Abstract
Herpes simplex virus (HSV) has evolved multiple strategies to modulate host immune responses. In a screen of HSV open reading frames to identify additional HSV-encoded proteins that affect NF-κ B signaling, we identified the viral US3 tegument protein as an inhibitor of NF-κB signaling. We found that the US3 protein is required for inhibition of TLR2 signaling induced by viral infection and that this inhibition occurs at very early times post-infection. Expression of US3 in transfected cells inhibits TLR2 signaling induced by Zymosan, and this inhibition occurs at or downstream of MyD88 and upstream of p65. Polyubiquitination of TRAF6 is critical for its function in TLR2 signaling. Using US3-null and US3 kinase-defective mutant viruses, we demonstrate that HSV US3 reduces TRAF6 polyubiquitination and that the kinase activity of US3 is necessary for this effect. Therefore, US3 is necessary and sufficient for inhibiting TLR2 signaling at or before the stage of TRAF6 ubiquitination.
Keywords: HSV, US3, Tegument protein, TRAF6, NF-kB
Introduction
One of the first critical lines of defense by a host organism against an invading virus is its innate immune system. The earliest events of innate immune responses include sensing of virus components by host pattern recognition receptors (PRRs), which initiate signaling cascades and transcriptional activation of genes encoding type I interferons (IFNs) and pro-inflammatory cytokines. These signaling pathways are complex mechanisms that engage a variety of cell types (inflammatory cells, dendritic cells and lymphocytes) to control viral infection and are tightly regulated. In addition to type I IFNs, which mediate the early antiviral response to a large extent, cytokines (like IL-1β, IL-6, IL-8, IL-18 and IL-12) induced by innate immune cells are also critical for an effective early antiviral defense. Pathogen sensing triggers a cascade of intracellular signaling events involving a large number of adaptor proteins. Sequential steps of post-translational modifications on these proteins, such as phosphorylation and ubiquitination, result in the translocation of transcription factors such as NF-κB, AP-1, or JNK to the nucleus where they stimulate the transcription of antiviral and pro-inflammatory genes. These events function to curb early events in productive viral infection as well as to program the adaptive immune response. Not surprisingly, viruses have also evolved numerous mechanisms to blunt or evade these protective measures elicited by the host.
NF-κB is a major transcriptional activator for pro-inflammatory cytokine genes (Hayden et al., 2006), and herpes simplex virus (HSV) infection activates the NF-κB signaling pathway by both TLR-dependent and -independent pathways resulting in the induction of cytokines IL-6 and IL-8 (Hilton et al., 1995; Kurt-Jones et al., 2005, 2004). Upon microbial recognition, TLR2 dimerizes with either TLR1 or TLR6 and recruits MyD88 and Mal/TIRAP through TIR domain interactions. This complex then recruits the IRAKs (IL-1 receptor-associated kinases). IRAK4 is recruited first, becomes activated and phosphorylates IRAK-1, which activates IRAK-2. IRAK activation leads to an interaction with TRAF6 (tumor necrosis factor receptor-associated factor 6) and oligomerization of TRAF6 and its autoubiquitination. TRAF6, an E3 ubiquitin ligase, catalyzes the synthesis of polyubiquitin chains (polyUb) bound to itself and TAK1, thereby activating TAK1. The polyUb chains bind to NEMO, the regulatory component of the IKK complex. The resulting complex leads to phosphorylation of IKKβ by TAK1, leading to activation of the IKK complex, which phosphorylates the α subunit of IκB (inhibitor of κB), causing its ubiquitination and degradation, release of NF-κB and its translocation into the nucleus. Nuclear NF-κB binds to κB elements in enhancers and promoters and also to the basal transcriptional machinery to activate transcription (Oliveira-Nascimento et al., 2012; Rathinam and Fitzgerald, 2011).
The TLR2 dependence for HSV induction of NF-κB signaling is cell type-specific (Rathinam and Fitzgerald, 2011). We have shown that infection with HSV-1 wild-type (WT) strains KOS and F can activate TLR2 signaling in mouse macrophages and human cells expressing TLR2 (Kurt-Jones et al., 2005, 2004). Further, while TLR2 is essential for the recognition of HSV and induction of pro-inflammatory cytokines by macrophages, microglial cells and myeloid dendritic cells (Aravalli et al., 2007, 2005; Lima et al., 2010), plasmacytoid dendritic cells (pDCs) can sense HSV in a TLR2-independent fashion (Rasmussen et al., 2007; Sato et al., 2006). Recently, it has also been reported that in response to HSV infection, type I interferon production in inflammatory monocytes is partially dependent on TLR2 (Barbalat et al., 2009). Moreover, TLR2 recognition of HSV in vivo appears to depend on route of inoculation and virus subtype. In the case of HSV-2 infection in mice, although TLR2 appears to be nonessential for the control of viral spread following intraperitoneal or vaginal infection, an effective cytokine response in the brain following natural vaginal infection is dependent on a synergistic role of TLR2 and TLR9 (Sorensen et al., 2008). In the corneal and intraperitoneal infection models in mice, TLR2 sensing of HSV has been shown to mount an excessive immune response that can be detrimental to the host (Kurt-Jones et al., 2004; Sarangi et al., 2007). Interestingly, in humans, two polymorphisms in TLR2 are associated with increased HSV-2 viral shedding and increased lesions (Bochud et al., 2007), supporting a role for TLR2 in the control of virus infection. In addition, work done by Iwasaki and colleagues indicated that TLR2 sensing of HSV-1 is virus strain/clone-dependent (Sato et al., 2006), although the molecular mechanism underlying this phenomenon is not known. It has been recently demonstrated that HSV gB and gH/gL proteins interact with TLR2, but gH/gL alone are capable of triggering NF-κB activation (Leoni et al., 2012).
HSV gene products have been shown to regulate NF-κB signaling in a number of ways. HSV infection activates NF-κB signaling, which is essential for optimal viral replication (Amici et al., 2001; Patel et al., 1998). It has been demonstrated that ICP27 is essential for NF-κB induction (Hargett et al., 2006). The virion UL37 protein was shown to activate NF-κB signaling by interacting with and activating TRAF6 (Liu et al., 2008). Infection with UV-inactivated virus and binding of gD to HVEM can also lead to activation of NF-κB (Medici et al., 2003; Sciortino et al., 2008). In contrast, HSV-1 ICP0 inhibited NF-κB signaling by reducing levels of adaptor proteins (van Lint et al., 2010). Therefore, the net induction of NF-κB signaling by HSV is the result of the combined activities of HSV proteins that both activate and inhibit NF-κB signaling.
In this study, in a screen of the HSV open reading frames (ORFs) to identify additional proteins that modulate NF-κB activity, we identified the HSV US3 tegument protein as an inhibitor of the NF-κB signaling pathway. The HSV-1 US3 protein kinase is a virion tegument protein that has been implicated in a variety of processes during productive virus infection. Most notable among them are its roles in virion maturation and egress (Favoreel et al., 2005; Matsuzaki et al., 2005; Mou et al., 2007; Reynolds et al., 2002; Wisner et al., 2009), in preventing virus-induced apoptosis (Benetti and Roizman, 2004; Hata et al., 1999; Leopardi et al., 1997; Munger and Roizman, 2001; Ogg et al., 2004), enhancing histone acetylation (Gu et al., 2005; Poon et al., 2006), suppression of the IFNγ response by down-regulating the IFNAR-1 receptor (Liang and Roizman, 2008), and more recently, in stimulating mRNA translation (Chuluunbaatar et al., 2010). In this study we show that US3 also down-regulates TLR2-dependent NF-κB signaling at very early times post infection, and we found that this inhibition of TLR2 signaling occurs before or at the stage of TRAF6 ubiquitination.
Results
Construction of an HSV-1 ORF expression plasmid library and identification of US3 as an inhibitor of NF-κB signaling
HSV-1 encodes at least 84 ORFs (Roizman et al., 2007) and has the ability to activate the NF-κB signaling pathway (Patel et al., 1998; Santoro et al., 2003). To identify additional HSV proteins that modulate NF-κB activity, we constructed an HSV-1 cDNA library and performed a reporter gene assay-based screen of the HSV proteome. The HSV-1 ORFs were PCR-amplified from genomic DNA of low passage HSV-1 KOS strain. Each PCR product was inserted into the pcDNA3.1 plasmid vector so that the ORF was tagged with a C-terminus V5-epitope and 6XHis motif for easy detection of protein expression. A total of 66 ORF-expressing plasmids were constructed, and following verification by sequencing, protein expression was confirmed by western blotting (Liu and Knipe, unpublished results).
To screen the effects of the viral gene products on NF-κB activity, we performed an NF-κB-dependent luciferase reporter assay, which measures overall NF-κB activity directly as described before (Liu et al., 2008). Briefly, HEK293T cells were cotransfected with an NF-κB-dependent firefly luciferase reporter plasmid, a CMV promoter/enhancer β-galactosidase reporter plasmid as a transfection efficiency control, and a plasmid expressing an HSV-1 ORF. Induction of NF-κB activity was measured by the expression of firefly luciferase and normalized to β-gal activity. Over-expression of p65, which enhances the basal level of reporter-gene activity, was used as a positive control. Among the 66 HSV-1 ORFs screened for their ability to affect NF-κB -reporter activity in HEK293T cells, the majority had minimal effect, a few ORFs stimulated NF-κB activity (e.g., UL37, (Liu et al., 2008)), while a few HSV-1 proteins reduced the basal level of reporter gene activity (results not shown). In the latter group, we identified the HSV-1 tegument protein US3 as a potential inhibitor of the NF-κB activation pathway. This observation led us to hypothesize that US3 may play a role in immune evasion by suppression of NF-κB activity. To confirm this result, we used HEK293 cells stably expressing TLR2 (H2.14.12 cells) to determine whether US3 could block NF-κB signaling triggered by Zymosan, a well-characterized TLR2 agonist. H2.14.12 cells were transfected with various amounts of US3 expression plasmid together with NF-κB-luciferase reporter and TK-Renilla control plasmids. At 24 h post-transfection the cells were treated with Zymosan or mock treated for 6 h, and then the NF-κB-driven firefly luciferase and Renilla luciferase activities were measured in the cell lysates. Zymosan stimulation led to a robust TLR2-driven luciferase activity compared to the empty vector transfected mock-treated sample, but expression of US3 reduced luciferase activity significantly (almost to basal level) and in a dose-dependent manner (Fig. 1). These results argued for an inhibitory role for US3 in TLR2 signaling.
Fig. 1.
HSV-1 US3 inhibits Zymosan activation of TLR2. TLR2-expressing cells (H2.14.12 cells) were transfected with NF-κB-driven firefly luciferase reporter and thymidine kinase promoter-driven Renilla luciferase (TK-Renilla) plasmids, together with increasing amounts of US3 plasmid and pcDNA3.1 empty vector to keep the total plasmid amount constant. After 24 h, transfected cells were treated with Zymosan or mock-treated (without Zymosan), and at 6 h post stimulation firefly and Renilla luciferase activities were measured using the Promega dual-glo luciferase assay system. Results are presented as normalized luciferase activity.
US3 inhibits NF-κB signaling at or downstream of MyD88 but upstream of p65
To identify the step of the NF-κB activation pathway targeted by US3, we tested the effect of US3 on NF-κB induction with various stimuli. Over-expression of individual components of the signaling pathway downstream of TLR2 activation, for example MyD88, TRAF6 or a subunit of NF-κB (p65), is sufficient to trigger NF-κB signaling (Fitzgerald et al., 2001). Therefore, we investigated whether US3 could block the stimulatory signal induced by over-expression of MyD88 or p65. HEK293 T cells were transfected with the NF-κB-luciferase and TK-Renilla plasmids and either MyD88 or p65 plasmid with or without the US3 plasmid and empty vector to keep the total DNA amount constant. The empty vector transfected sample was used as a control and luciferase activity was measured at 24 h post-transfection. As expected, expression of MyD88 or p65 alone was sufficient to activate NF-κB, resulting in robust luciferase activity (Fig. 2A). Co-expression of US3 resulted in a significant reduction in the MyD88-induced luciferase activity, showing that ectopic expression of US3 alone was capable of inhibiting NF-κB activation. In contrast, p65-driven NF-κB activity was not affected by co-expression of US3, arguing that the US3 effect is upstream of nuclear translocation of activated p65 and its binding to DNA. Taken together, these results showed that US3 functions downstream of MyD88 but upstream of p65.
Fig. 2.
(A) HSV-1 US3 inhibits NF-κB signaling downstream of MyD88 and upstream of p65. Cells were transfected with NF-κB-luciferase and TK-Renilla plasmids along with either MyD88 or p65 plasmids, with or without US3 plasmid and empty vector to keep the plasmid amount constant. Luciferase activity was measured at 24 h post-transfection. Results are presented as fold induction of luciferase activity over empty vector-transfected control. (B) HSV-1 US3 does not inhibit TRAF2- and TBK1-mediated signaling. Cells were transfected with NF-κB-luciferase and TK-Renilla plasmids along with either TRAF2 or TBK1 plasmid, with or without US3 plasmid and empty vector to keep the plasmid amount constant. Luciferase activity was measured at 24 h post transfection. Results are presented as fold induction of luciferase activity relative to empty vector-transfected control.
To test the specificity of US3, we examined the effect of US3 on other signaling pathways. US3 did not affect TBK-1-driven activation of ISRE-luciferase reporter levels and led to only a small reduction in TRAF2-driven NF-κB activation (Fig. 2B). This inhibition was much smaller than what we observed for signaling downstream of MyD88 and could be due to an indirect effect of US3 overexpression in the cell, especially because this viral kinase is known to be a multifunctional protein. This demonstrated that the inhibitory effect of US3 shows at least some specificity for the MyD88-TRAF6-NF-κ cascade.
US3-mediated inhibition of NF-κB signaling occurs upon HSV-triggered TLR2 activation
To extend the transfection studies to virus infection, we assessed induction of NF-κB activity after virus infection in TLR2 + HEK293 (H2.14.12) cells by measuring the levels of IL-8, which is an NF-κB-activated pro-inflammatory cytokine, in cells infected with the R7041 mutant virus strain with a deletion in the US3 gene or its rescued viral strain, R7306 (Purves et al., 1991). We collected extracellular supernatants at 6 h post-infection (hpi) and analyzed them for levels of IL-8 by ELISA. We observed that the amount of IL-8 secreted into the medium was significantly higher in the US3 deletion virus-infected cells compared to the WT or US3 rescued virus-infected cells (Fig. 3A). Importantly, in HEK293 T cells that do not express TLR2, there was no detectable increase in IL-8 level in the cell supernatant, showing that the induction was via TLR2. The inhibition of TLR2 signaling involving US3 was apparent starting at very early times post-infection (Fig. 3B). Significantly higher levels of IL-8 were detected in the cell supernatant as early as 2–3 hpi with R7041 compared with WT virus infection, and this difference was maintained at least through 7 hpi. Moreover, when TLR2+ cells were infected at different MOIs, we observed that the induction of IL-8 was virus dose-dependent (Fig. 3C). Similar results were observed in murine macrophages, which are known to play a critical role in the early stages of the antiviral response, in part by releasing proinflammatory cytokines upon activation. In RAW264.7 cells, a murine macrophage-like cell line derived from Balb/C mice, a similar trend was observed for NF-κB-induced proinflammatory cytokine genes (Fig. 3D). RAW264.7 cells were infected with either WT or US3 deletion mutant virus, and at 6 hpi the levels of IL-6 and CCL2 mRNA were measured by RT-PCR. In comparison to WT virus infection, infection of RAW cells with the US3 deletion virus resulted in significantly higher levels of IL-6 mRNA. Induction of CCL2 mRNA was also higher in deletion virus-infected cells, although to a somewhat lower extent. Because the US3 deletion virus showed significantly higher NF-κB activity downstream of TLR2 activation compared to both WT and US3 rescued viruses, we concluded that the mutant phenotype was due to the absence of US3.
Fig. 3.
(A) US3-mediated inhibition of IL-8 induction in infected cells is TLR2-dependent. H2.14.12 cells (TLR2+) and HEK293T cells (TLR2−) were infected with wild-type (WT), R7041 (US3−) or R7306 (US3R) virus, and at 6 hpi accumulation of IL-8 in the cell supernatant was measured by ELISA. (B) US3-mediated inhibition of the TLR2 signaling pathway occurs very early after virus infection. TLR2 + cells were infected with wild-type (WT) or R7041 (US3−) virus at an MOI of 20. At various time points postinfection, levels of IL-8 secreted into the cell supernatant were measured by ELISA. (C) US3-mediated inhibition of NF-κB activation is dose-dependent. TLR2 + cells were infected with wild-type (WT), R7041 (US3−) or R7306 (US3R) virus at an MOI of 0.2, 2 or 20, and at 6 hpi, accumulation of IL-8 in the cell supernatant was measured by ELISA. (D). Relative cytokine mRNA levels in infected murine macrophage cells. At 6 hpi with the indicated viruses, mRNA levels were measured by real time PCR. (E) Expression of HSV-1 kCP0 protein in virus infected Vero cells. Cells were infected with wild-type (WT), R7041 (US3−) or R7306 (US3R) viruses at an MOI of 20, and ICP0 expression was evaluated by Western blotting of infected cell lysates harvested at 2, 3 and 4 hpi. (F) Relative amounts of tegument proteins in viral particles. Equivalent numbers of infectious viral particles were resolved on SDS-PAGE, and virion proteins were detected by Western blot. Wild-type (WT), R7041 (US3−), R7306 (US3R).
Because HSV-1 US3 is a component of the virion tegument and is carried into host cells at the time of infection along with other tegument proteins, we determined whether equivalent amounts of virion tegument proteins like VP16 and UL37 were being introduced into the cells upon infection with WT, R7041 and R7306 viruses. We therefore analyzed equivalent numbers of infectious virus particles (based upon equal numbers of PFUs) by SDS-PAGE and Western blotting to confirm that comparable quantities of virion tegument proteins were present in the virus stock used to infect the cells. We observed that the WT, R7041 and R7306 virus stocks had comparable levels of VP16, another tegument protein (Fig. 3F). Furthermore, we observed that comparable levels of the immediate-early ICP0 protein were expressed by 3 hpi in Vero cells infected with these viruses (Fig. 3E).
US3 inhibits nuclear accumulation of p65
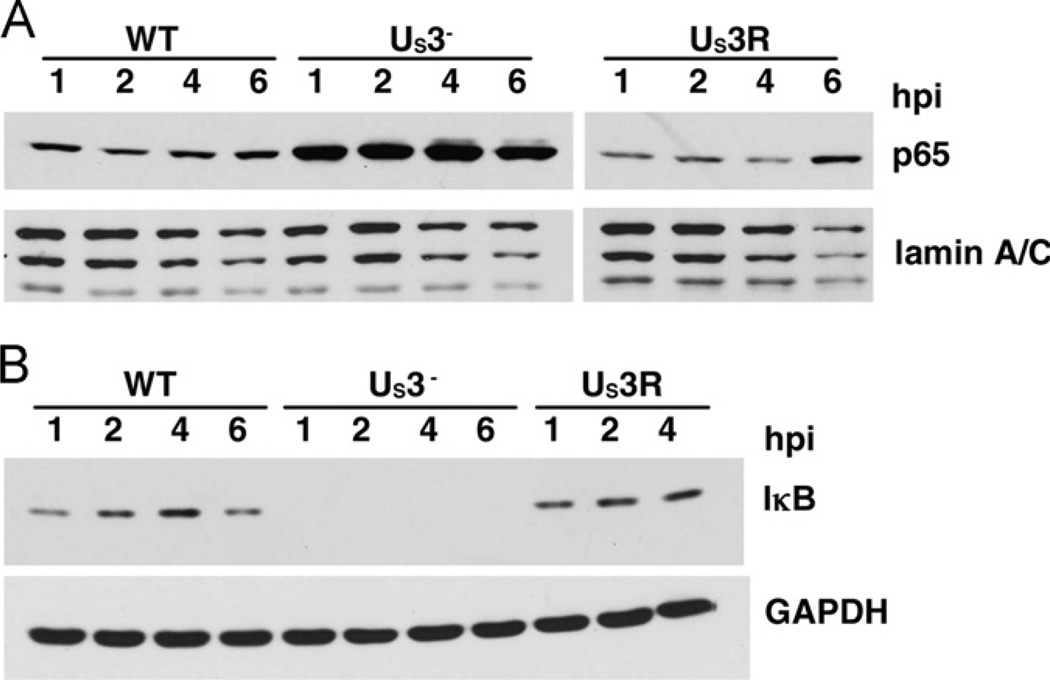
We have shown that US3 inhibits NF-κB activity upstream of p65 and that the US3-mediated effect occurs early during infection, i.e., by 2–3 hpi. This suggested that the US3 protein carried in with the virion tegument might bring about the observed inhibitory effects. In unstimulated cells, the IκB protein sequesters NF-κB in the cytoplasm. Upon TLR2 stimulation, IκB is phosphorylated, ubiquitinated and degraded, allowing active NF-κB to translocate to the nucleus. Therefore, the increased nuclear accumulation of the NF-κB subunit p65 provides a direct and quantitative measure of NF-κB activation. To determine if there was differential nuclear translocation of p65 at early times after infection with WT or US3 deletion mutant viruses, we infected TLR2+ HEK293 cells with WT, R7041 or R7306 virus strains and carried out cellular fractionation at various times to compare nuclear versus cytoplasmic NF-κB levels. By 1 hpi, there was increased nuclear accumulation of p65 in the US3 null (R7041) virus-infected cells compared to WT virus-infected cells, and this continued through 6 hpi (Fig. 4A). Consistent with increased nuclear p65 levels, there was a decrease in cytosolic IκB levels in R7041 virus-infected cells (Fig. 4B). In cells infected with the US3 rescued virus (R7306), the level of nuclear NF-κB was comparable to that of the WT virus-infected cells, further arguing that the increased nuclear translocation of NF-κB was specifically due to the absence of US3. Moreover, because this effect was observed at a time when there was little or no late gene expression, it seemed likely that virion US3 acts to inhibit the canonical NF-κB activation pathway.
Fig. 4.
Effects of infection on localization of NF-κB and levels of IκB. TLR2+ HEK293 cells were infected with wild-type (WT), US3 deletion (US3−) or US3 rescued (US3R) virus. At the indicated time points, cellular fractionation and Western blot analyses were performed. (A) Nuclear levels of NF-κB. (B) Cytoplasmic levels of IκB.
US3 inhibits TRAF6 ubiquitination
Having established that HSV US3 dampens TLR2 signaling by causing inhibition of nuclear translocation of NF-κB, we then investigated how US3 might exert this effect. We have demonstrated that HSV ICP0 modulates innate responses by reducing the levels of sensor or adaptor components of innate signaling pathways within the host cell (Orzalli et al., 2012; van Lint et al., 2010). To examine the effect of US3 on TLR2-activated NF-κB signaling, we transfected HEK293 T cells with HA-MyD88, Flag-IRAK-1, Flag-TRAF6 and Flag-TAK1 plasmids with or without a Flag-US3 plasmid, and measured the levels of MyD88, IRAK-1, TRAF6 and TAK1 proteins in cell lysates in the presence or absence of US3. Co-expression of US3 had no detectable effect on the adaptor protein expression levels (Fig. 5A–C). Thus, there was no evidence that levels of signaling proteins were altered by US3.
Fig. 5.
Effects of US3 on adaptor proteins in transfected cells. HEK293T cells were transfected with (A) HA-Myd88 with (lane 3) or without (lane 2) US3-expressing plasmid, (B) Flag-TRAF6 with (lane 3) or without US3 (lane 2), (C) Flag-IRAK1 with (lane 1) or without (lane 2) US3 and Flag-TAK1 with (lane 3) or without (lane 4) US3, as indicated. Empty vector transfected cells were used as control (mock, lane 1 in (A) and (B)). Cell lysates were analyzed by Western blotting using anti-HA antibody (for MyD88) and anti-Flag antibody (for TRAF6, IRAKI, TAK1 and US3). (D) Effect of US3 on TRAF6 ubiquitination. HEK293T cells were transfected with Flag-TRAF6 and HA-Ubiquitin plasmids with (lane 4) or without (lane 3) the US3 plasmid. Empty vector transfected cells were used as control (lane 1). Lysates were immunoprecipitated with TRAF6 antibody and analyzed by Western blotting using the anti-ubiquitin antibody. TRAF6 and US3 were detected using the anti-Flag antibody (lower panel).
A pivotal step in the TRAF6 signaling pathway is the ubiquitination of TRAF6 and recruitment of signalosome protein components like TAK1, TAB2, and TAB3 (Chen, 2005). It has also been shown recently that inhibition of TRAF6 ubiquitination or the deubiquitination of TRAF6 results in inhibition of downstream NF-κB signaling (Shembade et al., 2010). We hypothesized that HSV US3 interferes with TRAF6 ubiquitination and therefore examined its effect on TRAF6 ubiquitination. To test our hypothesis, we transfected HEK293 T cells with Flag-TRAF6 and HA-Ubiquitin plasmids with or without the Flag-US3 plasmid. We observed that US3 expression dramatically reduced the levels of TRAF6 polyubiquitination in cotransfected cells (Fig. 5D). This argued that US3 modulates NF-κB signaling by inhibiting the polyubiquitination of TRAF6.
To study a more biologically relevant situation, we then looked at the effects of viral infection on endogenous TRAF6 ubiquitination. We infected TLR2+ HEK293 cells with WT or US3 deletion (R7041) virus strains. Because the US3 inhibitory effects occurred at early times post infection, we harvested and prepared infected cell lysates at 1 and 2 hpi and immunoprecipitated endogenous TRAF6 protein. Similar to the transfection experiments described above, levels of endogenous TRAF6 were comparable in cells infected with WT or US3 deletion virus (Fig. 6). However, we observed that by as early as 1 hpi, R7041 virus-infected cells had higher levels of polyubiquitinated TRAF6 compared to WT virus-infected cells (Fig. 6), suggesting that in the presence of US3 polyubiquitination of endogenous TRAF6 was inhibited. Therefore, at very early times post-infection HSV US3 inhibits the signaling pathway at or prior to TRAF6 ubiquitination.
Fig. 6.
Effect of US3 on TRAF6 ubiquitination in infected cells. H2.14.12 cells were infected with wild-type (WT, lanes 2,3) or US3 deletion (US3−, lanes 4,5) virus. At the indicated time points, cell lysates were prepared as described in Materials and Methods and analyzed by IP of TRAF6, followed by Western blot for Ub.
US3-inhibition of NF-κB is dependent on its kinase function
HSV US3 protein is a kinase with a broad specificity for both cellular and viral proteins. To determine whether the US3 Ser/Thr kinase activity was required for inhibition of NF-κB activity downstream of TLR2 activation, we mock-infected or infected TLR2+ HEK293 cells with R7041 US3 deletion virus, the K220A mutant virus expressing catalytically inactive US3, the R7306 US3 rescued virus, or WT virus. When we analyzed infected cell supernatants for levels of IL-6 and IL-8 by ELISA, we observed that R7041 and K220A recombinant viruses induced IL-8 and IL-6 secretion to much higher levels than WT or R7306 viruses (Fig. 7A), consistent with previous results obtained with the R7041 virus. Moreover, the R7041 and K220A viruses induced comparable levels of IL-6 and IL-8, indicating that the inhibition of NF-κB activation is dependent on the kinase activity of US3. We then determined the effect on TRAF6 polyubiquitination in K220A-infected H2.14.12 cells. As in our previous experiments, endogenous TRAF6 was immunoprecipitated from mock or infected cell lysates and TRAF6 polyubiquitination level was determined by Western blotting using an anti-Ubiquitin antibody. We observed that both R7041 (US3 deletion) and K220A (US3 kinase-inactive) viruses led to significantly higher levels of polyubiquitination of endogenous TRAF6, compared to either WT or R7306 (US3 rescued) virus (Fig. 7B). This observation was also consistent with the IL-6 and IL-8 ELISA assays, which measured active NF-κB downstream of TRAF6 ubiquitination and activation.
Fig. 7.
Role of the US3 protein kinase activity in inhibition of NF-κB. (A) Role in cytokine induction. TLR2+ cells were mock infected or infected with wild-type (WT), R7041 (US3−), R7306 (US3 deletion) or K220A (US3 kinase-deficient) virus. At 6 hpi IL-6 and IL-8 levels in the cell supernatant were measured by ELISA. (B) Role in TRAF6 ubiquitination. TLR2+ cells were mock infected (Lane 1) or infected with WT (Lane 2), US3– (Lane 3), K220A (Lane 4) and R7306 (Lane 5) virus. At 2 hpi, cell lysates were analyzed by IP-Western. Endogenous TRAF6 was immunoprecipitated with anti-TRAF6 antibody, and polyubiquitination was detected using the anti-ubiquitin antibody.
Discussion
In a screen of HSV ORFs to identify viral proteins that modulate NF-κB signaling, we identified the US3 virion tegument protein as an additional viral-encoded inhibitor of NF-κB signaling. Transfection studies showed that US3 alone is sufficient to block NF-κB signaling at or between MyD88 and TRAF6 adaptor proteins. Further studies in cells infected with a US3 deletion mutant virus and rescued virus showed that US3 is required for a viral mechanism that restricts TLR2 signaling. This inhibition occurs at or prior to TRAF6 ubiquitination because the rescued virus and WT viruses showed lower TRAF6 ubiquitination than the US3 null mutant virus. Furthermore, the inhibition of p65 nuclear localization occurred as early as 1–2 hpi, consistent with a possible role for the virion tegument US3 protein in this inhibition. A kinase-dead US3 mutant virus also showed elevated NF-κB signaling, arguing for a role for the kinase activity in the US3 inhibitory effect. This work adds to the growing list of HSV proteins that modulate NF-κB and TLR2 signaling.
Mechanism of US3-mediated NF-κB inhibition
The HSV US3 gene encodes a serine/threonine protein kinase with an amino acid sequence that is conserved in members of the Alphaherpesvirinae sub-family (Frame et al., 1987; McGeoch and Davison, 1986). We found no evidence that US3 affected the levels of signaling proteins; therefore, US3 could modulate this signaling pathway by affecting the activities of the signaling adaptor proteins by phosphorylation of any of the components from TLR2 to TRAF6. Inhibition of signaling could be due to (1) phosphorylation of adaptor proteins directly, which could lead to an inhibition of signaling, (2) phosphorylations blocking the interaction of the protein with other adaptor proteins in the pathway, or (3) phosphorylations that recruit other enzymes such as cellular or viral deubiquitinases that reverse the ubiquitination of TRAF6. The US3 kinase targets a broad range of substrates within the cell, and several studies have implicated US3 in a variety of processes during the virus life cycle as reviewed in the introduction. None of the known substrates for US3 provide a ready explanation for its NF-κB inhibitory activity as none are known to affect NF-κB signaling. Interestingly, phosphorylation of the retinoic acid-inducible gene I (RIG-I) prevents its ubiquitination by TRIM25 (Gack et al., 2010); thus, a similar mechanism could be operative here in which phosphorylation of TRAF6 by US3 prevents the autoubiquitination of TRAF6. The substrate specificity of the US3 kinase is similar to that of protein kinase A of the host cell (Benetti and Roizman, 2007). There are precedents for PKA phosphorylation modulating the activities of other proteins in that an inhibitory phosphorylation by PKA has been shown to modulate the activity of Na+–K+– ATPase in response to beta-adrenergic hormone (Cheng et al., 1997). PKA is known to affect NF-κB signaling, but the documented effects are all at the level of IKK or post-translational modifications of p65/Rel (Gerlo et al., 2011). Therefore, these effects would not be candidates for modification of TRAF6 ubiquitination.
US3 may also tap into normal cellular mechanisms for regulation of TRAF6 ubiquitination. It has been demonstrated recently that the cellular USP25 protein negatively regulates IL-17-mediated TRAF6 signaling by deubiquitinating TRAF6 (Zhong et al., 2012), and SYK-mediated phosphorylation of USP25 alters cellular levels of USP25 (Cholay et al., 2010). Because US3 has diverse phosphorylation targets, it is worthwhile to test whether USP25 is a target of US3 kinase activity or is recruited to TRAF6 by US3. Further experiments are necessary to dissect out these potential mechanisms of US3-mediated inhibition, and experiments to test these hypotheses are currently underway.
Regulation of NF-κB signaling by HSV
It is noteworthy that HSV encodes multiple proteins that appear to modulate NF-κB signaling in various ways. The incoming virion contains both the UL37 protein, which stimulates NF-κB signaling through its interaction with TRAF6 (Liu et al., 2008), and the US3 protein, which inhibits NF-κB signaling (this report). We show here that US3 leads to decreased TRAF6 ubiquitination while other studies have shown that UL37 leads to increased ubiquitination of TRAF6 (Yan, Liu and Knipe, manuscript in preparation). The virion gD is also thought to stimulate NF-κB signaling (Medici et al., 2003; Sciortino et al., 2008) so multiple virion proteins affect NF-κB signaling. Once the immediate-early proteins are expressed, the ICP0 protein can inhibit TLR2 signaling (van Lint et al., 2010), and the ICP27 protein leads to a stimulation of NF-κB signaling in cells that do not express TLR2 (Hargett et al., 2006). This complex regulation and the opposing effects of these proteins may have evolved to provide some NF-κB stimulation to allow optimal replication and infected cell survival while restricting the NF-κB stimulation so that a strong antiviral innate response is not induced. This regulatory network may also have evolved to allow the virus to differentially regulate NF-κ signaling in cells that express TLR2 (Kurt-Jones et al., 2004), versus those that do not express TLR2 (Hilton et al., 1995).
Variability in HSV strains (Sato et al., 2006) or in different stocks (van Lint, Sen and Knipe, manuscript κpreparation) to activate TLR2 has been observed. Two viral factors have now been shown to regulate NF-κB signaling: (1) ICP0 inhibits TLR2 signaling, possibly by affecting the levels of adaptor proteins (van Lint et al., 2010), and (2) US3 has been shown here to inhibit TLR2 signaling at or before the stage of TRAF6 ubiquitination. The effects of both of these proteins are exerted early following infection and therefore could be exerted through virion proteins or proteins expressed very early in the infection process. Therefore, epigenetic effects could lead to variability in virion protein content or modification or levels of expression during the initial stages of infection and explain the variability of HSV-1 stocks to activate NF-κB signaling.
HSV-1 is the causative agent of severe diseases like keratitis and neonatal encephalitis, and HSV-2, the causative agent of genital herpes, has also been implicated in augmenting the risk of HIV transmission. Moreover, HSV also has clinical importance as a gene delivery and vaccine vector agent. The complete set of HSV gene products that potentiate or modulate innate immune responses is still unknown and therefore, a thorough mechanistic understanding of host anti-viral responses is central to the development not only of anti-viral therapeutics and vaccines but also to improve the safety of viral vectors in gene therapies.
Materials and methods
Cell lines and viruses
The HEK293 cell line stably expressing TLR2 (H2.14.12) was described previously (Kurt-Jones et al., 2002). The wild-type (WT) HSV-1 F strain was propagated and viral stock titers were determined on Vero cells. The HSV-1 US3 null (R7041) and US3 rescued (R7306) virus strains (Purves et al., 1991) were provided by Dr. Bernard Roizman (University of Chicago, Chicago, IL). R7041, R7306 and the US3 kinase-dead K220A (Ryckman and Roller, 2004) virus strains were propagated and titered on Vero cells. For all experiments, cell-free virus stocks were prepared from infected cell supernatants, and virus titers were determined on Vero cells by standard plaque assay. The HEK293T, H2.14.12 and murine macrophage cells (RAW264.7) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS (DMEM-10).
Plasmids
HSV expression plasmids used in this study were constructed by PCR amplification of individual ORFs from genomic DNA isolated from low passage HSV-1 KOS virus and subcloning into the pcDNA3.1 vector (Invitrogen). These constructs express the HSV proteins in-frame with a V5 and 6xHis tag (or Flag tag in some cases), for easy detection of protein expression. All plasmid inserts were sequenced at the Dana Farber Cancer Center Sequencing Facility, and HSV protein expression was confirmed by transfection and Western blot analysis using anti-V5 or Flag antibodies.
Luciferase, IL-6 and IL-8 cytokine assays
Luciferase reporter assays were carried out as described previously (Liu et al., 2008). For the HSV ORF screen, HEK293 T cells were transfected in 96-well plates with NF-κB-driven firefly luciferase (NF-κB-luciferase) reporter plasmid, β-galactosidase (β-gal) expressing plasmid as transfection control, and each of the plasmids encoding HSV proteins. At 24 h post-transfection the luciferase activity was measured in cell lysates. Luciferase levels were normalized to β-galactosidase activity, and fold-induction values were calculated relative to the normalized activity of empty vector transfected sample. In other luciferase assays, HEK293T cells were plated in 96-well plates at a density of 2 × 104 cells/well. Twenty-four hours later, the cells were transfected with the NF-κB-luciferase and thymidine kinase promoter-driven Renilla luciferase (TK-Renilla) reporter plasmids, 50 ng of MyD88, TRAF6, p65, TBK1 or TRAF2 expression plasmids, with or without US3 plasmid and pcDNA3.1 empty vector to keep the total plasmid amount constant. Transfected cells were incubated for 24 h at 37 °C before being analyzed for luciferase activity. To determine luciferase expression, cells were lysed in 100 µ1 of reporter lysis buffer, and firefly luciferase activity was measured using the dual-glo luciferase assay system (Promega). Results are presented as fold induction of luciferase activity of transfected samples relative to the empty vector transfected control sample, after normalizing the firefly luciferase activity of each sample to its Renilla luciferase activity. For the US3 dose-dependence reporter assay, TLR2-expressing cells (H2.14.12 cells) were transfected with NF-κB-luciferase and TK-Renilla plasmids, together with increasing amounts of US3-plasmid and pcDNA3.1 empty vector to keep the total plasmid amount constant. After 24 h, transfected cells were treated with Zymosan, and at 6 h post stimulation firefly and Renilla luciferase activities were measured using the Promega dual-glo luciferase assay system.
To measure IL-6 or IL-8 production, H2.14.12 or RAW cells were infected with virus diluted in DMEM containing 1% calf serum (DMEV) at the indicated MOI for 1 h at 37 °C. The virus inoculum was replaced with DMEV and incubated at 37 °C. Cell supernatants were collected at the indicated time points, and IL-6 or IL-8 levels were measured by ELISA using the OptEIA human IL-6 or IL-8 ELISA kit (BD Biosciences, San Diego, CA) according to the manufacturer’s protocol.
Cell fractionation
Virus-infected cells were washed with ice-cold PBS and lysed in low-salt sucrose buffer (10 mM HEPES pH 7.9, 50 mM NaCl, 0.5 M sucrose, 0.1 mM EDTA, 0.5% Triton X-100 supplemented with protease inhibitor cocktail) on ice for 10 min. Lysates were clarified by centrifugation at 1500 rpm at 4 °C for 5 min, and the supernatant was saved as the cytoplasmic extract. Pellets were washed once with low-salt buffer without sucrose, and the pellet was further extracted with high-salt buffer (10 mM HEPES pH 7.9, 500 mM NaCl, 0.1 mM EDTA, 0.1% NP-40 supplemented with protease inhibitor cocktail) to obtain nuclear extracts. Protein levels in the cytoplasmic and nuclear fractions were determined using the Bradford method of protein quantitation (Bio-Rad Bradford reagent), and equivalent amounts of total protein in lysate samples were resolved by SDS-PAGE and analyzed by Western blotting to detect cytoplasmic and nuclear proteins.
Transfection and Immunoprecipitation
HEK293 T cells plated in 10 cm dishes were transfected with the indicated plasmids using the calcium phosphate precipitation method. At 24 h post transfection, cells were washed with ice-cold PBS and harvested in RIPA buffer containing 1% NP-40, 0.5% sodium deoxycholate, protease inhibitors and 20 mM iodoacetate. For detecting endogenous TRAF6, H2.14.12 cells were infected in 10 cm culture plates, and cells were lysed in RIPA buffer as described above. Aliquots of lysate containing equal amounts of total protein were incubated with anti-TRAF6 antibody, immunoprecipitated with Protein-A-agarose beads and washed in RIPA buffer. Bound proteins were eluted with Laemmli sample buffer, resolved by SDS-PAGE, and transferred onto PVDF membrane.
Western blot analysis and antibodies used
PVDF membranes were blocked in 5% milk/TBST solution and probed with anti-TRAF6, anti-Ubiquitin, anti-lκBα (Santa Cruz Biotech), anti-p65 (Abcam), anti-HA (Clonetech), anti-V5 (Invitrogen) or anti-FLAG (SIGMA) antibodies. Secondary antibodies used were HRP-conjugated anti-mouse and anti-rabbit antibodies from Bio-Rad Laboratories. Blots were developed using enhanced chemiluminescence (ECL) Western blotting reagents (Pierce).
RNA extraction and real-time PCR
RNA was isolated from RAW264.7 cells using the Qiagen RNeasy Kit as per the manufacturer’s protocol. After quantification by spectrophotometry, equal amounts of RNA were subjected to DNAse treatment (Ambion), reverse-transcribed using the high capacity cDNA reverse transcription kit (Applied Biosystems), and then quantified by real-time PCR using Sybr Green and the following primers: mIL-6-F (5′’-GAGGATACCACTCCCAACAGACC-3′), mIL-6-R (5′-AAGTGCATCGGTGGTCATACA-3′) (Koga et al., 2008), mCCL2-F (5′-TGACCCGTAAATCGTAAGC-3′), mCCL2-R (5′-CGAGTCACACTAGTTCACTG-3′) (Keepers et al., 2007). The abundance of mRNA was normalized to that of GAPDH mRNA and fold increase in RNA levels in infected cells compared to that in mock infected samples was calculated using the ΔΔCt method (Livak and Schmittgen, 2001).
Acknowledgments
We thank Lisa Holik and Fernando Diaz for assistance with the manuscript and Emily Chandler and Jeho Shin for technical support. We thank Kate Fitzgerald, Evelyn Kurt-Jones, and Robert Finberg for their helpful comments on this research. We thank Bernard Roizman for providing the mutant and rescued viruses. This research was supported by National Institutes of Health grants AI39576 and AI057552.
References
- Amici C, Belardo G, Rossi A, Santoro MG. Activation of IkB kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 2001;276:28759–28766. doi: 10.1074/jbc.M103408200. [DOI] [PubMed] [Google Scholar]
- Aravalli RN, Hu S, Lokensgard JR. Toll-like receptor 2 signaling is a mediator of apoptosis in herpes simplex virus-infected microglia. J. Neuroinflamm. 2007;4:11. doi: 10.1186/1742-2094-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J. Immunol. 2005;175(7):4189–4193. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti L, Roizman B. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc. Nat. Acad. Sci. U.S.A. 2004;101(25):9411–9416. doi: 10.1073/pnas.0403160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetti L, Roizman B. In transduced cells, the US3 protein kinase of herpes simplex virus 1 precludes activation and induction of apoptosis by transfected procaspase 3. J. Virol. 2007;81:10242–10248. doi: 10.1128/JVI.00820-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochud PY, Magaret AS, Koelle DM, Aderem A, Wald A. Polymorphisms in TLR2 are associated with increased viral shedding and lesional rate in patients with genital herpes simplex virus Type 2 infection. J. Infect. Dis. 2007;196:505–509. doi: 10.1086/519693. [DOI] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XJ, Fisone G, Aizman O, Aizman R, Levenson R, Greengard P, Aperia A. PKA-mediated phosphorylation and inhibition of Na( + )–K( + )– ATPase in response to beta-adrenergic hormone. Am. J. Physiol. 1997;273:C893–C901. doi: 10.1152/ajpcell.1997.273.3.C893. [DOI] [PubMed] [Google Scholar]
- Cholay M, Reverdy C, Benarous R, Colland F, Daviet L. Functional interaction between the ubiquitin-specific protease 25 and the SYK tyrosine kinase. Exp. Cell. Res. 2010;316:667–675. doi: 10.1016/j.yexcr.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Chuluunbaatar U, Roller R, Feldman ME, Brown S, Shokat KM, Mohr I. Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev. 2010;24:2627–2639. doi: 10.1101/gad.1978310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favoreel HW, Van Minnebruggen G, Adriaensen D, Nauwynck HJ. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc. Nat. Acad. Sci. U.S.A. 2005;102:8990–8995. doi: 10.1073/pnas.0409099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O’Neill LA. Mai (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Frame MC, Purves FC, McGeoch DJ, Marsden HS, Leader DP. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J. Gen. Virol. 1987;68(10):2699–2704. doi: 10.1099/0022-1317-68-10-2699. [DOI] [PubMed] [Google Scholar]
- Gack MU, Nistal-Villán E, Inn KS, García-Sastre A, Jung JU. Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J. Virol. 2010;84:3220–3229. doi: 10.1128/JVI.02241-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlo S, Kooijman R, Beck IM, Kolmus K, Spooren A, Haegeman G. Cyclic AMP: a selective modulator of NF-κB action. Cell. Mol. Life Sci. 2011;68:3823–3841. doi: 10.1007/s00018-011-0757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Liang Y, Mandel G, Roizman B. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Nat. Acad. Sci. U.S.A. 2005;102:7571–7576. doi: 10.1073/pnas.0502658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargett D, Rice S, Bachenheimer SL. Herpes simplex virus type 1 ICP27-dependent activation of NF{kappa}B. J. Virol. 2006;80:10565–10578. doi: 10.1128/JVI.01119-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S, Koyama AH, Shiota H, Adachi A, Goshima F, Nishiyama Y. Antiapoptotic activity of herpes simplex virus type 2: the role of US3 protein kinase gene. 1999;1:601–607. doi: 10.1016/s1286-4579(99)80059-8. [DOI] [PubMed] [Google Scholar]
- Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- Hilton MJ, Mounghane D, McLean T, Contractor NV, O’Neil J, Carpenter K, Bachenheimer SL. Induction by herpes simplex virus of free and heteromeric forms of E2F transcription factor. Virology. 1995;213:624–638. doi: 10.1006/viro.1995.0034. [DOI] [PubMed] [Google Scholar]
- Keepers TR, Gross LK, Obrig TG. Monocyte chemoattractant protein 1, macrophage inflammatory protein 1 alpha, and RANTES recruit macrophages to the kidney in a mouse model of hemolytic-uremic syndrome. Infect. Immun. 2007;75:1229–1236. doi: 10.1128/IAI.01663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Lim JH, Jono H, Ha UH, Xu H, Ishinaga H, Morino S, Xu X, Yan C, Kai H, Li JD. Tumor suppressor cylindromatosis acts as a negative regulator for Streptococcus pneumoniae-induced NFAT signaling. J. Biol. Chem. 2008;283:12546–12554. doi: 10.1074/jbc.M710518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Belko J, Yu C, Newburger PE, Wang J, Chan M, Knipe DM, Finberg RW. The role of toll-like receptors in herpes simplex infection in neonates. J. Infect. Dis. 2005;191:746–748. doi: 10.1086/427339. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Nat. Acad. Sci. U.S.A. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Mandell L, Whitney C, Padgett A, Gosselin K, Newburger PE, Finberg RW. Role of toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100:1860–1868. [PubMed] [Google Scholar]
- Leoni V, Gianni T, Salvioli S, Campadelli-Fiume G. Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-kappaB. J. Virol. 2012;86:6555–6562. doi: 10.1128/JVI.00295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Nat. Acad. Sci. U.S.A. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Roizman B. Expression of gamma interferon-dependent genes is blocked independently by virion host shutoff RNase and by US3 protein kinase. J. Virol. 2008;82:4688–4696. doi: 10.1128/JVI.02763-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima GK, Zolini GP, Mansur DS, Freire Lima BH, Wischhoff U, Astigarraga RG, Dias MF, das Gracas Almeida Silva M, Bela SR, do Valle Antonelli LR, Arantes RM, Gazzinelli RT, Bafica A, Kroon EG, Campos MA. Toll-like receptor (TLR) 2 and TLR9 expressed in trigeminal ganglia are critical to viral control during herpes simplex virus 1 infection. Am. J. Pathol. 2010;177(5):2433–2445. doi: 10.2353/ajpath.2010.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Fitzgerald K, Kurt-Jones E, Finberg R, Knipe DM. Herpesvirus tegument protein activates NF-kappaB signaling through the TRAF6 adaptor protein. Proc. Nat. Acad. Sci. U.S.A. 2008;105:11335–11339. doi: 10.1073/pnas.0801617105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matsuzaki A, Yamauchi Y, Kato A, Goshima F, Kawaguchi Y, Yoshikawa T, Nishiyama Y. US3 protein kinase of herpes simplex virus type 2 is required for the stability of the UL46-encoded tegument protein and its association with virus particles. J. Gen. Virol. 2005;86:1979–1985. doi: 10.1099/vir.0.80949-0. [DOI] [PubMed] [Google Scholar]
- McGeoch DJ, Davison AJ. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 1986;14(4):1765–1777. doi: 10.1093/nar/14.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici MA, Sciortino MT, Perri D, Amid C, Avitabile E, Ciotti M, Balestrieri E, De Smaele E, Franzoso G, Mastino A. Protection by herpes simplex virus glycoprotein d against Fas-mediated apoptosis: role of nuclear factor kappaB. J. Biol. Chem. 2003;278:36059–36067. doi: 10.1074/jbc.M306198200. [DOI] [PubMed] [Google Scholar]
- Mou F, Forest T, Baines JD. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J. Virol. 2007;81:6459–6470. doi: 10.1128/JVI.00380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger J, Roizman B. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Nat Acad. Sci. U.S.A. 2001;98:1410–1415. doi: 10.1073/pnas.181344498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg PD, McDonell PJ, Ryckman BJ, Knudson CM, Roller RJ. The HSV-1 US3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology. 2004;319:212–224. doi: 10.1016/j.virol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Oliveira-Nascimento L, Massari P, Wetzler LM. The role of TLR2 in infection and immunity. Front. Immunol. 2012;3:79. doi: 10.3389/fimmu.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzalli MJH, DeLuca NA, Knipe DM. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection: degradation of IFI16 by nuclear HSV ICP0 protein. Proc. Nat. Acad. Sci. U.S.A. 2012;109:E3008–E3017. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Hanson J, McLean RI, Olgiate J, Hilton M, Miller WE, Bachenheimer SL. Herpes simplex virus type 1 induction of persistent NF-κB nuclear translocation increases the efficiency of virus replication Virology. 1998;247:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- Poon AP, Benetti L, Roizman B. US3 and US3.5 protein kinases of herpes simplex virus 1 differ with respect to their functions in blocking apoptosis and in virion maturation and egress. J. Virol. 2006;80:3752–3764. doi: 10.1128/JVI.80.8.3752-3764.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves FC, Spector D, Roizman B. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J. Virol. 1991;65:5757–5764. doi: 10.1128/jvi.65.11.5757-5764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SB, Sorensen LN, Malmgaard L, Ank N, Baines JD, Chen ZJ, Paludan SR. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J. Virol. 2007;81:13315–13324. doi: 10.1128/JVI.01167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Fitzgerald KA. Innate immune sensing of DNA viruses. Virology. 2011;411:153–162. doi: 10.1016/j.virol.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Wills EG, Roller RJ, Ryckman BJ, Baines JD. Ultrastructural localization of the herpes simplex virus type 1 u(l)31, u(l)34, and u(s)3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 2002;76:8939–8952. doi: 10.1128/JVI.76.17.8939-8952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, editors. Fields Virology Fifth edition. Philadelphia: Lippincott, Williams and Wilkins; 2007. pp. 2501–2602. [Google Scholar]
- Ryckman BJ, Roller RJ. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J. Virol. 2004;78:399–412. doi: 10.1128/JVI.78.1.399-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MG, Rossi A, Amici C. NF-κB and virus infection: who controls whom. EMBO J. 2003;22:2552–2260. doi: 10.1093/emboj/cdg267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangi PP, Kim B, Kurt-Jones E, Rouse BT. Innate recognition network driving herpes simplex virus-induced corneal immunopathology: role of the toll pathway in early inflammatory events in stromal keratitis. J. Virol. 2007;81:11128–11138. doi: 10.1128/JVI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc. Nat. Acad. Sci. U.S.A. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino MT, Medici MA, Marino-Merlo F, Zaccaria D, Giuffre-Cuculletto M, Venuti A, Grelli S, Bramanti P, Mastino A. Involvement of gD/HVEM interaction in NF-κB-dependent inhibition of apoptosis by HSV-1 gD. Biochem. Pharmacol. 2008;76:1522–1532. doi: 10.1016/j.bcp.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J. Immunol. 2008;181:8604–8612. doi: 10.4049/jimmunol.181.12.8604. [DOI] [PubMed] [Google Scholar]
- van Lint AL, Murawski MR, Goodbody RE, Severa M, Fitzgerald KA, Finberg RW, Knipe DM, Kurt-Jones EA. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-kappaB signaling. J. Virol. 2010;84:10802–10811. doi: 10.1128/JVI.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner TW, Wright CC, Kato A, Kawaguchi Y, Mou f, Baines JD, Roller RJ, Johnson DC. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J. Virol. 2009;83:3115–3126. doi: 10.1128/JVI.01462-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B, Liu X, Wang X, Chang SH, Liu X, Wang A, Reynolds JM, Dong C. Negative regulation of IL-17-mediated signaling and inflammation by the ubiquitin-specific protease USP25. Nat. Immunol. 2012;13:1110–1117. doi: 10.1038/ni.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]