Abstract
We examined the relationship of elevated depressive symptoms with antioxidant status. Cross-sectional data from the National Health and Nutrition Examination Surveys 2005–06 on US adults aged 20–85 years were analyzed. Depressive symptoms were measured using the Patient Health Questionnaire with a score cutpoint of 10 to define “elevated depressive symptoms”. Serum antioxidant status was measured by serum levels of carotenoids, retinol (free and retinyl esters), vitamin C and vitamin E. The main analyses consisted of multiple logistic and zero-inflated poisson regression models, taking into account sampling design complexity. The final sample consisted of 1,798 US adults with complete data. Higher total carotenoid serum level was associated with lower likelihood of elevated depressive symptoms with a reduction in the odds by 37% overall with each SD increase in exposure, and by 34% among women (p<0.05). A dose-response relationship was observed when serum total carotenoids were expressed as quartiles [Q4 (1.62–10.1 μmol/L) vs. Q1(0.06–0.86 μmol/L): OR=0.41; 95% CI: 0.23–0.76, P<0.001; p-value for trend=0.035], though no significant associations were found with other antioxidant levels. Among carotenoids, β-carotene (men and women combined) and lutein+zeaxanthins (women only, after control for dietary lutein+zeaxanthin intake and supplement use) had an independent inverse association with elevated depressive symptoms among US adults. None of the other serum antioxidants had a significant association with depressive symptoms, independently of total carotenoids and other covariates. In conclusion, total carotenoids (mainly β-carotene and lutein+zeaxanthins) in serum were associated with reduced levels of depressive symptoms among community-dwelling US adults.
Keywords: Depressive symptoms, antioxidants, carotenoids, adults
1. Introduction
Depressive symptoms are the most common outcomes of stress-induced reactions in humans (1) and have been studied in relation to a wide array of diseases and conditions including cardiovascular disease(2–3), cancer(4–5), type 2 diabetes mellitus(6–7) and allergic responses(8–9). Recent research has pointed to a link between oxidative stress and psychological stressors conducive to elevated depressive symptoms(10).
Dietary antioxidants include a number of micronutrients that were shown to reduce oxidative stress triggered by injury characterizing pathogenesis of many chronic diseases, including type 2 diabetes, CVD, rheumatological conditions, carcinogenesis(11). In fact, serum levels of antioxidants, reflected their dietary intakes based on several studies(12–13). One class of antioxidants, known as carotenoids are found primarily in fruits and vegetables (FV), while their secondary sources are bread, eggs, beverages, fats, and oils(14). Even though the human diet contains over 40 different types of carotenoids, only β-carotene, α-carotene, β-cryptoxanthin, lycopene, lutein and zeaxanthin have detectable concentrations in human serum (14). Other micronutrients with well-established antioxidative properties include vitamin C, retinol (sparing pro-vitamin A) and vitamin E(15–17).
Evidence from basic experimental and epidemiological studies have triggered a number of hypotheses regarding the possible direction of association between serum antioxidant levels and depressive symptoms in humans as follows: (1) Depression is the outcome of low serum antioxidant status; (2) Antioxidant status is the outcome of poor diet resulting from depression; (3) Both depression and antioxidant status are markers of a third factor (e.g. a genetic variant), which makes them non-causally related. However, assuming the first scenario is true, one possible hypothesis (Hypothesis A) is that depressive symptoms caused by external stressors trigger oxidative stress which in turn causes reduced concentrations of certain antioxidants in serum, independently of dietary intake of those antioxidants(18–22). Another hypothesis (Hypothesis B) is that increased intake of antioxidants in the diet reduces oxidative stress by increasing serum antioxidant level(12–13), thereby reducing the incidence of elevated depressive symptoms by reducing lipid peroxidation in the brain(23–25). While both hypotheses are possible, Hypothesis B is more amenable to dietary intervention. In order to test Hypothesis B, it is important to allow dietary intakes of the specific antioxidant to vary while adjusting for other potentially confounding covariates. To test Hypothesis A, additional adjustment for dietary intake of antioxidants is required.
To date, however, few epidemiological studies have directly assessed the association between antioxidant status (or dietary intakes of antioxidants) and elevated depressive symptoms among adults(22, 26–32) and none so far have used nationally representative US data.
Our present study uses national data on US adults to examine the relationship of elevated depressive symptoms with antioxidant status. We hypothesize that there is an inverse association between serum antioxidant status and the number and severity of depressive symptoms among US adult men and women, that is partially accounted for by dietary intake of those antioxidants (Hypotheses A and B).
2. Materials and methods
2.1 Database and study subjects
The National Health and Nutrition Examination Surveys (NHANES) include a series of cross-sectional surveys providing nationally representative data on the nutrition and health status of the U.S. civilian population. The National Center for Health Statistics (NCHS), Centers for Disease Control (CDC), conducted three earlier waves of NHANES surveys (NHANES I, II, and III) in 1971–1975, 1976–1980, and 1988–1994, respectively. Since 1999, NHANES became a continuous survey. Using a stratified, multistage probability cluster sampling design, the NHANES survey consists of an in-home interview for demographic and basic health information and a health examination in a mobile examination center (MEC). Household interviews were conducted by trained staff and the MEC consists of physicians, medical and health technicians, and dietary and health interviewers. Detailed descriptions of the sample design, interview procedures, and physical examinations conducted were published elsewhere (33). This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Institutional Review Board of the CDC, NCHS. Written, or verbal informed consent was obtained from all subjects/patients. Verbal consent was witnessed and formally recorded. (34).
We analyzed NHANES data from adults aged 20–85 years from 2005–2006. Among a sample of 4,979 adult subjects (2,387 men and 2,592 women) with complete basic demographic data (Sample 1), 3,097 had complete serum antioxidant status and dietary data. Among those, complete data on other covariates of interest including physical activity (PA), smoking status, supplement use, weight and height, serum 25(OH)D, folate, vitamin B-12, homocysteine and total cholesterol were available for 2,859 participants (Sample 2). Among those in Sample 2, complete data on depressive symptoms was available for 1,798 participants (i.e. study sample: Sample 3). Sample 3 differed from Sample 1 on some basic demographic variables. Specifically, Sample 3 participants were younger, more likely to be women, Non-Hispanic black or Mexican-Americans, with >High School level of education and to be current or former smokers compared to those selected only in Sample 1.
2.2. Outcome assessment
The questionnaire section of NHANES 2005–06 included the Patient Health Questionnaire (PHQ). This set of 10 questions reflects self-reported depressive signs and symptoms that are derived from the diagnostic and statistical manual (DSM) IV criteria. There are nine signs and symptoms in DSM IV that were scored between zero (not at all) and 3 (nearly every day), and an additional follow-up question to assess overall impairment ascribed to depressive symptoms. PHQ was validated in primary care settings and shown as a reliable tool for depression diagnosis (35–36). Summing scores on 10 PHQ items potentially yields a total score between 0 and 30. In our sample, the 90th percentile on total PHQ score corresponded to a value of 10. This cutoff point was similarly used elsewhere and had a sensitivity of 88% and a specificity of 88% for major depression.(35–36)
2.3. Exposure assessment
Using high performance liquid chromatography with photodiode array detection, serum concentrations of key antioxidants were measured. In this study, retinol and retinyl esters (defined as the sum of retinyl palmitate and retinyl stearate) were of interest separately and as their sum. Additionally, carotenoids grouped as α-carotene, β-carotene (cis+trans), β-cryptoxanthin, lutein+zeaxanthin, total lycopene and total carotenoids were considered as exposures. Vitamin E was defined as the sum of α- and γ-tocopherol. Vitamin C was also measured with high performance liquid chromatography with electrochemical detection.(37–38)
2.4. Covariates
Socio-demographic covariates included age, sex, race/ethnicity, education, marital status and family income, measured by the poverty income ratio (PIR<100% of the poverty line, 100%-<200%, ≥200%). An objective measure for physical activity was constructed based on individual leisure-time activities with an intensity score assessed by metabolic equivalent (MET) multiplied by duration of the activity and frequency converted to per week unit. This MET×hr/week value was summed for each subject depending on the number of leisure-time physical activities elicited, with participants not eliciting any activity being considered as sedentary (score=0)(39–41). This continuous score was categorized as “0–<5”; “5–10”; “>10”, in the main part of the analysis. Cigarette smoking status was defined as never, former or current smoker. All NHANES participants were eligible for two 24 hr recalls in the 2005–06 wave. The first one was administered during MEC exams and the second 3–10 days later by telephone interview. The average of two 24 hr recalls was examined from which nutrient intakes were estimated using a revised nutrient database(42). Total dietary intakes of alcohol, selected antioxidants (α-carotene, β-carotene, β-cryptoxanthin, lutein+zeaxanthin, lycopene, total carotenoids (i.e. sum of previously listed micronutrients in μg/d), vitamin C and vitamin E in mg/d) and n-3 polyunsaturated fatty acids (n-3 PUFA, sum of DHA+EPA+n-3 DPA; (C≥20), in g/d) were considered as potential confounders, given their putative associations with elevated depressive symptoms(26, 43–45). Moreover, total energy intake was added into statistical models as a covariate to obtain energy-adjusted associations between nutrients and outcome. Using another database to obtain mypyramid equivalents (46) of each food group, cup equivalents of fruits and vegetables were estimated per individual and averaged out between the two 24-hr recalls. Participants with only one 24-hr recall were excluded in the final analysis. A general measure of dietary supplement use over the past 30 days was computed as follows: 0: non-users; 1: using one supplement; 2: using two supplements or more. Another measure of anti-depressant use was obtained from the medication data file with first level category being “242” and second-level category being “249”, using Multum’s therapeutic classification system(47). The final variable was coded as “having used an anti-depressant” (1=yes; 0=no) over the past 30 days.
Serum folate and vitamin B-12 were measured by Bio-Rad Laboratories “Quantaphase II Folate” radioassay kit(48–49). tHcy in serum was measured using “Abbott Homocysteine (HCY) assay”, a fully automated technique (50–51). Serum 25(OH)D was measured by radioimmunoassay (Diasorin Inc., Stillwater, Minnesota)(52). Those nutritional biomarkers were associated with mood disorders and cognitive function in a number of previous studies(53–69).
Moreover, because depressive symptoms are usually increased in number and severity in the presence of co-morbid chronic conditions, we created three measures using self-reports for history of “type 2 diabetes”, “cardiovascular disease” (i.e. congestive heart failure, coronary heart disease, angina, heart attack or stroke) and “cancer of any type”. Finally, many of the lipophillic vitamins are highly correlated with serum lipids and thus total cholesterol was also adjusted for in the analysis as was done in a previous study(70).
2.5. Statistical Analysis
Using Stata release 11.0(71), we first described NHANES 2005–06 study sample characteristics by sex and elevated depressive symptoms status. Differences in means across groups and associations between categorical variables were tested with t- and χ2 tests, respectively. Second, we used Pearson’s correlations to examine associations between Loge transformed serum antioxidant variables, first between each others and second with selected dietary factors and the continuous PHQ score. Partial correlations were additionally conducted to examine the degree to which the association between serum antioxidants and the corresponding dietary antioxidant is explained by dietary intakes of fruits and vegetables and by dietary supplement use. A full multiple regression model of covariates and dietary factors as predictors of serum antioxidants has been published elsewhere using NHANES 2001–06 data(70). Third, multiple logistic regression models were conducted to test associations between antioxidant status (standardized z-scores computed from the total NHANES sample aged 20–85y with available antioxidant data) and “elevated depressive symptoms”, controlling for potential confounders (i.e. all covariates as described earlier, entered simultaneously) and stratifying by sex, as was done in a previous study(69). These associations were tested for depressive symptoms count score (range: 0–30) as well using multiple zero-inflated Poisson (ZIP) regression models (72). The choice of the ZIP model over poisson and negative binomial models was based on a previous similar analysis that compared the three models(73). Two sets of models were carried out to test Hypotheses A and B, as described earlier. One set controlled for all factors except for dietary antioxidants while another set, in addition to all factors, controlled for dietary antioxidants. Models were compared between Hypotheses B and A, in terms of change-in-estimate(74), when dietary antioxidants and supplement use were entered into the model.
To test dose-response relationships, quartiles of main antioxidants (Q2, Q3, Q4 vs. Q1) were entered into multiple logistic regression models and p-values for trend were computed. In all analyses, sampling design complexity was taken into account and adequate sampling weights, strata and primary sampling units were specified using Stata survey commands. Two-year MEC exam weights were used for most sample estimations and masked variance units were used to estimate variances using the Taylor series linearization method(75).
To additionally account for potential selection bias in all main analyses (logistic and zero-inflated poisson regression models), given missing data on the outcome variable compared to the MEC exam sample, we constructed a two-stage Heckman selection model(76), as was done previously in another study using a similar sample(69). To this end, a probit model was conducted at first in which the main selection variable (i.e. belonging to Sample 3 vs. not, among those in Sample 1) were modeled against basic sociodemographic variables that were pseudo-complete for the total NHANES adult sample (i.e. Sample 1). These variables included age, sex, race/ethnicity, marital status, education, poverty income ratio and smoking status. A dummy variable for missing data on those variables was included. From the probit model, the conditional probability of being selected was predicted from which an inverse mills ratio, a function of that predicted probability, was computed and entered at a second stage as a covariate into the main statistical models(77).
In addition, confounding effects of various covariates included in the model were explored using a change-in-estimate type of analysis, in which all main exposures were retained in the model while other covariates were backward eliminated sequentially from each model. The effect of eliminating each covariate on the main effects of interest was assessed accordingly by examining the % change in the estimate of OR associated with each exposure when each covariate was eliminated(78). This analysis, however, did not take design complexity into account.
To test for sex differences in main associations, multiple logistic and ZIP regression models were re-ran in non-stratified model, adding a main effect for sex and an interaction term for each of the antioxidant exposure with sex. Sex difference in the association between each serum antioxidant and PHQ was deemed significant if the interaction term was significant at a level of 0.10, given the lower power of interaction terms compared to main effects (74). All other p-values presented were 2-tailed with p < 0.05 was considered statistically significant and p<0.10 considered as marginally significant, before correction for multiple testing. Multiple testing correction was done by considering a p-value<0.001 as statistically significant when comparing one quartile of a predictor to the referent category (e.g. Q4 vs. Q1) or examining standardized predictor variables in relation to the outcome (i.e. per SD). The same cutoff was applied to the p-value for trend and p-value for interaction.
3. Results
3.1. Study sample characteristics
Table 1 presents the distribution of sample characteristics by sex and depressive symptoms status based on PHQ score. Mean PHQ score was significantly higher among women compared to men (4.5±0.2 vs. 3.9±0.2, p<0.05). Participants with elevated depressive symptoms were generally less educated, more likely to belong to PIR<100% category, be unmarried, less physically active and had higher proportion with history of cancer. Women with elevated depressive symptoms were more likely to be current smokers and had a higher mean BMI. Men and women with elevated depressive symptoms had consistently lower serum levels of β-carotene and total carotenoids combined compared to their non-depressed counterparts and were more likely to use anti-depressants. Women with elevated depressive symptoms had lower serum levels of vitamin E and C as well as all carotenoids compared to their non-depressed counterparts. They additionally reported lower intakes of β-carotene, β-cryptoxanthin, lutein+zeaxanthin, total carotenoids, vitamin C, vitamin E, n-3 PUFA and lower consumption of fruits and vegetables. Moreover, they exhibited lower serum folate and 25-hydroxyvitamin D status. Men with elevated depressive symptoms had higher mean total homocysteine compared to their non-depressed counterparts. Gender differences in dietary intakes and other covariates were also noted, including in intakes of carotenoids, vitamin E, n-3 PUFA, anti-depressant use, dietary supplement use, consumption of fruits and vegetables as well as serum levels of folate and tHcy.
TABLE 1.
Selected baseline characteristics of NHANES 2005–06 participants by sex and “elevated depressive symptoms” status (n=1,798)a
| Men |
Women |
Pb
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHQ score<10 (n=675) | PHQ score≥10 (n =75) | All men (n=750) | PHQ score<10 (n=928) | PHQ score≥10 (n =120) | All Women (n=1,048) | Men vs. women | PHQ ≥10 vs. PHQ <10 (men) | PHQ ≥10 vs. PHQ <10 (women) | |||||||
| Mean or % | SEM/SEP | Mean or % | SEM/SEP | Mean or % | SEM/SEP | Mean or % | SEM/SEP | Mean or % | SEM/SEP | Mean or % | SEM/SEP | ||||
| Socio-demographic, lifestyle factors | |||||||||||||||
| Age (y) | 45.5 | 1.0 | 46.3 | 2.6 | 45.5 | 1.0 | 47.3 | 1.0 | 45.7 | 1.8 | 47.1 | 1.0 | 0.011 | 0.759 | 0.447 |
| Race/ethnicity, % | |||||||||||||||
| Non-Hispanic White | 74.9 | 3.1 | 68.3 | 5.7 | 74.3 | 73.1 | 73 | 2.8 | 65.7 | 4.9 | 72.2 | 2.8 | 0.269 | 0.458 | 0.27 |
| Non-Hispanic black | 8.6 | 1.5 | 13.9 | 3.2 | 9 | 1.5 | 11 | 2.1 | 14.5 | 3.8 | 11.4 | 2.1 | |||
| Mexican-American | 8.3 | 1.4 | 7.5 | 3.2 | 8.2 | 1.4 | 6.9 | 0.8 | 8.4 | 1.9 | 7.1 | 0.8 | |||
| Other ethnicity | 8.1 | 1.8 | 10.3 | 4.4 | 8.3 | 1.6 | 9.1 | 1.8 | 11.4 | 3.1 | 9.3 | 1.8 | |||
| Married, % | 61.8 | 2.2 | 46.9 | 6.4 | 60.6 | 2.3 | 57.2 | 2.6 | 45.5 | 4.3 | 56 | 2.5 | 0.048 | 0.024 | 0.019 |
| Education, % | |||||||||||||||
| <High School | 6.0 | 0.8 | 7.6 | 2.6 | 6.1 | 0.7 | 4.7 | 0.7 | 7.3 | 1.7 | 4.9 | 0.7 | 0.067 | 0.036 | <0.001 |
| High School | 34.8 | 2.3 | 47.5 | 5.3 | 35.9 | 2.3 | 31.3 | 2.2 | 50.7 | 45.1 | 33.4 | 2.2 | |||
| >High School | 59.2 | 2.4 | 44.9 | 4.7 | 58 | 2.4 | 64.1 | 2.6 | 41.9 | 4.4 | 61.6 | 2.5 | |||
| Poverty Income Ratio, % | |||||||||||||||
| <100% | 8.7 | 1.4 | 16.1 | 4.4 | 9.3 | 1.4 | 10 | 1.1 | 26.3 | 4.7 | 11.8 | 1.2 | 0.14 | 0.043 | <0.001 |
| 100%–<200% | 18.6 | 2.3 | 24.3 | 4.8 | 19.1 | 2.3 | 20.7 | 1.8 | 29.6 | 5.2 | 21.7 | 1.9 | |||
| ≥200% | 72.7 | 2.7 | 59.7 | 6.7 | 71.6 | 2.8 | 69.3 | 2.7 | 44.1 | 5.6 | 66.6 | 2.7 | |||
| Smoking status, % | |||||||||||||||
| Never smoker | 42 | 2.7 | 27.9 | 10.6 | 40.8 | 2.9 | 57.1 | 2.5 | 45.4 | 5.6 | 55.9 | 2.4 | <0.001 | 0.271 | 0.018 |
| Former smoker | 30 | 1.5 | 32.9 | 7.1 | 30.3 | 1.7 | 22.4 | 1.9 | 19.1 | 5.3 | 22.1 | 1.9 | |||
| Current smoker | 27.9 | 2.4 | 39.2 | 9.8 | 28.9 | 2.6 | 20.4 | 1.8 | 35.4 | 4.5 | 22.1 | 1.9 | |||
| Physical activity, Mets.hr.wk−1 | 7.8 | 0.6 | 4.0 | 0.7 | 7.5 | 0.6 | 6.1 | 0.5 | 4.2 | 0.9 | 5.9 | 0.4 | 0.001 | 0.033 | 0.045 |
| Body mass index, kg.m−2 | 28.7 | 0.3 | 29.1 | 1.2 | 28.7 | 0.3 | 28.3 | 0.3 | 30.8 | 1.0 | 28.6 | 0.3 | 0.167 | 0.846 | 0.039 |
| History of selected chronic conditions | |||||||||||||||
| Type 2 diabetes, % | 7.4 | 1.1 | 12.6 | 3.5 | 7.9 | 1.0 | 8.6 | 1.1 | 9.7 | 2.5 | 8.8 | 1.1 | 0.613 | 0.154 | 0.656 |
| Cardiovascular disease, % | 7.4 | 0.8 | 9.7 | 3.0 | 7.6 | 0.7 | 7.9 | 1.2 | 12.1 | 3.6 | 8.4 | 1.3 | 0.548 | 0.434 | 0.125 |
| Cancer, % | 4.7 | 1.0 | 16.7 | 4.3 | 5.8 | 0.9 | 9.5 | 1.1 | 11.8 | 3.9 | 9.7 | 0.9 | 0.002 | 0.004 | 0.582 |
| Antioxidant status | |||||||||||||||
| Retinol, μmol/L | 2.23 | 0.03 | 2.4 | 0.08 | 2.24 | 0.03 | 2.00 | 0.04 | 1.9 | 0.08 | 1.99 | 0.03 | <0.001 | <0.001 | 0.835 |
| Serum retinyl esters, μmol/L | 0.121 | 0.003 | 0.106 | 0.009 | 0.120 | 0.003 | 0.116 | 0.004 | 0.08 | 0.004 | 0.112 | 0.003 | 0.075 | 0.531 | <0.001 |
| Total retinol+retinyl esters, μmol/L | 2.35 | 0.03 | 2.51 | 0.08 | 2.36 | 0.03 | 2.12 | 0.04 | 1.98 | 0.04 | 2.1 | 0.04 | <0.001 | 0.001 | 0.5 |
| α-Carotene, μmol/L | 0.07 | 0.01 | 0.04 | 0.00 | 0.07 | 0.01 | 0.11 | 0.09 | 0.06 | 0.00 | 0.11 | 0.01 | <0.001 | 0.065 | 0.001 |
| β-Carotene, μmol/L | 0.3 | 0.01 | 0.19 | 0.02 | 0.29 | 0.01 | 0.51 | 0.03 | 0.24 | 0.02 | 0.48 | 0.01 | <0.001 | 0.003 | <0.001 |
| β-Cryptoxanthin, μmol/L | 0.17 | 0.01 | 0.15 | 0.02 | 0.17 | 0.01 | 0.19 | 0.01 | 0.14 | 0.02 | 0.18 | 0.01 | 0.001 | 0.135 | 0.045 |
| Lutein+zeaxanthin, μmol/L | 0.28 | 0.01 | 0.24 | 0.02 | 0.28 | 0.01 | 0.24 | 0.02 | 0.31 | 0.01 | 0.3 | 0.01 | 0.091 | 0.067 | <0.001 |
| Lycopene, μmol/L | 0.48 | 0.02 | 0.41 | 0.02 | 0.47 | 0.01 | 0.44 | 0.01 | 0.39 | 0.02 | 0.43 | 0.01 | 0.04 | 0.063 | 0.009 |
| Total carotenoids, μmol/L | 1.3 | 0.03 | 1.03 | 0.07 | 1.28 | 0.03 | 1.56 | 0.05 | 1.05 | 0.04 | 1.5 | 0.04 | <0.001 | 0.006 | <0.001 |
| Vitamin E, μmol/L | 28.9 | 0.4 | 28.4 | 2.0 | 28.8 | 0.4 | 30.4 | 0.7 | 26.3 | 1.5 | 30 | 0.6 | 0.059 | 0.742 | 0.005 |
| Vitamin C, μmol/L | 48.3 | 1.1 | 46.1 | 5.2 | 48.2 | 1.15 | 60.3 | 1.7 | 42.9 | 3.8 | 58.4 | 1.6 | <0.001 | 0.413 | <0.001 |
| Dietary Intake | |||||||||||||||
| Total Energy Intake, MJ/d | 11.74 | 0.28 | 11.44 | 0.82 | 11.71 | 0.3 | 8.03 | 0.11 | 6.94 | 0.3 | 7.54 | 0.11 | <0.001 | 0.849 | 0.073 |
| Alcohol intake, g/d | 15.3 | 1.7 | 20.6 | 7.0 | 15.8 | 1.5 | 5.6 | 0.9 | 3.7 | 1.1 | 5.4 | 0.8 | <0.001 | 0.535 | 0.535 |
| α-carotene, μg/d | 442.9 | 52.7 | 296 | 70.7 | 430.3 | 47.1 | 443.9 | 31.0 | 266.2 | 45.8 | 424.3 | 29.1 | 0.813 | 0.83 | 0.207 |
| β-carotene, μg/d | 2190.2 | 160.2 | 1535.7 | 156.9 | 2134.2 | 141.8 | 2364.5 | 120.7 | 1368.4 | 258.1 | 2254.8 | 117.5 | 0.715 | 0.577 | 0.004 |
| β-Cryptoxanthin, μg/d | 137.9 | 10.2 | 156 | 43.1 | 139.4 | 9.8 | 123.9 | 5.3 | 77.8 | 10.6 | 118.8 | 4.9 | 0.335 | 0.979 | 0.008 |
| Lutein+zeaxanthin, μg/d | 1414.7 | 139.4 | 1203.3 | 145.6 | 1396 | 135.1 | 1603.4 | 88.4 | 1001.2 | 158.3 | 1537.1 | 80.8 | 0.35 | 0.724 | 0.031 |
| Lycopene, μg/d | 6520.8 | 474.9 | 6180.8 | 1349 | 6491.7 | 453.6 | 5039.9 | 320.6 | 4253.6 | 1134 | 4953.3 | 311 | <0.001 | 0.596 | 0.628 |
| Total carotenoids, μg/d | 12453 | 727 | 10611 | 1471 | 12296 | 625 | 11496 | 538 | 8069 | 1592 | 11118 | 553 | 0.017 | 0.471 | 0.013 |
| vitamin C, mg/d | 91.6 | 4.0 | 101.4 | 13.7 | 92.4 | 3.4 | 82.7 | 2.6 | 67.1 | 7.6 | 81 | 2.6 | 0.07 | 0.353 | 0.039 |
| vitamin E, mg/d | 8.6 | 0.3 | 6.9 | 0.6 | 8.4 | 0.3 | 6.9 | 0.1 | 5.5 | 0.4 | 6.7 | 0.1 | <0.001 | 0.147 | 0.002 |
| n-3 PUFA, g/d | 0.19 | 0.01 | 0.14 | 0.03 | 0.19 | 0.01 | 0.13 | 0.01 | 0.06 | 0.01 | 0.13 | 0.01 | <0.001 | 0.366 | 0.031 |
| Dietary supplement use, past 30 d | |||||||||||||||
| None | 43.7 | 1.9 | 37.9 | 6.6 | 43.2 | 2.1 | 32.1 | 2.3 | 41.5 | 5.7 | 33.1 | 2.4 | <0.001 | 0.326 | 0.147 |
| One | 30 | 2.1 | 28.8 | 5.6 | 29.9 | 1.9 | 28.3 | 1.0 | 25.8 | 4.1 | 28 | 1.1 | |||
| Two or more | 26.3 | 1.9 | 33.3 | 3.4 | 26.9 | 1.9 | 39.6 | 2.6 | 32.7 | 5.6 | 38.8 | 2.6 | |||
| Fruits and vegetable intake, cup equivalent/d | 2.78 | 0.09 | 2.63 | 0.22 | 2.77 | 0.09 | 2.5 | 0.1 | 1.88 | 0.13 | 2.43± | 0.1 | 0.003 | 0.502 | <0.001 |
| Anti-depressant medication use, % yes | 6.8 | 1.2 | 18 | 4.4 | 7.7 | 1.2 | 15.9 | 0.8 | 38.6 | 6.4 | 18.4 | 1.0 | <0.001 | 0.005 | <0.001 |
| Folate, B-12, tHcy and 25-hydroxyvitamin D in serum | |||||||||||||||
| Folate, nmol/L | 27.7 | 0.7 | 26.2 | 2.1 | 27.6 | 0.7 | 33.9 | 1.0 | 25.5 | 1.6 | 33 | 0.9 | <0.001 | 0.505 | <0.001 |
| Vitamin B-12, pmol/L | 376.4 | 10.9 | 356.9 | 31.3 | 374.8 | 10.8 | 393.7 | 9.6 | 377.4 | 24.7 | 391.9 | 8.6 | 0.137 | 0.384 | 0.804 |
| Total homocysteine, μmol/L | 8.9 | 0.2 | 10.1 | 0.5 | 8.9 | 0.1 | 7.6 | 0.2 | 8.3 | 0.5 | 7.7 | 0.2 | <0.001 | 0.03 | 0.106 |
| 25-hydroxyvitamin D, ng/mL | 21.8 | 0.5 | 20.9 | 0.9 | 21.2 | 0.5 | 21.8 | 0.5 | 18.3 | 0.9 | 21.4 | 0.4 | 0.269 | 0.792 | <0.001 |
Abbreviations: DEP=Depressive symptoms; n-3 PUFA=n-3 highly unsaturated fatty acids; NHANES=National Health and Nutrition Examination Survey; PHQ=Patient Health Questionnaire; SEM=Standard error of the mean; SEP=Standard Error of the Proportion; tHcy=Total homocysteine.
Values are mean±SEM or percent±SEP. Sampling design complexity is taken into account in all analyses. This analysis is done among participants with complete data for PHQ and other key variables of interest (n=1,798).
P-value was based on t-test when row variable is continuous and design-based χ2 test when row variable is categorical.
3.2. Correlation between key study variables
Pearson’s correlation coefficients between Loge transformed serum antioxidant status variables (I. between each others; II. With other dietary variables and PHQ continuous score) are presented in Table 2. For analysis I., correlation coefficients between serum antioxidant variables ranged between <0.10 and non-significant (e.g. Lycopene and retinol+retinyl esters), and >0.70 (e.g. α-carotene and β-carotene). For analysis II., most correlations were weak to moderate (<0.10–0.40) with the strongest coefficients being 0.42 (supplement use vs. serum vitamin E). As expected, PHQ score was inversely related to most serum antioxidant variables. Partial correlations of serum antioxidants with their corresponding dietary intake variable, controlling for fruits and vegetable consumption and supplement use indicated that both sources were important in explaining those correlations, with partial correlation being attenuated by 14% (retinol) to 77% (vitamin E).
TABLE 2.
Pearson’s correlation coefficients between: (I) Loge transformed serum antioxidant status variables and (II) Loge transformed serum antioxidant status variables vs. selected dietary intake variables and continuous PHQ score; NHANES 2005–06 (n=1,798)
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| I. Correlations between Loge transformed serum antioxidant status variables | |||||||||
|
| |||||||||
| Retinol+retinyl esters | Vitamin E | Vitamin C | Total carotenoids | α-carotene | β-carotene | β-cryptoxanthin | Lutein +zeaxanthin | Lycopene | |
| Loge transformed serum antioxidants, μmol/L | |||||||||
| Retinol+retinyl esters | 1 | ||||||||
| Vitamin E | 0.38 | 1 | |||||||
| Vitamin C | 0.10 | 0.28 | 1 | ||||||
| Total carotenoids | 0.11 | 0.42 | 0.42 | 1 | |||||
| α-carotene | 0.10 | 0.32 | 0.41 | 0.76 | 1 | ||||
| β-carotene | 0.12 | 0.40 | 0.45 | 0.86 | 0.79 | 1 | |||
| β-cryptoxanthin | 0.02b | 0.27 | 0.45 | 0.74 | 0.59 | 0.59 | 1 | ||
| Lutein+zeaxathin | 0.09 | 0.40 | 0.35 | 0.75 | 0.54 | 0.56 | 0.60 | 1 | |
| Lycopene | 0.04 b | 0.15 | 0.05 | 0.57 | 0.22 | 0.25 | 0.31 | 0.29 | 1 |
|
| |||||||||
| II. Correlations of
Loge transformed serum antioxidants with selected dietary
intake variables and PHQ score |
|||||||||
| Retinol+retinyl esters | Vitamin E | Vitamin C | Total carotenoids | α-carotene | β-carotene | β-cryptoxanthin | Lutein +zeaxanthin | Lycopene | |
|
|
|||||||||
| Loge transformed dietary intake | |||||||||
| Retinol, μg/d | 0.14;0.12 | 0.10 | 0.08 | −0.00* | 0.05 | 0.05 | −0.02* | −0.00* | −0.01* |
| vitamin E, mg/d | 0.10 | 0.13;0.03* | 0.13 | 0.17 | 0.16 | 0.14 | 0.09 | 0.17 | 0.14 |
| vitamin C, mg/d | 0.02* | 0.11 | 0.36;0.26 | 0.27 | 0.26 | 0.21 | 0.35 | 0.24 | 0.03* |
| Total carotenoids, μg/d | 0.08 | 0.10 | 0.18 | 0.29;0.16 | 0.27 | 0.26 | 0.18 | 0.25 | 0.16 |
| α-carotene, μg/d | 0.11 | 0.15 | 0.18 | 0.22 | 0.36;0.24 | 0.28 | 0.18 | 0.19 | −0.05* |
| β-carotene, μg/d | 0.12 | 0.16 | 0.21 | 0.30 | 0.37 | 0.34;0.22 | 0.18 | 0.28 | −0.01* |
| β-Cryptoxanthin, μg/d | 0.04* | 0.12 | 0.31 | 0.27 | 0.22 | 0.21 | 0.39;0.28 | 0.23 | 0.03* |
| Lutein+zeaxanthin, μg/d | 0.09 | 0.14 | 0.21 | 0.29 | 0.26 | 0.25 | 0.20 | 0.33;0.19 | 0.04* |
| Lycopene, μg/d | 0.01 | 0.03* | 0.05* | 0.13 | 0.06 | 0.05 | 0.08 | 0.06 | 0.25;0.24 |
| Fruits and vegetable intake, cup equivalent/d | 0.08 | 0.14 | 0.28 | 0.30 | 0.31 | 0.26 | 0.29 | 0.26 | 0.09 |
| Supplement use, past 30 days (0, 1, 2) | 0.16 | 0.42 | 0.29 | 0.19 | 0.21 | 0.28 | 0.06 | 0.14 | −0.00 |
| Loge transformed (PHQ score) | −0.06 | −0.10 | −0.07 | −0.07 | −0.08 | −0.09 | −0.09 | −0.09 | −0.04* |
Abbreviations: NHANES=National Health and Nutrition Examination Survey; PHQ=Patient Health Questionnaire; SEE=Standard error of the estimate.
Values are Pearson’s correlation coefficients. Italicized correlation coefficients are partial correlations between serum antioxidant and its corresponding dietary antioxidant (both Loge transformed), controlling for Loge transformed fruit and vegetable intake and supplement use (0, 1, 2 or more).
P>0.05 for null hypothesis that r=0; All other correlations were statistically significant.
3.3. Antioxidant status and depressive symptoms
Findings from a series of multiple logistic and ZIP models examining associations between selected serum antioxidant status variables and depressive symptoms (measured by PHQ) are presented in Table 3. In all presented models, dietary antioxidants and supplement use were not among the covariates that were adjusted for. In a first series of models (Model 1), serum carotenoids, retinol, retinyl esters, vitamins C and E were entered separately as predictors for depressive symptoms along with potential confounding covariates. Significant inverse associations were found between elevated depressive symptoms and three different carotenoids (β-carotene (all, women), lutein+zeaxanthin (all, women) and lycopene (all)) and with retinyl esters in women only. Among those, only β-carotene remained significantly inversely related to elevated depressive symptoms after correction for multiple testing (all: OR=0.52, 95% CI: 0.39–0.70 p<0.001).
TABLE 3.
Associations between selected serum antioxidant status (per 1 SD increase) and depressive symptoms: Multiple logistic and zero-inflated poisson regression models, uncontrolled for dietary antioxidant intakes or supplement use (Hypothesis B): 2-stage Heckman selection models; NHANES 2005–06a
| PHQ score≥10 Vs. PHQ
score<10 |
PHQ count score |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Men | Women | All | Men | Women | ||||
|
|
|
||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | β±SEE | β±SEE | β±SEE | |
|
|
|
||||||||
| MODEL 1b | (n=1,776) | (n=736) | (n=1,040) | (n=1,776) | (n=736) | (n=1,040) | |||
| Retinol, per 0.63 μmol/L | 1.05 | (0.82;1.35) | 1.20 | (0.90;1.60) | 0.97 | (0.60;1.56) | +0.01±0.02 | +0.04±0.04 | −0.02±0.04 |
| Retinyl esters, per 0.09 μmol/L | 0.63 | (0.37;1.08) | 0.87 | (0.54;1.40) | 0.46* | (0.21;0.99) | −0.15±0.03┼ | −0.11±0.05* | −0.18±0.04┼ |
| α-Carotene, per 0.11 μmol/L | 0.70* | (0.50;0.97) | 0.57 | (0.26;1.24) | 0.79 | (0.61;1.03) | −0.05±0.02* | −0.05±0.03 | −0.05±0.03 |
| β-Carotene, per 0.41 μmol/L | 0.52┼ | (0.39;0.70) | 0.57 | (0.26;1.23) | 0.58* | (0.42;0.79) | −0.06±0.04 | −0.09±0.06 | −0.04±0.04 |
| β-Cryptoxanthin, per 0.16 μmol/L | 0.95 | (0.76;1.19) | 0.99 | (0.72;1.37) | 1.00 | (0.70;1.42) | −0.02±0.02 | −0.07±0.03 | +0.01±0.03 |
| Lutein+zeaxanthin, per 0.16 μmol/L | 0.67* | (0.49;0.91) | 0.79 | (0.49;1.28) | 0.65* | (0.44;0.97) | −0.07±0.02* | −0.04±0.05 | −0.07±0.02* |
| Lycopene, per 0.20 μmol/L | 0.81* | (0.70;0.94) | 0.79 | (0.60;1.04) | 0.84 | (0.68;1.04) | −0.08±0.03* | −0.09±0.05 | −0.05±0.03 |
| Vitamin E, per 14.6 μmol/L | 0.83 | (0.55;1.23) | 1.16 | (0.53;2.53) | 0.85 | (0.45;1.60) | −0.00±0.04 | −0.00±0.08 | +0.04±0.04 |
| Vitamin C, per 28.5 μmol/L | 0.85 | (0.58;1.26) | 1.04 | (0.67;1.62) | 0.82 | (0.50;1.36) | −0.03±0.04 | −0.01±0.06 | −0.03±0.06 |
| MODEL 2c | (n=1,776) | (n=736) | (n=1,040) | (n=1,776) | (n=736) | (n=1,040) | |||
| Retinol, per 0.63 μmol/L | 1.07 | (0.83;1.39) | 1.18 | (0.85;1.63) | 0.99 | (0.58;1.68) | +0.01±0.02 | +0.04±0.04 | −0.03±0.04 |
| Retinyl esters, per 0.09 μmol/L | 0.81 | (0.48;1.39) | 0.88 | (0.44;1.78) | 0.60 | (0.28;1.29) | −0.13±0.04* | −0.10±0.05* | −0.16±0.06* |
| α-Carotene, per 0.11 μmol/L | 1.02 | (0.63;1.65) | 0.66 | (0.26;1.69) | 1.16 | (0.72;1.87) | −0.01±0.02 | −0.01±0.04 | −0.01±0.03 |
| β-Carotene, per 0.41 μmol/L | 0.58* | (0.36;0.94) | 0.78 | (0.30;1.65) | 0.60* | (0.39;0.92) | −0.02±0.04 | −0.03±0.08 | −0.01±0.05 |
| β-Cryptoxanthin, per 0.16 μmol/L | 1.31* | (1.10;1.56) | 1.27 | (0.97;1.65) | 1.36 | (1.00;1.85) | +0.03±0.02 | −0.04±0.03 | +0.05±0.03 |
| Lutein+zeaxanthin, per 0.16 μmol/L | 0.75 | (0.54;1.04) | 0.80 | (0.50;1.28) | 0.72 | (0.50;1.04) | −0.04±0.03 | +0.00±0.05 | −0.06±0.03* |
| Lycopene, per 0.20 μmol/L | 0.92 | (0.76;1.10) | 0.89 | (0.62;1.26) | 0.99 | (0.77;1.28) | −0.04±0.02 | −0.06±0.05 | −0.00±0.03 |
| Vitamin E, per 14.6 μmol/L | 0.97 | (0.62;1.39) | 1.19 | (0.67;1.80) | 1.04 | (0.56;1.94) | +0.04±0.04 | +0.04±0.08 | +0.09±0.04 |
| Vitamin C, per 28.5 μmol/L | 0.97 | (0.67;1.39) | 1.10 | (0.67;1.80) | 0.93 | (0.61;1.41) | −0.01±0.05 | +0.01±0.08 | −0.01±0.05 |
| MODEL 3d | (n=1,776) | (n=736) | (n=1,040) | (n=1,776) | (n=736) | (n=1,040) | |||
| Total retinol+retinyl esters, per 0.66 μmol/L | 1.06 | (0.80; 1.42) | 1.15 | (0.80;1.65) | 0.95 | (0.55;1.65) | −0.01±0.02 | +0.02±0.05 | −0.05±0.04 |
| Total carotenoids, per 0.73 μmol/L | 0.62┼ | (0.51; 0.76) | 0.62 | (0.37;1.03) | 0.68┼ | (0.55;0.83) | −0.08±0.03* | −0.12±0.06 | −0.06±0.03 |
| Vitamin E, per 14.6 μmol/L | 0.89 | (0.60;1.32) | 1.12 | (0.48;2.60) | 0.95 | (0.51;1.74) | +0.01±0.04 | −0.00±0.08 | +0.07±0.04 |
| Vitamin C, per 28.5 μmol/L | 0.96 | (0.66; 1.40) | 1.10 | (0.67;1.83) | 0.90 | (0.59;1.40) | −0.01±0.05 | +0.01±0.07 | −0.02±0.06 |
Abbreviations: BMI=Body Mass Index; CI=confidence interval; Met=Metabolic Equivalent; NHANES=National Health and Nutrition Examination Survey; OR=odds ratio; PHQ=Patient Health Questionnaire; SEE=Standard error of the estimate.
Values are odds ratios with 95% confidence intervals or β±SEE. Sampling design complexity is taken into account in all analyses.
P<0.05;
P<0.001 for null hypothesis that β=0 or Loge(OR)=0 based on Wald test.
Model 1 included each antioxidant exposure separately and adjusted for socio-demographic factors: age, sex, race/ethnicity, marital status, educational level and poverty income ratio, and other potential confounders: Lifestyle and health-related factors (smoking status, BMI, physical activity: Mets.hr.wk−1, recoded as “0–<5”; “5–10”; “>10”, history of selected chronic conditions (i.e. type 2 diabetes, CVD and cancer)), anti-depressant use and dietary intakes (total energy intake, alcohol, n-3 PUFA), serum levels folate, total homocysteine, vitamin B-12, 25-hydroxyvitamin D and serum total cholesterol, anti-depressant use, and the inverse mills ratio, 2-stage Heckman selection model.
Model 2 included all serum antioxidant exposures simultaneously, controlling for the same covariate as above.
Model 3 is model 2 (i.e. controlling for the same covariates as above) but with main exposures being the total retinol+retinyl esters, total carotenoids and vitamin E.
Similar patterns of association were found when PHQ count (Model 1) was the outcome in the series of ZIP models, though only the inverse relationship between retinyl esters and PHQ count was deemed significant after correction for multiple testing, in both the total population and among women.
In model 2, when all antioxidants were entered into the same model simultaneously along with potential confounding covariates, some of the previously observed inverse associations remained significant (though slightly attenuated) but not others. In particular, while the association between lycopene and elevated depressive symptoms became non-significant, the significance of the inverse association was retained for β-carotene (all, women), while β-cryptoxanthin became associated with elevated depressive symptoms in the total population (OR=1.31; 95% CI: 1.10–1.56). In ZIP models, an inverse relationship between lutein+zeaxanthin and PHQ count score was found only among women. In addition, those models indicated that retinyl esters were inversely related to the PHQ count score among both men and women. None of these associations were deemed statistically significant when correcting for multiple testing (p>0.001).
In model 3, all antioxidants were similarly entered simultaneously while combining total carotenoids and retinol+retinyl esters together as two main exposures and adjusting for the same covariates as in model 1. In logistic regression models, among both sexes combined and in women, there was an inverse association between total carotenoid level and elevated depressive symptoms with a reduction in the odds by 38% overall and 32% in women (p<0.001). In ZIP models, this inverse association was observed only in both sexes combined, but did not reach significant after correction for multiple testing (p>0.001). None of the other antioxidants had a significant association with depressive symptoms independently of total carotenoids and other covariates. Although some of the associations were found to be significant in women and not in men, it is worth noting that sex differences as tested in a separate model with interaction terms were not statistically significant at a 0.10 level.
In Table 4 models, when additional control was made on dietary antioxidants and supplement use, most of the findings remained consistent with those of Table 3 corresponding models, even after correction for multiple testing.
TABLE 4.
Associations between selected serum antioxidant status (per 1 SD increase) and depressive symptoms: Multiple logistic and zero-inflated poisson regression models, controlled for dietary antioxidant intakes and supplement use (Hypothesis A): 2-stage Heckman selection models; NHANES 2005–06a
| PHQ score≥10 Vs. PHQ
score<10 |
PHQ count score |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Men | Women | All | Men | Women | ||||
|
|
|
||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | β±SEE | β±SEE | β±SEE | |
|
|
|
||||||||
| MODEL 1b | (n=1,776) | (n=736) | (n=1,040) | (n=1,776) | (n=736) | (n=1,040) | |||
| Retinol, per 0.63 μmol/L | 1.06 | (0.81;1.37) | 1.18 | (0.87;1.63) | 0.97 | (0.61;1.56) | 0.00±0.02 | +0.03±0.05 | −0.03±0.04 |
| Retinyl esters, per 0.09 μmol/L | 0.60 | (0.34;1.06) | 0.82 | (0.49;1.37) | 0.42* | (0.19;0.90) | −0.17±0.03┼ | −0.13±0.05* | −0.20±0.04┼ |
| α-Carotene, per 0.11 μmol/L | 0.71* | (0.52;0.97) | 0.59 | (0.26;1.33) | 0.79 | (0.62;1.00) | −0.06±0.02* | −0.05±0.04 | −0.05±0.03 |
| β-Carotene, per 0.41 μmol/L | 0.52┼ | (0.37;0.74) | 0.55 | (0.25;1.21) | 0.58* | (0.37;0.90) | −0.06±0.04 | −0.10±0.06 | −0.04±0.05 |
| β-Cryptoxanthin, per 0.16 μmol/L | 0.94 | (0.73;1.21) | 0.86 | (0.55;1.33) | 1.07 | (0.78;1.47) | −0.02±0.02 | −0.10±0.05 | 0.02±0.03 |
| Lutein+zeaxanthin, per 0.16 μmol/L | 0.66* | (0.48;0.92) | 0.74 | (0.45;1.24) | 0.65* | (0.43;0.99) | −0.07±0.02* | −0.06±0.05 | −0.08±0.03* |
| Lycopene, per 0.20 μmol/L | 0.80* | (0.70;0.92) | 0.79 | (0.60;1.04) | 0.83 | (0.64;1.07) | −0.08±0.02* | −0.09±0.05 | −0.06±0.03 |
| Vitamin E, per 14.6 μmol/L | 0.75 | (0.47;1.18) | 0.98 | (0.41;2.34) | 0.78 | (0.38;1.61) | −0.03±0.04 | −0.05±0.08 | 0.01±0.05 |
| Vitamin C, per 28.5 μmol/L | 0.78 | (0.51;1.22) | 0.87 | (0.50;1.50) | 0.80 | (0.46;1.39) | −0.06±0.05 | −0.05±0.07 | −0.04±0.06 |
| MODEL 2c | |||||||||
| Retinol, per 0.63 μmol/L | 1.08 | (0.82;1.43) | 1.12 | (0.80;1.58) | 1.02 | (0.59;1.74) | 0.01±0.02 | 0.04±0.05 | −0.03±0.04 |
| Retinyl esters, per 0.09 μmol/L | 0.83 | (0.47;1.47) | 0.91 | (0.42;1.97) | 0.54 | (0.24;1.21) | −0.13±0.03* | −0.09±0.05 | −0.17±0.06* |
| α-Carotene, per 0.11 μmol/L | 1.08 | (0.68;1.72) | 0.77 | (0.29;2.01) | 1.19 | (0.75;1.90) | −0.01±0.02 | +0.00±0.04 | −0.01±0.03 |
| β-Carotene, per 0.41 μmol/L | 0.56* | (0.32;0.97) | 0.80 | (0.35;1.86) | 0.60* | (0.38;0.93) | −0.03±0.04 | −0.04±0.08 | −0.02±0.05 |
| β-Cryptoxanthin, per 0.16 μmol/L | 1.37* | (1.07;1.75) | 1.15 | (0.79;1.68) | 1.54* | (1.11;2.13) | +0.04±0.02* | −0.06±0.04 | +0.08±0.03* |
| Lutein+zeaxanthin, per 0.16 μmol/L | 0.74 | (0.54;1.03) | 0.86 | (0.54;1.37) | 0.67* | (0.46;0.98) | −0.04±0.02 | +0.01±0.05 | −0.06±0.03* |
| Lycopene, per 0.20 μmol/L | 0.91 | (0.79;1.06) | 0.84 | (0.60;1.18) | 0.99 | (0.74;1.34) | −0.04±0.02 | −0.06±0.05 | −0.01±0.03 |
| Vitamin E, per 14.6 μmol/L | 0.84 | (0.52;1.34) | 0.99 | (0.31;3.13) | 0.86 | (0.46;1.62) | 0.01±0.04 | −0.02±0.08 | 0.06±0.05 |
| Vitamin C, per 28.5 μmol/L | 0.89 | (0.58;1.35) | 0.91 | (0.46;1.82) | 0.93 | (0.60;1.43) | −0.03±0.05 | −0.02±0.08 | −0.02±0.06 |
| MODEL 3d | |||||||||
| Total retinol+retinyl esters, per 0.66 μmol/L | 1.07 | (0.81;1.42) | 1.13 | (0.79;1.63) | 0.97 | (0.58;1.60) | −0.00±0.02 | +0.02±0.05 | −0.05±0.04 |
| Total carotenoids, per 0.73 μmol/L | 0.62┼ | (0.51;0.76) | 0.63 | (0.36;1.08) | 0.65┼ | (0.47;0.89) | −0.09±0.03* | −0.12±0.07 | −0.07±0.04 |
| Vitamin E, per 14.6 μmol/L | 0.79 | (0.51;1.22) | 0.97 | (0.39;2.42) | 0.84 | (0.46;1.55) | −0.01±0.04 | −0.05±0.08 | +0.04±0.05 |
| Vitamin C, per 28.5 μmol/L | 0.88 | (0.57;1.35) | 0.92 | (0.47;1.80) | 0.88 | (0.55;1.42) | −0.03±0.05 | −0.02±0.07 | −0.03±0.06 |
Abbreviations: BMI=Body Mass Index; CI=confidence interval; Met=Metabolic Equivalent; NHANES=National Health and Nutrition Examination Survey; OR=odds ratio; PHQ=Patient Health Questionnaire; SEE=Standard error of the estimate.
Values are odds ratios with 95% confidence intervals or β±SEE. Sampling design complexity is taken into account in all analyses.
P<0.05;
P<0.01 for null hypothesis that β=0 or Loge(OR)=0 based on Wald test.
Model 1 included each antioxidant exposure separately and adjusted for socio-demographic factors: age, sex, race/ethnicity, marital status, educational level and poverty income ratio, and other potential confounders: Lifestyle and health-related factors (smoking status, BMI, physical activity: Mets.hr.wk−1, recoded as “0–<5”; “5–10”; “>10”, history of selected chronic conditions (i.e. type 2 diabetes, CVD and cancer)) and dietary intakes (total energy intake, alcohol, dietary antioxidant (or group of antioxidants), n-3 PUFA, dietary supplement use), serum levels folate, total homocysteine, vitamin B-12, 25-hydroxyvitamin D and serum total cholesterol, anti-depressant use, and the inverse mills ratio, 2-stage Heckman selection model.
Model 2 included all antioxidant exposures simultaneously, controlling for the same covariate as above.
Model 3 is model 2 (i.e. controlling for the same covariates as above) but with main exposures being the total retinol+retinyl esters, total carotenoids and vitamin E.
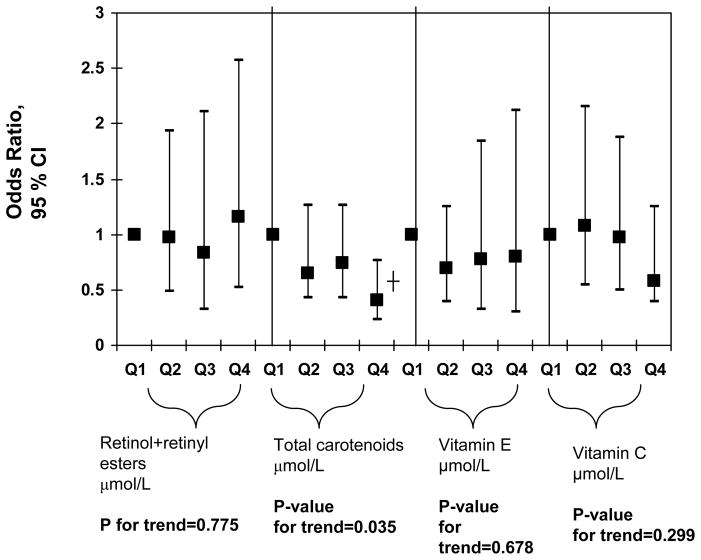
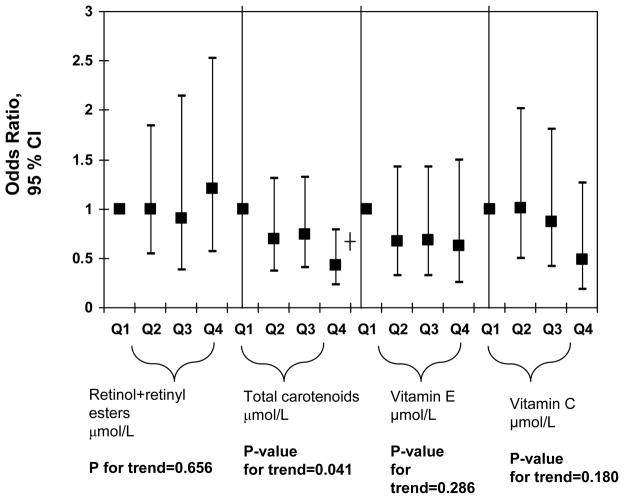
In order to further examine dose-response relationships between antioxidant status and elevated depressive symptoms, quartiles of main exposures were used and p-value for trend was obtained (Figure 1) for men and women combined. This particular analysis was uncontrolled for dietary antioxidants or supplement use. Among all serum antioxidant exposures entered into the model, only total carotenoids showed an apparent linear dose-response relationship with a p-value for trend of 0.035 and the OR for elevated depressive symptoms and quartiles of carotenoids was only significant when comparing Q4 (1.62–10.1 μmol/L) with Q1(0.06–0.86 μmol/L) [OR=0.41; 95% CI: 0.23–0.76; p<0.001], suggesting a possible threshold of 1.62 μmol/L before a significant association can be detected between serum carotenoids and elevated depressive symptoms. When dietary antioxidants and supplement use were introduced into the same model (Figure 2), the results remained similar, with a p-value for trend of 0.041 for serum total carotenoids expressed as quartiles. However, linear dose-response was no longer significant in both Figures 1 and 2 when taking multiple testing into account (p-value for trend>0.001). A sensitivity analysis that additionally controlled for employment status and medical insurance coverage resulted in a slight attenuation of effects though the OR for Q4 vs. Q1 for total carotenoids in relation to elevated depressive symptoms remained statistcally significant in both Figures 1 and 2 analyses (p=0.02). Another sensitivity analysis in which subjects with inadequate dietary intakes of antioxidants were excluded did not alter our findings appreciably.
FIGURE 1.
Adjusted odds ratios (with 95% CI) of major serum antioxidant level (expressed as quartiles, Q2, Q3, Q4 vs. Q1) and elevated depressive symptoms among US adults, uncontrolled for dietary antioxidant intakes or supplement use; NHANES 2005–06
Notes: CI=Confidence Interval. Ranges for each antioxidant quartile is as follows in μmol/L: Retinol+retinyl esters (Q1: 0.07–1.7; Q2: 1.7–2.1; Q3: 2.1–2.5; Q4: 2.5–8.9); Total carotenoids (Q1: 0.06–0.86; Q2: 0.86–1.18; Q3: 1.18–1.62; Q4: 1.62–10.1); Vitamin E (Q1: 0.2–26.7; Q2: 21.7–27.3; Q3: 27.4–35.9; Q4: 35.9–303.8); Vitamin C (Q1: 0.6–34.6; Q2: 35.2–54.5; Q3: 55.1–70.4; Q4: 71.0–274.2). Analyses were based on multiple logistic regression models that included all antioxidant exposures simultaneously adjusted for socio-demographic factors: Lifestyle and health-related factors (smoking status, BMI, physical activity: Mets.hr.wk−1, recoded as “0–<5”; “5–10”; “>10”, history of selected chronic conditions (i.e. type 2 diabetes, CVD and cancer)), anti-depressant use and dietary intakes (total energy intake, alcohol, n-3 PUFA), serum levels folate, total homocysteine, vitamin B-12, 25-hydroxyvitamin D and serum total cholesterol, anti-depressant use, and the inverse mills ratio, 2-stage Heckman selection model.
*P<0.05; ┼P<0.001 for null hypothesis that Loge(OR)=0.
FIGURE 2.
Adjusted odds ratios (with 95% CI) of major serum antioxidant level (expressed as quartiles, Q2, Q3, Q4 vs. Q1) and elevated depressive symptoms among US adults, controlled for dietary antioxidant intakes and supplement use; NHANES 2005–06
Notes: CI=Confidence Interval. Ranges for each antioxidant quartile is as follows in μmol/L: Retinol+retinyl esters (Q1: 0.07–1.7; Q2: 1.7–2.1; Q3: 2.1–2.5; Q4: 2.5–8.9); Total carotenoids (Q1: 0.06–0.86; Q2: 0.86–1.18; Q3: 1.18–1.62; Q4: 1.62–10.1); Vitamin E (Q1: 0.2–26.7; Q2: 21.7–27.3; Q3: 27.4–35.9; Q4: 35.9–303.8); Vitamin C (Q1: 0.6–34.6; Q2: 35.2–54.5; Q3: 55.1–70.4; Q4: 71.0–274.2). Analyses were based on multiple logistic regression models that included all antioxidant exposures simultaneously adjusted for socio-demographic factors: age, sex, race/ethnicity, marital status, educational level and poverty income ratio, and other potential confounders: Lifestyle and health-related factors (smoking status, BMI, physical activity: Mets.hr.wk−1, recoded as “0–<5”; “5–10”; “>10”, history of selected chronic conditions (i.e. type 2 diabetes, CVD and cancer)) and dietary intakes (total energy intake, alcohol, dietary antioxidant (or group of antioxidants), n-3 PUFA, dietary supplement use), serum levels folate, total homocysteine, vitamin B-12, 25-hydroxyvitamin D and serum total cholesterol, anti-depressant use, and the inverse mills ratio, 2-stage Heckman selection model.
*P<0.05 ┼P<0.001 for null hypothesis that Loge(OR)=0.
Potential confounding by various covariates entered into the main statistical models, particularly logistic regression model of Figure 2, was assessed using a change-in-estimate analysis, which did not take into account sampling design complexity (data not shown). Our findings suggest that when covariates aside from main antioxidant exposures were backward eliminated (Figure 2 model, with antioxidant quartiles entered as an ordinal variable to assess dose-response), change-in-estimate of the OR did not exceed 10% (for serum total carotenoids as a covariate, when main exposure was serum vitamin C), suggesting weak confounding effect exerted by each of the other antioxidants and the remaining covariates in the model. More notably, dietary antioxidants did not act as significant confounders in the association between serum antioxidants and elevated depressive symptoms.
4. Discussion
Our present study used national data on US adults to examine the relationship of elevated depressive symptoms with antioxidant status as measured by serum levels of carotenoids, retinol (free and retinyl esters), vitamin C and vitamin E. There were several key findings. First, we found an inverse association between serum total carotenoid level and elevated depressive symptoms with a reduction in the odds by 38% overall with each SD increase in exposure, and by 32% among women (p<0.05). Second, we found a dose-response relationship when total carotenoids were expressed as quartiles [Q4(1.62–10.1 μmol/L) vs. Q1(0.06–0.86 μmol/L): OR=0.41; 95% CI: 0.23–0.76, P<0.001; p-value for trend=0.035], though no significant associations were found with other antioxidant levels. Third, among carotenoids, β-carotene (both sexes combined) and lutein+zeaxanthins (among women, after control for dietary intake and supplement use) were associated inversely with elevated depressive symptoms among US adults.
To date, only a handful of studies have evaluated the role played by antioxidants in depressive symptoms (22, 26, 30, 32) or depression (22, 26–28, 30–31). The majority of those studies focused on elderly populations (26, 30–32) and the most frequently studied antioxidant was vitamin E (26–28, 30–31). Moreover, one study (26) examined carotene and vitamin C in relation to depressive symptoms, another focused on total serum carotenoids(32), while a thrid study (22) tested several antioxidants simultaneously. In a case-control study, Maes et al. (27) found significantly lower serum levels of vitamin E among 42 patients with major depressive disorder compared to 26 healthy volunteers. Owen et al. measured both serum and dietary levels of vitamin E among depressed adults based on Beck Depression Inventory. Their results suggested no significant association between dietary level of vitamin E and major depression, but a significantly lower serum vitamin E level among depressed adults compared to the general population (28). Shibata et al. used the short version of the Geriatric Depression Scale to assess cross-sectional and longitudinal effects of blood levels of vitamin E on depressive status among 504 elderly residents of a rural community. While cross-sectional analysis revealed no significant findings, longitudinal analysis showed a protective effect of vitamin E on depressive status among men only, after adjustment for age, education and baseline GDS score (31). In the Rotterdam study, Tiemeir et al. investigated the association of blood vitamin E level with depressive symptoms as determined by the Center for Epidemiologic Studies Depression (CES-D) scale, comparing 262 cases to 459 randomly selected controls. After adjustment for biological and behavioral factors, no significant associations were noted between blood vitamin E level and depressive symptoms or depression among elderly men and women (30). Oishi et al. conducted a cross-sectional study of 279 community-dwelling Japanese elderly persons to evaluate dietary factors, including several antioxidants, in relation to depressive symptoms measured using the CES-D. Among males, the observed odds ratios (the 95% confidence intervals) for the depressive state were 0.36 (95% CI: 0.13–0.98) in the highest tertile of carotene intake, 0.33 (95% CI: 0.12–0.93) in the highest tertile of vitamin C intake and 0.33 (95% CI: 0.12–0.92) in the medium tertile of vitamin E intake (26). Among females, similar results were observed, but these results were not statistically significant (26). Recently, a longitudinal study using InChianti data found a clear inverse relationship between serum total carotenoids at baseline and incidence of elevated depressive symptoms after a 6yr follow-up period (OR=0.72, 95% CI=0.52–0.99, P = 0.04), adjusting for important confounders. This association was shown to be partially mediated by inflammatory markers such as interleukin-1 receptor antagonist(32).
Our findings of an assocation between serum total carotenoids, β-carotene and lutein+zeaxathin and elevated depressive symptoms are similar to that study(32), and unlike the study by Oishi et al. (26), we did not observe a strong association between depressive symptoms and dietary carotenoids. In addition, while our unadjusted analyses indicated that depressed women had lower levels of vitamins C and E compared to non-depressed women, this association became null after adjustment for potentially confounding factors and other serum antioxidants. These findings indicates that only total carotenoids are associated with a reduced risk of depression especially after reaching a threshold corresponding to the uppermost quartile in our sample (>1.62 μmol/L). Since this effect was not attenuated by dietary or supplemental intake, this suggests that hypothesis A is more likely than hypothesis B. In other words, depressive symptoms caused by external stressors (10) trigger oxidative stress which in turn causes reduced concentrations of total carotenoids in serum, independently of dietary intake of this class of antioxidants. The differential effect of total carotenoids as opposed to vitamins C and E should be studied further. Nevertheless, reverse causation whereby lower carotenoid levels among depressed individuals is due to poor diet can only be ruled out in a longitudinal study.
The biological mechanisms behind the putative causal association between antioxidant status and depression in general can be outlined as follows: the brain is considered particularly vulnerable to oxidative stress due to its high oxygen consumption, its modest antioxidant defenses, and its lipid-rich constitution (79–80). The brain is also susceptible to secondary and self-perpetuating damage from oxidative cellular injury via the neurotoxic effects of released excitatory amines (mainly glutamate) and iron, and the activated inflammatory response(79). Oxidative stress, an imbalance between the production of reactive oxygen species (ROS) and the cell’s ability to scavenge those species with various antioxidants, has been implicated in the pathogenesis of many chronic diseases, including type 2 diabetes mellitus, cardiovascular disease, rheumatologic disorders and carcinogenesis(11). Oxidative stress induces damage to DNA and cell membranes in both animal experiments(17) and human epidemiological studies(81). Many studies found evidence of an increase in ROS with age and their deleterious effects on lipids, especially polyunsaturated fatty acids. The increase in lipid peroxidation affects the oxidation of structurally important proteins disrupting transmembrane ion movements and cellular metabolic processes(82–83), the most notable one of which is brain synaptic function.
Furthermore, oxidative damage may cause an autoimmune response by changing the chemical structures of otherwise ubiquitous molecules to generate a variety of new, highly immunogenic epitopes (84). Overall, these oxidative insults lead to a decrease in membrane fluidity, an inactivation of enzymes, ion channels and receptors, and as a result, alterations of neurotransmission, neuronal function and general brain activity(82–83).
In animal studies, adding immobilization stress on rats triggered increased levels of lipid peroxidation and a weakened endogenous antioxidant system in plasma (18–19). In mice, adding another form of stressor, a communication box paradigm with electric stress, was shown to increase lipid peroxidation activity in the brain (20). Those stress-induced changes in lipid peroxidation and levels of antioxidants have also been shown recently in humans(21–22).
Our study has notable strengths, which include its selection of a large nationally representative sample, the collection of antioxidant status biomarker data and use of a validated questionnaire for assessment of depressive symptoms. However, our study also suffers from some limitations. First, although use of dietary supplements over a 30-days period was considered a proxy or crude measure for individual micronutrient supplementation, it was previously shown to be directly associated with each of the serum antioxidant status measures, with a clear dose-response relationship, independently of socio-demographic, lifestyle and health-related factors(70). Second, because of the study’s cross-sectional design, it was not possible to make causal inference through ascertaining temporality of associations. Hence, we were not able to ascertain the temporality of the associations between serum antioxidants, depressive symptoms and oxidative stress, though it was clear that serum carotenoids were a direct reflection of an increased consumption of fruits and vegetables as well as dietary supplement use. Lower intake of fruits, vegetables and fibers may mediate the relationship between lower carotenoid levels and depression. Indeed, depression is directly associated with reduction in dietary quality, thus reduction in the consumption of antioxidants. (70) Finally, residual confounding by unmeasured covariates cannot be totally discounted.
In conclusion, we found a relation between carotenoids and depressive symptoms with some dose-response. Further investigation is needed through RCTs and longitudinal studies to examine the nature of the temporality of the relation. Currently, there is limited evidence to support the role of antioxidants in general and carotenoids in particular for the prevention of depression or depressive symptoms. Thus, additional intervention research is needed before making any prescriptive or policy recommendations for antioxidants in prevention of depression.
Acknowledgments
All sources of funding: This study was fully supported by the Intramural Research Program of the National Institute on Aging, NIA/NIH/IRP.
ABBREVIATIONS
- CES-D
Center for Epidemiologic Studies Depression
- CVD
cardiovascular disease
- DEP
Depressive symptoms
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- DSM
Diagnostic and Statistical Manual
- EPA
eicosapentaenoic acid
- GSH
plasma-reduced glutathione
- GSSG
glutathione disulfide
- MEC
Mobile examination center
- n-3 PUFA
n-3 polyunsaturated fatty acids
- NHANES
National Health and Nutrition Examination Survey
- PHQ
Patient Health Questionnaire
- ROS
Reactive oxygen species
- SEM
Standard error of the mean
- SEP
Standard Error of the Proportion
- tHcy
Total homocysteine
Footnotes
Contribution of each author:
MAB: Conceptualization, literature review, plan of analysis, data management and statistical analysis, write-up of the manuscript, revision of the manuscript.
HAB: Literature search and review, plan of analysis, write-up of parts of the manuscript, revision of the manuscript.
AB: Literature search and review, write-up of parts of the manuscript, revision of the manuscript.
MRS: Literature search, plan of analysis, write-up of parts of the manuscript, revision of the manuscript.
ABZ: Plan of anlysis, write-up of parts of the manuscript, revision of the manuscript
Conflict of interest statement: None declared.
References
- 1.Leonard BE. Stress, norepinephrine and depression. J Psychiatry Neurosci. 2001;26( Suppl):S11–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Lesperance F, Frasure-Smith N. Depression in patients with cardiac disease: a practical review. J Psychosom Res. 2000 Apr-May;48(4–5):379–91. doi: 10.1016/s0022-3999(99)00102-6. [DOI] [PubMed] [Google Scholar]
- 3.Ketterer MW, Mahr G, Goldberg AD. Psychological factors affecting a medical condition: ischemic coronary heart disease. J Psychosom Res. 2000 Apr-May;48(4–5):357–67. doi: 10.1016/s0022-3999(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 4.Newport DJ, Nemeroff CB. Assessment and treatment of depression in the cancer patient. J Psychosom Res. 1998 Sep;45(3):215–37. doi: 10.1016/s0022-3999(98)00011-7. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel D. Cancer and depression. Br J Psychiatry Suppl. 1996 Jun;(30):109–16. [PubMed] [Google Scholar]
- 6.Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults: is there a link? Diabetes Care. 2000 Oct;23(10):1556–62. doi: 10.2337/diacare.23.10.1556. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami N, Takatsuka N, Shimizu H, et al. Depressive symptoms and occurrence of type 2 diabetes among Japanese men. Diabetes Care. 1999 Jul;22(7):1071–6. doi: 10.2337/diacare.22.7.1071. [DOI] [PubMed] [Google Scholar]
- 8.Cuffel B, Wamboldt M, Borish L, et al. Economic consequences of comorbid depression, anxiety, and allergic rhinitis. Psychosomatics. 1999 Nov-Dec;40(6):491–6. doi: 10.1016/S0033-3182(99)71187-4. [DOI] [PubMed] [Google Scholar]
- 9.Bell IR, Jasnoski ML, Kagan J, et al. Depression and allergies: survey of a nonclinical population. Psychother Psychosom. 1991;55(1):24–31. doi: 10.1159/000288404. [DOI] [PubMed] [Google Scholar]
- 10.Moller P, Wallin H, Knudsen LE. Oxidative stress associated with exercise, psychological stress and life-style factors. Chem Biol Interact. 1996 Sep 27;102(1):17–36. doi: 10.1016/0009-2797(96)03729-5. [DOI] [PubMed] [Google Scholar]
- 11.Soory M. Relevance of nutritional antioxidants in metabolic syndrome, ageing and cancer: potential for therapeutic targeting. Infect Disord Drug Targets. 2009 Aug;9(4):400–14. doi: 10.2174/187152609788922537. [DOI] [PubMed] [Google Scholar]
- 12.Campbell DR, Gross MD, Martini MC, et al. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol Biomarkers Prev. 1994 Sep;3(6):493–500. [PubMed] [Google Scholar]
- 13.Stryker WS, Kaplan LA, Stein EA, et al. The relation of diet, cigarette smoking, and alcohol consumption to plasma beta-carotene and alpha-tocopherol levels. Am J Epidemiol. 1988 Feb;127(2):283–96. doi: 10.1093/oxfordjournals.aje.a114804. [DOI] [PubMed] [Google Scholar]
- 14.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007 Mar;55(3):207–16. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Paolisso G, D’Amore A, Balbi V, et al. Plasma vitamin C affects glucose homeostasis in healthy subjects and in non-insulin-dependent diabetics. Am J Physiol Endocrinol Metab. 1994 Feb 1;266(2):E261–8. doi: 10.1152/ajpendo.1994.266.2.E261. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Mokdad AH, Giles WH, et al. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003 Sep;52(9):2346–52. doi: 10.2337/diabetes.52.9.2346. [DOI] [PubMed] [Google Scholar]
- 17.Sies H, Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr. 1995 Dec;62(6 Suppl):1315S–21S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Wang X, Mori A. Immobilization stress-induced antioxidant defense changes in rat plasma: effect of treatment with reduced glutathione. Int J Biochem. 1994 Apr;26(4):511–7. doi: 10.1016/0020-711x(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 19.Davydov VV, Shvets VN. Lipid peroxidation in the heart of adult and old rats during immobilization stress. Exp Gerontol. 2001 Jul;36(7):1155–60. doi: 10.1016/s0531-5565(01)00086-9. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto K, Yobimoto K, Huong NT, et al. Psychological stress-induced enhancement of brain lipid peroxidation via nitric oxide systems and its modulation by anxiolytic and anxiogenic drugs in mice. Brain Res. 1999 Aug 21;839(1):74–84. doi: 10.1016/s0006-8993(99)01715-1. [DOI] [PubMed] [Google Scholar]
- 21.Cernak I, Savic V, Kotur J, et al. Alterations in magnesium and oxidative status during chronic emotional stress. Magnes Res. 2000 Mar;13(1):29–36. [PubMed] [Google Scholar]
- 22.Tsuboi H, Shimoi K, Kinae N, et al. Depressive symptoms are independently correlated with lipid peroxidation in a female population: comparison with vitamins and carotenoids. J Psychosom Res. 2004 Jan;56(1):53–8. doi: 10.1016/S0022-3999(03)00567-1. [DOI] [PubMed] [Google Scholar]
- 23.Irwin M. Immune correlates of depression. Adv Exp Med Biol. 1999;461:1–24. doi: 10.1007/978-0-585-37970-8_1. [DOI] [PubMed] [Google Scholar]
- 24.Maddock C, Pariante CM. How does stress affect you? An overview of stress, immunity, depression and disease. Epidemiol Psichiatr Soc. 2001 Jul-Sep;10(3):153–62. doi: 10.1017/s1121189x00005285. [DOI] [PubMed] [Google Scholar]
- 25.Craft NE, Haitema TB, Garnett KM, et al. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J Nutr Health Aging. 2004;8(3):156–62. [PubMed] [Google Scholar]
- 26.Oishi J, Doi H, Kawakami N. Nutrition and depressive symptoms in community-dwelling elderly persons in Japan. Acta Med Okayama. 2009 Feb;63(1):9–17. doi: 10.18926/AMO/31854. [DOI] [PubMed] [Google Scholar]
- 27.Maes M, De Vos N, Pioli R, et al. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000 Jun;58(3):241–6. doi: 10.1016/s0165-0327(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 28.Owen AJ, Batterham MJ, Probst YC, et al. Low plasma vitamin E levels in major depression: diet or disease? Eur J Clin Nutr. 2005 Feb;59(2):304–6. doi: 10.1038/sj.ejcn.1602072. [DOI] [PubMed] [Google Scholar]
- 29.Sher L. Depression and suicidal behavior in alcohol abusing adolescents: possible role of selenium deficiency. Minerva Pediatr. 2008 Apr;60(2):201–9. [PubMed] [Google Scholar]
- 30.Tiemeier H, Hofman A, Kiliaan AJ, et al. Vitamin E and depressive symptoms are not related. The Rotterdam Study. J Affect Disord. 2002 Oct;72(1):79–83. doi: 10.1016/s0165-0327(01)00427-x. [DOI] [PubMed] [Google Scholar]
- 31.Shibata H, Kumagai S, Watanabe S, et al. Relationship of serum cholesterols and vitamin E to depressive status in the elderly. J Epidemiol. 1999 Aug;9(4):261–7. doi: 10.2188/jea.9.261. [DOI] [PubMed] [Google Scholar]
- 32.Milaneschi Y, Bandinelli S, Penninx BW, et al. The relationship between plasma carotenoids and depressive symptoms in older persons. World J Biol Psychiatry. 2011 Sep 20; doi: 10.3109/15622975.2011.597876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006 Apr 5;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 34.Center for Disease Control and Prevention (CDC) [Access date: September 25th, 2006];National Health and Nutrition Examination Survey. 2006 http://www.cdc.gov/nchs/nhanes.htm. [updated 2006; cited 2006 September 25th]; Available from.
- 35.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov 10;282(18):1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Surveys (NHANES 2005–06): Laboratory Procedure Manual: Fat Soluble Micronutrients (Vitamins A, E and Carotenoids) –UV-visible Detection. Hyatsville, MD: 2009. [updated 2009; cited]; Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/vitaec_d_met_aecar.pdf. [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Surveys (NHANES 2005–06): Laboratory Procedure Manual: Vitamin C. Hyatsville, MD: 2009. [updated 2009; cited]; Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/vic_d_met.pdf. [Google Scholar]
- 39.Cheng YJ, Gregg EW, De Rekeneire N, et al. Muscle-strengthening activity and its association with insulin sensitivity. Diabetes Care. 2007 Sep;30(9):2264–70. doi: 10.2337/dc07-0372. [DOI] [PubMed] [Google Scholar]
- 40.Lagerros YT, Lagiou P. Assessment of physical activity and energy expenditure in epidemiological research of chronic diseases. Eur J Epidemiol. 2007;22(6):353–62. doi: 10.1007/s10654-007-9154-x. [DOI] [PubMed] [Google Scholar]
- 41.McCullough ML, Feskanich D, Rimm EB, et al. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in men. Am J Clin Nutr. 2000 Nov;72(5):1223–31. doi: 10.1093/ajcn/72.5.1223. [DOI] [PubMed] [Google Scholar]
- 42.United States Department of Agriculture (USDA), Agriculture Research Service FSRG. Food and Nutrient Database for Dietary Studies, 3.0. Beltsville, MD: USDA; [Accessed: March, 2008]. 2008. http://www.ars.usda.gov/Services/docs.htm?docid=17031. [updated 2008; cited]; Available from. [Google Scholar]
- 43.Silvers KM, Scott KM. Fish consumption and self-reported physical and mental health status. Public Health Nutr. 2002 Jun;5(3):427–31. doi: 10.1079/phn2001308. [DOI] [PubMed] [Google Scholar]
- 44.Hasin DS, Glick H. Depressive symptoms and DSM-III-R alcohol dependence: general population results. Addiction. 1993 Oct;88(10):1431–6. doi: 10.1111/j.1360-0443.1993.tb02030.x. [DOI] [PubMed] [Google Scholar]
- 45.Raimo EB, Schuckit MA. Alcohol dependence and mood disorders. Addict Behav. 1998 Nov-Dec;23(6):933–46. doi: 10.1016/s0306-4603(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 46.United States Department of Agriculture (USDA), Agriculture Research Service. MyPyramid Equivalents Database for USDA Survey Food Codes Version 1.0. USDA; [Accessed: July, 2007]. 2007. http://www.ars.usda.gov/Services/docs.htm?docid=8503. [updated 2007; cited]; Available from. [Google Scholar]
- 47.Center for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey 1988 – 2008 Data Documentation, Codebook, and Frequencies. 2010 [updated 2010; cited 2011 April 25th ]; Available from: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/RXQ_RX_D.htm.
- 48.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Surveys (NHANES 2005–06): Description of Laboratory Methodology: Vitamin B-12. Hyatsville, MD: 2008. [updated 2008; cited]; Available from: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/B12_D.htm#Description_of_Laboratory_Methodology. [Google Scholar]
- 49.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Surveys (NHANES 2005–06): Description of Laboratory Methodology: Folate. Hyatsville, MD: 2008. [updated 2008; cited]; Available from: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/FOLATE_D.htm#Description_of_Laboratory_Methodology. [Google Scholar]
- 50.Abbott Homocysteine (HCY) assay package insert fo IMX Analyzer, inventor.
- 51.Pernet P, Lasnier E, Vaubourdolle M. Evaluation of the AxSYM homocysteine assay and comparison with the IMx homocysteine assay. Clin Chem. 2000 Sep;46(9):1440–1. [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Surveys (NHANES 2005–06): Description of Laboratory Methodology: Vitamin D. Hyatsville, MD: 2008. [updated 2008; cited]; Available from: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/VID_D.htm#Description_of_Laboratory_Methodology. [Google Scholar]
- 53.Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006 Dec;14(12):1032–40. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 54.Forti P, Rietti E, Pisacane N, et al. Blood homocysteine and risk of depression in the elderly. Arch Gerontol Geriatr. 2009 Jul 29; doi: 10.1016/j.archger.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 55.Almeida OP, McCaul K, Hankey GJ, et al. Homocysteine and depression in later life. Arch Gen Psychiatry. 2008 Nov;65(11):1286–94. doi: 10.1001/archpsyc.65.11.1286. [DOI] [PubMed] [Google Scholar]
- 56.Tiemeier H, van Tuijl HR, Hofman A, et al. Vitamin B12, folate, and homocysteine in depression: the Rotterdam Study. Am J Psychiatry. 2002 Dec;159(12):2099–101. doi: 10.1176/appi.ajp.159.12.2099. [DOI] [PubMed] [Google Scholar]
- 57.Bottiglieri T, Laundy M, Crellin R, et al. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000 Aug;69(2):228–32. doi: 10.1136/jnnp.69.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dimopoulos N, Piperi C, Salonicioti A, et al. Correlation of folate, vitamin B12 and homocysteine plasma levels with depression in an elderly Greek population. Clin Biochem. 2007 Jun;40(9–10):604–8. doi: 10.1016/j.clinbiochem.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Kim JM, Stewart R, Kim SW, et al. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br J Psychiatry. 2008 Apr;192(4):268–74. doi: 10.1192/bjp.bp.107.039511. [DOI] [PubMed] [Google Scholar]
- 60.Nanri A, Mizoue T, Matsushita Y, et al. Serum folate and homocysteine and depressive symptoms among Japanese men and women. Eur J Clin Nutr. 2010 Jan 20; doi: 10.1038/ejcn.2009.143. [DOI] [PubMed] [Google Scholar]
- 61.Kendrick T, Dunn N, Robinson S, et al. A longitudinal study of blood folate levels and depressive symptoms among young women in the Southampton Women’s Survey. J Epidemiol Community Health. 2008 Nov;62(11):966–72. doi: 10.1136/jech.2007.069765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, et al. The sex-specific role of plasma folate in mediating the association of dietary quality with depressive symptoms. J Nutr. 2010 Feb;140(2):338–47. doi: 10.3945/jn.109.113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sachdev PS, Parslow RA, Lux O, et al. Relationship of homocysteine, folic acid and vitamin B12 with depression in a middle-aged community sample. Psychol Med. 2005 Apr;35(4):529–38. doi: 10.1017/s0033291704003721. [DOI] [PubMed] [Google Scholar]
- 64.Bjelland I, Tell GS, Vollset SE, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003 Jun;60(6):618–26. doi: 10.1001/archpsyc.60.6.618. [DOI] [PubMed] [Google Scholar]
- 65.Kim JM, Kim SW, Shin IS, et al. Folate, vitamin b(12), and homocysteine as risk factors for cognitive decline in the elderly. Psychiatry Investig. 2008 Mar;5(1):36–40. doi: 10.4306/pi.2008.5.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Astorg P, Couthouis A, de Courcy GP, et al. Association of folate intake with the occurrence of depressive episodes in middle-aged French men and women. Br J Nutr. 2008 Jul;100(1):183–7. doi: 10.1017/S0007114507873612. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez-Villegas A, Doreste J, Schlatter J, et al. Association between folate, vitamin B(6) and vitamin B(12) intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009 Apr;22(2):122–33. doi: 10.1111/j.1365-277X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 68.Tolmunen T, Hintikka J, Ruusunen A, et al. Dietary folate and the risk of depression in Finnish middle-aged men. A prospective follow-up study. Psychother Psychosom. 2004 Nov-Dec;73(6):334–9. doi: 10.1159/000080385. [DOI] [PubMed] [Google Scholar]
- 69.Beydoun MA, Shroff MR, Beydoun HA, et al. Serum folate, vitamin B-12, and homocysteine and their association with depressive symptoms among U.S. adults. Psychosom Med. 2010 Nov;72(9):862–73. doi: 10.1097/PSY.0b013e3181f61863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beydoun MA, Shroff MR, Chen X, et al. Serum Antioxidant Status Is Associated with Metabolic Syndrome among U.S. Adults in Recent National Surveys. J Nutr. 2011 May;141(5):903–13. doi: 10.3945/jn.110.136580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.STATA. Statistics/Data Analysis: Release 11.0. College Station, TX: Stata Corporation; 2009. [Google Scholar]
- 72.Long JS. Regression Models for Categorical and Limited Dependent Variables. Thousand Oaks, CA: Sage; 1997. [Google Scholar]
- 73.Bandiera FC, Arheart KL, Caban-Martinez AJ, et al. Secondhand Smoke Exposure and Depressive Symptoms. Psychosom Med. 2009 Nov 30; doi: 10.1097/PSY.0b013e3181c6c8b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Selvin S. Statistical Analysis of Epidemiologic Data. 3. Oxford University Press; 2004. [Google Scholar]
- 75.Lohr SL. Sampling: Design and Analysis. 2. 2010. [Google Scholar]
- 76.Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–61. [Google Scholar]
- 77.Puhani P. The Heckman Correction for sample selection and its critique. Journal of Economic Surveys. 2000;14(1):53–68. [Google Scholar]
- 78.Wang Z. Two postestimation commands for assessing confounding effects in epidemiological studies. Stata Journal. 2007;7(2):183–96. [Google Scholar]
- 79.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006 Jun;97(6):1634–58. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 80.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Florence TM. The role of free radicals in disease. Aust N Z J Ophthalmol. 1995 Feb;23(1):3–7. doi: 10.1111/j.1442-9071.1995.tb01638.x. [DOI] [PubMed] [Google Scholar]
- 82.Mason RP, Walter MF, Mason PE. Effect of oxidative stress on membrane structure: small-angle X-ray diffraction analysis. Free Radic Biol Med. 1997;23(3):419–25. doi: 10.1016/s0891-5849(97)00101-9. [DOI] [PubMed] [Google Scholar]
- 83.Tirosh O, Kohen R, Katzhendler J, et al. Oxidative stress effect on the integrity of lipid bilayers is modulated by cholesterol level of bilayers. Chem Phys Lipids. 1997 May 30;87(1):17–22. doi: 10.1016/s0009-3084(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 84.Maes M, Galecki P, Chang YS, et al. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2010 May 12; doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]