Abstract
Pregnancy establishment and maintenance represents a challenge for the maternal immune system because it has to be alert against pathogens while tolerating paternal alloantigens expressed in fetal structures. Regulatory T cells (Tregs) are important for successful implantation and involved in allotolerance towards paternal antigens. The origin and mechanisms leading to Treg generation during pregnancy at different stages remain under discussion. We report an accumulation of Helios+ Tregs in thymus and in the lymph nodes draining the uterus at early pregnancy. At later pregnancy stages an expanded population of Foxp3+ Tregs was generated in the periphery as we showed in a Rag-1-/- model of cell transfer. Our data suggest that Tregs, predominantly of thymic origin, are needed for pregnancy establishment. At later pregnancy stages an extra thymic Treg population contributes to the Treg pool in the periphery. Our data provides new insights in the origin of Tregs during pregnancy that are essential to understand natural mechanisms of tolerance acquisition.
Keywords: Regulatory T cells, thymic Tregs, peripheral Tregs, pregnancy
Introduction
Maternal and fetal CD4+CD25+Foxp3+ regulatory T cells (Tregs) have been reported to contribute to the acquisition and maintenance of tolerance during pregnancy by suppressing maternal alloreactive immune responses against paternal structures in fetal cells [1,2]. The levels of this unique T cell subpopulation in pregnant individuals increase immediately after insemination in several lymphoid organs as well as directly at the fetal-maternal interface as shown in human and mouse [1,3,4]. While it is clear that Tregs positively influence the pregnancy outcome, it is not clear which origin they have and the precise mechanisms underlying their generation.
There are contradictory data regarding the participation of antigen-dependent and independent mechanisms for Treg expansion during pregnancy [5-7]. Aluvihare and colleagues [8] showed that the increase in the number of CD4+CD25+ T cells in syngeneically mated female mice is comparable to that of allogeneically mated females. Due to the strong hormonal changes taking place during pregnancy the authors suggested a hormone-driven effect on Treg expansion. This suggestion is supported by another study in mice showing that physiological doses of estrogen mediate Treg expansion as well as the conversion of naïve T cells in Tregs [9]. Furthermore, Arruvito et al. [10] also described oscillations in the Treg population during the human menstrual cycle that were attributed to hormonal changes. Contradicting these data, Zhao and colleagues demonstrated that neither estrogen nor progesterone alone or in combination had an influence on the number of Tregs in ovariectomized mice [11]. They further provided evidence that Treg levels are elevated in allogeneic matings compared to syngeneic matings supporting the assumption that Treg expansion during pregnancy is rather caused by alloantigens than by hormones [11]. Additional work from Tilburgs et al. [12] strengthens this hypothesis by suggesting that human decidual T cells specifically recognize fetal HLA-C at the fetal-maternal interface following by an increased percentage of CD4(+)CD25(dim) activated T cells in decidual tissue.
We observed that the number of Tregs increased in lymph nodes of pregnant females mated with intact males and in pseudopregnant females that were mated with vasectomized male mice. No Treg expansion was observed however after pseudo-pregnancy was induced mechanically [13]. Thus, hormonal changes induced by pseudopregnancy in the absence of paternal antigens were not strong enough to induce changes in the Treg population. Paternal-derived antigens could be detected at early pregnancy stages not only in vaginal lumen from pregnant female mice but also in several lymphoid organs and other organs such as the lungs [14,15]. Thus, it is clear that paternally-derived antigens present in fetal structures can be processed by maternal APCs [15] and prone a specific Treg population which would then allow the presence of foreign antigens without immune rejection by the maternal immune system. Recent papers with mouse models broaden this concept and show that Tregs are important for preventing rejection of male fetuses [7] and that peripheral converted Tregs are necessary to prevent fetal death due to allogenicity [16]. It was also suggested that pregnancy imprints Tregs capable of sustaining protective regulatory memory to fetal antigens [17]. The nature of the antigens responsible for the generation of a specific tolerant immune response is a subject of debate. It is possible that the implanting blastocyst shed some cellular structures upon nidation. However, previous studies report Treg expansion at earlier time points (e.g. day 2.5) [13] and a protective effect of Tregs is achieved only when they are transferred immediately after pregnancy onset [1,18]. Additionally, it has been suggested that depletion of Tregs by using a CD25 antibody only impairs pregnancy if applied at periimplantation period, having no effect once implantation occurred [1,19]. Guerin and colleagues [20] showed that seminal fluid regulates the accumulation of Foxp3+ Tregs in the mouse uterus previous to implantation. Recently, we were able to show that Tregs are important even before mating. Their absence is associated with a hostile uterine microenvironment and impaired implantation rate in both syngeneic and allogeneic pairings [21]. Similarly, abnormally low Foxp3 levels were found in endometrium of infertile women compared to fertile women [22]. In the light of these contradictory reports it is very important to clarify where and at which time points of pregnancy Tregs are generated as well as to understand if they are thymus-derived or converted in the periphery upon pregnancy-induced changes.
Material and methods
Mice
Foxp3GFP mice [23] in C57BL/6 background were originally provided by Jocelyne Demengeot (Oeiras, Portugal) upon MT agreement with Alexander Rudensky. This strain was bred and maintained at the Animal Facilities of the Medical Faculty, Otto-von-Guericke University, Magdeburg. C57BL/6 and BALB/c males as well as BALB/c females were purchased from Charles River, Germany. Rag-1-/- mice were purchased from Jackson laboratory.
All animals were housed in a barrier facility in a 12-h light/dark cycle and received food and water ad libitum. The animals experiments were performed according to the principles expressed in the Declaration of Helsinki. The experiments were previously authorized by the local authorities (AZ 42502-2-868, Landesverwaltungsamt Sachsen-Anhalt, Halle and 0070/03 LaGeSo Berlin) and followed the guidelines of Use of Agricultural Animals in Agricultural Research and Teaching, USA. Exact number of animals used for each experiment is stated in the Figure Legends.
After mating, females were checked daily for vaginal plugs. The day at which the vaginal plug was detected was considered day 0 of pregnancy. Animals were sacrificed at different pregnancy days as indicated in results and figure legends by cervical dislocation under anaesthesia.
Antibodies and flow cytometry
The following rat anti-mouse monoclonal antibodies were used; FITC- and Alexa Fluor 647-conjugated anti-CD4 (Clones RM4-4 and RM4-5 respectively; BD Pharmingen), PE-conjugated anti-Foxp3 (Clone NRRF-30; eBioscience), APC-conjugated anti-Helios (Clone 22F6; BioLegend).
For flow cytometry analysis, blood was acquired by cardiac puncture. Uterine or decidual samples were cut in small pieces and collected in Hanks’ balanced salt solution free of Ca2+ or Mg2+ (Sigma, Taufkirchen, Germany). Spleen, thymus and lymph nodes were collected in RPMI medium. Samples were processed as previously described [1] and stained with the antibodies listed above. Foxp3 and Helios intracellular staining was performed by using the eBioscience staining set. Acquisition was performed using a FACSCalibur (BD Biosciences) and the analysis was done with CellQuest Pro software (BD Biosciences).
Adoptive cell transfer
According with previous work from Sun et al. [24], cell suspensions of total lymph nodes from either Foxp3GFP or congenic C57BL/6 mice were enriched for CD4+ T cells by a negative selection kit (Miltenyi Biotec). CD4+Foxp3GFP T cells or congenic CD4+ T cells were further labelled with rat anti mouse Alexa Fluor 647-conjugated anti-CD4 and PE-conjugated anti-CD25 monoclonal antibodies and sorted by flow cytometry using a Diva cell sorter or FACSAria (BD Biosciences). The purity of the population of eGFP- cells after sorting was of 99.1% (Figure S1). An amount of 3×105 CD4+eGFP- purified cells from the Foxp3GFP mice and 1×105 CD4+CD25+ purified cells from the congenic C57BL/6 mice were transferred intravenously into Rag-1-/- mice. The possible conversion of CD4+GFP- cells into CD4+eGFP+ cells was assessed 4-16 weeks later by flow cytometric detection of GFP.
1 mg of anti TGFβ mAb (clone 1D11 kindly provided by Prof Hideo Yagita, Japan) or Rat IgG (Sigma Aldrich) was injected subcutaneously to the reconstituted animals on days 0 and 7 of pregnancy. For the experiments comprising thymus-derived cells, total thymocytes from Foxp3GFP animals were transferred intravenously into Rag-1-/- mice.
Statistical analysis
The normality of the distribution was assessed by using the D’Agostino-Pearson omnibus test. Statistical analyses were performed by using Prism software (GraphPad) and the tests used for each experiment are detailed in the Figure legends of each graph.
Results
Thymic-derived Foxp3+ cells is the dominant Treg population before implantation
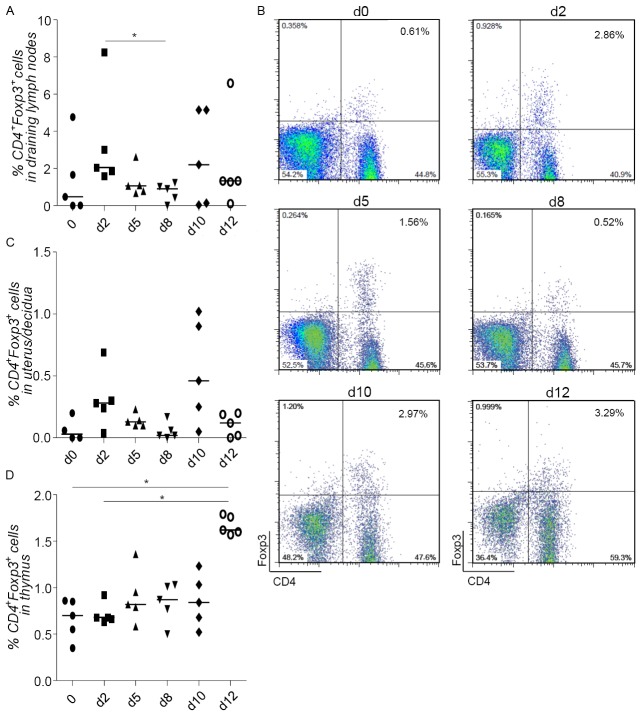
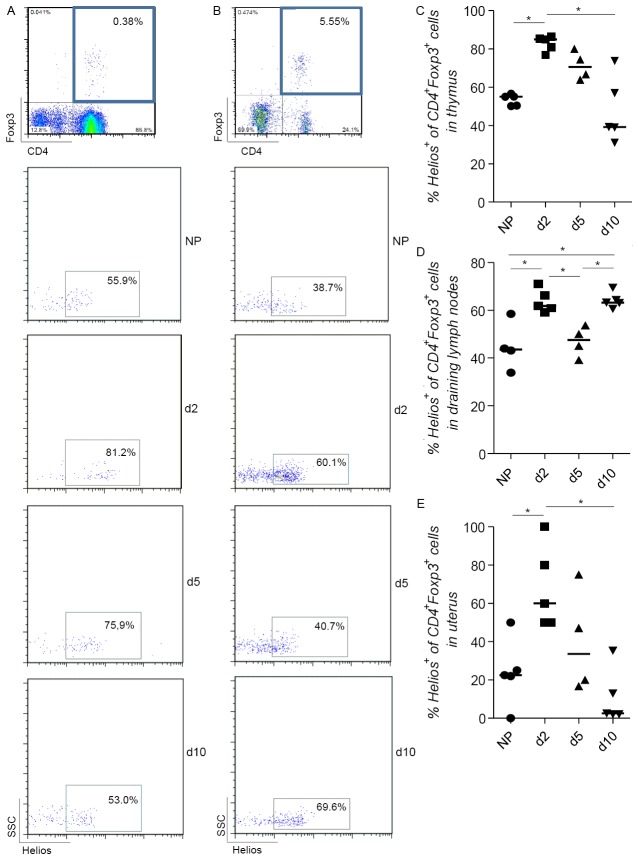
We first confirmed that the frequency of CD4+Foxp3+ Tregs oscillates during allogeneic pregnancy (BALB/c-mated C57BL/6 females). We observed an initial augmentation in the proportion of double positive CD4+Foxp3+ cells in the lymph nodes draining the uterus as early as day 2 of pregnancy (Figure 1A and 1B). This trend was also observed in the pregnant uterus (Figure 1C). In the thymus this augmentation was not as evident (Figure 1D). The frequency of Tregs diminished at implantation and was again augmented at day 10 of pregnancy (Figure 1A-C). To understand the origin of these cells, we took advantage of new marker called Helios that was previously reported to detect Tregs of thymic origin in mice [25]. We therefore analyzed the expression of Helios within the CD4+Foxp3+ population in thymus, uterine draining lymph nodes and uterus and found that this was elevated at day 2 of pregnancy and diminished at day 5 (Figure 2A-E).
Figure 1.
Treg kinetics shows local oscillatory changes during pregnancy. BALB/c females were mated with C57/BL6 males and the population of CD4+Foxp3+ Treg was analyzed in the draining lymph nodes (A, B), uterus (C), and thymus (D) on days 0, 2, 5, 8, 10 and 12 of pregnancy (d0, d2, d5, d8, d10 and d12) (n=5). B shows representative dot plots of the analyzed frequency of CD4+Foxp3+ cells in draining lymph nodes from one animal representative of each group. Data are expressed as single dots with medians and were analyzed by using the one way ANOVA, Kruskal-Wallis test, followed by a Dunn’s multiple comparison test (*: P ≤0.05).
Figure 2.
Thymic and peripheral origin of the population of CD4+Foxp3+ cells. The percentage of Helios+ of CD4+Foxp3+ cells was analyzed in the thymus (A and B), uterine draining lymph nodes (C and D) and uterus (E) of both non pregnant (NP) C57/B6 females and BALB/c mated C57/B6 females on days 2, 5 and 10 of pregnancy (n=4-5). B and C show representative dot plots of one individual mouse of each group as how we analyzed the frequency of CD4+Foxp3+Helios+ cells in thymus and draining lymph nodes. Data are expressed as single dots with medians and were analyzed by using the one way ANOVA, Kruskal-Wallis test, followed by a Dunn’s multiple comparison test (*: P ≤0.05).
Foxp3+ cells convert from Foxp3- cells after implantation
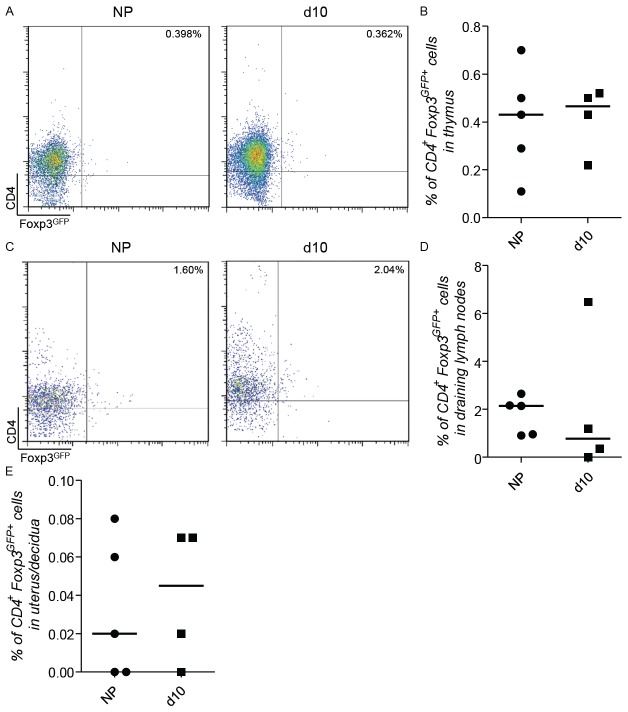
Implantation is finished at day 5 of pregnancy. At this day, we observed that total and Helios+ Tregs begin to decline in frequency in the draining lymph nodes (Figures 1A, 1B, 2B and 2D) and uterus (Figures 1C and 2E). Additionally, there was a second augmentation in the frequency of total Tregs in the draining lymph nodes and at the feto-maternal interface and at day 10 of pregnancy (Figure 1A-C). Additionally, there was a second augmentation in the frequency of total Tregs in the draining lymph nodes and at the feto-maternal interface and at day 10 of pregnancy (Figure 1A and 1C). Because of the low Helios expression within the Foxp3+ population at the feto-maternal interface (Figure 2B), we speculated that the contribution of thymic Tregs to the Treg population is minimal. To confirm this, we transferred thymocytes from 8 days-pregnant Foxp3GFP mice into Rag-1-/- animals at the same day of pregnancy and traced their localization at day 10 of pregnancy. We were not able to find any differences in the frequency of GFP+ cells between pregnant and non-pregnant recipients (Figure 3A-E). Thus, we speculate that at this later pregnancy stage no thymic Treg reach the uterus.
Figure 3.
After reconstitution of Rag-1-/- mice with thymocytes from Foxp3GFP mice Tregs presented the same migratory phenotype in both non-pregnant and mice at day 10 of pregnancy. Thymocytes isolated from 8 days pregnant Foxp3GFP mice were transferred into Rag-1-/- animals at the same day of pregnancy and their localization was traced on day 10 (d10) of pregnancy. As control, thymocytes isolated from non-pregnant Foxp3GFP mice were transferred into non pregnant (NP) Rag-1-/- animals and traced two days after transference. CD4+ Foxp3GFP+ cells were analyzed in thymus (A and B), uterine draining lymph nodes (C and D) and uterus/decidua (E) (n=4-5 animals per group) A and C show representative dot plots of the analyzed frequency of CD4+Foxp3GFP+ cells from one animal representative of each group. Data are expressed as single dots with medians and were analyzed by using the Mann-Whitney-U test.
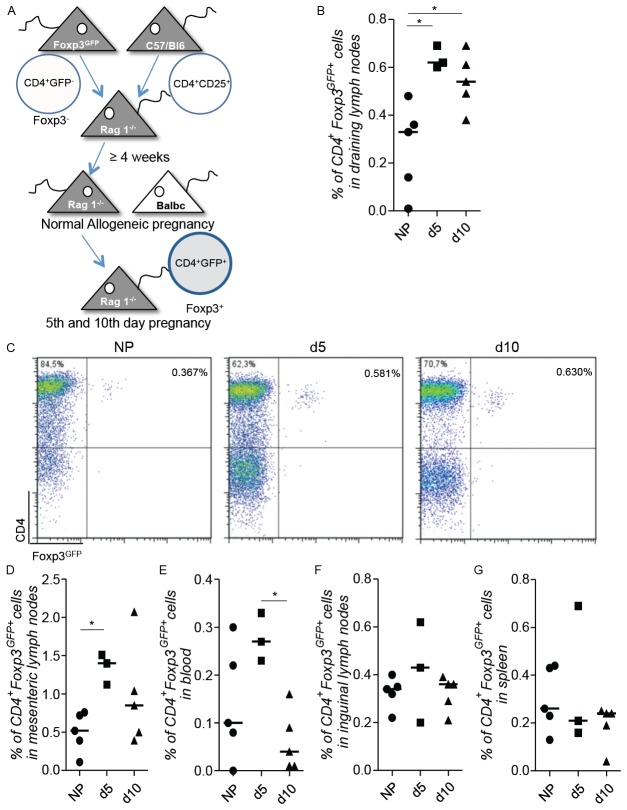
To test whether a peripheral conversion of Treg takes place, we took advantage of a model where the conversion of Foxp3- cells into Foxp3+ cells can be visualized by the appearance of GFP expression [23,24]. We reconstituted Rag-1-/- mice with CD4+Foxp3- cells from Foxp3GFP mice and added CD4+CD25+ cells from wild type mice for avoiding autoimmune colitis [21,26] (Figure 4A). The animals were paired 4 weeks after reconstitution. We confirmed the existence of a Foxp3GFP+ population in the draining lymph nodes at both days 5 and 10 of pregnancy (Figure 4B and 4C). Conversion from Foxp3- into Foxp3+ cells was significantly higher in pregnant than in non-pregnant animals. Thus, at implantation and later, Tregs are originated in the periphery from Foxp3- cells. Pregnancy-induced Foxp3+ cells were detected in draining lymph nodes and also in the mesenteric lymph nodes and blood but not in other lymph nodes or the spleen (Figure 4D-G). To test whether TGF-β was implied in the conversion of Foxp3+ from Foxp3- cells as it is in other models [27,28], we administered anti-TGFβ to the pregnant animals. Treg conversion was not significantly affected by the application of TGF-β (Figure S2).
Figure 4.
An expansion/de novo conversion of Tregs occurs in peripheral organs at days 5 and 10 of pregnancy. Rag-1-/- female mice were transferred with CD4+Foxp3GFP- cells from Foxp3GFP mice and CD4+CD25+ cells from normal C57/BL6 WT mice were added to avoid autoimmunity. After allowing reconstitution for a period of 4 weeks, the animals were paired with BALB/c males and possible conversion of CD4+Foxp3- into CD4-Foxp3+ cells was traced by de novo expression of CD4+Foxp3GFP Tregs in samples of reconstituted mice at days 5 (d5) and 10 (d10) of pregnancy (A) in the draining lymph nodes (B and C), mesenteric lymph nodes (D), blood (E), inguinal lymph nodes (F) and spleen (G) (n=3-5). Non-pregnant reconstituted animals were used as a control. C shows representative dot plots of the analyzed frequency of CD4+Foxp3GFP+ cells in draining lymph nodes from one animal representative of each group. Data are expressed as single dots with medians and were analyzed by using the one way ANOVA, Kruskal-Wallis test, followed by a Dunn’s multiple comparison test (*: P <0.05).
Expansion of Foxp3+ cells is due to the presence of paternal alloantigens
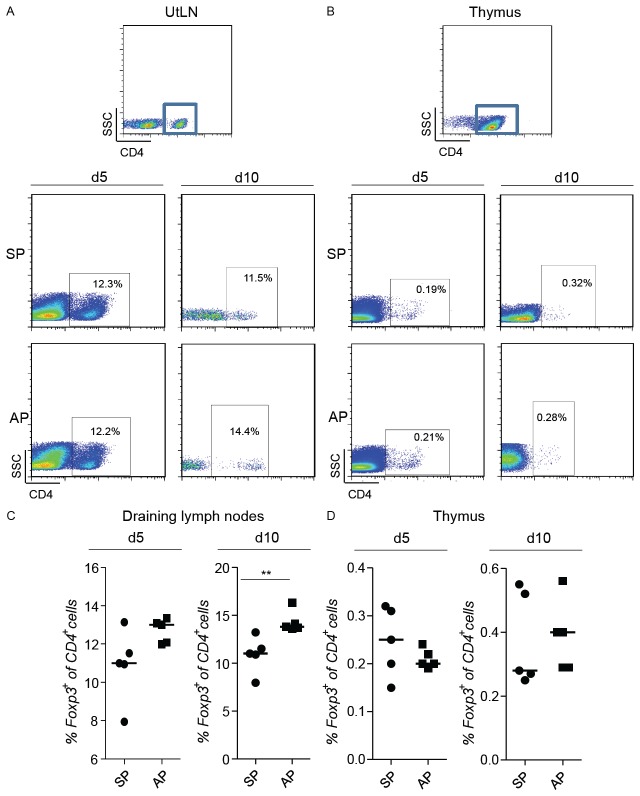
To determine whether paternal alloantigens are responsible for the expansion of Treg during pregnancy, we next analyzed the frequency of CD4+Foxp3+ cells (measured as Foxp3+ cells within the population of CD4+ cells) in organs of allogeneic versus syngeneic pregnant females at days 5 and 10 of pregnancy (Figure 5A-D). In both, elevated Treg frequency was observed compared to non-pregnant individuals. At day 10, this raise was statistically significant. Allogeneically mated animals presented however elevated Treg numbers when compared to females that were mated syngeneically (Figure 5A and 5C). This suggests an alloantigen-driven generation of Foxp3+ cells induced by the presence of paternal antigens at this time point of pregnancy.
Figure 5.
The population of Tregs expands differently in allogeneic and syngeneic pregnancies. Foxp3GFP mice were paired with C57/BL6 or BALB/c males and the expression of Foxp3GFP+ was analyzed in the CD4+ cell population in the uterine draining lymph nodes (A and C) and thymus (B and D) on day 5 of pregnancy (d5). The same procedure was done with C57/BL6 females and the population of CD4+Foxp3+ cells was analyzed on day 10 of pregnancy (d10) (A-D). (n=5) A and B shows representative dot plots of the analyzed frequency of Foxp3+ in the gated population of CD4+ cells from one animal representative of each group. Data are expressed as single dots with medians and were analyzed by using the Mann-Whitney-U test (**: P ≤0.01).
Discussion
Several groups highlighted the importance of Treg for pregnancy. It is known that they expand upon pregnancy establishment [13,18,29]. Their transfer can protect from immunological rejection of allogeneic fetuses [1,30]. Depleting Treg in female mice provoked the generation of a hostile uterine environment and hindered implantation [21]. Shima et al. [19] reported that depletion of CD25+ cells around implantation impaired pregnancy while depletion at later time points did not compromise pregnancy. There are reports however that Tregs may also be needed at later time points [16]. Saito et al. [31] recently suggested to clearly identifying the type of Treg necessary at each time point of pregnancy to better understand their role in pregnancy and implantation. Here, we investigated the frequency of total Tregs during murine pregnancy and whether Tregs derive from the thymus or are converted at the periphery.
The frequency of total Tregs oscillates during pregnancy, peaking as early as day 2 of pregnancy and decreasing shortly after implantation (day 5). We have recently reported that the frequency of Treg oscillates before pregnancy occurs, namely during the oestrus cycle of female mice, peaking at the receptive phase, the estrus, in the vaginal lumen and uterus [21]. We have recently reported that the frequency of Treg oscillates before pregnancy occurs, namely during the oestrus cycle of female mice, peaking at the receptive phase, the estrus in vaginal lumen and the uterus [21]. We interpreted this as a preparation for nidation, which is further supported by our observations in vivo by 2-photon microscopy of the uterus; Treg accumulate at defined areas in the mesometrial region of the uterus [21]. These Treg are likely originated from the thymus and migrate to the uterus, being CCR7 and hormones important for this [13,21]. As Treg augmented at day 2 of pregnancy we speculated that the augmentation in the total Treg population will owe to the expansion of thymic Treg already expanded and present at critical sites as the uterus and the draining lymph nodes [13,21]. The discrimination between thymic derived and peripherally converted Tregs is a difficult issue. Thornton and colleagues [25] recently proposed a marker called Helios for identifying thymic-derived Tregs. Based on the expression of Helios on Tregs we could define the origin of these cells. Most of the Tregs at day 2 of pregnancy were Helios+ indicating that thymic derived Tregs are the main Treg population present in pregnant mice at the pre-implantation period. It is to remark that the frequency of Helios+ Tregs in both thymus and draining lymph nodes of the non-pregnant controls were smaller than the populations observed by Thornton et al. [25], whose data show that approximately 100% of thymic and 70% of peripheral Foxp3+ Tregs were Helios+. Thus, our results point to a thymic origin of Tregs and their migration to the uterus previous to implantation. This population, which remains augmented upon pregnancy onset, declines quite rapidly around implantation, at day 5 of pregnancy. The decrease in the Tregs population at this time point can be explained through the adaptation changes needed to allow angiogenesis to occur, which is per se similar to an inflammatory process [32,33]. This phenomenon was accompanied by a decrease in the population of Helios+ Tregs suggesting that Tregs at this stage of pregnancy are not exclusively of thymic origin and many of them originate as well in the periphery. Tregs of peripheral origin can thus replace Treg of thymic origin once pregnancy is established. A recent report [34] describes an enhanced suppressive potential of Foxp3+Helios+ in comparison with Foxp3+Hellos- cells associated with the expression of CD103 and GITR. In addition, another study shows that both populations differ in terms of their capacity to produce cytokines and the stability of their Foxp3 expression in vitro [35]. This suggests that the differences we observed in the expression of Helios in the Treg population might also mirror different suppressive potentials and stability of Tregs at different time points of pregnancy. We have however not tested this.
In the last years it has become clear that besides naturally occurring Tregs, other cells possess suppressive capacities and can overtake the role of naturally occurring Tregs, e.g. de novo converted Tregs. To address a possible conversion of Tregs in the periphery during pregnancy, we transferred CD4+Foxp3- cells from Foxp3GFP animals into Rag-1-/- animals, lacking mature B and T cells. With this model, putative Foxp3+ cells converted from Foxp3- cells would express GFP and be detectable by flow cytometry. We observed a population of Foxp3+ cells that was augmented at days 5 and 10 of pregnancy in allogeneic pregnancies as compared to virgin females. It is tempting to speculate that this population results from a peripheral conversion from Foxp3- into Foxp3+ cells; however we can not discard the possibility of an expansion of a residual population of Foxp3GFP+ cells that constituted 0.86% of the sorted population of eGFP- that was transferred into the Rag-1-/- mice and that could expand during the 4 weeks before mating the animals. As a significant difference was observed regarding the non-pregnant controls, we assume that allogeneic pregnancy drove a conversion of Foxp3+ cells from Foxp3- cells so to protect the fetus from a possible attack as paternal antigens are present in the maternal body [15]. In a recently published paper, Samstein could elegantly show that peripheral converted Treg and not thymic derived Treg are responsible for the avoidance of some fetal resorption while the importance of peripheral converted Tregs or thymic derived Tregs for implantation was not studied in the mentioned study [16]. This reinforces our hypothesis of a peripheral conversion at days 5 and 10. Whether this is relevant for pregnancy maintenance is questionable as these authors only observed around 10% of abortion in the absence of converted Tregs while 90% of the fetuses were viable [16]. Unlike in other models [24] the blockage of TGF-β did not exert any effect on the de novo conversion of Tregs on the uterine draining lymph nodes during pregnancy suggesting that it occurs independently of this molecule.
By comparing the proportion of Foxp3+ of CD4+ cells in allogeneically vs syngeneically pregnant mice we could show that the presence of alloantigens drives a bigger expansion of Treg in the uterine draining lymph nodes of allogeneic vs syngeneic pregnancies.
In summary, we could show that the frequency of Treg augments upon pregnancy establishment and oscillates during gestation. We observed that before implantation Treg are mainly of thymic origin and after implantation a peripheral expansion and/or conversion from Foxp3- into Foxp3+ cells takes place, which may be of importance for avoiding undesired immune reactions against the fetuses. Our data reveals unknown and useful information about Treg kinetic and importance for pregnancy that is very relevant for human pregnancies, especially when designing protocols for improving fertility.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, Volk HD. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811–822. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal Alloantigens Promote the Development of Tolerogenic Fetal Regulatory T Cells in Utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mjosberg J, Svensson J, Johansson E, Hellstrom L, Casas R, Jenmalm MC, Boij R, Matthiesen L, Jönsson JI, Berg G, Ernerudh J. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J Immunol. 2009;183:759–769. doi: 10.4049/jimmunol.0803654. [DOI] [PubMed] [Google Scholar]
- 5.Darrasse-Jèze G, Darasse-Jèze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102:106–109. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA, Claas FH. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180:5737–5745. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- 7.Kahn DA, Baltimore D. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A. 2010;107:9299–9304. doi: 10.1073/pnas.1003909107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 9.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, Wang B. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 10.Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+ and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572–2578. doi: 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Zeng Y, Liu Y. Fetal alloantigen is responsible for the expansion of the CD4+CD25+ regulatory T cell pool during pregnancy. J Reprod Immunol. 2007;75:71–81. doi: 10.1016/j.jri.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 12.Tilburgs T, Scherjon SA, van der Mast BJ, Haasnoot GW, Versteeg-V D Voort-Maarschalk M, Roelen DL, van Rood JJ, Claas FH. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol. 2009;82:148–157. doi: 10.1016/j.jri.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Thuere C, Zenclussen ML, Schumacher A, Langwisch S, Schulte-Wrede U, Teles A, Paeschke S, Volk HD, Zenclussen AC. Kinetics of Regulatory T Cells During Murine Pregnancy. Am J Reprod Immunol. 2007;58:514–523. doi: 10.1111/j.1600-0897.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 14.Khosrotehrani K, Johnson KL, Guégan S, Stroh H, Bianchi DW. Natural history of fetal cell microchimerism during and following murine pregnancy. J Reprod Immunol. 2005;66:1–12. doi: 10.1016/j.jri.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Zenclussen ML, Thuere C, Ahmad N, Wafula PO, Fest S, Teles A, Leber A, Casalis PA, Bechmann I, Priller J, Volk HD, Zenclussen AC. The Persistence of Paternal Antigens in the Maternal Body is Involved in Regulatory T-Cell Expansion and Fetal-Maternal Tolerance in Murine Pregnancy. Am J Reprod Immunol. 2010;63:200–208. doi: 10.1111/j.1600-0897.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 16.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacher A, Wafula PO, Bertoja AZ, Sollwedel A, Sollwedel A, Thuere C, Wollenberg I, Yagita H, Volk HD, Zenclussen AC. Mechanisms of action of regulatory T cells specific for paternal antigens during pregnancy. Obstet Gynecol. 2007;110:1137–1145. doi: 10.1097/01.AOG.0000284625.10175.31. [DOI] [PubMed] [Google Scholar]
- 19.Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J Reprod Immunol. 2010;85:121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19-mediated recruitment. Biol Reprod. 2011;85:397–408. doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- 21.Teles A, Schumacher A, Kühnle MC, Linzke N, Thuere C, Reichardt P, Tadokoro CE, Hämmerling GJ, Zenclussen AC. Control of uterine microenvironment by Foxp3+ cells facilitates embryo implantation. Front Immunol. 2013;4:158. doi: 10.3389/fimmu.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the regulatory T cell transcription factor FOXP3 in endometrial tissue. Mol Hum Reprod. 2006;12:301–308. doi: 10.1093/molehr/gal032. [DOI] [PubMed] [Google Scholar]
- 23.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Sun C, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3+ T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Express ion of Helios, an Ikaros Transcription Factor Family Member, Differentiates Thymic-Derived from Peripherally Induced Foxp3+ T Regulatory Cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denning TL, Kim G, Kronenberg M. Cutting Edge: CD4+CD25+ Regulatory T Cells Impaired for Intestinal Homing Can Prevent Colitis. J Immunol. 2005;174:7487–7491. doi: 10.4049/jimmunol.174.12.7487. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille J. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173:7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 29.Chen T, Darrasse-Jèze G, Bergot AS, Courau T, Churlaud G, Valdivia K, Strominger JL, Ruocco MG, Chaouat G, Klatzmann D. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol. 2013;191:2273–81. doi: 10.4049/jimmunol.1202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Y, Han X, Shi Q, Zhao Y, He Y. Adoptive transfer of CD4+CD25+ regulatory T cells for prevention and treatment of spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2012;161:177–81. doi: 10.1016/j.ejogrb.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Saito S, Shima T, Inada K, Nakashima A. Which types of regulatory T cells play important roles in implantation and pregnancy maintenance? Am J Reprod Immunol. 2013;69:340–5. doi: 10.1111/aji.12101. [DOI] [PubMed] [Google Scholar]
- 32.Sherer DM, Abulafia O. Angiogenesis during implantation, and placental and early embryonic development. Placenta. 2001;22:1–13. doi: 10.1053/plac.2000.0588. [DOI] [PubMed] [Google Scholar]
- 33.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 34.Zabransky DJ, Nirschl CJ, Durham NM, Park BV, Ceccato CM, Bruno TC, Tam AJ, Getnet D, Drake CG. Phenotypic and functional properties of Helios+ regulatory T cells. PLoS One. 2012;7:e34547. doi: 10.1371/journal.pone.0034547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YC, Bhairavabhotla R, Yoon J, Golding A, Thornton AM, Tran DQ, Shevach EM. Oligodeoxynucleotides stabilize Helios-expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood. 2012;22:2810–8. doi: 10.1182/blood-2011-09-377895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.