Abstract
Purpose
To assess integration of a biosynthetic corneal implant in dogs.
Methods
Three normal adult laboratory Beagles underwent ophthalmic examinations, including slit-lamp biomicroscopy, indirect ophthalmoscopy, applanation tonometry, and Cochet–Bonnet aesthesiometry. Biosynthetic corneas fabricated from glutaraldehyde crosslinked collagen and copolymers of collagen and poly (N-isopropylacrylamide-co-acrylic acid-co-acryloxysuccinimide, denoted as TERP) were implanted into dogs by a modified epikeratoplasty technique. Ophthalmic examinations and aesthesiometry were performed daily for 5 days and then weekly thereafter for 16 weeks. Corneal samples underwent histopathological and transmission electron microscopy examination at 16 weeks.
Results
Implants were epithelialized by 7 days. Intraocular pressure was within normal range throughout the study. Aesthesiometry values dropped from an average of 3.67 cm preoperatively to less than 1 mm for all dogs for the first postoperative weeks. By week 16, the average Cochet–Bonnet value was 1.67 cm, demonstrating partial recovery of functional innervation of the implant. No inflammation or rejection of the implant occurred, and minimal haze formation was noted. Light microscopy revealed thickened but normal epithelium over the implant with fibroblast migration into the scaffold. On transmission electron microscopy, the basement membrane was irregular but present and adhesion complexes were noted.
Conclusion
Biosynthetic corneal implantation is well tolerated in dogs, and the collagen–polymer hybrid construct holds promise for clinical application in animals and humans.
Keywords: biosynthetic cornea, bioengineering, corneal transplantation
Corneal disease is the second most common cause of vision loss and blindness.1 It is estimated that over 10 million people suffer from corneal blindness.2 The most successful treatment of corneal blindness to date is penetrating keratoplasty using donor corneal tissue; however, this approach is dependent on a readily available supply of donor corneas. Unfortunately, this supply may be limited by an aging population and by the increasing numbers of corneal refractive surgeries performed in developed countries. In developing countries, a lack of collection systems and eye banks limits the supply of healthy donor corneal tissue, despite efforts to develop corneal banking systems and distribution networks.3 Furthermore, the success rates for human corneal grafting procedures tend to be lower for many ocular surface diseases or problems such as chemical burns, ocular cicatricial pemphigoid, or Stevens–Johnson syndrome.4
The alternative to transplantation of human corneal tissues is implantation of a keratoprosthesis. An ideal artificial cornea would allow epithelialization of the surface without epithelial downgrowth and ingrowth of nerves, would prevent retrocorneal membranes, and would not elicit an immune or mutagenic response.5 The mechanical strength of the native cornea must also be approximated in any successful corneal replacement. Keratoprosthetic implantation thus far, however, has high complication rates, and such complications are usually blinding, including extrusion of the implant, epithelial downgrowth, glaucoma, and infection.3 The Boston keratoprosthesis, the most commonly implanted artificial cornea, has an excellent retention rate and improves visual acuity in many patients. The complication rate, however, remains high, with complications including retroprosthetic membranes, increased intraocular pressure, glaucoma progression, and endophthalmitis in a recent case series.6 Frequent follow-up is required to treat and minimize these complications. Another type of keratoprosthesis is constructed of a hydrogel with a porous skirt to promote integration into the host cornea.7 Limited clinical trials with this device resulted in significant complications, including corneal melts, extrusions, retroprosthetic membranes, retinal detachment, and deposits on the device that decreased optic clarity.7–9 An implant that could be seamlessly incorporated into host cornea would clearly result in better outcomes.
Tissue engineering, the integration of biomaterials and cells, offers a potential solution to these problems.10 Using an engineered collagen tissue matrix, in vitro construction of a complete multilayered cornea that has morphological characteristics similar to human eye bank corneas is now possible.11 More recent work has evolved to use a combination of collagens and copolymers that better mimic the mechanical, refractive, and wound healing properties of the cornea while still allowing cell ingrowth, including ingrowth of functional nerves and coverage with epithelial cells.12–15 Implantation of these biosynthetic biomimetic extracellular matrices in normal rabbits and pigs resulted in rapid epithelialization of the implant, nerve ingrowth with some return of corneal sensitivity, minimal inflammation, and a clear graft.16,17 The implants were well integrated into the host cornea, with a stratified squamous epithelium overlying the implant and activated stromal fibroblasts within the implant.16,17
In the present pilot study, biosynthetic extracellular matrices were implanted in dogs to determine integration, innervation, and tolerance of such biointeractive corneal matrices with a view toward future utilization in veterinary patients. Dogs were chosen because they not only provide a useful model for biosynthetic cornea implantation but may also serve as a model for the treatment of spontaneous corneal disease, once safety is established. Nonhealing erosions, vision-threatening corneal ulcers, blinding corneal perforations, and tumors in dogs are commonly seen in veterinary ophthalmology practices,18,19 and many of these diseases share characteristics with their human counterparts.20 Therefore, subsequent clinical studies in dogs have the potential of benefiting both human and veterinary patients. This pilot study was undertaken to ensure no adverse effects and to validate the use of this species as a model, given that species variation in response to corneal implants has been documented.21
MATERIALS AND METHODS
Preparation of Corneal Substitutes
Poly(N-isopropylacrylamide-co-acrylic acid-co-acryl-oxysuccinimide) (TERP)–cross linked collagen hydrogels were prepared and characterized according to previous procedures,16 but using a higher initial collagen concentration of 6% wt/wt bovine type I collagen and omitting the YIGSR incorporation, to determine as a secondary objective if nerve ingrowth would occur without incorporation of YIGSR. The final hydrogel was 12 mm in diameter and 350 ± 30-μm thick, with a radius of curvature of about 8 mm centrally (human corneal curvature) and contained 3.5% collagen and 1.4% TERP. White light transmission, measured as previously described, was 85%.22
Cross linked collagen was also prepared by cross linking 2 mL of 10% type I porcine collagen (Koken, Co, Ltd, Tokyo, Japan) with 0.9 mL 0.22% glutaraldehyde at room temperature for 7 days in curved molds with thickness of 350 μm. The resulting corneal substitutes, containing 7% collagen in the final hydrogels, were immersed in 0.5% glycine phosphate-buffered saline (PBS) solution overnight to bind up any unreacted glutaraldehyde. The constructs, 12 mm in diameter and 350 ± 30-μm thick were then washed in several changes of PBS over a 24-hour period. Transmission of white light was 90%.
All hydrogels were stored in chloroform-saturated PBS to ensure sterility before use. Before implantation, hydrogels were soaked in balanced salt solution with gentamicin.
Implantation
Three young adult laboratory Beagles underwent complete ophthalmic examinations, including slit-lamp biomicroscopy, indirect ophthalmoscopy, applanation tonometry, and Cochet–Bonnet aesthesiometry. Only 3 dogs were used so as to minimize the number of animals as this was essentially a safety study. Board-certified veterinary ophthalmologists performed all exams, ancillary tests, and surgery (E.B. and C.J.M.). After routine induction of anesthesia, biosynthetic matrices were implanted using a modified epikeratoplasty technique. Briefly, a 4 mm in diameter area of axial cornea of the right eye was trephined and undermined with a Martinez corneal dissector. Approximately 200 μm of superficial cornea was removed. A peripheral pocket in the corneal stroma at the base of the stromal wound was created using a Suarez corneal spreader, allowing placement of a 6.5-mm-diameter biosynthetic corneal disk into the peripheral pocket, covering the previously created defect. No sutures were placed because the implant did not easily retain suture. The left eye served as an untreated control.
Dogs received 1% prednisolone acetate and 0.3% ocufloxacin topically in both eyes 4 times daily for 21 days. An ophthalmic examination, including slit-lamp biomicroscopy, indirect ophthalmoscopy, applanation tonometry, and Cochet–Bonnet aesthesiometry was performed daily for 5 days postoperatively then once weekly for 16 weeks. Cochet–Bonnet measurements were taken in the center of the implant. Applanation tonometry was performed away from the surgical site in normal cornea. After 16 weeks, dogs were humanely euthanized (intravenous Beauthanasia; Schering Plough, Union, NJ; 1 mL/10 lbs per manufacturer’s instructions) and corneas from both eyes collected for histopathology, immunohistochemistry, and transmission electron microscopy (TEM). All dogs were treated in accordance with the tenets of the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research and the research was approved the Animal Care and Use Committee of the University of Wisconsin–Madison.
Samples for light microscopy were fixed in formalin and were routinely embedded in paraffin, sectioned at 6 μm, and stained with hematoxylin and eosin. Samples for TEM were fixed in 2% glutaraldehyde in phosphate buffer, embedded in Spurr resin, sectioned at 1 μm on a Leica UC6 microtome, poststained with urnayl acetate and lead citrate, and then examined by brightfield microscopy. Approximately 70-nm sections were then obtained from the area of the recipient/host junction and viewed on a FEI CM120 microscope, and digital images were collected using an Olympus-SIS MegaView III camera.
Immunohistochemistry
Tovisualize nerveswithin theimplantareas, cornealslices were cut using a straight-edged razor. Pieces were put into a 24-well plate wells containing 1 mL PBS and rocked gently at 37°C overnight. After washing 2 × 10 minutes with 0.2% Triton 100× in PBS, the pieces were blocked for 60 minutes in blocking solution (4% heat-inactivated fetal bovine serum [FBS] + 0.2% Triton 100× in PBS). Corneal pieces were then incubated with the primary antibody [monoclonal mouse anti-human neurofilament protein(clone 2F11 DAKOCat#M0762)] and diluted 1:100 in blocking solution at 4°C for 2 nights. Pieces werewashed 2 × 10 minutes with 0.2% Triton × 100 in PBS and incubated for 4 hours at room temperature with the goat anti-mouse Cy2 conjugate secondary antibody (Amersham Pharmacia Cat# PA42002) diluted 1:200 in blocking solution. Pieces were then washed for 2 × 10 minutes with 0.2% Triton × 100 in PBS, sandwiched between 2 glass slides, and imaged on a Zeiss Axiovert microscope.
RESULTS
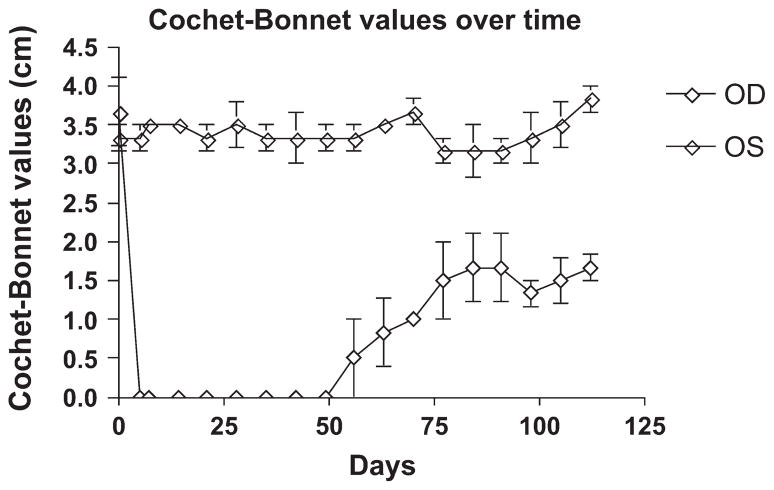
Mild conjunctival hyperemia was noted for several days postoperatively, but all dogs seemed comfortable (no blepharospasm or discharge) by 2 days postoperatively. Epithelial closure was achieved in 2 dogs by day 3 and in 1 dog by day 7 postoperatively. One implant became slightly thinner, as observed by slit-lamp biomicroscopy, at 7 days, but no further thinning or changes were noted in the implant at subsequent examinations. The epithelium remained firmly adherent to all implants throughout the remainder of the study. All implants developed a small amount of opacity around the peripheral edge of the implant where it had been inserted into the stromal pocket. All developed some haze in the implant in the first 4 weeks after surgery, which gradually cleared over the next 3 months (Figs. 1, 2). Over time, the epithelium thickened and filled in the step defect between the recipient stroma and the implant, resulting in a smooth corneal surface. Intraocular pressure remained within the normal range through the study. Cochet–Bonnet values initially dropped in the operated eyes to zero but gradually rose, beginning around day 56 postoperatively, although normal values were not reestablished before the end of the 4-month study (Fig. 3).
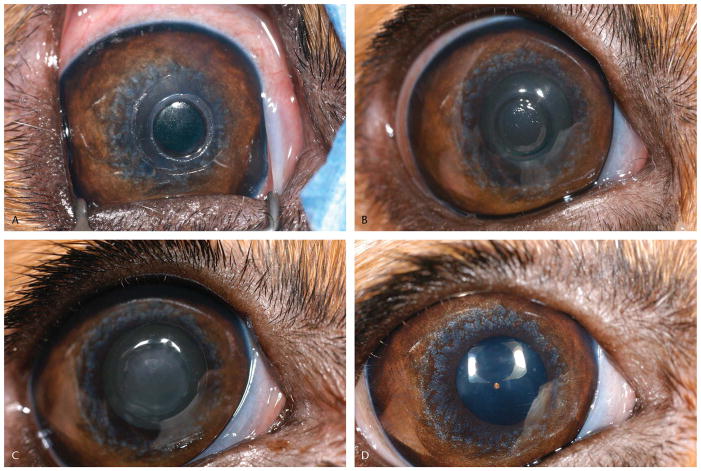
FIGURE 1.
Ocular photographs of dogs after use of a modified epikeratoplasty technique to place biosynthetic implants. Photographs demonstrate typical appearance immediately post-operatively (A), two days (B), 28 days (C), and 112 days (D).
FIGURE 2.
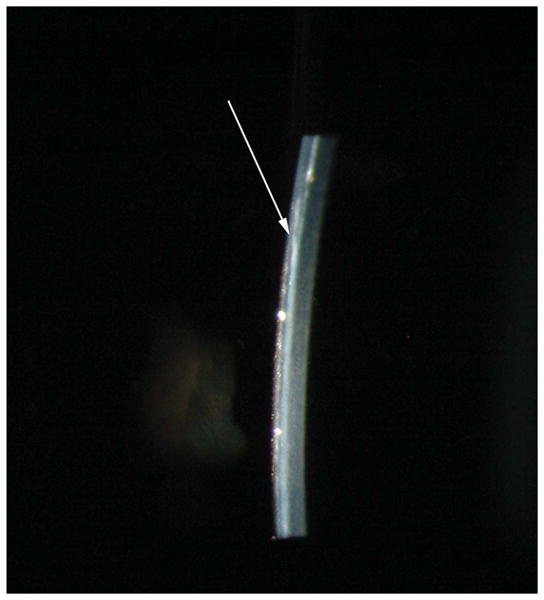
Slit lamp biomicroscopy photograph of a dog 16 weeks after use of modified epikeratoplasty technique to place a biosynthetic implant. Arrow denotes edge of biosynthetic implant.
FIGURE 3.
Graph demonstrating Cochet-Bonnet aesthesiometry values over time after implantation of a biosynthetic implant into the right eye of 3 dogs.
Histopathology revealed a normally differentiated but-slightly thicker epithelium over the implant (Fig. 4). Staining with alcian blue and periodic acid Schiff demonstrated normal staining characteristics of the newly formed basement membrane. Fibroblasts were noted populating the full thickness of all implants. The corneal equivalent seemed to be well integrated into the host cornea. Immunohistochemistry using neurofilament antibody to locate neurites showed the presence of nerves in both operated and unoperated eyes. Neurites extended into the implant zone in the operated eyes, correlating to clinical aesthesiometry findings of the return of touch sensitivity in the operated corneas (Fig. 5).
FIGURE 4.
Photomicrograph of biosynthetic cornea implanted into a normal dog 16 weeks postoperatively. Note the normally differentiated but slightly thicker epithelium over the implant (arrow denotes beginning of step defect, implant is below).
FIGURE 5.
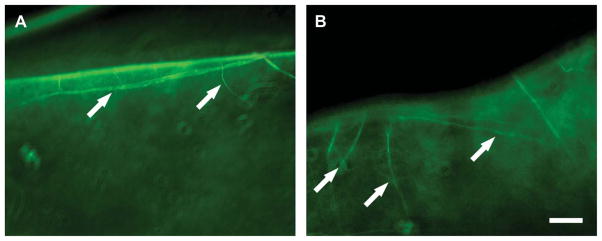
Immunohistochemistry demonstrating presence of neurites extending into a biosynthetic cornea implanted into a canine eye 16 weeks postoperatively. Nerves were seen entering the implant (arrows) similar to the control cornea, although a shorter distance. Scale=50 mm.
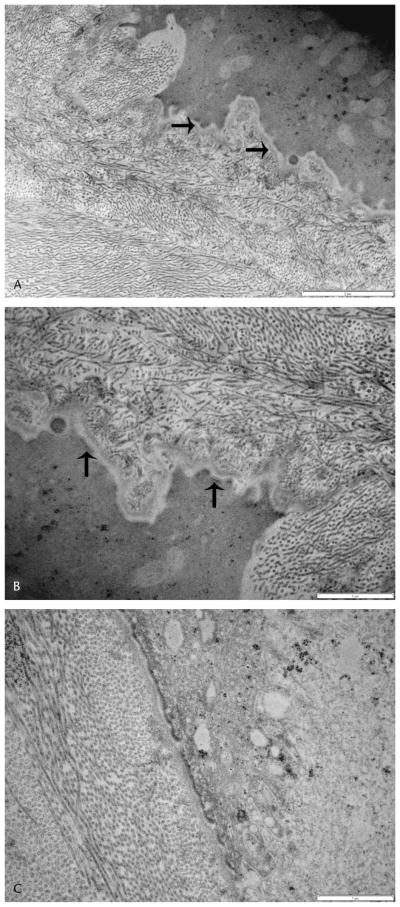
By TEM, the epithelial cells had a similar appearance to the unoperated eyes. The junction of the basal epithelial cells, basement membrane, and underlying implant was more interdigitated than in the unoperated control (Fig. 6). The basement membrane over the implant was present but irregular and appeared thin in places. In some areas, adhesion complexes were seen to be reforming. The implant could be identified by the presence of smaller disorganized collagen fibers. Spindle cells had populated the implant in all samples. The junction between the implant and the surrounding corneal stroma was difficult to identify.
FIGURE 6.
Transmission electron micrographs of a biosynthetic implant in a canine eye 16 weeks postoperatively (A, B). Note the increased interdigitation of the junction of the basal epithelial cells, basement membrane and underlying implant. Collagen fibers in the implant are disorganized compared to the native corneal stroma. Adhesion complexes are being replaced in the basement membrane over the implant (arrows). Normal canine cornea for comparison (C).
DISCUSSION
Biosynthetic corneal implants manufactured from glutaraldehyde cross linked collagen and TERP cross linked were well tolerated by dogs in this study. Although the surgery performed in this study only replaced the superficial portion of the cornea, the relative clarity and the lack of rejection is significant because dogs have a high rejection rate in response to even partial-thickness corneal surgeries.23,24 Even under experimental conditions, most keratoplasties in dogs induce a moderate to severe inflammatory and vascular response and quickly become opaque, in contrast to the very mild haze formation found in this study.25,26 The use of atelocollagen may have resulted in less reaction because it is the telopeptides in collagen that generally result in an inflammatory response.27
Although the implants were not rejected, some haze formation occurred. The exact source of the haze that developed in the implants in this study was not obvious but was likely because of keratocyte/fibroblast migration and myofibroblast differentiation. Remodeling of the implant resulting in deposition of other types of collagen that alter the light scattering properties of the implant and native cornea also likely played a role.28 It is possible over time that the area of the implant would clear further as the activated cells become quiescent.
The exact long-term effects of remodeling of the implant are not known, however, PNIPAAM poly(N-isopropylacrylamide) may be released, but it is considered to be biocompatible and nonbiodegradable. No toxic effects were noted after 4 months in this study or up to year in other animal models.29 As remodeling and degradation of the implant occur, the collagen network may be degraded but the PNIPAAM may be stable enough to remain as a scaffold for healing. Furthermore, recent work suggests that keratocytes migrate into the implant in patients with keratoconus and in patients with central scarring.30
Partial return of touch sensitivity, documented by Cochet–Bonnet aesthesiometry, began by 2 months postoperatively. Return to normal levels, however, did not occur by the end of the 4-month study period. This lack of complete return is consistent with studies in humans in which corneal innervation remains abnormal for over 12 months after lamellar refractive procedures.31,32 Any return of touch sensitivity, however, is novel in a potential artificial corneal implant and may serve to improve function and integration of the implant.
Previous work with this implant in pigs showed greater recovery of touch sensitivity in a shorter period than observed in the current study in dogs.16 However, in the pig study, the corneal substitutes prepared incorporated YIGSR peptide, which is known to promote neurite outgrowth. Omission of the peptide in this study gave a more natural course of reinnervation. Other possible causes for the discrepancy in the regrowth of nerves also exist. For example, in this study, Cochet–Bonnet aesthesiometry was performed only in the center of the implant, rather than centrally and peripherally as in the study in minipigs. Nerve regeneration typically occurs from the periphery of the wound, therefore, touch sensitivity would be expected to recover faster in the peripheral region of the implant.33,34 Second, the thickness of the original incision greatly impacts nerve regrowth. Chang-Ling et al33 demonstrated that recovery of sensitivity occurred faster in incisions that were less than 53% of corneal thickness, as this allowed sparing of corneal stromal nerves, which are the source of neural regeneration. With an estimated resection of 200 μm, approximately one third of the corneal thickness had been removed at surgery in these dogs. If the stromal nerves were truncated, as occurs with deeper stromal incisions, corneal nerve regeneration was impaired. Differences in the depth of the surgical incision between the current study and the previously reported studies may have resulted in the differences in recovery of corneal touch sensitivity, although allograft controls in the previous study in minipigs failed to show similar nerve regeneration. Finally, Cochet–Bonnet aesthesiometry can be difficult to perform and interpret in laboratory animals, leading to variations in technique between laboratories and therefore, variations in results.
Establishment of the safety of this implant will allow dogs with spontaneous ocular surface diseases such as nonhealing erosions, epibulbar melanomas, squamous cell carcinomas, or ulcerative keratitis to receive this implant, which will provide a useful clinical model for future applications in humans. Implants in diseased corneas will likely have very different results than implants placed in normal corneas. Most ocular surface diseases lead to or are a result of a greatly altered corneal environment, with levels of many factors up- or downregulated compared with the normal situation.35 Treatment of dogs with spontaneous disease will provide a much more accurate reflection of the ability of this biosynthetic implant to be integrated into a diseased human cornea and will be a better model for future clinical use than normal laboratory animals or artificially created models of disease.
Although reformation of basement membrane was obvious over the implant, the basement membrane did not have a normal morphology. Basement membrane was thin in appearance and also demonstrated a greater degree of interdigitation over the implant. The native corneal basement membrane of several species36–38 has been shown to possess a rich 3-dimensional nanoscale topography that has been shown to modulate fundamental epithelial cell behaviors such as adhesion, migration, alignment, and focal adhesion formation.39–42 Adding nanoscale topography to the surface of the implant could improve epithelial adhesion and regeneration of the basement membrane.
Future studies will involve an implant that can be sutured for full-thickness corneal replacement in a larger number of dogs. Future implants will be designed to incorporate nanoscale topography to mimic the native corneal basement membrane to improve epithelial regeneration. Implants will be optimized in terms of endothelial cell growth or prevention of endothelial cell growth and will need to be mechanically strong enough to permit suturing for full-thickness corneal defects.43,44 Newer implants based on interpenetrating networks of collagen and phosphorylcholine are much stronger than the implants used in this study, and studies are underway with these suturable implants.30,45 With stronger implants, full-to-partial thickness replacement will be performed in canine clinical patients to assess the performance of this implant in the diseased cornea.
Acknowledgments
Supported by Natural Sciences and Engineering Research of Canada grant STPGP 246418-01. National Eye Institute grant 1R01EY016134-01 to C. J. Murphy.
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Chirila TV. An overview of the development of artificial corneas with porous skirts and the use of PHEMA for such an application. Biomaterials. 2001;22:3311–3317. doi: 10.1016/s0142-9612(01)00168-5. [DOI] [PubMed] [Google Scholar]
- 3.Hicks C, Crawford G, Chirila T, et al. Development and clinical assessment of an artificial cornea. Prog Retin Eye Res. 2000;19:149–170. doi: 10.1016/s1350-9462(99)00013-0. [DOI] [PubMed] [Google Scholar]
- 4.Sutphin J. Indications and contraindications for penetrating keratoplasty. In: Brightbill F, editor. Corneal Surgery: Theory, Technique & Tissue. 3. St Louis, MO: Mosby; 1999. pp. 247–258. [Google Scholar]
- 5.Myung D, Duhamel PE, Cochran JR, et al. Development of hydrogel-based keratoprostheses: a materials perspective. Biotechnol Prog. 2008;24:735–741. doi: 10.1021/bp070476n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew H, Ayres B, Hammersmith M, et al. Boston Keratoprosthesis outcomes and complications. Cornea. 2009;28:989–996. doi: 10.1097/ICO.0b013e3181a186dc. [DOI] [PubMed] [Google Scholar]
- 7.Hicks CR, Crawford GJ, Lou X, et al. Corneal replacement using a synthetic hydrogel cornea, AlphaCor: device, preliminary outcomes and complications. Eye. 2003;17:385–392. doi: 10.1038/sj.eye.6700333. [DOI] [PubMed] [Google Scholar]
- 8.Hicks CR, Crawford GJ, Tan DT, et al. AlphaCor cases: comparative outcomes. Cornea. 2003;22:583–590. doi: 10.1097/00003226-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Hicks CR, Crawford GJ, Tan DT, et al. Outcomes of implantation of an artificial cornea, AlphaCor: effects of prior ocular herpes simplex infection. Cornea. 2002;21:685–690. doi: 10.1097/00003226-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Germain L, Carrier P, Auger FA, et al. Can we produce a human corneal equivalent by tissue engineering? Prog Retin Eye Res. 2000;19:497–527. doi: 10.1016/s1350-9462(00)00005-7. [DOI] [PubMed] [Google Scholar]
- 11.Griffith M, Osborne R, Munger R, et al. Functional human corneal equivalents constructed from cell lines. Science. 1999;286:2169–2172. doi: 10.1126/science.286.5447.2169. [DOI] [PubMed] [Google Scholar]
- 12.Doillon CJ, Watsky MA, Hakim M, et al. A collagen-based scaffold for a tissue engineered human cornea: physical and physiological properties. Int J Artif Organs. 2003;26:764–773. doi: 10.1177/039139880302600810. [DOI] [PubMed] [Google Scholar]
- 13.Griffith M, Hakim M, Shimmura S, et al. Artificial human corneas: scaffolds for transplantation and host regeneration. Cornea. 2002;21:S54–S61. doi: 10.1097/01.ico.0000263120.68768.f8. [DOI] [PubMed] [Google Scholar]
- 14.Suuronen EJ, McLaughlin CR, Stys PK, et al. Functional innervation in tissue engineered models for in vitro study and testing purposes. Toxicol Sci. 2004;82:525–533. doi: 10.1093/toxsci/kfh270. [DOI] [PubMed] [Google Scholar]
- 15.Suuronen EJ, Nakamura M, Watsky MA, et al. Innervated human corneal equivalents as in vitro models for nerve-target cell interactions. FASEB J. 2004;18:170–172. doi: 10.1096/fj.03-0043fje. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Carlsson D, Lohmann C, et al. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc Natl Acad Sci U S A. 2003;100:15346–15351. doi: 10.1073/pnas.2536767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimmura S, Doillon CJ, Griffith M, et al. Collagen-poly(N-isopropylacrylamide)-based membranes for corneal stroma scaffolds. Cornea. 2003;22:S81–S88. doi: 10.1097/00003226-200310001-00012. [DOI] [PubMed] [Google Scholar]
- 18.Gilger B, Bentley E, Ollivier F. Diseases and surgery of the canine cornea and sclera. In: Gelatt K, editor. Veterinary Ophthalmology. 4. Ames, IA: Blackwell Publishing; 2007. pp. 690–752. [Google Scholar]
- 19.Murphy C, Marfurt C, McDermott A, et al. Spontaneous chronic corneal epithelial defects (SCCED) in dogs: clinical features, innervation, and effect of topical SP, with or without IGF-1. Invest Ophthalmol Vis Sci. 2001;42:2252–2261. [PubMed] [Google Scholar]
- 20.Bentley E, Abrams G, Covitz D, et al. Morphology and immunohistochemistry of spontaneous chronic corneal epithelial defects (SCCED) in dogs. Invest Ophthalmol Vis Sci. 2001;42:2262–2269. [PubMed] [Google Scholar]
- 21.Acosta A, Espana E, Stoiber J, et al. Corneal stroma regeneration in felines after supradescemetic keratoprosthesis implantation. Cornea. 2006;25:830–838. doi: 10.1097/01.ico.0000220769.19402.86. [DOI] [PubMed] [Google Scholar]
- 22.Li F, Griffith M, Li Z, et al. Recruitment of multiple cell lines by collagen-synthetic copolymer matrices in corneal regeneration. Biomaterials. 2005;26:3093–3104. doi: 10.1016/j.biomaterials.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 23.Gilger B, Whitley R. Surgery of the cornea and sclera. In: Gelatt K, editor. Veterinary Ophthalmology. 3. Philadelphia, PA: Lippincott Williams and Wilkins; 1999. pp. 675–700. [Google Scholar]
- 24.Wilkie DA, Whittaker C. Surgery of the cornea. Vet Clin North Am Small Anim Pract. 1997;27:1067–1107. doi: 10.1016/s0195-5616(97)50104-5. [DOI] [PubMed] [Google Scholar]
- 25.Dice P, Severin G, Lumb W. Experimental autogenous and homologous corneal transplantation in the dog. J Am Anim Hosp Assoc. 1973;9:245–251. [Google Scholar]
- 26.McEntyre J. Experimental penetrating keratoplasty in the dog. Arch Ophthalmol. 1968;80:372–376. doi: 10.1001/archopht.1968.00980050374017. [DOI] [PubMed] [Google Scholar]
- 27.Lynn A, Yannas I, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res Part B Appl Biomater. 2004;71:343–354. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 28.van de Pol C, Soya K, Hwang DG. Objective assessment of transient corneal haze and its relation to visual performance after photorefractive keratectomy. Am J Ophthalmol. 2001;132:204–210. doi: 10.1016/s0002-9394(01)01003-0. [DOI] [PubMed] [Google Scholar]
- 29.Lageli N, Griffith M, Shinozaki N, et al. Innervation of tissue-engineered corneal implants in a porcine model: a 1-year in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2007;48:3537–3544. doi: 10.1167/iovs.06-1483. [DOI] [PubMed] [Google Scholar]
- 30.Fagerholm P, Lageli N, Carlsson D, et al. Corneal regeneration following implantation of a biomimetic tissue-engineered substitute. Clin Transl Sci. 2009;2:162–164. doi: 10.1111/j.1752-8062.2008.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy PJ, Corbett MC, O’Brart DP, et al. Loss and recovery of corneal sensitivity following photorefractive keratectomy for myopia. J Refract Surg. 1999;15:38–45. doi: 10.3928/1081-597X-19990101-07. [DOI] [PubMed] [Google Scholar]
- 32.Tervo K, Latvala TM, Tervo TM. Recovery of corneal innervation following photorefractive keratoablation. Arch Ophthalmol. 1994;112:1466–1470. doi: 10.1001/archopht.1994.01090230080025. [DOI] [PubMed] [Google Scholar]
- 33.Chang-Ling T, Vannas A, Holden BA, et al. Incision depth affects the recovery of corneal sensitivity and neural regeneration in the cat. Invest Ophthalmol Vis Sci. 1990;31:1533–1541. [PubMed] [Google Scholar]
- 34.Rozsa A, Guss R, Beuerman R. Neural remodeling following experimental surgery of the rabbit cornea. Invest Ophthalmol Vis Sci. 1983;24:1033–1051. [PubMed] [Google Scholar]
- 35.Bentley E, Murphy CJ. Topical therapeutic agents that modulate corneal wound healing. Vet Clin North Am Small Anim Pract. 2004;34:623–638. doi: 10.1016/j.cvsm.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Abrams G, Bentley E, Nealey P, et al. Nanoscale topography of the corneal epithelial basement membrane and Descemet’s membrane of the canine. Cells Tissues Organs. 2002;170:251–257. doi: 10.1159/000047929. [DOI] [PubMed] [Google Scholar]
- 37.Abrams G, Goodman S, Nealey P, et al. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 2000;299:39–46. doi: 10.1007/s004419900074. [DOI] [PubMed] [Google Scholar]
- 38.Abrams GA, Schaus SS, Goodman SL, et al. Nanoscale topography of the corneal epithelial basement membrane and Descemet’s membrane of the human. Cornea. 2000;19:57–64. doi: 10.1097/00003226-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Abrams G, Foley J, Nealey P, et al. Differential ativation of the small GTPase, Rho, in corneal epithelial cells plated on nanopatterned and smooth substrates[ARVO abstract] Invest Ophthalmol Vis Sci. 2003;44:nr1343. [Google Scholar]
- 40.Diehl K, Foley J, Zhang G, et al. Nanoscale topography modulates corneal epithelial cell migration[ARVO abstract] Invest Ophthalmol Vis Sci. 2003;44:nr3276. [Google Scholar]
- 41.Karuri NW, Liliensiek S, Teixeira AI, et al. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J Cell Sci. 2004;117:3153–3164. doi: 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teixeira AI, Abrams GA, Bertics PJ, et al. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J Cell Sci. 2003;116:1881–1892. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mclaughlin C, Tsai R, Latorre M, et al. Bioengineered corneas for transplantation and in vitro toxicology. Front Biosci. 2009;14:3326–3337. doi: 10.2741/3455. [DOI] [PubMed] [Google Scholar]
- 44.Rafat M, Matsura T, Li F, et al. Surface based modification of artificial cornea for reduced endothelization. J Biomed Mater Res. 2009;88:755–768. doi: 10.1002/jbm.a.31910. [DOI] [PubMed] [Google Scholar]
- 45.Liu W, Deng C, McLaughlin C, et al. Collagen-phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials. 2009;30:1551–1559. doi: 10.1016/j.biomaterials.2008.11.022. [DOI] [PubMed] [Google Scholar]