Significance
We describe the presence of essentially two functional tails in the Early Cretaceous Jeholornis (the second most primitive bird)—one like that of some modern birds with a fan-shaped tract of feathers over the proximal tail vertebrae and another distal frond like that of feathered dinosaurs such as Caudipteryx and Microraptor. We suggest that the unique “two-tail” plumage in Jeholornis probably evolved as the result of complex interactions between natural and sexual selective pressures and served both aerodynamic (flight and balance, etc.) and ornamental functions (communication/display, etc.). Our aerodynamic analysis also provides a plausible functional explanation for the elongation of the boney tail in Jeholornis relative to Archaeopteryx.
Keywords: Aves, Mesozoic, Jehol
Abstract
The Early Cretaceous bird Jeholornis was previously only known to have a distally restricted ornamental frond of tail feathers. We describe a previously unrecognized fan-shaped tract of feathers situated dorsal to the proximal caudal vertebrae. The position and morphology of these feathers is reminiscent of the specialized upper tail coverts observed in males of some sexually dimorphic neornithines. As in the neornithine tail, the unique “two-tail” plumage in Jeholornis probably evolved as the result of complex interactions between natural and sexual selective pressures and served both aerodynamic and ornamental functions. We suggest that the proximal fan would have helped to streamline the body and reduce drag whereas the distal frond was primarily ornamental. Jeholornis reveals that tail evolution was complex and not a simple progression from frond to fan.
Tail feathers are extraordinarily diverse in form and function in extant birds. A shallow forked tail is predicted by flight models to be aerodynamically optimal in the sense of having the highest possible moment:drag ratio, but the rectrices (flight feathers of the tail) and tectrices (upper tail coverts) that together form the “tail” have been modified for other functions in many taxa (1). The elaborate ornamental tail feathers seen in the males of some sexually dimorphic birds are the quintessential example of the power of sexual selection to dictate morphology (2). The Jehol Biota is recognized as the second oldest and most diverse Mesozoic avifauna but is most celebrated for its numerous specimens preserving integumentary structures and other soft tissues that are normally exceedingly rare in the fossil record. Preserved tail feathers are known for nearly every major avian clade in the Jehol (3), revealing patterns that strongly parallel those observed in the tails of living birds. The Jehol Biota contains the earliest known members of both Ornithuromorpha, the derived clade that includes living birds (3), and Enantiornithes, the dominant avians of the Cretaceous. These relatively derived birds lived alongside an array of more primitive groups including the basalmost pygostylian clades, Sapeornithiformes and Confuciusornithiformes, and the long boney-tailed Jeholornis. In the latter taxon, the tail was even longer (and contained more individual vertebrae) than in Archaeopteryx, despite most phylogenetic analyses resolving Jeholornis in a more derived position (4, 5).
The tails of enantiornithines and confuciusornithiforms commonly preserve a pair of elongate racket plumes, modified pennaceous feathers that carry barbs only near their distal ends. These feathers are the earliest record of an ornamental tail morphology within Aves and has been suggested to indicate sexual dimorphism in these two clades (6, 7). A recently described specimen of Jeholornis (SDM, Shandong Museum, 20090109) preserves the complete distal caudal integument, a palm-like frond of feathers near the tip of the tail (8). Unlike in Archaeopteryx, the tail feathers are restricted to the distal end of the tail and do not form a large cohesive surface capable of generating lift, and thus were interpreted as ornamental (8). Here, we present a more complete description of the caudal plumage of Jeholornis based on a revised study of SDM 20090109 and several other published and unpublished specimens (Table 1), some of which preserve a previously undocumented pteryla (feather tract) on the proximal tail. The new specimens indicate a previously unrecognized degree of diversity in the tail plumage configurations of Mesozoic birds and demonstrate that tail evolution did not follow a simple path from the “frond-like” arrangement seen in Archaeopteryx and some derived nonavian theropods to the “fan-like” arrangement seen in ornithuromorphs. We describe the complete tail of Jeholornis, consider its possible functions, and discuss trends in tail evolution in both Mesozoic and living birds.
Table 1.
A list of Jeholornis sp. specimens preserving tail plumage
| Specimen | Femur length | Remiges | Fan, no. of feathers | Preserved length, fan | Frond, no. of feathers | Preserved length, frond |
| SDM 20090109.1 | 57.9 | — | 4 | 90 | Complete - 11 | 90 |
| STM2-18 | 53 | Preserved | 4–6 | 103 | — | — |
| STM2-8 | 68.8 | Preserved | 4 | 83.7 | Incomplete | (45.9) |
| STM2-11 | 47.6 | Preserved | — | — | Ventral half, 6 | 72.6 |
| STM2-23 | 73* | Preserved | (1–3) | — | — | — |
| STM2-37 | 68.4 | Preserved | 4–6 | 113 | — | |
| STM3-3 | 66 | — | 4 | 105 | — | — |
| STM3-4 | 58 | — | 6 | (53–57) | Incomplete | (45) |
| STM3-30 | 56 | — | 4 | 72 | Dorsal half, 6–7 | (52) |
| IVPP V13350 | 55.6 | — | — | — | Incomplete | — |
| IVPP V13353 | 64 | Preserved | — | — | — | — |
All measurements are in millimeters. Parentheses indicate incomplete measurements. Preserved feather lengths were taken from the longest of the feathers forming the fan and frond respectively.
Estimates.
Description
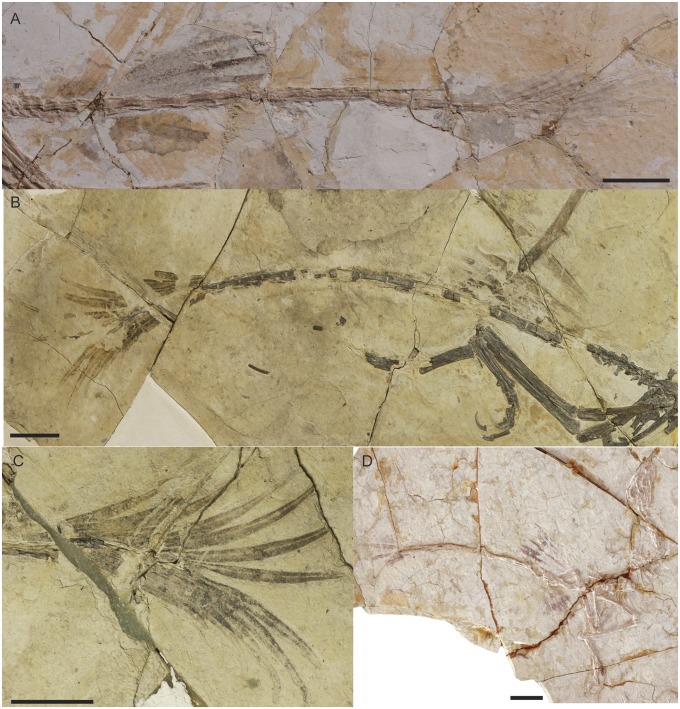
Several specimens of Jeholornis (Table 1) preserve elongate pennaceous feathers that appear to project from an area dorsal to the proximal caudal vertebrae (Figs. 1–3, Figs. S1 and S2). In one specimen, these feathers were previously identified as displaced remiges (8), but new additional specimens clearly indicate that the feathers represent a previously undescribed pteryla forming a proximal tail fan. Most specimens preserve four clear feathers, but these fans may well be incomplete. The feathers are shorter than the remiges but slightly longer and much broader than the distal tail feathers (Table 1). The feathers appear to attach to the dorsal surface of the tail above the proximal caudal vertebrae, close to the transition from short to elongate morphology that occurs at the fifth to sixth caudal vertebra and were presumably rooted in the soft tissues of this region. Based on their appearance in one specimen that is preserved in dorsal view (STM2-37; Fig. 1), the feathers are inferred to be arranged into a horizontal fan originating from a single area, a configuration broadly similar to the tail fans typical of living birds. However, in most specimens, the skeleton is preserved in lateral view, and the feathers are displaced and rotated so that their broad surfaces are parallel to the surface of the slab (Fig. 2 A and D). The medial feathers are longer than the lateral feathers, giving the fan a graded morphology.
Fig. 1.
Jeholornis sp. STM2-37 preserving the proximal tail fan in situ forming a horizontally oriented fan, or aerofoil. (A) Full slab. (Scale bar: 5 cm.) (B) Close up of the proximal fan. (Scale bar: 1 cm.)
Fig. 3.
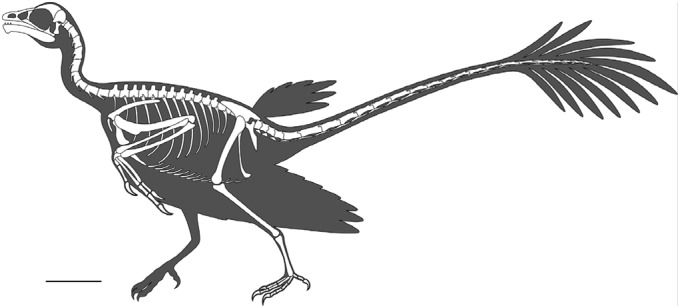
Reconstruction of the plumage of Jeholornis. (Scale bar: 5 cm.)
Fig. 2.
Tail feathers in Jeholornis sp. (A) Close-up of the tail in STM3-30 preserving both the proximal fan and distal frond. (B) Tail in Jeholornis palmapenis holotype SDM 20090109.1 preserving nearly complete caudal plumage. (C) Close-up of the distal tail frond in the counter slab of J. palmapenis SDM 20090109.2. (D) Full slab of STM3-4 preserving both the proximal fan and distal frond. (Scale bars: 3 cm.)
The fan is consistently preserved as a unit, suggesting that the calami of the individual feathers were bound together by ligaments. The absence of bending or distortion in any of the feathers indicates that the rachises were fairly stiff. The rachises are comparable in diameter with those of the remiges, rather than extremely wide as in the tail feathers of Confuciusornis and some enantiornithines (9). In STM2-37, the preserved rachises of the remiges range in diameter from 0.36 to 0.52 mm whereas those of the proximal tail feathers range from 0.32 to 0.44 mm, although poor preservation makes these values only approximations. In specimens preserved in lateral view, the vanes of the proximal tail feathers appear to be asymmetric and to taper distally (Fig. 2 A, B, and D). However, these characteristics probably result from distortion. In STM2-37, the feathers have symmetrical vanes, and one clearly preserved feather has a broad, rounded distal margin (Fig. 1). The amount of overlap between the feathers is unclear. In STM2-37, the preserved feathers are separated by small spaces, but the tail fan is probably incomplete. We interpret the feathers as having overlapped mediolaterally to some degree in life, forming a graded fan-shaped surface (Fig. 3).
The distal tail feathers form a frond rather than a fan, with feathers attaching to multiple successive vertebrae along the distalmost part of the tail. The frond is absent or incomplete, preserving only the proximal halves of the feathers (Table 1), in all known specimens other than the holotype of Jeholornis palmapenis (SDM 20090109) (8). Five to six pairs of feathers consistently line the dorsal and ventral surfaces of the seven distalmost caudal vertebrae, and an additional feather attached to the distal end of the caudalmost vertebra brings the total count to ∼11–13 feathers. All specimens with distal tail fronds are preserved in lateral view, and, in all cases, the frond appears dorsoventrally expanded in the sagittal plane. However, it is probable that the distal frond was laterally oriented, like the proximal fan of Jeholornis, the distal tail frond of the nonavian theropod Microraptor, and the frond extending along the entire length of the tail in Archaeopteryx (10, 11). Although the distal tail feathers are comparable in rachis thickness with the remiges and proximal tail feathers (0.366–0.398 mm), their vanes are narrow and curved and taper sharply along their distal thirds. The vanes are preserved in only two specimens, and it is unclear whether they are symmetrical. In STM2-11, only the caudal half of each vane is preserved whereas, in SDM 20090109, the feathers appear symmetrical on one side of the tail and asymmetrical on the other.
Notably, contour feathers are preserved in only a single specimen (STM2-11), and, even in this specimen, they are present only along the neck and the proximal part of the forelimb (Fig. S3). The feathers are fairly long (27 mm) and appear to lack distinct rachises, as in the primitive contour feathers of Confuciusornis, enantiornithines, and some nonavian dinosaurs (12), but their true morphology is uncertain owing to poor preservation. The absence of contour feathers from most parts of the body is most likely a preservational artifact, and it is presently unclear whether specific areas (such as the crus) were feathered (13).
Potential Aerodynamic Function.
Although the tail plumage of Jeholornis is visually striking, the potential aerodynamic benefits of the tail feathers merit investigation. At least in the proximal fan, the thick rachises of the individual feathers suggest that their ability to withstand aerodynamic forces was probably comparable with that of the remiges. The distal frond feathers combine stiff rachises with a narrow, distally tapering morphology that would reduce their contribution to the drag generated by the tail but also compromise the ability of the frond as a whole to produce lift. Although the lift:drag ratio might still be favorable, the frond feathers also have a foreswept curvature that would make them individually prone to stalling. It is probable that these distal feathers evolved from ones that were previously better-adapted to an aerodynamic role.
We infer that the feathers of the proximal fan overlapped to some extent, forming a continuous surface capable of generating lift, but the exact geometry of this surface is uncertain owing to poor preservation. The delta-wing model of tail function in modern birds (1, 14) predicts that the ability of the tail to produce lift is proportional to the square of the maximum continuous width (MCW) of the surface formed by the tail plumage for a given air density (ρ), airstream velocity (U), and angle of attack (α). For ease of comparison, we adopt the same values of these parameters (ρ = 1.23 kg/m3, U = 5 m/s, α = 15°) considered by Gatesy and Dial (10) in comparing the lift-generating function of the tail of Archaeopteryx to that of an extant pigeon (Columba livia). We reconstructed the proximal tail fan based on STM2-37 (Fig. S4). Because of poor preservation, we considered a range of possible fan shapes, varying in MCW from 80 to 103 mm and in fan angle from 45° to 65°. The results suggest that the proximal tail fan would have generated between 0.04 and 0.07 N of lift under the specified conditions. These results are comparable to the 0.05 N estimated for the entire tail of Archaeopteryx, which bore pairs of elongate, caudolaterally directed pennaceous feathers upon all but the most proximal caudal segments, and 0.42 N for the triangular tail of a pigeon (10). The surface area of the tail plumage is much greater in Archaeopteryx than in Jeholornis because pennaceous feathers are present essentially along the entire length of the tail in the former, but the similar tail widths of the two taxa (90 mm in Archaeopteryx) lead to comparable predicted lift values in the framework of the delta-wing model. The tail of the pigeon, despite being craniocaudally short, is broad enough (26 cm when fully fanned) to produce much greater lift.
Although the delta-wing model yields inaccurate predictions when applied to modern avian tails at large fan angles and large angles of attack (15) and does not account for the tail’s aerodynamic interactions with the wings and body (16), experimental data support its accuracy in gliding birds with narrowly spread tails held at angles of attack up to about 20° (15). Regardless, the delta-wing model strongly suggests that the amount of lift produced by the proximal fan was comparable with that generated by the entire tail of Archaeopteryx, and much less than that generated by the tail of an extant pigeon. We estimate the distal frond to have an MCW of 28–34 mm in SDM 20090109 (Fig. S5), and scaling based on femoral length implies that the MCW of the frond would be 33–40 mm in an individual the size of STM2-37. Even using the scaled-up values, the delta-wing model indicates that the distal frond could have generated only negligible lift (no more than about 0.01 N).
Thomas (14) made a strong, explicit case for the applicability of the delta-wing model to avian tails. However, an alternative approach to estimating the lift produced by the tail plumage of Jeholornis is to consider the frond and fan not as delta wings but as generic thin, flat aerodynamic surfaces of low aspect ratio (17) (see SI Methods for details). Lift estimates then increase to some extent with tail area, as well as being influenced by MCW. The large area of the tail frond of Archaeopteryx results in flat-plate lift estimates (0.08–0.09 N) that slightly exceed the highest ones generated for the short fan of Jeholornis (0.05–0.08 N). However, even the flat-plate approach suggests that the more extensive tail plumage of Archaeopteryx could generate only a little more lift than that of Jeholornis (Table S1).
Aerodynamic properties of the tail other than lift are less amenable to quantitative evaluation on the basis of theoretical models. Both the frond and the fan of Jeholornis would inevitably have generated some amount of drag. In extant birds, however, the presence of a short tail can streamline the shape of the body in a way that reduces drag overall, and even considerable ornamental elaboration of the tail feathers may have only subtle aerodynamic consequences because the tail lies in the aerodynamic wake of the body (18). It is possible that these effects reduced the contribution of the fan and frond to the total drag experienced by Jeholornis. Furthermore, a major component of drag affecting the tail of an extant bird is “skin friction,” which is proportional to surface area (19). Relative to the extensive tail frond of Archaeopteryx, the fan-and-frond tail plumage of Jeholornis had a smaller surface area and would accordingly have produced less skin friction, perhaps at the cost of a slight decrease in lift-generating capability. The individual feathers of the distal frond of Jeholornis extend caudally and laterally well beyond the margins of the cohesive and potentially aerodynamically effective surface formed by their overlapping proximal portions, but it is notable that their tapering tips would have reduced the surface area of the frond and contributed to streamlining, both drag-reducing features that would have minimized the aerodynamic cost of this ornament that did not produce significant lift.
Finally, it is possible that the frond and fan acted to some degree as control surfaces contributing to stability and/or mobility. Raising or depressing the tail, for example, could have allowed both the frond and the fan to generate pitching moments about the center of gravity, increasing stability and helping to control the position of the body in maneuvers such as landing. Despite the small size of the frond, the length of the tail would have provided this structure with a substantial moment arm about the center of gravity, perhaps allowing it to produce aerodynamically significant moments. Optimization of the latter function may also explain the elongation of the tail in the more derived Jeholornis relative to Archaeopteryx (4).
Discussion
Adaptive Value of the Tail Plumage of Jeholornis.
Although detailed, quantitative analysis will ultimately be needed to understand the aerodynamic costs and benefits of the caudal fan and frond in Jeholornis, the potential of the tail to generate lift is clear. However, tail feathers also serve a social function in many extant birds, and this may also have applied to Jeholornis. Particularly if they bore striking color patterns, as was the case in some known Mesozoic paravian feathers (20), the feathers would undoubtedly have been visually conspicuous and could have been used in sexual displays or other forms of social signaling. The elongation of the distal frond feathers beyond the margins of the cohesive, potentially aerodynamic surface formed by their overlapping proximal portions implies that they may have been enlarged under sexual selection pressure to a greater degree than natural selection pressure driven by aerodynamics would have dictated (8). Although the newly discovered proximal fan does not appear enlarged beyond the point of aerodynamic necessity as in the elaborate display tails of some extant birds, if in fact the feathers did not overlap, we would consider the proximal fan to be purely ornamental. Accordingly, Jeholornis potentially represents the first Mesozoic bird in which multiple ornamental structures have been documented. Because of the cost of growing feathers, elaborate plumage is usually restricted to males of a species, and multiple ornaments are common in polygamous birds. Thus, the presence of two tails may suggest that Jeholornis species were sexually dimorphic, polygamous, and/or had lekking social behaviors, as has been suggested for Confuciusornis (6). However, even among living birds, the relationship between dimorphism and social behavior is not straightforward. Furthermore, given the potential aerodynamic benefits of the tail plumage of Jeholornis over that of the more primitive Archaeopteryx (less drag), the tail plumage may well have been present in both genders.
Display and aerodynamics may appear to represent competing hypotheses for the adaptive value of the caudal fan and frond in Jeholornis, but the two functions are not mutually exclusive except at extremes of either ornamentation or flight performance. Modern avian tail feathers may certainly be enlarged and elaborated to an aerodynamically disadvantageous degree owing to sexual selection. Among living birds, the largest known feathers are tail rectrices of the crested argus (Rheinardia ocellata) (21), and tectrices are commonly modified into extravagant display structures, the most striking example being the feather train of the peafowl (Pavo cristatus). However, experiments suggest that elaborate ornaments become aerodynamically detrimental only at high flight speeds (19). Conversely, some birds are optimized for efficient flight with little ornamental modification to their plumage. The frond and fan of Jeholornis are integumentary structures of modest size, and living birds in forested environments comparable with the Jehol Biota do not typically engage in high-speed flight (20). Potentially ornamental modifications such as elongation of the distal frond feathers may have had little aerodynamic disadvantage, particularly given the position of the tail in the wake of the body (18), or indeed carried some aerodynamic benefit such as improved mobility resulting from the ability to generate pitching moments. Feathers are energetically costly structures to grow and maintain and often combine aerodynamic and social functions in modern birds (1). Indeed, recent studies show that extant avian tail morphology is driven by complex interactions between natural and sexual selection and that the cost of most ornaments is either minimal or offset by additional “compensatory traits” (22, 23). These considerations suggest that the “ornamental” distal tail frond of Jeholornis (8) may easily have been both a naturally selected aerodynamic surface and a sexually selected ornament.
Tail Evolution in Mesozoic Paravians.
The “two-tail” plumage of Jeholornis is unique among known avian and nonavian theropods. Archaeopteryx is dissimilar to Jeholornis in lacking a proximal fan and having a much more proximally extensive frond. The volant dromaeosaurid Microraptor and flightless oviraptorosaur Caudipteryx resemble Jeholornis in possessing a distal frond of laterally oriented pennaceous tail feathers (8). However, there is no evidence for a proximal fan in these taxa, and we interpret the proximal three-quarters of the tail as being covered in primitive contour feathers in both cases. Microraptor differs from Caudipteryx in that the feathers are shorter, and both taxa differ from Jeholornis in that, as in Archaeopteryx, their tail feathers appear to create a more extensive planar surface and are less distally restricted. Furthermore, fan-shaped tails among Mesozoic birds have been previously clearly documented only within Ornithuromorpha, in association with a small, plow-shaped pygostyle of essentially modern appearance, and it is probable that the proximal fan and the pygostyle coevolved in this lineage (24). One Jehol ornithuromorph even preserves a forked tail, but none display ornamental caudal designs, indicating that members of this clade used the tail in flight (25). One enantiornithine bird potentially preserves a fan-shaped tail formed by at least four rectrices, suggesting that aerodynamic tail morphologies paralleling those of ornithuromorphs evolved in at least one enantiornithine lineage (26). Jeholornis is unique among all known birds in possessing a proximally positioned tail fan that is not associated with a pygostyle. Gatesy and Dial (10) described the evolution of the avian tail in terms of a transition from a frond supported by a long boney-tail (Archaeopteryx) to a fan supported by a pygostyle (Ornithuromorpha). However, the fan-and-frond arrangement seen in Jeholornis, combined with the fact that this taxon possesses up to 27 caudal vertebrae compared with five fewer in Archaeopteryx (4), emphatically indicates that tail reduction (10) was not a simple linear progression.
Given the lack of evidence for fans in other nonornithuromorph birds, including many that are phylogenetically closer to ornithuromorphs than is Jeholornis, the proximal tail fan of Jeholornis is probably autapomorphic. Discovery of this feather tract demonstrates that fan-like, proximally located, presumably aerodynamically valuable tails were not limited to birds with derived pygostyle morphology and that primitive birds also experimented with this type of caudal airfoil. The distal tail frond, by contrast, appears to have been primarily ornamental (8), despite being a presumed homolog of the larger and slightly more aerodynamically potent tail frond of taxa such as Archaeopteryx. Reduction of this distal flight surface may reflect a transfer of aerodynamic functionality to the proximal fan, leaving the distal frond free to undergo reduction and ornamental modification. However, even this smaller frond may have resembled many ornaments in living birds in retaining some aerodynamic utility (18, 23), particularly given the long moment arm about the center of gravity provided by the characteristically elongated tail of Jeholornis.
Feather ornaments in Mesozoic birds are common: the basal bird Confuciusornis and several species of enantiornithine possess racket plumes (9), with no other pennaceous tail feathers (9, 12). Although the length, morphology, and probable dimorphic nature (6, 12) of these racket plumes preclude a purely aerodynamic function, their aerodynamic costs may have been limited for reasons parallel to those given above for the distal frond of Jeholornis. The partly ornamental nature of the tail plumage of Jeholornis, and of the racket plumes known in some other Mesozoic taxa, indicates that sexual selection was an important influence from almost the earliest stages of avian evolution. Documentation of the unique frond-and-fan tail of Jeholornis adds to the growing body of evidence that basal birds resembled their living counterparts in using a remarkably diverse and advanced array of feather types and configurations to optimize flight and engage in social signaling, with many integumentary features likely playing key roles in both flight and display.
Methods
Nine previously undescribed specimens of Jeholornis (Maniraptora: Aves: Jeholornithiformes) housed in the Shandong Tianyu Museum of Natural History (STM) were incorporated in this study (Table 1). These specimens were identified as belonging to the genus Jeholornis based on their short and deep skull with small peg-like reduced dentition and triangular mandibles, elongate boney tail composed of ∼27 free vertebrae with transition point at the fifth/sixth caudal, curved scapula, and unreduced manus with three claws (4, 5). All specimens were thoroughly examined for authenticity and showed no sign of tampering. The specimens were studied using a Motic K-500L stereo microscope.
Supplementary Material
Acknowledgments
We thank A.-J. Shi for the reconstruction, F. Huchzermeyer and R. Dudley for useful discussions, and three reviewers for their comments. The research was supported by the National Basic Research Program of China (973 Program, 2012CB821906); National Natural Science Foundation of China Grants 41372014, 41172020, and 41172016; and the Chinese Academy of Sciences.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316979110/-/DCSupplemental.
References
- 1.Thomas ALR. On the tails of birds. Bioscience. 1997;47:215–225. [Google Scholar]
- 2.Balmford A, Jones IL, Thomas ALR. How to compensate for sexually-selected tails: The evolution of sexual dimorphism in wing length in long-tailed birds. Evolution. 1994;48:1062–1070. doi: 10.1111/j.1558-5646.1994.tb05293.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Z-H, Zhang F-C. Mesozoic birds of China: A synoptic review. Vertebr Palasiat. 2006;44:74–98. [Google Scholar]
- 4.Zhou Z, Zhang F. Jeholornis compared to Archaeopteryx, with a new understanding of the earliest avian evolution. Naturwissenschaften. 2003;90(5):220–225. doi: 10.1007/s00114-003-0416-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z, Zhang F. A long-tailed, seed-eating bird from the Early Cretaceous of China. Nature. 2002;418(6896):405–409. doi: 10.1038/nature00930. [DOI] [PubMed] [Google Scholar]
- 6.Hou L, Martin LD, Zhou Z, Feduccia A. Early adaptive radiation of birds: Evidence from fossils from northeastern China. Science. 1996;274(5290):1164–1167. doi: 10.1126/science.274.5290.1164. [DOI] [PubMed] [Google Scholar]
- 7. Chinsamy A, Chiappe LM, Marugán-Lobón J, Gao C-H, Zhang F-J (2013) Gender identification of the Mesozoic bird Confuciusornis sanctus. Nat Commun 4:1381. [DOI] [PubMed]
- 8.O'Connor JK, Sun C-K, Xu X, Wang X-L, Zhou Z-H. A new species of Jeholornis with complete caudal integument. Hist Biol. 2012;24:29–41. [Google Scholar]
- 9.O’Connor JK, Chiappe LM, Chuong C-M, Bottjer DJ, You H-L. Homology and potential cellular and molecular mechanisms for the development of unique feather morphologies in early birds. Geosciences (Basel) 2012;2(3):157–177. doi: 10.3390/geosciences2030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gatesy SM, Dial KP. From frond to fan: Archaeopteryx and the evolution of short-tailed birds. Evolution. 1996;50:2037–2048. doi: 10.1111/j.1558-5646.1996.tb03590.x. [DOI] [PubMed] [Google Scholar]
- 11. Li Q, et al. (2012) Reconstruction of Microraptor and the evolution of iridescent plumage. Science 335(6073):1215–1219. [DOI] [PubMed]
- 12. O'Connor JK (2009) A systematic review of Enantiornithes (Aves: Ornithothoraces). PhD thesis (University of Southern California, Los Angeles)
- 13.Zhang F, Zhou Z. Palaeontology: Leg feathers in an Early Cretaceous bird. Nature. 2004;431(7011):925. doi: 10.1038/431925a. [DOI] [PubMed] [Google Scholar]
- 14.Thomas ALR. On the aerodynamics of birds’ tails. Philos Trans R Soc Lond B Biol Sci. 1993;340:361–380. [Google Scholar]
- 15. Evans MR (2003) Birds' tails do act like delta wings but delta-wing theory does not always predict the forces they generate. Proc R Soc Biol Sci Ser B 270:1379-1385. [DOI] [PMC free article] [PubMed]
- 16.Clark CJ. The evolution of tail shape in hummingbirds. Auk. 2010;127:44–56. [Google Scholar]
- 17.Pennycuick CJ. Modelling the Flying Bird. Vol 4. New York: Academic; 2008. [Google Scholar]
- 18. Clark JC, Dudley R (2009) Flight costs of long, sexually selected tails in hummingbirds. Proc R Soc Biol Sci Ser B 276:2109-2115. [DOI] [PMC free article] [PubMed]
- 19.Balmford A, Thomas ALR, Jones IL. Aerodynamics and the evolution of long tails in birds. Nature. 1993;361:628–631. [Google Scholar]
- 20.Li Q-G, et al. Plumage color patterns of an extinct dinosaur. Science. 2010;327(5971):1369–1372. doi: 10.1126/science.1186290. [DOI] [PubMed] [Google Scholar]
- 21.Lucas SG, Stettenheim PR. Avian Anatomy: Integument II. Vol 362. Washington, DC: US Department of Agriculture; 1972. [Google Scholar]
- 22.Buchanan KL, Evans MR. The effect of tail streamer length on aerodynamic performance in the barn swallow. Behav Ecol. 2000;11:228–238. [Google Scholar]
- 23. Norberg RA (1994) Swallow tail streamer is a mechanical device for self deflection of tail leading edge, enhancing aerodynamic efficiency and flight manoeuvrability. Proc R Soc Biol Sci Ser B 257:227-233.
- 24.Clarke JA, Zhou Z, Zhang F. Insight into the evolution of avian flight from a new clade of Early Cretaceous ornithurines from China and the morphology of Yixianornis grabaui. J Anat. 2006;208(3):287–308. doi: 10.1111/j.1469-7580.2006.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S, Zhou Z-H, O'Connor JK. A new toothless ornithurine bird (Schizooura lii gen. et sp. nov.) from the Lower Cretaceous of China. Vertebr Palasiat. 2012;50:9–24. [Google Scholar]
- 26.O'Connor JK, et al. Phylogenetic support for a specialized clade of Cretaceous enantiornithine birds with information from a new species. J Vertebr Paleontol. 2009;29:188–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.