Abstract
Background
IgE-mediated allergic reactions to cashews and other nuts can trigger life-threatening anaphylaxis. Proactive therapies to decrease reaction severity do not exist.
Objectives
We aimed to determine the efficacy of pepsin-digested cashew proteins used as immunotherapy in a murine model of cashew allergy.
Methods
Mice were sensitized to cashew and then underwent challenges with digested or native cashew allergens to assess the allergenicity of the protein preparations. Using native or pepsinized cashew proteins, mice underwent oral or intraperitoneal sensitization protocols to determine the immunogenic properties of the protein preparations. Finally, cashew-sensitized mice underwent an immunotherapy protocol with native or pepsinized cashew proteins and subsequent provocation challenges.
Results
Pepsinized cashew proteins elicited weaker allergic reactions than native cashew proteins but importantly retained the ability to stimulate cellular proliferation and cytokine production. Mice sensitized with pepsinized proteins reacted on challenge with native allergens, demonstrating that pepsinized allergens retain immunogenicity in vivo. Immunotherapy with pepsinized cashew allergens significantly decreased allergic symptoms and body temperature decrease relative to placebo after challenge with native and pepsinized proteins.
Immunologic changes were comparable after immunotherapy with native or pepsinized allergens: TH2-type cytokine secretion from splenocytes was decreased, whereas specific IgG1 and IgG2a levels were increased.
Conclusions
Pepsinized cashew proteins are effective in treating cashew allergy in mice and appear to work through the same mechanisms as native protein immunotherapy.
Keywords: Food allergy, tree nut allergy, cashew, immunotherapy, pepsin, murine model
Food allergies affect approximately 6% to 8% of children and 4% of adults in the United States and other westernized societies.1 Allergic reactions to foods range in severity from mild skin symptoms to anaphylactic shock and account for an estimated 200,000 emergency department visits annually.2 Peanuts and tree nuts appear to cause the most severe reactions and are implicated in the majority of fatal food-induced reactions.3 An estimated 1.4% of the US population is allergic to peanuts or tree nuts, with an apparent doubling of those allergic to tree nuts in the last 5 years.4 Allergic reactions to cashew have been described as particularly severe in nature.5
Typically, IgE-mediated allergic disease can be treated by using subcutaneous administration of allergen extracts, as is routinely done for aeroallergen and insect venom allergies. However, administration of intact allergens for peanut allergy was deemed unsafe, leaving researchers to find alternate therapeutic strategies for food allergy.6 Oral immunotherapy,7-9 sublingual immunotherapy,10 anti-IgE injections,11 and Chinese herbs12,13 are all being tested in human subjects with peanut and other food allergies. Other approaches have targeted the unique properties of allergens that serve to reduce allergenicity while retaining immunogenicity. For example, mutated engineered peanut proteins were generated such that IgE binding was diminished but T cell–stimulating properties were maintained.14 Peptide immunotherapy has shown promise in clinical trials for bee venom15 and cat allergy,16,17 as well as in animal models of food allergy.18
Previous studies have shown that pepsin and trypsin digestion products of peanut allergens generated in vitro retain immunogenic properties both in human cell assays19,20 and in animal models.21 The ability of these digestion products to stimulate T cells makes them interesting immunotherapy candidates for food allergy. We hypothesized that pepsin-digested nut allergens used as immunotherapy would downregulate allergic responses in sensitized mice. In this study we used 2 murine models of cashew allergy to investigate the immunogenic and allergenic properties of pepsin-digested cashew allergens. In an oral sensitization model cashew proteins were fed intragastrically to mimic the route of sensitization in human subjects. In a second murine model we used intraperitoneal injections to induce hypersensitivity, which bypasses gastroduodenal digestion. After our initial studies, we administered pepsinized cashew proteins in orally sensitized mice using an established immunotherapy protocol.22
METHODS
Pepsin digestion of cashew proteins
Native cashew protein extract (nCSH) was prepared as previously described23 and then diluted to a working concentration of 10 mg/mL in PBS. The pH of the cashew protein solution was adjusted to 2.0 with 6 mol/L HCl, and then pepsin (porcine derived, 3000 U/mg; Calbiochem, San Diego, Calif) was added at a final concentration of 100 μg/mL. The pepsin protein constituted 1% (wt/wt) of the total protein in solution. The reaction was allowed to proceed for 30 minutes at 37°C, at which time the pH was adjusted to 7.5 with 6 mol/L NaOH to inactivate pepsin. The resulting products were analyzed by using SDS-PAGE. The pepsin-digested cashew proteins will be referred to as pepsin-digested cashew protein extract (pCSH).
Mice
C3H/HeJ female mice were obtained from Jackson Laboratories (Bar Harbor, Me) at 3 weeks of age and then allowed to acclimate to their new housing for 2 weeks before beginning experimental protocols. Mice were housed under pathogen-free conditions with free access to water and food while being kept on a diet free of any tree nuts (eg, cashews, walnuts, and almonds) during the course of the study.
Murine models of cashew allergy
We used 2 previously reported murine models of food allergy. In the oral sensitization model24 mice were fed 2 mg of nCSH or pCSH (protein antigen) with 10 μg of cholera toxin (List Biologics, Campbell, Calif) on days 1, 8, and 15 and then boosted on day 22 with 5 mg of protein antigen plus 10 μg of cholera toxin. In the intraperitoneal model mice were injected with 0.5 mg of protein antigen with 2 mg of aluminum hydroxide (Alum; Pierce, Rockford, Ill) on days 1, 8, and 22.22 Mice were bled on day 36 to measure cashew-specific IgE levels. All procedures were approved by the Institutional Animal Care and Use Committee at Duke University Medical Center.
Food challenges in mice
Mice were challenged by means of intraperitoneal injection of nCSH or pCSH. In both the oral and intraperitoneal sensitization models, mice were challenged with up to 1.0 mg of nCSH or pCSH. After injection, mice were monitored for allergic symptoms and scored on a 0- to 5-point scale (0, no symptoms; 1, scratching around the nose and head; 2, puffiness around the eyes and mouth with reduced activity; 3, labored respiration, cyanosis around the mouth and tail, or both; 4, no activity after prodding or tremor and convulsion; and 5, death) at 30 minutes after challenge.24 Body temperatures were measured with a rectal probe before the challenge and at 30 minutes after the challenge. Body temperatures are reported as the change in body temperature (ie, 30 minute-reading minus baseline reading).
Immunotherapy in sensitized mice
After mice were sensitized as described above, they underwent 3 intraperitoneal injections per week (ie, Monday, Wednesday, and Friday) over a 4-week immunotherapy protocol similar to those previously described.14,22 The model thus represents immunotherapy administration to animals with an established hypersensitivity to cashew. By using the oral sensitization model, treatment began at 50 μg per injection of nCSH or pCSH during week 1, 125 μg per injection during week 2, and maintenance dosing at 200 μg per injection during weeks 3 and 4. Mice were bled 10 days after completion of the immunotherapy protocol to measure immunoglobulin levels and challenged 4 days later. Naive mice did not receive cashew during sensitization and only received PBS during the immunotherapy phase and thus represent a nonsensitized control group that was expected not to react to nCSH or pCSH challenges.
Cytokines, proliferations, and immunoglobulins
Splenocyte cultures were carried out as previously described.23 Briefly, 5 million cells were cultured in the presence of 100 μg/mL protein antigen in 2 mL of culture medium for 96 hours. Culture supernatants were collected, and cytokines were quantified by using ELISA, according to the manufacturer’s recommendations (R&D Systems, Minneapolis, Minn). Proliferations were measured with a tritiated thymidine incorporation assay after 96 hours in culture, as previously described.23 Cashew-specific IgE, IgG1, and IgG2a levels were quantified, as previously reported.23
Statistical analyses
Statistical analyses were performed with GraphPad Prism 5 software (GraphPad Software, La Jolla, Calif). Allergic symptom scores were compared with the Mann-Whitney test. Body temperatures, cytokine levels, and immunoglobulin levels were compared with the unpaired 2-tailed t test. P values of less than .05 were considered significant.
RESULTS
pCSH is hypoallergenic in vivo compared with nCSH
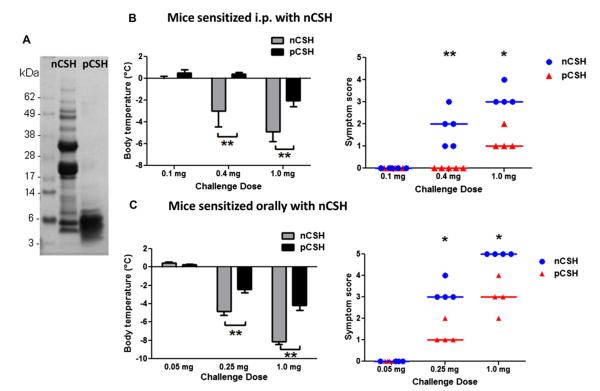
Native cashew proteins digested with pepsin for 30 minutes generated decreased molecular weight protein fragments. Intact nCSH proteins range in molecular weight from 4.5 to greater than 70 kDa, whereas pCSH fragments are less than 10 kDa, with the majority of peptides in the 3- to 6-kDa range (Fig 1, A). The large vicilin and legumin proteins are obviously hydrolyzed by pepsin. To determine whether pCSH would result in decreased allergic reactions, we used 2 murine models of cashew allergy.
FIG 1.
Allergic reactions to native or pepsin-digested proteins in mice. A, SDS-PAGE gel showing nCSH and pCSH. B, Mice sensitized by means of intraperitoneal injection of nCSH were challenged with nCSH or pCSH at increasing doses and assessed for body temperatures and symptom scores. C, Orally sensitized mice challenged with nCSH or pCSH at increasing doses were assessed for body temperatures and symptom scores. Circles or triangles represent individual mice. Bars represent means with SDs. *P < .05 and **P < .01. Data shown are results after initial experiments to determine optimal challenge doses in groups consisting of 3 mice each.
Mice sensitized by means of intraperitoneal injection with nCSH had significantly reduced allergic reactions to pCSH compared with reactions seen after nCSH challenges. A dose-response study demonstrated that neither protein preparation causes reactions at 0.1 mg, but at the 0.4- and 1.0-mg challenges, nCSH causes significantly more severe allergic symptoms and body temperature decreases than pCSH (Fig 1, B). Mice sensitized by means of oral gavage with nCSH also underwent a dose-response study and were similarly shown to require a much larger challenge dose of pCSH than nCSH to induce comparable allergic symptoms and body temperature decreases (Fig 1, C). Approximately 4 times the amount of pCSH is required to induce the same reaction severity as a nCSH challenge.
Splenocytes from nCSH-sensitized mice are responsive to pCSH
Spleen cells from mice sensitized either orally or intraperitoneally with nCSH were used to study T-cell responses. Mice sensitized by means of oral gavage with nCSH had very similar T-cell responses irrespective of whether cells were cultured in the presence of nCSH or pCSH (Fig 2, A). Levels of the TH2-type cytokines IL-4, IL-13, and IL-5, as well as the TH1-type cytokine IFN-γ, were not significantly different between the 2 cashew preparations. These results indicate that pCSH retains critical T-cell epitopes within the peptide fragments. In mice sensitized intraperitoneally with nCSH, pCSH and nCSH both caused cellular proliferations that were not significantly different (data not shown). Comparable levels of IL-4 and IFN-γ were found for cells cultured with either nCSH or pCSH proteins in the intraperitoneal sensitization model as well (Fig 2, B).
FIG 2.

Cellular responses to nCSH and pCSH. Splenocytes from orally or intraperitoneally sensitized mice (A and B, respectively) were cultured with nCSH or pCSH and assessed for TH2/TH1 cytokine production. Bars represent means with SDs by using 4 individual mice per group. Data shown are representative of 2 separate experiments.
pCSH retains immunogenicity in vivo and primes mice for hypersensitivity reactions
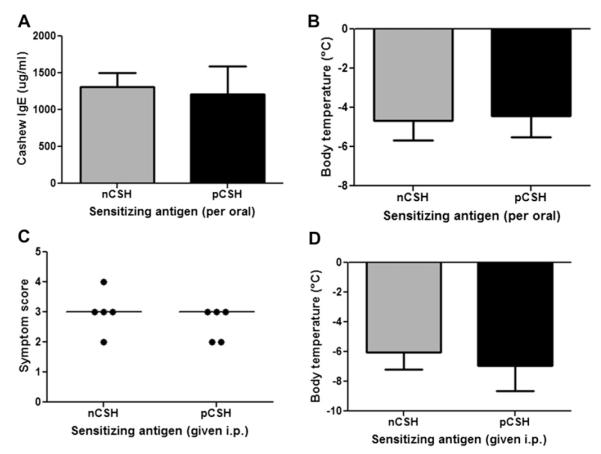
Mice were sensitized orally or by means of intraperitoneal injection with pCSH and then studied for immunologic parameters. Mice administered pCSH by means of oral gavage had comparable levels of cashew-specific IgE as mice sensitized with nCSH (Fig 3, A). Secreted cytokines from splenocytes of these mice demonstrated that pCSH sensitization results in a strong TH2-skewed response, which is not different than that seen after sensitization with nCSH (data not shown). After sensitization with either nCSH or pCSH, mice were challenged with nCSH. Challenge outcomes demonstrated that mice sensitized with pCSH have comparable allergic reactions, as measured based on both symptoms and body temperature (Fig 3, B), as mice sensitized with nCSH.
FIG 3.
In vivo immunogenicity of nCSH and pCSH. Mice were sensitized with nCSH or pCSH by means of oral or intraperitoneal (i.p.) administration. Orally sensitized mice were assessed for specific IgE (A) and challenged with nCSH to determine allergic status by change in body temperature (B). Mice sensitized intraperitoneally were challenged with nCSH and assessed for symptom scores (C) and body temperature changes (D). Circles represent individual mice. Bars represent means with SDs.
Sensitization in mice by means of intraperitoneal injection is distinct from sensitization through the oral route in that no gastric digestion occurs. Because it is possible that mice fed orally with nCSH and pCSH end up being sensitized to very similar protein fragments in vivo, we tested whether intraperitoneal injection of nCSH or pCSH would produce the same outcomes observed in the oral sensitization model. The intraperitoneally sensitized mice were challenged with nCSH, and both groups of mice experienced severe reactions, with no differences in symptom scores and body temperatures (Fig 3, C and D). Cytokine responses again demonstrated a TH2-skewed response in both groups of mice (data not shown).
Sera from mice sensitized by means of intraperitoneal injection with nCSH or sensitized orally with nCSH were compared by using ELISA to assess differences in the binding preferences of IgE to intact or digested linearized proteins. Ratios of anti-nCSH to anti-pCSH IgE levels were calculated for the 2 different methods of allergic sensitization. The ratio of IgE binding nCSH/pCSH was significantly higher in the mice sensitized by means of intraperitoneal injection compared with that seen in orally sensitized mice (Fig 4). These findings indicate that intraperitoneal sensitization produces IgE that preferentially binds structurally intact cashew proteins more so than linear peptides. Mice sensitized by means of oral gavage, in contrast, have IgE that binds nCSH and pCSH with less preference, providing evidence that mice sensitized orally have IgE that recognizes both structural and linear epitopes of cashew proteins more so than IgE from mice sensitized by means of intraperitoneal injection.
FIG 4.
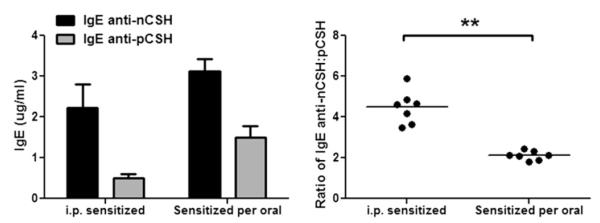
Comparison of IgE binding to nCSH and pCSH in mice sensitized by means of intraperitoneal (i.p.) injection or oral gavage. Mice were sensitized with nCSH by means of either intraperitoneal injection or oral gavage, and serum IgE levels were measured against nCSH or pCSH by using ELISA. Absolute levels of IgE are shown, as well as ratios of anti-nCSH/anti-pCSH IgE. Bars represent means with SDs. Circles represent individual murine sera. **P < .01.
Immunotherapy with pCSH reduces allergic reactions in orally sensitized mice
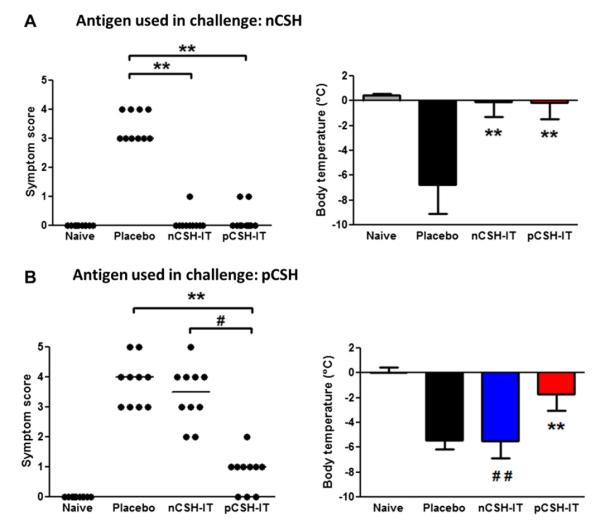
Our initial findings that pCSH is hypoallergenic but strongly immunogenic led us to hypothesize that pCSH would be a useful therapeutic reagent. Cashew hypersensitivity was established in mice and then immunotherapy was initiated to test this hypothesis. Orally sensitized mice treated with pCSH or nCSH immunotherapy displayed no differences in allergic reactions during nCSH challenge (Fig 5, A). Both mice undergoing pCSH immunotherapy and those undergoing nCSH immunotherapy had median symptom scores of zero, whereas the placebo group had scores and body temperature decreases indicative of severe allergic reactions. However, when these groups of mice were challenged with pCSH, the nCSH immunotherapy group reacted as severely as the placebo group, whereas pCSH immunotherapy mice had significantly less body temperature decrease and significantly lower symptom scores (Fig 5, B). These findings indicate that pCSH immunotherapy can prevent anaphylaxis after challenges with both nCSH and pCSH, whereas nCSH immunotherapy is only effective in preventing reactions induced by nCSH challenge.
FIG 5.
Immunotherapy (IT) with nCSH and pCSH in orally sensitized mice. Mice were sensitized with nCSH and then administered nCSH, pCSH, or placebo immunotherapy. Naive mice are nonsensitized control animals. A, Challenges with 0.25 mg of nCSH were assessed for symptom scores and body temperatures. B, Challenges with 1.0 mg of pCSH were assessed for symptom scores and body temperatures. Circles represent individual mice. Bars represent means with SDs. **P < .01 versus placebo, #P < .05 versus pCSH immunotherapy, and ##P < .01 versus pCSH immunotherapy. Data shown are combined results from 2 independent experiments using 5 mice per group.
pCSH immunotherapy induces IgG production and decreases TH2 cytokine responses in orally sensitized mice
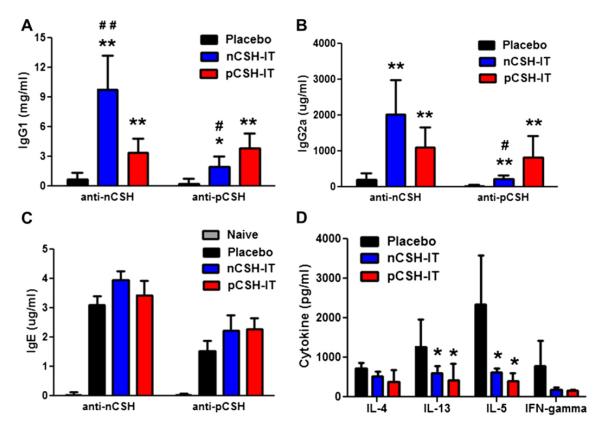
Immunologic changes associated with pCSH and nCSH immunotherapy were studied in orally sensitized mice. nCSH immunotherapy elicited dramatic increases in levels of IgG1 and IgG2a specific for nCSH; however, much lower levels were found that were anti-pCSH (Fig 6, A and B). pCSH immunotherapy induced approximately equal levels of anti-nCSH and anti-pCSH IgG1 or IgG2a. The IgG responses reflect the outcomes measured in nCSH and pCSH challenges. No significant differences were found between groups for IgE levels (Fig 6, C). Cytokine responses were greatly affected by both pCSH and nCSH immunotherapy because both led to significant decreases in IL-13 and IL-5 levels compared with those seen after placebo treatment (Fig 6, D).
FIG 6.
Humoral and cellular profiles after immunotherapy (IT) in orally sensitized mice. Mice sensitized orally with nCSH were given nCSH, pCSH, or placebo immunotherapy. Serum levels of anti-nCSH and anti-pCSH IgG1 (A), IgG2a (B), and IgE (C) after immunotherapy are shown. Cytokines secreted from splenocytes of the immunotherapy groups are also shown (D). Bars represent means with SDs. *P < .05 versus placebo, **P < .01 versus placebo, #P < .05 versus pCSH immunotherapy, and ##P < .01 versus pCSH immunotherapy. Data shown are representative of 2 experiments and include 5 mice per group.
DISCUSSION
Producing protein allergens that are hypoallergenic and immunogenic is a typical strategy when developing immunotherapy reagents for IgE-mediated allergies. Much work has focused on manipulating allergens with recombinant DNA technology. For example, site-directed mutation of allergens can destroy IgE-binding properties while retaining T-cell epitopes, as has been shown for the major peanut allergens.14,25 Another manipulation used in pollen allergy is to rearrange the domains of structural epitopes, as was done to produce a hypoallergenic Bet v 1 molecule.26 This approach, although often effective, is labor intensive with regard to identifying allergen IgE epitopes, manipulating genetic material of the allergens, and then producing and purifying the modified allergens from in vitro cell cultures. Peptide immunotherapy takes this strategy a step further by using small peptides incapable of cross-linking IgE on mast cells, but T cell–stimulating epitopes remain because the entire protein sequence of the allergen is represented.16
Pepsin digestion of allergens has been shown to decrease allergenicity, particularly in the context of oral allergy syndrome27 but also for food allergens.28 We hypothesized that pepsin-digested cashew allergens would elicit milder allergic reactions in mice sensitized to cashew proteins. A drastic difference in the molecular weight of cashew seed storage proteins is apparent after 30 minutes of pepsin digestion (Fig 1, A). The majority of cashew protein fragments in pCSH end up in the molecular weight range of 3 to 6 kDa. These peptides can still bind and cross-link IgE molecules, as evidenced by in vivo challenges; however, we found that pepsin digestion was enough to significantly decrease the allergenicity in mice sensitized by means of oral gavage or intraperitoneal injection. In both sensitization models approximately 4 to 5 times higher doses of pCSH were required to induce the same severity of reactions as nCSH. In our models pCSH is thus hypoallergenic compared with nCSH.
To determine the immunogenic potential of pCSH, we first studied T-cell responses in cell cultures. Splenocytes from mice sensitized to cashew by means of oral gavage or intraperitoneal injection responded to both nCSH and pCSH with no significant differences in terms of TH2- and TH1-type cytokines and cellular proliferation. Encouraged by the outcomes of pCSH stimulation in vitro, we determined the in vivo immunogenic potential of this protein preparation. pCSH administered orally induced IgE, TH2-type cytokine responses, and primed mice for allergic reactions to the same extent as nCSH sensitization. Additionally, pCSH administered intraperitoneally produced the same outcomes as nCSH administered intraperitoneally, clearly demonstrating that pCSH retains immunogenic properties in vivo and primes mice for allergic reactions. Importantly, Western blotting demonstrated that both forms of the sensitizing antigen, nCSH and pCSH, administered either orally or by means of intraperitoneal injection resulted in reactivity with the same cashew allergens, indicating that digestion products of cashew extract contain protein fragments with both B- and T-cell epitopes of vicilin and legumin allergens (data not shown). These findings collectively argue that for cashew antigens in C3H/HeJ mice, simulated gastric digestion does not significantly alter the antigen’s immunogenic potential.
The overall goal of these experiments was to determine whether pepsin could achieve similar results as other methods (ie, recombinant DNA technology) for producing immunotherapy reagents. Once we achieved both of the aforementioned criteria, reducing IgE-mediated reactions and retaining T cell–stimulating abilities, with simple pepsin digestion, we determined the efficacy of pCSH as an immunotherapy for cashew allergy. As shown in Fig 5, pCSH is highly effective for treating cashew allergy, with pCSH immunotherapy working as effectively as nCSH immunotherapy to decrease allergic reactions on challenge with nCSH. Both pCSH and nCSH immunotherapy resulted in significantly increased IgG1 and IgG2a anti-nCSH levels relative to placebo. Additionally, both immunotherapy reagents decreased TH2-type cytokine levels from cultured spleen cells, indicating that similar mechanisms are invoked by pCSH immunotherapy and nCSH immunotherapy.
Orally administered antigens do not always induce consistent anaphylactic reactions in this model,29 prompting us to mimic an oral challenge by injecting pCSH to produce more pronounced clinical symptoms. Interestingly, pCSH immunotherapy prevented allergic reactions to pCSH challenge, although nCSH immunotherapy did not (Fig 5, B). We hypothesize that this reflects the nature of the sensitizing antigen, which is likely pepsin digested, at least in part, in the oral sensitization model. The idea is that nCSH immunotherapy drives protective IgG responses to structural epitopes (surface exposed) of the allergens, whereas pCSH drives production of IgG against linearized epitopes (buried within the allergens), which likely mimic the sensitizing epitope’s B cells used to produce IgE. Indeed, mice sensitized by means of oral gavage have IgE that binds to pepsin-digested linearized cashew peptides with greater frequency than IgE from mice sensitized by means of intraperitoneal injection, which have IgE that preferentially binds structurally intact cashew proteins (Fig 4). This explanation fits with experimental data for the 2 immunotherapy groups’ IgG1 and IgG2a levels specific for pCSH, in which levels after pCSH immunotherapy are significantly higher than those after nCSH immunotherapy (Fig 6, A and B). This is an interesting and unexpected finding that highlights the potential importance of producing blocking IgG antibodies directed at the same epitopes that were used to produce IgE. This might be an important observation for clinical immunotherapy for food allergy in which both structural and linear epitopes have been reported30-32 and nonoral routes of sensitization, such as the epicutaneous route, might exist.33
Although these preclinical results in mice demonstrate the hypoallergenicity and efficacy of immunotherapy, future studies should focus on human cellular responses. For example, digested cashew proteins should be tested in basophil activation assays by using blood from patients with cashew allergy. T-cell responses also need to be investigated to determine whether pCSH can cause proliferation and cytokine secretion from human T cells. If successful, possible routes of administration could be through subcutaneous injection, sublingual dosing, or epicutaneous application. Furthermore, additional studies with cashew or other food allergens should also focus on optimization of enzymatic digestion times and use of other enzymes to strike an ideal balance of hypoallergenicity and capacity to stimulate T cells. In terms of utility for human studies, an enzymatic digestion approach might be more practical than approaches aimed at identifying all T-cell epitopes for each HLA type (ie, peptide immunotherapy) because the digested allergens might provide enough T cell–stimulating peptides for any patient with cashew allergy. Clearly, further studies are required before immunotherapy with pepsin-digested allergens should be attempted in clinical trials.
In conclusion, we have demonstrated that pCSH is an effective immunotherapy in a murine model of cashew allergy because pCSH can drive T- and B-cell responses with the added benefit of hypoallergenicity. This might represent a novel approach of immunotherapy for cashew allergy.
Clinical implications.
Immunotherapy with hypoallergenic digested food allergens does not require identification of individual T-cell epitopes and might generate beneficial IgG responses that are distinct from native allergen immunotherapy.
Acknowledgments
Supported by an NRSA F32 Fellowship to M.K. (1F32AI084332-01).
M. Kulis has received research support from the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) and is employed by Duke University. R. Guo is employed by Duke University. X.-P. Zhong has received research support from the NIH, the American Cancer Society, and the Food Allergy & Anaphylaxis Network (FAAN). A. W. Burks has received research support from the NIAID/NIH, the FAAN, the Food Allergy Initiative, the NIH, the National Peanut Board, Scientific Hospital Supplies, and the Wallace Research Foundation; has received travel support from the NIAID/NIH, the American Academy of Allergy, Asthma & Immunology (AAAAI), the American College of Allergy, Asthma & Immunology, and the European Academy of Allergy and Clinical Immunology; is on the board for the AAAAI, the FAAN, the US Food and Drug Administration, the Journal of Allergy and Clinical Immunology, and the NIH HAI; has received consultancy fees from Dannon Co Probiotics, ExploraMed Development, Intelliject, McNeil Nutritionals, Merck & Co, Novartis, Nutricia, Pfizer, Portola Pharmaceuticals, and Schering-Plough; is employed by the Duke University Medical Center and UNC North Carolina Children’s Hospital; receives royalties from UpToDate; has received payment for educational presentations development from Current Views; and has stock/stock options in Allertein and MastCell.
Abbreviations used
- nCSH
Native cashew protein extract
- pCSH
Pepsin-digested cashew protein extract
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(suppl):S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark S, Espinola JA, Rudders SA, Banerji A, Camargo CA., Jr. Frequency of US emergency department visits for food-related acute allergic reactions. J Allergy Clin Immunol. 2011;127:682–3. doi: 10.1016/j.jaci.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;119:1016–8. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–6. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Clark AT, Anagnostou K, Ewan PW. Cashew nut causes more severe reactions than peanut: case-matched comparison in 141 children. Allergy. 2007;62:913–6. doi: 10.1111/j.1398-9995.2007.01447.x. [DOI] [PubMed] [Google Scholar]
- 6.Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol. 1992;90:256–62. doi: 10.1016/0091-6749(92)90080-l. [DOI] [PubMed] [Google Scholar]
- 7.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. e291–7. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveria LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91. e81. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemoer A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–6. e641. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson HA, Leung DY, Burks AW, Lack G, Bahna SL, Jones SM, et al. A phase II, randomized, doubleblind, parallelgroup, placebocontrolled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127:1309–10. e1. doi: 10.1016/j.jaci.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Qu C, Srivastava K, Yang N, Busse P, Zhao W, et al. Food allergy herbal formula 2 protection against peanut anaphylactic reaction is via inhibition of mast cells and basophils. J Allergy Clin Immunol. 2010;126:1208–17. e1203. doi: 10.1016/j.jaci.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Patil SP, Yang N, Ko J, Lee J, Noone S, et al. Safety, tolerability, and immunologic effects of a food allergy herbal formula in food allergic individuals: a randomized, double-blinded, placebo-controlled, dose escalation, phase 1 study. Ann Allergy Asthma Immunol. 2010;105:75–84. doi: 10.1016/j.anai.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks W, et al. Persistent protective effect of heat-killed Escherichia coli producing “engineered,” recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol. 2003;112:159–67. doi: 10.1067/mai.2003.1622. [DOI] [PubMed] [Google Scholar]
- 15.Muller U, Akdis CA, Fricker M, Akdis M, Blesken T, Bettens F, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998;101:747–54. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 16.Larche M. Immunotherapy with allergen peptides. Allergy Asthma Clin Immunol. 2007;3:53–9. doi: 10.1186/1710-1492-3-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Worm M, Lee HH, Kleine-Tebbe J, Hafner RP, Laidier P, Healey D, et al. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J Allergy Clin Immunol. 2011;127:89–97. e81–14. doi: 10.1016/j.jaci.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Yang M, Yang C, Mine Y. Multiple T cell epitope peptides suppress allergic responses in an egg allergy mouse model by the elicitation of forkhead box transcription factor 3- and transforming growth factor-beta-associated mechanisms. Clin Exp Allergy. 2010;40:668–78. doi: 10.1111/j.1365-2222.2009.03442.x. [DOI] [PubMed] [Google Scholar]
- 19.Hong SJ, Michael JG, Fehringer A, Leung DY. Pepsin-digested peanut contains T-cell epitopes but no IgE epitopes. J Allergy Clin Immunol. 1999;104:473–8. doi: 10.1016/s0091-6749(99)70396-9. [DOI] [PubMed] [Google Scholar]
- 20.Eiwegger T, Rigby N, Mondoulet L, Bernard H, Krauth MT, Boehm A, et al. Gastro-duodenal digestion products of the major peanut allergen Ara h 1 retain an allergenic potential. Clin Exp Allergy. 2006;36:1281–8. doi: 10.1111/j.1365-2222.2006.02565.x. [DOI] [PubMed] [Google Scholar]
- 21.Bogh KL, Kroghsbo S, Dahl L, Rigby NM, Barkholt V, Mills EN, et al. Digested Ara h 1 has sensitizing capacity in Brown Norway rats. Clin Exp Allergy. 2009;39:1611–21. doi: 10.1111/j.1365-2222.2009.03333.x. [DOI] [PubMed] [Google Scholar]
- 22.Kulis M, Li Y, Lane H, Pons L, Burks W. Single-tree nut immunotherapy attenuates allergic reactions in mice with hypersensitivity to multiple tree nuts. J Allergy Clin Immunol. 2011;127:81–8. doi: 10.1016/j.jaci.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Kulis M, Pons L, Burks AW. In vivo and T cell cross-reactivity between walnut, cashew and peanut. Int Arch Allergy Immunol. 2009;148:109–17. doi: 10.1159/000155741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–8. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 25.King N, Helm R, Stanley JS, Vieths S, Luttkopf D, Hatahet L, et al. Allergenic characteristics of a modified peanut allergen. Mol Nutr Food Res. 2005;49:963–71. doi: 10.1002/mnfr.200500073. [DOI] [PubMed] [Google Scholar]
- 26.Campana R, Vrtala S, Maderegger B, Jertschin P, Stegfellner G, Swoboda I, et al. Hypoallergenic derivatives of the major birch pollen allergen Bet v 1 obtained by rational sequence reassembly. J Allergy Clin Immunol. 2010;126:1024–31. e1021–8. doi: 10.1016/j.jaci.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Schimek EM, Zwolfer B, Briza P, Jahn-Schmid B, Vogel L, Vieths S, et al. Gastrointestinal digestion of Bet v 1-homologous food allergens destroys their mediator-releasing, but not T cell-activating, capacity. J Allergy Clin Immunol. 2005;116:1327–33. doi: 10.1016/j.jaci.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Untersmayr E, Vestergaard H, Malling HJ, Jensen LB, Platzer MH, Boltz-Nitulescu G, et al. Incomplete digestion of codfish represents a risk factor for anaphylaxis in patients with allergy. J Allergy Clin Immunol. 2007;119:711–7. doi: 10.1016/j.jaci.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Gonhcarova S, et al. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J Immunol. 2007;179:6696–703. doi: 10.4049/jimmunol.179.10.6696. [DOI] [PubMed] [Google Scholar]
- 30.Shreffler WG, Beyer K, Chu TH, Burks AW, Sampson HA. Microarray immunoassay: association of clinical history, in vitro IgE function, and heterogeneity of allergenic peanut epitopes. J Allergy Clin Immunol. 2004;113:776–82. doi: 10.1016/j.jaci.2003.12.588. [DOI] [PubMed] [Google Scholar]
- 31.Ditto AM, Neilsen CV, Neerukonda S, Shreffler WG, Bryce PJ. Clinical reactivity to raw peanut correlates with IgE binding to conformational epitopes of Ara h 1: a case report. Allergy. 2010;65:1485–6. doi: 10.1111/j.1398-9995.2010.02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albrecht M, Kuhne Y, Ballmer-Weber BK, Becker WM, Holzhauser T, Lauer I, et al. Relevance of IgE binding to short peptides for the allergenic activity of food allergens. J Allergy Clin Immunol. 2009;124:328–36. e321–6. doi: 10.1016/j.jaci.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005;35:757–66. doi: 10.1111/j.1365-2222.2005.02260.x. [DOI] [PubMed] [Google Scholar]