Mammalian blood has numerous essential and well-known functions, including oxygen and nutrient delivery. This elixir is recognized by blood-feeding species of mosquitos, ticks, fleas, lice, leeches, and bats that rely on blood meals for nutrition, life cycle progression and survival. To obtain these blood meals that require minutes to a week or longer to complete1, these bloodsucking creatures must thwart endogenous defense systems contained within blood—immune and procoagulant cells and plasma proteins that rapidly clot (within 3–4 minutes) to provide first-line defense against breaches in vascular integrity. In a fascinating display of evolutionary agility, hemovores have adapted elegant mechanisms to evade detection and prevent blood coagulation by synthesizing an extensive armament of molecules with anesthetic, immunosuppressive, vasodilatory, anticoagulant, and profibrinolytic properties in mammals.1–3 Research characterizing the molecules generated by hemovores to bypass mammalian defense pathways has revealed exciting new mechanisms and in some cases, novel therapeutic approaches for anticoagulation.
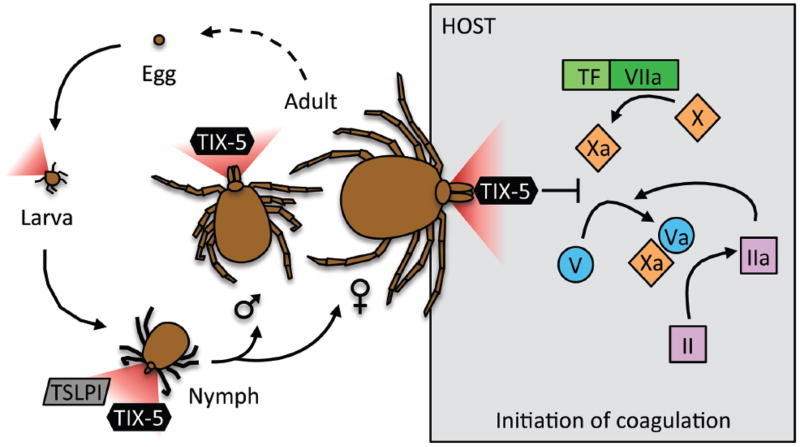
In particular, ticks have received considerable attention for their remarkable evolutionary adaptations to life as obligate hemovores. Briefly, the typical tick lifecycle includes four stages: egg, six-legged larva, eight-legged nymph, and adult (Figure 1), and takes 1–3 years to complete this full cycle. Ticks must consume blood at the larval, nymph and adult stages to survive, and die if they do not find a host. Interestingly, while neither larva nor nymphs have overt sexual differentiation, adults are fully differentiated into males and females, and compared to males, longer nymph feeding is required for expression of female characteristics.4 Consequently, tick saliva contains multiple proteins that maintain blood fluidity to enable feeding, and therefore tick survival.
Figure 1.
Lifecycle of a typical tick. Larvae, nymphs, and adults require blood meals for survival, and secrete anti-complement (Tick Salivary Lectin Pathway Inhibitor, TSLPI) and anticoagulant (Tick Inhibitor of factor Xa towards factor V, TIX-5) proteins to accomplish the blood meal. Schuijt et al.7 have now described the function of TIX-5 as an inhibitor of factor Xa-mediated factor V activation during the initiation of coagulation.
Using yeast surface display, Schuijt and colleagues5 previously identified several novel tick salivary proteins that promote feeding. Characterization of these proteins revealed anti-complement (P8, later termed Tick Salivary Lectin Pathway Inhibitor [TSLPI])6 and anticoagulant (P23) activities. Interestingly, when rabbits were immunized against a cocktail containing these recombinant proteins, nymph feeding was reduced, and this reduction had fascinating consequences. Compared to tick nymphs fed on control rabbits, nymphs fed on rabbits immunized against tick salivary proteins were significantly smaller. Further, this reduction in weight had a profound effect on the sexual maturation of ticks into adults. Schuijt et al6 observed that nymphs that reached 3.4 mg or greater molted into female adults; whereas, nymphs 3.3 mg and smaller molted into male adults.6 Notably, the smaller “male” nymphs were composed of two distinct populations, prompting speculation that the larger of these populations were “failed females” unable to reach sexual maturation. These data suggest that altering feeding in a way that even subtly decreases mean nymph size could profoundly alter adult sex ratios and decrease tick numbers in subsequent generations. These findings suggest anti-complement and anti-coagulant proteins in tick saliva are potential vaccine candidates for reducing tick populations, as well as reducing transmission of tick-borne illnesses.
In the current issue of Circulation, Schuijt et al7 have now extended their work by characterizing the biological target of the anticoagulant protein P23 (now termed Tick Inhibitor of factor Xa towards factor V [TIX-5]), revealing additional features in this already intriguing story. Recombinant TIX-5 (rTIX-5) delays thrombin generation in human plasma in which clotting is initiated via intrinsic or extrinsic activators, and in factor VIII- or factor XI-deficient plasmas, suggesting its molecular target lies within the common pathway. Accordingly, rTIX-5 is unable to inhibit thrombin generation in the presence of pre-activated factor V (FV), indicating rTIX-5 inhibits clotting by delaying FV activation. Unexpectedly, however, the inhibitory effects of rTIX-5 on FV activation were not observed in reactions triggered by thrombin or meizothrombin, the hypothesized activators of FV. Rather, rTIX-5 inhibits factor Xa/phospholipid-mediated generation of factor Va (FVa). Schuijt et al7 further interrogated the nature of this mechanism and showed that rTIX-5 does not inhibit factor Xa substrate cleavage or even bind directly to factor Xa, but instead specifically blocks factor Xa-mediated activation of FV in a FV B-domain-dependent fashion. Thus, this study has not only identified a novel anticoagulant protein in ticks, but importantly, the identification of TIX-5’s biological target reveals the physiological importance of a pathway not previously described during mammalian coagulation in vivo.
This work has important implications for several hot topics in coagulation research. First, the identification of the molecular target of TIX-5 sheds light on a long-standing question regarding the origin of FVa during the initiation of coagulation. Although several proteases including α-thrombin8, meizothrombin9, calpain10, plasmin11, elastase and cathepsin G12, and factor Xa13 can cleave FV to generate the active cofactor (FVa) in purified systems, the primary activator of FV during the initiation of coagulation has remained elusive. This activity has primarily been attributed to trace amounts of meizothrombin and/or α-thrombin generated by the extrinsic activation of factor Xa. Since rTIX-5 specifically delays factor Xa-, but not thrombin- or meizothrombin-mediated activation of FV, this protein provides a unique tool to study the role of factor Xa-mediated activation of FV during coagulation. Schuijt et al7 show that rTIX-5 prolongs the lag time to thrombin generation in both human and rabbit plasma, as well as in human whole blood. Importantly, Schuijt et al show that when rabbits are immunized with rTIX-5, post-feeding weights of adult ticks are significantly reduced compared to controls, indicating that inhibiting TIX-5 prevents the natural anticoagulation mechanism needed for optimal feeding. This simple, but elegant, assay shows for the first time, the relevance of this pathway during coagulation in vivo.
Second, the identification of rTIX-5 yields a valuable new tool to characterize the molecular mechanisms that maintain the procofactor state of circulating FV and the conversion of FV to active cofactor FVa. During coagulation, proteolytic removal of the large central B-domain of FV eliminates steric constraints provided by the B-domain that block factor V(a) activity; however, the nature of the steric inhibition mechanism has been elusive because FV(a) does not require proteolysis to acquire its activity.14 Bos and Camire15 recently identified two evolutionarily-conserved sequences in the B-domain, one acidic and one basic, that define minimal sequence requirements for FV’s autoinhibitory function. The ability of rTIX-5 to inhibit FV molecules with mutations in the cleavage sites, but not inhibit B-domain-deleted FV, demonstrates that TIX-5 does not simply block proteolysis, but instead interferes with this B-domain-dependent inhibitory mechanism. Notably, rTIX-5 can bind to both the basic and acidic regions of the FV B-domain, as well as phospholipids, and the inhibitory activity of rTIX-5 is supported by the presence of both the B-domain acidic and basic regions. These data suggest rTIX-5 forms a complex with the FV B-domain and the phospholipid surface that blocks the accessibility of factor Xa to FV. Further, since factor Xa activates the FV QIQQQ variant that lacks all specific factor Xa activation sites, other arginine residues in the B-domain or heavy and light chains must also support partial activation of FV. Future studies to model these interactions on the molecular level promise critical information regarding the role of the B-domain in mediating the procofactor to cofactor transition during FV activation, and potentially, a means to modulate this transition and control coagulation.
Third, it is interesting that ticks utilize both anti-complement and anti-coagulant strategies to facilitate feeding and maturation over their life cycles. Coagulation and complement pathways are both ancient serine protease defense mechanisms, components of which have been in existence since the divergence of lamprey eels from jawed vertebrates over 600 million years ago, and several studies have linked these pathways in modern physiology. For example, factor XIIa can activate complement factor C1r16, a subcomponent of C1 which initiates the classical complement pathway, and both thrombin and factor Xa can activate complement factors C3 and C5, members of the common complement pathway17, 18. The lectin pathway, which is inhibited by the tick salivary protein TSLPI6, has been shown to promote prothrombin activation (reviewed in 19). These observations suggest multiple levels of cross-talk between these systems, such that ticks must inhibit these pathways at several points during certain life stages to accomplish their blood meal. Indeed, nymphs can inhibit either coagulation or complement to obtain a blood meal and mature to adult size5; whereas, adults must block the coagulation pathway for sufficient feeding (Figure 1). The use of these and other tick salivary proteins may therefore yield additional information about cross-talk between complement and coagulation, with important implications for both thrombotic and inflammatory disorders.
Identification of TIX-5 anticoagulant activity and procoagulant pathway gives rise to exciting questions regarding the role of this pathway in coagulation and future studies to evaluate its potential as a therapeutic target. First, in contrast to ticks fed on TIX-5-immunized rabbits, the mean weight of ticks fed on TIX-5-immunized mice is not reduced20, suggesting mice do not make antibodies against TIX-5, or that the coagulation pathway inhibited by TIX-5 is less important in mice than in humans or rabbits. Since mice are a primary go-to model for human coagulation studies, these data suggest caution in the interpretation of murine studies evaluating initiating events in coagulation. Second, recent studies have shown that in contrast to platelets, when prothrombinase is assembled on the surface of erythrocytes, thrombin generation proceeds via the meizothrombin intermediate21, indicating that procoagulant pathways are determined by both plasma protein composition and the nature of the cell surface. The observation that rTIX-5 can bind directly to phospholipids suggests the composition of the cell surface could also influence TIX-5’s ability to block factor Xa activation of FV. It will be interesting to determine the role of the factor Xa/FV pathway on different cells and under different initiating circumstances.
Finally, and perhaps most attractive, by demonstrating the physiological relevance of factor Xa-mediated activation of FV in vivo and the ability of rTIX-5 to inhibit this reaction, this work has yielded a potential new antithrombotic approach. Both hemovores and clinicians share a goal with regard to anticoagulation – the need to reduce clotting in a highly-controlled way that does not cause excessive bleeding. Indeed, anticoagulant mechanisms developed by nature have previously been exploited for the development of other drugs, including hirudin and analogs from leeches and defibrinating enzymes from snakes.3 In this case, ticks have identified and inhibited a procoagulant pathway that researchers had not yet characterized in vivo. Can we once again exploit nature’s efforts to regulate coagulation in the clinic? This exciting possibility warrants studies to test the antithrombotic potential of TIX-5 and similar molecules in “traditional” thrombosis models that have shown clinical relevance during the development of other antithrombotic drugs. Since the inhibitory effect of rTIX-5 is reduced in plasmas with reduced anticoagulant levels, targeting this pathway may be less effective in certain types of coagulopathies stemming from deficiency in anticoagulant pathways (e.g. protein C deficiency). It will be critical to explicitly test the ability of TIX-5 and similar molecules to prevent thrombosis in specific models of plasma hypercoagulability and vascular dysfunction in future studies.
Acknowledgments
Funding Sources: This work was partially supported by National Institutes of Health grants R01HL094740 (to ASW) and F31HL112608 (to MMA).
This is a commentary on article Schuijt TJ, Bakhtiari K, Daffre S, Deponte K, Wielders SJ, Marquart JA, Hovius JW, van der Poll T, Fikrig E, Bunce MW, Camire RM, Nicolaes GA, Meijers JC, van 't Veer C. Factor Xa activation of factor V is of paramount importance in initiating the coagulation system: lessons from a tick salivary protein. Circulation. 2013 July 16;128(3):254-66.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Mans BJ, Neitz AW. Adaptation of ticks to a blood-feeding environment: Evolution from a functional perspective. Insect Biochem Mol Biol. 2004;34:1–17. doi: 10.1016/j.ibmb.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Jones D. The neglected saliva: Medically important toxins in the saliva of human lice. Parasitology. 1998;116 (Suppl):S73–81. doi: 10.1017/s0031182000084961. [DOI] [PubMed] [Google Scholar]
- 3.Koh CY, Kini RM. Molecular diversity of anticoagulants from haematophagous animals. Thromb Haemost. 2009;102:437–453. doi: 10.1160/TH09-04-0221. [DOI] [PubMed] [Google Scholar]
- 4.Dusbabek F. Nymphal sexual dimorphism in the sheep tick ixodes ricinus (acari: Ixodidae) Folia Parasitol. 1996;43:75–79. [PubMed] [Google Scholar]
- 5.Schuijt TJ, Narasimhan S, Daffre S, DePonte K, Hovius JW, Van’t Veer C, van der Poll T, Bakhtiari K, Meijers JC, Boder ET, van Dam AP, Fikrig E. Identification and characterization of ixodes scapularis antigens that elicit tick immunity using yeast surface display. PLoS One. 2011;6:e15926. doi: 10.1371/journal.pone.0015926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuijt TJ, Coumou J, Narasimhan S, Dai J, Deponte K, Wouters D, Brouwer M, Oei A, Roelofs JJ, van Dam AP, van der Poll T, Van’t Veer C, Hovius JW, Fikrig E. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell Host Microbe. 2011;10:136–146. doi: 10.1016/j.chom.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuijt TJ, Bakhtiari K, Daffre S, DePonte K, Wielders SJH, Marquart JA, Hovius JW, van der Poll T, Fikrig E, Bunce MW, Camire RM, Nicolaes GA, Meijers JC, van’t Veer C. Factor xa activation of factor v is of paramount importance in initiating the coagulation system: Lessons from a tick salivary protein. Circulation. 2013;128:XX–XXX. doi: 10.1161/CIRCULATIONAHA.113.003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nesheim ME, Mann KG. Thrombin-catalyzed activation of single chain bovine factor V. J Biol Chem. 1979;254:1326–1334. [PubMed] [Google Scholar]
- 9.Tans G, Nicolaes GA, Thomassen MC, Hemker HC, van Zonneveld AJ, Pannekoek H, Rosing J. Activation of human factor v by meizothrombin. J Biol Chem. 1994;269:15969–15972. [PubMed] [Google Scholar]
- 10.Bradford HN, Annamalai A, Doshi K, Colman RW. Factor v is activated and cleaved by platelet calpain: Comparison with thrombin proteolysis. Blood. 1988;71:388–394. [PubMed] [Google Scholar]
- 11.Lee CD, Mann KG. Activation/inactivation of human factor v by plasmin. Blood. 1989;73:185–190. [PubMed] [Google Scholar]
- 12.Allen DH, Tracy PB. Human coagulation factor v is activated to the functional cofactor by elastase and cathepsin g expressed at the monocyte surface. J Biol Chem. 1995;270:1408–1415. doi: 10.1074/jbc.270.3.1408. [DOI] [PubMed] [Google Scholar]
- 13.Foster WB, Nesheim ME, Mann KG. The factor xa-catalyzed activation of factor v. J Biol Chem. 1983;258:13970–13977. [PubMed] [Google Scholar]
- 14.Toso R, Camire RM. Removal of b-domain sequences from factor v rather than specific proteolysis underlies the mechanism by which cofactor function is realized. J Biol Chem. 2004;279:21643–21650. doi: 10.1074/jbc.M402107200. [DOI] [PubMed] [Google Scholar]
- 15.Bos MH, Camire RM. A bipartite autoinhibitory region within the b-domain suppresses function in factor v. J Biol Chem. 2012;287:26342–26351. doi: 10.1074/jbc.M112.377168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghebrehiwet B, Silverberg M, Kaplan AP. Activation of the classical pathway of complement by hageman factor fragment. J Exp Med. 1981;153:665–676. doi: 10.1084/jem.153.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of c5a in the absence of c3: A new complement activation pathway. Nat Med. 2006;12:682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 18.Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Bruckner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: A key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wikel SK, Ramachandra RN, Bergman DK, Burkot TR, Piesman J. Infestation with pathogen-free nymphs of the tick ixodes scapularis induces host resistance to transmission of borrelia burgdorferi by ticks. Infect Immun. 1997;65:335–338. doi: 10.1128/iai.65.1.335-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whelihan MF, Zachary V, Orfeo T, Mann KG. Prothrombin activation in blood coagulation: The erythrocyte contribution to thrombin generation. Blood. 2012;120:3837–3845. doi: 10.1182/blood-2012-05-427856. [DOI] [PMC free article] [PubMed] [Google Scholar]