Abstract
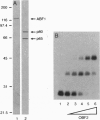
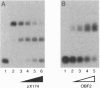
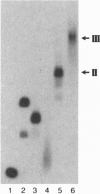
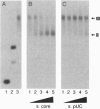
We have previously shown that three distinct DNA-binding activities, in crude form, are necessary for the ATP-dependent assembly of a specific and stable multiprotein complex at a yeast origin of replication. Here we show the purification of one of these DNA binding activities, referred to as origin binding factor 2 (OBF2). The purified protein is a heterodimer composed of two polypeptides with molecular mass values of 65 and 80 kDa as determined by SDS/PAGE. Purified OBF2 not only binds DNA but also supports the formation of a protein complex at essential sequences within the ARS121 origin of replication. Interestingly, OBF2 binds tightly and nonspecifically to both duplex DNA and single-stranded DNA. The interaction with duplex DNA occurs at the termini. N-terminal sequencing of the 65-kDa subunit has revealed that this polypeptide is identical to the previously identified HDF1 peptide, a yeast homolog of the small subunit of the mammalian Ku autoantigen. Although the potential involvement of Ku in DNA metabolic events has been proposed, this is the first requirement for a Ku-like protein in the assembly of a protein complex at essential sequences within a eukaryotic origin of replication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell S. P., Stillman B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature. 1992 May 14;357(6374):128–134. doi: 10.1038/357128a0. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987 Nov 6;51(3):463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- Cao Q. P., Pitt S., Leszyk J., Baril E. F. DNA-dependent ATPase from HeLa cells is related to human Ku autoantigen. Biochemistry. 1994 Jul 19;33(28):8548–8557. doi: 10.1021/bi00194a021. [DOI] [PubMed] [Google Scholar]
- Christ C., Tye B. K. Functional domains of the yeast transcription/replication factor MCM1. Genes Dev. 1991 May;5(5):751–763. doi: 10.1101/gad.5.5.751. [DOI] [PubMed] [Google Scholar]
- Deshpande A. M., Newlon C. S. The ARS consensus sequence is required for chromosomal origin function in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Oct;12(10):4305–4313. doi: 10.1128/mcb.12.10.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell S. J., Romanowski P., Diffley J. F. Interaction of Dbf4, the Cdc7 protein kinase regulatory subunit, with yeast replication origins in vivo. Science. 1994 Aug 26;265(5176):1243–1246. doi: 10.1126/science.8066465. [DOI] [PubMed] [Google Scholar]
- Dvir A., Peterson S. R., Knuth M. W., Lu H., Dynan W. S. Ku autoantigen is the regulatory component of a template-associated protein kinase that phosphorylates RNA polymerase II. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11920–11924. doi: 10.1073/pnas.89.24.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Francesconi S. C., Civalier C., Walker S. S. Purification of DNA-binding proteins by site-specific DNA affinity chromatography. Methods Enzymol. 1990;182:521–529. doi: 10.1016/0076-6879(90)82041-y. [DOI] [PubMed] [Google Scholar]
- Estes H. G., Robinson B. S., Eisenberg S. At least three distinct proteins are necessary for the reconstitution of a specific multiprotein complex at a eukaryotic chromosomal origin of replication. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11156–11160. doi: 10.1073/pnas.89.23.11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H., Winnacker E. L. A putative homologue of the human autoantigen Ku from Saccharomyces cerevisiae. J Biol Chem. 1993 Jun 15;268(17):12895–12900. [PubMed] [Google Scholar]
- Ferguson B. M., Brewer B. J., Reynolds A. E., Fangman W. L. A yeast origin of replication is activated late in S phase. Cell. 1991 May 3;65(3):507–515. doi: 10.1016/0092-8674(91)90468-e. [DOI] [PubMed] [Google Scholar]
- Francesconi S. C., Eisenberg S. Purification and characterization of OBF1: a Saccharomyces cerevisiae protein that binds to autonomously replicating sequences. Mol Cell Biol. 1989 Jul;9(7):2906–2913. doi: 10.1128/mcb.9.7.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffin W., Torrance H., Rodda D. J., Préfontaine G. G., Pope L., Hache R. J. Sequence-specific DNA binding by Ku autoantigen and its effects on transcription. Nature. 1996 Mar 21;380(6571):265–268. doi: 10.1038/380265a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb T. M., Jackson S. P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993 Jan 15;72(1):131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Hennessy K. M., Clark C. D., Botstein D. Subcellular localization of yeast CDC46 varies with the cell cycle. Genes Dev. 1990 Dec;4(12B):2252–2263. doi: 10.1101/gad.4.12b.2252. [DOI] [PubMed] [Google Scholar]
- Huang R. Y., Kowalski D. Multiple DNA elements in ARS305 determine replication origin activity in a yeast chromosome. Nucleic Acids Res. 1996 Mar 1;24(5):816–823. doi: 10.1093/nar/24.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Spotila L. D., Nawotka K. A., el-Assouli S. M., Davis L. R. The in vivo replication origin of the yeast 2 microns plasmid. Cell. 1987 Nov 6;51(3):473–481. doi: 10.1016/0092-8674(87)90643-x. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Zhu J. G., Davis L. R., Newlon C. S. Close association of a DNA replication origin and an ARS element on chromosome III of the yeast, Saccharomyces cerevisiae. Nucleic Acids Res. 1988 Jul 25;16(14A):6373–6384. doi: 10.1093/nar/16.14.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Herskowitz I. Isolation of ORC6, a component of the yeast origin recognition complex by a one-hybrid system. Science. 1993 Dec 17;262(5141):1870–1874. doi: 10.1126/science.8266075. [DOI] [PubMed] [Google Scholar]
- Liang C., Weinreich M., Stillman B. ORC and Cdc6p interact and determine the frequency of initiation of DNA replication in the genome. Cell. 1995 Jun 2;81(5):667–676. doi: 10.1016/0092-8674(95)90528-6. [DOI] [PubMed] [Google Scholar]
- Mages G. J., Feldmann H. M., Winnacker E. L. Involvement of the Saccharomyces cerevisiae HDF1 gene in DNA double-strand break repair and recombination. J Biol Chem. 1996 Apr 5;271(14):7910–7915. doi: 10.1074/jbc.271.14.7910. [DOI] [PubMed] [Google Scholar]
- Marahrens Y., Stillman B. A yeast chromosomal origin of DNA replication defined by multiple functional elements. Science. 1992 Feb 14;255(5046):817–823. doi: 10.1126/science.1536007. [DOI] [PubMed] [Google Scholar]
- Mimori T., Akizuki M., Yamagata H., Inada S., Yoshida S., Homma M. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J Clin Invest. 1981 Sep;68(3):611–620. doi: 10.1172/JCI110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A. Mechanism of interaction between Ku protein and DNA. J Biol Chem. 1986 Aug 5;261(22):10375–10379. [PubMed] [Google Scholar]
- Paillard S., Strauss F. Analysis of the mechanism of interaction of simian Ku protein with DNA. Nucleic Acids Res. 1991 Oct 25;19(20):5619–5624. doi: 10.1093/nar/19.20.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S. E., Greenwell P. W., Ritchie K. B., Petes T. D. The DNA-binding protein Hdf1p (a putative Ku homologue) is required for maintaining normal telomere length in Saccharomyces cerevisiae. Nucleic Acids Res. 1996 Feb 15;24(4):582–585. doi: 10.1093/nar/24.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W. H. Use of monoclonal antibodies for the characterization of novel DNA-binding proteins recognized by human autoimmune sera. J Exp Med. 1985 Jan 1;161(1):18–39. doi: 10.1084/jem.161.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier D. H., Rine J. An origin of DNA replication and a transcription silencer require a common element. Science. 1992 May 1;256(5057):659–663. doi: 10.1126/science.1585179. [DOI] [PubMed] [Google Scholar]
- Siede W., Friedl A. A., Dianova I., Eckardt-Schupp F., Friedberg E. C. The Saccharomyces cerevisiae Ku autoantigen homologue affects radiosensitivity only in the absence of homologous recombination. Genetics. 1996 Jan;142(1):91–102. doi: 10.1093/genetics/142.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb D. T., Struhl K., Davis R. W. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979 Nov 1;282(5734):39–43. doi: 10.1038/282039a0. [DOI] [PubMed] [Google Scholar]
- Suwa A., Hirakata M., Takeda Y., Jesch S. A., Mimori T., Hardin J. A. DNA-dependent protein kinase (Ku protein-p350 complex) assembles on double-stranded DNA. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6904–6908. doi: 10.1073/pnas.91.15.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis J. F., Newlon C. S. Domain B of ARS307 contains two functional elements and contributes to chromosomal replication origin function. Mol Cell Biol. 1994 Nov;14(11):7652–7659. doi: 10.1128/mcb.14.11.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troelstra C., Jaspers N. G. Recombination and repair. Ku starts at the end. Curr Biol. 1994 Dec 1;4(12):1149–1151. doi: 10.1016/s0960-9822(00)00260-8. [DOI] [PubMed] [Google Scholar]
- Tuteja N., Tuteja R., Ochem A., Taneja P., Huang N. W., Simoncsits A., Susic S., Rahman K., Marusic L., Chen J. Human DNA helicase II: a novel DNA unwinding enzyme identified as the Ku autoantigen. EMBO J. 1994 Oct 17;13(20):4991–5001. doi: 10.1002/j.1460-2075.1994.tb06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umek R. M., Kowalski D. The ease of DNA unwinding as a determinant of initiation at yeast replication origins. Cell. 1988 Feb 26;52(4):559–567. doi: 10.1016/0092-8674(88)90469-2. [DOI] [PubMed] [Google Scholar]
- Walker S. S., Francesconi S. C., Eisenberg S. A DNA replication enhancer in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4665–4669. doi: 10.1073/pnas.87.12.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. S., Malik A. K., Eisenberg S. Analysis of the interactions of functional domains of a nuclear origin of replication from Saccharomyces cerevisiae. Nucleic Acids Res. 1991 Nov 25;19(22):6255–6262. doi: 10.1093/nar/19.22.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Gibson S., Tye B. K. Mcm2 and Mcm3, two proteins important for ARS activity, are related in structure and function. Genes Dev. 1991 Jun;5(6):944–957. doi: 10.1101/gad.5.6.944. [DOI] [PubMed] [Google Scholar]
- Yaneva M., Busch H. A 10S particle released from deoxyribonuclease-sensitive regions of HeLa cell nuclei contains the 86-kilodalton-70-kilodalton protein complex. Biochemistry. 1986 Sep 9;25(18):5057–5063. doi: 10.1021/bi00366a013. [DOI] [PubMed] [Google Scholar]