Abstract
Background
Conventional therapy with β-blockers is incompletely effective in preventing arrhythmic events in patients with catecholaminergic polymorphic ventricular tachycardia (CPVT). We have previously discovered that flecainide in addition to conventional drug therapy prevents ventricular arrhythmias in genotype-positive CPVT patients.
Objective
To study the efficacy of flecainide in genotype-negative CPVT patients.
Methods
We studied the efficacy of flecainide for reducing ventricular arrhythmias during exercise testing and preventing arrhythmia events during long-term follow-up.
Results
Twelve genotype-negative CPVT patients were treated with flecainide. Conventional therapy failed to control ventricular arrhythmias in all patients. Flecainide was initiated because of significant ventricular arrhythmias (n=8), syncope (n=3), or cardiac arrest (n=1). At the baseline exercise test before flecainide, 6 patients had ventricular tachycardia and 5 patients had bigeminal or frequent ventricular premature beats. Flecainide reduced ventricular arrhythmias at the exercise test in 8 patients compared to conventional therapy, similarly to genotype-positive patients in our previous report. Notably, flecainide completely prevented ventricular arrhythmias in 7 of the patients. Flecainide was continued in all patients except for one who had ventricular tachycardia at the exercise test on flecainide. During a follow-up of 48±94 months, arrhythmia events (sudden cardiac death and aborted cardiac arrest) associated with noncompliance occurred in two patients. Flecainide was not discontinued due to side effects in any of the patients.
Conclusion
Flecainide was effective in genotype-negative CPVT patients, suggesting that spontaneous Ca2+ release from ryanodine channels plays a role in arrhythmia susceptibility, similarly to genotype-positive patients.
Keywords: catecholaminergic polymorphic ventricular tachycardia, arrhythmia, sudden death, genetics, antiarrhythmic drugs, flecainide, beta-blockers
Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited arrhythmia syndrome characterized by bidirectional or polymorphic VT induced by adrenergic stress in the absence of structural heart disease.1-3 Three causative genes have been identified for CPVT: RYR2, which encodes the cardiac ryanodine receptor Ca2+ release channel, CASQ2, which encodes cardiac calsequestrin, and TRDN, which encodes triadin, and all of them are constitutive proteins of the macromolecular Ca2+ release complex in the sarcoplasmic reticulum.4-7 A mutation in these genes is identified in approximately 60-70% of patients with CPVT.2, 8 A locus on chromosome 7p14-p22 has also been linked to CPVT, but a corresponding gene has not been identified yet.9 Furthermore, mutations in KCNJ2 encoding the potassium inwardly-rectifying channel Kir2.1, which generally are associated with Andersen-Tawil syndrome, may phenocopy CPVT.10
Treatment with β-adrenergic blockers reduces ventricular arrhythmia burden and mortality in patients with CPVT. However, the efficacy of β-blockers is not sufficiently protective and an estimated 8-year rate of fatal or near-fatal events on β-blocker therapy is 15%.2, 3 Although the beneficial effects of the calcium channel blocker verapamil in combination with a β-blocker have been reported,11-14 the role of verapamil has not been well assessed.2 Left cardiac sympathetic denervation has been reported to be highly effective in severely affected patients, but requires surgery and is not universally available.15 Implantable cardioverter defibrillators (ICDs) are recommended for prevention of sudden death in patients with CPVT.16 However, painful shocks can trigger further adrenergic stress and arrhythmias, and deaths have occurred despite appropriate ICD shocks.17, 18 We have recently discovered that the antiarrhythmic agent flecainide inhibits Ca2+ release from ryanodine receptor19 and that flecainide in addition to conventional drug therapy prevents ventricular arrhythmias in CPVT patients carrying a mutation in RYR2 or CASQ2.3 Here, we studied the efficacy and safety of flecainide in CPVT patients with no mutations in RYR2, CASQ2 and KCNJ2, who have similar risk of arrhythmia events to genotype-positive patients.2
Methods
Study population
This study included all consecutive genotype-negative CPVT patients in whom flecainide was started because of the insufficient efficacy of conventional therapy with β-blockers ± verapamil at 5 tertiary referral centers in the Netherlands, the United Kingdom, Israel, Japan, and Germany. All patients had a clinical diagnosis of CPVT based on adrenergic stress-induced bidirectional or polymorphic VT in the absence of structural heart abnormalities by echocardiography.20 All families received genetic counseling and all investigated individuals consented to both cardiologic evaluation and genetic testing. Patients were screened for a putative pathogenic mutation in all exons of RYR2, CASQ2, and KCNJ2. Only individuals who were not carrying proven or putative pathogenic mutations in all of these genes were included. Decisions on administration of flecainide and its dose were made by the local cardiologists. Data collection and analysis were done retrospectively by chart review. Patients in whom the dose of β-blocker or verapamil was increased after the initiation of flecainide were not included in this study.
Outcome measures
The efficacy of flecainide on CPVT was compared between the last exercise test on conventional therapy and the first exercise test on a stable dose of flecainide after ≥5 days of the initiation. Exercise testing was performed using treadmill (standard or modified Bruce protocols) or bicycle ergometer depending on the institutions. Ventricular arrhythmias during exercise testing were quantified using the ventricular arrhythmia score defined by the worst ventricular arrhythmia observed: 1=no or isolated ventricular premature beats (VPBs), 2=bigeminal VPBs and/or >10 VPBs per minute, 3=couplet, 4=NSVT.20 In addition, the presence of either of the parameters of the ventricular arrhythmia score, sinus rate at the onset of ventricular ectopy, most often an isolated VPB, the maximum number of VPBs during a 10-seconds period, and the ratio of VPBs/sinus beats during the 10-seconds period with the maximum number of VPBs were analyzed. The effects of flecainide on arrhythmic events including syncope, aborted cardiac arrest, appropriate ICD shocks, and sudden cardiac death during follow-up were also assessed.
Data analysis
Values are expressed as mean±SD. To study the effects of flecainide during exercise testing, related data were compared using paired Wilcoxon signed-rank test for continuous and ordinal variables and McNemar test for dichotomous variables. All statistical analyses were performed with SPSS, version 20.0 (SPSS Inc., Chicago, IL). A two-sided P <0.05 was considered statistically significant.
Results
Patient characteristics
We identified 12 genotype-negative CPVT patients who received flecainide (Table 1). Mean age at baseline was 22±11 years and 6 patients were female. Our cohort included 7 patients with a family history of CPVT (6 probands and 1 family member). Nine patients had a history of syncope and/or cardiac arrest. In patient #6, a common variant of unknown significance (VUS), p.Val507Ile, was identified in RYR2. Patient #11 tested heterozygote positive for two new VUS in CASQ2: c.158G>T (p.Cys53Phe) and c.838+3A>G. The c.158G>T VUS was once detected in 13000 control alleles, whereas the c.838+3A>G may affect splicing at the donorsite of exon 8 and was not detected in 13000 control alleles. Before the initiation of flecainide, β-blockers failed to suppress ventricular arrhythmias in all patients. In 7 patients, multiple drugs including β-blockers, verapamil, and other antiarrhythmic drugs failed to suppress ventricular arrhythmias before flecainide was initiated.
Table 1. Characteristics of genotype-negative CPVT patients who received flecainide.
| Patient No. | Sex | Age at onset, years | Family history | Negative gene | Presenting symptom | Cardiac arrest | Age at baseline, years | Drug therapy at baseline exercise test, mg (mg/kg body weight) | Indication for flecainide | Flecainide dose, mg (mg/kg body weight) | Baseline exercise test | Effects of flecainide | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||||
| Sudden death | CPVT | Exercise test | Follow-up, months | Arrhythmia events | Side effects* | |||||||||||
| 1 | M | 7 | No | No | RYR2, CASQ2, KCNJ2 | Syncope | Yes | 15 | Metoprolol 200 (3.4) | Cardiac arrest | 200 (3.4) | Bigeminal VPBs | Bigeminal VPBs | 328 | Yes† | No |
| 2 | F | 12 | No | Yes | RYR2, CASQ2, KCNJ2 | Syncope | Yes | 31 | Metoprolol 400 (4.7), Verapamil 240 (2.8) | Bigeminal/frequent VPBs | 200 (2.4) | Bigeminal VPBs | No arrhythmia | 41 | No | No |
| 3 | M | 6 | No | Yes | RYR2, CASQ2, KCNJ2 | Syncope | No | 9 | Propranolol 160 (2.3), Verapamil 120 (1.7) | Syncope | 150 (2.1) | No arrhythmia | Isolated VPBs | 41 | No | No |
| 4 | F | 8 | Yes | Yes | RYR2, CASQ2, KCNJ2 | Syncope | No | 15 | Propranolol 120 (3.0), Verapamil 120 (3.0) | VT | 100 (2.5) | Bigeminal VPBs | No arrhythmia | 41 | No | No |
| 5 | F | 15 | No | No | RYR2, CASQ2, KCNJ2 | Cardiac arrest | Yes | 35 | Metoprolol 100 (2.0) | VT | 100 (2.0) | VT | No arrhythmia | 34 | No | No |
| 6 | M | 10 | Yes | Yes | RYR2, CASQ2, KCNJ2 | Syncope | No | 21 | Nadolol 120 (2.1) | Syncope | 150 (2.7) | VT | Bigeminal VPBs | 4 | Yes† | No |
| 7 | F | 40 | Yes | Yes | RYR2, CASQ2, KCNJ2 | Palpitation | No | 43 | Nadolol 20 (0.2)‡ | VT | 200 (2.0) | VT | VT | 18 | No | No |
| 8 | M | 11 | Yes | Yes | RYR2, CASQ2, KCNJ2 | None (positive family history) | No | 20 | Propranolol 80 (1.8) | VT during exercise testing | 150 (3.3) | VT | No arrhythmia | 6 | No | No |
| 9 | M | 16 | No | No | RYR2, CASQ2, KCNJ2 | Cardiac arrest | No | 19 | Bisoprolol 10 (0.13) | VT | 100 (1.3) | Bigeminal VPBs | No arrhythmia | 8 | No | No |
| 10 | F | 10 | No | Yes | RYR2, CASQ2, KCNJ2 | Syncope | No | 15 | Nadolol 120 (2.1), Bisopolol 10 (0.18) | VT during exercise testing | 200 (3.6) | VT | VT | Discontinued after exercise testing | ||
| 11 | M | 9 | No | No | RYR2, CASQ2, KCNJ2 | Syncope | No | 11 | Nadolol 80 (2.3) | Syncope | 225 (6.5) | Couplet VPBs | No arrhythmia | 5 | No | No |
| 12 | F | 39 | No | No | RYR2, CASQ2, KCNJ2 | Palpitation | No | 39 | Bisoprolol 5 (0.07) | VT | 200 (2.9) | VT | No arrhythmia | 7 | No | No |
|
| ||||||||||||||||
| Total | M: 6 | 15±11 | Yes: 4 (33%) | Yes: 7 (58%) | RYR2: 12 (100%), CASQ2: 12 (100%), KCNJ2: 12 (100%) | Symptoms: 11 (92%) | Yes: 3 (25%) | 22±11 | β-blocker: 12 (100%) Verapamil: 3 (25%) | VT: 8 (67%) | 165±46 (2.9±1.3) | VT: 6 (50%) | VT: 2 (17%) | 48±94 | Yes: 2 (17%) | Yes: 0 (0%) |
CPVT denotes catecholaminergic polymorphic ventricular tachycardia; VPB, ventricular premature beat.
Side effects requiring discontinuation of flecainide.
Episode occured after noncompliance of flecainide.
The patient could not tolerate larger dose.
Effects of flecainide on exercise-induced ventricular arrhythmias
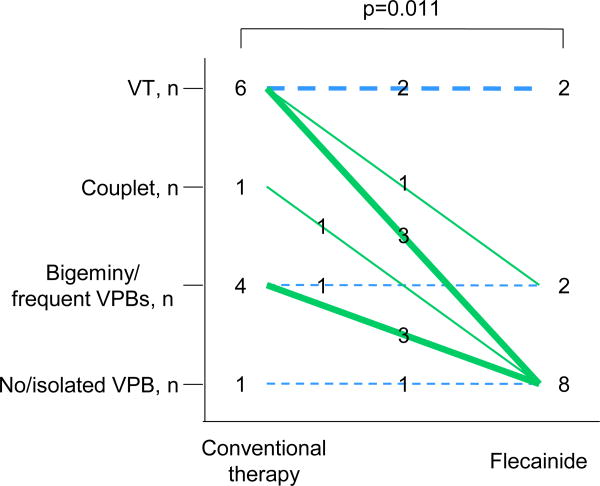
Among genotype-negative patients, 6 patients (50%) had VT and 5 patients (42%) had couplet or bigeminal VPBs at the baseline exercise testing before flecainide administration (Tables 1 and 2). In a remaining patient who had syncope during a combination therapy with propranolol and verapamil (patient #3), there was no ventricular arrhythmia during the baseline exercise testing. Flecainide improved the ventricular arrhythmia score at exercise testing in 8 patients (67%) compared to that during conventional therapy (Figure), while the maximum workload during flecainide therapy was increased compared to that during conventional drug therapy (Table 2). The effects of flecainide seemed similar to that in genotype-positive patients in our previously report (76%).20 Flecainide completely suppressed ventricular arrhythmias in 7 patients (58%) and suppressed VT in 4 of the 6 patients who developed VT during the baseline testing. Compared to the baseline exercise test, flecainide reduced the maximum number of VPBs during a 10-seconds period. In one patient (patient #10), flecainide was discontinued because of VT during exercise testing.
Table 2. Effects of flecainide on ventricular arrhythmias during exercise testing.
| Standard therapy | Flecainide | P-value | |
|---|---|---|---|
| Sinus rate at baseline, beats/min | 68±14 | 72±28 | 0.82 |
| Sinus rate at maximal exercise, beats/min | 148±26 | 136±23 | 0.33 |
| Maximum workload attained, METs | 9±3 | 11±3 | 0.19 |
| Sinus rate at onset of ventricular arrhythmias, beats/min | 114±34 | 117±23 | 0.50 |
| Maximum no. of VPBs during a 10 seconds | 12±7 | 6±7 | 0.02 |
| Ratio of VPBs to sinus beats during 10 seconds with the maximum no. of VPBs | 1.5±2.6 | 0.5±0.6 | 0.21 |
| Isolated VPB | 11 (92) | 6 (50) | 0.03 |
| Bigeminal VPBs | 10 (83) | 5 (42) | 0.03 |
| Frequent VPBs (>10/min) | 9 (75) | 5 (42) | 0.10 |
| Couplet | 8 (67) | 3 (25) | 0.10 |
| Nonsustained ventricular tachycardia | 6 (50) | 2 (17) | 0.10 |
| Longest ventricular salvo, VPBs | 9.5 (3-16) | 5 (4-6) | 0.13 |
| Bidirectional NSVT | 4 (33) | 1 (8) | 0.08 |
Values are mean±SD or number (%).
Figure.
Effects of flecainide on exercise-induced ventricular arrhythmias. Ventricular arrhythmias during exercise testing were compared between conventional therapy and flecainide in genotype-negative patients. Green line indicates suppression of ventricular arrhythmias by flecainide; blue line, no change. VT denotes ventricular tachycardia; VPB, ventricular premature beat.
Effects of flecainide on arrhythmia events
A total of 11 patients (92%) continued to receive flecainide and were included in the further analysis of the incidence of arrhythmic events. During a mean follow-up of 48±94 months, arrhythmia events associated with noncompliance occurred in 2 of 11 genotype-negative patients. Patients #6 died suddenly while playing soccer. Although flecainide combined with nadolol suppressed ventricular tachycardias during exercise testing, the patient self-discontinued the drug therapy after his last visit to the clinic. Patient #1 collapsed and was resuscitated by his relatives. The patient had not taken flecainide from the night before the collapse, and the serum level of flecainide was low (0.13 mg/L; therapeutic range, 0.4-0.9 mg/L) at the event. Metoprolol and flecainide were resumed at the previous doses, and no event occurred thereafter. In the remaining 9 patients, there was no arrhythmia event during the follow-up period, while 7 of the 9 patients had arrhythmia events despite conventional drug therapy before the initiation of flecainide. Flecainide was not discontinued due to side effect in any of the patients.
Discussion
In this study, flecainide suppressed ventricular arrhythmias during exercise testing in genotype-negative patients similar to genotype-positive CPVT patients. Flecainide was highly effective in preventing arrhythmia events during a long-term follow-up.
CPVT has been associated with mutations in RYR2, CASQ2, and TRDN.4-7 Mutations in these genes destabilize the ryanodine channel complex in the sarcoplasmic reticulum and result in spontaneous Ca2+ release through the ryanodine channel leading to delayed after depolarizations, triggered activity, and VT.21-24 Recently, we and others have identified the therapeutic effects of flecainide in CPVT.19, 25, 26 Flecainide directly inhibits the ryanodine channel and suppresses delayed afterdepolarizations and triggered activity in mutant cardiomyocytes in which Ryr2 or Casq2 loci are modified.19, 26, 27 In our mouse model of CPVT, flecainide prevents spontaneous VT and inducible VT by exercise or isoproterenol.19 Yet, we have recently discovered that flecainide prevents CPVT in patients carrying a mutation in RYR2 or CASQ2, possibly resulting in spontaneous Ca2+ release.19, 20 In this study, flecainide was also effective in genotype-negative CPVT patients, similarly in genotype-positive patients in our prior study.20 Notably, all arrhythmia events during flecainide therapy were associated with noncompliance in our present and prior studies.20
The efficacy of flecainide in genotype-negative CPVT patients suggests that uncontrolled Ca2+ release through the ryanodine channel is also important as the underlying mechanisms in patients without an identified mutation in RYR2 or CASQ2, that account for 30-40% of patients with CPVT.3, 28 There are a certain number of CPVT patients with positive family history but negative genotyping results, suggesting the presence of other causative genes that have not been identified yet. Unknown genetic backgrounds of CPVT may include genes encoding the constitutive proteins and the modifiers of macromolecular Ca2+ release complex. For example, homozygosity for mutations in TRDN encoding triadin, an anchoring protein of calsequestrin to ryanodine channel, has very recently been identified in families affected by CPVT.7 Furthermore, flecainide may suppress CPVT through sodium channel blocking effects as we and others have previously proposed.19, 26
Genotype-phenotype correlations have been studied in inherited arrhythmia syndromes. The genotype contributes to risk stratification and impacts on therapeutic decisions in long QT syndrome; the most studied arrhythmia syndrome.29 In CPVT, the risk of arrhythmia events and the efficacy of β-blocker seem to be similar in genotype-positive and genotype-negative patients.2 Our findings indicate that flecainide can be used regardless of genotyping results in CPVT patients in whom β-blockers fail to control their ventricular arrhythmias or symptoms.
Our study has several limitations. The number of patients was limited because CPVT is a very rare disease. Therefore, the efficacy of flecainide could not be systematically assessed and further studies, probably randomized controlled studies, are needed.30 Patients with CPVT whose arrhythmias are refractory to the conventional drug therapy were included in this study and our previous study.20 Therefore, the efficacy of flecainide in patients whose arrhythmias are well responded to the conventional therapy is not known. Exercise testing is used to assess the effects of therapies in CPVT and our previous study showed that ventricular arrhythmia scores at exercise testing are reproducible measures of drug efficacy in CPVT20 However, the predictive ability of ventricular arrhythmia scores for arrhythmia events is not clear. Finally, the patients in this study were not tested for mutations in TRDN.7 However, in this study, none of the patients were from a consanguineous family, so the possibility of identifying mutations in TRDN seems very low.
In conclusion, we found that flecainide was effective in suppressing ventricular arrhythmias during exercise and preventing arrhythmia events in genotype-negative CPVT patients, similarly to genotype-positive patients. Our results suggest that flecainide can be added, regardless of genotype, to control ventricular arrhythmias or symptoms, when they are insufficiently controlled by conventional therapy with β-blockers.
Acknowledgments
This work was supported by a grant from Ministry of Education, Culture, Sports, Science and Technology, Japan (2012-24591038) (HW), by ZorgOnderzoek Nederland Medische Wetenschappen (ZonMW, grant 120610013 to C.W. and A.A.M.W.), and in part by NIH grant R01HL88635 (BCK) and American Heart Association Established Investigator Award 0840071N (BCK).
Abbreviations
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- ICD
implantable cardioverter defibrillators
- NSVT
non-sustained ventricular tachycardia
- VPB
ventricular premature beats
Footnotes
Disclosures: None declared
References
- 1.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995 Mar 1;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi M, Denjoy I, Extramiana F, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009 May 12;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 3.van der Werf C, Zwinderman AH, Wilde AA. Therapeutic approach for patients with catecholaminergic polymorphic ventricular tachycardia: state of the art and future developments. Europace. 2011 Sep 4;14:175–183. doi: 10.1093/europace/eur277. [DOI] [PubMed] [Google Scholar]
- 4.Laitinen PJ, Brown KM, Piippo K, et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001 Jan 30;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- 5.Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002 Jul 2;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 6.Lahat H, Pras E, Olender T, et al. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet. 2001 Dec;69:1378–1384. doi: 10.1086/324565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roux-Buisson N, Cacheux M, Fourest-Lieuvin A, et al. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Human molecular genetics. 2012 Jun 15;21:2759–2767. doi: 10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, et al. The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis. J Am Coll Cardiol. 2009 Nov 24;54:2065–2074. doi: 10.1016/j.jacc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhuiyan ZA, Hamdan MA, Shamsi ET, et al. A novel early onset lethal form of catecholaminergic polymorphic ventricular tachycardia maps to chromosome 7p14-p22. J Cardiovasc Electrophysiol. 2007 Sep;18:1060–1066. doi: 10.1111/j.1540-8167.2007.00913.x. [DOI] [PubMed] [Google Scholar]
- 10.Kimura H, Zhou J, Kawamura M, et al. Phenotype Variability in Patients Carrying KCNJ2 Mutations. Circ Cardiovasc Genet. 2012 Jun 1;5:344–353. doi: 10.1161/CIRCGENETICS.111.962316. [DOI] [PubMed] [Google Scholar]
- 11.Sumitomo N, Harada K, Nagashima M, et al. Catecholaminergic polymorphic ventricular tachycardia: electrocardiographic characteristics and optimal therapeutic strategies to prevent sudden death. Heart. 2003 Jan;89:66–70. doi: 10.1136/heart.89.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosso R, Kalman JM, Rogowski O, et al. Calcium channel blockers and beta-blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2007 Sep;4:1149–1154. doi: 10.1016/j.hrthm.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Swan H, Laitinen P, Kontula K, Toivonen L. Calcium channel antagonism reduces exercise-induced ventricular arrhythmias in catecholaminergic polymorphic ventricular tachycardia patients with RyR2 mutations. J Cardiovasc Electrophysiol. 2005 Feb;16:162–166. doi: 10.1046/j.1540-8167.2005.40516.x. [DOI] [PubMed] [Google Scholar]
- 14.Katz G, Khoury A, Kurtzwald E, et al. Optimizing catecholaminergic polymorphic ventricular tachycardia therapy in calsequestrin-mutant mice. Heart Rhythm. 2010 Nov;7:1676–1682. doi: 10.1016/j.hrthm.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilde AA, Bhuiyan ZA, Crotti L, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008 May 8;358:2024–2029. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 16.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008 May 27;117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed U, Gollob MH, Gow RM, Krahn AD. Sudden cardiac death despite an implantable cardioverter-defibrillator in a young female with catecholaminergic ventricular tachycardia. Heart Rhythm. 2006 Dec;3:1486–1489. doi: 10.1016/j.hrthm.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Pizzale S, Gollob MH, Gow R, Birnie DH. Sudden Death in a Young Man with Catecholaminergic Polymorphic Ventricular Tachycardia and Paroxysmal Atrial Fibrillation. J Cardiovasc Electrophysiol. 2008 Jun 12; doi: 10.1111/j.1540-8167.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Chopra N, Laver D, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009 Apr;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Werf C, Kannankeril PJ, Sacher F, et al. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011 May 31;57:2244–2254. doi: 10.1016/j.jacc.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang D, Xiao B, Yang D, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004 Aug 31;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.di Barletta MR, Viatchenko-Karpinski S, Nori A, et al. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2006 Sep 5;114:1012–1019. doi: 10.1161/CIRCULATIONAHA.106.623793. [DOI] [PubMed] [Google Scholar]
- 23.Knollmann BC, Chopra N, Hlaing T, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006 Sep;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerrone M, Noujaim SF, Tolkacheva EG, et al. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2007 Nov 9;101:1039–1048. doi: 10.1161/CIRCRESAHA.107.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilliard FA, Steele DS, Laver D, et al. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol. 2010 Feb;48:293–301. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang G, Giovannone SF, Liu N, et al. Purkinje cells from RyR2 mutant mice are highly arrhythmogenic but responsive to targeted therapy. Circ Res. 2010 Aug 20;107:512–519. doi: 10.1161/CIRCRESAHA.110.221481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Q, Xiao J, Jiang D, et al. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca(2+) release. Nat Med. 2011;17:1003–1009. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leenhardt A, Denjoy I, Guicheney P. Catecholaminergic Polymorphic Ventricular Tachycardia. Circulation: Arrhythmia and Electrophysiology. 2012 Sep 27; doi: 10.1161/CIRCEP.111.962027. 2012. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz PJ. The congenital long QT syndromes from genotype to phenotype: clinical implications. J Intern Med. 2006 Jan;259:39–47. doi: 10.1111/j.1365-2796.2005.01583.x. [DOI] [PubMed] [Google Scholar]
- 30.Knollmann BC. Power and pitfalls of using transgenic mice to optimize therapy for CPVT: a need for prospective placebo-controlled clinical trials in genetic arrhythmia disorders. Heart Rhythm. 2010 Nov;7:1683–1685. doi: 10.1016/j.hrthm.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]