Abstract
Oxidative stress causes transient actin cytoskeleton depolarization and also provokes vacuole fragmentation in wild-type cells. Under conditions of oxidative stress induced by hydrogen peroxide, the Slt2 protein is required to repolarize the actin cytoskeleton and to promote vacuole fusion. In this study, we show that grx3 grx4 and grx5 mutants are cellular models of endogenous oxidative stress. This stress is the result of alterations in iron homeostasis that lead to impairment of vacuolar function and also to disorganization of the actin cytoskeleton. Slt2 overexpression suppresses defects in vacuolar function and actin cytoskeleton organization in the grx3 grx4 mutant. Slt2 exerts this effect independently of the intracellular levels of reactive oxygen species (ROS) and of iron homeostasis. The deletion of SLT2 in the grx3 grx4 mutant results in synthetic lethality related to vacuolar function with substantial vacuole fragmentation. The observation that both Vps4 and Vps73 (two proteins related to vacuole sorting) suppress vacuole fragmentation and actin depolarization in the grx3 grx4 slt2 triple mutant strengthens the hypothesis that Slt2 plays a role in vacuole homeostasis related to actin dynamics. Here we show that in sod1, grx5, and grx3 grx4 slt2 mutants, all of which are affected by chronic oxidative stress, the overexpression of Slt2 favors vacuole fusion through a mechanism dependent on an active actin cytoskeleton.
INTRODUCTION
Oxidative stress is a common feature of cells living in aerobic environments. Saccharomyces cerevisiae is a model organism used to study the multiple cellular responses that take place in order to overcome oxidation (1–3). Reactive oxygen species (ROS) oxidize and damage all kinds of biological molecules (4). The presence of ROS alters normal cellular metabolism; therefore, cells must be ready to react rapidly to oxidation through early and late responses (5, 6). Mitogen-activating protein kinase (MAPK) cascades are among the early responses in charge of the rapid transmission of the oxidative signal to the nucleus and to other parts of the cell.
Among the different signaling pathways of Saccharomyces cerevisiae, the cell wall integrity (CWI) pathway plays an important role in the oxidative stress response (7–10). This pathway is constituted by a number of cell wall receptors (11–14) that transmit the signals to the GTPase Rho1. This protein, in turn, is regulated by different GDP/GTP exchange factor (GEF) proteins and GTPase-activating proteins (GAPs). Rom2 is the most relevant GEF involved in the oxidative response (15). Rho1 interacts with and activates the protein kinase C Pkc1 (12, 13). Pkc1 phosphorylates the MAPK module, whose first representative is the MAPK kinase kinase (MAPKKK) Bck1 (16). Bck1 activates the redundant MAPK kinases Mkk1 and Mkk1 (17) and finally phosphorylates Slt2/Mpk1, the last kinase of the pathway (18). The oxidative signal is sensed by Mtl1 and transmitted through Rom2 to the CWI (7, 9, 16) and to other pathways, such as the TOR and RAS-cyclic AMP pathways (9). Pkc1 is required for cell survival under oxidative stress, in part by remodeling of the actin cytoskeleton (7). Rho1 has recently been characterized to be a requirement for the oxidative stress responses in a manner associated with vacuole membrane fusion (19).
Cells also synthesize a number of proteins to detoxify ROS and to repair other possibly oxidized proteins. Among those proteins are the glutaredoxins, known to play a relevant role in reducing and repairing oxidized proteins (20). Grx3 and Grx4 are two monothiol glutaredoxins with a principal role in regulating the function of Aft1 (21, 22), a transcription factor involved in iron homeostasis (23, 24). The Grx3 and Grx4 redundant proteins also participate in actin dynamics independently of the function of Aft1. However, the absence of these two proteins leads to the accumulation of high levels of ROS as a result of iron homeostasis misregulation (25). Grx5 is another monothiol glutaredoxin located in the mitochondria and is required for the activity of iron/sulfur enzymes (26, 27). The absence of Grx5 promotes iron accumulation and oxidative damage (26, 27).
ROS might play different roles in the damaging or the signaling of molecules. Little is known about the specific roles that certain MAPKs play in the regulation of cellular functions with regard to oxidative stress. Many studies are based on exogenous oxidative stress; however, here we used S. cerevisiae yeast mutants with chronic oxidative stress as biological models of endogenous oxidative stress. Iron accumulates in cells as a result of the deregulation of iron homeostasis. Iron is a highly reactive metal that reacts with hydrogen peroxide in the cells, generating the hydroxyl radical through the so-called Fenton reaction (4). Consequently, defects in iron homeostasis provoke chronic oxidative stress that has occasionally been associated with vacuole fragmentation, as in the case of the sod1 mutant (28). In general, ROS have been associated with vacuole fragmentation (28). To characterize the roles that both Slt2 and Bck1 play in the oxidative response, we have used three different strains: grx3 grx4, grx5, and sod1 mutants. All of them present with chronic oxidative stress as a consequence of iron homeostasis deregulation. Our study suggests a role for the MAPK module of the CWI pathway in actin remodeling and vesicular trafficking under conditions of oxidative stress, promoting cell survival.
MATERIALS AND METHODS
Media, growth conditions, and chemicals.
Yeasts were grown at 25°C in Sabouraud dextrose (SD) minimum medium (2% glucose, 0.67% yeast nitrogen base, and the required amino acids) (29). Sporulation medium consisted of potassium acetate (1%) and yeast extract (0.1%). The final concentrations of the reagents used in the plates were as follows: hydrogen peroxide, 1 mM; calcofluor white, 20 μg/ml; Congo red, 6.5 μg/ml; and caffeine, 3 mM.
All of these reagents, along with dihydroethidium and manganese sulfate monohydrate, were purchased from Sigma. Dihydroethidium was prepared at 5 mg/ml in sterilized Milli-Q water. Latrunculin B (LatB; Molecular Probes) was prepared in dimethyl sulfoxide (DMSO) at a concentration of 100 mM.
Yeast strains and plasmids.
The strains with the wild-type, grx4, grx3, aft1, grx3 grx4, and grx3 grx4 aft1 genotypes and constructions have been described by Pujol-Carrion et al. (21). The grx5 strain was kindly provided by E. Herrero (MML100 in reference 27), whereas the sod1 strain (strain DJY118) was kindly provided by D. J. Jamieson (30). The grx3 grx4 slt2 triple mutant (strain GSL36) was constructed for this study as follows: the grx3 grx4 double mutant (21) was disrupted in the SLT2 locus by the one-step disruption method by using the LEU2 marker, as described by Vilella et al. (7).
Plasmid pBck1-20 is a pRS413 derivative bearing the constitutively activated BCK1-20 allele (16). Plasmid pSlt2 is a YEp352 derivative that contains the SLT2 open reading frame under the control of its own promoter and that is tagged with hemagglutinin at the C terminus. pSlt2 was kindly provided by Maria Molina (7). Plasmids carrying VPS73 and VPS4 were constructed by cloning these genes in the YEplac195 vector carrying URA3 as a marker and were a gift from Joaquim Ariño.
Cell viability.
We followed two different approaches to analyze cell viability. One of them consisted of the use of serial dilutions (dilution steps of 1/10) of cultures exponentially growing at an optical density at 600 nm of 0.6. The inoculum was 5 μl for each of the dilution steps plated onto SD medium or yeast extract-peptone-dextrose (YPD) plates containing reagents, where needed. The results are shown as the means of three independent experiments, each of which was conducted in triplicate. For the second approach, we plated 1,000 cells onto the corresponding plates and then incubated them at 30°C for 3 days. Upon growth, we considered 100% survival to be the number of colonies counted in untreated wild-type cultures for each of the experimental conditions used in this study. The viability of the mutants transformed with the empty vectors used in this study was scored as well. In all cases, the viability of the strains transformed with empty vectors was totally equivalent to that of the strains transformed with empty plasmids (data not shown for simplification).
Detection of ROS through quantification of superoxide anion.
For detection of ROS through quantification of superoxide anion, we basically used an adaptation of a previously described method (31, 32). Cells were grown to logarithmic phase in SD minimum medium with the required amino acids at 25°C. We collected 1 ml of culture for each time point. After centrifugation at 6,000 rpm for 4 min at room temperature, cells were resuspended in 1 ml of phosphate buffer containing 0.1% glucose. This suspension was then transferred to quartz cuvettes, to which dihydroethidine (DHE) was added to a final concentration of 5 μg/ml. We used an RF-5000 Shimadzu spectrophotometer to register the fluorescence released by ethidine upon the oxidation of the DHE. Readings were registered at 590 nm at 5-min intervals for 30 min. The wavelengths used for this assay were 520 nm for excitation and 590 nm for emission.
Actin staining.
Cells growing exponentially at 25°C were stained with rhodamine-phalloidin as previously described (33). For quantification, we counted 1,000 budded cells in which the bud was smaller than the mother cell. We considered the cells to be depolarized when no actin cables were microscopically detected. We repeated this experiment three times to obtain average values for the experiments, and the corresponding error bars are plotted in the histograms shown in the relevant figures.
Vacuole staining.
We used the FM4-64 dye purchased from Molecular Probes (4 mM stock solution in DMSO). We basically adapted the protocol described by Vida and Emr (34). We grew cells in SD medium to log phase. Samples of 1 ml were collected, centrifuged at 3,000 rpm for 5 min, and resuspended in 50 μl of fresh medium, to which 1 μl of FM4-64 was added. Samples were incubated at 30°C for 30 min and then washed 4 times with fresh medium by centrifuging at 3,000 rpm for 5 min. Finally, the pellet was resuspended in 50 μl of fresh medium for microscopic observation.
Internalization of lucifer yellow.
Lucifer yellow internalization allows the characterization of fluid-phase endocytosis. We followed the protocol described by Belmont et al. (35). Cells were exponentially grown in SD medium with amino acids at 25°C. Aliquots of 2 ml were collected and centrifuged at 3,000 rpm for 5 min at room temperature. Pellets were resuspended in 40 μl of fresh medium, to which 5 μl of lucifer yellow was added, and the mixture was incubated for 2 h at 30°C. After this period, samples were washed three times and resuspended in azide-succinate buffer (50 mM succinic acid, 20 mM NaN3, pH 5) at 4°C. Lucifer yellow was purchased from Molecular Probes and prepared in a stock solution at 40 mg/ml in sterile Milli-Q water.
Fusion protocol.
We used the strategy described by Jones et al. (36) to examine mutants that could be affected in vacuole fusion.
(i) Hypotonic shock.
Vacuoles were stained with the dye FM4-64 and subsequently centrifuged for 2 min at 3,000 rpm and washed twice in hypotonic medium (SD medium previously diluted 1/10 in Milli-Q water). The final pellet was resuspended in the same medium to be visualized under a fluorescence microscope.
(ii) Hypertonic shock.
In a second step, cells were washed twice with hypertonic medium (SD medium containing 0.4 M NaCl). Upon the last wash, the pellet was resuspended in hypertonic medium for observation under a microscope. The second hypotonic shock was performed identically to the first one. All the washes were carried out by centrifuging at 3,000 rpm for 2 min.
Yeast extraction and immunoblot analyses.
Both yeast extraction and immunoblot analyses were performed as described by Petkova et al. (9). The anti-carboxypeptidase Y (anti-CPY) monoclonal antibody was used at a 1:100 dilution in TBST (Tris-buffered saline-Tween 20) buffer containing 5% nonfat milk; as the secondary antibody, we used antimouse horseradish peroxidase-linked antibody (NA931V; Amersham Biosciences) at a 1:10,000 dilution in TBST buffer containing 0.25% nonfat milk. In all cases, chemiluminescent detection was performed using the SuperSignal substrate (Pierce) in a Lumi-Imager (Roche Molecular Biochemicals).
RNA preparation and Northern blot analyses.
RNA purification, Northern blotting, and probe labeling with digoxigenin were carried out as described by Gallego et al. (37). Probes covering the entire open reading frame, without adjacent sequences, were generated by PCR from genomic DNA.
RESULTS
Slt2 is required to remodel the actin cytoskeleton and vacuole morphology in the adaptive response to oxidative stress.
Our group has previously demonstrated that Pkc1, a protein member of the cell wall integrity (CWI) pathway, plays an essential role in cell survival in response to oxidative stress (7). One of the functions of this kinase is to favor actin repolarization and repolymerization under oxidative conditions (7, 8). In the context of the CWI pathway, Pkc1 signals to a MAPK module whose representative MAPK is Slt2. Therefore, we sought to determine whether Slt2 is also involved in the process of actin remodeling in response to oxidizing agents. We observed that slt2 cells growing exponentially presented an actin organization similar to that observed in wild-type cells (Fig. 1A). Hydrogen peroxide treatment provoked a marked actin cytoskeleton depolarization similar to that observed in wild-type cells. However, upon 3 h of treatment, wild-type cells became almost totally repolarized, with clear detection of actin cables, whereas in the slt2 mutant, actin repolarization was undetectable (Fig. 1A), even upon 6 h of treatment (not shown). These results suggest that Slt2 is also required for the correct repolarization of the actin cytoskeleton under conditions of oxidative stress.
Fig 1.
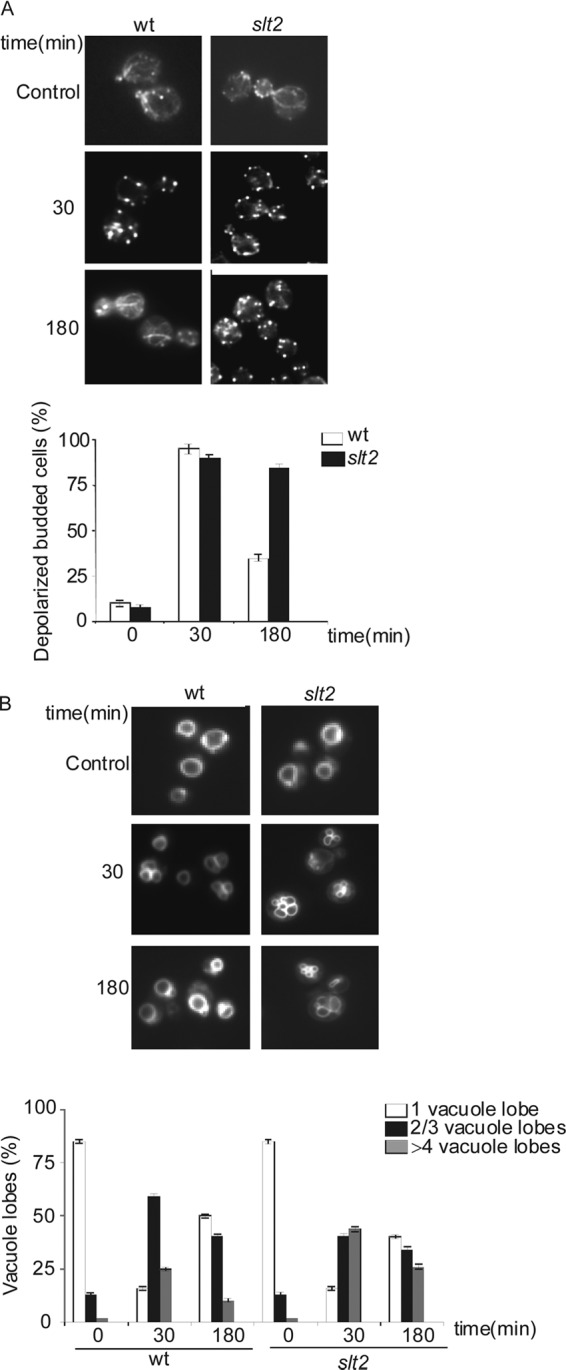
Slt2 is required to repolarize the actin cytoskeleton and also favors correct vacuole fusion under conditions of oxidative stress. Cells exponentially growing in SD complete medium were treated with 1 mM hydrogen peroxide for the indicated times. Samples were harvested and prepared for either actin (A) or vacuole (B) staining with the dye FM4-64. Five hundred cells were analyzed for each experiment. The histograms represent the averages of three independent experiments. The percentage of depolarized budded cells was plotted. Vacuole fragmentation was recorded as the number of cells that presented 1 normal vacuole or 2, 3, or 4 fragmented lobes. wt, wild type.
Actin mutations affect vacuole fusion (38). We observed that treatment with the oxidizing agent hydrogen peroxide provoked transient vacuole fragmentation in both the wild type and the slt2 mutant concomitantly with the transient depolarization of the actin cytoskeleton (Fig. 1B). In slt2 mutants, we could observe the maintenance of higher levels of vacuole fragmentation during the course of treatment, and again, this was correlated with the disorganization of the actin cytoskeleton (Fig. 1A and B). These results suggest a possible role for Slt2 kinase in actin dynamics related to vacuolar function under oxidative conditions.
The grx3 grx4, sod1, and grx5 mutants are cellular models of endogenous oxidative stress which present aberrant vacuole morphology and function connected to deregulation of iron homeostasis.
In order to better understand the function in the oxidative stress response that members of the CWI pathway could play, we decided to use yeast mutants as cellular models of endogenous oxidative stress. We selected grx3 grx4, sod1, and grx5 mutants. All of them share a characteristic: they present altered iron homeostasis which results in intracellular iron deposition and, consequently, the accumulation of ROS in the cells.
The sod1 mutant also presents vacuole fragmentation. This abnormal vacuole morphology has been described to be a consequence of iron homeostasis deregulation, leading to clear defects in vacuolar function (28). The grx5 mutant is deficient in the synthesis of Fe/S clusters and also accumulates high concentrations of iron (27, 28). Finally, the grx3 grx4 mutant is unable to regulate the proper location of Aft1, leading to upregulation of genes involved in iron internalization. This provokes accumulation of high concentrations of iron in the cells, constitutive oxidative stress, and actin cytoskeleton disorganization (22, 26). Given that Corson et al. (28) demonstrated that mutants affected in iron homeostasis presented ROS accumulation and vacuole alterations, we decided to study vacuole morphology and function in the grx3 grx4 double mutant. We observed that the grx3 grx4 double mutant presented an abnormal vacuole morphology compared to wild-type cells (Fig. 2A). Vacuoles are compartments required to store nutrients, to maintain pH homeostasis, and to store metals, calcium, and other cations (28, 39). Vacuolar function is important for cell survival when nutritional conditions are adverse, such as during stationary phase or in sporulation medium (40, 41). We observed that the grx3 grx4 mutant lost viability upon growth either in sporulation medium (a loss of viability of 60% relative to wild-type cells; Fig. 2B) or in SD medium at pH 2.5 or 7.5 (a reduction in viability of about 50% in comparison with wild-type cells; Fig. 2C). Wild-type cells were 100% viable under the same conditions (Fig. 2B and C). These results suggest that a defect in pH homeostasis is probably related to vacuolar function and that vacuolar function must be compromised upon the simultaneous absence of both Grx3 and Grx4.
Fig 2.
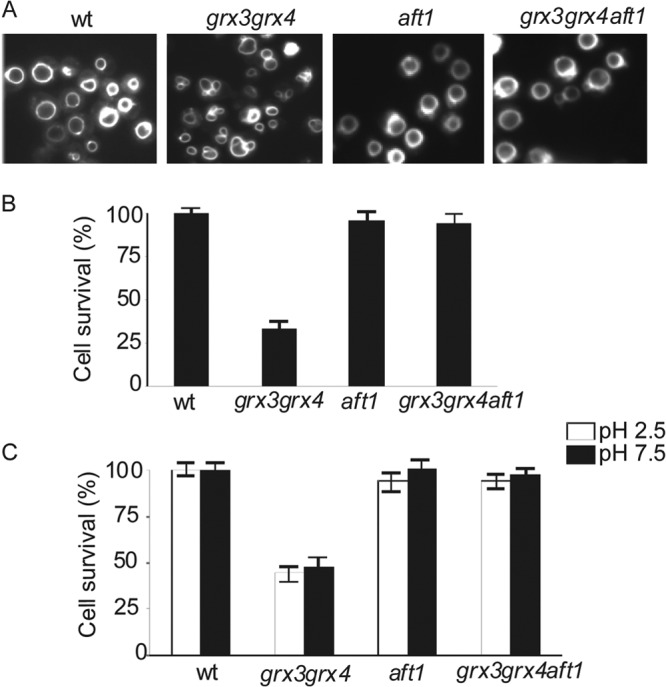
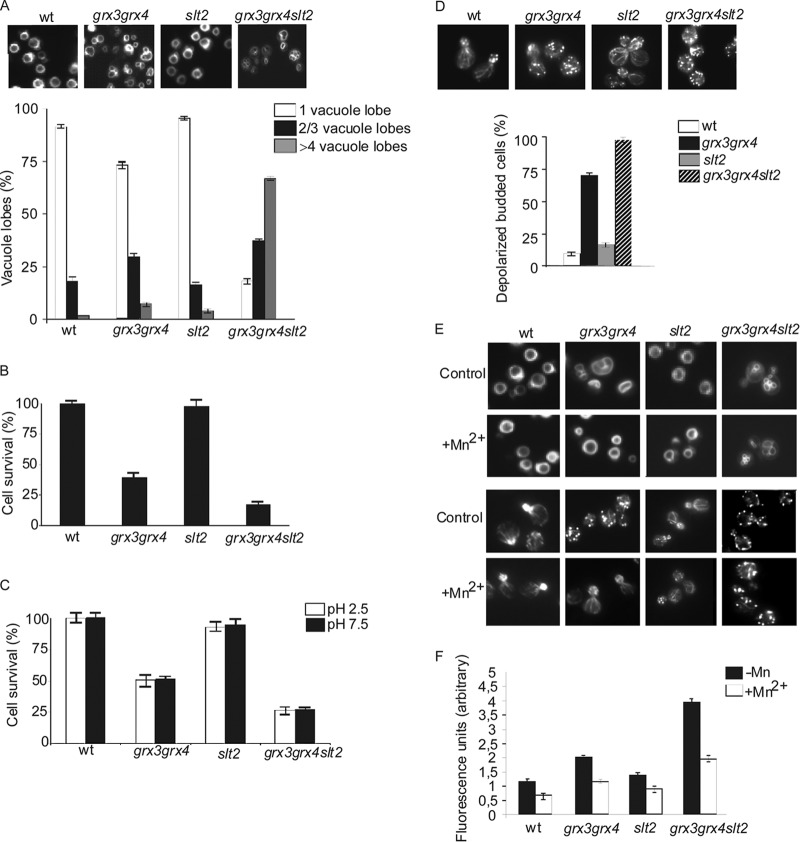
grx3 grx4 mutants present abnormal vacuole morphology and function due to deregulation of iron homeostasis. Cultures of wild-type, grx3 grx4, aft1, and grx3 grx4 aft1 strains were exponentially grown in SD complete medium. (A) Aliquots were collected and stained with the dye FM4-64 to visualize vacuole morphology. Cultures of each of the above-mentioned strains were exponentially grown in SD complete medium, and different aliquots were taken. One aliquot was transferred and incubated in sporulation medium for 7 days (B), whereas the other two aliquots were transferred to SD medium at low pH (pH 2.5) or high pH (pH 7.5), respectively, and grown for 1 day (C). Cell survival was scored upon plating 1,000 cells on YPD plates in triplicate (see Materials and Methods). Plates were incubated for 3 days. The control medium was SD medium at pH 5.2.
Because iron homeostasis is severely altered in the grx3 grx4 mutant and Corson et al. (28) have demonstrated that iron is involved in the aberrant vacuole morphology, we decided to investigate whether iron is the main cause of vacuole dysfunction in the grx3 grx4 double mutant. For this, we studied the grx3 grx4 aft1 triple mutant. This mutant does not accumulate iron in the cells and does not have high ROS levels either (26). As shown in Fig. 2A, the vacuole morphology and function of the grx3 grx4 aft1 mutant turned out to be the same as those of the wild type, as the triple mutant presented improved growth in sporulation medium (Fig. 2B) or in response to either alkaline or acidic pH (Fig. 2C). Consequently, we suggest that vacuole impairment in the grx3 grx4 mutant is mainly due to Fe accumulation caused by Aft1 misregulation. We also used ferrozine, an iron chelator, and obtained results identical to those described upon AFT1 deletion in the grx3 grx4 double mutant (not shown). We also wondered whether endogenous oxidative stress might provoke vacuole problems in the grx3 grx4 double mutant. In order to ascertain this, we treated cultures with MnSO4, which is known to be an efficient ROS chelator (28, 42). We have already demonstrated that MnSO4 treatment induced a remarkable decrease of ROS levels in the grx3 grx4 double mutant (26). Our results indicate that MnSO4 detoxifies the grx3 grx4 mutant of ROS concomitantly with restoration of the vacuole morphology so that it is similar to that in wild-type cells (see Fig. 5F). Therefore, we conclude that the vacuole defects of the grx3 grx4 mutant are mainly a consequence of iron and ROS accumulation in the cytoplasm. These results indicate that the grx3 grx4 mutant is an appropriate model to study endogenous oxidative stress caused by iron homeostasis misregulation.
Fig 5.
The absence of SLT2 in the grx3 grx4 mutant provokes vacuole fragmentation and exacerbates the impairment in vacuolar function and actin cytoskeleton depolarization of the grx3 grx4 double mutant. Cultures of grx3 grx4 slt2 mutant, grx3 grx4 mutant, slt2 mutant, and wild-type cells were exponentially grown in SD medium at 25°C. (A) One aliquot of each culture was harvested to observe and quantify vacuole morphology, as described in the legend to Fig. 2. The histograms reflect the number of cells with 1 normal vacuole or with 2, 3, or 4 fragmented lobes. Five hundred cells were counted per experiment. Other aliquots were used to determine cell survival in sporulation medium (B) or SD medium at high and low pH (C). (D) Aliquots were collected, fixed, and stained to analyze the actin cytoskeleton. Five hundred cells were counted to estimate the percentage of depolarized budded cells. (E) MnSO4 (2 mM) was added to cultures of the above-mentioned strains exponentially growing in SD medium at 25°C for 2 h, and the cultures were stained with FM4-64 to observe vacuole morphology. (F) Aliquots (1 ml) were removed from each culture to quantify ROS levels using the method described in Materials and Methods. The histograms represent arbitrary units of fluorescence, calculated as the slope of the graph registered, and represent the results of an average of three independent experiments.
Slt2 and Bck1, two kinases from the CWI pathway, suppress grx3 grx4 mutant defects in vacuole homeostasis and actin cytoskeleton organization.
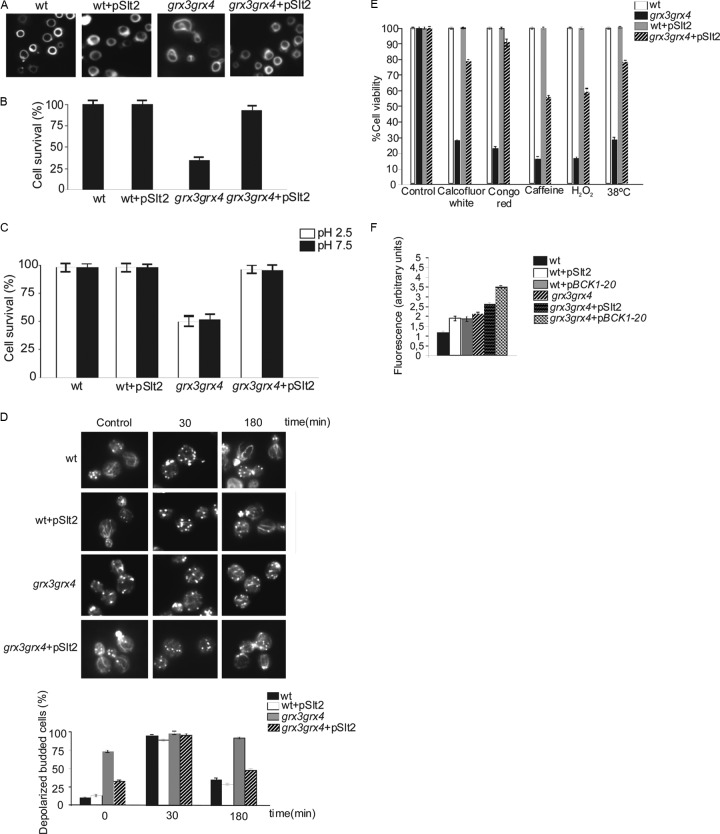
We next investigated the role that the MAPK module downstream of Pkc1 could play in vacuole homeostasis under oxidative stress conditions. To do this, we analyzed whether Slt2 is able to suppress some of the grx3 grx4 mutant phenotypes as a consequence of its endogenous oxidative stress. We observed that Slt2 overexpression was able to restore both vacuole morphology (Fig. 3A) and vacuolar function in the grx3 grx4 mutant, determined by measurement of cell viability in sporulation medium (Fig. 3B) or in medium with an acidic or alkaline pH (Fig. 3C). Slt2 overproduction was also capable of restoring the organization of the actin cytoskeleton (Fig. 3D). In addition, Slt2 also rescued grx3 grx4 mutant sensitivity to calcofluor white, Congo red, hydrogen peroxide, caffeine, and heat shock at 38°C (Fig. 3E). Slt2 restored the vacuole morphology (not shown) and actin cytoskeleton organization (Fig. 3D) in response to treatment with hydrogen peroxide, suggesting a relevant role for Slt2 in cell survival under conditions of endogenous oxidative stress. When we used the constitutively active allele BCK1-20, we obtained results equivalent to those shown for Slt2 (not shown).
Fig 3.
Activation of the MAPK module downstream of Pkc1 suppresses all the described phenotypic defects of the grx3 grx4 mutant, with the exception of cellular accumulation of iron and ROS. Cultures of wild-type cells and cells of the grx3 grx4 mutant transformed with a plasmid containing SLT2 or with the empty plasmid were grown as described in the legend to Fig. 2 to determine vacuole morphology (A) and function by means of the study of cell survival in sporulation medium (B) and in SD medium at low or high pH (C). (D) Aliquots from each culture were treated with 1 mM hydrogen peroxide for the indicated times and processed for actin staining. Five hundred cells were counted to determine the percentage of depolarized budded cells. Histograms represent the results of an average of three independent experiments. (E) Cultures of the same strains growing exponentially were serially diluted, and 1,000 cells were plated on the indicated media containing calcofluor white (20 μg/ml), Congo red (6.5 μg/ml), caffeine (3 mM), or H2O2 (1 mM) or were heat shocked at 38°C. All the cultures were grown at 30°C for 3 days. These experiments were performed in triplicate, as described in Materials and Methods. Error bars represent standard deviations. (F) Cultures of wild-type, wild-type(pSlt2), wild-type(pBck1-20), grx3 grx4 mutant, grx3 grx4(pSlt2) mutant, and grx3 grx4(pBck1-20) mutant cells were grown exponentially. Aliquots (1 ml) from each culture were collected to measure ROS levels using the dihydroethidine method, as described in Materials and Methods. The histograms represent arbitrary units of fluorescence, calculated as the slope of the graph registered, and represent the results of an average of three independent experiments.
We discarded the possibility that Slt2 detoxified grx3 grx4 mutant cells of the ROS because, far from suppressing ROS accumulation, both the Slt2 and Bck1 kinases caused severe increases of these species in both wild-type and grx3 grx4 mutant cells (Fig. 3F). Moreover, the Slt2 suppression function was not a consequence of iron homeostasis restoration, since upon Slt2 overexpression expression of FET3 and FIT3 (two representative genes regulated by Aft1) did not change in comparison to that observed in the grx3 grx4 double mutant (Fig. 4). We conclude that the role that Slt2 plays in the restoration of the vacuole morphology is not directly related to iron homeostasis. These results suggest that Slt2 plays a role in repairing vacuolar function through a mechanism that is directly dependent on actin dynamics in the grx3 grx4 mutant but independent of iron homeostasis and ROS accumulation. The relevance of the Slt2 function in the grx3 grx4 mutant is highlighted by the fact that the kinase is also able to restore the defects in viability that the double mutant presents upon treatment with different stress reagents (Fig. 3E). We suggest that in the grx3 grx4 mutant, the defective vacuolar function can be restored through at least two independent mechanisms. The first one takes place throughout the induction of actin cytoskeleton repolarization and dynamics, in a manner dependent on the activity of the CWI pathway and in the context of a high ROS concentration. The second one occurs by promoting the detoxification of ROS independently of the CWI pathway. Taking our results altogether, we suggest a function for Slt2 and the CWI pathway in vacuole homeostasis in the context of oxidative stress caused by iron accumulation.
Fig 4.
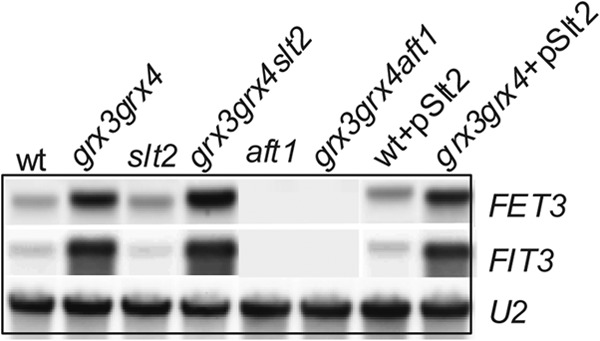
Exponentially growing cultures of the wild type, grx3 grx4 mutant, slt2 mutant, grx3 grx4 slt2 mutant, aft1 mutant, grx3 grx4 aft1 mutant, grx3 grx4(pSlt2) mutant, and wild type(pSlt2) were harvested and processed for Northern blot analysis. Northern blots were hybridized with FET3, FIT3, and U1 probes. U1 was used as a loading control.
Slt2 induces vacuole fusion in the grx3 grx4 slt2 mutant in a manner dependent on actin cytoskeleton dynamics and independent of iron or ROS detoxification.
We next decided to construct a grx3 grx4 slt2 triple mutant and to investigate both possible genetic interactions related to vacuole biogenesis and the role of Slt2 in vacuolar function.
We first screened the vacuole morphology and observed a remarkable fragmentation in the grx3 grx4 slt2 mutant (Fig. 5A). We next analyzed cell viability upon growth in the different media selected to test vacuolar function (sporulation medium, acidic and alkaline media) and in response to hydrogen peroxide treatment. We detected a remarkable loss of viability of the grx3 grx4 slt2 mutant compared to that of the grx3 grx4 double mutant (Fig. 3B and C and 5B and C), reflecting a pronounced loss of vacuolar function. Associated with these phenotypes was the increment in actin cytoskeleton depolarization (Fig. 5D) and the increase in ROS accumulation (Fig. 5F). Taken altogether, we can conclude that deletion of SLT2 in the grx3 grx4 mutant leads to synthetic lethality related to vacuole homeostasis, ROS accumulation, and disorganization of the actin cytoskeleton.
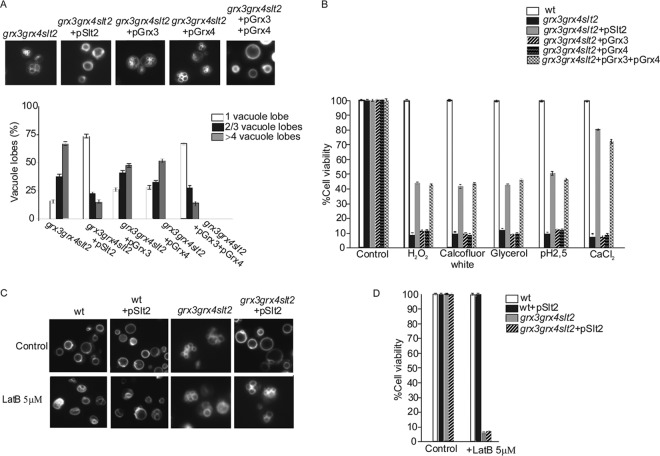
However, addition of MnSO4 neither completely reduced ROS accumulation (Fig. 5F) nor even restored vacuole biogenesis or actin dynamics in the grx3 grx4 slt2 triple mutant (Fig. 5E). Moreover, only Slt2 overexpression or the simultaneous overexpression of both Grx3 and Grx4 was able to restore both vacuolar function and morphology in the grx3 grx4 slt2 triple mutant (Fig. 6A and B). These results suggest a direct role for Slt2 in actin dynamics and vacuole organization under oxidative conditions and stress the relevance of Slt2 in oxidative defense.
Fig 6.
Only Slt2 and not Grx3 or Grx4 restored the defects in vacuole morphology and function of the grx3 grx4 slt2 triple mutant. (A) Cultures of the grx3 grx4 slt2, grx3 grx4 slt2(pSlt2), grx3 grx4 slt2(pGrx3), grx3 grx4 slt2(pGrx4), and grx3 grx4 slt2(pGrx4/pGrx3) strains were exponentially grown and stained with FM4-64 to visualize the vacuoles. (B) Serial dilutions of the above-indicated strains were performed, and 1,000 cells were spread onto SD medium plates at pH 2.5 or plates containing the following reagents: H2O2 (1 mM), calcofluor white (20 μg/ml), glycerol (2%), and CaCl2 (0.2 M). All the plates were incubated at 30°C for 3 days. (C) Exponentially growing cultures from the strains used for the experiment whose results are presented in panel A were incubated in SD medium plates in the presence or absence of 5 μM LatB for 1 day. After that, samples were removed and stained with FM4-64 to observe vacuolar morphology. (D) Cultures from the above-mentioned strains were exponentially grown in liquid SD medium, after which the cultures were serially diluted and 1,000 cells were plated in triplicate onto SD medium plates containing or not 5 μM LatB. Histograms represent the average of three independent experiments, and error bars represent standard deviations. Survival experiments were performed as described in Materials and Methods.
Therefore, Slt2 is required for vacuole fusion under conditions of oxidative stress. One question that arises is whether cell wall defects might be affecting vacuolar function. In order to ascertain this, we grew cells in the presence of sorbitol, which is known to be a cell wall stabilizer. Even under these conditions the triple mutant displayed vacuole fragmentation identical to that of the wild type (not shown). We next wondered whether actin polymerization was required for Slt2 to undergo efficient vacuolar fusion in the oxidative environment of the grx3 grx4 mutant. We treated cells with concentrations of latrunculin B that did not affect the vacuolar function or the viability of wild-type cells. Interestingly, upon the use of this reagent, neither SLT2 overexpression (Fig. 6C and D) nor BCK1-1 overexpression (not shown) was able to restore the vacuolar morphology or function in the grx3 grx4 slt2 mutant. Since the terminal phenotype of the grx3 grx4 slt2 triple mutant was pronounced vacuole fragmentation (Fig. 6A), we examined whether it could be due to either defects in vacuole inheritance or, alternatively, problems with vacuole fusion. We observed that the grx3 grx4 slt2 mutant was not deficient in vacuolar inheritance (see Fig. S1 in the supplemental material). We next decided to check whether vacuole fragmentation was due to defects in vacuole fusion. For that, we used a simple assay, proposed by Jones et al. (36), based on the observation that hypotonic stress triggers rapid in vivo vacuole fusion (43), whereas hypertonic stress induces in vivo vacuole fission (44). As shown in Fig. 7A and B, the triple mutant displayed a clear defect in vacuole fusion upon the second hypotonic stress compared to the wild type. These results suggest that Slt2 function is required for vacuole fusion under oxidative conditions and this process is dependent on correct actin cytoskeleton dynamics.
Fig 7.
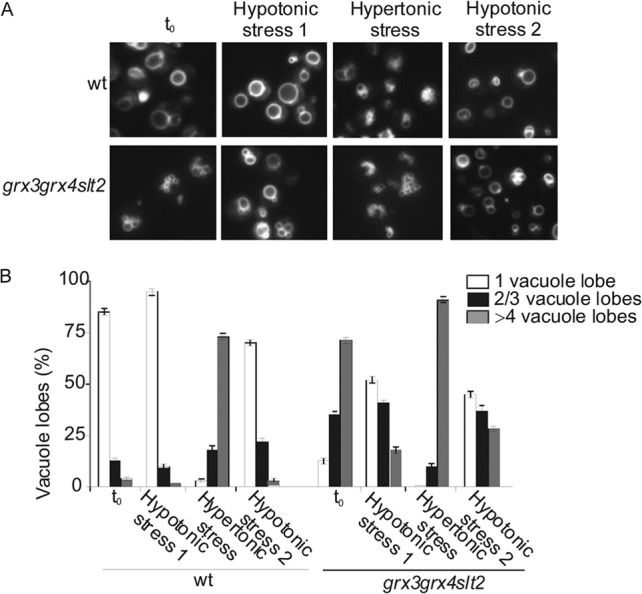
Deletion of Slt2 in the grx3 grx4 double mutant affects vacuole fusion. (A) Vacuole morphology was quantified in the wild-type and grx3 grx4 slt2 strains grown in SD medium with amino acids after hypotonic stress via 1/10 dilution in water, after hypertonic stress via addition of NaCl to 0.4 M, or after a second hypotonic stress treatment. t0, time zero. (B) Five hundred cells were scored in three independent experiments. The histograms reflect the numbers of cells with 1 normal vacuole or with 2, 3, or 4 fragmented lobes.
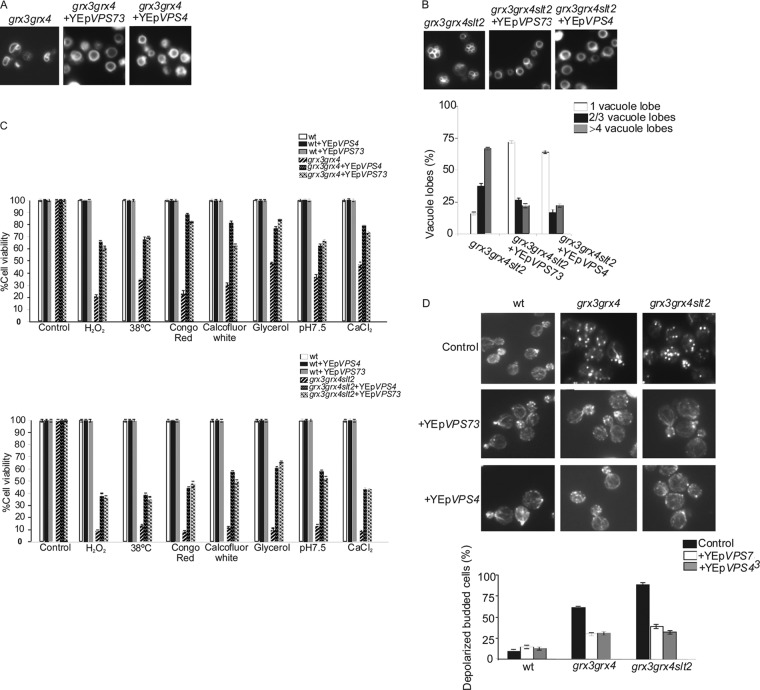
Both the Vps4 and the Vps73 proteins, involved in vacuolar protein sorting, rescue the alterations in vacuolar function and actin cytoskeleton observed in both the grx3 grx4 and grx3 grx4 slt2 mutants.
In order to investigate whether the grx3 grx4 mutant and especially the grx3 grx4 slt2 mutant could have a defect in vesicular traffic and sorting which could explain their altered vacuolar morphology, we transformed the mutants with plasmids harboring the VPS73 and VPS4 genes. Vps73 is a mitochondrial protein involved in vacuolar protein sorting, whereas Vps4 is involved in multivesicular (MVS) body protein sorting. Both proteins were able to restore the vacuolar morphology to wild-type levels (Fig. 8A and B) in both the grx3 grx4 and grx3 grx4 slt2 mutants, as well as the defects in cell viability of these mutants in response to different stresses (Fig. 8C). One interesting observation was that both Vps4 and Vps73 were able to suppress the abnormal actin cytoskeleton organization in both mutants (Fig. 8D). As in the case of Slt2 protein, neither of the Vps proteins tested in this study was able to reduce ROS accumulation in both the grx3 grx4 and the grx3 grx4 slt2 mutants (not shown). These results demonstrate that both the grx3 grx4 and the grx3 grx4 slt2 mutants present a defect in actin remodeling (Fig. 8D) that is tightly associated with vacuole morphogenesis. This defect is much more pronounced in the absence of Slt2 function. These results strengthen our hypothesis of the role of Slt2 in vacuole and actin functions and potentially connect Slt2 with MVS protein sorting under conditions of oxidative stress.
Fig 8.
The vacuole proteins Vps4 and Vps73 suppress grx3 grx4 and grx3 grx4 slt2 mutant phenotypic defects associated with vacuole morphology and function and also with the organization of the actin cytoskeleton. (A and B) Exponentially growing cultures of the grx3 grx4 and grx3 grx4 slt2 mutants transformed with plasmids harboring either the VPS4 or VPS73 gene were used to analyze vacuole morphology, as described in the legend to Fig. 2. Samples from these cultures were serially diluted, and subsequently, 1,000 cells were spotted onto plates containing different reagents (C) and processed for actin staining (D). Cell viability was calculated as described in Materials and Methods and in the legend to Fig. 6D. Actin staining was performed as described in the legend to Fig. 1A.
The grx3 grx4 slt2 triple mutant is not significantly defective in the process of endocytosis or secretion.
The absence of the Slt2 function in the grx3 grx4 mutant provoked marked vacuole fragmentation, reminiscent of that seen for certain vacuolar mutants (45), in which processing of CPY was altered. For this reason, we decided to analyze CPY maturation in the slt2, grx3 grx4, and grx3 grx4 slt2 strains, and we observed that all of them presented levels of the mature form of CPY equivalent to those determined in wild-type cells (Fig. 9A). These results ruled out potential defects in the secretory pathway to be the main cause of vacuole fragmentation or of the impairment in vacuolar function detected in the triple mutant. Alternatively, we considered that the process of endocytosis could be affected, thus explaining some of the phenotypes of the grx3 grx4 slt2 mutant. We checked endocytosis by analyzing the internalization of lucifer yellow, and we observed that the triple mutant internalized the dye as efficiently as the wild type. Consequently, we also discarded endocytosis as a function affected in the slt2, grx3 grx4, and grx3 grx4 slt2 mutants (Fig. 9B).
Fig 9.
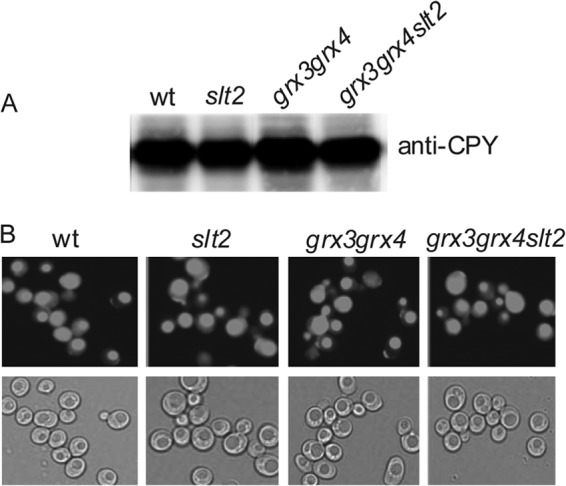
Chronic oxidative stress caused by iron accumulation and the lack of Slt2 function does not affect CPY maturation. Cultures from the wild-type, slt2, grx3 grx4, and grx3 grx4 slt2 strains were grown to log phase in SD medium with amino acids at 25°C. (A) Samples were collected to prepare total protein extracts, and the extracts were analyzed by Western blotting and detected with anti-CPY antibody, as described in Materials and Methods. (B) Aliquots were collected and were treated with the dye lucifer yellow (see Materials and Methods) to detect defects in endocytosis.
Slt2 and Bck1 also rescue vacuolar problems in grx5 and sod1 mutants through a mechanism dependent on actin dynamics.
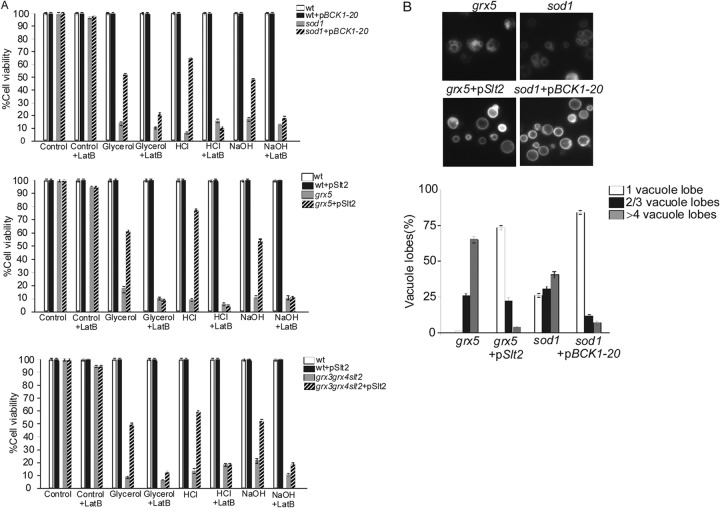
We decided to investigate whether Slt2 and Bck1 also promote vacuole fusion and increase cell survival in the other two mutants selected to be cellular models of endogenous oxidative stress in this study. We observed that both the Slt2 and Bck1-20 proteins were able to restore sod1 and grx5 viability in the different culture media used to check vacuolar function in this study (Fig. 10A). Upon Slt2 and Bck1-20 overexpression, we observed restoration of vacuole morphology concomitantly with the restoration of vacuolar function (Fig. 10A and B). These results indicate that proteins of the CWI pathway are also capable to restore vacuolar function and morphology in other yeast mutants affected in iron homeostasis. When we analyzed the organization of the actin cytoskeleton, we observed that the sod1 mutant presented a normal organization, whereas the grx5 mutant presented a depolarized actin cytoskeleton. The Slt2 or Bck1-20 protein did not induce actin cable formation in the grx5 mutants (not shown). Actin cables represent the highest level of actin polarization. However, the fact that we could not detect actin cables did not necessarily mean that Slt2 or Bck1-20 was not promoting a certain degree of actin polymerization. In order to elucidate this, we used a sublethal concentration of LatB (5 μM) that did not provoke any significant growth impairment in the wild-type strain. We observed that the usage of this agent precluded the restoration of vacuolar function and morphology in the grx3 grx4 slt2, grx5, or sod1 mutant by the function of Slt2 or Bck1 (Fig. 10A). These results revealed that both Slt2 and Bck1-20 activate vacuole fusion, restore vacuolar function, and induce actin polymerization in three mutants affected by endogenous oxidative stress provoked by abnormal iron accumulation.
Fig 10.
Slt2 and Bck1 promote vacuole fusion and cell survival in grx5 and sod1 mutants, both of which are affected by chronic oxidative stress. (A) Cultures of the grx3 grx4 slt2 and grx5 mutants were transformed with a plasmid harboring SLT2, whereas sod1 cells were transformed with a plasmid containing BCK1-1. These cultures were grown exponentially in SD medium. Samples were taken and serially diluted to plate 1,000 cells onto two parallel sets of plates, both of which contained each of the following reagents: glycerol as the sole carbon source and HCl (1 M) or NaOH (1.5 M). LatB (5 μM) was additionally added to only one set. Cells were grown for 3 days at 30°C. The histograms represent cell viability calculated as described in Materials and Methods and in the legend to Fig. 6D. (B) Cultures of the sod1, sod1(pBck1-20), grx5, and grx5(pSlt2) strains were stained with the dye FM4-64 to observe the vacuolar morphology. To quantify vacuole fragmentation, the results for 500 cells were recorded in three independent experiments.
DISCUSSION
In this study, we characterized a function for Slt2 in the oxidative response. Here we provide evidence demonstrating that Slt2 is involved in the vacuolar function associated with activation of actin polymerization. All these effects take place under conditions of endogenous oxidative stress provoked by deregulation of iron homeostasis. Slt2 and Bck1 kinases are able to rescue the alterations in vacuolar function and viability present in grx3 grx4, grx3 grx4 slt2, sod1, and grx5 mutants affected by chronic oxidative stress.
Loss of iron homeostasis regulation and the consequent ROS accumulation in cells provoke endogenous oxidative stress (21, 25–28). The vacuole is a very important organelle regarding iron homeostasis (46). Oxidative damage caused by compromised iron metabolism provokes an increase in vacuole iron transport (47). Vacuoles in yeast play a role equivalent to that played by lysosomes in human cells (48). Abnormal iron accumulation can lead to ROS production through the Fenton reaction (4). Both iron accumulation and oxidative stress provoke impairment in both the vacuolar function and morphology (28). In grx3 grx4 mutant cells, iron accumulates inside cells as a consequence of Aft1 misregulation (21), thus generating ROS, possibly through the Fenton reaction (25). In this study, we show that these ROS alter the vacuolar function.
This is in agreement with the observations published by Corson et al. (28), who elegantly demonstrated that in sod1 mutants, superoxide accumulation causes oxidative stress due to the fact that the ROS oxidized Fe/S proteins, releasing large amounts of iron to the cytoplasm.
Vacuole mutants present sensitivity to both acidic and alkaline media, to nutritional stress, and to the accumulation of metals and cations, and they also present severe growth problems when incubated in nonfermentable carbon sources (49–52). In this study, we show evidence demonstrating that the grx3 grx4, grx5, and, especially, grx3 grx4 slt2 mutants present high sensitivity to changes in the pH in the culture medium and that they lose viability when grown in nonfermentable carbon sources or in sporulation medium.
The grx3 grx4 (25), sod1 (28), and grx5 (26) mutants share some important characteristics: all of them present high levels of intracellular ROS, which is correlated with the deregulation of iron homeostasis and vacuole alterations.
Taking into consideration these characteristics, we decided to use the three mutants as cellular models of endogenous oxidative stress to study the role of Slt2 and Bck1 in the response to oxidation. We had previously described the role of the Pkc1 pathway in repolarizing and repolymerizing the actin cytoskeleton under oxidative stress conditions (7, 8). The sole absence of elements of the MAPK module does not provoke any evident problem either in actin organization or in vacuolar function in cells growing exponentially. However, the absence of Slt2 function (as already demonstrated for Pkc1 [7]) impinges upon actin cytoskeleton repolarization upon treatment with the oxidizing agent hydrogen peroxide. Here we present some evidence demonstrating that Slt2 and Bck1 participate in actin remodeling not only in response to exogenous oxidative stress but also under conditions of endogenous oxidative stress. Supporting this conclusion is the observation that these two MAPKs partly suppress other phenotypic defects detected in these mutants. Another important observation derived from this study is the involvement of Slt2 in vacuole fusion under oxidative conditions. Both Slt2 overexpression and the use of Bck1-20 partly suppress the phenotype of vacuole fragmentation in the sod1, grx3 grx4 slt2, and grx5 mutants. It is relevant to point out that this suppression is linked to actin dynamics, leading to a considerable increase in cell survival under conditions in which the vacuolar function is essential (53–56). This is not unexpected, since Eitzen et al. (38) demonstrated that mutants affected in actin dynamics present problems in vacuole morphology and/or vacuole fusion. In line with this, our results demonstrate that the grx3 grx4, grx3 grx4 slt2, and grx5 mutants (not shown) present evident defects in actin polarization. The fact that in all the mutants tested in this study (the grx3 grx4 slt2, sod1, and grx5 mutants) LatB inhibited Slt2 and Bck1-20 function in the restoration of both vacuole fusion and function suggests two interpretations: (i) vacuole fusion and actin dynamics are two processes simultaneously regulated by Slt2, and (ii) actin dynamics is required for Slt2 and Bck1 to regulate correct vacuole fusion and function under conditions of endogenous oxidative stress caused by iron accumulation. We propose that cross talk between iron homeostasis, vacuolar function, actin cytoskeleton organization, and oxidative status is likely.
Our results strengthen the theory that the CWI pathway plays a role in vacuole fusion under conditions of oxidative stress caused by iron accumulation. Supporting this hypothesis is the observation that the deletion of SLT2 in the grx3 grx4 double mutant provokes a severe defect in vacuolar function and fragmentation linked to actin disorganization. These defects in the grx3 grx4 slt2 mutant are not due to either ROS or iron homeostasis deregulation, since the use of antioxidants or iron chelators does not rescue the vacuole defects observed in the triple mutant. Again, in agreement with the data shown for sod1 by Corson et al. (28), our results indicate that the vacuole fragmentation observed in the grx3 grx4 or grx3 grx4 slt2 mutant is not associated with trafficking to the vacuole or with traffic assembly. In fact, some vps (vacuolar protein sorting) mutants are impaired in CPY maturation (45). Robinson et al. (57) demonstrated that the presence of immature CPY forms is associated with a compromised secretory pathway. However, this was not the case for the grx3 grx4 slt2 mutant, given that the vacuole fragmentation detected in this triple mutant was mainly a consequence of a defective process of fusion. In addition, Slt2 plays a role in promoting actin remodeling and vacuolar homeostasis by a mechanism different from and independent of the mechanism of either ROS detoxification or iron homeostasis restoration. In fact, Slt2 overexpression provokes ROS accumulation by promoting the increase of cellular oxidation in the context of iron levels higher than those detected in wild-type cells. Consequently, the activation of the CWI pathway can be an alternative mechanism to induce vacuole fusion and actin polymerization, thus promoting cell survival under specific conditions when cells cannot be detoxified of ROS and iron.
Cdc42 and Rho1 GTPases play an important role in vacuole fusion (58). The vacuole membrane fusion mechanism seems to be involved in the activation of both proteins (19). Rho proteins also participate in the process of vacuole fusion connected to actin remodeling (38, 59). Recently, a role for Rho1 in oxidative protection through a mechanism involving a physical interaction with a vacuole transporter has been reported (60). Other members of the Pkc1-MAPK pathway play a role in the oxidative stress response through regulatory signaling pathways, such as those described for Mtl1 (7, 9) or for Rom2 (15). The CWI pathway is also involved in the activation of actin cytoskeleton dynamics in the context of the oxidative stress response, as described for the Pkc1 protein (7, 8), which also affects the secretory machinery (10). Since Rho1 is upstream of Slt2 in the Pkc1-MAPK pathway, the observation that Slt2 plays a role in actin dynamics and vacuole fusion under oxidative conditions is not unexpected. Although it is yet to be demonstrated, Slt2 might receive the signal for this function from Rho1 and possibly through the CWI pathway.
The data that we present here do not allow us to establish which mechanism, either actin polymerization driving vacuole fusion or, alternatively, vacuole fusion driving actin polymerization, occurs first. Our results suggest that vacuole fusion and actin polymerization are processes tightly connected and might occur simultaneously under conditions of oxidative stress. The vacuole fragmentation observed in grx3 grx4 slt2 mutants is similar to that described for some class B vacuolar proteins (Vps) (53). In addition, we demonstrate that Vps4 (vacuolar protein sorting 4), a protein related to the multivesicular body sorting pathway (61), also partly restores vacuolar function in the grx3 grx4 mutant and promotes vacuolar function and fusion in the grx3 grx4 slt2 mutant. This mechanism occurs concomitantly with repolymerization of the actin cytoskeleton. Again, and similar to Slt2 and Bck1, Vps4 and Vps73 promote vacuolar fusion leading to a correct vacuolar function in association with actin remodeling but in a manner independent of either the ROS concentration or iron homeostasis. Taking together all the results shown in this study, we suggest the following model: the increase of ROS could damages vacuoles, inducing their fragmentation and, consequently, impairing their function. In the absence of Slt2 and in the presence of ROS, vacuole fragmentation increases. This leads to an inability of the cell to store iron, which increases oxidation and, consequently, ROS accumulation and, finally, provokes damage to cellular structures, such as actin (Fig. 11). Alternatively, Slt2 and CWI pathway activity are required to repolarize and repair the actin cytoskeleton in an oxidized environment (provoked by iron accumulation, as shown in this study). In line with this, actin dynamics would favor vacuole fusion and function, leading to correct cellular homeostasis and, hence, cellular detoxification of ROS and iron (Fig. 11). Our results are more in line with the second hypothesis, since Slt2 is unable to suppress vacuolar functions if actin dynamics become impaired by an external agent, such as LatB, as occurred with the mutants used in this study. In addition, we have observed that Pfy1 overexpression promotes vacuole fusion and rescues the lack of viability of the grx3 grx4 slt2 mutant when grown in the medium used to study vacuolar function. Interestingly, these effects disappeared upon the addition of sublethal concentrations of LatB (not shown). Profilin activates actin polymerization in yeast upon oxidative stress (8); therefore, our results are consistent with the hypothesis that a cellular system with endogenous oxidative stress caused by iron homeostasis deregulation has both its vacuolar function and actin cytoskeleton severely affected. Our results suggest the existence of two different molecular mechanisms to circumvent the problem: the first involves ROS detoxification and regulation of iron homeostasis, whereas the second one, which is independent of the first one, takes place through the activation of actin cytoskeleton dynamics concomitantly with the restoration of vacuole homeostasis. The latter mechanism is independent of the accumulation of iron and of the ROS concentration. Recently, Diab and Kane (62) published the findings of a study which provide interesting data linking vacuolar function, pH, oxidative stress, and Aft1 function. They demonstrate that vacuolar ATPase mutants regulate the Aft1 function in iron homeostasis, with a consequent effect on endogenous oxidative stress. The results of that study are in accordance with our results and with the models proposed here. The objective of our study was to show evidence indicating that two MAPKs, Slt2 and Bck1, both members of the CWI pathway, contribute to the second proposed mechanism and thus promote cell survival. Many questions remain open for future studies; one of them is the potential connection between the CWI pathway, Pfy, and Vps in these cellular responses to endogenous oxidative stress.
Fig 11.
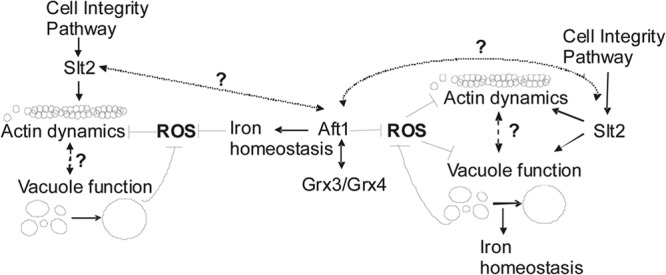
Our results suggest the existence of two possible models linking oxidative stress, vacuolar function, iron homeostasis, and the CWI pathway.
Supplementary Material
ACKNOWLEDGMENTS
We thank Enrique Herrero for kindly providing the grx5 strain. We are very grateful to Joaquim Ariño for plasmids YEplac195-Vps73 and YEplac195-Vps4 and to Maria Molina for plasmid pSlt2.
This research was supported by grants BFU2009-11215 and BFU2012-31407 from the MEC (Spanish Government). Nuria Pujol-Carrion was supported by a contract associated with grant BFU2009-11215, and Mima I. Petkova was supported by a fellowship from the Generalitat de Catalunya (Spain) and a contract funded by grant 2009SGR196 from the Generalitat de Catalunya (Spain).
Footnotes
Published ahead of print 16 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01692-13.
REFERENCES
- 1.Moradas-Ferreira P, Costa V. 2000. Adaptive response of the yeast Saccharomyces cerevisiae to reactive oxygen species: defences, damage and death. Redox Rep. 5:277–285 [DOI] [PubMed] [Google Scholar]
- 2.Costa V, Quintanilha A, Moradas-Ferreira P. 2007. Protein oxidation, repair mechanisms and proteolysis in Saccharomyces cerevisiae. UBMB Life 59:293–298 [DOI] [PubMed] [Google Scholar]
- 3.de la Torre-Ruiz MA, Mozo-Villarías A, Pujol N, Petkova MI. 2010. How budding yeast sense and transduce the oxidative stress signal and the impact in cell growth and morphogenesis. Curr. Protein Pept. Sci. 11:669–679 [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B, Gutteridge JMC. 1999. Free radicals in biology and medicine, 3rd ed. Clarendon Press, Oxford, United Kingdom [Google Scholar]
- 5.Costa V, Moradas-Ferreira P. 2001. Oxidative stress and signal transduction in Saccharomyces cerevisiae: insights into ageing, apoptosis and diseases. Mol. Aspects Med. 22:217–246 [DOI] [PubMed] [Google Scholar]
- 6.Toledano MB, Delaunay A, Biteau B, Spector D, Azevedo D. 2003. Oxidative stress responses in yeast, p 242–303 In Hohmann S, Mager WH. (ed), Topics in current genetics. 1. Yeast stress responses. Springer-Verlag, Berlin, Germany [Google Scholar]
- 7.Vilella F, Herrero E, Torres J, de la Torre Ruiz MA. 2005. Pkc1 and the elements of the cell integrity pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. J. Biol. Chem. 280:9149–9159 [DOI] [PubMed] [Google Scholar]
- 8.Pujol N, Bonet C, Vilella F, Petkova MI, Mozo-Villarías A, de la Torre-Ruiz MA. 2009. Two proteins from Saccharomyces cerevisiae: Pfy1 and Pkc1, play a dual role in activating actin polymerization and in increasing cell viability in the adaptive response to oxidative stress. FEMS Yeast Res. 9:1196–1207 [DOI] [PubMed] [Google Scholar]
- 9.Petkova MI, Pujol-Carrion N, Arroyo J, García-Cantalejo J, de la Torre-Ruiz MA. 2010. Mtl1 is required to activate general responses through Tor1 and Ras2 inhibition under conditions of glucose starvation and oxidative stress. J. Biol. Chem. 285:19521–19531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitjana FV, Petkova MI, Pujol-Carrion N, de la Torre-Ruiz MA. 2011. Pkc1 and actin polymerisation activities play a role in ribosomal gene repression associated with secretion impairment caused by oxidative stress. FEMS Yeast Res. 11:656–659 [DOI] [PubMed] [Google Scholar]
- 11.Verna J, Lodder A, Lee K, Vagts A, Ballester R. 1997. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 94:13804–13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ketela T, Green R, Bussey H. 1999. Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181:3330–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delley PA, Hall MN. 1999. Cell wall stress depolarises cell growth via hyperactivation of RHO1. J. Cell Biol. 147:163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petkova MI, Pujol-Carrion N, de la Torre-Ruiz MA. 2012. Mtl1 O-mannosylation mediated by both Pmt1 and Pmt2 is important for cell survival under oxidative conditions and TOR blockade. Fungal Genet. Biol. 49:903–914 [DOI] [PubMed] [Google Scholar]
- 15.Park JL, Collinson EJ, Grant CM, Dawes I. 2005. Rom2p, the Rho1 GTP/GDP exchange factor of Saccharomyces cerevisiae, can mediate stress responses via the Ras-cAMP pathway. J. Biol. Chem. 280:2529–2535 [DOI] [PubMed] [Google Scholar]
- 16.Lee KS, Levin DE. 1992. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol. Cell. Biol. 12:172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irie K, Takase M, Lee KS, Levin DE, Araki H, Matsumoto K, Oshima Y. 1993. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 13:3076–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres L, Martin H, Garcia-Saez MI, Arroyo J, Molina M, Sánchez M, Nombela C. 1991. A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signalling by protein kinase C. Mol. Microbiol. 5:2845–2854 [DOI] [PubMed] [Google Scholar]
- 19.Logan MR, Jones L, Eitzen G. 2010. Cdc42p and Rho1p are sequentially activated and mechanistically linked to vacuole membrane fusion. Biochem. Biophys. Res. Comm. 394:64–69 [DOI] [PubMed] [Google Scholar]
- 20.Herrero E, de la Torre-Ruiz MA. 2007. Monothiol glutaredoxins: a common domain for multiple functions. Cell. Mol. Life Sci. 64:1518–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pujol-Carrion N, Bellí G, Herrero E, Nogues A, de la Torre-Ruiz MA. 2006. Glutaredoxins Grx3 and Grx4 regulate nuclear localisation of Aft1 and the oxidative stress response in Saccharomyces cerevisiae. J. Cell Sci. 119:4554–4564 [DOI] [PubMed] [Google Scholar]
- 22.Ojeda L, Keller G, Mühlenhoff U, Rutherford JC, Lill R, Winge DR. 2006. Role of glutaredoxin-3 and glutaredoxin-4 in the iron-regulation of the Aft1 transcriptional activator in Saccharomyces cerevisiae J. Biol. Chem. 281:17661–17669 [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi-Iwai Y, Stearman R, Dancis A, Klausner RD. 1996. Iron-regulated DNA binding by the AFT1 protein controls the iron regulon in yeast. EMBO J. 15:3377–3384 [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi-Iwai Y, Ueta R, Fukunaka A, Sasaki R. 2002. Subcellular localisation of Aft1 transcription factor responds to iron status in Saccharomyces cerevisiae. J. Biol. Chem. 277:18914–18918 [DOI] [PubMed] [Google Scholar]
- 25.Pujol-Carrion N, de la Torre-Ruiz MA. 2010. Glutaredoxins Grx4 and Grx3 of Saccharomyces cerevisiae play a role in actin dynamics through their Trx domains, which contributes to oxidative stress resistance. Appl. Environ. Microbiol. 76:7826–7835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Manzaneque MT, Ros J, Cabiscol E, Sorribas A, Herrero E. 1999. Grx5 glutaredoxin plays a central role in protection against protein oxidative damage in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:8180–8190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodríguez-Manzaneque MT, Tamarit J, Bellí G, Ros J, Herrero E. 2002. Grx5 is a mitochondrial glutaredoxin required for the activity of iron/sulfur enzymes. Mol. Biol. Cell 13:1109–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corson LB, Folmer J, Strain JJ, Culotta VC, Cleveland DW. 1999. Oxidative stress and iron are implicated in fragmenting vacuoles of Saccharomyces cerevisiae lacking Cu, Zn-superoxide dismutase. J. Biol. Chem. 274:27590–27596 [DOI] [PubMed] [Google Scholar]
- 29.Kaiser C, Michaelis S, Mitchell A. 1994. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 30.Jamieson DJ, Rivers SL, Stephen DW. 1994. Analysis of Saccharomyces cerevisiae proteins induced by peroxide and superoxide stress. Microbiology 140:3277–3283 [DOI] [PubMed] [Google Scholar]
- 31.Bencsath FA, Shartava-Monteiro A, Goodman SR. 1996. Identification of the disulfide-linked peptide in irreversibly sickled cell beta-actin. Biochemistry 35:4403–4408 [DOI] [PubMed] [Google Scholar]
- 32.Aguilaniu H, Gustafsson L, Rigoulet M, Nyström T. 2003. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299:1751–1753 [DOI] [PubMed] [Google Scholar]
- 33.Torres J, Di Como CJ, Herrero E, de la Torre-Ruiz MA. 2002. Regulation of the cell integrity pathway by rapamycin sensitive TOR function in budding yeast. J. Biol. Chem. 277:42495–42504 [DOI] [PubMed] [Google Scholar]
- 34.Vida TA, Emr SD. 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128:779–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belmont LD, Patterson GM, Drubin DG. 1999. New actin mutants allow further characterization of the nucleotide binding cleft and drug binding sites. J. Cell Sci. 112:1325–1336 [DOI] [PubMed] [Google Scholar]
- 36.Jones L, Tedrick K, Baier A, Logan MR, Eitzen G. 2010. Cdc42p is activated during vacuole membrane fusion in a sterol-dependent subreaction of priming. J. Biol. Chem. 285:4298–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallego C, Garí E, Colomina N, Herrero E, Aldea M. 1997. The Cln3-cyclin is down-regulated by translational repression and degradation during the G1 arrest caused by nitrogen deprivation in budding yeast. EMBO J. 16:7196–7206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eitzen G, Wang L, Thorngren N, Wickner W. 2002. Remodeling of organelle-bound actin is required for yeast vacuole fusion. J. Cell Biol. 158:669–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claire-Lee S, Kane PM. 2009. The yeast lysosome-like vacuole: endpoint and crossroads. Biochim. Biophys. Acta 1793:650–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Achstetter T, Wolf DH. 1985. Proteinases, proteolysis and biological control in the yeast Saccharomyces cerevisiae. Yeast 1:139–157 [DOI] [PubMed] [Google Scholar]
- 41.Teichert U, Mechler B, Muller H, Wolf DH. 1989. Lysosomal (vacuolar) proteinases of yeast are essential catalysts for protein degradation, differentiation, and cell survival. J. Biol. Chem. 264:16037–16045 [PubMed] [Google Scholar]
- 42.Aguirre D, Culotta VC. 2012. Battles with iron: manganese in oxidative stress protection. J. Biol. Chem. 287:13541–13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang YX, Kauffman EJ, Duex JE, Weisman LS. 2001. Fusion of docked membranes requires the armadillo repeat protein Vac8p. J. Biol. Chem. 276:35133–35140 [DOI] [PubMed] [Google Scholar]
- 44.LaGrassa TJ, Ungermann C. 2005. The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J. Cell Biol. 168:401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonangelino CJ, Chavez EM, Bonifacino JS. 2002. Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol. Biol. Cell 13:2482–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raguzzi F, Lesuisse E, Crichton RR. 1988. Iron storage in Saccharomyces cerevisiae. FEBS Lett. 231:253–258 [DOI] [PubMed] [Google Scholar]
- 47.Li L, Murdock G, Bagley D, Jia X, Ward DM, Kaplan J. 2010. Genetic dissection of a mitochondria-vacuole signalling pathway in yeast reveals a link between chronic oxidative stress and vacuolar iron transport. J. Cell Biol. 285:10232–10242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostrowicz CW, Meiringer CT, Ungermann C. 2008. Yeast vacuole fusion: a model system for eukaryotic endomembrane dynamics. Autophagy 4:5–19 [DOI] [PubMed] [Google Scholar]
- 49.Nelson H, Nelson N. 1990. Disruption of genes encoding subunits of yeast vacuolar H(+)-ATPase causes conditional lethality. Proc. Natl. Acad. Sci. U. S. A. 87:3503–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohya Y, Umemoto N, Tanida I, Ohta A, Iida H, Anraku Y. 1991. Calcium-sensitive cls mutants of Saccharomyces cerevisiae showing a Pet− phenotype are ascribable to defects of vacuolar membrane H(+)-ATPase activity. J. Biol. Chem. 266:13971–13977 [PubMed] [Google Scholar]
- 51.Serrano R, Bernal D, Simon E, Ariño J. 2004. Copper and iron are the limiting factors for growth of the yeast Saccharomyces cerevisiae in an alkaline environment. J. Biol. Chem. 279:19698–19704 [DOI] [PubMed] [Google Scholar]
- 52.Kane PM. 2006. The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol. Mol. Biol. Rev. 70:177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banta LM, Robinson JS, Klionsky DJ, Erm SD. 1988. Organelle assembly in yeast: characterisation of yeast mutants defective in vacuolar biogenesis and protein sorting. J. Cell Biol. 107:1369–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klionsky DJ, Herman PK, Emr SD. 1990. The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev. 54:266–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Preston RA, Manolson MF, Becherer K, Weidenhammer E, Kirkpatrick D, Wright R, Jones EW. 1991. Isolation and characterization of PEP3, a gene required for vacuolar biogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:5801–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szczypka MS, Zhu Z, Silar P, Thiele DJ. 1997. Saccharomyces cerevisiae mutants altered in vacuole function are defective in copper detoxification and iron-responsive gene transcription. Yeast 13:1423–1435 [DOI] [PubMed] [Google Scholar]
- 57.Robinson JS, Klionsky DJ, Banta LM, Erm SD. 1988. Protein sorting in yeast: isolation of mutants defective in the processing and sorting of multiple vacuolar hydrolases. Mol. Cell. Biol. 8:4936–4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wickner W. 2002. Yeast vacuoles and membrane fusion pathways. EMBO J. 1241:1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isgandarova S, Jones L, Forsberg D, Loncar A, Dawson J, Tedrick K, Eitzen G. 2007. Stimulation of actin polymerization by vacuoles via Cdc42p-dependent signaling. J. Biol. Chem. 282:30466–30475 [DOI] [PubMed] [Google Scholar]
- 60.Lee ME, Singh K, Snider J, Shenoy A, Paumi CM, Stagljar I, Park HO. 2011. The Rho1 GTPase acts together with a vacuolar glutathione S-conjugate transporter to protect yeast cells from oxidative stress. Genetics 188:859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davies BA, Azmi IF, Katzmann DJ. 2009. Regulation of Vps4 ATPase activity by ESCRT-III. Biochem. Soc. Trans. 37:143–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diab HI, Kane PM. 2013. Loss of vacuolar H+-ATPase (V-ATPase) activity in yeast generates an iron deprivation signal that is moderated by induction of the peroxiredoxin TSA2. J. Biol. Chem. 288:11366–11377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.