Abstract
Background
Atrial fibrillation (AF) is a major global public health problem. Observational studies are necessary to understand patient characteristics, management, and outcomes of this common arrhythmia. Accordingly, our objective was to describe the current status of published prospective observational studies of AF.
Methods and results
MEDLINE and EMBASE (to June 2012) and reference lists of eligible studies were searched for English-language prospective observational registries of AF (n ⩾ 100 and follow-up ⩾6 months). Two reviewers independently extracted data. Disagreements were resolved by consensus. Eight prospective studies enrolled a total of 17,924 patients with AF (total 41,306 patient-years of exposure; follow-up 11 months to 9.9 years). The majority of subjects were enrolled in Europe (74%) or North America (21%), and 0.3% had rheumatic AF. The most consistently reported comorbidities were diabetes mellitus (range 5–18%), hypertension (39–68%), heart failure (5–58%), and prior stroke (4–17%). Three studies did not report all the variables necessary to calculate the currently recommended stroke risk assessment score, and no study reported all the variables required to calculate a recently validated bleeding risk score. The most consistently reported management features were oral anticoagulation (32–64%) and aspirin (28–61%) use. Calcium channel blockers were less frequently used than other rate controlling agents, and digoxin was most common in the single study from Africa (63%). Total mortality was reported in all studies, while data on stroke/systemic embolism, hospitalizations, and major hemorrhage rates were not always reported.
Conclusions
Current literature on real-world management of AF is relatively limited with inadequate data to allow detailed comparisons among reports. Data on rheumatic AF and from Africa and the developing world in general are sparse.
Keywords: Atrial fibrillation, Systematic review
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice, affecting from 1% to 2% of the population, with an increasing incidence and prevalence as the global population ages [1,2]. It is estimated that 1 in 4 persons who reach 40 years of age will develop AF during their lifetime [3]. AF is often a progressive disease, and has a significant impact on morbidity, mortality, quality of life, and healthcare resources [4,5].
Studies have shown racial and geographical differences in the prevalence and incidence of AF, with 1 study reporting an incidence in white subjects twice that in African-Americans [6]. Although similar prevalence and incidence have been reported in Europe and the US, the epidemiology of AF may be different in Asia [7].
Key strategies in the management of AF have been tested in large randomized controlled trials including stroke prevention, rate versus rhythm control, and optimal rate control targets [8–10]. Evidence from these trials has informed international guidelines for AF management. The characteristics of patients enrolled in clinical trials typically differ in important ways from patients treated in real-life. Therefore, well-designed observational studies are necessary to understand patient characteristics and their outcomes, and the effectiveness of different management strategies in the uncontrolled setting of daily practice. Nearly a quarter of a century ago, AF was considered an emerging epidemic in the larger battle against cardiovascular disease [11]. More recently, in the United States, an Institute of Medicine report identified the management of AF as a top national priority for comparative effectiveness research [12]. Therefore, to assess the status of research designed specifically to study AF in real-life practice we performed a systematic review of prospective observational studies of patients with AF that reported baseline characteristics, management, and outcomes.
Material and methods
Information sources and search
We searched MEDLINE (1966 to June 2012) and EMBASE (1988 to June 2012) for English publications of observational studies of patients with AF. We also screened the bibliography of eligible articles for additional studies that fit the inclusion criteria. The database search strategy is described in Appendix A.
Protocol and eligibility criteria
We used a pre-defined protocol for study selection and data extraction, and were guided by the PRISMA checklist for organizing this report [13]. Eligible studies were prospective observational AF registries enrolling a minimum of 100 patients with AF that reported outcomes over a follow-up of at least 6 months. We excluded secondary reports and subgroup analyses from epidemiologic studies that were not originally designed as AF registries.
Study selection and data items and extraction
The four co-authors (all cardiologists) independently reviewed the title and the abstract of retrieved articles for potential eligibility. Articles determined to be eligible were read by all authors, and disagreements were resolved by consensus.
A pre-defined list of items to be extracted was developed, which included data on study characteristics (design, single versus multicenter, geographic origin); patient characteristics, with specific attention to variables included in widely used risk models for thromboembolism or bleeding (e.g., age, gender, hypertension, diabetes mellitus, heart failure); management patterns (e.g., use of rate control medications, antiarrhythmic drugs, cardioversions); and patient outcomes (e.g., death, stroke, hospital admissions, bleeding). Data extraction was done independently by two investigators (AH and AA), with each investigator verifying the extractions of the other against the primary paper. Any discrepancies in data extraction were resolved by discussion between the two extracting investigators, including a third arbiter (MZ) when warranted.
Statistical analysis and data synthesis
We graphically summarized the temporal accrual of enrolled patients per geographic region by plotting the cumulative number of patients enrolled in each region over time. For studies not reporting the starting year of patient enrollment we estimated the start year based on a linear regression that used follow-up duration and publication year as predictor variables. We summarized the reported variables by calculating median and 25th and 75th percentiles. Since follow-up varied by study, we calculated annualized probabilities for the reported outcomes using the following formula:
Because of the expected heterogeneity in study design, patient characteristics, and management, we did not calculate pooled estimates of any variables.
Results
Study selection
The Medline and EMBASE searches yielded a total of 11,521 citations (8877 from MEDLINE and 2644 from EMBASE). Of these, 8 unique studies were eligible for inclusion [14–21] (Fig. 1).
Figure 1.
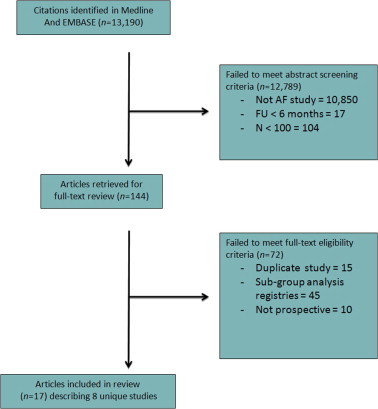
Flowchart.
Study characteristics
Over nearly 2 decades (1991–2008), 8 prospective studies enrolled a total of 17,924 patients with AF (total 41,306 patient-years of exposure; Table 1). Completeness of follow-up ranged from 51.2% to 100%. Follow-up ranged from 11 months to 9.9 years (median 1 year [2.6,4.8]). Studies were done in Europe (n = 4), North America (n = 2), Africa (n = 1), and multi-continents (Europe, Americas, Asia; n = 1). The majority of subjects were enrolled from European centers (74%), and the rest were predominantly from North America (21%). A minority of patients were enrolled from Asia (2.8%), South America (1.3%) or Africa (0.9%) (Fig. 2). Most studies (5/8) declared funding from the pharmaceutical industry, which was the sole sponsor in 3 studies. Government funding was declared in 2 studies, one of which was exclusively government-funded.
Table 1.
Characteristics of eligible studies.
| Study | Years of Enrollment | Country | Number of Patients | Follow-up(months) | Number of Centers | Completeness of Follow-Up | Funding |
|---|---|---|---|---|---|---|---|
| Canadian Registry of AF [14] | 1991–1995 | Canada | 1,086 | 50 | 7 | 99% | Industry |
| Stockholm Cohort Study of AF [15] | 2002 | Sweden | 2824 | 55 | 2 | 100% | Industry + Government |
| Pappone et al. [16] | 2002–2007 | Italy | 106 | 60 | 1 | 100% | None declared |
| Euro Heart Survey [17] | 2003–2004 | Europe (35 countries) | 5333 | 12 | 182 | 80% | Industry |
| AFIB Cameroon [18] | 2006–2007 | Cameroon | 172 | 11 | 10 | 51% | Non-profit foundation |
| RecordAF [19] | 2007–2008 | Europe, Americas, Asia(21 countries) | 5814 | 12 | 532 | 90% | Industry |
| Belgrade Atrial Fibrillation Study [20] | 1992–2007 | Serbia | 1056 | 119 | 1 | 100% | Government |
| AFFECTS [21] | 2005–2007 | USA | 1531 | 12 | 248 | 55% | Industry + University |
Canadian Registry of AF: Completeness of follow-up of 99% is in the 899 with AF not precipitated by cardiothoracic surgery with a median follow-up of 4.1 years. Three-year visits were completed in 86%. Euro Heart Survey: 80% refers to proportion of patients with known survival status at 1-year. RecordAF: 90% refers to proportion of patients with known cardiovascular death status at 1-year. Belgrade AF Study: 100% refers to proportion of patients with known vitals status during follow-up. AFFECTS: 55% refers to proportion of patients who completed 1-year follow-up. Completeness of follow-up of 100% in the Stockholm Cohort Study of AF, Pappone et al., and Belgrade AF Study was not explicitly stated in the manuscript but implied by the data presentation in the results sections.
Figure 2.
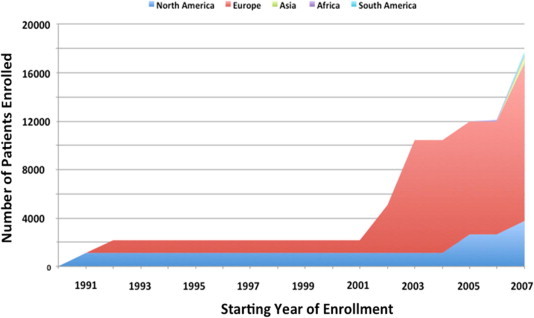
Cumulative enrollment of subjects into prospective registries of atrial fibrillation by geographic region.
AF and patient characteristics
The proportion of patients with each type of AF varied among studies, with paroxysmal AF reported in 23–84% of patients, persistent AF in 19–48%, and chronic/permanent AF in 19–56% (Table 2). Enrollment was limited to ambulatory/outpatient settings in 3 studies (n = 2758), emergency room in 1 study (n = 106), and was not restricted in the remainder (n = 15,060). Two studies exclusively enrolled patients with first presentation of AF, but then reported AF classification (paroxysmal, persistent, or permanent) during follow-up. Duration of AF was reported in 3 studies. The proportion of patients with lone AF, reported in 6 studies, ranged from 5% to 51%. One study clearly stated the exclusion of patients with rheumatic AF, and only 1 study reported the proportion of patients with rheumatic AF. The overall reported proportion of patients with rheumatic AF in the 8 studies was 0.3%.
Table 2.
Characteristics of atrial fibrillation in eligible studies.
| Study | Setting | Type of AF | Duration of AF (months) | Paroxysmal N (%) | Persistent N (%) | Permanent/chronic N (%) | Lone AF N (%) | Rheumatic AF N (%) |
|---|---|---|---|---|---|---|---|---|
| Canadian Registry of AF [14] | Mixed | First diagnosis | 12 | 757 (84) | – | 142 (16) | – | – |
| Stockholm Cohort Study of AF [15] | Mixed | Mixed | 23 | 888 (31) | 618 (22) | 1186 (42) | 141 (5) | – |
| Pappone et al. [16] | Emergency room | First diagnosis | – | 56 (53) | 24 (23) | 16 (15) | 54 (51) | – |
| Euro Heart Survey [17] | Mixed | Mixed | – | 1517 (28) | 1167 (22) | 1541 (29) | – | – |
| AFIB Cameroon [18] | Outpatient | Mixed | – | 39 (23) | 37 (22) | 96 (56) | 16 (9) | 44 (26) |
| RecordAF [19] | Mixed | Mixed | – | 2748 (52) | 2506 (48) | 0 (0) | 1044 (19) | – |
| Belgrade Atrial Fibrillation Study [20] | Outpatient | Mixed | – | 647 (61) | 212 (20) | 197 (19) | 440 (42) | – |
| AFFECTS [21] | Outpatient | Mixed | 26 | 1165 (80) | 273 (19) | 0 (0) | 226 (16) | Excluded |
Denominators may vary among cells. In the Canadian Registry of AF, the numbers refer to a denominator of 899 (out of the original 1086 shown in Table 1), as 189 patients with post-operative AF were excluded. For studies that enrolled patients with first diagnosis of AF (Canadian Registry of AF and Pappone et al.), AF classification (e.g. paroxysmal versus persistent) refers to reported classification during follow-up.
Reported mean or median ages for enrolled patients ranged from 53 to 74 years (median 66 years [60, 67]), and the proportion of female patients ranged from 35% to 56% (overall proportion of females 42%) (Table 3). The proportion of patients with diabetes mellitus (range 5–18%) or hypertension (range 39–68%) was reported for 7 studies. Most studies also reported the proportion of patients with heart failure (range 5–58%), stroke/TIA (range 4–17%), and coronary artery disease (range 5–32%). History of smoking was not consistently reported (available only in 2 of 8 studies). Three of the 8 studies did not report all the variables necessary to calculate the CHADS2 stroke risk assessment score, and no study reported all the variables to calculate the HAS-BLED bleeding risk score.
Table 3.
Characteristics of patients enrolled in eligible studies.
| Study | Age | Women N (%) | Diabetes Mellitus N (%) | Hypertension N (%) | Heart failure N (%) | Stroke/TIA N (%) | CAD N (%) | Smoking N (%) |
|---|---|---|---|---|---|---|---|---|
| Canadian Registry of AF [14] | 62 | 339 (38) | 81 (9) | 354 (39) | 158 (18) | 40 (4) | – | – |
| Stockholm Cohort Study of AF [15] | 74 | 1271 (45) | 480 (17) | 1356 (48) | 1271 (45) | 452 (16) | – | – |
| Pappone et al. [16] | 58 | 38 (36) | 5 (5) | 48 (45) | 13 (12) | – | 10 (9) | – |
| Euro Heart Survey [17] | 67 | 2189 (41) | 941 (18) | 3318 (62) | 1751 (33) | 556 (10) | 1704 (32) | 633 (12) |
| AFIB Cameroon [18] | 66 | 97 (56) | 18 (10) | 111 (65) | 100 (58) | 30 (17) | 11 (6) | – |
| RecordAF [19] | 66 | 2396 (43) | 879 (16)‘ | 3833 (68) | 1452 (26) | 317 (6) | 961 (18) | 2358 (43) |
| Belgrade Atrial Fibrillation Study [20] | 53 | 365 (35) | 74 (7) | 530 (50) | 57 (5) | – | 49 (5) | – |
| AFFECTS [21] | 66 | 677 (46) | – | – | – | – | – | – |
Proportion of patients with prior TIA in RecordAF was 4%.
Management and outcomes
The most consistently reported features of AF management were oral anticoagulation and aspirin use, which were reported in 6 and 5 studies, respectively (Table 4). Use of oral anticoagulation therapy ranged from 32% to 64% of patients, while aspirin use was reported in 28–61% of patients. Direct current cardioversion use was reported in only half the studies and ranged from 2% to 29%. Pharmacologic cardioversion and rate of anti-arrhythmic drug use was reported in 2 and 5 studies, respectively. When data on rate controlling agents were available, calcium channel blockers were consistently used less frequently than other drugs. Digoxin use was more common in the early Canadian study that recruited patients between 1991 and 1995 (43%) and in the 1 study from Africa (63%), which also had the highest proportion of patients with heart failure (58%).
Table 4.
Management characteristics of patients with atrial fibrillation.
| Study | DCCV N (%) | Pharmacologic CV N (%) | AAD N (%) | Beta-blocker N (%) | CCB N (%) | Digoxin N (%) | Rhythm control N (%) | Rate control N (%) | OAT N (%) | ASA N (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Canadian Registry of AF [14] | 155 (17) | 611 (68) | 315 (35) | 120 (31) | 142 (16) | 385 (43) | – | – | 284 (32) | – |
| Stockholm Cohort Study of AF [15] | – | – | – | 50 (2) | – | – | – | – | 1130 (40) | 1158 (41) |
| Pappone et al. [16] | 31 (29) | – | 56 (53) | – | – | – | –– | – | – | – |
| Euro Heart Survey [17] | 900 (17) | 1128 (21) | 2089 (39) | 2236 (42) | 463(9) | 1369 (26) | – | – | 3271 (61) | 1490 (28) |
| AFIB Cameroon [18] | 4 (2) | – | – | 20 (12) | 15 (9) | 108 (63) | 28 (16) | 144 (84) | 57 (33) | 105 (61) |
| RecordAF [19] | – | – | 616 (11) | 4035 (72) | 841 (15) | 1905 (34) | 3076 (55) | 2528 (45) | 2914 (52) | 2353 (42) |
| Belgrade Atrial Fibrillation Study [20] | – | – | – | – | – | – | – | – | – | – |
| AFFECTS [21] | – | – | 412 (28) | 632 (43) | 345 (24) | 323 (22) | 942 (65) | 519 (35) | 930 (64) | 464 (32) |
AAD - antiarrhythmic drug, ASA - aspirin, CCB - calcium channel blocker, DCCV - direct current cardioversion, OAT - oral anticoagulation therapy.
Total mortality was reported in all studies, while data on stroke/systemic embolism, hospitalizations, and major hemorrhage rates were lacking for 1, 5, and 3 studies, respectively (Table 5). Annual probabilities of these events varied widely from 0.6% to 28% for death, 0.5% to 17% for stroke, 15.4% to 29.9% for hospitalization, and 0.3% to 3.7% for major hemorrhage.
Table 5.
Outcomes of patients with atrial fibrillation.
| Study | Death N (% 1 year probability) | Stroke/SSE N (% 1 year probability) | Hospitalization N (% 1 year probability) | Major Hemorrhage N (% 1 year probability) |
|---|---|---|---|---|
| Canadian Registry of AF [14] | 149 (3.9) | 63 (1.7) | – | 36 (1.0) |
| Stockholm Cohort Study of AF [15] | 1038 (7.7) | – | – | – |
| Pappone et al. [16] | 3 (0.6) | 3 (0.6) | – | – |
| Euro Heart Survey [17] | 213 (3.9) | 117 (2.2) | 1895 (29.9) | 68 (1.3) |
| AFIB Cameroon [18] | 26 (28) | 10 (17) | 13 (15.4) | 1 (1.8) |
| RecordAF [19] | 154 (3.0) | 106 (2.1) | 831 (16.7) | 182 (3.7) |
| Belgrade Atrial Fibrillation Study [20] | 73 (0.7) | 82 (0.8) | – | – |
| AFFECTS [21] | 30 (2) | 7 (0.5) | – | 4 (0.3) |
AFIB Cameroon: Death rate is based on denominator of 88 patients with available data. Stroke/SSE rate refers to non-fatal stroke among 62 survivors. Hospitalization rate is based events for uncontrolled AF among 88 patients with available data. RECORDAF: The figure 3% is for all-cause death as reported in the study. Rate of cardiovascular death was 1.7%. The rate of 2.1% is stroke/TIA. The rate of hospitalization refers to hospitalization for a cardiovascular event. Major hemorrhage rate refers to bleeding related to oral anticoagulant. Denominators vary by outcome. Belgrade Atrial Fibrillation Study: The rate of ischemic stroke was 0.5%. AFFECTS: The rate of 0.5% refers to stroke/TIA. Major hemorrhage refers to intracerebral and intracranial hemorrhages only.
Discussion
Our systematic review of English-language observational studies that followed a minimum of 100 patients with AF for at least 6 months identified 8 eligible studies that enrolled a total of almost 18,000 patients. Nearly three-quarters of the patients were enrolled in studies conducted in Europe, and only 1% of patients were from studies conducted in the developing world.
While controlled clinical trials remain the gold standard for evaluating the efficacy of interventions, the value of registries in providing important insights into clinical care is increasingly acknowledged [22]. Real-world studies are essential to understand disease progression and outcomes outside the restrictions imposed by randomized trial designs, and they allow assessment of the effectiveness of treatments that had proven efficacy in clinical trials. Excellent resources are available to guide the development of effective registries [23]. Assuring the scope and rigor of acquired data are adequate is particularly important in the dynamic field of AF management.
This review identified several gaps in the literature describing the characteristics, management, and outcomes of patients with AF in clinical practice. Reports from registries dedicated to AF patients did not consistently include the same information, complicating comparisons among studies. Some ongoing studies are expected to produce additional reports; however, providing basic information on patient and disease characteristics, management, and outcomes initially for the entire cohort would facilitate assessment of disease status as well as allow comparisons with other populations studied.
Data on major co-morbidities was not consistently reported across studies. The lack of data on factors known to be associated with AF, such as heart failure, CAD, and smoking, is surprising, One-third of the studies did not report all the variables necessary to calculate the CHADS2 stroke risk assessment, which was introduced as a validated tool in stoke risk stratification in 2001 [24]. Although none of the studies reported all the variables required to calculate risk score of bleeding with either HEMORR2HAGES or HAS-BLED, the introduction of these tools postdated most of the enrollment periods for the studies in this review. HAS-BLED, introduced in 2010, was developed using real-world data, and its use was incorporated into recommendations in the European guidelines for the management of AF published that same year [25]. Availability of such data from ongoing and future AF registries is essential to better understand the utility of using risk scores in common practice.
In addition, several studies have not reported data on guideline recommended therapies and in particular AF ablation therapy which is considered a state -of-art-management strategy in the AHA/ACC/ESC AF management guidelines. Although studies with longer enrollment and/or follow-up periods may be complicated by changes in definitions and practices, they may also be able to provide valuable trend data. However, enrollment time frames for 4 of the studies pre-date important developments in management of AF, including landmark clinical trials that redefined how AF is managed (e.g., rate control versus rhythm control, targets for rate control), therapeutic advances in treating the arrhythmia (catheter ablation and novel anticoagulants), and the most recent comprehensive guidelines from professional societies. While absence of prospectively collected data on the newer developments can be explained by the fact that some of the studies in this review pre-date incorporation of these management strategies into guidelines and routine practice, it reflects a gap in the current literature that is likely to be addressed by ongoing AF registries.
With the exception of RecordAF [19], these studies were geographically restrictive, which contributes to a skewed representation of the global status of AF. For example, although rheumatic heart disease has decreased substantially in the developed world, it remains a serious health issue in the developing world, which is home to two-thirds of the world population [26]. The global burden of rheumatic heart disease is estimated at more than15 million patients [27]. Rheumatic AF, a common complication of rheumatic heart disease, was present in 26% of AF patients in the Cameroon study in this review, compared with 0% and 7% of patients in Italy and France, respectively. Rheumatic AF is not restricted to lower income developing countries, however, with 16% of 2043 AF patients in the Gulf SAFE registry in Middle Eastern Gulf countries having rheumatic valve disease [28]. Other characteristics of AF patients, particularly age and diabetes status, are known to vary considerably among ethnic, cultural, and geographic populations [28]. Finally, treatment approaches outside areas from which guidelines originate can be driven by patient practices and local physician attitudes.
Given the significant and global burden of AF, it can be argued that the total number of patients enrolled in the prospective studies reviewed here is relatively small. For example, the Global Registry of Acute Coronary Events (GRACE) (also a major public health problem) has enrolled >100,000 patients from 30 countries who present with acute coronary syndromes (ACS) [29].
In addition, four recently published clinical trials randomized and followed nearly 60,000 patients with AF; that is, more than three times that enrolled in the registries in this review [30–33]. Some of these trials included patients from nearly 1000 centers in more than 40 countries around the world. The size and scope of ACS registries and clinical trials of AF highlight the feasibility of conducting large and rigorous global registries of AF.
In response to the need for larger studies, the Global Anticoagulant Registry in the FIELD (GARFIELD) study enrolled the first of a targeted 55,000 patients in 2009 [34]. The study includes >1000 centers in 50 countries, with a planned 4-year recruitment interval and minimum 2-year follow-up. The GARFIELD registry will evaluate management and outcomes of patients with newly diagnosed non-valvular AF who are at risk for stroke.
In addition, other regional and national registries have been established that will supplement the information acquired from the global registries, and expand our knowledge of AF in the context of varying cultures and geographical locations. For example, Gulf-SAFE, the Gulf Survey of Atrial Fibrillation Events, enrolled approximately 2000 emergency room patients in 9 months between 2009 and 2010, with 1-year follow-up data expected to add valuable information on AF management and outcomes in these Middle Eastern countries [35]. AFNET is a German AF registry with over 9000 patients enrolled between 2004 and 2006, which plans long-term follow-up to determine guideline compliance and outcomes [36]. The ORBIT-AF registry has a target enrollment of 10,000 patients from approximately 200 outpatient practices in the United States [37].
Our systematic review has several limitations. Search strategy limitations can produce common biases in systematic reviews, including lack of comprehensiveness of databases searched, publication bias, and publication language restrictions [38]. Our protocol required reports to be based on dedicated AF registry data; therefore, we believed Medline and EMBASE, supplemented with a manual bibliography search, were adequate. Registry data should not be prone to publication bias, as there are no positive or negative results in the real world. Finally, our search did not reveal any titles or abstracts of articles in languages other than English that suggested they were reporting AF registry data.
By restricting study eligibility to AF registries, we did not include reports from studies that extracted data from other sources, such as the longitudinal data being acquired in the Framingham study [3], and data obtained from national health registries. Our objective, however, was to summarize the comprehensiveness of data acquired by dedicated AF registries, while advocating the establishment of registries that assure relevant data are captured that can provide accurate assessment of patient characteristics, management, and outcomes, and that are amenable to comparisons among registries.
Conclusion
In summary, current literature based on prospective observational studies of real-world management of AF is relatively limited, with inadequate data to allow detailed comparisons among reports. Data on rheumatic AF and from Africa and the developing world in general are sparse. The need for this information is being addressed by ongoing studies from which more complete, globally representative data are expected.
Funding sources
No funding was obtained for this work. Boehringer Ingelheim provided logistical support for electronic retrieval of some articles through an independent librarian. The four authors solely conceived the study, collected and analyzed the data, wrote the paper and approved the final version for submission.
Footnotes
Peer review under responsibility of King Saud University.
Appendix A. Electronic search strategy to identify eligible studies
-
1.
Atrial fibrillation.mp. or exp Atrial Fibrillation/
-
2.
Atrial flutter.mp. or exp Atrial Flutter/
-
3.
1 or 2
-
4.
Epidemiologic studies/
-
5.
exp case control studies/
-
6.
exp cohort studies/
-
7.
Case control.tw.
-
8.
(cohort adj (study or studies)).tw.
-
9.
Cohort analy$.tw.
-
10.
(Follow up adj (study or studies)).tw.
-
11.
(observational adj (study or studies)).tw.
-
12.
Longitudinal.tw.
-
13.
Retrospective.tw.
-
14.
Cross sectional.tw.
-
15.
Cross-sectional studies/
-
16.
or/4-15
-
17.
3 and 16
-
18.
limit 17 to humans
References
- 1.Stewart S., Hart C.L., Hole D.J., McMurray J.J. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86(5):516–521. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go A.S., Hylek E.M., Phillips K.A., Chang Y., Henault L.E., Selby J.V. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA: the Journal of the American Medical Association. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones Donald M., Wang Thomas J., Leip Eric P., Larson Martin G., Levy Daniel, Vasan Ramachandran S. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 4.Camm A.J., Savelieva I., Lip G.Y. Rate control in the medical management of atrial fibrillation. Heart. 2007;93(1):35–38. doi: 10.1136/hrt.2006.099903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyne K.S., Paramore C., Grandy S., Mercader M., Reynolds M., Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9(5):348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 6.Psaty Bruce M., Manolio Teri A., Kuller Lewis H., Kronmal Richard A., Cushman Mary, Fried Linda P. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96(7):2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 7.Ryder K.M., Benjamin E.J. Epidemiology and significance of atrial fibrillation. American Journal of Cardiology. 1999;84(9A):131R–138R. doi: 10.1016/s0002-9149(99)00713-4. [DOI] [PubMed] [Google Scholar]
- 8.Wyse D.G., Waldo A.L., DiMarco J.P., Domanski M.J., Rosenberg Y., Schron E.B. A comparison of rate control and rhythm control in patients with atrial fibrillation. The New England Journal of Medicine. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 9.De Denus S., Sanoski C.A., Carlsson J., Opolski G., Spinler S.A. Rate vs. rhythm control in patients with atrial fibrillation: a meta-analysis. Archives of Internal Medicine. 2005;165:258–262. doi: 10.1001/archinte.165.3.258. [DOI] [PubMed] [Google Scholar]
- 10.Van Gelder I.C., Wyse D.G., Chandler M.L., Cooper H.A., Olshansky B., Hagens V.E. Does intensity of rate-control influence outcome in atrial fibrillation? An analysis of pooled data from the RACE and AFFIRM studies. Europace. 2006;8:935–942. doi: 10.1093/europace/eul106. [DOI] [PubMed] [Google Scholar]
- 11.Braunwald E. Shattuck lecture-cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. The New England Journal of Medicine (NEJM) 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Comparative Effectiveness Research Prioritization Institute of Medicine . The National Academics Press; Washington, DC: 2009. Initial National Priorities for Comparative effectiveness Research. IOM (Institute of Medicine) [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G. The PRISMA group: preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International Journal of Surgery. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Kerr C.R., Humphries K.H., Talajic M., Klein G.J., Connolly S.J., Green M. The Canadian registry of atrial fibrillation: a noninterventional follow-up of patients after the first diagnosis of atrial fibrillation. The American Journal of Cardiology. 1998;82(7–1):82N–85N. doi: 10.1016/s0002-9149(98)00589-x. [DOI] [PubMed] [Google Scholar]
- 15.Friberg L., Hammar Niklas, Pettersson Hans, Rosenqvist Marten. Increased mortality in paroxysmal atrial fibrillation: report from the Stockholm Cohort-Study of Atrial Fibrillation (SCAF) European Heart Journal. 2007;28:2346–2353. doi: 10.1093/eurheartj/ehm308. [DOI] [PubMed] [Google Scholar]
- 16.Pappone Carlo, Radinovic Andrea, Manguso Francesco, Vicedomini Gabriele, Ciconte Giuseppe, Sacchi Stefania. Atrial fibrillation progression and management: a 5-year prospective follow-up study. Heart Rhythm. 2008;5(11):1501–1507. doi: 10.1016/j.hrthm.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwlaat R., Capucci A., Camm A.J., Olsson S.B., Andresen D., Davies D.W. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. European Heart Journal. 2005;26(22):2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 18.Ntep-Gweth M., Zimmermann M., Meiltz A., Kingue S., Ndobo P., Urban P. Atrial fibrillation in Africa: clinical characteristics, prognosis, and adherence to guidelines in Cameroon. Europace. 2010;12(4):482–487. doi: 10.1093/europace/euq006. [DOI] [PubMed] [Google Scholar]
- 19.Camm A.J., Breithardt G., Crijns H., Dorian P., Kowey P., Le Heuzey J.Y. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation) Journal of the American College of Cardiology. 2011;58(5):493–501. doi: 10.1016/j.jacc.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Potpara T.S., Vasiljevic Z.M., Vujisic-Tesic B.D., Marinkovic J.M., Polovina M.M., Stepanovic J.M. Mitral annular calcification predicts cardiovascular morbidity and mortality in middle-aged patients with atrial fibrillation: the Belgrade Atrial Fibrillation Study. Chest. 2011;140(4):902–910. doi: 10.1378/chest.10-2963. [DOI] [PubMed] [Google Scholar]
- 21.Kowey P.R., Reiffel J.A., Myerburg R., Naccarelli G.V., Packer D.L., Pratt C.M. Practice patterns among United States cardiologists for managing adults with atrial fibrillation (AFFECTS) The American Journal of Cardiology. 2010;105(8):1122–1129. doi: 10.1016/j.amjcard.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 22.Vandenbroucke J.P. Observational research, randomised trials, and two views of medical science. PLoS Medicine. 2008;5(3):e67. doi: 10.1371/journal.pmed.0050067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Registries for Evaluating Patient Outcomes: A User’s Guide. Agency for Healthcare Research and Quality (AHRQ). 2010; second ed., Publication No. 10-EHC049. [PubMed]
- 24.Gage B.F., Waterman A.D., Shannon W., Boechler M., Rich M.W., Radford M.J. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA: the Journal of the American Medical Association. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 25.Camm A.J., Kirchhof P., Lip G.Y., Schotten U., Savelieva I., Ernst S. European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC), Heart Rhythm Society, European Association for Cardio-Thoracic Surgery. Europace. 2010;12(10):1360–1420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 26.Chandrashekhar Y., Westaby S., Narula J. Mitral stenosis. Lancet. 2009;374(9697):1271–1283. doi: 10.1016/S0140-6736(09)60994-6. [DOI] [PubMed] [Google Scholar]
- 27.Carapetis J.R., Steer A.C., Mulholland E.K., Weber M. The global burden of group A streptococcal diseases. The Lancet Infectious Diseases. 2005;5(11):685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 28.Zubaid M., Rashed W.A., Alsheikh-Ali A.A., Almahmeed W., Shehab A., Sulaiman K. Gulf Survey of Atrial Fibrillation Events (Gulf SAFE) investigators. Circulation: Cardiovascular Quality and Outcomes. 2011;4(4):477–482. doi: 10.1161/CIRCOUTCOMES.110.959700. [DOI] [PubMed] [Google Scholar]
- 29.Global Registry of Acute Coronary Events (GRACE) 2012. Scientific Advisory Committee. [Cited 2012 Mar 6]. Available from: <http://www.outcomes-umassmed.org/grace/>.
- 30.Granger Christopher B., Alexander John H., McMurray John J.V., Lopes Renato D., Hylek Elaine M., Hanna Michael. Apixaban versus Warfarin in Patients with Atrial Fibrillation (ARISTOTLE) The New England Journal of Medicine. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 31.Connolly S.J., Ezekowitz M.D., Yusuf S., Eikelboom J., Oldgren J., Parekh A. Dabigatran versus warfarin in patients with atrial fibrillation (RE-LY) The New England Journal of Medicine. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 32.Patel M.R., Mahaffey K.W., Garg J., Pan G., Singer D.E., Hacke W. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation (ROCKET AF) The New England Journal of Medicine. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 33.Connolly S.J., Eikelboom J., Joyner C., Diener H.C., Hart R., Golitsyn S. Apixaban in patients with atrial fibrillation (AVERROES) The New England Journal of Medicine. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 34.Kakkar A.K., Mueller I., Bassand J.P., Fitzmaurice D.A., Goldhaber S.Z., Goto S. International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD) American Heart Journal. 2012;163(1):13–19. doi: 10.1016/j.ahj.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 35.Zubaid M., Rashed W.A., Alsheikh-Ali A.A., Almahmeed W., Shehab A., Sulaiman K. Gulf Survey of Atrial Fibrillation Events (Gulf SAFE): design and baseline characteristics of patients with atrial fibrillation in the Arab Middle East. Circulation: Cardiovascular Quality and Outcomes. 2011;4(4):477–482. doi: 10.1161/CIRCOUTCOMES.110.959700. [DOI] [PubMed] [Google Scholar]
- 36.Nabauer M., Gerth A., Limbourg T., Schneider S., Oeff M., Kirchhof P. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11(4):423–434. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piccini J.P., Fraulo E.S., Ansell J.E., Fonarow G.C., Gersh B.J., Go A.S. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. American Heart Journal. 2011;162(4):606–612. doi: 10.1016/j.ahj.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Parekh-Bhurke S., Kwok C.S., Pang C., Hooper L., Loke Y.K., Ryder J.J. Uptake of methods to deal with publication bias in systematic reviews has increased over time, but there is still much scope for improvement. Journal of Clinical Epidemiology. 2011;64(4):349–357. doi: 10.1016/j.jclinepi.2010.04.022. [DOI] [PubMed] [Google Scholar]



