Abstract
Terpenes comprise a distinct class of natural products that serve a diverse range of physiological functions, provide for interactions between plants and their environment and represent a resource for many kinds of practical applications. To better appreciate the importance of terpenes to overall growth and development, and to create a production capacity for specific terpenes of industrial interest, we have pioneered the development of strategies for diverting carbon flow from the native terpene biosynthetic pathways operating in the cytosol and plastid compartments of tobacco for the generation of specific classes of terpenes. In the current work, we demonstrate how difficult it is to divert the 5-carbon intermediates DMAPP and IPP from the mevalonate pathway operating in the cytoplasm for triterpene biosynthesis, yet diversion of the same intermediates from the methylerythritol phosphate pathway operating in the plastid compartment leads to the accumulation of very high levels of the triterpene squalene. This was assessed by the co-expression of an avian farnesyl diphosphate synthase and yeast squalene synthase genes targeting metabolism in the cytoplasm or chloroplast. We also evaluated the possibility of directing this metabolism to the secretory trichomes of tobacco by comparing the effects of trichome-specific gene promoters to strong, constitutive viral promoters. Surprisingly, when transgene expression was directed to trichomes, high-level squalene accumulation was observed, but overall plant growth and physiology were reduced up to 80 % of the non-transgenic controls. Our results support the notion that the biosynthesis of a desired terpene can be dramatically improved by directing that metabolism to a non-native cellular compartment, thus avoiding regulatory mechanisms that might attenuate carbon flux within an engineered pathway.
Keywords: Triterpenes, Squalene, Metabolic engineering
Introduction
Terpenes are a structurally diverse class of compounds in plants that contribute to an equally diverse array of physiological and ecological functions. The structural diversity is most readily recognized in the classification of terpene families with repeating units of 5-carbon building blocks, like sesquiterpenes with 15 carbons and triterpenes derived from a 30-carbon scaffold. Terpene chemical diversity, however, extends much beyond polymer size or linear versus cyclized forms to the substituent decorations like hydroxylation, acylation, aroylation, methylation, and glycosylation. Given such chemical richness, it is not too surprising, how recent efforts have uncovered unique roles for terpenes in general growth and developmental processes. For instance, the essentiality of brassinolides (30 carbon triterpenes derivatives) for overall plant growth (Clouse 2011) and strigolactones (15 carbon sesquiterpenes arising from the breakdown of 40 carbon carotenoids) for the control of axillary bud dormancy and root architecture (Kohlen et al. 2011) are two such examples. Our appreciation for the specialized roles of terpenes in mediating ecological interactions between plants with other plants (Kegge and Pierik 2010), insects (Keeling and Bohlmann 2006) and microbes (Huffaker et al. 2011) has also grown in parallel with our understanding for the structural diversity of plant-biosynthesized terpenes. Plant-derived terpenes have also played a large role in various industrial applications ranging from flavors and fragrances (Schwab et al. 2008) to medicines (Shelar and Shirote 2011), and more recently to their utility for biofuels (Niehaus et al. 2011).
With the increased recognition of terpene contributions to physiological functions and evolving industrial uses, a parallel effort has been to manipulate the biosynthesis and accumulation of these compounds in plants for a variety of reasons. First, genetic and molecular genetic technologies to abolish or ectopically produce specific terpenes have been used to identify the biochemical and physiological function of genes, thus providing a gene annotation capability (Tholl et al. 2005). This has also been important for testing our understanding for biochemical processes in general and understanding the genetic and biochemical components associated with terpene biosynthetic enzymes (Mandel et al. 1996). Second, generating transgenic plants accumulating altered amounts of a specific terpene or suite of terpenes can be evaluated for their health-promoting properties (Sawai and Saito 2011) or could provide new means for the sustainable production of high-value chemicals for industrial uses (Krings and Berger 2010).
Engineering terpene metabolism in microbial hosts has advanced significantly in the recent past with much of the emphasis on providing a higher yield and recovery of high-valued terpenes. Much of the early success took advantage of the innate biosynthetic machinery in E. coli, the methylerythritol phosphate or MEP pathway, and introduced a limited number of carotenoid biosynthetic genes to yield visibly distinct lines (Schmidt-Dannert et al. 2000). Additional efforts to up-regulate putative rate-limiting steps early in the MEP have also improved carotenoid yields (Kim and Keasling 2001). Significant gains in the production of sesquiterpenes was dramatically realized when the complement of the eukaryotic mevalonate, MVA, pathway from yeast was mobilized into E. coli (Martin et al. 2003). Yields improved from μg/l to mg/l for the sesquiterpene hydrocarbon amorphadiene upon heterologous expression of these yeast genes in E. coli. Complementary to these prokaryotic studies, investigators also desired production platforms for more highly deco-rated terpenes and especially hydroxylated forms. Because the eukaryotic enzymes for terpene hydroxylation and their associated cofactors like cytochrome P450 reductases require internal membrane systems unique to eukaryotic cells, development of terpene production in yeast has also been advanced. In contrast to E. coli, yeast only possess the MVA pathway, which directs a significant amount of car-bon down this cytosolic pathway to ergosterol biosynthesis, the dominant sterol required for normal growth of yeast. Introducing additional mutations in yeast allowing them to utilize exogenous ergosterol under aerobic conditions frees up intermediates that can be redirected in desired ways. Ro et al. (2006) and Takahashi et al. (2007), for instance, demonstrated that such a strategy allowed for the development of yeast strains producing greater than 50 mg/l of oxygenated sesquiterpenes.
The manipulation of terpene metabolism in plants as a means for investigating key biochemical processes as well as for developing plants as production platforms for high-value terpenes has also been advanced significantly. Notable examples include molecular breeding efforts to enhance carotenoid (Harjes et al. 2008) and artemisinin (Graham et al. 2010) metabolism in maize and Artemisia annua, respectively. Other investigators have focused on augmenting terpene metabolism by the ectopic expression of terpene biosynthetic activities in different cellular compartments. Monoterpene biosynthesis naturally occurs in the chloroplast compartment of plant cells, but several investigations have documented that over-expression of a single monoterpene synthase to either the chloroplast or cytoplasm compartment resulted in the accumulation of new monoterpenes and in some cases novel derivatives like glyco-conjugates (Aharoni et al. 2003; Lewinsohn et al. 2001; Lucker et al. 2001; Ohara et al. 2003). Re-directing the biosynthesis of sesquiterpene metabolism to the mitochondria and plastid compartment has had an equal, if not greater, impact on overall terpene metabolism. Kappers et al. (2005) demonstrated the ability of plants to synthesize unusual sesquiterpenes in the mitochondrial compartment led to plants able to attract predatory insects as a biocontrol mechanism. Likewise, Wu et al. (2006) demonstrated that engineering a more robust sesquiterpene metabolism normally associated with the cytoplasm to the chloroplast compartment over-rode any innate regulatory mechanisms and yielded robust production of industrially valued sesquiterpenes. More recently, Kumar et al. (2012) extended such ectopic engineering strategies by inserting genes coding for the normal cytoplasmic MVA pathway into the chloroplast genome to affect high-level expression of the enzymes leading up to mevalonate. These transgenic plant lines accumulated mevalonate while no such accumulation in control plants was evident and exhibited a twofold increase in their main sterol level and a tenfold increase in squalene, but only a 20 % increase in β-carotene content. The results of Kumar et al. (2012) suggested that a mevalonate biosynthetic pathway engineered into the chloroplast was able to complement and augment overall terpene biosynthetic processes occurring both within and outside the chloroplast compartment.
In the present effort, our objective was to determine if our previous strategy for engineering high-level sesquiterpene accumulation was applicable to larger terpenes, and in particular to the triterpene class of compounds. We also aimed to evaluate the possibility of targeting this metabolism to trichomes such that the biosynthesis of a target molecule might be secreted as per the suggestions of Wang et al. (2004) and Ennajdaoui et al. (2010).
Materials and methods
Expression vector construction and plant transformation
Construct design and assembly were based on the work previously described by Wu et al. (2006) using standard molecular methodologies (see Fig. 2). Gene constructs consisted of a truncated form of the yeast squalene synthase (ySQS) gene (ERG9, GenBank accession NM 001179321) (Zhang et al. 1993) and the avian farnesyl diphosphate synthase (FPS) gene (P08836) (Tarshis et al. 1994). The truncated SQS was created by PCR amplifying the yeast SQS mRNA from its start codon to nucleotide 1260, thus deleting the DNA encoding for the carboxy-terminal 24 amino acids. These carboxy-terminal amino acids are predicted to tether the SQS protein to endomembrane systems in vivo. Hence, deletion of these amino acids creates a functionally soluble enzyme (Zhang et al. 1993). The ySQS and FPS genes were inserted downstream of strong constitutive promoters [(Pca, 35S cauliflower mosaic viral promoter (Benfey and Chua 1990); Pcv, cassava vein mosaic viral promoter (Verdaguer et al. 1996)], or trichome-specific promoters [(Pcbt, the cembratrien-ol synthase promoter (Ennajdaoui et al. 2010) or the Pcyp16, cembratrieneol hydroxylase promoter (Wang et al. 2002)]. The Pcbt and Pcyp16 promoters were further augmented with duplicated CAMV 35S enhancer elements (Benfey et al. 1990) fused to the 5' termini of the promoters. Where indicated, a plastid targeting signal sequence (tp) encoding for the first 58 amino acids of the Arabidopsis Rubisco small subunit gene (NM23202) (Lee et al. 2006) was fused onto the 5' end of the respective genes.
Fig. 2.
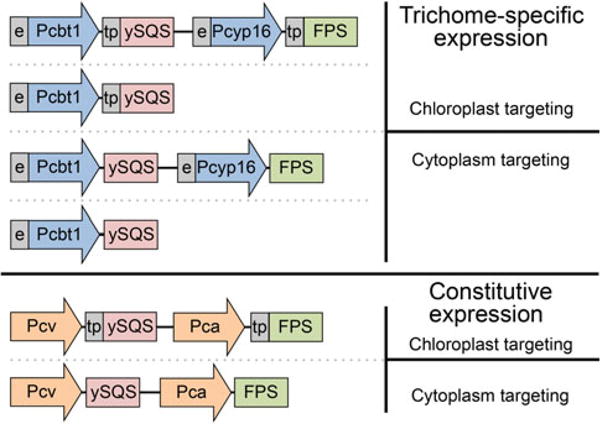
Gene constructs used to introduce squalene synthase (SQS) and farnesyl diphosphate synthase (FPS) genes into the genome of transgenic plants, and to target the encoded catalytic activities to the cytoplasm or chloroplast (tp) compartments. Promoters: cbt1 cembratrienol synthase (Ennajdaoui et al. 2010), cyp16 cytochrome P450 71D16 (Wang et al. 2002), e duplicated transcriptional enhancer sequence found in the CaMV 35S promoter (Benfey et al. 1990), cv 35S cassava mosaic virus (Verdaguer et al. 1998), ca 35S cauliflower mosaic virus (Benfey and Chua 1990). Genes; ySQS the yeast squalene synthase with a truncation of 168 bp at the 3′ end (Zhang et al. 1993), FPS the chicken farnesyl diphosphate synthase (Tarshis et al. 1994), tp the chloroplast targeting signal sequence from the Arabidopsis Rubisco small subunit gene (Lee et al. 2006)
The DNA sequences were assembled together using standard molecular biology methods and the various elements verified by DNA sequencing. The expression cassettes were then introduced into pBDON (Wu et al. 2006), a modified pBI101 Ti plasmid vector harboring a hygromycin selection marker and a recombination cloning cassette. In some cases, simple substitution cloning of the desired DNA elements into previously constructed inter-mediate helper vectors was performed as described by Wu et al. (2006), (see Online Resources Supplementary Fig. 1). The engineered Ti plasmid vectors were then introduced into Agrobacterium tumefaciens GV3850, and the resulting Agrobacterium lines used to genetically engineer Nicotiana tabacum (tobacco) TI accession 1068 (Nielsen et al. 1982) as previously described previously by Wu et al. (2006).
Leaf explants were transformed with the respective gene constructs and the resulting calli selected for hygromycin resistance (15 μg/ml) under tissue culture conditions to regenerate plantlets. The selected T0 plantlets were then propagated in the greenhouse and assessed for squalene accumulation by GC–MS analyses.
Plant propagation and field tests
All the T0 plantlets selected for hygromycin resistance were grown in common commercial vermiculite/soil blends in the greenhouse and fertilized weekly with commercially available high nitrogen, phosphorus, and potassium fertilizer. Insect control was performed on an as-needed basis. The T0 plants were allowed to flower in the greenhouse and the T1 seed collected for subsequent cycles of propagation. Segregation of the hygromycin resistance trait in the T1 seed lines was also evaluated by germinating sterilized seeds on 50 μg/ ml hygromycin in T— tissue culture media (4.2 g MS salts (Phytotechnology Laboratories, Overland Park, KS), 0.112 g B5 vitamins (Phytotechnology Laboratories), 30 g sucrose, 8 g agar). For field evaluation, T1 seeds were sown directly in propagation trays in the greenhouse 6 weeks prior to trans-planting in the field and were not pre-selected for antibiotic resistance. To determine squalene accumulation, leaf discs of 2 cm diameter were collected from the upper most, fully expanded leaves. Photosynthetic gas exchange measurements of first fully expanded leaves were determined at atmospheric concentrations of CO2 and a saturating irradiance of 1,500 micromoles photons m−2 s−1 using a LI-COR 6400 portable photosynthesis system according to Salvucci and Crafts-Brandner (2004). At the time of harvest, plant height was taken, the plants cut at the soil interface, weighed, and all the stripped leaves combined for leaf area determinations. All transgenic work was done in accordance with regulations and permits provided by the APHIS Division of the USDA.
Squalene determinations
One hundred to 500 mg of transgenic leaf material was collected for chemical analyses using a 2-cm-diameter cork borer tool to obtain leaf discs of approximately 100 mg each. Each sample was ground in liquid nitrogen, then extracted with 2–3 ml of a hexane:ethyl acetate mixture (v/v 85:15) containing 200 ng of α-cedrene as an external standard for quantification and calculations of recovery. The extracts were carefully concentrated to 500 μl under a nitrogen stream without drying the sample. The concentrated extracts were then partially purified by passing through a silica column (500 mg, prepared in glass wool plugged glass pipette) and further eluted with 1 ml of the hexane solvent. After concentration of the combined eluate under a stream of nitrogen, aliquots were injected onto a GC-MS equipped with a Rtx-5 capillary column (30 m × 0.32 mm, 0.25 um phase thickness) with the following temperature program of 70 °C for 1 min, followed by a 4 °C per min gradient to 250 °C. Mass spectra were recorded at 70 eV, scanning from 35 to 500 atomic mass units, and experimental samples were compared with authentic standards of squalene for verification (see Online Resources Supplementary Fig. 2).
The structure of purified squalene from tobacco was determined by 1H-NMR and 13C-NMR spectral analyses. Squalene was extracted from greenhouse grown plants as described earlier, except additional purification was afforded by a silica HPLC methodology. Essentially, 60 g leaf material of homozygous line #5 expressing plastid target SQS and FPS under the direction of the constitutive promoters was ground in liquid nitrogen, then extracted with 1.2 l of hexane:ethyl acetate (85:15), the extract concentrated to 5 ml, and the extract fractionated on a silica column with 5 ml aliquots of hexane as the eluting solvent. Fractions were monitored by TLC (silica plates, hexane solvent, iodine vapor stain) and GC for the desired triterpene compound. Enriched fractions were pooled, concentrated under nitrogen, and the entire sample processed by silica HPLC–PDA using hexane as the eluting solvent (Niehaus et al. 2012). Recovery of 4 mg of purified squalene sample with a 50 % yield was obtained. 1H-NMR and 13C-NMR spectra were recorded on a 500-MHz Varian J-NMR spectrometer at 300 K, and chemical shifts were referenced relative to solvent peaks, namely δH 7.24 and δC 77.0 for CDCl3.
Results
Experimental approach
Terpene metabolism in plant cells is divided between the mevalonate (MVA) pathway operating in the cytoplasm and the methylerythritol phosphate (MEP) pathway occurring in the chloroplast (Fig. 1). Interestingly, a convenient division of labor between these two pathways has been established with the MVA pathway largely dedicated to sesquiterpene, triterpenes, and polyprenol biosynthesis in association with the ER endomembrane system, while the MEP pathway is responsible for monoterpenes, diterpenes, carotenoids (tetraterpenes), and long-chain phytol biosyn-thesis occurring in the chloroplast stroma. Given this sort of organizational complexity, we reasoned it would be best to evaluate several different strategies for engineering triterpene metabolism and specifically squalene accumulation.
Fig. 1.
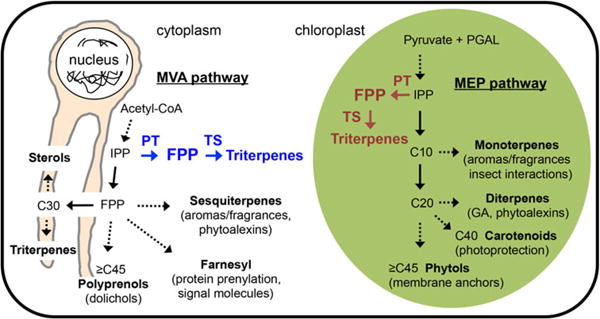
A depiction of the mevalonate (MVA) and methylerythritol phosphate (MEP) pathways operating natively in plants and their contributions to the biosynthesis of particular classes of terpenes (black), along with a conceptualization for targeting novel triterpene metabolism to the cytoplasm (blue) or to the chloroplast (red) compartments. PT prenyl transferase (i.e. farnesyl diphosphate synthase), TS triterpene synthase (i.e. squalene synthase)
The first approach was to compare squalene accumulation in transgenic plants expressing a heterologous squalene synthase directed to the cytosolic compartment versus the chloroplast compartment (Fig. 2). The squalene synthase gene used in all these constructs is from yeast and has a 3′ truncation of the DNA sequence coding for a carboxy-terminal, membrane-spanning domain, and hence yielding a functionally soluble squalene synthase enzyme activity (ySQS) (Zhang et al. 1993). This deletion was important to assure catalytic activity of the squalene synthase vectorially imported into the chloroplast compartment via an amino terminal targeting signal sequence (tp) from the Rubisco small subunit gene from Arabidopsis (Lee et al. 2006). The yeast squalene synthase gene was also chosen because it was assumed to be devoid of sequences subject to transcriptional to post-translational regulation that another plant squalene synthase gene/enzyme might be. Expression of these initial constructs were directed by tri-chome-specific promoters as described by Ennajdaoui et al. (2010) and Wang et al. (2002) to potentially provide for the secretion of the trichome synthesized squalene onto the leaf surface, as well as the fairly conventional constitutive promoters from caulimoviruses (Benfey and Chua 1990; Verdaguer et al. 1998).
These first constructs assumed that an introduced squalene synthase could compete for any available FPP in the cytoplasm or FPP that could arise in the chloroplast compartment as an intermediate released from the MEP path-way or imported from the cytoplasm. Cytosolic FPP levels are, however, generally low, and FPP is thought to serve a regulatory role in controlling carbon flux into the MVA pathway (Closa et al. 2010). There is also little evidence, if any, for all trans-FPP being formed in chloroplasts (Sallaud et al. 2009). The second construct iterations thus included a chicken gene encoding for a well-characterized farnesyl diphosphate synthase (FPS) (Tarshis et al. 1994) in addition to the yeast squalene synthase (Fig. 2). Expression of these constructs varied by using either strong constitutive promoters or trichome-specific promoters, plus/minus amino terminal sequences targeting the SQS and FPS enzymes to the cytoplasm or chloroplast compartments.
Screening of the T0 and T1 transgenic lines
The constructs of Fig. 2 were then used to generate approximately 20 independent transgenic lines per construct. The particular cultivar of tobacco, TI 1068, used for these experiments is an accession line identified for its high density of secretory trichomes (Nielsen et al. 1982). The various transgenic lines were first evaluated for squalene accumulation while still in their final stages of plantlet develop in tissue cultures, then re-screened as the T0 generation plants were propagated in the greenhouse (Table 1).
Table 1. Screen of T0 transgenic lines for their squalene content.
| Construct | # of lines evaluated | Ave (μg/g fw) | Min | Max | Dwarf | Chlorotic | Dwarf and chlorotic |
|---|---|---|---|---|---|---|---|
| Wild type (control) | 15 | 3.5 | 0.5 | 7.4 | 0 | 0 | 0 |
| Trichome SQS only cytosolic | 24 | 7.6 | 1.0 | 34.2 | 1 | 0 | 0 |
| Trichome SQS only plastidic | 18 | 6.7 | 0.6 | 30.7 | 0 | 0 | 0 |
| Constitutive SQS + FPS cytosolic | 29 | 5.5 | 0.8 | 38.7 | 5 | 0 | 0 |
| Trichome SQS +FPS cytosolic | 16 | 8.1 | 1 | 20.1 | 1 | 0 | 0 |
| Constitutive SQS + FPS plastidic | 26 | 63.9 | 1.4 | 659.7 | 6 | 0 | 0 |
| Trichome SQS + FPS plastidic | 17 | 101.8 | 3.6 | 203.5 | 6 | 3 | 5 |
Transgenic plantlets generated with the indicated construct (expression promoter, gene(s) and intracellular targeting information) were propagated under sterile conditions until they established root systems and were ready for growth in the greenhouse. The greenhouse-grown plants were screened several independent times for their squalene content. In the data shown, plants were from 40 to 60 cm tall and the first fully expanded leaf was sampled. Data represent the average from all the independent plant lines sampled twice, as well as the minimum (min) and maximum (max) observed. Plants were scored as dwarf if their height was 25 % less than their sibling plants. Plants were scored as chlorotic if there was obvious yellowing within 3 or more leaves
Plants expressing only the squalene synthase gene tended to accumulate only marginally higher levels of squalene than observed in the control, non-transgenic lines, approximately twofold. Nonetheless, whether the squalene synthase was targeted to the cytoplasm or chloroplast, 1–2 transgenic plants within each group accumulated squalene levels 4–5 times the maximum level observed for the control plants. Surprisingly, plants engineered with both SQS and FPS targeted to the cytoplasm and directed by either strong constitutive or trichome specific promoters also did not accumulate squalene much beyond those lines engineered with the SQS gene by itself.
In contrast, plants engineered with SQS and FPS tar-geted to the chloroplast compartment demonstrated an overall average accumulation of squalene 20- to 30-fold greater than the non-transgenic control lines. More impressive, individual lines accumulated 200–600 μg of squalene per g fresh weight, 27–90 times greater than the non-transgenic controls. Nonetheless, abnormal growth characteristics were observed for several of these lines. For the constitutive expressed forms of SQS and FPS, 30 % of the lines exhibited a noticeable dwarfing phenotype, whereas more than 85 % of the regenerated lines with the trichome-specific expression cassette demonstrated some degree of dwarfing (35 %), chlorosis (17 %), or some combination of both (30 %) (Fig. 3).
Fig. 3.

Example phenotypes of plants engineered with genes encoding for SQS and FPS targeted to the chloroplast and directed by trichome specific promoter (left hand pictures) or constitutive viral promoters (center pictures), relative to wild type plants (right hand pictures). T2 seed from the trichome promoter line 31 and constitutive promoter line 5 were germinated in the presence of hygromycin, while the wild type control seed was germinated on medium without antibiotic. Six week old plants were transferred to the greenhouse and grown for several months before representative plants were chosen for these pictures
Similar trends in squalene accumulation were noted for individual plants examined in the T1 generation (Table 2). The T1 screen also attempted to correlate squalene accumulation with plant development; hence samples representing young, developing, and mature stages of leaf development were evaluated. Plant lines engineered for SQS targeted to the cytoplasm exhibited little or no difference in their ability to accumulate squalene relative the non-transgenic control. Cytosolic targeting of SQS and FPS, regardless of the expression promoters used, also did not accumulate significantly more squalene than found in the control plant. Transgenic plants with SQS and FPS targeted to the chloroplast compartment, however, demonstrated a very significant accumulation of squalene in a developmental-dependent manner. Levels of squalene accumulation were 300–1,000-fold greater than those levels measured in control plant lines.
Table 2. Developmental accumulation of squalene in T1 greenhouse grown plants.
| Construct | Line | Leaf | Squalene |
|---|---|---|---|
| designation | development | (μg/g fw) | |
| Wild type (control) | # 21 | Young | 1.9 |
| Developing | 3.4 | ||
| Mature | 2.3 | ||
| Trichome | # 21 | Young | 1.3 |
| SQS | Developing | 4.8 | |
| cytosolic | Mature | 9.8 | |
| # 44 | Young | 3.6 | |
| Developing | 4.1 | ||
| Mature | 2.3 | ||
| Trichome | # 39 | Young | 7.1 |
| SQS | Developing | 16.7 | |
| plastidic | Mature | 26.3 | |
| Constitutive | # 16 | Young | 6.4 |
| SQS ? FPS | Developing | 5.5 | |
| cytosolic | Mature | 7.4 | |
| # 204 | Young | 2.6 | |
| Developing | 1.9 | ||
| Mature | 5.6 | ||
| Trichome | # 27 | Young | 5.7 |
| SQS + FPS | Developing | 8.4 | |
| cytosolic | Mature | 5.3 | |
| Constitutive | #7 | Young | 30.1 |
| SQS + FPS | Developing | 121.15 | |
| plastidic | Mature | 147.4 | |
| #15 | Young | 329.3 | |
| Developing | 450.4 | ||
| Mature | 667.5 | ||
| Trichome | #21 dwarf | Young | 90.0 |
| SQS + FPS | Developing | 74.3 | |
| plastidic | Mature | 256.7 | |
| #31 mosaic | Young | 527.6 | |
| Developing | 594.7 | ||
| Mature | 1,760.2 |
Individual, second-generation (T1) plants were propagated in the greenhouse and identified on the basis of preliminary chemical pro-filing screens. On average, young leaves were 5–7.5 cm in length, developing leaves were 10–15 cm in length, and mature leaves were greater than 20 cm long. Distinct phenotypes are noted for the plants harboring the constructs for trichome specifically expression of SQS and FPS targeted to the plastid compartment (see Fig. 3 for examples)
While squalene is a relatively stable compound, it could be subject to both secondary metabolism occurring in planta, as well as environmental-induced changes like oxidation. To examine the integrity of the squalene accumulating by plants grown in the greenhouse, 60 g of leaves from a plant constitutively expressing plastid targeted SQS and FPS was extracted with hexane, the putative squalene compound purified by successive rounds of silica chromatography, and the isolated compound then analyzed by GC–MS and NMR analyses. When evaluated by GC–MS, the squalene purified from tobacco leaves exhibited an identical retention time and mass spectrum to an authentic squalene standard, as did its 1H-NMR and 13C-NMR spectra (see Online Resources Supplementary Figs. 3 and 4).
Assessment of field grown plants
To gain a better appreciation for the robustness of the squalene accumulation trait and its impact on overall growth performance, segregating populations of transgenic lines expressing plastid targeted SQS and FPS enzymes under the direction of the trichome-specific promoters (line #32) or the constitutive promoters (line #42) were propagated in outdoor field conditions. These lines were chosen because the squalene levels determined for these lines during the initial T0 generation screens appeared more typical for this class of transgenic plants, rather than representing an extreme. In the T0 greenhouse screens, the line expressing SQS and FPS targeted to the plastid compartment with trichome specific promoters (line 32) accumulated 192 lg squalene/g fresh weight, whereas 150 lg squalene/g fresh weight was recorded for the constitutive expressing line 42.
T1 seeds for both lines were germinated without any selection for the transgenes, hence representing a segregating population, and grown for approximately 6 weeks in a greenhouse before transplanting the plantlets in the field. Two replicate rows of each line were grown with standard plant and row spacing, along with independent rows of the non-transgenic parental line. Plantlets were randomly selected from the greenhouse propagation trays for planting, watered once to twice a week for a couple of weeks to support their initial establishment, and then allowed to grow without any additional treatments (i.e. no fertilizer or pesticide treatments) for a 60-day growing period. Agronomic performance characteristics and chemical profiles were measured twice for each plant and data for plants accumulating squalene (presumably homozygous and heterozygous for the transgenes) as well as those not accumulating squalene were clustered for quantitative comparisons. The data in Table 3 were obtained from plants at the end of field growth cycle with photosynthetic measurements and squalene accumulation determined for the uppermost, fully expanded leaf. For line 32, 6 plants accumulated squalene and 18 plants did not. Of the 22 plants of line 42 examined, 14 plants accumulated squalene.
Table 3. Field performance and squalene accumulation by select, segregating transgenic lines.
| Plant line | Height (cm) | Weight (kg) | Leaf area (cm2) | Photosynthesis (μmol CO2/m2•sec) | Conductance (mol H2O/m2•sec) | Ci (μmol CO2/mol air) | Squalene (μg/g fw) |
|---|---|---|---|---|---|---|---|
| Wild type (control) | 46.8 ± 13.6 | 1.0 ± 0.4 | 13,887 ± 4,586 | 23.4 ± 2.2 | 0.6 ± 0.1 | 254.0 ± 8.6 | nd |
| 32– | 50.2 ± 12.9 | 1.0 ± 0.3 | 11,313 ± 3,356 | 21.5 ± 3.1 | 0.5 ± 0.1 | 249.3 ± 19.0 | nd |
| 32+ | 18.9 ± 5.8 | 0.2 ± 0.1 | 2,398 ± 1,128 | 6.9 ± 2.2 | 0.6 ± 0.2 | 327.8 ± 11.2 | 79.9 ± 36.6 |
| 42− | 58.4 ± 8.1 | 1.1 ± 0.3 | 11,704 ± 2,956 | 21.3 ± 2.1 | 0.5 ± 0.1 | 249.6 ± 15.6 | nd |
| 42+ | 39.5 ± 6.3 | 0.6 ± 0.1 | 7,776 ± 1,650 | 21.4 ± 2.5 | 0.4 ± 0.1 | 243.2 ± 20.5 | 112.0 ± 27.8 |
Segregating (T1 generation) seed for transgenic lines targeting SQS and FPS to the chloroplast under the direction of trichome-specific promoters (line 32) or constitutive promoters (line 42) were grown under greenhouse conditions for approximately 6 weeks prior to transplanting them to the field. Plants were chosen randomly for field planting and the plants grown for a total of 60 days, which is approximately two-thirds of a full growth cycle. The plants were screened several times throughout the growing season, but the data shown are for the final data collection at the end of the field season. For squalene determinations, two leaf discs of 2 cm diameter collected from the first fully expanded leaf from the top of each plant were extracted with organic solvent and their squalene content determined by GC–MS. For line 32, 6 plants accumulated significant squalene (denoted as +), while18 others did not (−). For line 42, 14 plants accumulated squalene (+) and 8 did not (−). Six control plants were evaluated. nd no squalene detected, below the detection limit of ∼ 0.5 μg/g fw. Measurements of photosynthetic gas exchanges were conducted between 10AM and 12PM on a cloudless day with light intensity of 1,500 μmol/m2•sec with a LI-COR 6400 portable instrument
Of the non-squalene accumulating plants segregating out of lines 32 and 42, these plants performed directly comparable to the wild-type check plants with regard to any of the agronomic indicators (height, biomass accumulation, leaf area) or photosynthetic measurements (CO2 fixation rates, transpiration and internal CO2 levels). The only modest impact on performance was for their total leaf area measurements with the non-squalene accumulators within line 42 having 84 % and those within line 32 having only 72 % of that for the control plants.
In contrast, for the squalene accumulating plants of line 32 (SQS and FPS targeted to the chloroplast and expression directed by the trichome-specific promoters), their overall agronomic performance was only 20, 21, and 37 % that of their non-squalene accumulating siblings for biomass accumulation, leaf area, and height, respectively. The squalene accumulating plants within line 32 also only exhibited about 32 % the photosynthetic rate of their non-squalene accumulating siblings. Growth was also impacted for the squalene accumulating plants within transgenic line 42, though the average squalene accumulation within these plants exceeds that observed for line 32 (112 versus 80 μg/ g fresh weight). Leaf area, total biomass accumulation, and plant height of the squalene accumulators were 66, 54, and 68 % of that for their non-squalene accumulating siblings. However, their photosynthesis rates, stomatal conductance, and their internal CO2 levels were almost identical to their non-squalene accumulating siblings and the wild-type control plants.
Given the relative normal growth appearance of the squalene accumulating plants within line 42 (they appear to grow slower and hence appear smaller than the control check plants), we also examined the squalene accumulation in leaves of different developmental stages in several of these plants. The importance of this measurement became obvious when we noted that the squalene level of these plants after about 4 weeks in the field was 26.5 μg/g fresh weight and over 230 μg/g fresh weight after an additional four more weeks. As shown in Table 4, squalene levels in the very young leaf tissues was quite low, almost below detection limits for the just emerging leaves, but exhibited a significant increase in accumulation with leaf maturation. In lower, more senescent leaves, the amount of extractable squalene was significantly lower than that in the mature leaves. Also evident in the data of Table 4 is the variability between individual plants and absolute leaf position, some of which might be related to the zygosity of the particular plant as well as difficulty in attaining an absolute standardization of leaf development between plants.
Table 4. Developmental accumulation of squalene in field-grown plants.
| Construct | Plant | Leaf development | Squalene (μg/g fw) |
|---|---|---|---|
| Constitutive | # 10 | Young (4th) | 91.8 |
| SQS + FPS | Developing (7th) | 235.1 | |
| plastidic | Mature (9th) | 349.4 | |
| Senescing (11th) | 51.1 | ||
| # 21 | Young (2nd) | nd | |
| Developing (5th) | 83.7 | ||
| Maturing (8th) | 163.3 | ||
| Mature (11th) | 219.5 | ||
| Senescing | 263.2 |
Leaves at various positions of two plants within line 42 were sampled for their squalene levels. Plants were grown for 60 days in the field and appeared visually comparable to one another. nd not detected, below detection limit of 0.5 μg/g fw
Discussion
The current work extends earlier efforts to engineer terpene metabolism in plants in several significant ways. Investigators, including ourselves, have successfully engineered relatively robust monoterpene (Aharoni et al. 2003; Lewinsohn et al. 2001; Lucker et al. 2001; Ohara et al. 2003) and ses-quiterpene (Kappers et al. 2005; Wu et al. 2006) biosynthesis in transgenic plants, and more modest manipulations in the level of diterpenes (Besumbes et al. 2004) and triterpenes (Kumar et al. 2012; Lee et al. 2004; Seo et al. 2005). Interestingly, many of the early efforts were focused on introducing enzymes to effectively compete for substrates or intermediates in distinct cellular compartments where this metabolism occurs naturally, or were attempts to overcome prospective rate-limiting steps by over-expressing a gene coding for the suspected limiting enzyme. We, and other investigators, have demonstrated that a more successful strategy is to divert carbon flux at earlier upstream inter-mediates to build particular terpene compounds in compartments where this metabolism does not normally occur. That was indeed the case here in the successful engineering of triterpene metabolism. For example, when SQS was targeted to the cytoplasm and thus potentially accessing FPP synthesized by the MVA pathway, only a twofold increase in squalene levels relative to the wild-type controls was observed. When the SQS was targeted to the chloroplast where FPP biosynthesis is not known to occur, the levels of squalene observed were again, on average, twofold greater than the control, non-transgenic plants. There were, of course, a few exceptions where squalene accumulation in transgenic lines having SQS targeted to the cytoplasm or chloroplast were more than fourfold greater than the levels in the control plants, but these were single transgenic events and not general observations.
Explanations for why such a narrow window in the enhancement of squalene accumulation is observed when attempting to directly divert the normally produced FPP include possible channeling of this intermediate within metabolons or stringent regulation imposed upon carbon flux down these pathways by metabolite feedback regulatory networks (Gardner and Hampton 1999; Manzano et al. 2004; Masferrer et al. 2002; Munoz-Bertomeu et al. 2007; Sawai and Saito 2011), thus limiting the availability of FPP. More difficult to understand is how chloroplast targeted SQS by itself would even come into contact with FPP because all trans-FPP is not known to be synthesized in the chloroplast. Perhaps some cytoplasmic produced FPP can diffuse or be transported into the chloroplast. Alternatively, or some of the chloroplast targeted SQS could gain access to the cytosolic FPP during its movement from its site of synthesis in the cytosol to the chloroplast. Equally possible, at least for the plastid targeted SQS, is that some of the targeted enzyme may not actually be making its way to the chloroplast compartment and is simply diverting FPP formed in the cytoplasmic compartment. But then, one would expect cytosolic targeted SQS to have an equal effect and it does not. Nonetheless, when FPS and SQS are co-expressed and targeted to the chloroplast compartment, the FPS appears able to divert significant DMAPP and IPP (the substrates for FPS) from the MEP pathway for the biosynthesis of FPP, which in turn is available to SQS for the novel and very robust biosynthesis of squalene in the chloroplast compartment.
Interestingly, the same is not true for FPS and SQS co-expressed and targeted to the cytosolic compartment where the MVA pathway operates. This result suggests that DMAPP and IPP are not as readily available in the cytoplasmic compartment, or that metabolic flux down the MVA pathway is much more regulated by unknown factors than is observed for these metabolites in the chloroplast. Similar conclusions were reached by Wu et al. (2006) when FPS was co-expressed with sesquiterpene synthases targeted to either the chloroplast or the cytoplasmic compartments.
An equally surprising observation was that targeted expression of the FPS and SQS using trichome-specific promoters resulted in physiologically impaired plants, while use of strong, constitutive promoters were much less so. The first indication of this effect was evident in the T0 generation plants. Greater than 80 % of the transgenic lines utilizing the trichome-specific promoters were either dwarf, chlorotic, or a combination of both. In comparison, only 23 % of the T0 transgenic lines using the constitutive viral promoters showed some signs of dwarfism, but no chlorosis. These differences were even more accentuated when the plants were grown in field conditions. While the tri-chome-specific expressing plants accumulated relatively modest levels of squalene, overall growth, and physiological functioning were reduced 60–80 %. The impact of constitutively expressing FPS and SQS targeted to the chloroplast was a growth reduction of 30–40 % without any adverse effects on photosynthetic parameters, even though these plants accumulated greater amounts of squalene than the trichome-specific expressing plant line. Why the trichome-specific expressing transgenic plants are more impacted than the constitutively expressing plants is currently unknown. It may be that trichome-directed expression simply distorts biochemical processes in such a manner that trichome-derived cues or signals alter metabolism occurring elsewhere in the plant. Or, the enhancer elements used to improve trichome expression may cause ectopic expression in meristematic or progenitor cells distorting their contributions to normal physiological growth features. We are not aware of any other reports of similar growth distortion when attempting to engineer trichome metabolism, although the number of such engineering efforts are rather limited at this time. Additional screening of more independent transgenic lines created with these and other trichome-specific constructs, and unbiased metabolomics profiling might help resolve these issues.
Finally, comparison of the developmental squalene accumulation profiles by the field grown plants and separate transgenic lines grown under greenhouse conditions suggests there is certainly variability by leaf position, which is expected. If the novel squalene biosynthetic machinery is produced constitutively over developmental time, then squalene accumulation should continue as leaves develop and expand over time. Importantly, the levels of squalene accumulating in the field grown plants harboring the constitutively expressing FPS and SQS targeted to the chloroplast were equal to or higher than those levels measured in greenhouse grown plants, an indication of the robustness of this engineering approach for plants grown under a variety of conditions and the utility of such a platform for high value triterpene production.
Supplementary Material
Acknowledgments
We thank Scott Kinison and Madison Wallace for their assistance with all aspects of the experimental work and the entire Chappell laboratory for their critical consideration of this work. This work was supported, in part, by a NIFA/USDA grant 2010-04025 and the UK College of Agriculture Experiment Station.
Abbreviations
- FPS
Farnesyl diphosphate synthase
- SQS
Squalene synthase
- MVA
Mevalonate
- MEP
Methylerythritol phosphate
- DMAPP
Dimethylallyl diphosphate
- IPP
Isopentenyl diphosphate
- FPP
Farnesyl diphosphate
Footnotes
A contribution to the Special Issue on Metabolic Plant Biology.
Electronic supplementary material The online version of this article (doi:10.1007/s00425-012-1680-4) contains supplementary material, which is available to authorized users.
References
- Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel WJ, Verstappen FWA, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell. 2003;15:2866–2884. doi: 10.1105/tpc.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfey PN, Chua NH. The cauliflower mosaic virus-35s promoter—combinatorial regulation of transcription in plants. Science. 1990;250:959–966. doi: 10.1126/science.250.4983.959. [DOI] [PubMed] [Google Scholar]
- Benfey PN, Ren L, Chua NH. Combinatorial and synergistic properties of camv 35s enhancer subdomains. EMBO J. 1990;9:1685–1696. doi: 10.1002/j.1460-2075.1990.tb08292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besumbes O, Sauret-Gueto S, Phillips MA, Imperial S, Rodriguez-Concepcion M, Boronat A. Metabolic engineering of isoprenoid biosynthesis in Arabidopsis for the production of taxadiene, the first committed precursor of Taxol. Biotech Bioeng. 2004;88:168–175. doi: 10.1002/bit.20237. [DOI] [PubMed] [Google Scholar]
- Closa M, Vranova E, Bortolotti C, Bigler L, Arro M, Ferrer A, Gruissem W. The Arabidopsis thaliana FPP synthase isozymes have overlapping and specific functions in isoprenoid biosynthesis, and complete loss of FPP synthase activity causes early developmental arrest. Plant J. 2010;63:512–525. doi: 10.1111/j.1365-313X.2010.04253.x. [DOI] [PubMed] [Google Scholar]
- Clouse SD. Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell. 2011;23:1219–1230. doi: 10.1105/tpc.111.084475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennajdaoui H, Vachon G, Giacalone C, Besse I, Sallaud C, Herzog M, Tissier A. Trichome specific expression of the tobacco (Nicotiana sylvestris) cembratrien-ol synthase genes is controlled by both activating and repressing cisregions. Plant Mol Biol. 2010;73:673–685. doi: 10.1007/s11103-010-9648-x. [DOI] [PubMed] [Google Scholar]
- Gardner RG, Hampton RY. A highly conserved signal controls degradation of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase in eukaryotes. J Biol Chem. 1999;274:31671–31678. doi: 10.1074/jbc.274.44.31671. [DOI] [PubMed] [Google Scholar]
- Graham IA, Besser K, Blumer S, Branigan CA, Czechowski T, Elias L, Guterman I, Harvey D, Isaac PG, Khan AM, Larson TR, Li Y, Pawson T, Penfield T, Rae AM, Rathbone DA, Reid S, Ross J, Smallwood MF, Segura V, Townsend T, Vyas D, Winzer T, Bowles D. The genetic map of Artemisia annua l. identifies loci affecting yield of the antimalarial drug artemis-inin. Science. 2010;327:328–331. doi: 10.1126/science.1182612. [DOI] [PubMed] [Google Scholar]
- Harjes CE, Rocheford TR, Bai L, Brutnell TP, Kandianis CB, Sowinski SG, Stapleton AE, Vallabhaneni R, Williams M, Wurtzel ET, Yan JB, Buckler ES. Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science. 2008;319:330–333. doi: 10.1126/science.1150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker A, Kaplan F, Vaughan MM, Dafoe NJ, Ni X, Rocca JR, Alborn HT, Teal PEA, Schmelz EA. Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize. Plant Physiol. 2011;156:2082–2097. doi: 10.1104/pp.111.179457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappers IF, Aharoni A, van Herpen TWJM, Luckerhoff LLP, Dicke M, Bouwmeester HJ. Genetic engineering of terpenoid metabolism attracts, bodyguards to Arabidopsis. Science. 2005;309:2070–2072. doi: 10.1126/science.1116232. [DOI] [PubMed] [Google Scholar]
- Keeling CI, Bohlmann J. Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol. 2006;170:657–675. doi: 10.1111/j.1469-8137.2006.01716.x. [DOI] [PubMed] [Google Scholar]
- Kegge W, Pierik R. Biogenic volatile organic compounds and plant competition. Trends Plant Sci. 2010;15:126–132. doi: 10.1016/j.tplants.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Kim SW, Keasling JD. Metabolic engineering of the nonme-valonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotech Bioeng. 2001;72:408–415. doi: 10.1002/1097-0290(20000220)72:4<408::aid-bit1003>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kohlen W, Ruyter-Spira C, Bouwmeester HJ. Strigolactones: a new musician in the orchestra of plant hormones. Botany-Botanique. 2011;89:827–840. [Google Scholar]
- Krings U, Berger RG. Terpene bioconversion - How does its future look? Nat Prod Comm. 2010;5:1507–1522. [PubMed] [Google Scholar]
- Kumar S, Hahn FM, Baidoo E, Kahlon TS, Wood DF, McMahan CM, Cornish K, Keasling JD, Daniell H, Whalen MC. Remodeling the isoprenoid pathway in tobacco by expressing the cytoplasmic mevalonate pathway in chloroplasts. Metab Eng. 2012;14:19–28. doi: 10.1016/j.ymben.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Jeong JH, Seo JW, Shin CG, Kim YS, In JG, Yang DC, Yi JS, Choi YE. Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol. 2004;45:976–984. doi: 10.1093/pcp/pch126. [DOI] [PubMed] [Google Scholar]
- Lee DW, Lee S, Lee GJ, Lee KH, Kim S, Cheong GW, Hwang I. Functional characterization of sequence motifs in the transit peptide of Arabidopsis small subunit of Rubisco. Plant Physiol. 2006;140:466–483. doi: 10.1104/pp.105.074575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn E, Schalechet F, Wilkinson J, Matsui K, Tadmor Y, Nam KH, Amar O, Lastochkin E, Larkov O, Ravid U, Hiatt W, Gepstein S, Pichersky E. Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol. 2001;127:1256–1265. [PMC free article] [PubMed] [Google Scholar]
- Lucker J, Bouwmeester HJ, Schwab W, Blaas J, van der Plas LHW, Verhoeven HA. Expression of Clarkia S-linalool synthase in transgenic petunia plants results in the accumulation of S-linalyl-beta-D-glucopyranoside. Plant J. 2001;27:315–324. doi: 10.1046/j.1365-313x.2001.01097.x. [DOI] [PubMed] [Google Scholar]
- Mandel MA, Feldmann KA, HerreraEstrella L, RochaSosa M, Leon P. CLA1, a novel gene required for chloroplast development, is highly conserved in evolution. Plant J. 1996;9:649–658. doi: 10.1046/j.1365-313x.1996.9050649.x. [DOI] [PubMed] [Google Scholar]
- Manzano D, Fernandez-Busquets X, Schaller H, Gonzalez V, Boronat A, Arro M, Ferrer A. The metabolic imbalance underlying lesion formation in Arabidopsis thaliana overexpressing farnesyl diphosphate synthase (isoform 1S) leads to oxidative stress and is triggered by the developmental decline of endogenous HMGR activity. Planta. 2004;219:982–992. doi: 10.1007/s00425-004-1301-y. [DOI] [PubMed] [Google Scholar]
- Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotech. 2003;21:796–802. doi: 10.1038/nbt833. [DOI] [PubMed] [Google Scholar]
- Masferrer A, Arro M, Manzano D, Schaller H, Fernandez-Busquets X, Moncalean P, Fernandez B, Cunillera N, Boronat A, Ferrer A. Overexpression of Arabidopsis thaliana farnesyl diphosphate synthase (FPS1S) in transgenic Arabidopsis induces a cell death/senescence-like response and reduced cytokinin levels. Plant J. 2002;30:123–132. doi: 10.1046/j.1365-313x.2002.01273.x. [DOI] [PubMed] [Google Scholar]
- Munoz-Bertomeu J, Sales E, Ros R, Arrillaga I, Segura J. Up-regulation of an N-terminal truncated 3-hydroxy-3-methylglut-aryl CoA reductase enhances production of essential oils and sterols in transgenic Lavandula latifolia. Plant Biotech J. 2007;5:746–758. doi: 10.1111/j.1467-7652.2007.00286.x. [DOI] [PubMed] [Google Scholar]
- Niehaus TD, Okada S, Devarenne TP, Watt DS, Sviripa V, Chappell J. Identification of unique mechanisms for triterpene biosynthesis in Botryococcus braunii. Proc Natl Acad Sci USA. 2011;108:12260–12265. doi: 10.1073/pnas.1106222108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus T, Kinison S, Okada S, Yeo Y-s, Bell SA, Cui P, Devarenne TP, Chappell J. Functional identification of triterpene methyltransferases from Botryococcus braunii race B. J Biol Chem. 2012;287:8163–8173. doi: 10.1074/jbc.M111.316059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MT, Jones GA, Collins GB. Inheritance pattern for secreting and non-secreting glandular trichomes in tobacco. Crop Sci. 1982;22:1051–1053. [Google Scholar]
- Ohara K, Ujihara T, Endo T, Sato F, Yazaki K. Limonene production in tobacco with Perilla limonene synthase cDNA. J Exp Bot. 2003;54:2635–2642. doi: 10.1093/jxb/erg300. [DOI] [PubMed] [Google Scholar]
- Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- Sallaud C, Rontein D, Onillon S, Jabes F, Duffe P, Giacalone C, Thoraval S, Escoffier C, Herbette G, Leonhardt N, Causse M, Tissier A. A novel pathway for sesquiterpene biosynthesis from z, z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. Plant Cell. 2009;21:301–317. doi: 10.1105/tpc.107.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Crafts-Brandner SJ. Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol. 2004;134:1460–1470. doi: 10.1104/pp.103.038323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai S, Saito K. Triterpenoid biosynthesis and engineering in plants. Front Plant Sci. 2011;2:25. doi: 10.3389/fpls.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Dannert C, Umeno D, Arnold FH. Molecular breeding of carotenoid biosynthetic pathways. Nat Biotech. 2000;18:750–753. doi: 10.1038/77319. [DOI] [PubMed] [Google Scholar]
- Schwab W, Davidovich-Rikanati R, Lewinsohn E. Biosynthe-sis of plant-derived flavor compounds. Plant J. 2008;54:712–732. doi: 10.1111/j.1365-313X.2008.03446.x. [DOI] [PubMed] [Google Scholar]
- Seo JW, Jeong JH, Shin CG, Lo SC, Han SS, Yu KW, Harada E, Han JY, Choi YE. Overexpression of squalene synthase in Eleutherococcus senticosus increases phytosterol and triterpene accumulation. Phytochemistry. 2005;66:869–877. doi: 10.1016/j.phytochem.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Shelar DB, Shirote PJ. Natural product in drug discovery: back to future. Biomed Pharm J. 2011;4:141–146. [Google Scholar]
- Takahashi S, Yeo Y, Greenhagen BT, McMullin T, Song L, Maurina-Brunker J, Rosson R, Noel JP, Chappell J. Metabolic engineering of sesquiterpene metabolism in yeast. Biotech Bioeng. 2007;97:170–181. doi: 10.1002/bit.21216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarshis LC, Yan MJ, Poulter CD, Sacchettini JC. Crystal-structure of recombinant farnesyl diphosphate synthase at 2.6-angstrom resolution. Biochem. 1994;33:10871–10877. doi: 10.1021/bi00202a004. [DOI] [PubMed] [Google Scholar]
- Tholl D, Chen F, Petri J, Gershenzon J, Pichersky E. Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. Plant J. 2005;42:757–771. doi: 10.1111/j.1365-313X.2005.02417.x. [DOI] [PubMed] [Google Scholar]
- Verdaguer B, deKochko A, Beachy RN, Fauquet C. Isolation and expression in transgenic tobacco and rice plants, of the cassava vein mosaic virus (CVMV) promoter. Plant Mol Biol. 1996;31:1129–1139. doi: 10.1007/BF00040830. [DOI] [PubMed] [Google Scholar]
- Verdaguer B, de Kochko A, Fux CI, Beachy RN, Fauquet C. Functional organization of the cassava vein mosaic virus (CsVMV) promoter. Plant Mol Biol. 1998;37:1055–1067. doi: 10.1023/a:1006004819398. [DOI] [PubMed] [Google Scholar]
- Wang EM, Gan SS, Wagner GJ. Isolation and characterization of the CYP71D16 trichome-specific promoter from Nicotiana tabacum L. J Exp Bot. 2002;53:1891–1897. doi: 10.1093/jxb/erf054. [DOI] [PubMed] [Google Scholar]
- Wang EM, Hall JT, Wagner GJ. Transgenic Nicotiana tabacum L. with enhanced trichome exudate cembratrieneols has reduced aphid infestation in the field. Mol Breed. 2004;13:49–57. [Google Scholar]
- Wu SQ, Schalk M, Clark A, Miles RB, Coates R, Chappell J. Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nat Biotech. 2006;24:1441–1447. doi: 10.1038/nbt1251. [DOI] [PubMed] [Google Scholar]
- Zhang DL, Jennings SM, Robinson GW, Poulter CD. Yeast squalene synthase—expression, purification, and characterization of soluble recombinant enzyme. Arch Biochem Biophys. 1993;304:133–143. doi: 10.1006/abbi.1993.1331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


