Abstract
Elevated in vivo markers of presynaptic striatal dopamine activity have been a consistent finding in schizophrenia, and include a large effect size elevation in dopamine synthesis capacity. However, it is not known if the dopaminergic dysfunction is limited to the striatal terminals of dopamine neurons, or is also evident in the dopamine neuron cell bodies, which mostly originate in the substantia nigra. The aim of our studies was therefore to determine whether dopamine synthesis capacity is altered in the substantia nigra of people with schizophrenia, and how this relates to symptoms. In a post-mortem study, a semi-quantitative analysis of tyrosine hydroxylase staining was conducted in nigral dopaminergic cells from post-mortem tissue from patients with schizophrenia (n = 12), major depressive disorder (n = 13) and matched control subjects (n = 13). In an in vivo imaging study, nigral and striatal dopaminergic function was measured in patients with schizophrenia (n = 29) and matched healthy control subjects (n = 29) using 18F-dihydroxyphenyl-l-alanine (18F-DOPA) positron emission tomography. In the post-mortem study we found that tyrosine hydroxylase staining was significantly increased in nigral dopaminergic neurons in schizophrenia compared with both control subjects (P < 0.001) and major depressive disorder (P < 0.001). There was no significant difference in tyrosine hydroxylase staining between control subjects and patients with major depressive disorder, indicating that the elevation in schizophrenia is not a non-specific indicator of psychiatric illness. In the in vivo imaging study we found that 18F-dihydroxyphenyl-l-alanine uptake was elevated in both the substantia nigra and in the striatum of patients with schizophrenia (effect sizes = 0.85, P = 0.003 and 1.14, P < 0.0001, respectively) and, in the voxel-based analysis, was elevated in the right nigra (P < 0.05 corrected for family wise-error). Furthermore, nigral 18F-dihydroxyphenyl-l-alanine uptake was positively related with the severity of symptoms (r = 0.39, P = 0.035) in patients. However, whereas nigral and striatal 18F-dihydroxyphenyl-l-alanine uptake were positively related in control subjects (r = 0.63, P < 0.001), this was not the case in patients (r = 0.30, P = 0.11). These findings indicate that elevated dopamine synthesis capacity is seen in the nigral origin of dopamine neurons as well as their striatal terminals in schizophrenia, and is linked to symptom severity in patients.
Keywords: dopamine, psychosis, depression, substantia nigra, schizophrenia, F-DOPA, dihydroxyphenyl-l-alanine, brain imaging, striatum, tyrosine, post-mortem
Introduction
The dopamine hypothesis of schizophrenia proposes that subcortical dopamine dysfunction underlies the illness (Davis et al., 1991; Howes and Kapur, 2009). Recent revisions of the hypothesis have specified a critical role for presynaptic dopamine dysfunction, in contrast to the earlier focus on postsynaptic dopamine D2 receptors (Howes and Kapur, 2009; Lyon et al., 2011). Supporting this there is now a large body of evidence for presynaptic dopaminergic dysfunction in the striatum in schizophrenia (Howes et al., 2007; Meisenzahl et al., 2007; Lyon et al., 2011). Specifically schizophrenia is associated with elevations in striatal dopamine synthesis capacity, as measured using the PET radiotracers L-[β-11C]- dihydroxyphenyl-l-alanine or 6-[18F]fluoro-dihydroxyphenyl-l-alanine (18F-DOPA; Reith et al., 1994; Hietala et al., 1995, 1999; Dao-Castellana et al., 1997; Lindstrom et al., 1999; Elkashef et al., 2000; Meyer-Lindenberg et al., 2002; McGowan et al., 2004; Kumakura et al., 2007; Bose et al., 2008; Howes et al., 2009b; Nozaki et al., 2009; Shotbolt et al., 2011; Demjaha et al., 2012), and elevated baseline and stimulated striatal dopamine levels (Laruelle et al., 1996; Breier et al., 1997; Abi-Dargham et al., 1998, 2000, 2009; Mizrahi et al., 2012). Increased striatal dopamine synthesis capacity is also evident in individuals with prodromal symptoms of schizophrenia, suggesting that the dopaminergic abnormality predates the onset of frank psychosis (Howes et al., 2009b, 2011a,b; Egerton et al., 2013). However, it is not elevated in non-psychotic depression (Martinot et al., 2001) or in people with persistent subclinical psychotic symptoms who have not developed a psychotic disorder (Howes et al., 2011b, 2013), suggesting specificity to psychotic illness (Howes et al., 2007, 2009a).
Recent evidence indicates that these alterations in dopamine function are particularly apparent in the associative subdivision of the striatum (Howes et al., 2009b; Kegeles et al., 2010), which predominantly receives projections from the substantia nigra (Haber et al., 2000, 2006; Joel and Weiner, 2000).
This suggests that they may reflect changes to dopaminergic neurons in the substantia nigra. Dopamine is synthesized from tyrosine in a two-step process: tyrosine hydroxylase converts tyrosine into dihydroxyphenyl-l-alanine (DOPA), which is then converted by aromatic acid decarboxylase into dopamine (Fernstrom and Fernstrom, 2007). Both enzymes are regulated, but the rate limiting step in dopamine synthesis is the conversion of tyrosine to dihydroxyphenyl-l-alanine (Zhu et al., 1993; Cumming et al., 1995, 1997; Fernstrom and Fernstrom, 2007).
Post-mortem studies have found altered tyrosine hydroxylase messenger RNA levels, increased tyrosine hydroxylase activity and increased variability in tyrosine hydroxylase levels in the substantia nigra of patients with schizophrenia compared to control subjects in some (Toru et al., 1988; Mueller et al., 2004; Perez-Costas et al., 2012) but not all studies (Ichinose et al., 1994). In addition one PET study has reported increased turnover of dopamine in the midbrain of patients with schizophrenia compared with matched control subjects (Kumakura et al., 2007). However, as the midbrain region of interest was an order of magnitude larger than the substantia nigra (Ahsan et al., 2007; Duzel et al., 2009), it is unclear from this study whether the abnormalities reported represent dysregulated nigral dopaminergic function or dysfunction in other midbrain neurons. We have recently shown that it is possible to reliably index dopaminergic function in the substantia nigra in vivo using 18F-DOPA-PET in humans (Egerton et al., 2009), enabling it to be specifically studied in schizophrenia.
We hypothesized that dopamine synthesis capacity in schizophrenia will be increased in the substantia nigra as well as in the striatum. We tested this hypothesis in two complementary studies of different aspects of dopamine synthesis in the substantia nigra. The first study used post-mortem tissue to investigate tyrosine hydroxylase levels ex vivo. Non-disorder specific factors, such as suicide as cause of death, are potential confounds in post-mortem studies of psychiatric disorders (Biegon and Fieldust, 1992). For this reason we also included a psychiatric comparator group comprising samples from patients diagnosed with major depressive disorder. We hypothesized that tyrosine hydroxylase levels would be elevated in schizophrenia in comparison with both healthy control and major depression groups. The second study used 18F-DOPA imaging in vivo, which measures the uptake and conversion of 18F-DOPA into 18F-fluorodopamine by aromatic acid decarboxylase in dopamine neurons (Kumakura and Cumming, 2009). We hypothesized that this would also be elevated in the substantia nigra in schizophrenia in comparison with healthy control subjects.
Materials and methods
Post-mortem study
Sample
This study was approved by the London South West Local Ethics Committee. Post-mortem tissue from 12 patients with schizophrenia was compared with that from 13 patients with major depressive disorder and 13 healthy control subjects matched for age and post-mortem interval (see Supplementary Tables 1 and 2 for case details). To be included, patients had to have met International Classification of Disease-10 criteria for schizophrenia or recurrent major depressive disorder. Controls were required to have no history of psychiatric or neurological disorder. Exclusion criteria for all subjects were: any evidence of neurodegenerative, neurovascular or infectious pathology, including Parkinson’s disease, at post-mortem conducted by a consultant pathologist, or any recorded history of alcohol or drug abuse. Samples were selected at random from the Corsellis Tissue Bank (Supplementary material).
Tissue preparation and immunohistochemical staining
Transverse sections of the substantia nigra at the level of the superior colliculus were obtained from formalin-fixed, paraffin-embedded tissue (Supplementary Fig. 2). Each block was sectioned in coronal plane at 10 µm thickness using a microtome (Thermo Shandon Finesse) and mounted onto electrostatic glass slides. The first mounted 10 µm section of each specimen was stained with haematoxylin and eosin for anatomical identification of the substantia nigra as described in the Supplementary material. The adjacent section in each hemisphere, giving two sections per case, was then prepared for immunohistochemical staining (Supplementary material), which was conducted by incubation with horseradish peroxidase-conjugated secondary antibody (Dako K4002) for 30 min at room temperature before further washing (Supplementary material), counterstaining and mounting. Slides were randomized and blinded by an independent investigator before image capture.
Image capture and quantification
Images were captured using an Olympus Vanox AHBT 3 microscope with Q Imaging Micropublisher RTV 3.3 camera and analysed using Image-Pro Plus 5.1 software (Media Cybernetics). The nigra was identified to ensure measures incorporated the entire nucleus (Supplementary material). Sample cells showing no staining, moderate staining and heavy staining were captured at ×400 magnification and used as standards (Supplementary Fig. 1). The medial region of each tyrosine hydroxylase-stained nigral section was imaged at ×40 magnification. All dopaminergic cells (identified as large, oval stained neurons containing neuromelanin and a counterstained nucleus) were tagged. A random number generator (www.graphpad.com) was used to select eight unique neurons which were imaged at ×400 magnification and semi-quantitatively assessed for tyrosine hydroxylase expression using the following scale: 0 = no staining visible in the cytoplasm; 1 = moderate staining visible in the cytoplasm distinct from neuromelanin; 2 = heavy staining forming a clear halo around the nucleus and intense staining observed elsewhere in the cytoplasm (Supplementary Fig. 1).
In vivo imaging study
Subjects
The study was approved by the Hammersmith Hospital research ethics committee. Following complete description of the study, all subjects gave written informed consent to participate. Twenty-nine subjects meeting International Classification of Disease-10 criteria for schizophrenia were recruited from outpatient clinics in London, and included 13 antipsychotic-free patients (no antipsychotic treatment for at least 3 months before scanning, drug free n = 8; drug naive n = 5) and 16 antipsychotic-treated patients (drugs listed in Supplementary material). They were compared with 29 healthy control subjects recruited contemporaneously from the same geographical area of London. See Supplementary material for further details on the subjects.
Clinical assessment
Subjects were assessed at the time of scanning using the Comprehensive Assessment of Symptoms and History to assess current symptoms and clinical characteristics (Andreasen, 1987). Symptom severity scores were calculated for positive, negative and affective symptoms by summing the scores for symptoms in each domain, and for all symptoms combined (total symptom severity score). Additionally all subjects received assessment of recreational exposure to psychoactive substances by semi-structured questionnaire [modified from Barkus et al. (2006)].
Positron emission tomography scanning
The PET data were acquired using the 966 ECAT/EXACT3D PET scanner (Siemens/CTI). All subjects were positioned in the tomograph so that the orbitomeatal line was parallel to the transaxial plane of the tomography, and head position was monitored via laser crosshairs and a camera. A 5-min transmission scan was carried out using a 150 MBq cesium-137 rotating point source for attenuation and scatter correction. Data were acquired in list mode for 95 min.
Image analysis
All PET scan image preprocessing and analysis was performed using fully automated methods and blind to group status using a region of interest approach to test the primary hypothesis. Standardized regions in Montreal Neurologic Institute (MNI) space were defined in the cerebellum (the reference region) and regions of interest: substantia nigra (left and right sides combined; Supplementary Fig. 2A); striatum (left and right sides combined) using the HamNet atlas, a probabilistic atlas of standard brain regions (Ahsan et al., 2007). This approach has shown good reliability in delineating these structures, including after spatial normalization (Egerton et al., 2009). Although it was not the focus of this study, as recent findings suggest that dopaminergic alterations are most marked in the associative subdivision of the striatum (Howes et al., 2009b; Kegeles et al., 2010), we also conducted exploratory analyses of the striatal functional subdivisions defined as previously described (Howes et al., 2009b). The region of interest map was normalized together with an 18F-DOPA template (which aids normalization) to each individual PET summation image using statistical parametric mapping (Statistical Parametric Mapping 5; Wellcome Department of Cognitive Neurology, London, England) and then used to sample the dynamic PET images. The positioning of the nigral region of interest following normalization to individual dynamic images is illustrated in Supplementary Fig. 2B. A Patlak graphical analysis was used to calculate the influx constant ( value) for the specific uptake of 18F-DOPA in the regions of interest relative to uptake in the reference region (Patlak et al., 1983). To investigate subregional and whole-brain differences, a voxel-wise analysis was conducted using statistical parametric mapping (Supplementary material).
value) for the specific uptake of 18F-DOPA in the regions of interest relative to uptake in the reference region (Patlak et al., 1983). To investigate subregional and whole-brain differences, a voxel-wise analysis was conducted using statistical parametric mapping (Supplementary material).
Statistical analysis
All statistical analyses were performed using Windows SPSS 18.0.
Post-mortem study
Analysis of variance was used to determine if there was an effect of group on age, sex or post-mortem interval. The primary analysis used a Kruskal-Wallis test to determine if there was an effect of diagnostic group (control, schizophrenia, major depressive disorder) on staining score. Where there was a significant effect of group, planned post hoc Mann-Whitney U-tests adjusted for multiple comparisons using Bonferroni correction, were used to determine if there was a significant difference between the schizophrenia, major depressive disorder and control groups. To determine if age, sex, or post-mortem interval moderated the effect, a secondary analysis of variance was used to determine the effect of diagnostic group on staining score co-varying for these variables.
In vivo imaging study
All variables showed a normal distribution and equal variances with the exception of cigarette smoking and symptom scores. Group differences in radiochemistry, demographic and clinical measures were tested using independent t-tests for parametric variables and Mann-Whitney U-tests for non-normally distributed data. Regional 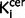 values in schizophrenic patients versus control subjects were compared using independent t-tests. The relationship between nigral
values in schizophrenic patients versus control subjects were compared using independent t-tests. The relationship between nigral 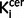 values and clinical variables was explored using Pearson’s correlation coefficients. Where there was a group difference in nigral or striatal
values and clinical variables was explored using Pearson’s correlation coefficients. Where there was a group difference in nigral or striatal  values, planned post hoc independent t-tests with Bonferroni correction for multiple comparisons were used to determine if this was also the case when the analysis was restricted to the subgroup of patients who were drug-free compared with control subjects. All tests were two-tailed and P < 0.05 was considered significant. Glass’ delta effect sizes were calculated for significant differences between groups. Exploratory analyses compared
values, planned post hoc independent t-tests with Bonferroni correction for multiple comparisons were used to determine if this was also the case when the analysis was restricted to the subgroup of patients who were drug-free compared with control subjects. All tests were two-tailed and P < 0.05 was considered significant. Glass’ delta effect sizes were calculated for significant differences between groups. Exploratory analyses compared 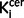 values in striatal subdivisions in schizophrenic patients versus control subjects using independent t-tests.
values in striatal subdivisions in schizophrenic patients versus control subjects using independent t-tests.
Results
Post-mortem study
There was no significant effect of group on age [F(2,37) = 2.9; P = 0.07], sex [F(2,37) = 3.1; P = 0.06], or post-mortem interval [F(2,37 = 2.4; P = 0.11]; see Supplementary Tables 1 and 2 for details.
There was a significant main effect of group on tyrosine hydroxylase staining score (Χ2 = 21.9, df = 2, P < 0.001, Fig. 1). Post hoc tests showed that tyrosine hydroxylase staining score was significantly greater in the schizophrenia group compared with both the control group [median (interquartile range, IQR) score for control group = 0.38 (0.31); schizophrenia group = 1.56 (0.51); Bonferroni corrected P < 0.001] and the major depressive disorder group [median (IQR) score major depressive disorder group = 0.37 (0.31); Bonferroni corrected P < 0.001]. There was no significant difference in staining score between the control and major depressive disorder groups (P > 0.9). The effect of group on tyrosine hydroxylase staining score was also highly significant after co-varying for age, sex and post-mortem interval [F(2,37 = 30.3; P < 0.001].
Figure 1.
Median (IQR) staining scores for tyrosine hydroxylase levels in substantia nigra for control subjects (n = 13); major depressive disorder (MDD, n = 13); and schizophrenia (n = 12).
In vivo imaging study
Radiochemistry, demographic and clinical characteristics
There were no significant differences between groups in the dose of 18F-DOPA injected [mean (SD) for control subjects = 136.8 (17.8) and schizophrenia = 129.6 (19.3) MBq; t(54) = 1.5, P = 0.15], or its specific activity [mean (SD) for control subjects = 27.8 (17.8) and schizophrenia = 20.2 (14.3) MBq/µmol; t(53) = 1.7, P = 0.09].
There was no significant difference in age or sex between groups [mean age (SD): control subjects = 29.3 (7.5), schizophrenia = 33.7 (10.6) years, t(56) = 1.8, P = 0.07; sex: control subjects = 22 males, 75.8%; schizophrenia = 26 males, 89.7%; z = 1.4, P = 0.17].
There was no significant difference between groups in cigarette consumption or alcohol use [median (IQR) cigarette use for control subjects = 0.1 (7) and schizophrenia < 0.1 (10) cigarettes/day, Z = 0.6, P = 0.54; median (IQR) alcohol use for control subjects = 6 (10) and schizophrenia = 2 (8) units/week, Z = 1.2, P = 0.24].
In the schizophrenia group the mean (SD) symptom severity scores were: total symptoms = 77.6 (47.6); positive symptoms = 38.3 (30); affective symptoms = 2.0 (3.9); negative symptoms = 31.9 (22.9).
Nigral and striatal dopaminergic function
Region of interest analysis
The mean  value in the substantia nigra was significantly elevated in the schizophrenic group compared with the control group with an effect size of 0.85 [t = 3.2 (56), P = 0.003; Fig. 2 and Table 1]. Post hoc tests showed that this remained the case when the analysis was restricted to the drug-free schizophrenic subjects (n = 13; Bonferroni corrected P = 0.016; effect size = 1.02, Table 1). However, there was no significant difference between control subjects and drug-treated patients (n = 16; Bonferroni corrected P = 0.086, Table 1).
value in the substantia nigra was significantly elevated in the schizophrenic group compared with the control group with an effect size of 0.85 [t = 3.2 (56), P = 0.003; Fig. 2 and Table 1]. Post hoc tests showed that this remained the case when the analysis was restricted to the drug-free schizophrenic subjects (n = 13; Bonferroni corrected P = 0.016; effect size = 1.02, Table 1). However, there was no significant difference between control subjects and drug-treated patients (n = 16; Bonferroni corrected P = 0.086, Table 1).
Figure 2.
Mean (SD) dopamine synthesis capacity ( /min) in the substantia nigra in patients with schizophrenia and control subjects. There was a significant elevation in dopamine synthesis capacity in the schizophrenia group compared with control subjects.
/min) in the substantia nigra in patients with schizophrenia and control subjects. There was a significant elevation in dopamine synthesis capacity in the schizophrenia group compared with control subjects.
Table 1.
In vivo dopamine synthesis capacity in control and schizophrenia groups
| Region | Control subjects n = 29 | Schizophrenia total sample, n = 29 | Schizophrenia drug-treated, n = 16 | Schizophrenia drug free, n = 13 |
|---|---|---|---|---|
| Substantia nigra | 0.0066 (0.0017) | 0.0075** (0.0009) | 0.0073 (0.0009) | 0.0077* (0.0009) |
| Whole striatum | 0.0134 (0.0015) | 0.0152*** (0.0016) | 0.0148* (0.0017) | 0.0156*** (0.0013) |
Mean [(SD) in min−1] 18F-DOPA  values in the substantia nigra and striatum. Significant differences between control and schizophrenia groups are indicated: ***P < 0.0001, **P < 0.005, *P < 0.05.
values in the substantia nigra and striatum. Significant differences between control and schizophrenia groups are indicated: ***P < 0.0001, **P < 0.005, *P < 0.05.
The mean 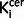 value in the striatum was also significantly elevated in the schizophrenic group compared to the control group with an effect size of 1.14 [t = 4.4 (56), P < 0.0001; Table 1]. This remained the case when the analysis was restricted to the drug-free schizophrenic subjects (n = 13; Bonferroni corrected P < 0.0001; effect size = 1.57; Table 1), and when restricted to drug-treated patients (n = 16; Bonferroni corrected P = 0.012; effect size = 0.87; Table 1). There was no significant difference between
value in the striatum was also significantly elevated in the schizophrenic group compared to the control group with an effect size of 1.14 [t = 4.4 (56), P < 0.0001; Table 1]. This remained the case when the analysis was restricted to the drug-free schizophrenic subjects (n = 13; Bonferroni corrected P < 0.0001; effect size = 1.57; Table 1), and when restricted to drug-treated patients (n = 16; Bonferroni corrected P = 0.012; effect size = 0.87; Table 1). There was no significant difference between 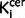 values in drug-free and drug treated patients in either the nigra (P = 0.28) or striatum (P = 0.20). In the patients who were receiving antipsychotic treatment, there was no relationship between antipsychotic treatment duration and nigral (r = 0.35, P = 0.21) or striatal (r = 0.05, P = 0.85)
values in drug-free and drug treated patients in either the nigra (P = 0.28) or striatum (P = 0.20). In the patients who were receiving antipsychotic treatment, there was no relationship between antipsychotic treatment duration and nigral (r = 0.35, P = 0.21) or striatal (r = 0.05, P = 0.85) 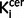 values.
values.
Exploratory analyses of the striatal subdivisions indicated that there was a significant elevation in schizophrenia in the associative [mean (SD) 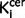 value/min for control subjects = 0.0131 (0.0015), schizophrenia = 0.0146 (0.0017); P = 0.001], sensorimotor [mean (SD)
value/min for control subjects = 0.0131 (0.0015), schizophrenia = 0.0146 (0.0017); P = 0.001], sensorimotor [mean (SD) 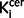 value/min for control subjects = 0.0149 (0.0019), schizophrenia = 0.0167 (0.0020); P = 0.001] and limbic [mean (SD)
value/min for control subjects = 0.0149 (0.0019), schizophrenia = 0.0167 (0.0020); P = 0.001] and limbic [mean (SD)  value/min for control subjects = 0.0138 (0.0016), schizophrenia = 0.0148 (0.0015); P = 0.017] subdivisions.
value/min for control subjects = 0.0138 (0.0016), schizophrenia = 0.0148 (0.0015); P = 0.017] subdivisions.
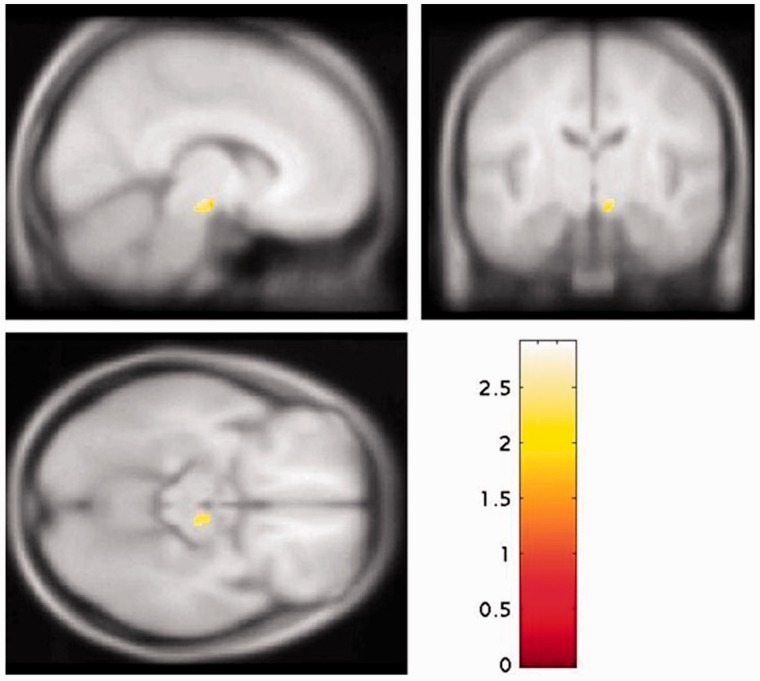
Voxel-based analysis
The voxel-based analysis within the nigra identified a significant elevation in schizophrenia in the right substantia nigra (Fig. 3, peak: family-wise error corrected P = 0.031; MNI coordinates 12, −18, −10), and no voxels where the control subjects were greater than the schizophrenic group (even at an uncorrected P < 0.05). The whole brain voxel-based analysis supported the region of interest analyses, showing significantly elevated dopaminergic function in schizophrenia in two clusters, one including the striatum and the other with a peak in the nigra (Supplementary Fig. 5A; peak right nigra: MNI coordinates 12, −18, −10), whereas the contrast control subjects > patients did not identify any regions where there were significant elevations in control subjects (Supplementary Fig. 5B).
Figure 3.
Statistical parametric map of increased 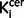 values in schizophrenia relative to control subjects for the substantia nigra rendered on a T1 template (peak at MNI coordinates: 12, −18, −10; FWE P < 0.05).
values in schizophrenia relative to control subjects for the substantia nigra rendered on a T1 template (peak at MNI coordinates: 12, −18, −10; FWE P < 0.05).
The relationship between nigral and striatal 18F-DOPA uptake
There was a significant positive relationship between nigral 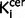 values and striatal
values and striatal  values in the control subjects (r = 0.631, P < 0.001, Supplementary Fig. 3) that was not evident in the schizophrenic group as a whole (r = 0.303, P = 0.11), or in either the drug-free (r = 0.28, P = 0.36) or drug-treated subgroups (r = 0.26, P = 0.33). However, there was no significant difference between these correlation coefficients (Z > 1.1, P > 0.11 in all cases).
values in the control subjects (r = 0.631, P < 0.001, Supplementary Fig. 3) that was not evident in the schizophrenic group as a whole (r = 0.303, P = 0.11), or in either the drug-free (r = 0.28, P = 0.36) or drug-treated subgroups (r = 0.26, P = 0.33). However, there was no significant difference between these correlation coefficients (Z > 1.1, P > 0.11 in all cases).
The relationship between nigral dopaminergic function and symptoms
There was a positive relationship between total symptom scores and nigral 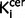 values (r = 0.39, P = 0.035, Supplementary Fig. 4A) but not with striatal
values (r = 0.39, P = 0.035, Supplementary Fig. 4A) but not with striatal 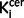 value (r = 0.27, P = 0.16). Sub-analyses found there was a significant direct relationship between nigral
value (r = 0.27, P = 0.16). Sub-analyses found there was a significant direct relationship between nigral 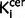 values and positive symptom subscale score (r = 0.37, P = 0.048), but this did not survive correction for multiple comparisons and was not significant when the sample was restricted to the drug-free or drug-treated patients. There was also a significant direct relationship between striatal
values and positive symptom subscale score (r = 0.37, P = 0.048), but this did not survive correction for multiple comparisons and was not significant when the sample was restricted to the drug-free or drug-treated patients. There was also a significant direct relationship between striatal  values and positive symptom subscale score in the drug-free patients (r = 0.60, P = 0.029), although this did not survive correction for multiple comparisons and was not significant in the whole sample or drug-treated patients alone. There were no significant relationships between nigral or striatal
values and positive symptom subscale score in the drug-free patients (r = 0.60, P = 0.029), although this did not survive correction for multiple comparisons and was not significant in the whole sample or drug-treated patients alone. There were no significant relationships between nigral or striatal 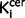 values and negative symptom subscale scores in the whole sample or either sub-group.
values and negative symptom subscale scores in the whole sample or either sub-group.
Discussion
Our major findings are that two measures of dopamine synthesis capacity, one ex vivo and one in vivo, were elevated in the substantia nigra in schizophrenia. In addition, greater nigral 18F-DOPA uptake was linked to greater symptom severity, indicating a link to the clinical expression of the disorder. These findings indicate that elevated dopamine synthesis capacity is evident in the nigral cell bodies of dopamine neurons, and not just in their striatal terminals (Howes et al., 2012a). They extend a previous finding of increased dopamine turnover in the midbrain (Kumakura et al., 2007) to indicate that dopamine synthesis capacity is elevated specifically in the substantia nigra in schizophrenia. Taken with evidence for increased dopamine D2, but not D3 or dopamine transporter, availability in the substantia nigra (Arakawa et al., 2009; Graff-Guerrero et al., 2009; Kessler et al., 2009; Mizrahi et al., 2011), these findings indicate that particular aspects of nigral dopaminergic function are abnormal in schizophrenia.
We found a direct relationship between striatal and nigral 18F-DOPA uptake in control subjects. This extends to human findings in non-human primates, which have also shown this relationship between striatal and nigral 18F-DOPA uptake (Brown et al., 2013). In contrast, the relationship between striatal and nigral 18F-DOPA uptake was not present in schizophrenia, suggesting a decoupling of the normal relationship between dopamine synthesis capacity in the cell bodies and that in the terminal region. Whereas a non-human primate model of dopamine loss also found a loss of this normal coupling between striatal and nigral 18F-DOPA uptake (Brown et al., 2013), studies in preclinical models are required to determine the significance of this decoupling in the context of increased 18F-DOPA uptake, as is the case in schizophrenia. Finally our finding of no alteration in nigral tyrosine hydroxylase staining in patients with major depression extends previous findings in this patient population of altered tyrosine hydroxylase levels in the locus coeruleus (Baumann et al., 1999; Zhu et al., 1999; Gos et al., 2008). It suggests that, although tyrosine hydroxylase alterations in depressive disorder are present in a norepinephrinergic region, they are not present in a dopaminergic region, at least in our sample where the cause of death was predominantly suicide.
Methodological considerations
Some of the schizophrenic patients we studied were taking antipsychotic treatment. Although we cannot exclude an effect of treatment on the post-mortem tyrosine hydroxylase levels (as all the schizophrenia subjects had taken antipsychotics), rodent studies indicate that antipsychotic treatment either does not alter (Perez-Costas et al., 2012) or reduces tyrosine hydroxylase levels (Tejedor-Real et al., 2003). Furthermore the elevation in nigral and striatal 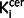 values in our in vivo imaging study was also observed in untreated patients, indicating that the elevation is not secondary to current antipsychotic treatment. Whereas the subgroup analyses of nigral
values in our in vivo imaging study was also observed in untreated patients, indicating that the elevation is not secondary to current antipsychotic treatment. Whereas the subgroup analyses of nigral 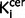 values found that there was a significant elevation in in the drug free patients compared to control subjects, there was no significant difference between the drug-treated group and either the drug free or control groups, suggesting the drug-treated group may be intermediate between the other groups. This potential effect of treatment (Grunder et al., 2003) warrants further investigation in a larger sample. Nevertheless tyrosine hydroxylase staining was significantly elevated in patients who had received antipsychotics ante-mortem. This could indicate that there is a greater elevation in tyrosine hydroxylase than aromatic acid decarboxylase, but is probably because of the greater sensitivity of the tyrosine hydroxylase staining and a lack of statistical power in the sub-analysis of treated patients in the imaging study. A study in the midbrain did not find increased
values found that there was a significant elevation in in the drug free patients compared to control subjects, there was no significant difference between the drug-treated group and either the drug free or control groups, suggesting the drug-treated group may be intermediate between the other groups. This potential effect of treatment (Grunder et al., 2003) warrants further investigation in a larger sample. Nevertheless tyrosine hydroxylase staining was significantly elevated in patients who had received antipsychotics ante-mortem. This could indicate that there is a greater elevation in tyrosine hydroxylase than aromatic acid decarboxylase, but is probably because of the greater sensitivity of the tyrosine hydroxylase staining and a lack of statistical power in the sub-analysis of treated patients in the imaging study. A study in the midbrain did not find increased 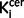 values in schizophrenia (Kumakura et al., 2007). This apparent discrepancy with our findings could be because, in contrast to our study, this study did not use entacapone to block the formation of the radiometabolite 6-[F-18]fluoro-3-O-methyl-L-dihydroxyphenyl-l-alanine, which can cross the blood–brain barrier and reduce the specific signal. Supporting this interpretation, they did find a significant elevation in 18F-DOPA uptake in the midbrain after they corrected for this plasma metabolite, in line with our findings in the nigra.
values in schizophrenia (Kumakura et al., 2007). This apparent discrepancy with our findings could be because, in contrast to our study, this study did not use entacapone to block the formation of the radiometabolite 6-[F-18]fluoro-3-O-methyl-L-dihydroxyphenyl-l-alanine, which can cross the blood–brain barrier and reduce the specific signal. Supporting this interpretation, they did find a significant elevation in 18F-DOPA uptake in the midbrain after they corrected for this plasma metabolite, in line with our findings in the nigra.
A general limitation of post-mortem studies is that clinical information is retrospectively obtained from medical notes. To reduce this risk, in the post-mortem study we only included patients with well documented symptoms who clearly met criteria for either schizophrenia or major depressive disorder, and control subjects where these disorders were positively excluded. Semi-quantitative analysis of staining, as used here, does not provide absolute protein levels and may be less sensitive than fully quantitative approaches, although, as lower sensitivity would militate against finding an elevation in schizophrenia, this is unlikely to change our findings. Our finding of increased nigral tyrosine hydroxylase staining is in agreement with previous findings of increased nigral tyrosine hydroxylase activity (Toru et al., 1988) and tyrosine hydroxylase messenger RNA levels in schizophrenia (Mueller et al., 2004). However, it is important to note that messenger RNA levels have been found to be unaltered in two studies, and, additionally tyrosine hydroxylase protein levels were reduced in one of these studies (Ichinose et al., 1994; Perez-Costas et al., 2012). This discrepancy may reflect the fact that our analysis specifically examined dopamine neurons in the nigra, whereas the other study used homogenates of tissue from the nigra, which, if the tissue samples contained different numbers of dopamine neurons, may have introduced sampling effects.
The cause of death was suicide in the majority (9 of 13) of the major depression cases. This suggests that they had severe illness. Although this serves as a useful psychiatric control for the schizophrenia group, indicating that the findings in the schizophrenia group are unlikely to be due to suicide and/or psychiatric illness per se, it may limit the generalizability of the findings to less severe cases of depression.
Bias in the positioning of region of interests may influence results in neuroimaging studies, but this is unlikely to have been a factor in the in vivo imaging, as the image analysis was automated and conducted blind to diagnosis. Our automatic atlas-based approach could be affected by a systematic group difference in the transformation of the standardized atlas to native space. However, the whole brain analysis identified an elevation in the same regions in schizophrenia and did not identify any regions where there was a significant elevation in 18F-DOPA uptake in control subjects, supporting our region of interest findings. An abnormality in the reference region is also unlikely to explain the results as previous studies have reported elevated striatal dopamine synthesis capacity in schizophrenia using other reference regions (Hietala et al., 1995; McGowan et al., 2004). Partial volume and spill-over effects can affect measurements in small regions of interest but are unlikely to account for the elevation in the nigra as nigral volumes are, if anything, reduced in schizophrenia (Bogerts et al., 1983), and these effects would thus tend to underestimate the 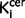 values. In contrast to recent findings that the striatal dopamine elevation is most marked in the associative subregion (Howes et al., 2009b, 2011b; Kegeles et al., 2010), we found significant elevations in all three striatal subregions. This may reflect the greater power of the larger sample size in our current study to detect small differences. Additionally we cannot exclude spill-over effects, which are more influential for small regions such as the limbic striatum (Kim et al., 2013b). Nevertheless, the smallest alteration was seen in the limbic striatum, in keeping with the previous studies (Howes et al., 2009b, 2011b; Kegeles et al., 2010). Finally, it is possible that less efficient blockade of peripheral 18F-DOPA metabolism in the schizophrenia group could have resulted in elevated brain penetrant radiometabolite levels (Asselin et al., 2007). However, as this would serve to mask an elevation (Asselin et al., 2007), it would not explain our findings. Furthermore, no difference in 18F-DOPA metabolism under carbidopa blockade has been found between patients with schizophrenia and control subjects (Hietala et al., 1999). Nevertheless it would be useful to measure metabolite levels in future studies.
values. In contrast to recent findings that the striatal dopamine elevation is most marked in the associative subregion (Howes et al., 2009b, 2011b; Kegeles et al., 2010), we found significant elevations in all three striatal subregions. This may reflect the greater power of the larger sample size in our current study to detect small differences. Additionally we cannot exclude spill-over effects, which are more influential for small regions such as the limbic striatum (Kim et al., 2013b). Nevertheless, the smallest alteration was seen in the limbic striatum, in keeping with the previous studies (Howes et al., 2009b, 2011b; Kegeles et al., 2010). Finally, it is possible that less efficient blockade of peripheral 18F-DOPA metabolism in the schizophrenia group could have resulted in elevated brain penetrant radiometabolite levels (Asselin et al., 2007). However, as this would serve to mask an elevation (Asselin et al., 2007), it would not explain our findings. Furthermore, no difference in 18F-DOPA metabolism under carbidopa blockade has been found between patients with schizophrenia and control subjects (Hietala et al., 1999). Nevertheless it would be useful to measure metabolite levels in future studies.
Implications for understanding schizophrenia
The elevated staining for an antibody specific to tyrosine hydroxylase in our post-mortem study indicates increased tyrosine hydroxylase protein levels in schizophrenia that were not seen in matched patients with major depression, suggesting it is not a non-specific indicator of psychiatric illness. Although there is also tyrosine hydroxylase and aromatic-l-amino acid decarboxylase activity in other monoaminergic neurons, most monoaminergic neurons in the nigra are dopaminergic and aromatic-l-amino acid decarboxylase activity in the substantia nigra is abolished by selective dopamine neuron toxin (Yee et al., 2000; Smith and Villalba, 2008). Furthermore there is also a close, direct relationship between nigral 18F-DOPA uptake and both nigral aromatic-l-amino acid decarboxylase activity and nigral dopamine cell numbers (Yee et al., 2000; Forsback et al., 2004; Brown et al., 2013). Our measures are therefore likely to substantially reflect dopaminergic function in the nigra. Taken together our results thus indicate increased activity in the final steps of dopamine synthesis in the nigra in schizophrenia. As post-mortem findings show that the number of dopamine neurons in the nigra is unaltered in schizophrenia (Bogerts et al., 1983), this elevation is likely to reflect increased capacity to produce dopamine rather than an increase in the number of dopamine neurons.
Given the functional MRI evidence of abnormal nigro-striatal activation in schizophrenia and its prodrome in the context of reward/conditioning tasks (Murray et al., 2008; Waltz et al., 2009; Romaniuk et al., 2010; Gradin et al., 2011; Allen et al., 2012a, b; Nielsen et al., 2012; Roiser et al., 2012; Yoon et al., 2013) and the consistent evidence for increased dopamine release in the striatal terminal regions [see (Laruelle et al., 1996; Breier et al., 1997; Abi-Dargham et al., 2000) and meta-analysis (Howes et al., 2012b)], it seems likely that this reflects an increase in midbrain dopamine neuron population activity, in line with that seen in preclinical developmental models of schizophrenia (Lodge and Grace, 2007, 2011). If this is the case, then it supports the development of treatments that target the control of midbrain dopamine neurons as alternatives to antipsychotics, which target the postsynaptic postsynaptic dopaminergic system (Kim et al., 2012, 2013a). A proof-of-concept for this has been shown using a GABA-alpha 5 receptor selective agent, which altered the hippocampal drive to the midbrain and was found to reverse aberrant midbrain dopamine neuronal functioning in rodent models of schizophrenia (Gill et al., 2011). Nevertheless future studies are needed to confirm our findings and to examine the breakdown pathways to determine if there is abnormal regulation of dopamine by catabolic processes.
Conclusion
Dopamine synthesis capacity is elevated in the substantia nigra in patients with schizophrenia, as well as in the striatum. This indicates that the pathophysiology of schizophrenia involves the cell bodies of dopamine neurons in addition to their striatal terminals. Nigral tyrosine hydroxylase staining was unaltered in major depression, suggesting that the alterations seen in schizophrenia are not non-specific indicators of psychiatric illness.
Supplementary Material
Acknowledgements
The authors acknowledge and are very grateful for the assistance of the staff of Hammersmith Imanet, Professor Steve Gentleman and the research subjects.
Post-mortem tissue was obtained from the Corsellis collection, which is supported by the Starr foundation and the West London Mental Health Trust and the research supported by funding from the MRC-UK PET Methodology Programme Grant G1100809/1.
Glossary
Abbreviations
- DOPA
dihydroxyphenyl-l-alanine
Funding
This work was funded by a Medical Research Council (UK) grant to Dr Howes (grant number: MC-A656-5QD30) and the National Institute of Health Research Biomedical Research Council grant to King’s College London.
Supplementary material
Supplementary material is available at Brain online.
References
- Abi-Dargham A, Giessen EV, Slifstein M, Kegeles LS, Laruelle M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol Psychiatry. 2009;65:1091–3. doi: 10.1016/j.biopsych.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155:761–7. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of d2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–9. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan RL, Allom R, Gousias IS, Habib H, Turkheimer FE, Free S, et al. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. Neuroimage. 2007;38:261–70. doi: 10.1016/j.neuroimage.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Allen P, Chaddock CA, Howes OD, Egerton A, Seal ML, Fusar-Poli P, et al. Abnormal relationship between medial temporal lobe and subcortical dopamine function in people with an ultra high risk for psychosis. Schizophr Bull. 2012a;38:1040–9. doi: 10.1093/schbul/sbr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P, Luigjes J, Howes OD, Egerton A, Hirao K, Valli I, et al. Transition to psychosis associated with prefrontal and subcortical dysfunction in ultra high-risk individuals. Schizophr Bull. 2012b;38:1268–76. doi: 10.1093/schbul/sbr194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. Comprehensive assessment of symptoms and history. Iowa: University of Iowa College of Medicine; 1987. [Google Scholar]
- Arakawa R, Ichimiya T, Ito H, Takano A, Okumura M, Takahashi H, et al. Increase in thalamic binding of [(11)c]pe2i in patients with schizophrenia: a positron emission tomography study of dopamine transporter. J Psychiatr Res. 2009;43:1219–23. doi: 10.1016/j.jpsychires.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Asselin MCH, Howes OD, Osman S, Yau K, Grasby PM. Increase in striatal f-DOPA influx constants in patients at ultra high risk of psychosis are not due to changes in plasma OMFD concentrations. J Cereb Blood Flow Metab. 2007;27:PO01–03M. [Google Scholar]
- Barkus EJ, Stirling J, Hopkins RS, Lewis S. Cannabis-induced psychosis-like experiences are associated with high schizotypy. Psychopathology. 2006;39:175–78. doi: 10.1159/000092678. [DOI] [PubMed] [Google Scholar]
- Baumann B, Danos P, Diekmann S, Krell D, Bielau H, Geretsegger C, et al. Tyrosine hydroxylase immunoreactivity in the locus coeruleus is reduced in depressed non-suicidal patients but normal in depressed suicide patients. Eur Arch Psychiatry Clin Neurosci. 1999;249:212–9. doi: 10.1007/s004060050089. [DOI] [PubMed] [Google Scholar]
- Biegon A, Fieldust S. Reduced tyrosine hydroxylase immunoreactivity in locus coeruleus of suicide victims. Synapse. 1992;10:79–82. doi: 10.1002/syn.890100111. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Hantsch J, Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol Psychiatry. 1983;18:951–69. [PubMed] [Google Scholar]
- Bose SK, Turkheimer FE, Howes OD, Mehta MA, Cunliffe R, Stokes PR, et al. Classification of schizophrenic patients and healthy controls using [18f] fluorodopa pet imaging. Schizophr Res. 2008;106:148–55. doi: 10.1016/j.schres.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–74. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CA, Karimi MK, Tian L, Flores H, Su Y, Tabbal SD, et al. Validation of midbrain PET measures for nigrostriatal neurons in macaques. Ann Neurol. 2013 doi: 10.1002/ana.23939. May 20 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming P, Ase A, Laliberte C, Kuwabara H, Gjedde A. In vivo regulation of dopa decarboxylase by dopamine receptors in rat brain. J Cereb Blood Flow Metab. 1997;17:1254–60. doi: 10.1097/00004647-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Cumming P, Kuwabara H, Ase A, Gjedde A. Regulation of DOPA decarboxylase activity in brain of living rat. J Neurochem. 1995;65:1381–90. doi: 10.1046/j.1471-4159.1995.65031381.x. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Paillere-Martinot ML, Hantraye P, Ttar-Levy D, Remy P, Crouzel C, et al. Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophr Res. 1997;23:167–74. doi: 10.1016/S0920-9964(96)00102-8. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–86. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Demjaha A, Murray RM, McGuire PK, Kapur S, Howes OD. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am J Psychiatry. 2012;169:1203–10. doi: 10.1176/appi.ajp.2012.12010144. [DOI] [PubMed] [Google Scholar]
- Duzel E, Bunzeck N, Guitart-Masip M, Wittmann B, Schott BH, Tobler PN. Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 2009;32:321–8. doi: 10.1016/j.tins.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Egerton A, Chaddock CA, Winton-Brown TT, Bloomfield MA, Bhattacharyya S, Allen P, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2013;74:106–12. doi: 10.1016/j.biopsych.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Egerton A, Demjaha A, McGuire P, Mehta MA, Howes OD. The test-retest reliability of 18f-dopa pet in assessing striatal and extrastriatal presynaptic dopaminergic function. Neuroimage. 2009;50:524–31. doi: 10.1016/j.neuroimage.2009.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef AM, Doudet D, Bryant T, Cohen RM, Li SH, Wyatt RJ. 6-(18)f-DOPA pet study in patients with schizophrenia. Positron emission tomography. Psychiatry Res. 2000;100:1–11. doi: 10.1016/s0925-4927(00)00064-0. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr. 2007;137:1539S–47S; discussion 48S. doi: 10.1093/jn/137.6.1539S. [DOI] [PubMed] [Google Scholar]
- Forsback S, Niemi R, Marjamaki P, Eskola O, Bergman J, Gronroos T, et al. Uptake of 6-[18f]fluoro-l-dopa and [18f]cft reflect nigral neuronal loss in a rat model of Parkinson's disease. Synapse. 2004;51:119–27. doi: 10.1002/syn.10293. [DOI] [PubMed] [Google Scholar]
- Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel alpha5GABA(a)r-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36:1903–11. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gos T, Krell D, Bielau H, Brisch R, Trubner K, Steiner J, et al. Tyrosine hydroxylase immunoreactivity in the locus coeruleus is elevated in violent suicidal depressive patients. Eur Arch Psychiatry Clin Neurosci. 2008;258:513–20. doi: 10.1007/s00406-008-0825-8. [DOI] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–64. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mizrahi R, Agid O, Marcon H, Barsoum P, Rusjan P, et al. The dopamine d2 receptors in high-affinity state and d3 receptors in schizophrenia: a clinical [11c]-(+)-phno pet study. Neuropsychopharmacology. 2009;34:1078–86. doi: 10.1038/npp.2008.199. [DOI] [PubMed] [Google Scholar]
- Grunder G, Vernaleken I, Muller MJ, Davids E, Heydari N, Buchholz HG, et al. Subchronic haloperidol downregulates dopamine synthesis capacity in the brain of schizophrenic patients in vivo. Neuropsychopharmacology. 2003;28:787–94. doi: 10.1038/sj.npp.1300103. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–76. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietala J, Syvalahti E, Vilkman H, Vuorio K, Rakkolainen V, Bergman J, et al. Depressive symptoms and presynaptic dopamine function in neuroleptic-naive schizophrenia. Schizophr Res. 1999;35:41–50. doi: 10.1016/s0920-9964(98)00113-3. [DOI] [PubMed] [Google Scholar]
- Hietala J, Syvalahti E, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–31. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry. 2011a;16:885–6. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Bose S, Valli I, Turkheimer F, Egerton A, Valmaggia L, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18]-DOPA pet imaging study. Am J Psychiatry. 2011b;168:1311–7. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Egerton A, Allan V, McGuire P, Stokes P, Kapur S. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009a;15:2550–9. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012a;69:776–86. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment: meta-analysis of imaging studies. Arch Gen Psychiatry. 2012b;69:776–86. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version iii–the final common pathway. Schizophr Bull. 2009;35:549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry Suppl. 2007;51:s13–8. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009b;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Howes OD, Shotbolt P, Bloomfield M, Daalman K, Demjaha A, Diederen KM, et al. Dopaminergic function in the psychosis spectrum: an [18f]-DOPA imaging study in healthy individuals with auditory hallucinations. Schizophr Bull. 2013;39:807–14. doi: 10.1093/schbul/sbr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose H, Ohye T, Fujita K, Pantucek F, Lange K, Riederer P, et al. Quantification of mRNA of tyrosine hydroxylase and aromatic l-amino acid decarboxylase in the substantia nigra in Parkinson's disease and schizophrenia. J Neural Transm Park Dis Dement Sect. 1994;8:149–58. doi: 10.1007/BF02250926. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience. 2000;96:451–74. doi: 10.1016/s0306-4522(99)00575-8. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–9. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- Kessler RM, Woodward ND, Riccardi P, Li R, Ansari MS, Anderson S, et al. Dopamine d2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol Psychiatry. 2009;65:1024–31. doi: 10.1016/j.biopsych.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Howes OD, Kim BH, Jeong JM, Lee JS, Jang IJ, et al. Predicting brain occupancy from plasma levels using PET: superiority of combining pharmacokinetics with pharmacodynamics while modeling the relationship. J Cereb Blood Flow Metab. 2012;32:759–68. doi: 10.1038/jcbfm.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Howes OD, Turkheimer FE, Kim BH, Jeong JM, Kim JW, et al. The relationship between antipsychotic d(2) occupancy and change in frontal metabolism and working memory : a dual [(11)c]raclopride and [(18) f]FDG imaging study with aripiprazole. Psychopharmacology. 2013a;227:221–9. doi: 10.1007/s00213-012-2953-0. [DOI] [PubMed] [Google Scholar]
- Kim E, Shidahara M, Tsoumpas C, McGinnity CJ, Kwon JS, Howes OD, et al. Partial volume correction using structural-functional synergistic resolution recovery: comparison with geometric transfer matrix method. J Cereb Blood Flow Metab. 2013b;33:914–20. doi: 10.1038/jcbfm.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumakura Y, Cumming P. Pet studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist. 2009;15:635–50. doi: 10.1177/1073858409338217. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Cumming P, Vernaleken I, Buchholz HG, Siessmeier T, Heinz A, et al. Elevated [18f]fluorodopamine turnover in brain of patients with schizophrenia: an [18f]fluorodopa/positron emission tomography study. J Neurosci. 2007;27:8080–87. doi: 10.1523/JNEUROSCI.0805-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA. 1996;93:9235–40. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom LH, Gefvert O, Hagberg G, Lundberg T, Bergstrom M, Hartvig P, et al. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by l-(beta-11c) DOPA and PET. Biol Psychiatry. 1999;46:681–88. doi: 10.1016/s0006-3223(99)00109-2. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–30. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Divergent activation of ventromedial and ventrolateral dopamine systems in animal models of amphetamine sensitization and schizophrenia. Int J Neuropsychopharmacol. 2011 doi: 10.1017/S1461145711000113. Feb 18:1–8 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GJ, Abi-Dargham A, Moore H, Lieberman JA, Javitch JA, Sulzer D. Presynaptic regulation of dopamine transmission in schizophrenia. Schizophr Bull. 2011;37:108–17. doi: 10.1093/schbul/sbp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinot M, Bragulat V, Artiges E, Dolle F, Hinnen F, Jouvent R, et al. Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am J Psychiatry. 2001;158:314–16. doi: 10.1176/appi.ajp.158.2.314. [DOI] [PubMed] [Google Scholar]
- McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18f]fluorodopa study. Arch Gen Psychiatry. 2004;61:134–42. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Schmitt GJ, Scheuerecker J, Moller HJ. The role of dopamine for the pathophysiology of schizophrenia. Int Rev Psychiatry. 2007;19:337–45. doi: 10.1080/09540260701502468. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–71. doi: 10.1038/nn804. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, et al. Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71:561–7. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Agid O, Borlido C, Suridjan I, Rusjan P, Houle S, et al. Effects of antipsychotics on d3 receptors: a clinical pet study in first episode antipsychotic naive patients with schizophrenia using [11c]-(+)-phno. Schizophr Res. 2011;131:63–8. doi: 10.1016/j.schres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Mueller HT, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res Mol Brain Res. 2004;121:60–9. doi: 10.1016/j.molbrainres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13:239, 67–39, 76. doi: 10.1038/sj.mp.4002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MO, Rostrup E, Wulff S, Bak N, Lublin H, Kapur S, et al. Alterations of the brain reward system in antipsychotic naive schizophrenia patients. Biol Psychiatry. 2012;71:898–905. doi: 10.1016/j.biopsych.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Kato M, Takano H, Ito H, Takahashi H, Arakawa R, et al. Regional dopamine synthesis in patients with schizophrenia using l-[beta-(11)c]DOPA PET. Schizophr Res. 2009;108:78–84. doi: 10.1016/j.schres.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Perez-Costas E, Melendez-Ferro M, Rice MW, Conley RR, Roberts RC. Dopamine pathology in schizophrenia: analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Front Psychiatry. 2012;3:31. doi: 10.3389/fpsyt.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, et al. Elevated DOPA decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci USA. 1994;91:11651–4. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, Howes OD, Chaddock CA, Joyce EM, McGuire P. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr Bull. 2012 doi: 10.1093/schbul/sbs147. Dec 12 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk L, Honey GD, King JR, Whalley HC, McIntosh AM, Levita L, et al. Midbrain activation during pavlovian conditioning and delusional symptoms in schizophrenia. Arch Gen Psychiatry. 2010;67:1246–54. doi: 10.1001/archgenpsychiatry.2010.169. [DOI] [PubMed] [Google Scholar]
- Shotbolt P, Stokes PR, Owens SF, Toulopoulou T, Picchioni MM, Bose SK, et al. Striatal dopamine synthesis capacity in twins discordant for schizophrenia. Psychol Med. 2011;41:2331–8. doi: 10.1017/S0033291711000341. [DOI] [PubMed] [Google Scholar]
- Smith Y, Villalba R. Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains. Mov Disord. 2008;23(Suppl. 3):S534–S47. doi: 10.1002/mds.22027. [DOI] [PubMed] [Google Scholar]
- Tejedor-Real P, Faucon BN, Dumas S, Mallet J. Tyrosine hydroxylase mRNA and protein are down-regulated by chronic clozapine in both the mesocorticolimbic and the nigrostriatal systems. J Neurosci Res. 2003;72:105–15. doi: 10.1002/jnr.10555. [DOI] [PubMed] [Google Scholar]
- Toru M, Watanabe S, Shibuya H, Nishikawa T, Noda K, Mitsushio H, et al. Neurotransmitters, receptors and neuropeptides in post-mortem brains of chronic schizophrenic patients. Acta Psychiatr Scand. 1988;78:121–37. doi: 10.1111/j.1600-0447.1988.tb06312.x. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Schweitzer JB, Gold JM, Kurup PK, Ross TJ, Salmeron BJ, et al. Patients with schizophrenia have a reduced neural response to both unpredictable and predictable primary reinforcers. Neuropsychopharmacology. 2009;34:1567–77. doi: 10.1038/npp.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee RE, Huang SC, Stout DB, Irwin I, Shoghi-Jadid K, Togaski DM, et al. Nigrostriatal reduction of aromatic L-amino acid decarboxylase activity in MPTP-treated squirrel monkeys: in vivo and in vitro investigations. J Neurochem. 2000;74:1147–57. doi: 10.1046/j.1471-4159.2000.741147.x. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Raouf S, D'Esposito M, Carter CS. Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biol Psychiatry. 2013;74:122–9. doi: 10.1016/j.biopsych.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Juorio AV, Paterson IA, Boulton AA. Regulation of striatal aromatic l-amino acid decarboxylase: effects of blockade or activation of dopamine receptors. Eur J Pharmacol. 1993;238:157–64. doi: 10.1016/0014-2999(93)90843-7. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Klimek V, Dilley GE, Haycock JW, Stockmeier C, Overholser JC, et al. Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol Psychiatry. 1999;46:1275–86. doi: 10.1016/s0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.