected hand-over-hand stepping but additional, less well-understood processes like “foot stomping” (21, 22), where one head detaches and rebinds to the same site. Thus, a comprehensive picture of MyoV motility needs to account for all the kinetic pathways, including back-stepping and foot stomping, how they vary under load, and their relationship to the structural and chemical parameters of the motor.
pN (4, 12, 16–19). Although MyoV is among the most extensively studied motor proteins, improvements in experimental resolution continue to provide new and surprising insights into the details of its dynamics. A beautiful recent example is the high-speed atomic force microscopy (AFM) of Kodera et al. (20), which was used to visualize not only the expected hand-over-hand stepping but additional, less well-understood processes like “foot stomping” (21, 22), where one head detaches and rebinds to the same site. Thus, a comprehensive picture of MyoV motility needs to account for all the kinetic pathways, including back-stepping and foot stomping, how they vary under load, and their relationship to the structural and chemical parameters of the motor.
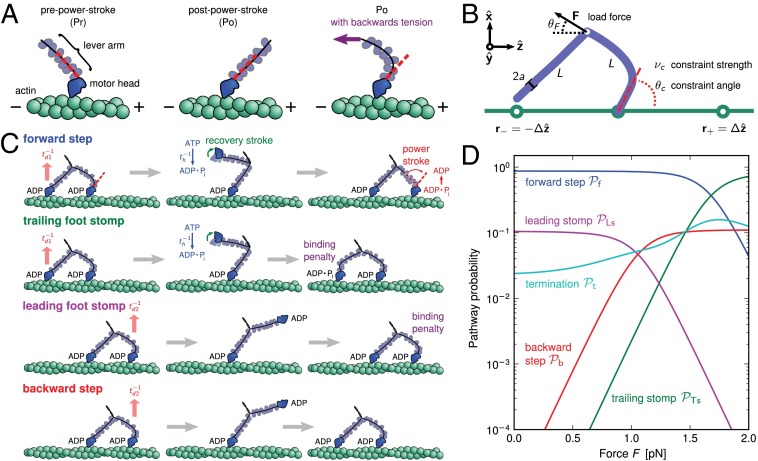
Fig. 1.
(A) Orientational states of the MyoV head with respect to its lever arm. For clarity, only one leg of the two-legged motor is shown, although the states are the same for both legs. (Left) Pr. (Middle) Po. For each state, the relaxed orientation (in the absence of tension on the lever arm end) is marked by a dashed red line. (Right) Po state with backward tension on the arm end, causing the lever arm to bend backward away from its relaxed direction. (B) Coarse-grained polymer representation of MyoV. (C) Schematic view of four MyoV kinetic pathways. For simplicity, the nucleotide-free state, following ADP release from the TH and before ATP binding, is not shown. (D) Probability of each kinetic pathway as a function of backward force F (with 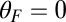 ) calculated from the theory using the parameter set in Table 1.
) calculated from the theory using the parameter set in Table 1.
To address these issues, we introduce a minimal model of MyoV dynamics, focusing on the stochastic fluctuations of the motor head during the diffusive search of the detached head for a binding site, whose importance has been illuminated by various experiments (22–25). The large persistence length lp of the lever arms (26–28) allows us to propose a coarse-grained polymer model for the reaction-diffusion problem, which, in turn, yields approximate analytical expressions for all the physical observables, including binding times, run length, velocity, and stall force. We have built on the insights of earlier theoretical works (28–34), which focused on modeling a reaction network of discrete states in the mechanochemical cycle of the motor heads. Our work supplements the reaction network with an explicit treatment of the diffusive search, which has been studied using insightful Brownian dynamics simulations of forward stepping in MyoV (35). An important aspect of our theory is that it allows us to tackle not just forward steps but the full complexity of foot stomping and back-stepping across the entire force spectrum up to the stall point. In our framework, the load dependence of the MyoV behavior enters naturally, because pulling on the molecule shifts the speed and likelihood of the detached head reaching the forward or backward binding site. The competition between the time scales of first passage to the sites, and how they compare with the detachment rates of the heads, determines the partitioning of the kinetic pathways. Significantly, polymer theory gives us a direct connection between the kinetics and the structural features of the motor, like the bending elasticity of the lever arms and the orientational bias due to the power stroke. The result is a theory with only three fitting parameters that have not been previously determined through experiment, all of which have simple physical interpretations. The theoretical fit quantitatively reproduces a variety of experimental data, like the time-dependent mean trajectories of the detached head (23) and the force dependence of the backward-to-forward step ratio (16) and run length/velocity (4, 16–18, 36). We also explore more broadly the design space of MyoV structural parameters, allowing us to predict the essential requirements for the observed dynamical behavior and to answer the following questions. Is the structure of the motor dictated by certain natural constraints? How robust is the motility of MyoV to perturbations in the parameters? What are the relative contributions of head chemistry (resulting from changes in the nucleotide states) and the structural features to the measured stall force? The answers to these questions, which are provided in terms of phase diagrams, lead to testable predictions.
Results
Polymer Model for MyoV.
In our model for MyoV (Fig. 1B), the motor and lever arm domains of each head are represented as a single semiflexible polymer chain with contour length L and persistence length lp. The two polymer legs are connected at a freely rotating joint. The parameter values characterizing our model are listed in Table 1. Although the tail domain of MyoV, attached to the cargo, is not explicitly included, its effect is to transmit a load force F to the joint. The force is oriented in the 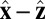 plane, at an angle
plane, at an angle 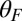 , measured clockwise from
, measured clockwise from 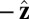 . The axis
. The axis 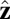 runs parallel to the actin filament, pointing toward the plus end. Our focus here is to study backward or resistive load
runs parallel to the actin filament, pointing toward the plus end. Our focus here is to study backward or resistive load 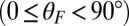 at force magnitudes smaller or close to the stall,
at force magnitudes smaller or close to the stall, 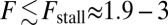 pN (4, 12, 16–19). The polymer end points can bind to the actin filament at discrete binding sites, which are evenly spaced at a distance
pN (4, 12, 16–19). The polymer end points can bind to the actin filament at discrete binding sites, which are evenly spaced at a distance 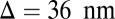 along the filament, corresponding approximately to the half-pitch of the actin double-helical structure (13 G-actin subunits). Although the model can be extended to incorporate a distribution of Δ values, reflecting binding to subunits neighboring the primary binding sites, in the simplest approximation, we keep Δ fixed. Because the first passage times to the primary binding site and its neighbors are similar, the effect of this approximation is small.
along the filament, corresponding approximately to the half-pitch of the actin double-helical structure (13 G-actin subunits). Although the model can be extended to incorporate a distribution of Δ values, reflecting binding to subunits neighboring the primary binding sites, in the simplest approximation, we keep Δ fixed. Because the first passage times to the primary binding site and its neighbors are similar, the effect of this approximation is small.
Table 1.
MyoV model parameters
| Parameter | Value | Notes |
| Mechanical parameters | ||
| Leg contour length, L | 35 nm | (35) |
| Leg persistence length, lp | 310 nm | (27) |
| Head diffusivity, Dh | 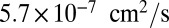 |
(40, 41) |
Constraint angle, 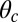
|
60° | Fit to experiment (23) |
Constraint strength, 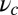
|
184 | Fit to experiment (16) |
| Binding parameters | ||
| Binding site separation, Δ | 36 nm | (35) |
| Capture radius, a | 1 nm | |
| Binding penalty, b | 0.065 | Fit to experiment (6, 10, 11) |
| Chemical rates | ||
Hydrolysis rate, 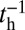
|
750 s−1 | (38) |
TH detachment rate, 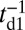
|
12 s−1 | (38) |
LH detachment rate, 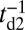
|
1.5 s−1 | (15) |
Gating ratio, 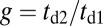
|
8 | |
For each leg, the lever arm can adopt different preferred configurations with respect to the motor head during the course of the stepping cycle: the prepower-stroke (Pr) and postpower-stroke (Po) states. When the motor head is bound to actin and there is no tension on the end of the lever arm transmitted through the junction, the two states have relaxed configurations, as illustrated in Fig. 1A (Left and Middle). In the Pr state, the lever arm relaxes to an orientation tilting toward the actin-minus end, whereas in the Po state, it tilts toward the actin-plus end. In our model, the tilting preference of the Po state enters as a harmonic constraint on the end tangent of the bound leg: If 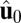 is the unit tangent vector at the point where the polymer leg attaches to actin, we have a potential of
is the unit tangent vector at the point where the polymer leg attaches to actin, we have a potential of 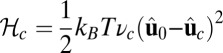 , with a constraint strength
, with a constraint strength 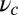 and direction
and direction 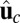 . Here, kB is Boltzmann's constant, and T is temperature. The vector
. Here, kB is Boltzmann's constant, and T is temperature. The vector 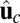 is in the
is in the 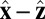 plane at an angle
plane at an angle 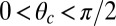 , where
, where 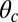 is the constraint angle, measured counterclockwise from the
is the constraint angle, measured counterclockwise from the 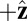 axis (the
axis (the 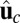 direction is marked by a red dashed line in Fig. 1 A and B). In principle, the Pr state is analogous but with distinct values of
direction is marked by a red dashed line in Fig. 1 A and B). In principle, the Pr state is analogous but with distinct values of 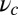 and
and 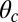 , with the latter in the range of
, with the latter in the range of 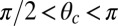 . However, as we will see below, all the kinetic pathways involve diffusion while the bound leg is in the Po state, so the parameters of the Pr state do not explicitly enter into the calculation. Hence, both
. However, as we will see below, all the kinetic pathways involve diffusion while the bound leg is in the Po state, so the parameters of the Pr state do not explicitly enter into the calculation. Hence, both 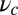 and
and 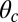 will refer only to the Po state.
will refer only to the Po state.
If there is tension propagated through the junction on the end of the lever arm (i.e., due to load or to the fact that both motor heads are bound to actin), the lever arm contour will be bent away from its relaxed conformation. Fig. 1A (Right) shows the Po state under backward tension on the arm: The lever arm is bent, adopting a shape that reflects several competing physical effects. The Po constraint of strength 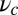 tries to keep the head–arm angle near
tries to keep the head–arm angle near 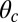 , the bending stiffness lp favors a straight lever arm contour, and the tension tries to pull the end of the arm backward. The polymer model naturally incorporates the interplay of these effects, which we will show is crucial in determining the dynamical response of the motor to load.
, the bending stiffness lp favors a straight lever arm contour, and the tension tries to pull the end of the arm backward. The polymer model naturally incorporates the interplay of these effects, which we will show is crucial in determining the dynamical response of the motor to load.
Kinetic Pathways.
The starting point for all MyoV kinetic pathways (Fig. 1C, Left) is the waiting state, where both heads have ADP, are strongly bound to actin, and are in the Po state. Because the leading (L) leg is connected to the trailing (T) leg at the junction, the L leg is under backward tension, and it bends in the manner discussed above. The resulting strained “telemark” or “reverse arrowhead” stance has been observed directly in both EM (37) and AFM (20) images. The waiting state leads to four possible kinetic pathways (Fig. 1C).
1. Forward step.
ADP is released from the trailing head (TH), followed by ATP binding, which makes association of the head with actin weak, leading to detachment. We assume saturating ATP concentrations 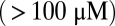 , where ATP binding and subsequent TH detachment are very fast compared with ADP release; hence, the entire detachment process for the TH is modeled with a single rate of
, where ATP binding and subsequent TH detachment are very fast compared with ADP release; hence, the entire detachment process for the TH is modeled with a single rate of 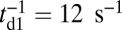 , equal to the experimentally measured ADP release rate (38). If we set the origin
, equal to the experimentally measured ADP release rate (38). If we set the origin  at the position of the bound leading head (LH), the free end of MyoV can diffuse and potentially rebind at one of two sites,
at the position of the bound leading head (LH), the free end of MyoV can diffuse and potentially rebind at one of two sites, 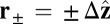 along the actin filament (Fig. 1B). Binding at
along the actin filament (Fig. 1B). Binding at 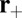 leads to a forward step (Fig. 1C, row 1). However, successful binding is dependent on two conditions: (i) reaching the capture radius a around the binding site and (ii) the motor head having already hydrolyzed its bound ATP.
leads to a forward step (Fig. 1C, row 1). However, successful binding is dependent on two conditions: (i) reaching the capture radius a around the binding site and (ii) the motor head having already hydrolyzed its bound ATP.
During the diffusive search, the entire two-legged polymer structure fluctuates in three dimensions, subject only to the end-tangent constraint at the bound leg attachment point. First passage to a given binding site 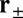 , which occurs at a mean time interval
, which occurs at a mean time interval 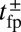 after detachment, is the first arrival of the detached head to any point within a radius a of the binding site. The capture radius a, which reflects the distance at which the free MyoV head can appreciably interact with the actin-binding site (35), is set to
after detachment, is the first arrival of the detached head to any point within a radius a of the binding site. The capture radius a, which reflects the distance at which the free MyoV head can appreciably interact with the actin-binding site (35), is set to 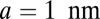 , comparable to the Debye screening length
, comparable to the Debye screening length 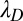 in physiological and in vitro conditions (i.e., for KCl concentrations of
in physiological and in vitro conditions (i.e., for KCl concentrations of 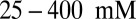 ,
, 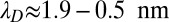 ).
).
The second condition for successful binding is the chemical state of the detached head. In order for the head to strongly associate and bind to actin, ATP must hydrolyze to ADP + inorganic phosphate (Pi), which occurs at a hydrolysis rate of 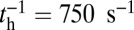 (38). Along with hydrolysis, the detached head also undergoes a recovery stroke, which reverses the power stroke, changing the orientation of the head with respect to the lever arm (Po→Pr). For simplicity, we combine the nucleotide/head–arm orientation states of the detached head into two possibilities: ATP/Po (A) and ADP + Pi/Pr (B). Unless otherwise specified, we assume the transition A→B occurs irreversibly at a rate of
(38). Along with hydrolysis, the detached head also undergoes a recovery stroke, which reverses the power stroke, changing the orientation of the head with respect to the lever arm (Po→Pr). For simplicity, we combine the nucleotide/head–arm orientation states of the detached head into two possibilities: ATP/Po (A) and ADP + Pi/Pr (B). Unless otherwise specified, we assume the transition A→B occurs irreversibly at a rate of 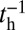 . (We will discuss one experimental variant of MyoV with modified light chain composition in the section on zero load binding kinetics, where there is a nonnegligible reverse hydrolysis rate of
. (We will discuss one experimental variant of MyoV with modified light chain composition in the section on zero load binding kinetics, where there is a nonnegligible reverse hydrolysis rate of 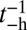 ). Binding can only occur in state B, so if the detached TH has reached the capture radius of one of the sites and the system is still in state A, it has a zero probability of binding, resulting in the TH continuing its diffusive trajectory. For forward stepping to occur, the TH must reach the capture radius of
). Binding can only occur in state B, so if the detached TH has reached the capture radius of one of the sites and the system is still in state A, it has a zero probability of binding, resulting in the TH continuing its diffusive trajectory. For forward stepping to occur, the TH must reach the capture radius of 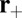 in state B, and then it can bind with a probability of 1.
in state B, and then it can bind with a probability of 1.
After successful binding, Pi is rapidly released from the bound head, which then results in a Pr→Po transition, returning the motor to its waiting state, with both the heads being in the Po state. Release of Pi and the power stroke is much faster than the detachment time scale td1 (13), so we can assume that the motor with two bound heads spends nearly all its time waiting in the telemark stance.
2. T foot stomp.
This kinetic pathway (Fig. 1C, row 2) is similar to the forward step, except that the detached TH diffuses to the site  rather than
rather than 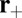 . Rebinding at
. Rebinding at  brings the center of mass of the motor back to its original location, without any net movement along the actin. For the binding to be successful, the head must be in state B within the capture radius a of
brings the center of mass of the motor back to its original location, without any net movement along the actin. For the binding to be successful, the head must be in state B within the capture radius a of  , in which case it will bind with a probability of
, in which case it will bind with a probability of 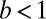 . The reduced probability of binding is a crucial difference between the forward step and T foot stomp pathways. The binding penalty b arises because the head in state B, after the recovery stroke, is in the Pr orientation, which is believed to favor binding to the forward target site
. The reduced probability of binding is a crucial difference between the forward step and T foot stomp pathways. The binding penalty b arises because the head in state B, after the recovery stroke, is in the Pr orientation, which is believed to favor binding to the forward target site 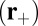 over the backward site
over the backward site 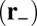 (3). Forward binding involves the detached head going in front of its lever arm, which has to tilt back toward the actin-minus end (the relaxed configuration of the Pr state). Backward binding has the opposite arrangement, with the lever arm bent toward the actin-plus end, which is an unnatural configuration in the Pr state, resulting in a strained back leg, as illustrated in Fig. 1C (Right), row 2. We model this effective extra energy barrier in the binding process through the probability b. The greater the barrier, the smaller is the value of b. The hypothesis that the recovery stroke is important in favoring forward binding has found support in a recent single-molecule study on single-headed MyoV (3), which established that the Pr orientation is highly kinetically and energetically stable (with an energy barrier of at least 5 kBT with respect to Po).
(3). Forward binding involves the detached head going in front of its lever arm, which has to tilt back toward the actin-minus end (the relaxed configuration of the Pr state). Backward binding has the opposite arrangement, with the lever arm bent toward the actin-plus end, which is an unnatural configuration in the Pr state, resulting in a strained back leg, as illustrated in Fig. 1C (Right), row 2. We model this effective extra energy barrier in the binding process through the probability b. The greater the barrier, the smaller is the value of b. The hypothesis that the recovery stroke is important in favoring forward binding has found support in a recent single-molecule study on single-headed MyoV (3), which established that the Pr orientation is highly kinetically and energetically stable (with an energy barrier of at least 5 kBT with respect to Po).
3. L foot stomp.
In addition to the two kinetic pathways above, initiated by TH detachment, there are two other possibilities that occur upon detachment of the LH. The first of these is the L foot stomp, where the LH unbinds and then rebinds to its original site (Fig. 1C, row 3). The detachment of the LH occurs at a slower rate than TH detachment, 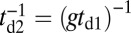 , where we denote the factor
, where we denote the factor 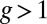 as the gating ratio. This asymmetry arises from the intramolecular strain within the two-legged MyoV structure bound to actin (12–15). The backward tension on the L lever arm in the waiting state slows down ADP release in the LH by 50- to 70-fold compared with the TH (13, 20), which makes detachment through the ADP-release/ATP-binding mechanism very rare. Rather, the LH under backward strain detaches primarily by means of an alternate pathway, where it retains ADP (15, 20), an assumption supported by the observation that single-headed MyoV under backward loads of
as the gating ratio. This asymmetry arises from the intramolecular strain within the two-legged MyoV structure bound to actin (12–15). The backward tension on the L lever arm in the waiting state slows down ADP release in the LH by 50- to 70-fold compared with the TH (13, 20), which makes detachment through the ADP-release/ATP-binding mechanism very rare. Rather, the LH under backward strain detaches primarily by means of an alternate pathway, where it retains ADP (15, 20), an assumption supported by the observation that single-headed MyoV under backward loads of 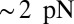 unbinds from actin at a slow rate of 1.5 s−1 independent of both ATP and ADP concentrations (15). As described below, the magnitude of the backward tension in the waiting state can also be directly estimated from the structural parameters of the polymer model, giving a value of 2.7 pN, sufficient to be in the slow unbinding regime. Based on these considerations, we set
unbinds from actin at a slow rate of 1.5 s−1 independent of both ATP and ADP concentrations (15). As described below, the magnitude of the backward tension in the waiting state can also be directly estimated from the structural parameters of the polymer model, giving a value of 2.7 pN, sufficient to be in the slow unbinding regime. Based on these considerations, we set 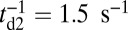 in our model, giving a gating ratio of
in our model, giving a gating ratio of 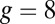 . In other words, the TH is eightfold more likely to detach than the LH per unit time. We also assume the LH always retains ADP upon detachment [staying in the Po state (20)], and thus no ATP hydrolysis needs to occur before rebinding.
. In other words, the TH is eightfold more likely to detach than the LH per unit time. We also assume the LH always retains ADP upon detachment [staying in the Po state (20)], and thus no ATP hydrolysis needs to occur before rebinding.
If we assign 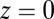 to be the position of the bound TH, then the L foot stomp involves reattachment to its original site
to be the position of the bound TH, then the L foot stomp involves reattachment to its original site 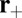 . Because the LH is Po, rebinding requires the lever arm to be bent backward, contrary to the plus-directed relaxed orientation of the Po state. We thus have a binding penalty analogous to the one for the T foot stomp: Successful binding will occur with a probability b within the capture radius a around
. Because the LH is Po, rebinding requires the lever arm to be bent backward, contrary to the plus-directed relaxed orientation of the Po state. We thus have a binding penalty analogous to the one for the T foot stomp: Successful binding will occur with a probability b within the capture radius a around 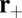 . There is no additional chemical requirement, because the LH is in an ADP state with high affinity to actin. Although it is possible to assign a distinct binding penalty b for the T and L foot stomps, this does not lead to any major qualitative differences in the analysis below, so we assume, for simplicity, a single value of b. After binding, MyoV returns to the waiting state.
. There is no additional chemical requirement, because the LH is in an ADP state with high affinity to actin. Although it is possible to assign a distinct binding penalty b for the T and L foot stomps, this does not lead to any major qualitative differences in the analysis below, so we assume, for simplicity, a single value of b. After binding, MyoV returns to the waiting state.
4. Backward step.
The final kinetic pathway proceeds analogously to the L foot stomp, but the detached LH diffuses and binds to the backward site  (Fig. 1C, row 4). MyoV thus steps backward, shifting the center of mass toward the minus-end of actin. The detached head retains ADP and stays in the Po state. Because a forward-tilted lever arm is the relaxed conformation in the Po state, there is no binding penalty. Therefore, upon reaching the capture radius a around
(Fig. 1C, row 4). MyoV thus steps backward, shifting the center of mass toward the minus-end of actin. The detached head retains ADP and stays in the Po state. Because a forward-tilted lever arm is the relaxed conformation in the Po state, there is no binding penalty. Therefore, upon reaching the capture radius a around  , the leg binds with a probability of 1, and MyoV returns to the waiting state. The fact that back-stepping in our model does not require ATP hydrolysis is consistent with observations of ATP-independent processive backward stepping in the superstall regime
, the leg binds with a probability of 1, and MyoV returns to the waiting state. The fact that back-stepping in our model does not require ATP hydrolysis is consistent with observations of ATP-independent processive backward stepping in the superstall regime 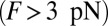 (18). For simplicity, we will not consider the superstall case in the present study. In principle, our model could be generalized to the superstall regime by including additional kinetic pathways that occur under extremely large backward loads, for example, power stroke reversal (39).
(18). For simplicity, we will not consider the superstall case in the present study. In principle, our model could be generalized to the superstall regime by including additional kinetic pathways that occur under extremely large backward loads, for example, power stroke reversal (39).
In all four kinetic pathways described above, only one leg is always bound to the actin during the diffusion step. If the bound leg detaches before the free leg binds, the processive run of MyoV is terminated. We assume a bound leg detachment rate of 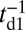 during this process. This completes the description of the model, where each MyoV waiting state ends in five possible outcomes: forward stepping, T/L foot stomps, backward stepping, or detachment of both heads from actin. The first four pathways bring the system back to the waiting state, where the entire mechanochemical cycle can be repeated, whereas the last ends the run. The only parameters for which we do not have direct experimental estimates are the strength and direction of the power stroke constraint,
during this process. This completes the description of the model, where each MyoV waiting state ends in five possible outcomes: forward stepping, T/L foot stomps, backward stepping, or detachment of both heads from actin. The first four pathways bring the system back to the waiting state, where the entire mechanochemical cycle can be repeated, whereas the last ends the run. The only parameters for which we do not have direct experimental estimates are the strength and direction of the power stroke constraint, 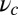 and
and 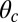 , respectively, and the binding penalty b. We will be able to fit these parameters by comparing the theoretical results with experimental data, as described below, resulting in the values listed in Table 1. Imaging studies (20, 37) suggest that the preferred Po orientation
, respectively, and the binding penalty b. We will be able to fit these parameters by comparing the theoretical results with experimental data, as described below, resulting in the values listed in Table 1. Imaging studies (20, 37) suggest that the preferred Po orientation 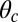 is likely to be in the vicinity of 60°, so this parameter could have been constrained from the outset. However, we have allowed it to be a free parameter because the angle
is likely to be in the vicinity of 60°, so this parameter could have been constrained from the outset. However, we have allowed it to be a free parameter because the angle 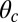 that appears in the potential function
that appears in the potential function 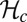 can, in principle, be slightly different from the observed orientation of the bound leg in any particular image, which is affected by both thermal fluctuations and any tension that is applied to the end of the bound leg. For the persistence length lp, there are estimates ranging from
can, in principle, be slightly different from the observed orientation of the bound leg in any particular image, which is affected by both thermal fluctuations and any tension that is applied to the end of the bound leg. For the persistence length lp, there are estimates ranging from 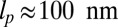 (26) up to
(26) up to 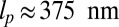 (28). We use the value
(28). We use the value 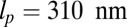 , based on the measurements of Moore et al. (27). From the point of view of the polymer model, the most important characteristic of the persistence length is that
, based on the measurements of Moore et al. (27). From the point of view of the polymer model, the most important characteristic of the persistence length is that 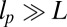 , so the legs behave almost as rigid rods. However, one of the major outcomes of our theory is that precise tuning of the parameters is not required to get efficient processive dynamics qualitatively similar to those seen in nature.
, so the legs behave almost as rigid rods. However, one of the major outcomes of our theory is that precise tuning of the parameters is not required to get efficient processive dynamics qualitatively similar to those seen in nature.
Analytical Theory for Diffusive Search Times.
The central physical quantity in our model is the first passage time to the binding site, 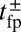 , which depends sensitively on the interplay of bending stiffness (lp), load force
, which depends sensitively on the interplay of bending stiffness (lp), load force 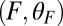 , and power stroke constraint
, and power stroke constraint 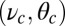 (Fig. 1B). The magnitude of
(Fig. 1B). The magnitude of 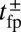 at a given F compared with the
at a given F compared with the 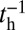 and detachment rate, along with the size of the binding penalty, determines exactly how the system partitions among the various kinetic pathways.
and detachment rate, along with the size of the binding penalty, determines exactly how the system partitions among the various kinetic pathways.
Remarkably, the polymer model allows us to derive an approximate analytical expression for 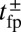 by exploiting the separation in time scales between polymer relaxation and the diffusive search (details are provided in Materials and Methods and SI Text). If tr is the relaxation time for the two-legged polymer structure to equilibrate after one of the legs detaches, then
by exploiting the separation in time scales between polymer relaxation and the diffusive search (details are provided in Materials and Methods and SI Text). If tr is the relaxation time for the two-legged polymer structure to equilibrate after one of the legs detaches, then 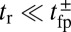 . Theory and simulations show that
. Theory and simulations show that 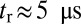 for nearly rigid legs at zero load and that it becomes even smaller as F increases (Fig. 2). The value of tr is two orders of magnitude smaller than the fastest times for first passage to the binding sites,
for nearly rigid legs at zero load and that it becomes even smaller as F increases (Fig. 2). The value of tr is two orders of magnitude smaller than the fastest times for first passage to the binding sites, 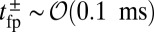 . Because
. Because 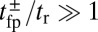 , we can relate
, we can relate 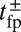 to the distribution
to the distribution 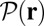 , the probability density of finding the MyoV free end at position r once the system has reached equilibrium after leg detachment:
, the probability density of finding the MyoV free end at position r once the system has reached equilibrium after leg detachment:
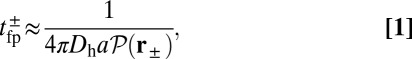 |
where 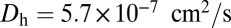 is the diffusion constant of the MyoV head, estimated using the program HYDROPRO (40) applied to the Protein Data Bank structure 1W8J (41). Eq. 1 transforms the dynamical problem of diffusive search time into one of calculating the equilibrium end-point distribution of a tethered, two-legged, semiflexible polymer structure. By adapting a mean field theory for individual semiflexible chains (42) and noting that contour fluctuations are small in the regime
is the diffusion constant of the MyoV head, estimated using the program HYDROPRO (40) applied to the Protein Data Bank structure 1W8J (41). Eq. 1 transforms the dynamical problem of diffusive search time into one of calculating the equilibrium end-point distribution of a tethered, two-legged, semiflexible polymer structure. By adapting a mean field theory for individual semiflexible chains (42) and noting that contour fluctuations are small in the regime 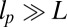 , we obtained an approximate but accurate analytical expression for
, we obtained an approximate but accurate analytical expression for 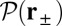 , taking into account both the load force on the joint and the end-tangent constraint (Materials and Methods and SI Text). Together with Eq. 1, we have a complete description of
, taking into account both the load force on the joint and the end-tangent constraint (Materials and Methods and SI Text). Together with Eq. 1, we have a complete description of 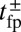 as a function of load and the MyoV structural parameters. If we assume that the other events in the mechanochemical cycle, hydrolysis and TH/LH detachment, are Poisson processes with respective rates of
as a function of load and the MyoV structural parameters. If we assume that the other events in the mechanochemical cycle, hydrolysis and TH/LH detachment, are Poisson processes with respective rates of 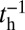 ,
, 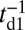 , and
, and 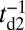 , the probability of each kinetic pathway can also be derived, together with related quantities like mean run length and velocity. The full set of analytical equations for our model is summarized in SI Text.
, the probability of each kinetic pathway can also be derived, together with related quantities like mean run length and velocity. The full set of analytical equations for our model is summarized in SI Text.
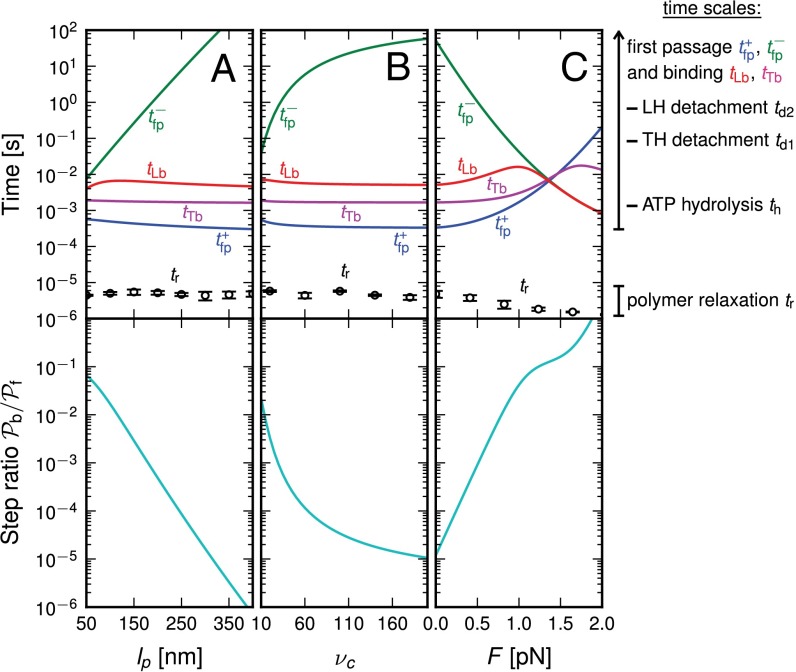
Fig. 2.
(Upper) Theoretical predictions for the mean first passage time 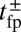 to the forward (+) and backward (−) sites; the mean binding times tLb and tTb for the L and T legs, respectively; and the polymer relaxation time tr for the MyoV structure to equilibrate after the detachment of one leg. All results except tr are derived from the analytical theory. The relaxation times are estimated using coarse-grained Brownian dynamics simulations (details are provided in SI Text). (Right) Main time scales in the problem are summarized, with their values (or ranges) indicated for comparison. (Lower) Ratio of backward to forward steps,
to the forward (+) and backward (−) sites; the mean binding times tLb and tTb for the L and T legs, respectively; and the polymer relaxation time tr for the MyoV structure to equilibrate after the detachment of one leg. All results except tr are derived from the analytical theory. The relaxation times are estimated using coarse-grained Brownian dynamics simulations (details are provided in SI Text). (Right) Main time scales in the problem are summarized, with their values (or ranges) indicated for comparison. (Lower) Ratio of backward to forward steps, 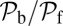 . For the three columns, the quantities are plotted as one parameter is varied, whereas all others are fixed at their Table 1 values: leg persistence length lp (A), power stroke constraint strength
. For the three columns, the quantities are plotted as one parameter is varied, whereas all others are fixed at their Table 1 values: leg persistence length lp (A), power stroke constraint strength 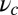 (B), and load force F (with
(B), and load force F (with 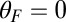 ) (C).
) (C).
Role of Diffusion in the Kinetic Pathway Probabilities at Zero Load.
To gain an understanding of how the structural features of MyoV influence its motility, it is instructive to start with 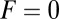 . Fig. 2 A (Upper) and B (Upper) shows the first passage times
. Fig. 2 A (Upper) and B (Upper) shows the first passage times 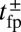 as a function of lp and
as a function of lp and 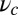 , respectively, with the other parameters being fixed at their Table 1 values. Because of the power stroke constraint, there is asymmetry in the first passage times:
, respectively, with the other parameters being fixed at their Table 1 values. Because of the power stroke constraint, there is asymmetry in the first passage times: 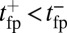 because the center of the
because the center of the 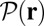 distribution is shifted toward the forward binding site at
distribution is shifted toward the forward binding site at 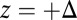 . At
. At 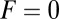 , the average z-axis location of the free leg,
, the average z-axis location of the free leg, 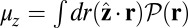 , is given by
, is given by
where 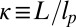 and the origin
and the origin 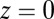 is at the binding site of the attached leg. With increasing lp and
is at the binding site of the attached leg. With increasing lp and 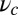 , the position
, the position 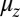 increases until it saturates at the limit of a rigid rod of length L with a fixed angle
increases until it saturates at the limit of a rigid rod of length L with a fixed angle 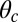 ,
, 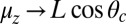 . In this limit,
. In this limit,  , because it is geometrically impossible to reach the backward binding site
, because it is geometrically impossible to reach the backward binding site 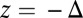 . In the opposite limit of small lp and
. In the opposite limit of small lp and 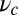 , the structure has greater flexibility, reaching the backward binding site is easier, and the asymmetry is smaller. For
, the structure has greater flexibility, reaching the backward binding site is easier, and the asymmetry is smaller. For 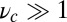 and
and 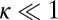 , the asymmetry parameter,
, the asymmetry parameter, 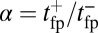 , has a simple relationship to the structural parameters:
, has a simple relationship to the structural parameters:
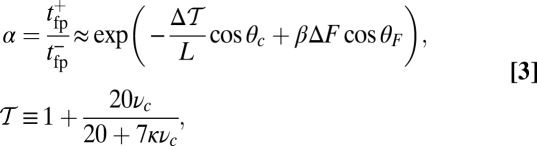 |
where 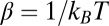 and
and 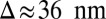 is the step size. At
is the step size. At 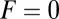 , the key role in determining the degree of asymmetry is the factor
, the key role in determining the degree of asymmetry is the factor  , which depends on lp and
, which depends on lp and 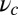 and is a dimensionless measure of the effectiveness of the power stroke constraint. A larger
and is a dimensionless measure of the effectiveness of the power stroke constraint. A larger  means a smaller α and greater asymmetry. The form of
means a smaller α and greater asymmetry. The form of  shows that the constraint strength
shows that the constraint strength 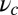 by itself is insufficient to guarantee a large
by itself is insufficient to guarantee a large  , because it can be counterbalanced by a small lp. In other words, the end-tangent constraint does not have a significant effect if the polymer leg is too flexible. Thus, both
, because it can be counterbalanced by a small lp. In other words, the end-tangent constraint does not have a significant effect if the polymer leg is too flexible. Thus, both 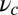 and lp have to be large to create significant asymmetry. In Discussion, we will highlight the relationship between
and lp have to be large to create significant asymmetry. In Discussion, we will highlight the relationship between  and important mechanical and energy scales in the system, including the overall compliance of the leg and the energy expended by the power stroke.
and important mechanical and energy scales in the system, including the overall compliance of the leg and the energy expended by the power stroke.
The asymmetry factor α influences kinetic pathway probabilities. At the end of each waiting stage, there is a probability of making a forward step 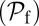 , a backward step
, a backward step 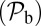 , an L foot stomp
, an L foot stomp 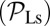 , and a T foot stomp
, and a T foot stomp 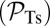 . We plot these probabilities in Fig. 1D for a range of F. When the time scale of leg detachment is much larger than the binding times, the ratios of the pathway probabilities can be expressed in terms of α as
. We plot these probabilities in Fig. 1D for a range of F. When the time scale of leg detachment is much larger than the binding times, the ratios of the pathway probabilities can be expressed in terms of α as
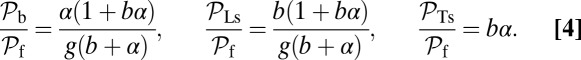 |
Note that 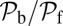 and
and 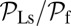 are inversely proportional to g, the ratio of TH to LH detachment, which is expected because backward steps and L foot stomps can only occur when the LH detaches. The binding penalty b enters into all the ratios because it influences the likelihood of T/L foot stomping, which competes with the backward/forward stepping pathways. Fig. 2 A (Lower) and B (Lower) shows the variation of
are inversely proportional to g, the ratio of TH to LH detachment, which is expected because backward steps and L foot stomps can only occur when the LH detaches. The binding penalty b enters into all the ratios because it influences the likelihood of T/L foot stomping, which competes with the backward/forward stepping pathways. Fig. 2 A (Lower) and B (Lower) shows the variation of 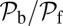 at
at 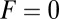 as lp and
as lp and 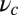 are varied. We find that
are varied. We find that 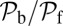 decreases as either variable increases, due to a larger
decreases as either variable increases, due to a larger  in Eq. 3, resulting in a smaller α. Experimentally, MyoV exhibits negligible back-stepping at zero load,
in Eq. 3, resulting in a smaller α. Experimentally, MyoV exhibits negligible back-stepping at zero load, 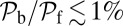 (16). To achieve this extreme unidirectionality,
(16). To achieve this extreme unidirectionality,  (or, equivalently, both
(or, equivalently, both 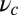 and lp) should be sufficiently large, an issue we will return to in Discussion when we examine the global constraints on the structural features of the motor. Along with back steps, T foot stomps are also negligible at
and lp) should be sufficiently large, an issue we will return to in Discussion when we examine the global constraints on the structural features of the motor. Along with back steps, T foot stomps are also negligible at 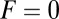 for small α, because
for small α, because 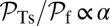 . Because
. Because 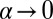 , the only ratio that has a nonzero limit is
, the only ratio that has a nonzero limit is 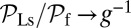 . Qualitatively similar behavior was observed in the high-speed AFM experiments (20), where the TH rarely detached without resulting in a forward step. On the other hand, essentially every time the LH detaches, it will rebind to its original location (L stomp) because the power stroke constraint prevents it from reaching the backward site. For example, in the
. Qualitatively similar behavior was observed in the high-speed AFM experiments (20), where the TH rarely detached without resulting in a forward step. On the other hand, essentially every time the LH detaches, it will rebind to its original location (L stomp) because the power stroke constraint prevents it from reaching the backward site. For example, in the 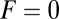 slice of Fig. 1D,
slice of Fig. 1D, 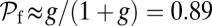 and
and 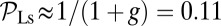 . The other pathways do not contribute significantly.
. The other pathways do not contribute significantly.
Binding Dynamics and the Average Step Trajectory at Zero Load.
The mean times tTb and tLb for the TH and LH to bind after detachment (irrespective of the binding site) are related to 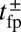 as
as
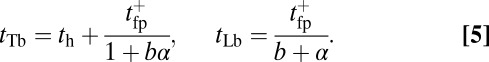 |
These binding times are plotted in Fig. 2 A (Upper) and B (Upper) as a function of lp and 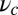 . The detached TH has to undergo hydrolysis before rebinding, so
. The detached TH has to undergo hydrolysis before rebinding, so 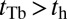 . For the parameters in Table 1,
. For the parameters in Table 1, 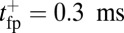 and
and 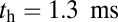 at
at 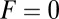 , so hydrolysis is the rate-limiting step for TH binding. As noted above, T foot stomping is infrequent in this case, so the binding events contributing to tTb are almost exclusively forward steps. We note, en passant, that our value for
, so hydrolysis is the rate-limiting step for TH binding. As noted above, T foot stomping is infrequent in this case, so the binding events contributing to tTb are almost exclusively forward steps. We note, en passant, that our value for 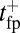 agrees well with the
agrees well with the 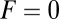 result of Brownian dynamics simulations (35), further validating the analytical model for the diffusive search.
result of Brownian dynamics simulations (35), further validating the analytical model for the diffusive search.
A closely related quantity to the mean binding time is the cumulative probability that the head has bound to a particular binding site at time t after detachment. For the TH, the probability 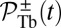 for the site
for the site 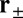 is given by
is given by
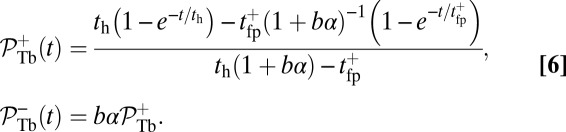 |
From 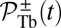 , we can calculate an experimentally measurable quantity, the average distance traveled by the free end along the z axis after detachment,
, we can calculate an experimentally measurable quantity, the average distance traveled by the free end along the z axis after detachment, 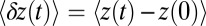 , where
, where  . The result is
. The result is
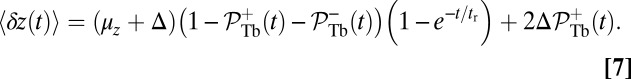 |
The first term represents the contribution from the ensemble of trajectories where the TH is still unbound: a fast polymer relaxation over time tr from the initial point at 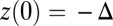 to the equilibrium average position
to the equilibrium average position 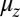 (Eq. 2). The second term represents the fraction of the ensemble where the TH has successfully bound to the forward site, which eventually corresponds to the entire ensemble for sufficiently large t. Thus,
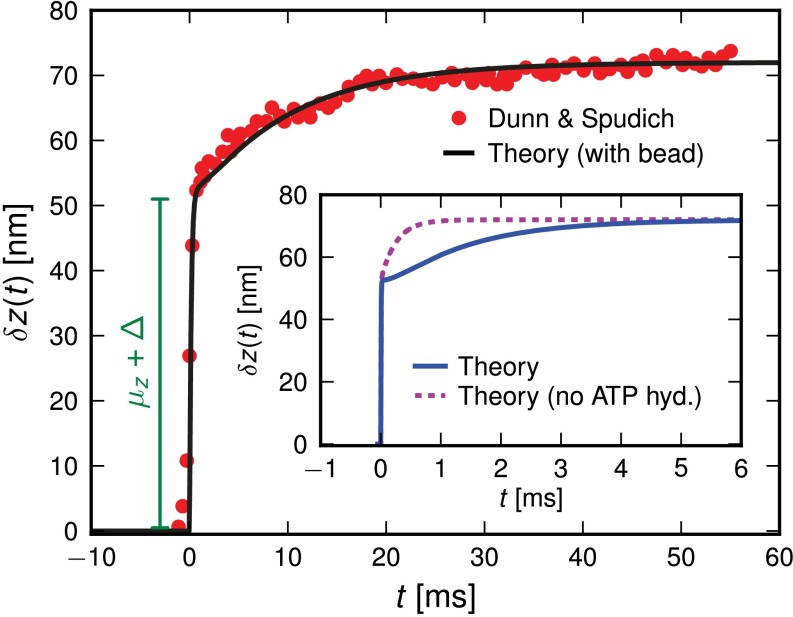
(Eq. 2). The second term represents the fraction of the ensemble where the TH has successfully bound to the forward site, which eventually corresponds to the entire ensemble for sufficiently large t. Thus, 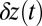 has two regimes, as shown in Fig. 3: a steep rise to
has two regimes, as shown in Fig. 3: a steep rise to 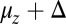 on time scales
on time scales 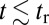 , followed by a slower ascent to the full step distance 2Δ. Dunn and Spudich (23) have measured
, followed by a slower ascent to the full step distance 2Δ. Dunn and Spudich (23) have measured 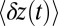 for MyoV by attaching a 40-nm diameter gold nanoparticle near the end of one lever arm. Observing the particle through dark-field imaging, they aligned and averaged 231 individual step trajectories to produce the
for MyoV by attaching a 40-nm diameter gold nanoparticle near the end of one lever arm. Observing the particle through dark-field imaging, they aligned and averaged 231 individual step trajectories to produce the 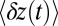 data points shown in Fig. 3. Because the nanoparticle is sufficiently large that its hydrodynamic drag will slow down the relaxation and diffusive dynamics, we included a time rescaling factor B into the theory to account for the effect of the bead:
data points shown in Fig. 3. Because the nanoparticle is sufficiently large that its hydrodynamic drag will slow down the relaxation and diffusive dynamics, we included a time rescaling factor B into the theory to account for the effect of the bead: 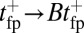 ,
, 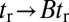 . The theory agrees well with the experiment for
. The theory agrees well with the experiment for 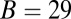 and
and 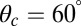 . The fitted value of
. The fitted value of 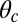 is based on setting the experimentally measured steep rise,
is based on setting the experimentally measured steep rise, 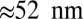 , equal to
, equal to 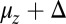 , with
, with 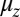 given by Eq. 2. The
given by Eq. 2. The 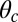 value is insensitive to the precise value of
value is insensitive to the precise value of 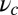 or lp (assuming we are in the
or lp (assuming we are in the 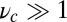 and
and 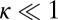 regime), as well as to the time rescaling B. In the experiment, the relaxation time for the steep rise was faster than the equipment time resolution of 320 ms. In our theory, the rescaled relaxation time is
regime), as well as to the time rescaling B. In the experiment, the relaxation time for the steep rise was faster than the equipment time resolution of 320 ms. In our theory, the rescaled relaxation time is 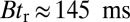 , which satisfies this upper bound. After the steep rise, the remaining ascent of
, which satisfies this upper bound. After the steep rise, the remaining ascent of  to the full step distance is determined by the diffusive search and binding to the forward site. According to Eqs. 6 and 7, this part of the step involves two time scales, th and
to the full step distance is determined by the diffusive search and binding to the forward site. According to Eqs. 6 and 7, this part of the step involves two time scales, th and 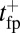 . Although
. Although 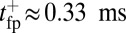 is smaller than
is smaller than 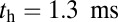 , the rescaled
, the rescaled 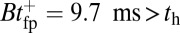 ; thus, in this particular case, hydrolysis is not rate-limiting.
; thus, in this particular case, hydrolysis is not rate-limiting.
Fig. 3.
Mean step trajectory 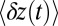 of the detached head along the actin filament at zero load. Red dots are the experimental results of Dunn and Spudich (23), obtained by tracking a gold nanoparticle attached near the end of the MyoV lever arm. A fast rise occurs over a distance
of the detached head along the actin filament at zero load. Red dots are the experimental results of Dunn and Spudich (23), obtained by tracking a gold nanoparticle attached near the end of the MyoV lever arm. A fast rise occurs over a distance 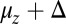 , resulting from the polymer structure relaxing to equilibrium after TH detachment. The more gradual rise that follows corresponds to the diffusive search for the forward binding site. The solid curve is the theoretical prediction, corrected for the slowing down of relaxation and first passage dynamics due to the particle. (Inset) Result of the original theory without the correction (solid curve), compared with a variant of the theory where ATP hydrolysis (hyd.) is removed as a condition for the TH to bind (dashed curve).
, resulting from the polymer structure relaxing to equilibrium after TH detachment. The more gradual rise that follows corresponds to the diffusive search for the forward binding site. The solid curve is the theoretical prediction, corrected for the slowing down of relaxation and first passage dynamics due to the particle. (Inset) Result of the original theory without the correction (solid curve), compared with a variant of the theory where ATP hydrolysis (hyd.) is removed as a condition for the TH to bind (dashed curve).
However, by changing the ATPase properties of the motor head, one can experimentally observe the role of hydrolysis in the binding kinetics. The nanoparticle tracking results described above are for MyoV with essential light chain LC1sa at the lever arm binding site closest to the motor head and calmodulin along the remainder of the arm. We will denote this type as MyoVelc. Dunn and Spudich (23) also studied a variant with only calmodulin (MyoVcam) that has very different ATPase rates. As shown in an earlier bulk study (43), for MyoVelc, the reverse 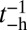 is negligible compared with the forward rate (
is negligible compared with the forward rate (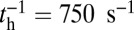 from Table 1) with
from Table 1) with 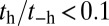 . In contrast, for MyoVcam, the forward rate is more than fourfold slower,
. In contrast, for MyoVcam, the forward rate is more than fourfold slower, 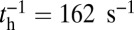 , and the reverse rate is substantial,
, and the reverse rate is substantial, 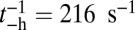 (43). With nonnegligible
(43). With nonnegligible 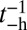 and the bead rescaling factor B, Eq. 5. for the TH binding time becomes
and the bead rescaling factor B, Eq. 5. for the TH binding time becomes
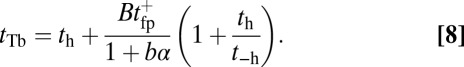 |
By substituting the 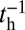 and
and 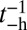 estimates for MyoVcam from ref. 43, while keeping all other parameters the same, Eq. 8. predicts a MyoVcam binding rate of
estimates for MyoVcam from ref. 43, while keeping all other parameters the same, Eq. 8. predicts a MyoVcam binding rate of 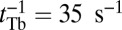 , which is about 2.6-fold slower than for MyoVelc, where
, which is about 2.6-fold slower than for MyoVelc, where 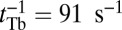 . Dunn and Spudich estimated the rebinding rates from the nanoparticle trajectories and found a similar threefold decrease between the MyoVelc and MyoVcam systems, from
. Dunn and Spudich estimated the rebinding rates from the nanoparticle trajectories and found a similar threefold decrease between the MyoVelc and MyoVcam systems, from 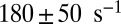 down to
down to 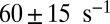 (23). The experimental rebinding rates are faster than the theoretical ones, which may be due, in part, to the fact that, experimentally, rebinding is not directly observed but only approximately inferred from where the
(23). The experimental rebinding rates are faster than the theoretical ones, which may be due, in part, to the fact that, experimentally, rebinding is not directly observed but only approximately inferred from where the 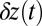 trajectory covers the full distance 2Δ to the forward site. The myosin head could still diffuse near 2Δ for some time without binding, and this could be indistinguishable from a binding event due to the intrinsic noise in the trajectory. However, the general slowdown seen in the experiment is reproduced in the theory and highlights the interplay of hydrolysis and diffusion times in the binding dynamics.
trajectory covers the full distance 2Δ to the forward site. The myosin head could still diffuse near 2Δ for some time without binding, and this could be indistinguishable from a binding event due to the intrinsic noise in the trajectory. However, the general slowdown seen in the experiment is reproduced in the theory and highlights the interplay of hydrolysis and diffusion times in the binding dynamics.
The hydrolysis rate would also play a greater role if the impediment of the attached bead were removed. For the MyoVelc case, with a bead factor 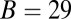 , the free end has enough time to hydrolyze before finding the forward binding site. Thus, the decay after the steep rise is mainly a single exponential in Fig. 3, with a characteristic time
, the free end has enough time to hydrolyze before finding the forward binding site. Thus, the decay after the steep rise is mainly a single exponential in Fig. 3, with a characteristic time 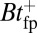 . If a future experiment were to measure
. If a future experiment were to measure 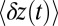 without slowing down the diffusion, we should see the average step shape shown in Fig. 3 (Inset), predicted by the theory for
without slowing down the diffusion, we should see the average step shape shown in Fig. 3 (Inset), predicted by the theory for 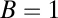 . There is a more gradual, double-exponential decay after the steep rise, reflecting both the th and
. There is a more gradual, double-exponential decay after the steep rise, reflecting both the th and 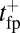 time scales. For comparison, we also show the results of the theory without ATP hydrolysis as a precondition for binding so as to emphasize the change in the
time scales. For comparison, we also show the results of the theory without ATP hydrolysis as a precondition for binding so as to emphasize the change in the 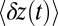 shape due to th.
shape due to th.
Experimentally, one can also study the average z-axis trajectory of the center of mass, for example, in a single-molecule bead assay (19). The results are essentially similar, but the above-cited distances are halved: We have a fast rise of 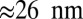 corresponding to the power stroke, detachment, and polymer relaxation, and a remaining slow ascent of
corresponding to the power stroke, detachment, and polymer relaxation, and a remaining slow ascent of 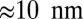 due to diffusive search and binding, giving a combined 36-nm center-of-mass step.
due to diffusive search and binding, giving a combined 36-nm center-of-mass step.
Run Length at Zero Load.
The final observable quantity of interest at 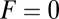 is the mean run length along the actin filament. Assuming
is the mean run length along the actin filament. Assuming 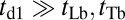 , the average run length zrun at any F is given by
, the average run length zrun at any F is given by
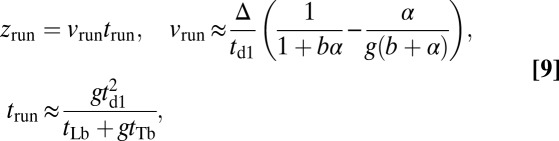 |
where vrun and trun are the mean run velocity and duration, respectively. The positive and negative terms in vrun are contributions from forward and backward stepping, respectively. Experimental estimates for zrun at 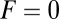 , plotted on the left edge of Fig. 4B, vary over a wide range from
, plotted on the left edge of Fig. 4B, vary over a wide range from 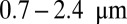 (6, 10, 11), most likely due to different measurement conditions (particularly the KCl concentration of the buffer). We choose as a representative value
(6, 10, 11), most likely due to different measurement conditions (particularly the KCl concentration of the buffer). We choose as a representative value 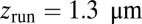 , which allows us to use Eq. 9 at
, which allows us to use Eq. 9 at 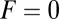 to solve for the binding penalty parameter,
to solve for the binding penalty parameter, 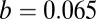 . This can be done because
. This can be done because 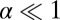 at zero load, and substituting
at zero load, and substituting 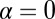 in Eq. 9 leads to an expression that is roughly independent of
in Eq. 9 leads to an expression that is roughly independent of 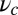 for large
for large 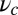 . Thus, we have fit two of the free parameters,
. Thus, we have fit two of the free parameters, 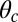 and b, by comparison with experimental values for the rise
and b, by comparison with experimental values for the rise 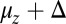 and the run length zrun, respectively. The final free parameter,
and the run length zrun, respectively. The final free parameter, 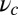 , will be fit by comparison with the stall force, which is discussed in the next section.
, will be fit by comparison with the stall force, which is discussed in the next section.
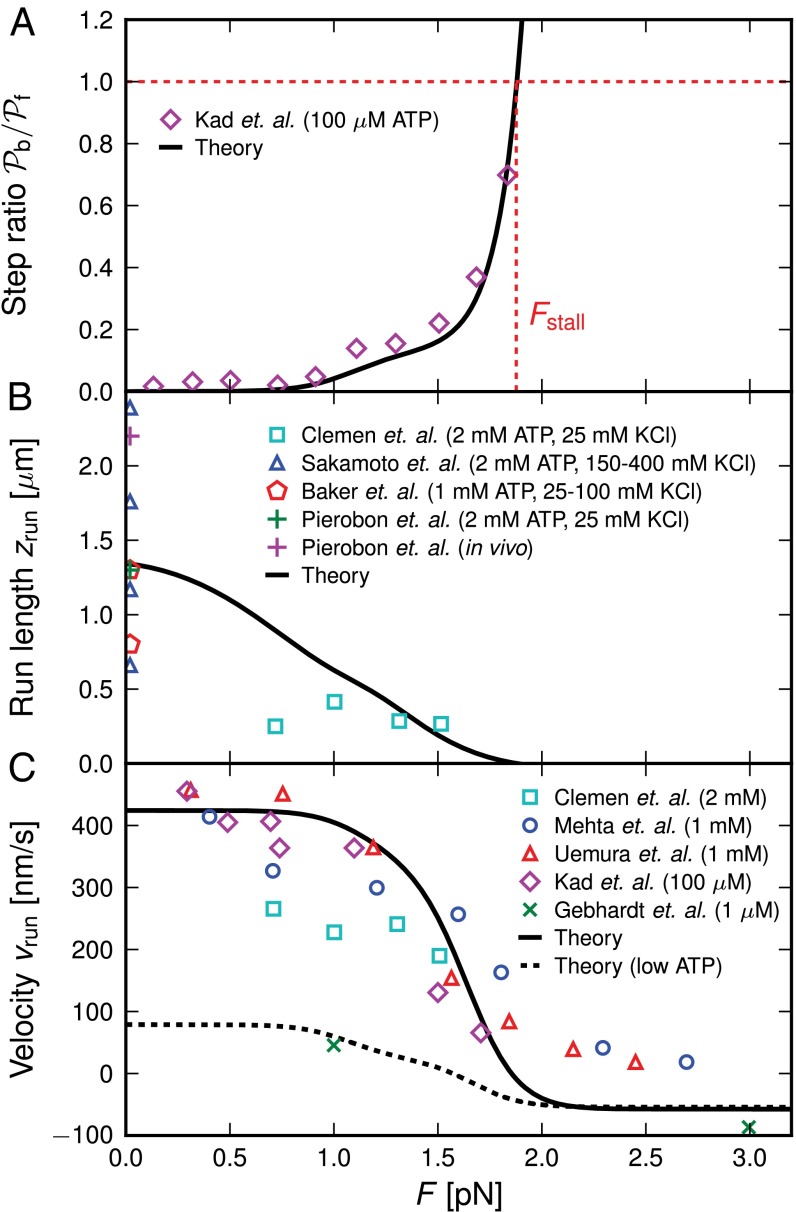
Fig. 4.
Comparison of the theory predictions (solid curves, with parameters in Table 1) to experimental results (symbols) as a function of load force F (with 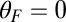 ). For the legends, the first and second terms in the parentheses correspond to experimental ATP and KCl concentrations, respectively. Where the KCl concentration is not indicated, the value is 25 mM. (A) Ratio of backward to forward steps,
). For the legends, the first and second terms in the parentheses correspond to experimental ATP and KCl concentrations, respectively. Where the KCl concentration is not indicated, the value is 25 mM. (A) Ratio of backward to forward steps, 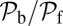 , compared with the data of Kad et al. (16). (B) Run length, zrun, compared with the data of Sakamoto et al. (6), Baker et al. (10), Pierobon et al. (11), and Clemen et al. (36). (C) Velocity, vrun, compared with the data of Mehta et al. (4), Kad et al. (16), Uemura et al. (17), Gebhardt et al. (18), and Clemen et al. (36). The dashed curve corresponds to a modified version of the theory, which accounts for the low ATP concentration in the experiment of Gebhardt et al. (18) (main text).
, compared with the data of Kad et al. (16). (B) Run length, zrun, compared with the data of Sakamoto et al. (6), Baker et al. (10), Pierobon et al. (11), and Clemen et al. (36). (C) Velocity, vrun, compared with the data of Mehta et al. (4), Kad et al. (16), Uemura et al. (17), Gebhardt et al. (18), and Clemen et al. (36). The dashed curve corresponds to a modified version of the theory, which accounts for the low ATP concentration in the experiment of Gebhardt et al. (18) (main text).
Load Dependence of the Kinetic Pathways and a Simple Formula for Stall Force.
When a backward force is applied to MyoV, it counteracts the bias due to the power stroke constraint, bending the bound leg and shifting the equilibrium away from the forward binding site. We see this directly in Eq. 3 for α, where the 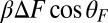 term in the exponential has the opposite sign of the
term in the exponential has the opposite sign of the 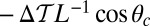 contribution from the constraint. Thus, α increases rapidly with increasing F, eventually becoming greater than 1, meaning that reaching the backward site is faster than reaching the forward one. Fig. 2C plots
contribution from the constraint. Thus, α increases rapidly with increasing F, eventually becoming greater than 1, meaning that reaching the backward site is faster than reaching the forward one. Fig. 2C plots 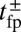 and the leg binding times as a function of F for the parameter set in Table 1. The changeover from
and the leg binding times as a function of F for the parameter set in Table 1. The changeover from 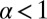 to
to 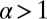 occurs near
occurs near 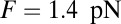 . The corresponding pathway probabilities are in Fig. 1D. With increasing force, each leg changes its primary kinetic pathway. TH detachment, which almost always leads to forward stepping at small F, instead leads to T foot stomping at high F. Similarly, LH detachment results in mainly L foot stomps at low F but leads to backward stepping at high F. Thus, application of a resistive load totally alters the partitioning between the kinetic pathways.
. The corresponding pathway probabilities are in Fig. 1D. With increasing force, each leg changes its primary kinetic pathway. TH detachment, which almost always leads to forward stepping at small F, instead leads to T foot stomping at high F. Similarly, LH detachment results in mainly L foot stomps at low F but leads to backward stepping at high F. Thus, application of a resistive load totally alters the partitioning between the kinetic pathways.
At the stall force, Fstall, the probabilities of backward and forward stepping are equal, and the mean MyoV velocity goes to zero. Setting 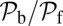 from Eq. 4 equal to 1, substituting α from Eq. 3, we obtain
from Eq. 4 equal to 1, substituting α from Eq. 3, we obtain
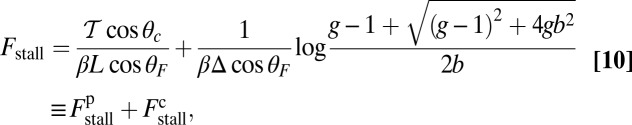 |
where the power stroke effectiveness  is defined in Eq. 3 in terms of lp, L, and
is defined in Eq. 3 in terms of lp, L, and 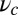 . The stall force has two main contributions. The first term
. The stall force has two main contributions. The first term 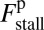 is due to the power stroke constraint, depending on
is due to the power stroke constraint, depending on  and
and 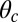 , and thus the structural parameters that determine
, and thus the structural parameters that determine  . Larger
. Larger  and smaller
and smaller 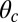 both act to shift the free end probability distribution closer to the forward site, impeding back-stepping and contributing to a larger
both act to shift the free end probability distribution closer to the forward site, impeding back-stepping and contributing to a larger 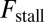 . The second term
. The second term 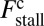 arises from two properties of MyoV head chemistry: the gating ratio g that controls how often the TH detaches relative to the LH and the binding penalty due to incorrect head orientation near the binding site. Increasing g makes detachment of the LH less common. Because back-stepping requires LH detachment, it will also become less probable. The importance of b is related to the Pr orientation penalty, which makes binding to the backward site less favorable. Larger g or smaller b reduces
arises from two properties of MyoV head chemistry: the gating ratio g that controls how often the TH detaches relative to the LH and the binding penalty due to incorrect head orientation near the binding site. Increasing g makes detachment of the LH less common. Because back-stepping requires LH detachment, it will also become less probable. The importance of b is related to the Pr orientation penalty, which makes binding to the backward site less favorable. Larger g or smaller b reduces 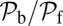 at any given F, thus increasing Fstall. If there were no gating asymmetry (the ratio
at any given F, thus increasing Fstall. If there were no gating asymmetry (the ratio 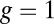 ), then the contribution
), then the contribution 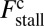 vanishes.
vanishes.
The optical trap experiment of Kad et al. (16) yielded 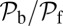 as a function of F. The data are plotted in Fig. 4A, corresponding to an estimated
as a function of F. The data are plotted in Fig. 4A, corresponding to an estimated 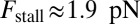 . Using this experimental value of Fstall and assuming, for simplicity, that
. Using this experimental value of Fstall and assuming, for simplicity, that 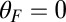 or a pure backward load, we get
or a pure backward load, we get 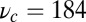 by solving Eq. 10. In Discussion, we will return to the magnitude of
by solving Eq. 10. In Discussion, we will return to the magnitude of 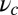 in the broader context of stiffness and energetics within the myosin motor family. The theoretical curve in Fig. 4A is in good agreement with the experimental data points over the entire measured F range. Back-stepping is mostly suppressed for
in the broader context of stiffness and energetics within the myosin motor family. The theoretical curve in Fig. 4A is in good agreement with the experimental data points over the entire measured F range. Back-stepping is mostly suppressed for 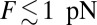 , and then rapidly increases until the stall point.
, and then rapidly increases until the stall point.
Run Length and Velocity Under Load.
The change in kinetic pathways with F manifests itself in two other observables, the mean run length zrun and velocity vrun, which both decrease to zero as the stall force is approached. In Fig. 4 B and C, we show various experimental results for these two quantities as a function of F, together with the theoretical prediction (Eq. 9). Aside from one exception mentioned below, all the experiments were done at saturating ATP 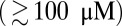 . Despite the scatter in the experimental values, the theory reproduces the overall trends well. The motor function nears its unloaded
. Despite the scatter in the experimental values, the theory reproduces the overall trends well. The motor function nears its unloaded 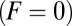 velocity of
velocity of 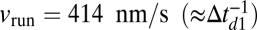 for small forces and then slows down noticeably for
for small forces and then slows down noticeably for 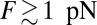 as the proportion of back steps increases. The extrapolated force at which the velocity goes to zero is another way to estimate the stall force, and the experiments show MyoV stalling in the range of
as the proportion of back steps increases. The extrapolated force at which the velocity goes to zero is another way to estimate the stall force, and the experiments show MyoV stalling in the range of 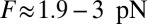 .
.
Above the stall force, the theory predicts a small net negative velocity, because back steps outnumber the forward steps. Although the present theory will likely require modifications at very high forces far into the superstall regime, we can tentatively compare our results with those of Gebhardt et al. (18) at 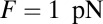 and
and 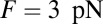 (green crosses in Fig. 4C), where the latter data point was just above stall and exhibited a small negative velocity
(green crosses in Fig. 4C), where the latter data point was just above stall and exhibited a small negative velocity 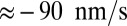 . In this case, the ATP concentration is 1 μM, which makes ATP binding the rate-limiting step in TH detachment. To accommodate this, we set
. In this case, the ATP concentration is 1 μM, which makes ATP binding the rate-limiting step in TH detachment. To accommodate this, we set 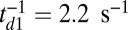 , which is the binding rate at 1 μM ATP estimated from the experimental kinetics (18). With this single modification, the theory gives the dashed curve in Fig. 4C, which roughly captures the velocities both below and above stall. Taken together, the comparison between the theory and a number of experimental results shows that our predictions agree with measurements remarkably well.
, which is the binding rate at 1 μM ATP estimated from the experimental kinetics (18). With this single modification, the theory gives the dashed curve in Fig. 4C, which roughly captures the velocities both below and above stall. Taken together, the comparison between the theory and a number of experimental results shows that our predictions agree with measurements remarkably well.
Discussion
Constraints on MyoV Structural and Binding Parameters.
MyoV walks nearly unidirectionally at zero load and can persist against backward loads up to the stall force. Is the system robust to variations in the parameter space? To make the question concrete, we can ask under what conditions does MyoV fulfill two requirements for processive motion and the ability to sustain load: (i) the backward-to-forward step ratio at zero load, 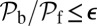 , and (ii) the stall force Fstall falls in some range from
, and (ii) the stall force Fstall falls in some range from 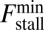 to
to 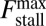 when the resistive load is applied parallel to the actin axis (
when the resistive load is applied parallel to the actin axis (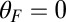 in Fig. 1B). We choose experimentally motivated values of
in Fig. 1B). We choose experimentally motivated values of 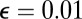 ,
, 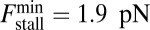 (16), and
(16), and 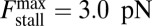 (4, 12, 17). From Eqs. 3, 4, and 10, these two conditions are satisfied within the blue-shaded area of Fig. 5A, which plots a
(4, 12, 17). From Eqs. 3, 4, and 10, these two conditions are satisfied within the blue-shaded area of Fig. 5A, which plots a  vs.
vs.  slice of the parameter space, with fixed
slice of the parameter space, with fixed  , Δ, and g. Along the
, Δ, and g. Along the  axis, the region has minimal and maximal boundaries:
axis, the region has minimal and maximal boundaries:
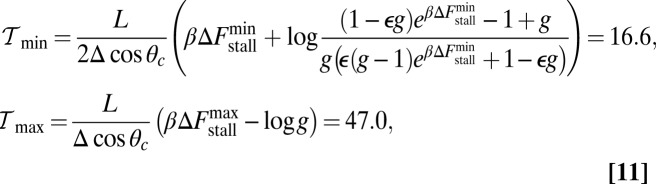 |
where the numerical values are computed for the specific parameters in Table 1. If 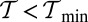 or
or 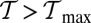 , there is no value of b where conditions i and ii are satisfied simultaneously. A density plot of
, there is no value of b where conditions i and ii are satisfied simultaneously. A density plot of  in terms of
in terms of 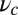 and lp is shown in Fig. 5B, with the
and lp is shown in Fig. 5B, with the 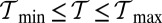 region shaded in green. Asymptotically, this region is bounded by a minimum persistence length
region shaded in green. Asymptotically, this region is bounded by a minimum persistence length 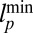 for
for  and minimum constraint strength
and minimum constraint strength 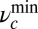 for
for 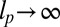 :
:
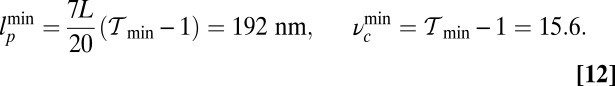 |
Having lp and 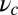 above these two minima constitutes necessary but not sufficient conditions for
above these two minima constitutes necessary but not sufficient conditions for  to fall between
to fall between 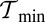 and
and 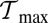 . Physically,
. Physically,  represents the effectiveness of the power stroke constraint, which is directly related to lp and
represents the effectiveness of the power stroke constraint, which is directly related to lp and 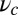 through Eq. 3. We thus see that motor function with the given specifications requires a certain minimal power stroke effectiveness, which cannot be achieved unless both the persistence length of the lever arms and the strength of the end-tangent constraint are large enough. If either lp or
through Eq. 3. We thus see that motor function with the given specifications requires a certain minimal power stroke effectiveness, which cannot be achieved unless both the persistence length of the lever arms and the strength of the end-tangent constraint are large enough. If either lp or 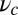 is too small, back-stepping becomes more frequent at zero load and it is easier to bend the bound leg backward, resulting in stall being reached at smaller force magnitudes.
is too small, back-stepping becomes more frequent at zero load and it is easier to bend the bound leg backward, resulting in stall being reached at smaller force magnitudes.
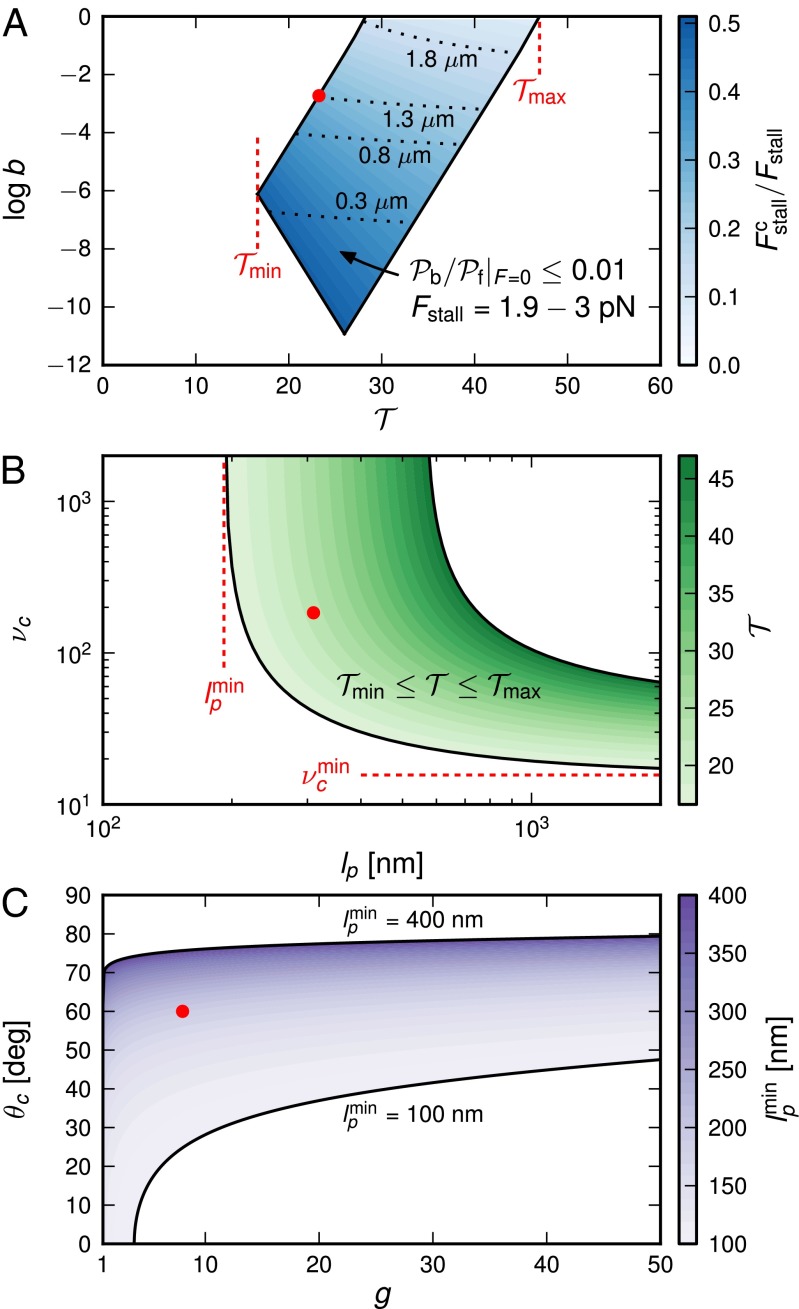
Fig. 5.
Exploring the design space for MyoV satisfying the constraints that 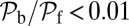 and that the stall force
and that the stall force 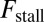 be in the range of
be in the range of 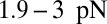 . The red dot in each panel corresponds to the parameter set in Table 1. (A) Blue-shaded region shows the allowed values for the binding penalty b and power stroke effectiveness
. The red dot in each panel corresponds to the parameter set in Table 1. (A) Blue-shaded region shows the allowed values for the binding penalty b and power stroke effectiveness  (Eq. 3). The intensity of the shading indicates the fraction
(Eq. 3). The intensity of the shading indicates the fraction 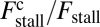 , where
, where 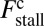 is the contribution of head chemistry to the total stall force (Eq. 10). The labeled black dotted lines correspond to loci of constant run length zrun. The blue-shaded region falls entirely within the range of
is the contribution of head chemistry to the total stall force (Eq. 10). The labeled black dotted lines correspond to loci of constant run length zrun. The blue-shaded region falls entirely within the range of 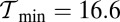 to
to 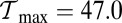 along the
along the  axis. (B) Green-shaded region corresponds to those values of persistence length lp and power stroke strength
axis. (B) Green-shaded region corresponds to those values of persistence length lp and power stroke strength 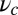 that yield
that yield  in the range of
in the range of 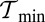 to
to 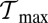 . The intensity of shading indicates the magnitude of
. The intensity of shading indicates the magnitude of  . Below the values
. Below the values 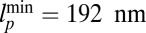 and
and 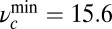 , it is impossible to satisfy the bounds on
, it is impossible to satisfy the bounds on  . (C) Purple-shaded region corresponds to values of the gating ratio g and power stroke constraint angle
. (C) Purple-shaded region corresponds to values of the gating ratio g and power stroke constraint angle 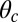 that yield
that yield 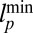 from
from 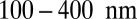 , with the shading intensity proportional to
, with the shading intensity proportional to 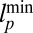 .
.
The bounds on  in Eq. 11 also depend on the gating ratio g and Po orientation
in Eq. 11 also depend on the gating ratio g and Po orientation 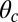 (Fig. 1B), which we illustrate in Fig. 5C by plotting the density of
(Fig. 1B), which we illustrate in Fig. 5C by plotting the density of 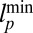 (related to
(related to 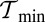 through Eq. 12) in terms of g and
through Eq. 12) in terms of g and 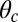 . By showing only the range
. By showing only the range 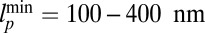 , comparable with estimates of the lever arm persistence length (26, 28), we see there are constraints on the angle
, comparable with estimates of the lever arm persistence length (26, 28), we see there are constraints on the angle 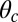 that vary depending on g. Angles too close to 90° give insufficient forward bias and have to be compensated for by an unrealistically stiff lever arm
that vary depending on g. Angles too close to 90° give insufficient forward bias and have to be compensated for by an unrealistically stiff lever arm 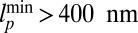 . As
. As 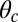 decreases,
decreases, 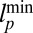 decreases, because the stronger forward bias means that one can use progressively more flexible lever arms and still get efficient motility and resistance to load.
decreases, because the stronger forward bias means that one can use progressively more flexible lever arms and still get efficient motility and resistance to load.
The parameter range where the motility conditions i and ii are simultaneously satisfied (the shaded region in Fig. 5A), is broad, encompassing a wide swathe of possible b values. To restrict the parameters further, we can specify that MyoV exhibit a certain run length. The dotted lines in Fig. 5A are loci of constant zrun, with the red dot marking the parameter set in Table 1 (where 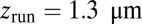 and
and 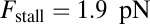 ). Even with this restriction, we still have a range of possible
). Even with this restriction, we still have a range of possible  values at each zrun, which corresponds to a region in the space of lp and
values at each zrun, which corresponds to a region in the space of lp and 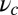 . Interestingly, the system has a degree of robustness against changes in the structural parameters and can meet the basic requirements for function with a high-duty ratio assuming lp and
. Interestingly, the system has a degree of robustness against changes in the structural parameters and can meet the basic requirements for function with a high-duty ratio assuming lp and 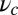 yield a
yield a  within the allowed range.
within the allowed range.
Relative Contributions of Power Stroke and Head Chemistry to the Stall Force Magnitude.
Although the emphasis in the preceding section has been on the structural parameters, it is important to note the complementary role of head chemistry (determined by the nucleotide state of MyoV) in producing the observed stall force. If TH and LH detachment were equally probable 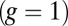 , the
, the 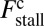 term in Eq. 10 would be zero, and
term in Eq. 10 would be zero, and 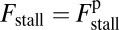 . From the definition of α in Eq. 3, one can see that
. From the definition of α in Eq. 3, one can see that 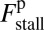 is the force magnitude at which
is the force magnitude at which 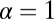 . In other words, at
. In other words, at 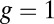 , the only condition for stall is that the first passage times to the forward and backward sites are equal. In fact, the value of
, the only condition for stall is that the first passage times to the forward and backward sites are equal. In fact, the value of 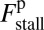 arises from a simple force balance: Stall occurs when the component
arises from a simple force balance: Stall occurs when the component 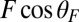 of the backward load along the z axis equals
of the backward load along the z axis equals 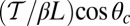 , the z component of an effective forward force
, the z component of an effective forward force 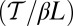 oriented along the power stroke constraint direction. This is another way of interpreting the power stroke parameter
oriented along the power stroke constraint direction. This is another way of interpreting the power stroke parameter  , relating it to a counteracting force on the joint to oppose the load. When the two forces are equal, there is no bias either forward or backward and
, relating it to a counteracting force on the joint to oppose the load. When the two forces are equal, there is no bias either forward or backward and 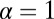 .
.
Head chemistry changes the picture, by making LH detachment less frequent 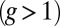 and introducing a binding penalty
and introducing a binding penalty 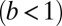 for the wrong head orientation at the binding site. A small b parameter reduces the probability of T foot stomping, which would otherwise compete more easily with forward stepping at large loads and reduce the likelihood of the latter. This is the beneficial role of the recovery stroke highlighted in the study by Shiroguchi et al. (3). The outcome is an additional contribution
for the wrong head orientation at the binding site. A small b parameter reduces the probability of T foot stomping, which would otherwise compete more easily with forward stepping at large loads and reduce the likelihood of the latter. This is the beneficial role of the recovery stroke highlighted in the study by Shiroguchi et al. (3). The outcome is an additional contribution 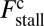 to Fstall, which means stall is delayed until we reach a value of
to Fstall, which means stall is delayed until we reach a value of 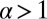 . In order for back-stepping to be as likely as forward stepping, it is not enough to make the first passage times to the two binding sites equal. We have to make
. In order for back-stepping to be as likely as forward stepping, it is not enough to make the first passage times to the two binding sites equal. We have to make 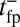 fast enough compared with
fast enough compared with 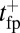 to compensate for the gating and binding biases. To illustrate the significance of the
to compensate for the gating and binding biases. To illustrate the significance of the 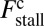 contribution over the allowed parameter range, we use the intensity of the shading in Fig. 5A to represent
contribution over the allowed parameter range, we use the intensity of the shading in Fig. 5A to represent 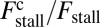 , the fraction of the stall force magnitude due to the head chemistry term. The fraction values vary from
, the fraction of the stall force magnitude due to the head chemistry term. The fraction values vary from 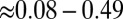 , with the parameter set in Table 1 giving
, with the parameter set in Table 1 giving 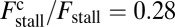 . Although the power stroke term always dominates, head chemistry has a smaller but nonnegligible role in helping MyoV move forward under load.
. Although the power stroke term always dominates, head chemistry has a smaller but nonnegligible role in helping MyoV move forward under load.
Relation of the Power Stroke Constraint Strength to Myosin Stiffness and Thermodynamic Efficiency.
The mechanical compliance of MyoV under load is determined both by the bending stiffness of the lever arm lp and the strength of the effective end-tangent constraint 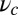 . The latter arises at a molecular level from the bending stiffness of the flexible joint between the motor head and lever arm domains. If we suppose this joint involves subdomains (i.e., the converter region of the motor head) on length scales of
. The latter arises at a molecular level from the bending stiffness of the flexible joint between the motor head and lever arm domains. If we suppose this joint involves subdomains (i.e., the converter region of the motor head) on length scales of 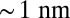 , then
, then 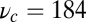 corresponds to a persistence length of
corresponds to a persistence length of 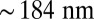 for the head–arm joint, which is reasonable because it is of the same order of magnitude as the persistence length,
for the head–arm joint, which is reasonable because it is of the same order of magnitude as the persistence length, 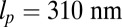 , of the lever arm itself.
, of the lever arm itself.
The complex coupling between these two different bending rigidities is reflected in the power stroke effectiveness parameter  , which depends nonlinearly on both lp and
, which depends nonlinearly on both lp and 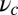 . In fact, one can approximately relate
. In fact, one can approximately relate  to the overall compliance of the head–arm system. For large lp, where the arms are nearly rigid rods, the backward force
to the overall compliance of the head–arm system. For large lp, where the arms are nearly rigid rods, the backward force 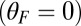 required to keep the end of the bound leg at an angle
required to keep the end of the bound leg at an angle 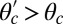 (Materials and Methods) is
(Materials and Methods) is
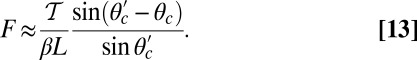 |
The horizontal 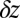 displacement corresponding to the angular displacement between
displacement corresponding to the angular displacement between 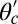 and
and 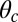 is
is 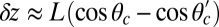 . For
. For  , the rough angular range during the motor cycle, F scales almost linearly with
, the rough angular range during the motor cycle, F scales almost linearly with 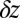 , with a slope
, with a slope 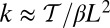 that gives an effective total spring constant of the bound leg. In the strained telemark stance of the waiting state, when both legs are bound and Po, and the L leg is bent backward from
that gives an effective total spring constant of the bound leg. In the strained telemark stance of the waiting state, when both legs are bound and Po, and the L leg is bent backward from 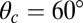 to about
to about 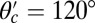 ,
, 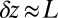 and the effective spring is loaded with a mechanical energy of
and the effective spring is loaded with a mechanical energy of 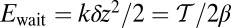 . This is essentially the energy necessary for the power stroke (Pr to Po) transition that loads the spring. For
. This is essentially the energy necessary for the power stroke (Pr to Po) transition that loads the spring. For 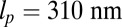 and
and 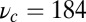 , we have
, we have 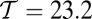 ,
, 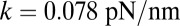 , and
, and 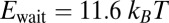 . If the total energy available from ATP hydrolysis is
. If the total energy available from ATP hydrolysis is 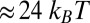 , then this corresponds to a thermodynamic efficiency of nearly 50%, similar to earlier estimates for MyoV (30) and myosin II (44). The tension in the waiting state associated with this stored mechanical energy is
, then this corresponds to a thermodynamic efficiency of nearly 50%, similar to earlier estimates for MyoV (30) and myosin II (44). The tension in the waiting state associated with this stored mechanical energy is 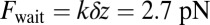 .
.
Myosin II offers an interesting point of comparison in terms of mechanical compliance. The stiffness k of its S1 domain is a key parameter in the swinging cross-bridge model of muscle contraction, with a range of 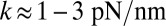 inferred from experimental measurements (26, 45, 46), which is an order of magnitude higher than our MyoV value above. The key factor underlying this difference is the length of the lever arm, with myosin II having an L about one-third that of MyoV. If one assumes that beyond this difference, the other structural factors (lp and
inferred from experimental measurements (26, 45, 46), which is an order of magnitude higher than our MyoV value above. The key factor underlying this difference is the length of the lever arm, with myosin II having an L about one-third that of MyoV. If one assumes that beyond this difference, the other structural factors (lp and  ) are similar between these two systems, then one can use our structural model with
) are similar between these two systems, then one can use our structural model with 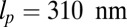 ,
, 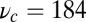 , and
, and 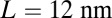 to predict a myosin II stiffness of
to predict a myosin II stiffness of 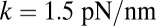 , which compares well with the experimental range.
, which compares well with the experimental range.
Conclusion
In conclusion, we have proposed a model of MyoV dynamics based on the polymeric nature of the lever arms and the probability distribution of their fluctuations during the diffusive search for actin-binding sites. Using only three experimentally unknown parameters, our theory quantitatively captures many experimental outcomes, such as the time dependence of the mean trajectory of the detached head and the force dependence of the probability ratio of forward to backward stepping. The theory, which allows us to explore the robustness of stepping to variations in the design of MyoV, also yields testable predictions for novel quantities, like the probabilities of foot stomping as a function of load. Although the unidirectionality of the motor and the stall force magnitude exhibit tolerance to variation in the structural parameters, the theory reveals constraints on the persistence length of the lever arms and power stroke bias. In the context of processive motors within the myosin superfamily, MyoV has the simplest lever arm structure, which can be approximated well by a stiff polymer. Myosins VI and X have evolved qualitatively different lever arms consisting of both stiff and flexible segments (47). The underlying theoretical ideas in our description of MyoV are quite general, and it will be interesting to extend them in the future to more complex geometries. How do the structural constraints change in a motor with heterogeneous persistence length, and can such an approach help resolve the competing hypotheses for the conformation of the myosin VI lever arm (48–50)?
From a broader perspective, the approach we have developed is also applicable in other motor systems, such as dynein and kinesin, provided the structural elements generating the power stroke can be modeled as suitable polymer chains. In addition, there are potential applications to other biological systems that transmit or generate force, such as microtubules and cytoskeletal structures.
Materials and Methods
First Passage Times to Binding Sites.
The derivation of Eq. 1 for the mean first passage times  is shown in detail in SI Text. The underlying approach is based on the renewal method for first passage problems (51); in the polymer context, this is equivalent to the Wilemski–Fixman theory for diffusion-controlled reactions (52). For analytical tractability, we ignore excluded volume interactions, which would likely lead to a small decrease in the first passage times but would not change the overall order of magnitude. Strictly speaking,
is shown in detail in SI Text. The underlying approach is based on the renewal method for first passage problems (51); in the polymer context, this is equivalent to the Wilemski–Fixman theory for diffusion-controlled reactions (52). For analytical tractability, we ignore excluded volume interactions, which would likely lead to a small decrease in the first passage times but would not change the overall order of magnitude. Strictly speaking, 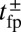 depends on the initial configuration of the polymer, but for MyoV dynamics,
depends on the initial configuration of the polymer, but for MyoV dynamics, 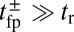 , the relaxation time of the polymer to equilibrium. Hence, the memory of the initial configuration is lost during the diffusive search, and the expression for
, the relaxation time of the polymer to equilibrium. Hence, the memory of the initial configuration is lost during the diffusive search, and the expression for 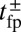 in Eq. 1 is valid assuming we do not start with the free end in the immediate vicinity of the target. When the latter condition is violated, for example, after failed binding attempts due to wrong head orientation or immediately following detachment from the actin, we assume fast relaxation to equilibrium before the head has a chance to rebind.
in Eq. 1 is valid assuming we do not start with the free end in the immediate vicinity of the target. When the latter condition is violated, for example, after failed binding attempts due to wrong head orientation or immediately following detachment from the actin, we assume fast relaxation to equilibrium before the head has a chance to rebind.
Mean Field Theory for Probability Distribution of MyoV Free End During Diffusive Search.
The key physical quantity in Eq. 1 that determines the average first passage time to a binding site is 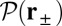 , the equilibrium probability density of finding the detached end of MyoV at
, the equilibrium probability density of finding the detached end of MyoV at 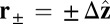 . For a structure of two semiflexible polymer legs, with one leg bound at the origin, the free end-point vector is
. For a structure of two semiflexible polymer legs, with one leg bound at the origin, the free end-point vector is 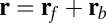 , where
, where 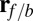 is the end-to-end vector of the free/bound leg. The distribution
is the end-to-end vector of the free/bound leg. The distribution 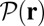 is a convolution of the individual leg distributions
is a convolution of the individual leg distributions 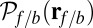 :
:
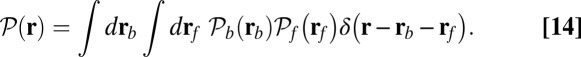 |
There is no exact closed form expression for the end-to-end distribution of a semiflexible polymer, although moments of the distribution can be calculated analytically (53, 54). For the free leg, which is not under tension, an earlier mean field theory (55) gives a useful approximation:
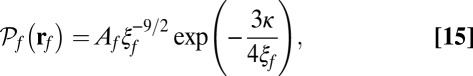 |
where 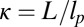 ,
, 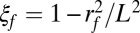 , and Af is a normalization constant:
, and Af is a normalization constant:
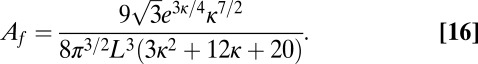 |
As shown in SI Text, this mean field approach can be generalized to include the end-tangent constraint and load force in the bound leg case, yielding
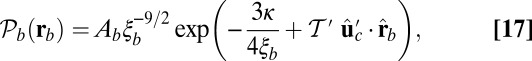 |
where 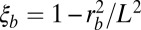 ,
, 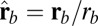 ,
, 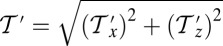 , and
, and
The power stroke effectiveness parameter  is defined in Eq. 3. The direction
is defined in Eq. 3. The direction 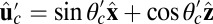 with an angle
with an angle 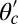 from the
from the 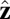 axis given by
axis given by
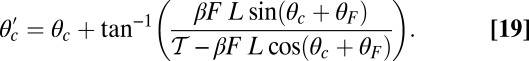 |
In the limit of large lp, the vector 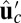 is approximately the average orientation of the bound leg, reflecting the combined influence of the load force F and the end-tangent constraint
is approximately the average orientation of the bound leg, reflecting the combined influence of the load force F and the end-tangent constraint 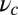 . In the case of a backward force
. In the case of a backward force 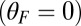 , we can invert Eq. 19 to find the force F required, on average, to maintain an orientation
, we can invert Eq. 19 to find the force F required, on average, to maintain an orientation 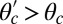 , as shown in Eq. 13. In both the free and bound leg cases, the analytical distributions
, as shown in Eq. 13. In both the free and bound leg cases, the analytical distributions 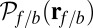 have excellent agreement with the exactly known moments. Carrying out the convolution in Eq. 14, we arrive at a final expression for
have excellent agreement with the exactly known moments. Carrying out the convolution in Eq. 14, we arrive at a final expression for 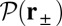 in the stiff regime
in the stiff regime 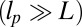 :
:
 |
where  is the zeroth-order modified Bessel function of the first kind.
is the zeroth-order modified Bessel function of the first kind.
Supplementary Material
Acknowledgments
M.H. was a Ruth L. Kirschstein National Research Service postdoctoral fellow, supported by the National Institute of General Medical Sciences (Grant 1 F32 GM 97756-1). D.T. was supported by the National Science Foundation (Grant CHE 09-10433) and National Institutes of Health (Grant GM 089685).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312393110/-/DCSupplemental.
References
- 1.Spudich JA, Sivaramakrishnan S. Myosin VI: An innovative motor that challenged the swinging lever arm hypothesis. Nat Rev Mol Cell Biol. 2010;11(2):128–137. doi: 10.1038/nrm2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reck-Peterson SL, Provance DW, Mooseker MS, Mercer JA. Class V myosins. Biochim Biophys Acta. 2000;1496(1):36–51. doi: 10.1016/s0167-4889(00)00007-0. [DOI] [PubMed] [Google Scholar]
- 3.Shiroguchi K, et al. Direct observation of the myosin Va recovery stroke that contributes to unidirectional stepping along actin. PLoS Biol. 2011;9(4):e1001031. doi: 10.1371/journal.pbio.1001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta AD, et al. Myosin-V is a processive actin-based motor. Nature. 1999;400(6744):590–593. doi: 10.1038/23072. [DOI] [PubMed] [Google Scholar]
- 5.Rief M, et al. Myosin-V stepping kinetics: A molecular model for processivity. Proc Natl Acad Sci USA. 2000;97(17):9482–9486. doi: 10.1073/pnas.97.17.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakamoto T, Amitani I, Yokota E, Ando T. Direct observation of processive movement by individual myosin V molecules. Biochem Biophys Res Commun. 2000;272(2):586–590. doi: 10.1006/bbrc.2000.2819. [DOI] [PubMed] [Google Scholar]
- 7.Yildiz A, et al. Myosin V walks hand-over-hand: Single fluorophore imaging with 1.5-nm localization. Science. 2003;300(5628):2061–2065. doi: 10.1126/science.1084398. [DOI] [PubMed] [Google Scholar]
- 8.Forkey JN, Quinlan ME, Shaw MA, Corrie JET, Goldman YE. Three-dimensional structural dynamics of myosin V by single-molecule fluorescence polarization. Nature. 2003;422(6930):399–404. doi: 10.1038/nature01529. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto T, Webb MR, Forgacs E, White HD, Sellers JR. Direct observation of the mechanochemical coupling in myosin Va during processive movement. Nature. 2008;455(7209):128–132. doi: 10.1038/nature07188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker JE, et al. Myosin V processivity: Multiple kinetic pathways for head-to-head coordination. Proc Natl Acad Sci USA. 2004;101(15):5542–5546. doi: 10.1073/pnas.0307247101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierobon P, et al. Velocity, processivity, and individual steps of single myosin V molecules in live cells. Biophys J. 2009;96(10):4268–4275. doi: 10.1016/j.bpj.2009.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veigel C, Wang F, Bartoo ML, Sellers JR, Molloy JE. The gated gait of the processive molecular motor, myosin V. Nat Cell Biol. 2002;4(1):59–65. doi: 10.1038/ncb732. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld SS, Sweeney HL. A model of myosin V processivity. J Biol Chem. 2004;279(38):40100–40111. doi: 10.1074/jbc.M402583200. [DOI] [PubMed] [Google Scholar]
- 14.Veigel C, Schmitz S, Wang F, Sellers JR. Load-dependent kinetics of myosin-V can explain its high processivity. Nat Cell Biol. 2005;7(9):861–869. doi: 10.1038/ncb1287. [DOI] [PubMed] [Google Scholar]
- 15.Purcell TJ, Sweeney HL, Spudich JA. A force-dependent state controls the coordination of processive myosin V. Proc Natl Acad Sci USA. 2005;102(39):13873–13878. doi: 10.1073/pnas.0506441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kad NM, Trybus KM, Warshaw DM. Load and Pi control flux through the branched kinetic cycle of myosin V. J Biol Chem. 2008;283(25):17477–17484. doi: 10.1074/jbc.M800539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uemura S, Higuchi H, Olivares AO, De La Cruz EM, Ishiwata S. Mechanochemical coupling of two substeps in a single myosin V motor. Nat Struct Mol Biol. 2004;11(9):877–883. doi: 10.1038/nsmb806. [DOI] [PubMed] [Google Scholar]
- 18.Gebhardt JCM, Clemen AEM, Jaud J, Rief M. Myosin-V is a mechanical ratchet. Proc Natl Acad Sci USA. 2006;103(23):8680–8685. doi: 10.1073/pnas.0510191103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cappello G, et al. Myosin V stepping mechanism. Proc Natl Acad Sci USA. 2007;104(39):15328–15333. doi: 10.1073/pnas.0706653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kodera N, Yamamoto D, Ishikawa R, Ando T. Video imaging of walking myosin V by high-speed atomic force microscopy. Nature. 2010;468(7320):72–76. doi: 10.1038/nature09450. [DOI] [PubMed] [Google Scholar]
- 21.Syed S, Snyder GE, Franzini-Armstrong C, Selvin PR, Goldman YE. Adaptability of myosin V studied by simultaneous detection of position and orientation. EMBO J. 2006;25(9):1795–1803. doi: 10.1038/sj.emboj.7601060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beausang JF, Shroder DY, Nelson PC, Goldman YE. Tilting and wobble of myosin V by high-speed single-molecule polarized fluorescence microscopy. Biophys J. 2013;104(6):1263–1273. doi: 10.1016/j.bpj.2013.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn AR, Spudich JA. Dynamics of the unbound head during myosin V processive translocation. Nat Struct Mol Biol. 2007;14(3):246–248. doi: 10.1038/nsmb1206. [DOI] [PubMed] [Google Scholar]
- 24.Shiroguchi K, Kinosita K., Jr Myosin V walks by lever action and Brownian motion. Science. 2007;316(5828):1208–1212. doi: 10.1126/science.1140468. [DOI] [PubMed] [Google Scholar]
- 25.Komori Y, Iwane AH, Yanagida T. Myosin-V makes two brownian 90 degrees rotations per 36-nm step. Nat Struct Mol Biol. 2007;14(10):968–973. doi: 10.1038/nsmb1298. [DOI] [PubMed] [Google Scholar]
- 26.Howard J, Spudich JA. Is the lever arm of myosin a molecular elastic element? Proc Natl Acad Sci USA. 1996;93(9):4462–4464. [PubMed] [Google Scholar]
- 27.Moore JR, Krementsova EB, Trybus KM, Warshaw DM. Does the myosin V neck region act as a lever? J Muscle Res Cell Motil. 2004;25(1):29–35. doi: 10.1023/b:jure.0000021394.48560.71. [DOI] [PubMed] [Google Scholar]
- 28.Vilfan A. Elastic lever-arm model for myosin V. Biophys J. 2005;88(6):3792–3805. doi: 10.1529/biophysj.104.046763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolomeisky AB, Fisher ME. A simple kinetic model describes the processivity of myosin-v. Biophys J. 2003;84(3):1642–1650. doi: 10.1016/S0006-3495(03)74973-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan GH, Sun SX. Dynamics of myosin-V processivity. Biophys J. 2005;88(2):999–1008. doi: 10.1529/biophysj.104.047662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skau KI, Hoyle RB, Turner MS. A kinetic model describing the processivity of myosin-V. Biophys J. 2006;91(7):2475–2489. doi: 10.1529/biophysj.105.070888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsygankov D, Fisher ME. Mechanoenzymes under superstall and large assisting loads reveal structural features. Proc Natl Acad Sci USA. 2007;104(49):19321–19326. doi: 10.1073/pnas.0709911104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu YZ, Wang ZS. Comprehensive physical mechanism of two-headed biomotor myosin V. J Chem Phys. 2009;131(24):245104. doi: 10.1063/1.3276283. [DOI] [PubMed] [Google Scholar]
- 34.Bierbaum V, Lipowsky R. Chemomechanical coupling and motor cycles of myosin V. Biophys J. 2011;100(7):1747–1755. doi: 10.1016/j.bpj.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig EM, Linke H. Mechanochemical model for myosin V. Proc Natl Acad Sci USA. 2009;106(43):18261–18266. doi: 10.1073/pnas.0908192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clemen AEM, et al. Force-dependent stepping kinetics of myosin-V. Biophys J. 2005;88(6):4402–4410. doi: 10.1529/biophysj.104.053504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker ML, et al. Two-headed binding of a processive myosin to F-actin. Nature. 2000;405(6788):804–807. doi: 10.1038/35015592. [DOI] [PubMed] [Google Scholar]
- 38.De La Cruz EM, Wells AL, Rosenfeld SS, Ostap EM, Sweeney HL. The kinetic mechanism of myosin V. Proc Natl Acad Sci USA. 1999;96(24):13726–13731. doi: 10.1073/pnas.96.24.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sellers JR, Veigel C. Direct observation of the myosin-Va power stroke and its reversal. Nat Struct Mol Biol. 2010;17(5):590–595. doi: 10.1038/nsmb.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortega A, Amorós D, García de la Torre J. Prediction of hydrodynamic and other solution properties of rigid proteins from atomic- and residue-level models. Biophys J. 2011;101(4):892–898. doi: 10.1016/j.bpj.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coureux PD, Sweeney HL, Houdusse A. Three myosin V structures delineate essential features of chemo-mechanical transduction. EMBO J. 2004;23(23):4527–4537. doi: 10.1038/sj.emboj.7600458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thirumalai D, Ha BY. In: Theoretical and Mathematical Methods in Polymer Research. Grosberg AY, editor. New York: Academic; 1998. pp. 1–35. [Google Scholar]
- 43.De La Cruz EM, Wells AL, Sweeney HL, Ostap EM. Actin and light chain isoform dependence of myosin V kinetics. Biochemistry. 2000;39(46):14196–14202. doi: 10.1021/bi001701b. [DOI] [PubMed] [Google Scholar]
- 44.Barclay CJ. Estimation of cross-bridge stiffness from maximum thermodynamic efficiency. J Muscle Res Cell Motil. 1998;19(8):855–864. doi: 10.1023/a:1005409708838. [DOI] [PubMed] [Google Scholar]
- 45.Decostre V, Bianco P, Lombardi V, Piazzesi G. Effect of temperature on the working stroke of muscle myosin. Proc Natl Acad Sci USA. 2005;102(39):13927–13932. doi: 10.1073/pnas.0506795102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewalle A, Steffen W, Stevenson O, Ouyang Z, Sleep J. Single-molecule measurement of the stiffness of the rigor myosin head. Biophys J. 2008;94(6):2160–2169. doi: 10.1529/biophysj.107.119396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun YJ, Goldman YE. Lever-arm mechanics of processive myosins. Biophys J. 2011;101(1):1–11. doi: 10.1016/j.bpj.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spink BJ, Sivaramakrishnan S, Lipfert J, Doniach S, Spudich JA. Long single alpha-helical tail domains bridge the gap between structure and function of myosin VI. Nat Struct Mol Biol. 2008;15(6):591–597. doi: 10.1038/nsmb.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukherjea M, et al. Myosin VI dimerization triggers an unfolding of a three-helix bundle in order to extend its reach. Mol Cell. 2009;35(3):305–315. doi: 10.1016/j.molcel.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thirumalai D, Zhang ZC. Myosin VI: How do charged tails exert control? Structure. 2010;18(11):1393–1394. doi: 10.1016/j.str.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 51.van Kampen NG. Stochastic Processes in Physics and Chemistry. 3rd ed. Amsterdam: North-Holland; 2007. [Google Scholar]
- 52.Wilemski G, Fixman M. Diffusion-controlled intrachain reactions of polymers. 1. Theory. J Chem Phys. 1974;60(3):866–877. [Google Scholar]
- 53.Kratky O, Porod G. Rontgenuntersuchung geloster fadenmolekule. Recueil Des Travaux Chimiques Des Pays Bas. 1949;68:1106–1122. Dutch. [Google Scholar]
- 54.Saito N, Takahashi W, Yunoki Y. Statistical mechanical theory of stiff chains. J Phys Soc Jpn. 1967;22:219–226. [Google Scholar]
- 55.Hyeon C, Thirumalai D. Kinetics of interior loop formation in semiflexible chains. J Chem Phys. 2006;124(10):104905. doi: 10.1063/1.2178805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.