Abstract
Purpose
UTL-5g is a novel small-molecule chemoprotector that lowers hepatotoxicity, nephrotoxicity, and myelotoxicity induced by cisplatin through TNF-α inhibition among other factors. The objective of this study was to investigate whether UTL-5g can reduce the overall acute toxicity of cisplatin and increase cisplatin tolerability in mice.
Materials and Methods
BDF1 female mice were treated individually with UTL-5g (suspended in Ora-Plus) by oral gavage at 60 mg/kg, 30 min before i.p. injection of cisplatin at 10, 15, and 20 mg/kg respectively on Day 0. Starting from Day 1, individual mice were again treated daily by the same dose of UTL-5g for 4 consecutive days. Survivals and bodyweights were monitored.
Results
UTL-5g treatment increased the survival rate and delayed the time to death for mice treated with 150% of the maximum tolerated dose (MTD) of cisplatin (15 mg/kg). Likewise, at 200% of the MTD of cisplatin (20 mg/kg), treatment of UTL-5g increased the survival rate and delayed the time to death. Treatment of UTL-5g did not have a significant effect on weight-loss induced by cisplatin indicating that bodyweight may not be a sensitive enough measure for chemoprotection of UTL-5g against cisplatin.
Conclusions
In summary, UTL-5g delayed deaths and increased survival rates of mice treated by high doses of cisplatin indicating that UTL-5g is capable of reducing the overall acute toxicity of cisplatin and increased cisplatin tolerability in mice; this is in line with the specific chemoprotective effects of UTL-5g previously reported. Further investigation of UTL-5g in combination with cisplatin is warranted.
Keywords: UTL-5g, cisplatin, maximum tolerated dose, toxicity, animal death/survival, bodyweight
Introduction
Cisplatin is associated with several side effects resulted from hepatotoxicity, nephrotoxicity, and myelotoxicity [1–4]. Therefore, it is of great interest to investigate/develop novel chemoprotective agents to reduce the overall toxicity associated with cisplatin and other platinum drugs.
A chemoprotective agent that reduces the side effects of cisplatin without affecting its therapeutic effect would have significant clinical benefit. Although a number of natural and synthetic compounds have been shown to be chemoprotective [5–7], the only FDA approved and generally accepted chemoprotective drug for cisplatin therapy is amifostine, which is a sulfur-containing agent that reduces toxicity due to various chemotherapy and radiotherapy regimens [8–11]. Amifostine has some chemoprotective effects against cisplatin-related renal toxicity and neutropenia due to cisplatin-cyclophosphamide combination therapy [11]. However, it might quench cisplatin’s activity and may lower the efficacy of cisplain [12,13]. In addition, amifostine by itself is associated with significant side effects, including hypotension, nausea, and vomiting [9]. Therefore, there is a continued interest in finding significantly improved chemoprotectors. Among the potential chemoprotective agents under investigation, UTL-5g is a promising compound.
UTL-5g is a novel small-molecule TNF-α inhibitor; in our previous report, we showed that UTL-5g reduced cisplatin-induced specific side effects on live, kidney, and platelets as indicated by lowering elevated levels of AST, ALT, creatinine, BUN, and TNF-α as well as by increasing the reduced platelet count [14]. UTL-5g also showed liver protection for acute liver injury induced by radiation as indicated by lowering elevated levels of AST, ALT, and TNF-α [13]. However, it is not clear whether UTL-5g can reduce the overall acute toxicity for animals treated by cisplatin. In this work, we set out to investigate whether UTL-5g can increase the tolerability of cisplatin and reduce the overall acute toxicity in mice treated with high doses of cisplatin. More specifically, we set out to monitor effects of UTL-5g on animal survival rates and survival times for mice treated with high doses of cisplatin.
Materials and methods
Animals
Female BDF1 (10 wk old, ~21 g each) were purchased from Charles River (Wilmington, MA). Principles of laboratory animal care (NIH publication No. 85–23, revised 1985) were followed and animal treatment was in full accordance with the Institutional Animal Care and Use Committee (IACUC) Guidelines for the care and management of laboratory animals.
Reagents
Cisplatin (Sigma-Aldrich) was dissolved in saline to make appropriate concentrations for i.v. injection (0.25 mL per injection). UTL-5g (Lot#1182-MEM-3D, Purity > 99%) was synthesized at Kalexsyn Medicinal Chemistry (Kalamazoo, Michigan). UTL-5g was weighed and compounded with Ora-Plus® (Paddock Laboratories, Minneapolis, Minnesota) in a mortar and pestle according to the instruction provided by the manufacturer to prepare a suspension of UTL-5g at 4.8 mg/mL (0.25 mL per administration, equivalent to 60 mg/kg); Ora-Plus® is an aqueous-based vehicle consisting of a blend of suspending agents having a high degree of colloidal activity. Other reagents/chemicals were purchased from Sigma-Aldrich unless otherwise specified.
Methods
Based on our preliminary experience, 10, 15, and 20 mg/kg of cisplatin were selected for this study and 60 mg/kg of oral UTL-5g was selected. Forty BDF1 female mice were randomly divided into 8 groups and treated (starting from Day 0) as described below:
Vehicle control: 0.25 mL of saline by oral gavage, daily × 5
UTL-5g by oral gavage, 60 mg/kg, daily × 5
Cisplatin by i.v. injection, 10 mg/kg
Cisplatin by i.v. injection, 15 mg/kg
Cisplaitn by i.v. injection, 20 mg/kg
UTL-5g, 60 mg/kg, by oral gavage; 30 min later, cisplatin by i.v. injection, 10 mg/kg. After that, each animal was treated by UTL-5g once a day for 4 more days.
UTL-5g, 60 mg/kg, by oral gavage; 30 min later, cisplatin by i.v. injection, 15 mg/kg. After that, each animal was treated by UTL-5g once a day for 4 more days.
UTL-5g, 60 mg/kg, by oral gavage; 30 min later, cisplatin by i.v. injection, 20 mg/kg. After that, each animal was treated by UTL-5g once a day for 4 more days.
Animal deaths were monitored and bodyweights were measured periodically during the study. Kaplan-Meier survival curve was used to show the survival patterns of different groups.
Results and discussion
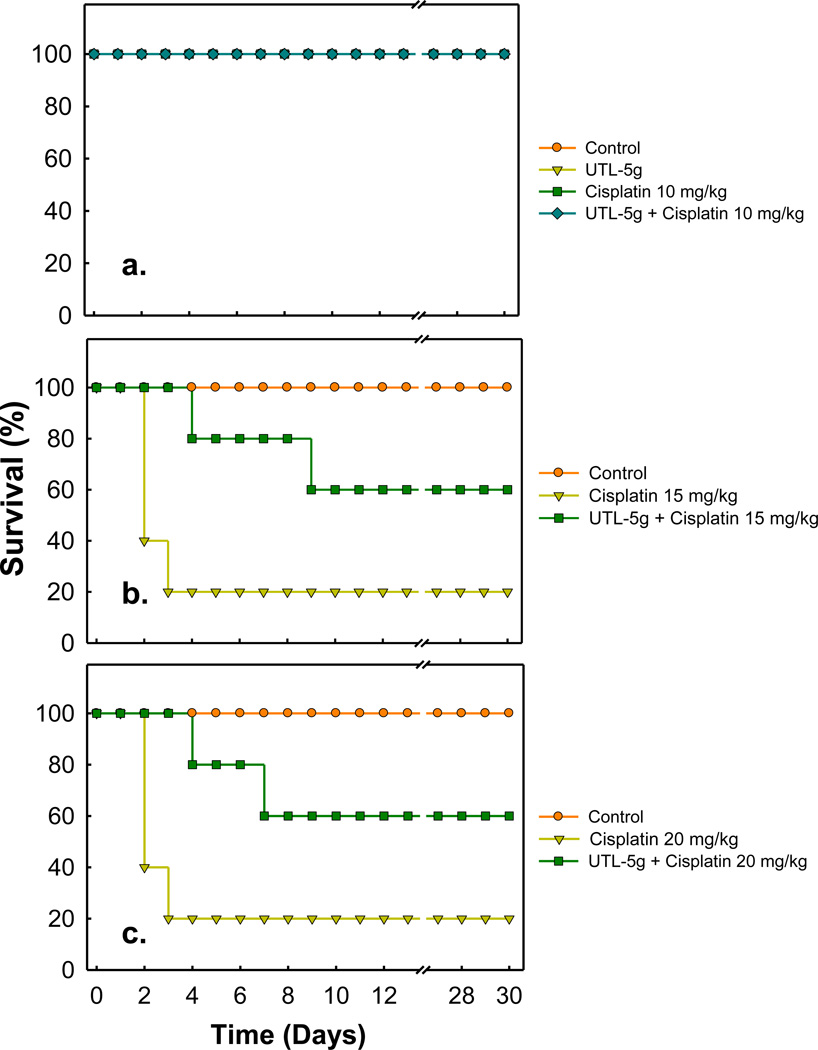
In the context of the present study, 10 mg/kg is determined as the MTD of cisplatin; the value is in the same order of the MTD (7.5 mg/kg) reported by Leite et al. [15]. As shown in Fig. 1a, at 10 mg/kg of cisplatin, all mice survived for 30 days and no effect was noted for UTL-5g treatment. At 150% MTD of cisplatin (15 mg/kg, Fig. 1b), 40% of the mice survived on day 2 and only 20% survived on day 3. UTL-5g treatment not only increased the survival rate but also delayed the time to death, 80% survival on day 4 and 60% survival on day 9. At 200% MTD of cisplatin (20 mg/kg, Fig. 1c), 40% of the mice survived on day 2 and only 20% survived on day 3. Again, treatment of UTL-5g not only increased the survival rate but also delayed the time to death, 80%/60% survival on day 4/7. The results are in line with our previous studies in which UTL-5g was shown to have a specific chemoprotective effect in liver, kidney, and platelets for mice treated with cisplatin [14]. In addition, in the previous chemoprotection study, UTL-5g was suspended in a mixture of dimethyl sulfoxide (DMSO), cremophor/propylene glycol (60/40 v/v), & saline, and administered by i.p. injection [14], whereas in the present study, UTL-5g was suspended in Ora-Plus and administered orally. Thus it can be concluded that oral administration of UTL-5g has sufficient bioavailability to show its chemoprotective effect.
Fig. 1.
Effect of UTL-5g on the survival rates for animals treated with cisplatin. BFD1 mice were randomly divided into eight groups and treated according to the following: (1) Vehicle control: 0.25 mL of saline by oral gavage, dailyx5; (2) UTL-5g by oral gavage, 60 mg/kg, daily × 5; (3) Cisplatin by i.v. injection, 10 mg/kg; (4) Cisplatin by i.v. injection, 15 mg/kg; (5) Cisplaitn by i.v. injection, 20 mg/kg; (6) UTL-5g, 60 mg/kg, by oral gavage; 30 min later, cisplatin by i.v. injection, 10 mg/kg. After that, each animal was treated by UTL-5g once a day for 4 more days. (7) UTL-5g, 60 mg/kg, by oral gavage; 30 min later, cisplatin by i.v. injection, 15 mg/kg. After that, each animal was treated by UTL-5g once a day for 4 more days; (8) UTL-5g, 60 mg/kg, by oral gavage; 30 min later, cisplatin by i.v. injection, 20 mg/kg. After that, each animal was treated by UTL-5g once a day for 4 more days.
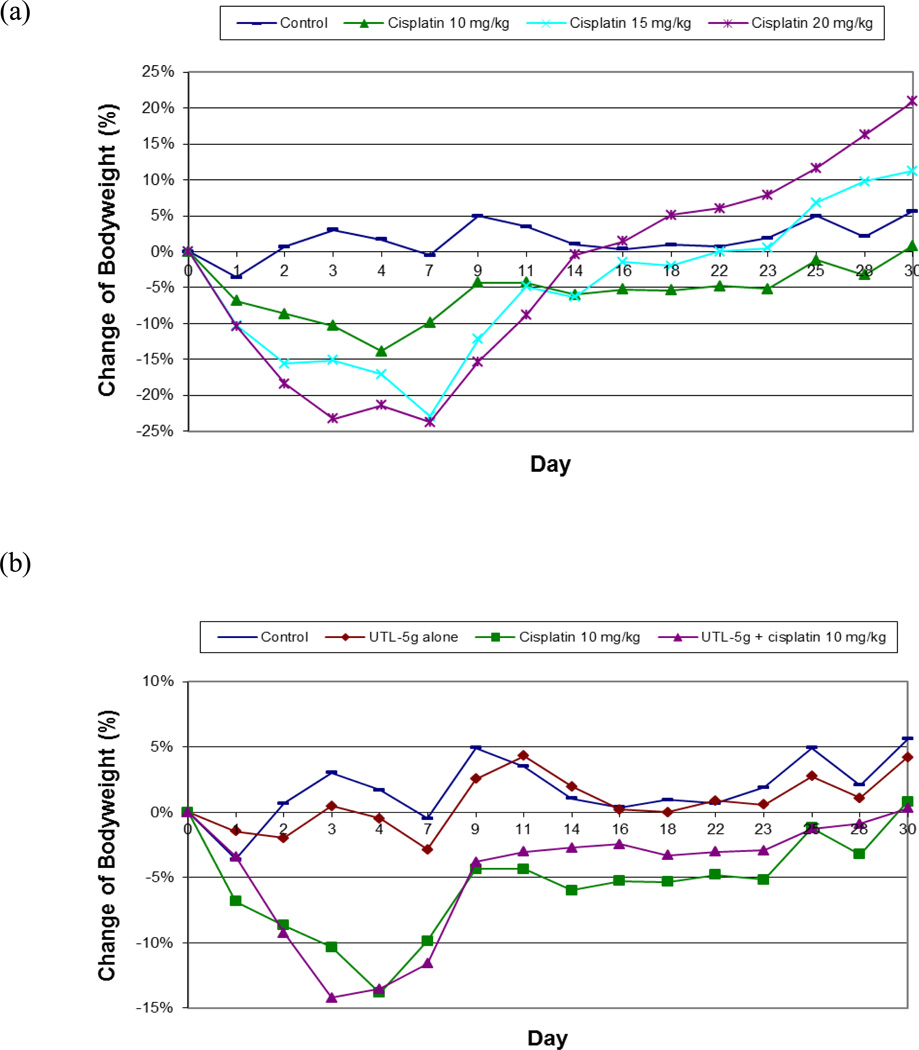
Weight loss could be used as a general toxicity marker in an MTD study. As shown in Fig. 2a, average weight loss of the mice in each group is generally in a cisplatin-dose dependent manner, animals lost more weights when treated with higher doses of cisplatin (15 and 20 mg/kg) as compared to animals treated with a lower dose of cisplatin (10 mg/kg). In addition, the average body weight in the 10 mg/kg group reached a nadir on Day 4 and, after that, mice started to recover the bodyweights and gradually returned to normal weight range. For the higher doses (15 and 20 mg/kg), mice continued to lose weights after Day 4 and reached the nadir on Day 7. After Day 7, surviving mice started to recover the bodyweights and returned to normal weight range, except the group treated by 20 mg/kg of cisplatin, which was from a single surviving mouse thus not representative. It can be concluded that animals treated with a lower dose of cisplatin (10 mg/kg) started to recover body weights earlier than those treated with higher doses of cisplatin (15 and 20 mg/kg).
Fig. 2.
(a) Comparison of the average body weights for 4 groups of BDF1 mice treated with (1) saline (Control), (2) cisplatin, 10 mg/kg, (3) cisplatin, 15 mg/kg, and (4) cisplatin, 20 mg/kg respectively
(b) Comparison of the average body weights for 4 groups of BDF1 mice treated with (1) Control (saline), (2) UTL-5g, (3) cisplatin, 10 mg/kg, and (4) UTL-5g and cisplatin respectively
Standard deviations are in the order of 10%, but not shown in order to make it easier to see the change pattern of bodyweights in individual groups. Student’s t-test shows p<0.005 comparing Control vs. (UTL-5g alone) and p<0.005 comparing (cisplatin 10 mg/kg) vs. (UTL-5g + cisplatin 10 mg/kg).
As to the effect of UTL-5g, when mice were treated with UTL-5g by itself, the animal bodyweights were not affected significantly over the study period indicating the low overall toxicity of UTL-5g (Fig. 2b). The two groups treated by 10 mg/kg of cisplatin, with and without UTL-5g, both showed the same pattern of weight loss. Similar weight-loss patterns were observed for the two groups treated by 15 mg/kg cisplatin, with and without UTL-5g. However, the survival rates are different (as shown in Fig. 1b) indicating that weight loss may not be a sensitive enough marker for the current study. For mice treated with 20 mg/kg of cisplatin and UTL-5g, the nadir was at Day 7, but the weight recovery rate was much slower as compared to mice treated with lower doses of cisplatin (15 and 10 mg/kg) and UTL-5g. This indicates that 20 mg/kg of cisplatin may be too high a dose for UTL-5g to show its protective effect in body weight.
Based on the current experimental conditions, pretreatment with UTL-5g (30 min before cisplatin) by oral administration at 60 mg/kg on Day 0, followed by UTL-5g (60 mg/kg, daily × 4 from Day 1), shows positive effects in increasing the survival rates and extending the survival times. Although the formulation and the route of administration of UTL-5g in this work are different from our previous study, the results complement previously reported specific chemoprotective effects of UTL-5g against the toxicity induced by cisplatin (hepatotoxicity, renal toxicity, and myelotoxicity). In addition, it may be worthwhile to further investigate the doses and administration regimens of UTL-5g to optimize its positive impact on the tolerability of cisplatin.
Acknowledgements
This work was supported by NIH/NCI grant 5R44CA141749-03.
Footnotes
Conflict of Interest
Disclosures: None
Contributor Information
Jiajiu Shaw, 21st Century Therapeutic, Inc., 1366 Hilton Rd., Ferndale, Michigan 48220, jiajiushaw@gmail.com.
Joseph Media, Henry Ford Health System, Detroit, Michigan 48202.
Ben Chen, 21st Century Therapeutics, Inc., Ferndale, Michigan 48220.
Fredrick Valeriote, Henry Ford Health System, Detroit, Michigan 48202.
References
- 1.Madias NE, Harrington JT. Platinum nephrotoxicity. Am J Med. 1978;65(2):307–314. doi: 10.1016/0002-9343(78)90825-2. [DOI] [PubMed] [Google Scholar]
- 2.Cavalli F, Tschopp L, Sonntag RW, Zimmermann A. A case of liver toxicity following cis-dichlorodiammineplatinum(II) treatment. Cancer Treat Rep. 1978;62(12):2125–2126. [PubMed] [Google Scholar]
- 3.Cersosimo RJ. Hepatotoxicity associated with cisplatin chemotherapy. Ann Pharmacother. 1993;27(4):438–441. doi: 10.1177/106002809302700408. [DOI] [PubMed] [Google Scholar]
- 4.Pollera CF, Ameglio F, Nardi M, Vitelli G, Marolla P. Cisplatin-induced hepatic toxicity. J Clin Oncol. 1987;5(2):318–319. doi: 10.1200/JCO.1987.5.2.318. [DOI] [PubMed] [Google Scholar]
- 5.Subbiah U, Raghunathan M. Chemoprotective action of resveratrol and genistein from apoptosis induced in human peripheral blood lymphocytes. J Biomol Struct Dyn. 2008;25(4):425–434. doi: 10.1080/07391102.2008.10507191. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Bianchet MA, Talalay P, Amzel LM. The three-dimensional structure of NAD(P)H:quinone reductase, a flavoprotein involved in cancer chemoprotection and chemotherapy: mechanism of the two-electron reduction. Proc Natl Acad Sci U S A. 1995;92(19):8846–8850. doi: 10.1073/pnas.92.19.8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Psotova J, Chlopcikova S, Miketova P, Hrbac J, Simanek V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part III. Apigenin, baicalelin, kaempherol, luteolin and quercetin. Phytother Res. 2004;18(7):516–521. doi: 10.1002/ptr.1462. [DOI] [PubMed] [Google Scholar]
- 8.Markman M. Amifostine in reducing cisplatin toxicity. Seminars in oncology. 1998;25(5):522–524. [PubMed] [Google Scholar]
- 9.Phillips KA, Tannock IF. Design and interpretation of clinical trials that evaluate agents that may offer protection from the toxic effects of cancer chemotherapy. J Clin Oncol. 1998;16(9):3179–3190. doi: 10.1200/JCO.1998.16.9.3179. [DOI] [PubMed] [Google Scholar]
- 10.Korst AE, Gall HE, Vermorken JB, van der Vijgh WJ. Pharmacokinetics of amifostine and its metabolites in the plasma and ascites of a cancer patient. Cancer Chemother Pharmacol. 1996;39(1–2):162–166. doi: 10.1007/s002800050553. [DOI] [PubMed] [Google Scholar]
- 11.Culy CR, Spencer CM. Amifostine: an update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. Drugs. 2001;61(5):641–684. doi: 10.2165/00003495-200161050-00012. [DOI] [PubMed] [Google Scholar]
- 12.Volckova E, Dudones LP, Bose RN. HPLC determination of binding of cisplatin to DNA in the presence of biological thiols: implications of dominant platinum-thiol binding to its anticancer action. Pharmaceutical research. 2002;19(2):124–131. doi: 10.1023/a:1014268729658. [DOI] [PubMed] [Google Scholar]
- 13.Sadowitz PD, Hubbard BA, Dabrowiak JC, Goodisman J, Tacka KA, Aktas MK, Cunningham MJ, Dubowy RL, Souid AK. Kinetics of cisplatin binding to cellular DNA and modulations by thiol-blocking agents and thiol drugs. Drug metabolism and disposition: the biological fate of chemicals. 2002;30(2):183–190. doi: 10.1124/dmd.30.2.183. [DOI] [PubMed] [Google Scholar]
- 14.Shaw J, Chen B, Huang W-H, Lee A-R, Media J, Valeriote F. The smalle-molecule TNF-A modulator, UTL-5g, reduces side effects induced by cisplaitn and enhances the therapeutic effect of cisplaitn in vivo. Journal of Experimental Therapeutics and Oncology. 2011;8 (in press) [PubMed] [Google Scholar]
- 15.Leite EA, Lana AM, Junior AD, Coelho LG, De Oliveira MC. Acute toxicity study of cisplatin loaded long-circulating and pH-sensitive liposomes administered in mice. Journal of biomedical nanotechnology. 2012;8(2):229–239. doi: 10.1166/jbn.2012.1388. [DOI] [PubMed] [Google Scholar]