Abstract
Polycomb group and trithorax group proteins maintain the memory of repressed and active chromatin states by regulating chromatin of their target genes via DNA sequences termed Polycomb- and trithorax response elements. Since these elements often overlap and are able to convey the memory of both silent and active chromatin through cell division, they were also defined as cellular memory modules (CMMs). We identify here a minimal CMM of 219 bp from the Drosophila Fab-7 region that regulates the homeotic gene Abdominal-B. This CMM conveys the inheritance of active chromatin states induced by an embryonic pulse of transcriptional activation via recruitment of the trithorax group proteins Trithorax (TRX) and Brahma (BRM), the Drosophila homologue of the SWI2/SNF2 ATPase involved in chromatin remodelling. Within this CMM, DNA-binding sites for the Zeste protein are necessary for the inheritance of active chromatin through Zeste-dependent recruitment of BRM, while TRX can bind the CMM even in their absence. Thus, epigenetic inheritance of active chromatin states involves the recruitment of multiple cooperative chromatin-modifying complexes at closely spaced but distinct sites within a CMM.
Keywords: cellular memory, chromatin, Polycomb group, trithorax group, Zeste
Introduction
The Polycomb and trithorax groups of genes (PcG and trxG) are essential regulatory factors that maintain the memory of expression states of developmental genes, such as homeotic genes, throughout development in Drosophila and other higher eukaryotes (Francis and Kingston, 2001). PcG products maintain the repressed state, while proteins of the trxG maintain memory of the active state. The maintenance of active states utilizes two kinds of trxG enzymatic activities. The first is ATP-dependent chromatin remodelling induced in Drosophila by the Brahma complex (Papoulas et al, 1998), the homologue of the yeast and mammalian SWI/SNF complex. The second is deposition of covalent histone tail modifications by TRX and ASH1 proteins and their cofactors (Bantignies et al, 2000; Petruk et al, 2001; Beisel et al, 2002; Czermin et al, 2002). These marks, together with chromatin remodelling, may be required to allow access of nucleosomal templates to transcriptional components (Narlikar et al, 2002). Conversely, silencing is induced by the association of PcG complexes to their target chromatin. This can inhibit ATP-dependent chromatin remodelling mediated by the BRM complex in vitro (Francis et al, 2001). Also, PcG complex association to chromatin in vivo is correlated with methylation of lysine 27 and 9 of histone H3 (Cao et al, 2002; Czermin et al, 2002; Muller et al, 2002).
PcG and trxG proteins act on their target chromatin by association to specific DNA regulatory elements named Polycomb and trithorax response elements (PREs and TREs). In many Drosophila PREs, the Pleiohomeotic protein (PHO) binds to a specific DNA motif that is required for PcG-mediated silencing (Brown et al, 1998; Mihaly et al, 1998; Busturia et al, 2001; Mishra et al, 2001). The GAGAG motif, which is bound by the Trithorax-like protein (Trl), also named GAGA factor (GAF) (Biggin and Tjian, 1988), and by the Pipsqueak protein (Psq) (Hodgson et al, 2001; Huang et al, 2002), also mediates PcG-dependent silencing at many Drosophila PREs (Mishra et al, 2001; Americo et al, 2002). Furthermore, several PREs contain clusters of binding motifs for the trxG protein Zeste. Mutation of such motifs in PREs has not been documented so far, but purification of the PcG complex PRC1 identified Zeste as a stoichiometric component, together with Polycomb (PC), Polyhomeotic (PH), dRing and Posterior Sex Comb (PSC) (Saurin et al, 2001). This suggests that Zeste might be involved in PcG-mediated repression.
Much less is known about TREs. Genetic studies show that transgenic PREs are also responsive to the products of trxG genes. This observation raised the idea that PRE and TRE sequences may act in a concerted manner. Consistently, the bxd regulatory element from the BX-C contains a PRE and a separable but overlapping TRE (Tillib et al, 1999). In addition, chromatin immunoprecipitation experiments showed that the Trithorax (TRX) protein and PC bind to largely overlapping regions (Orlando et al, 1998). Moreover, experiments using a transgenic system showed that a transient embryonic pulse of transactivator can switch a 3.6 kb Fab-7 chromosomal element from a PcG-dependent silencing state to a heritable, trxG-dependent active chromatin state (Cavalli and Paro, 1998, 1999). This element was therefore termed ‘cellular memory module' (CMM). More recently, other CMMs were identified (Maurange and Paro, 2002; Rank et al, 2002).
What are the factors responsible for the recruitment of trxG proteins? Tillib et al showed that an AACAA sequence in the bxd CMM was involved in regulation by trx, but no associated regulatory factors have been identified (Tillib et al, 1999). GAF binding motifs are also candidates, since GAF sites present in a bxd fragment can recruit in vitro a complex containing GAF and TRX (Poux et al, 2002). In addition, Trl (Trithorax-like) mutations lead to ectopic expression of homeotic genes (Farkas et al, 1994), suggesting that GAF might be involved not only in silencing but also in trxG functions. Finally, Zeste is another candidate recruiter of trxG proteins. Zeste can bind to the Ubx promoter and stimulates transcription of this gene in vitro (Biggin et al, 1988) and in vivo (Laney and Biggin, 1992). Moreover, Zeste interacts in vitro with several members of the BRM complex (Kal et al, 2000), and it was reported that it plays an activating role in the bxd PRE/TRE element in vivo (Horard et al, 2000).
Here, we investigated the function of the Fab-7 CMM. The 3.6 kb Fab-7 element previously described was sized down to a short element of 219 bp, the Ab-Fab core CMM. Ab-Fab recapitulates the ability of the larger element to maintain PcG-mediated silencing as well as trxG-dependent active chromatin states. The maintenance of active states depends on Zeste (Z) binding sites within Ab-Fab, and indeed Zeste and BRM proteins are recruited to the transgene, whereas the PcG protein PH is displaced. Thus, an important in vivo function of Zeste may be the maintenance of active chromatin via recruitment of the BRM complex at its target genes.
Results
The 219 bp fragment Ab-Fab is a minimal PRE
In a first approach to identify minimal requirements for Fab-7 CMM functions, we tried to reduce the DNA sequence required for PRE function. A region of 260 bp within a Fab-7 fragment that contains a cluster of binding sites for known PcG and trxG factors was reported to behave as a PRE (Mishra et al, 2001) (see Figure 1A). This region contains two GAF binding sites flanked by three PHO and four Zeste-binding motifs (see Figure 1A). We tested three fragments centred on the GAF sites: Midi-Fab (532 bp), Ab-Fab (219 bp) and Mini-Fab (93 bp). These shorter Fab-7 elements were inserted in a transgene at approximately 5 kb upstream of the mini-white gene (Cavalli and Paro, 1998; Rank et al, 2002).
Figure 1.
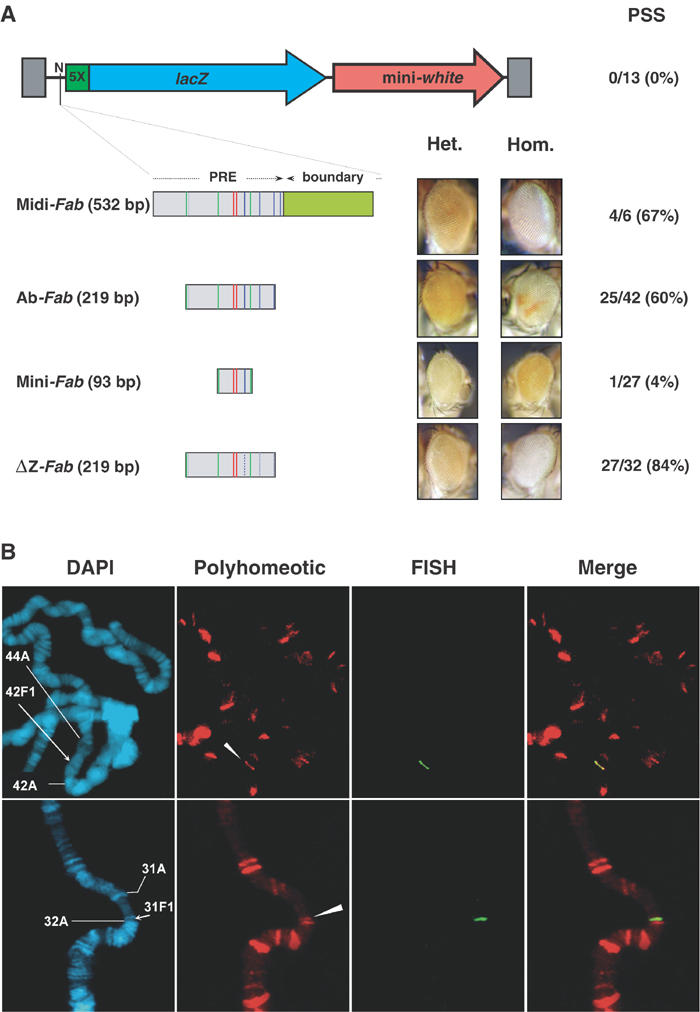
Midi- and Ab-Fab behave as PREs. (A) Schematic representation of the pUZ vector used in this study (not on scale), with the NotI (N) cloning site upstream of the lacZ and mini-white genes. LacZ expression is controlled by a UAS sequence containing five binding sites for GAL4 (5 ×). A control vector, containing 219 bp of irrelevant bacterial sequence cloned at the NotI site, was injected in flies and no PSS of mini-white could be observed in any of the 13 lines obtained. Below the vector, the candidate CMM inserts to be tested are shown. Green, red and blue bars in Fab-7-derived fragments represent binding sites for PHO, GAF and Zeste, respectively. Constructs are represented in a distal-to-proximal orientation and are inserted in pUZ in this orientation. For each construct, a representative example of eye colour obtained in heterozygous (Het.) and homozygous (Hom.) female flies for the transgene is shown. The number of PSS versus the total number of lines obtained for each construct is indicated. (B) Polytene chromosome immuno-FISH experiments performed on Ab-Fab (Ab14) and ΔZ-Fab (ΔZ14) lines using antibodies raised against PH protein. For each experiment, DAPI staining, immunostaining, FISH and a Merge between immunostaining and FISH are shown. In DAPI panels, the position of cytological landmarks is indicated by ticks, and the position of insertion of the transgene is shown by an arrow. In immunostaining panels, the position of the transgene is shown by arrowheads.
Four general PRE features were analysed. First, when PREs are inserted upstream of the mini-white gene, the eye colour of flies homozygous for the transgene is often lighter than the eye colour of heterozygous individuals. This phenomenon is termed pairing sensitive silencing (PSS). While Midi- and Ab-Fab were able to mediate PSS in 67 and 60% of the lines respectively, Mini-Fab did not show PSS (4%, one out of 27 lines, see Figure 1A). Second, PRE containing transgene leads to PcG recruitment at the site of insertion, that is, an additional PcG protein signal should be detected by immunostaining at the insertion locus of the transgene on polytene chromosomes. In order to analyse this, we developed an ‘immuno-FISH' technique that allows the identification of the transgene and associated proteins on the same chromosome preparation (see Methods and Lavrov et al, 2004). Midi-Fab and Ab-Fab fragments were able to recruit PH, PC and PHO proteins. As a control, staining of polytene chromosomes from w1118 flies showed that the Ab-Fab insertion was not located into an endogenous site for these proteins (Figure 1B and Supplementary Figures S1 and S2). In contrast to Midi- and Ab-Fab, Mini-Fab was unable to recruit PH, PHO or PC proteins. Third, PcG-mediated silencing of mini-white becomes more efficient at increasing growth temperatures. Indeed, mini-white repression increased greatly at higher temperature (see Supplementary Figure S3) in the three tested Midi- and Ab-Fab transgenic lines, while no effect was seen in three different Mini-Fab lines. Finally, PRE-mediated repression is affected in PcG mutant contexts, that is, in mini-white-containing transgenes, the eye colour is darker in a PcG-mutant genetic background. This was confirmed when an Ab-Fab transgenic line was analysed in the presence of a mutation in the gene polyhomeotic (ph410, see Supplementary Figure S3). Therefore, Midi-Fab and Ab-Fab, but not Mini-Fab, behave as PRE fragments. The identification of Ab-Fab as a minimal PRE is consistent with the previous identification of a 260 bp PRE (Mishra et al, 2001), which is located in the same region and is only slightly larger than Ab-Fab.
Ab-Fab is a minimal CMM
We then tested the ability of Midi- and Ab-Fab to maintain the cellular memory of an activated chromatin state with the GAL4/UAS dual system (Figure 2A) that was previously utilized to identify CMM (Cavalli and Paro, 1998). A GAL4 inducible lacZ reporter is placed between the CMM and mini-white. The yeast transcriptional activator GAL4 is expressed by a second construct under the control of the heat shock inducible hsp70 promoter. Using a 3.6 kb Fab-7 element, it was previously shown that an embryonic pulse of GAL4 protein erases silencing and potently activates the expression of lacZ. Although the activator disappears after about 1 day, adult flies emerging over 2 weeks after the embryonic GAL4 pulse maintain the active chromatin state, resulting in red-eyed progeny, while their noninduced siblings maintain the mini-white repressed state (Cavalli and Paro, 1998). With this assay, transgenic lines carrying Midi- and Ab-Fab were also able to maintain the active chromatin state throughout development until adulthood (Figure 2B). Thus, the 219 bp Ab-Fab fragment behaves as a core Fab-7 CMM. The Ab14 line depicted in Figure 2B showed the strongest activation between the two Ab-Fab lines tested, since nearly all the heat shocked population (approximately 95%) showed homogeneous red eyes or very large red patches of activation. As previously reported with the 3.6 kb Fab-7 CMM, maintenance of the active state required a pulse of GAL4 during embryogenesis, while pulses during larval development were not efficient (Figure 2B). As a control, a heat shock pulse applied to the Ab14 line without GAL4 driver did not affect mini-white expression (Figure 2B, Ab14 noGAL4). Unlike in the 3.6 kb Fab-7 CMM, memory of this active state was not transmitted through meiosis in the two lines tested. We believe that this inability is not due to weaker maintenance, since Ab14 and Midi1.2 lines showed an activation phenotype similar to the FLW-1 line, previously shown to convey meiotic inheritance (Cavalli and Paro, 1998). Other sequence elements present in the 3.6 kb Fab-7 CMM but not in Ab-Fab may contribute to meiotic transmission of derepressed chromatin, or alternatively the locus of insertion of the constructs may affect inheritance in the tested lines. Figure 2C depicts Fab-7 fragments that are able to convey the inheritance of active chromatin throughout development.
Figure 2.
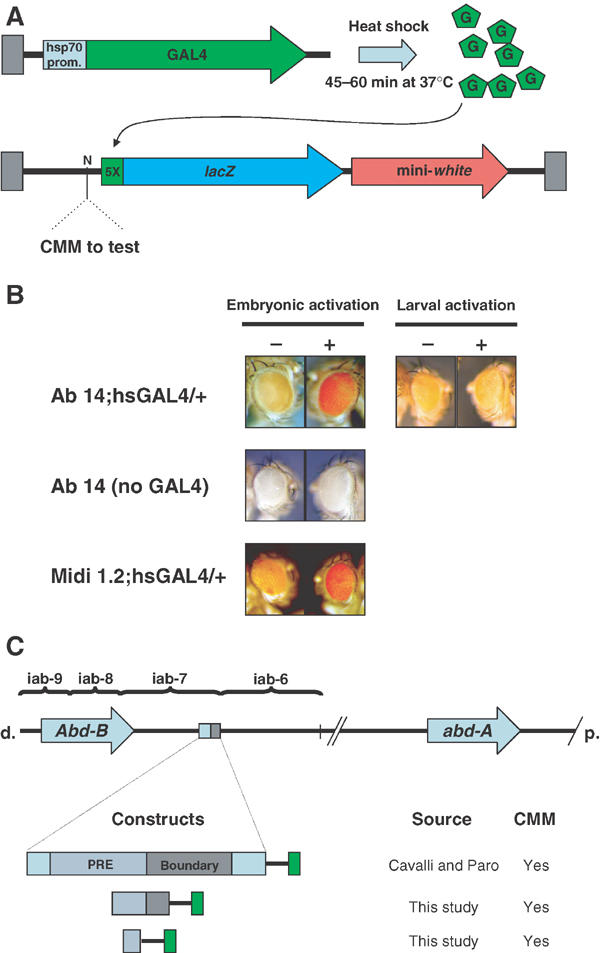
Ab-Fab is a minimal CMM. (A) Schematic representation of the GAL4 system used to test for CMM activity. (B) Midi1.2 and Ab14 lines show the memory of the active state after a pulse of embryonic activation. As controls, pulses of GAL4 in Ab14;hsGAL4/+ first and second instar larvae have no effect, as well as an embryonic heat shock in the line without the hsGAL4 driver. Female eyes are shown. (C) Schematic representation of Midi- and Ab-Fab CMM fragments.
In addition to the transgenic source of Fab-7 sequences, there is an endogenous copy of Fab-7 present at the BX-C. Multiple copies of the same sequence can interfere with each other in a phenomenon called cosuppression (Pal-Bhadra et al, 1997). This phenomenon depends on PcG, and on components involved in post-transcriptional silencing analogous to RNAi. Moreover, we recently found that transgenes containing a 3.6 kb Fab-7 sequence can physically associate with the BX-C (Bantignies et al, 2003). We therefore tested whether maintenance of activation may depend on the presence of the endogenous Fab-7. We generated an Ab14;hsGAL4 line carrying the Fab-71 deletion (Gyurkovics et al, 1990). This 4 kb deletion removes all sequence homology with the transgenes. Adult Ab14;hsGAL4,Fab-71 flies that were given a pulse of GAL4 as embryos displayed strong activation of mini-white, showing that maintenance of active chromatin does not require sequence homology at the endogenous Fab-7 (see Supplementary Figure S4). Another potential trigger of inheritance of active states induced by Fab-7 could be transcription running through the PRE and displacing PcG complexes (Rank et al, 2002). Therefore, we performed in situ hybridization experiments in the Ab14 line containing Ab-Fab. Cross-hybridization with the endogenous Fab-7 element was excluded by introducing in these lines the Fab-71 deletion in the homozygous state (Gyurkovics et al, 1990). No transcription was detected through Ab-Fab in either orientation while, with the same set of probes, we could detect a GAL4-dependent transcription in the 5F24 25,2 transgene, as recently described (Rank et al, 2002), as well as a transcript running through the endogenous Fab-7 in control w1118 flies (data not shown) (Rank et al, 2002). This result suggests that transcription through the Ab-Fab minimal CMM is not required for switching into an active state.
Role of Zeste-binding sites in the maintenance of active states by the Ab-Fab element
As mentioned above, Ab-Fab contains several binding motifs for PHO, GAF and Zeste proteins (Figure 1A). While PHO and GAF motifs have been studied in detail previously (Mishra et al, 2001; Poux et al, 2002), the role of Zeste is still unknown. We therefore analysed the role of Zeste-binding sites in Ab-Fab. Three Zeste consensus binding motifs (YGAGYG sequence, where Y can be C or T; Benson and Pirrotta, 1988) are present in this element (see Figure 1A). They were mutated by polymerase chain reaction (PCR) into YGCAYG to generate the ΔZ-Fab fragment. ΔZ-Fab-mediated repression of mini-white was not abolished and it was actually stronger than the one mediated by the Ab-Fab wild-type sequence. In total, 84% of ΔZ-Fab lines displayed PSS versus 60% of lines transformed with the Ab-Fab construct (Figure 1A). This was consistent with the recruitment of PH on polytene chromosomes in a ΔZ-Fab transgenic line (Figure 1B). ΔZ-Fab was also able to bind PC or PHO with no significant staining difference with Ab-Fab (see Supplementary Figure S5). This strongly suggests that PcG proteins are not recruited to Ab-Fab in vivo via its Zeste-binding sites.
We then tested whether mutation of Zeste sites might affect the maintenance of active chromatin states. We tested ΔZ-Fab lines in the GAL4 activation system. Five out of the six ΔZ-Fab lines tested could not maintain the active state of transcription (Figure 3A), while only one line maintained activation. Since polytene chromosome experiments in this line showed that the transgene is inserted at an endogenous TRX and BRM binding site, landing of the transgene in an active chromatin domain may explain maintenance of the active state in this case. Indeed, sensitivity of PRE/TRE to the locus of insertion of the transgene was previously reported in many cases, including in the case of activation (Poux et al, 2002). Thus, Zeste-binding sites play an important role in the maintenance of active states in the Ab-Fab CMM.
Figure 3.
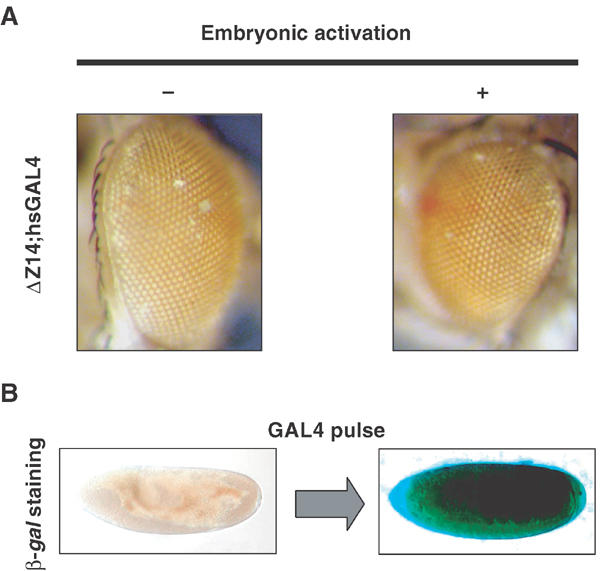
Zeste-binding sites are required for the inheritance of active chromatin states. (A) Eye colour from ΔZ-Fab flies of the ΔZ14 line carrying one copy of the GAL4 driver, either after a pulse of embryonic activation (+) or in its absence (−). (B) ß-Galactosidase staining in embryos of the ΔZ14 line upon a heat-shock-induced pulse of GAL4 expression.
This absence of maintenance upon mutation of Zeste sites may have two explanations. Zeste may be required for GAL4-mediated transactivation or, alternatively, it may be required for maintenance of the active state after decay of the primary transactivator. Figure 3B shows that a pulse of GAL4 induced strong transient lacZ activation, similar to lines carrying Ab-Fab, or other Fab-7-containing transgenes (Cavalli and Paro, 1998). This indicates that Zeste-binding sites are involved in the transmission of memory of active states rather than for transcriptional activation.
Minimal Fab-7 constructs do not recruit detectable amounts of trxG proteins in the repressed state
We compared the Ab14 (carrying Ab-Fab) and ΔZ14 lines (carrying ΔZ-Fab) with similar levels of mini-white expression in the absence of transcriptional stimulation (see wild-type Ab14 and ΔZ14, Figure 4A, eye pictures represent the average level of eye pigmentation of male populations at 20°C, while females display white eyes in both lines with rare little patches of activation). We first tested the effects of mutations in trxG genes on the two constructs. The trxE2 null mutation had a repressive effect on both Ab14 and ΔZ14 inserts (Figure 4A). In contrast, the null brm2 mutant context has a repressive effect on the Ab14 insertion, but had no detectable effect on the ΔZ14 line, suggesting that BRM protein may act via Zeste-binding sites (Figure 4A). Immuno-FISH on polytene chromosomes in the two lines was performed using antibodies directed against TRX or BRM proteins. We could not detect any TRX bound to the transgene in the ΔZ14 line, and only weak binding in some but not all the chromosomes examined was observed in the Ab14 line. We also could not detect a significant BRM signal in both insertions. The strong hypomorphic za mutation also had a repressive effect on mini-white in the Ab14 line (Figure 4C), suggesting that Zeste behaves as a trxG member at Ab-Fab (note that Zeste has no effect on mini-white in the absence of PRE (Qian et al, 1992; Horard et al, 2000)), but no Zeste staining was detected in immuno-FISH experiments (Figure 4C). The absence of detectable binding of trxG proteins on repressed transgenes may depend on the fact that, in the absence of transactivator, only weak amounts of trxG proteins associate to the CMM.
Figure 4.
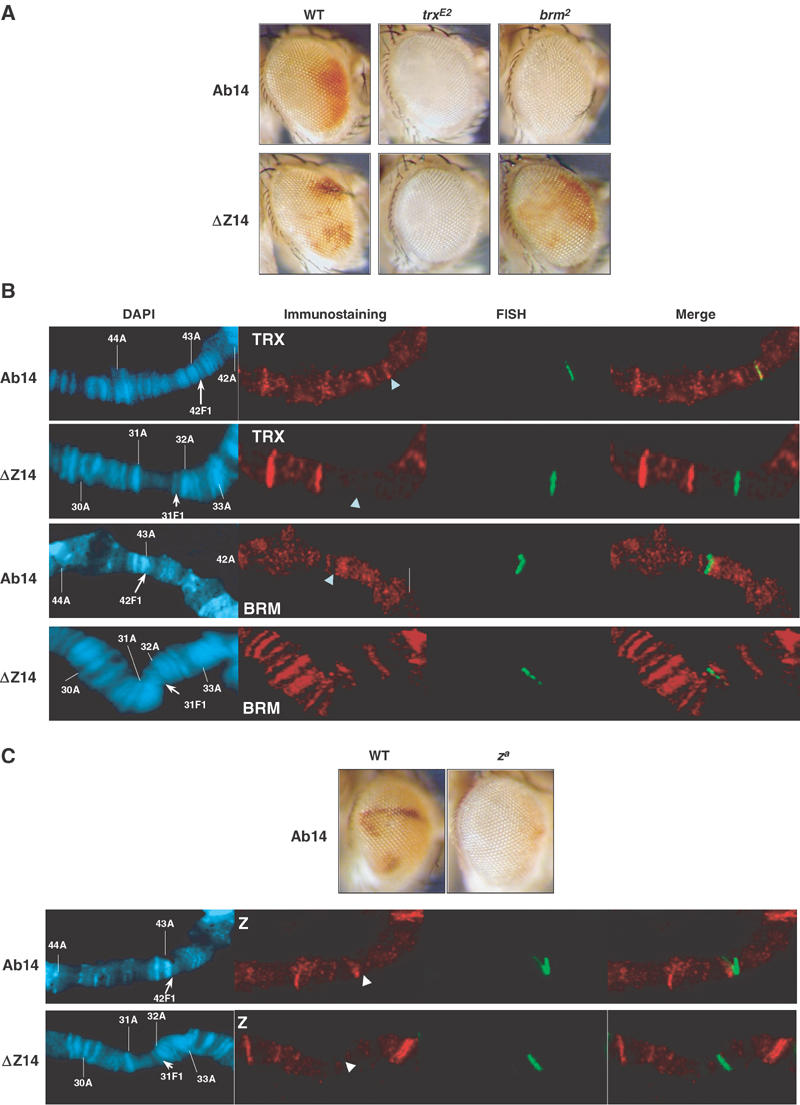
Role of trxG proteins in the absence of embryonic transcriptional activation. (A) Effects of heterozygous trxE2 and brm2 mutant backgrounds on mini-white expression in two lines carrying Ab-Fab (Ab14) or ΔZ-Fab (ΔZ14) transgenes. Male eyes are shown. (B) Immuno-FISH with anti-TRX and anti-BRM antibodies, of polytene chromosomes from Ab14 and ΔZ14 lines without GAL4 driver. Cytological landmarks and the position of the transgene are indicated as in Figure 1B. (C) Top: effect of a homozygous za mutant background on Ab-Fab-mediated mini-white expression in the Ab14 line. Bottom: immuno-FISH of Zeste in the Ab14 and ΔZ14 lines.
Trithorax is necessary but not sufficient for maintenance of the activated state
In order to study whether trxG proteins are required for the maintenance of the active chromatin state, we tested whether the Ab14 line could maintain an active chromatin state in the absence of these proteins. When a pulse of GAL4 was given to Ab14 embryos carrying a trxE2 mutation, the adult progeny showed no difference in eye colour compared to their noninduced siblings (Figure 5A). Therefore, TRX is required for maintenance of the activated state, consistent with previous findings (Cavalli and Paro, 1999; Poux et al, 2002).
Figure 5.
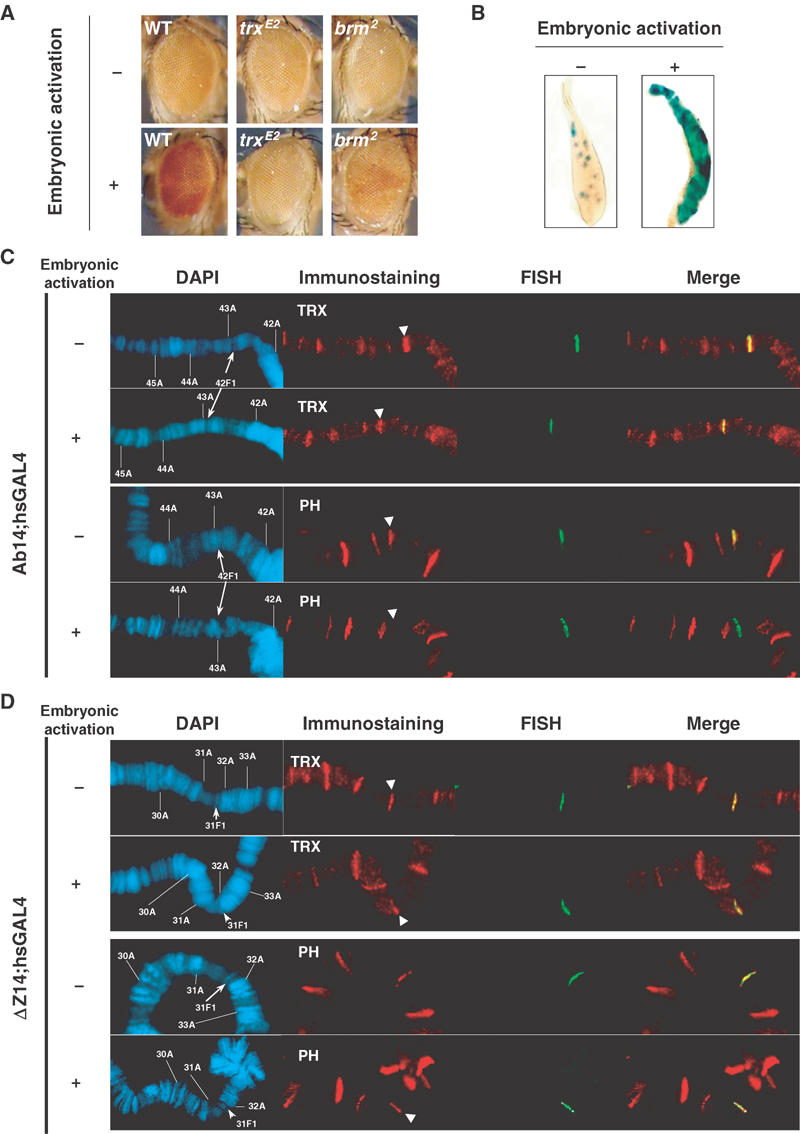
trxG-dependent maintenance of active states induces PH displacement. (A) Loss of memory of the active state in Ab14 flies carrying one copy of the GAL4 driver and analysed in heterozygous trxE2 or brm2 mutant backgrounds. (B) ß-Galactosidase staining of salivary glands from Ab14 larvae in the absence (−) or presence (+) of embryonic activation. (C) Immuno-FISH stainings of polytene chromosomes in the Ab14 line, carrying one copy of the GAL4 driver, in the absence (−) or presence (+) of an embryonic pulse of transcriptional activation. TRX and PH stainings are shown. (D) Same analysis as in (C) in the ΔZ14 line.
We then studied TRX association to Fab-7 transgenes in the presence of transactivator. Figure 5C shows TRX stainings of Ab-Fab and ΔZ-Fab transgenic lines in the presence of the hsp70-GAL4 driver. Without heat shock, basal levels of GAL4 drive the expression of a lacZ reporter in salivary glands in the absence of PRE, while PcG silencing induces variegation in this tissue (Cavalli and Paro, 1998). In this condition, both constructs were able to recruit large levels of TRX (Figure 5C and D). Thus, GAL4 binding to the UAS sequences is likely to promote TRX recruitment at Fab-7. However, silencing is not erased in the Ab14 line in this condition, as indicated by the variegated lacZ staining in salivary glands (Figure 5B), and by binding of PH protein at the transgene (Figure 5C). Thus, TRX and PH can colocalize at the same DNA element. Upon an embryonic pulse of GAL4, we could not observe any difference in TRX recruitment to both transgenes (Figure 5C and D). This indicates that maintenance of the memory of an active chromatin state does not strictly correlate with the amounts of TRX protein bound at the CMM. Therefore, TRX is necessary but not sufficient for the maintenance of active chromatin.
Maintenance of an activated state at Ab-Fab induces displacement of polyhomeotic protein
On the other hand, activated Ab14 chromosomes showed a dramatic decrease of PH staining compared to noninduced controls (Figure 5C). Strikingly, this was not found in the ΔZ14 line, where ΔZ-Fab maintained PH protein on the transgene (Figure 5D). Taken together with the data showing memory of chromatin states at the lacZ and the mini-white reporter genes, these results indicate that a switch between a silent and a derepressed state induced in embryos may correlate with loosening of the association of PcG proteins at Ab-Fab and that Zeste-binding sites may contribute to this diminished affinity.
Maintenance of the active chromatin state requires recruitment of Zeste and BRM
Since the mutation of Zeste-binding sites prevents the maintenance of activated states without impairing TRX recruitment by GAL4, we reasoned that they may be primarily involved in the recruitment of other trxG components. First, we tested Zeste itself. In the loss-of-function za mutant background, the maintenance of the active state is strongly affected (Figure 6A), although a residual activation could be observed (Figure 6A and B). This correlates with the binding of Zeste to Ab-Fab-containing transgenes. While not detectable at the silenced transgene in the Ab14 line, low amounts of Zeste were recruited by the expression of low levels of GAL4 (Figure 6C). Upon an embryonic pulse of GAL4 activation, larger amounts of Zeste were detected at the activated Ab-Fab transgene. On the other hand, Zeste was not detected in the ΔZ14 line, carrying the transgene with mutated Zeste sites (Figure 6C).
Figure 6.
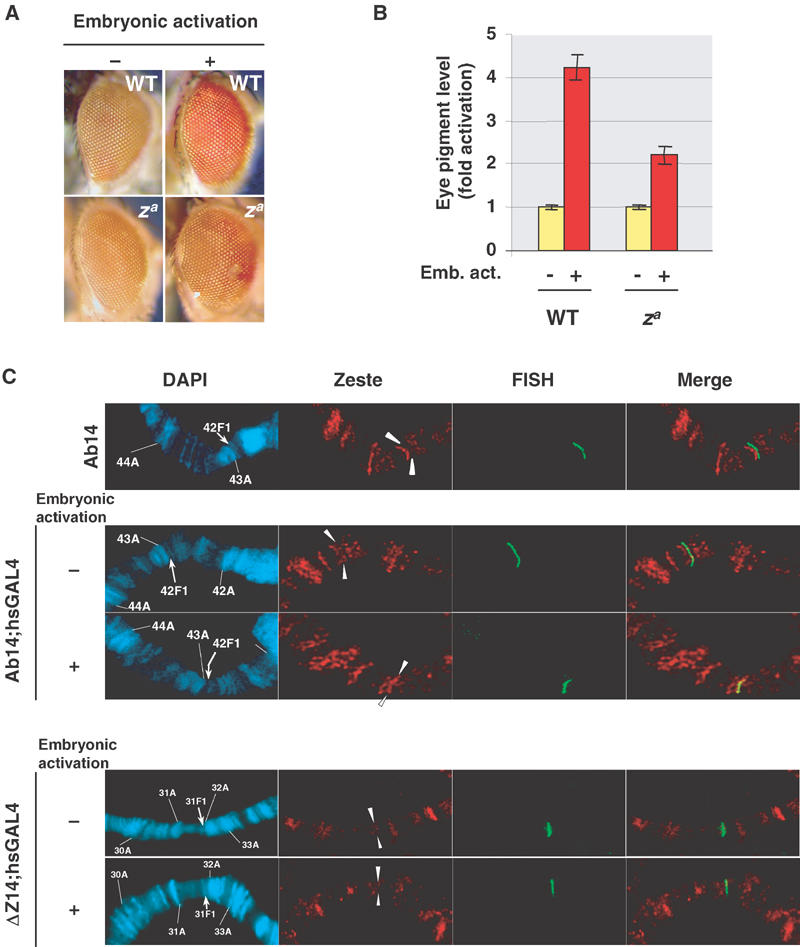
Zeste protein induces the maintenance of memory of active chromatin states. (A) Loss of memory of activated states in Ab14 in a za mutant background: eye pictures of males are shown in the presence (+) or absence (−) of an embryonic pulse of activation. Wild-type (WT) or homozygous za mutant Ab14 males are shown. (B) Eye pigment quantification from males in each condition. The average from five determinations is shown. Error bars represent the standard deviation. Similar results were obtained in females. (C) Immuno-FISH of Zeste recruitment to the CMM transgene. Top: Ab14 line, analysed in the absence of the GAL4 driver (see also Figure 4C), with low constitutive amounts of GAL4 (−) or upon an embryonic pulse of GAL4 (+). Bottom: ΔZ14 line analysed in the same conditions.
Zeste was shown to recruit the BRM complex as its transcription cofactor in vitro (Kal et al, 2000). Since we found that the brm2 mutation counteracts PcG-dependent silencing in Ab-Fab, but not in the derivative ΔZ-Fab construct with deleted Zeste sites, and since this mutation prevents the inheritance of active states at Ab-Fab (Figure 5A), we tested whether maintenance of active chromatin at Ab-Fab is linked to recruitment of BRM. Strikingly, the pattern of BRM followed exactly that of Zeste. BRM was absent from the transgene without GAL4 (Figure 7A); it was weakly recruited by low amounts of GAL4 (Figure 7B) and it was more strongly recruited at the Ab-Fab transgene activated by an embryonic pulse of GAL4 (Figure 7B). BRM staining at ΔZ-Fab was absent even upon an embryonic pulse of GAL4 activation (Figure 7C). These data strongly suggest that Zeste and BRM are directly involved in the maintenance of the active state and that BRM may be recruited at Ab-Fab by the binding of Zeste at its binding sites.
Figure 7.
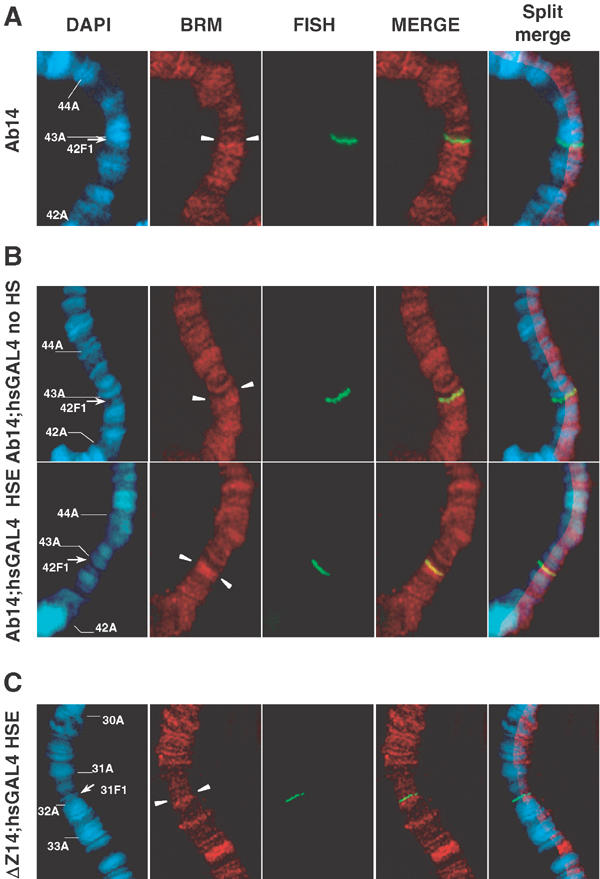
Zeste-dependent BRM recruitment at an activated CMM. Immuno-FISH of BRM in polytene chromosomes from larvae of the Ab14 and ΔZ14 lines. (A) Ab14 without GAL4 driver. (B) Ab14 in the presence of low amounts of GAL4 (Ab14;hsGAL4 no HS) or upon embryonic activation of the CMM (Ab14;hsGAL4 HSE). (C) ΔZ14 carrying one copy of the GAL4 driver, upon an embryonic pulse of GAL4. Split-merge panels are composite images showing that BRM is located at chromosomal interbands. This is apparent when BRM staining, as well as the FISH signal, are juxtaposed to DAPI staining of chromosomal DNA.
Discussion
In this work, we identified a short sequence, Ab-Fab, that is able to maintain either a PcG-dependent repressed state or a trxG-mediated active state of transcription. Within this element, mutation of Zeste-binding sites did not impair PcG-dependent silencing, indicating that Zeste is not the primary recruiter of PcG complexes. Rather, both the mutation of Zeste-binding sites or of z gene increase silencing, and the Ab-Fab CMM requires Zeste protein and Zeste DNA binding sites in order to maintain active chromatin throughout development. Finally, the maintenance of active states involves Zeste-dependent BRM recruitment at the Ab-Fab element.
A minimal CMM
A detailed analysis of homeotic PREs has identified short core PRE sequences containing PHO and GAF DNA binding motifs that are essential for silencing (Busturia et al, 2001; Hodgson et al, 2001; Mishra et al, 2001). Due to the less well-developed assays and the higher heterogeneity of trxG protein complexes, TRE elements are ill defined, but they seem to overlap with PREs. Our present finding identifies a short DNA element, Ab-Fab, that mediates inheritance of chromatin silencing or activation depending on early developmental cues. In earlier studies, CMM elements were rather large and minimal DNA regions for their function were not identified. This study shows that, within Ab-Fab, sequences required for the maintenance of silencing and activation are tightly associated, but they may be separable. Mutation of PHO and GAF sites leads to loss of silencing (Mishra et al, 2001, and our unpublished observations). In contrast, the mutation of neighbouring Zeste-binding sites does not prevent the recruitment of PcG proteins, while it prevents the maintenance of activated states. Moreover, PcG and trxG proteins can associate to this element in the same polytene chromosomes, but their strength of binding can be dynamically regulated depending on early regulatory cues. In the absence of activators, TRX binds weakly, while in the presence of activator, TRX and BRM are recruited to the CMM. When a strong pulse of activator is induced at embryogenesis, binding of the PcG protein PH is weakened. Thus, a dynamic modulation of the association of PcG and trxG members at CMMs may be an important determinant of the maintenance of chromatin states, both in our transgenic constructs and in a natural situation (Marchetti et al, 2003). This cross-talk may require physical proximity, and perhaps direct contacts between repressor and activator proteins and it would explain the close spacing of PcG and trxG recruiter elements along the chromosomes.
Role of Zeste and BRM in the maintenance of cellular memory
Our data suggest a central role for Zeste-dependent BRM recruitment for maintenance of active chromatin throughout development. The brahma gene was originally identified in a screen for extragenic suppressors of Polycomb and it was shown to encode for the Drosophila homologue of the yeast SWI2/SNF2 ATPase (Tamkun et al, 1992). Genes encoding for BRM complex components counteract PRE-mediated silencing in transgenic assays (Gindhart and Kaufmann, 1995; Cavalli and Paro, 1999). The BRM complex was subsequently shown to be generally involved in RNA polymerase-II-mediated transcriptional activation (Armstrong et al, 2002). Yeast and vertebrate SWI/SNF complexes are recruited to their target genes by direct contacts with a variety of transcriptional activators (Neely et al, 1999; Kadam et al, 2000). In Drosophila, Zeste recruits the BRM complex to chromatin in vitro via direct contacts with at least two of its subunits (Kal et al, 2000). Our data strongly suggest that this recruitment mediates the maintenance of active chromatin states at Ab-Fab and perhaps at other CMMs.
A second role of Zeste might be to weaken the association of PcG complexes to the Ab-Fab CMM. An embryonic pulse of activation induced a permanent loss of binding of PH to the transgene at Ab-Fab, which was not observed when Zeste-binding sites were mutated in ΔZ-Fab. This may depend on the ability of the BRM complex to compete with the PRC1 complex for chromatin association (Shao et al, 1999) or, alternatively, it may depend on Zeste itself. Notably however, PcG proteins were able to re-associate to a larger, activated 3.6 kb Fab-7 CMM (Cavalli and Paro, 1999), suggesting that displacement of PcG complexes is not strictly necessary for the maintenance of active chromatin, and that PcG complex recruitment at the larger element might be more stable, perhaps because it may depend on other DNA sequences flanking the core CMM. Indeed, adjacent sequence domains can drive PcG-dependent silencing at the bxd PRE (Horard et al, 2000).
Although we identified Zeste as an important component for the maintenance of active chromatin, previous biochemical studies identified Zeste within the PcG complex PRC1 (Saurin et al, 2001). In vivo, Zeste is not only involved in activation but also in repression at the promoter of the homeotic gene Ubx (Hur et al, 2002). Recent biochemical studies indicate that, while Zeste is able to enhance the function of a Polycomb core complex, this protein may not be important for recruitment of this complex to chromatin in vitro (Mulholland et al, 2003). This is consistent with the fact that Zeste-binding sites mutation does not affect PcG protein recruitment in vivo in our transgenic assay. Whether Zeste associated to the PRC1 complex stimulates silencing at a subset of PREs, or whether this association reflects a step in a mechanism weakening binding of PRC1 to its target chromatin during transcriptional activation remains to be elucidated.
The z gene is dispensable for fly viability and its mutation does not induce homeotic phenotypes by itself. How does one reconcile these findings with the role of Zeste in the maintenance of active states at the Ab-Fab CMM? In the context of endogenous target elements, other factors might substitute for Zeste absence in the recruitment of the BRM complex. These DNA elements may be located elsewhere in the regulatory DNA of the endogenous Abd-B gene. A redundant Zeste regulatory role is not unprecedented, since it was previously proposed in Zeste-dependent regulation of the Ubx gene (Laney and Biggin, 1996). Another line of evidence suggests that Zeste protein may not be the only BRM recruiter at Ab-Fab. While mutation of Zeste DNA binding sites in Ab-Fab abolished transmission of the memory of active states, partial inheritance was still observed when the wild-type Ab-Fab element was tested in the loss-of-function za mutant background. This could depend on the fact that the za mutant used still retains some z function, but it may also indicate that another unknown factor might partially replace Zeste protein in its absence at Zeste-binding sites, as previously suggested in the case of Ubx (Laney and Biggin, 1992). Thus, Zeste-mediated BRM complex recruitment may be part of a redundant mechanism required for the maintenance of cellular memory by trxG proteins.
Cooperation of multiple chromatin regulatory mechanisms in the maintenance of the memory of cell identity
In addition to Zeste and BRM, TRX and ASH1 protein complexes are also required for the maintenance of active states of homeotic gene expression. In particular, trx was shown to be involved in maintenance of activation at the Fab-7 CMM (Cavalli and Paro, 1999), and it is necessary, although not sufficient, for the inheritance of active states at Ab-Fab. The TRX complex TAC1, which is required in order to maintain active chromatin (Petruk et al, 2001), contains the histone acetyltransferase dCBP. In vitro, histone acetylation and the SWI/SNF complex can maintain an open state in chromatin templates activated by the transcription factor GAL4, even after disappearance of the activator (Hassan et al, 2001). Histone acetyltransferases and SWI/SNF complexes can also cooperate in vivo to drive gene expression (Krebs et al, 2000; Reinke et al, 2001). We suggest that recruitment of TRX and BRM complexes by distinct DNA binding proteins may be required for the inheritance of active states dependent on CMM activity. At Ab-Fab, one TRX recruiter may be the GAF protein. Poux et al (Poux et al, 2002) showed that the GAGAG motifs present in a bxd PRE fragment are important in vitro for GAF-mediated recruitment of TRX. Preliminary experiments indicate that mutation of the two GAF binding sites present at Ab-Fab prevents recruitment of TRX, both in the presence of low amounts of GAL4, and upon an embryonic pulse of GAL4 activator. Thus, the strong TRX recruitment observed in Ab-Fab or ΔZ-Fab constructs may rely on the presence of GAF binding sites.
Mutation of Zeste-binding sites does not prevent the recruitment of TRX protein at Ab-Fab, nor a genetic action of trx at the mutated transgene. This suggests that TRX and BRM complexes may be recruited by different mechanisms at their target DNA. However, they might act in concert. The presence of Zeste at Ab-Fab, while not necessary, might help GAF to recruit TRX. Indeed, we noticed that the amounts of TRX protein recruited at the Ab-Fab transgene were reproducibly higher than those recruited at ΔZ-Fab. This may depend on direct interactions between Zeste and components of the TRX complex, or on contacts between the TRX complex and the BRM complex recruited by Zeste (Rozenblatt-Rosen et al, 1998). Cooperation between Zeste-mediated BRM recruitment and GAF-dependent TRX recruitment is consistent with previous reports, showing that z mutations enhance Ubx phenotypes induced by Trl loss-of-function mutants (Laney and Biggin, 1996).
A further mechanism for the maintenance of active chromatin at CMMs may depend on transcription running through these elements (Hogga and Karch, 2002; Rank et al, 2002). In the case of our core CMM, no Ab-Fab transcripts could be detected under any condition, and thus transcription is unlikely to influence transmission of memory by this element in our assay. However, it is important to note that the endogenous Fab-7 element is located several tens of kb distal to its cognate promoter, while in our assay we placed this element close to the activated lacZ promoter. Thus, while the binding of a nearby activator might be sufficient to be sensed by the CMM, as was the case of Ab-Fab, this signal may not be efficient in the case of a CMM located distant from its promoter. In this case, transcription through the CMM may favour the maintenance of trxG-dependent active chromatin states. Although CMMs are far from promoters at homeotic genes, PcG/trxG target elements are located close to promoters in many other loci (Americo et al, 2002; Bloyer et al, 2003; Ringrose et al, 2003). In these cases, direct recruitment of proteins by DNA-binding activities may be sufficient to regulate these CMMs even in the absence of transcription.
Thus, CMMs may dispose of multiple, partially redundant mechanisms in order to sense chromatin silencing or activation, and to stably maintain these states. The exact combination of regulatory cues and maintenance factors used at different target genes may depend on the specific biology of these genes. This combinatorial use of different mechanisms for chromatin inheritance may offer the possibility of specific regulation of different PcG and trxG target genes, even though each of these mechanisms may be used at many genes, and may therefore not be strongly selective on its own.
Materials and methods
Transgenic constructs, P-element transformation, fly work and ß-galactosidase staining
Midi-Fab, Ab-Fab, Mini-Fab and ΔZ-Fab fragments were obtained by PCR using specific primers. The coordinates of Fab-7 sequences from these constructs relative to the previously published sequence of BX-C (Martin et al, 1995) are: Midi-Fab, nucleotides 83 283–83 814; Ab-Fab and ΔZ-Fab, 83 442–83 660; Mini-Fab: 83 495–83 587. Sequences of all primers are available upon request. All PCR fragments were subcloned into the pGEM-T Easy Vector System (Promega) and sequenced. Constructs were then excised from pGEM and cloned into the pUZ vector at the NotI restriction site. Insertion orientation and insert copy number were verified by PCR. Constructs were then injected in embryos from w1118 Canton-S strain using the classical transgenesis protocol (Spradling, 1986). The hsp70-GAL4 line used in this work is hsGAL42−1 (Brand et al, 1994) carrying the driver in chromosome 3. Details of crosses used to obtain transgenic lines into trxE2, brm2 or za mutant backgrounds are available upon request. Eye pictures were obtained using a Nikon Coolpix 990 CCD camera mounted on an MZFLIII binocular (Leica). For eye colour comparison, all individuals were of the same age and were imaged in the same frame. All eye pictures represent the average eye pigmentation from fly populations of 100–200 flies in each experiment, all experiments were repeated at least twice, and all results were clearly visible in both sexes. Eye pigment determination, ß-galactosidase staining and assays for the maintenance of active states at transgenes carrying CMMs were performed as previously described (Reuter and Wolff, 1981; Cavalli and Paro, 1998).
Antibodies
Immunostainings of polytene chromosomes were performed using affinity-purified rabbit polyclonal antibodies specific for PH, BRM, TRX. Rabbit anti-Z polyclonal antibody was a serum. Antibody anti-PH was a generous gift from R Paro. Antibodies against Z, TRX and PHO were a kind gift from V Pirrotta. Rabbit affipure anti-BRM 4449 polyclonal antibody is a kind gift from C Muchardt and displays an identical pattern with the one recently published (Armstrong et al, 2002). This antibody also recognizes a single band at 200 kDa on immunoblots.
Immuno-FISH
The detailed protocol for immuno-FISH is described in Lavrov et al (2004). Briefly, after immunostaining of polytene chromosomes performed using standard protocols, slides were submitted to a postfixation step in PBS–3.7% formaldehyde for 15 min at 37°C, washed twice in PBS for 5 min and a standard FISH protocol was applied, except that chromosomes were incubated in a 2 × SSC bath at 70°C for 30–45 min prior to alkali denaturation using NaOH 0.1 M for 10 min at room temperature. Chromosomes were counterstained with DAPI and mounted for fluorescence microscopy in Mowiol (Calbiochem). FISH probes (with the pUZ vector as a template) were labelled using the Bio-Nick nick-translation kit (Life Technologies) according to the manufacturer's instructions. Chromosomes were observed with a LEICA DMRA2 epifluorescence microscope coupled with a CoolSnap HQ high-resolution CCD camera (Roper Scientifics). Acquisitions were carried out using the Metamorph software (Universal Imaging Corporation) and images were processed using Adobe Photoshop. For each line in each condition, 10–20 chromosomes from two or more preparations were analysed.
Supplementary Material
Supplementary Figures S1
Supplementary Figures S2
Supplementary Figures S3
Supplementary Figures S4
Supplementary Figures S5
Acknowledgments
We thank all lab members for discussions during this work and R Paro for critically reading the manuscript. We also thank R Paro, V Pirrotta and C Muchardt for the generous gift of antibodies. We thank P Atger for artwork. JD was supported by a grant from Ministère de l'enseignement supérieur et de la recherche and by a grant from ‘La Ligue Nationale contre le cancer'. GC was supported by grants of the CNRS, the HFSPO, the Fondation pour la Recherche Médicale and the Association pour la Recherche sur le Cancer.
References
- Americo J, Whiteley M, Brown JL, Fujioka M, Jaynes JB, Kassis JA (2002) A complex array of DNA-binding proteins required for pairing-sensitive silencing by a Polycomb group response element from the Drosophila engrailed gene. Genetics 160: 1561–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JA, Papoulas O, Daubresse G, Sperling AS, Lis JT, Scott MP, Tamkun JW (2002) The Drosophila BRM complex facilitates global transcription by RNA polymerase II. EMBO J 21: 5245–5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantignies F, Goodman RH, Smolik SM (2000) Functional interaction between the coactivator Drosophila CREB-binding protein and ASH1, a member of the trithorax group of chromatin modifiers. Mol Cell Biol 20: 9317–9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantignies F, Grimaud C, Lavrov S, Gabut M, Cavalli G (2003) Inheritance of Polycomb-dependent chromosomal interactions in Drosophila. Genes Dev 17: 2406–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel C, Imhof A, Greene J, Kremmer E, Sauer F (2002) Histone methylation by the Drosophila epigenetic transcriptional regulator Ash1. Nature 419: 857–862 [DOI] [PubMed] [Google Scholar]
- Benson M, Pirrotta V (1988) The Drosophila Zeste protein binds cooperatively to sites in many gene regulatory regions: implications for transvection and gene regulation. EMBO J 7: 3907–3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin MD, Bickel S, Benson M, Pirrotta V, Tjian R (1988) Zeste encodes a sequence-specific transcription factor that activates the Ultrabithorax promoter in vitro. Cell 53: 713–722 [DOI] [PubMed] [Google Scholar]
- Biggin MD, Tjian R (1988) Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell 53: 699–711 [DOI] [PubMed] [Google Scholar]
- Bloyer S, Cavalli G, Brock HW, Dura JM (2003) Identification and characterization of polyhomeotic PREs and TREs. Dev Biol 261: 426–442 [DOI] [PubMed] [Google Scholar]
- Brand AH, Manoukian AS, Perrimon N (1994) Ectopic expression in Drosophila. Methods Cell Biol 44: 635–654 [DOI] [PubMed] [Google Scholar]
- Brown JL, Mucci D, Whiteley M, Dirksen M-L, Kassis JA (1998) The Drosophila Polycomb group gene pleiohomehotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol Cell 1: 1057–1064 [DOI] [PubMed] [Google Scholar]
- Busturia A, Lloyd A, Bejarano F, Zavortink M, Xin H, Sakonju S (2001) The MCP silencer of the Drosophila Abd-B gene requires both Pleiohomeotic and GAGA factor for the maintenance of repression. Development 128: 2163–2173 [DOI] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y (2002) Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298: 1039–1043 [DOI] [PubMed] [Google Scholar]
- Cavalli G, Paro R (1998) The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis. Cell 93: 505–518 [DOI] [PubMed] [Google Scholar]
- Cavalli G, Paro R (1999) Epigenetic inheritance of active chromatin after removal of the main transactivator. Science 286: 955–958 [DOI] [PubMed] [Google Scholar]
- Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V (2002) Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111: 185–196 [DOI] [PubMed] [Google Scholar]
- Farkas G, Gausz J, Galloni M, Reuter G, Gyurkovics H, Karch F (1994) The Trithorax-like gene encodes the Drosophila GAGA factor. Nature 371: 806–808 [DOI] [PubMed] [Google Scholar]
- Francis NJ, Kingston RE (2001) Mechanisms of transcriptional memory. Nat Rev Mol Cell Biol 2: 409–421 [DOI] [PubMed] [Google Scholar]
- Francis NJ, Saurin AJ, Shao Z, Kingston RE (2001) Reconstitution of a functional core polycomb repressive complex. Mol Cell 8: 545–556 [DOI] [PubMed] [Google Scholar]
- Gindhart JG, Kaufmann TC (1995) Identification of Polycomb and trithorax group responsive elements in the regulatory region of the Drosophila homeotic gene Sex combs reduced. Genetics 139: 797–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurkovics H, Gausz J, Kummer J, Karch F (1990) A new homeotic mutation in the Drosophila bithorax complex removes a boundary separating two domains of regulation. EMBO J 9: 2579–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AH, Neely KE, Workman JL (2001) Histone acetyltransferase complexes stabilize swi/snf binding to promoter nucleosomes. Cell 104: 817–827 [DOI] [PubMed] [Google Scholar]
- Hodgson JW, Argiropoulos B, Brock HW (2001) Site-specific recognition of a 70-base-pair element containing d(GA)(n) repeats mediates bithoraxoid polycomb group response element-dependent silencing. Mol Cell Biol 21: 4528–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogga I, Karch F (2002) Transcription through the iab-7 cis-regulatory domain of the bithorax complex interferes with maintenance of Polycomb-mediated silencing. Development 129: 4915–4922 [DOI] [PubMed] [Google Scholar]
- Horard B, Tatout C, Poux S, Pirrotta V (2000) Structure of a polycomb response element and in vitro binding of polycomb group complexes containing GAGA factor. Mol Cell Biol 20: 3187–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DH, Chang YL, Yang CC, Pan IC, King B (2002) pipsqueak encodes a factor essential for sequence-specific targeting of a polycomb group protein complex. Mol Cell Biol 22: 6261–6271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur MW, Laney JD, Jeon SH, Ali J, Biggin MD (2002) Zeste maintains repression of Ubx transgenes: support for a new model of Polycomb repression. Development 129: 1339–1343 [DOI] [PubMed] [Google Scholar]
- Kadam S, McAlpine GS, Phelan ML, Kingston RE, Jones KA, Emerson BM (2000) Functional selectivity of recombinant mammalian SWI/SNF subunits. Genes Dev 14: 2441–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kal AJ, Mahmoudi T, Zak NB, Verrijzer CP (2000) The Drosophila brahma complex is an essential coactivator for the trithorax group protein zeste. Genes Dev 14: 1058–1071 [PMC free article] [PubMed] [Google Scholar]
- Krebs JE, Fry CJ, Samuels ML, Peterson CL (2000) Global role for chromatin remodeling enzymes in mitotic gene expression. Cell 102: 587–598 [DOI] [PubMed] [Google Scholar]
- Laney JD, Biggin MD (1992) Zeste, a nonessential gene, potently activates Ultrabithorax transcription in the Drosophila embryo. Genes Dev 6: 1531–1541 [DOI] [PubMed] [Google Scholar]
- Laney JD, Biggin MD (1996) Redundant control of Ultrabithorax by zeste involves functional levels of zeste protein binding at the Ultrabithorax promoter. Development 122: 2303–2311 [DOI] [PubMed] [Google Scholar]
- Lavrov S, Déjardin J, Cavalli G (2004) Combined immunostaining and FISH analysis of polytene chromosomes. Methods Mol Biol 247: 289–308 [DOI] [PubMed] [Google Scholar]
- Marchetti M, Fanti L, Berloco M, Pimpinelli S (2003) Differential expression of the Drosophila BX-C in polytene chromosomes in cells of larval fat bodies: a cytological approach to identifying in vivo targets of the homeotic Ubx, Abd-A and Abd-B proteins. Development 130: 3683–3689 [DOI] [PubMed] [Google Scholar]
- Martin CH, Mayeda CA, Davis CA, Ericsson CL, Knafels JD, Mathog DR, Celniker SE, Lewis EB, Palazzolo MJ (1995) Complete sequence of the bithorax complex of Drosophila melanogaster. Proc Natl Acad Sci USA 92: 8398–8402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurange C, Paro R (2002) A cellular memory module conveys epigenetic inheritance of hedgehog expression during Drosophila wing imaginal disc development. Genes Dev 16: 2672–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly J, Mishra RK, Karch F (1998) A conserved sequence motif in Polycomb-response elements. Mol Cell 1: 1065–1066 [DOI] [PubMed] [Google Scholar]
- Mishra RK, Mihaly J, Barges S, Spierer A, Karch F, Hagstrom K, Schweinsberg SE, Schedl P (2001) The iab-7 Polycomb response element maps to a nucleosome-free region of chromatin and requires both GAGA and Pleiohomeotic for silencing activity. Mol Cell Biol 21: 1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland NM, King IF, Kingston RE (2003) Regulation of Polycomb group complexes by the sequence-specific DNA binding proteins Zeste and GAGA. Genes Dev 17: 2741–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, Miller EL, O'Connor MB, Kingston RE, Simon JA (2002) Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111: 197–208 [DOI] [PubMed] [Google Scholar]
- Narlikar GJ, Fan HY, Kingston RE (2002) Cooperation between complexes that regulate chromatin structure and transcription. Cell 108: 475–487 [DOI] [PubMed] [Google Scholar]
- Neely KE, Hassan AH, Wallberg AE, Steger DJ, Cairns BR, Wright AP, Workman JL (1999) Activation domain-mediated targeting of the SWI/SNF complex to promoters stimulates transcription from nucleosome arrays. Mol Cell 4: 649–655 [DOI] [PubMed] [Google Scholar]
- Orlando V, Jane EP, Chinwalla V, Harte PJ, Paro R (1998) Binding of trithorax and Polycomb proteins to the bithorax complex: dynamic changes during early Drosophila embryogenesis. EMBO J 17: 5141–5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Bhadra M, Bhadra U, Birchler JA (1997) Cosuppression in Drosophila: gene silencing of alcohol dehydrogenase by white-Adh transgenes is Polycomb dependent. Cell 90: 479–490 [DOI] [PubMed] [Google Scholar]
- Papoulas O, Beek SJ, Moseley SL, McCallum CM, Sarte M, Shearn A, Tamkun JW (1998) The Drosophila trithorax group proteins BRM, ASH1 and ASH2 are subunits of distinct protein complexes. Development 125: 3955–3966 [DOI] [PubMed] [Google Scholar]
- Petruk S, Sedkov Y, Smith S, Tillib S, Kraevski V, Nakamura T, Canaani E, Croce CM, Mazo A (2001) Trithorax and dCBP acting in a complex to maintain expression of a homeotic gene. Science 294: 1331–1334 [DOI] [PubMed] [Google Scholar]
- Poux S, Horard B, Sigrist CJ, Pirrotta V (2002) The Drosophila trithorax protein is a coactivator required to prevent re-establishment of polycomb silencing. Development 129: 2483–2493 [DOI] [PubMed] [Google Scholar]
- Qian S, Varjavand B, Pirrotta V (1992) Molecular analysis of the zeste–white interaction reveals a promoter-proximal element essential for distant enhancer–promoter communication. Genetics 131: 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank G, Prestel M, Paro R (2002) Transcription through intergenic chromosomal memory elements of the Drosophila bithorax complex correlates with an epigenetic switch. Mol Cell Biol 22: 8026–8034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke H, Gregory PD, Horz W (2001) A transient histone hyperacetylation signal marks nucleosomes for remodeling at the PHO8 promoter in vivo. Mol Cell 7: 529–538 [DOI] [PubMed] [Google Scholar]
- Reuter G, Wolff I (1981) Isolation of dominant suppressor mutations for position-effect variegation in Drosophila melanogaster. Mol Gen Genet 182: 516–519 [DOI] [PubMed] [Google Scholar]
- Ringrose L, Rehmsmeier M, Dura JM, Paro R (2003) Genome wide prediction of Polycomb/Trithorax response elements in Drosophila melanogaster. Dev Cell 5: 759–771 [DOI] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Rozovskaia T, Burakov D, Sedkov Y, Tillib S, Blechman J, Nakamura T, Croce CM, Mazo A, Canaani E (1998) The C-terminal SET domains of ALL-1 and Trithorax interact with the INI1 and SNR1 proteins, components of the SWI/SNF complex. Proc Natl Acad Sci USA 95: 4152–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurin AJ, Shao Z, Erdjument-Bromage H, Tempst P, Kingston RE (2001) A Drosophila Polycomb group complex includes Zeste and dTAFII proteins. Nature 412: 655–660 [DOI] [PubMed] [Google Scholar]
- Shao Z, Raible F, Mollaaghababa R, Guyon JR, Wu CT, Bender W, Kingston RE (1999) Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98: 37–46 [DOI] [PubMed] [Google Scholar]
- Spradling AC (1986) P element-mediated transformation. In Drosophila: A Practical Approach, Roberts, DB (ed), IRL Press: Oxford pp 175–197 [Google Scholar]
- Tamkun JW, Deuring R, Scott MP, Kissinger M, Pattatucci AM, Kaufman TC, Kennison JA (1992) Brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell 68: 561–572 [DOI] [PubMed] [Google Scholar]
- Tillib S, Petruk S, Sedkov Y, Kuzin A, Fujioka M, Goto T, Mazo A (1999) Trithorax- and Polycomb-group response elements within an Ultrabithorax transcription maintenance unit consist of closely situated but separable sequences. Mol Cell Biol 19: 5189–5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1
Supplementary Figures S2
Supplementary Figures S3
Supplementary Figures S4
Supplementary Figures S5


